Abstract
Background
Previous studies suggest that autonomic reactivity during encoding of emotional information could modulate the neural processes mediating mood-congruent memory. In this study, we use a point-process model to determine dynamic autonomic tone in response to negative emotions and its influence on long-term memory of major depressed subjects.
Methods
Forty-eight patients with major depression and 48 healthy controls were randomly assigned to either neutral or emotionally arousing audiovisual stimuli. An adaptive point-process algorithm was applied to compute instantaneous estimates of the spectral components of heart rate variability [Low frequency (LF), 0.04 to 0.15 Hz; High frequency (HF), 0.15 to 0.4 Hz]. Three days later subjects were submitted to a recall test.
Results
A significant increase in HF power was observed in depressed subjects in response to the emotionally arousing stimulus (p=0.03). The results of a multivariate analysis revealed that the HF power during the emotional segment of the stimulus was independently associated with the score of the recall test in depressed subjects, after adjusting for age, gender and educational level (Coef. 0.003, 95%CI, 0.0009-0.005, p=0.008).
Limitations
These results could only be interpreted as responses to elicitation of specific negative emotions, the relationship between HF changes and encoding/recall of positive stimuli should be further examined.
Conclusions
Alterations on parasympathetic response to emotion are involved in the mood-congruent cognitive bias observed in major depression. These findings are clinically relevant because it could constitute the mechanism by which depressed patients maintain maladaptive patterns of negative information processing that trigger and sustain depressed mood.
Keywords: Major depression, emotional memory, autonomic nervous system, vagus nerve, heart rate variability, point-process
INTRODUCTION1
Emotional events are preferentially encoded in mood states with a matching affective valence (Holland and Kensinger, 2010). For instance, a happy mood enhances retrieval of memories with positive attributes, while negative memories predominate in sad moods. This phenomenon is the basis for the negative bias in episodic memory observed in subjects with Major Depression (MD) (Matt et al., 1992).
The neurobiological mechanisms mediating the facilitation effects of emotion on episodic memory are only partly understood. Nevertheless, some studies have pointed out that activation of the autonomic nervous system (ANS) during encoding or retrieval of emotional information could modulate the neural processes mediating mood congruent memory (Critchley et al., 2013). Mood disorders have been previously associated with alterations in ANS functioning (Garcia-Gomez et al., 2007). Depressed subjects frequently present clinical symptoms related with autonomic dysfunction such as sleep pattern alterations, decreased appetite, gastrointestinal paresthesia and increased sweating (Tylee and Gandhi, 2005). In addition, multiple studies have reported decreased heart rate variability (HRV) and baroreflex sensitivity in these subjects (Kemp et al., 2010; Garcia et al., 2012). We hypothesize that in response to emotions with a negative valence, such as sadness or disgust, depressed subjects present alterations in ANS reactivity which could be one of the mechanisms for the increased encoding of negative events and the persistence of rumination associated to MD.
Since the ANS responds to emotional stimuli with rapid time-varying dynamics, computational tools able to discern these changes with high time resolution are the best candidates for providing optimal assessments. Standard HRV analysis does not comply with this requirement since it requires relatively long-time intervals (minutes) of electrocardiogram (ECG) acquisitions and is unable to detect rapid variations (European Society of Cardiology and North American Society of Pacing and Electrophysiology, 1996). To overcome these limitations, we propose the use of an effective statistical computational tool for the analysis of HRV series, based on the so-called point-process theory (Barbieri et al., 2005). Such a point-process analysis, in fact, represents a pioneering study in the field of mood assessment, as just being recently proposed for the characterization of heartbeat dynamics in patients with mood disorders (Valenza et al., 2014, 2015a, 2015b). The core of the model is the definition of the inter-heartbeat probability functions to predict the waiting time of the next heartbeat, i.e. the R- wave event, given a linear combination of the previous heartbeat events. In addition, the point-process model can be applied without using any prior interpolation method which might bias the HRV analysis (Barbieri et al., 2005). Finally, the model parameters are personalized and defined in an instantaneous fashion, and validated through a proper goodness of fit analysis (Barbieri et al., 2005).
In this study, we use this HRV point-process model to determine the relationship of dynamical vagal tone, as estimated through the instantaneous high frequency (HF) power, in response to negative emotions with the ability to encode and retrieve this information in depressed subjects compared to healthy controls.
METHODS
Subjects
Forty-eight Hispanic outpatients with MD (age: 22.6 ± 4.7 years) were included in the study. All subjects were screened from local universities by applying the Zung-self-rating depression scale (Zung, 1965). This scale is a 20-item questionnaire that measures the presence and severity of depressive symptomatology during the preceding two weeks. A score of 50/100 was considered compatible with a diagnosis of depression according to previous data in our population (Campo-Arias et al., 2006). A Spanish Structured Clinical Interview for DSM-IV Axis I disorders, Clinical version, was applied by qualified psychiatrists to confirm the MD diagnosis. All patients were experiencing their first depressive episode and had not received psychotherapeutic or pharmacological treatment. A control group consisting in 48 age- and gender-matched healthy control (HC) subjects was also included (age: 23.5 ± 4.9 years). Sixteen men (33.3%) and 32 women (66.6%) were included in each group. Exclusion criteria for both MD and HC subjects were known cardio-, cerebro-, or peripheral vascular disease, presence of neoplasm, diabetes mellitus, kidney or liver failure, infectious or systemic inflammatory disease and current neurological illness. All subjects received information about the study procedures and gave written informed consent approved by an Institutional Review Board.
Stimulus material
The stimulus used in the study consisted on a set of eleven slides accompanied by a narrative with two different versions: one emotionally neutral (N) and the other emotionally arousing (E). These stories had been previously adapted and validated in a Colombian sample (Oliveira et al., 2004) and were kept as close as possible to the originals (Cahill and McGaugh, 1995). Both set of slides show a mother taking her young son to see his father at a nearby hospital where he works. In the N version of the story, the mother and son witness a minor car accident, which attracts the attention of the child, whereas in the E version the child himself is critically injured and requires a surgical intervention at the hospital. The story's content can be divided into three phases, with the second period (slides 5-8) comprising the emotionally arousing elements (table 1). At the end of the presentation, each subject was asked to rank the story according to the emotional content (0 to 10, in which 0 corresponded to “not emotional” and 10 to “highly emotional”).
Table 1.
Narrative accompanying the slide presentation.
| Slide | Neutral version | Emotional version |
|---|---|---|
| 1. | A mother and her son are leaving home in the morning. | A mother and her son are leaving home in the morning. |
| 2. | She is taking him to visit his father's workplace | She is taking him to visit his father's workplace |
| 3. | The father is the chief laboratory technician at a nearby hospital. | The father is the chief laboratory technician at a nearby hospital. |
| 4. | They check before crossing a busy road. | They check before crossing a busy road. |
| 5. | While walking alone, the pass the scene of a minor accident, which the boy finds interesting. | While crossing the road, the boy is struck by a runway car, which critically injures him. |
| 6. | At the hospital, the staff are preparing for a practice disaster drill, which the boy will watch. | At the hospital, the staff prepare the emergency room, to which the boy is rushed. |
| 7. | All morning long, surgeons practiced the standard drill procedures. | All morning long, surgeons struggled to save the boy's life. |
| 8. | Special make-up artist were able to create realistic injuries on actors for the drill. | Specialized surgeons were able to succesfully re-atach the boy's severed feet. |
| 9. | After the drill, while the father stayed with the boy, the mother left to phone her other child's preeschool. | After the surgery, while the father stayed with the boy, the mother left to phone her other child's preschool. |
| 10. | Running late, she phones the preschool to tell them she will soon pick up her child. | Feeling distraught, she phones the preschool to tell them she will soon pick up her child. |
| 11. | Heading to pick up her child, she hails a taxi at the number nine bus stop. | Heading to pick up her child, she hails a taxi at the number nine bus stop. |
Procedures and recording of physiological variables
All recording sessions took place between 8 a.m. and 10 a.m. All tests were performed in a quiet, dimly lit room at a comfortable temperature (20–22°C). Participants abstained from smoking or consuming beverages containing caffeine, xanthines or alcohol the day before evaluation. Subjects from each group (MD, HC) were randomly assigned to the N or E stimulus version, resulting in 4 experimental groups (MD-N, MD-E, HC-N, HC-E). All subjects were told that the aim of the study was to evaluate how people pay attention to stories. It was explained that the slide presentation will be shown accompanied by a short narration. They were instructed to concentrate on each slide for the duration of its presentation and to watch the slide show as they would watch a movie. Images were presented on a 17 in. High-resolution color monitor at a rate of 10s per slide. Continuous ECG monitoring (lead II) was collected at 250Hz using a Finometer device (Finapress Medical System, The Netherlands). Data were digitized and stored in a PC computer using a signal acquisition system DATAQ 720-WINDAQ PRO (DataQ Instruments, Akron, OH, USA). Subjects were initially asked to rest for 10 minutes in a reclining position. This acclimation period was followed by a 3-minute baseline period and the exposure to the emotional stimulus previously described, followed by a 3-minute recovery period, during which participants rested quietly.
A second part of the experiment was performed three days later. On this occasion subjects were informed that their recall of the story they had seen would be tested. A multiple-choice questionnaire with 4-8 questions for each slide (65 in total) was administered. During this session the experimenter read the questions to the subjects and responses were recorded for a later analysis. A total score was calculated assigning one point for each correct response.
Heart Rate Variability Analysis
R-R series were detected from the ECG and corrected for ectopic beats using a previously developed algorithm (Citi et al., 2012). A point process algorithm (Barbieri et al., 2005) was applied to the R–R series to compute instantaneous estimates of mean R-R, and R-R standard deviation. Briefly, the stochastic structure of the R–R intervals is modeled as an inverse Gaussian renewal process where the R-R interval length is dependent on the recent history of parasympathetic and sympathetic inputs to the Sinoatrial node, and therefore is a function of the last p R-R intervals, where p is the model order.
The recursive, causal nature of the point-process estimation allows to predict each new heartbeat observation, given the previous history, independently at each iteration. The HRV point-process model and all its parameters are therefore also updated at each iteration. We determine the optimal model order p based on the Akaike Information Criterion and the model goodness-of-fit (obtained by prefitting the model to a subset of the data), which is based on the Kolmogorov-Smirnov (KS) test and associated KS statistic (Barbieri and Brown, 2006). Autocorrelation plots are also considered to test the independence of the model-transformed intervals. Once the order p is determined, the initial model coefficients are estimated by the method of least squares (Barbieri and Brown, 2006).
The point process algorithm is also able to separate the HRV dynamics in the classic spectral components [Low frequency (LF), 0.04 to 0.15 Hz; High frequency (HF), 0.15 to 0.4 Hz], updating their time-varying behavior at any time resolution (Barbieri and Brown, 2006). The spectral LF component is influenced by both cardiovagal and sympathetic activity, while the HF band is considered to be an index of parasympathetic modulation (European Society of Cardiology and North American Society of Pacing and Electrophysiology, 1996). After a preliminary evaluation of the KS statistics, a fixed order p=8 was chosen for the analysis of the spectral components, as well as the time domain measures, reported in this work. All indices were updated every 5 ms.
Statistical Analysis
A two-way ANOVA using a two group (MD vs HC) by 2 conditions (N vs E) design was used to evaluate differences in the emotional score in response to the test, and the score obtained in the recall session. Baseline characteristics of MD and HC subjects were compared using Student's t-test. To summarize the central tendency and dispersion, HRV features were averaged by using median and its respective absolute deviation (i.e. for a feature X, X = median(X) ± MAD(X) where MAD(X) =|X – median(X)|) for each phase of the emotional task (baseline, story, recovery). In addition, reactivity scores were used to calculate changes in spectral HF index during the segment of the story with most emotionally arousing elements (slides 5-8) (emotion HF index – baseline HF index) and during the recovery phase (recovery HF index – baseline HF index) in relation to baseline. A linear regression was performed to analyze the relationship between HF power during the emotional segment of the story and the recall session score adjusting by variables such as age, gender and educational level. Statistical significance was defined as p < 0.05. All analyses were conducted using Stata statistical software, release 13.0 (Stata Corporation, College Station, TX).
RESULTS
Emotional task results
Two-way ANOVA analysis showed a main effect of the version of the story in the rating of emotionality of the first session of the experiment (F1,92=15.18, p<0.001). Neither main group effect (F1,92=2.81, p=0.09) nor interaction group × version were identified (F1,92=0.68, p=0.41). Post-hoc analyses showed a higher emotionality rating in subjects with MD (6.4±2.1 vs 4.3± 2.8, p=0,005) and HC (6.8±1.9 vs 5.4± 1.5, p=0.01) exposed to the emotional version compared to those who observed the neutral version.
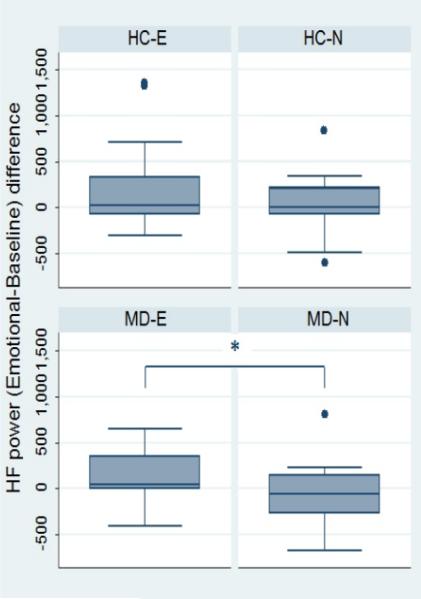
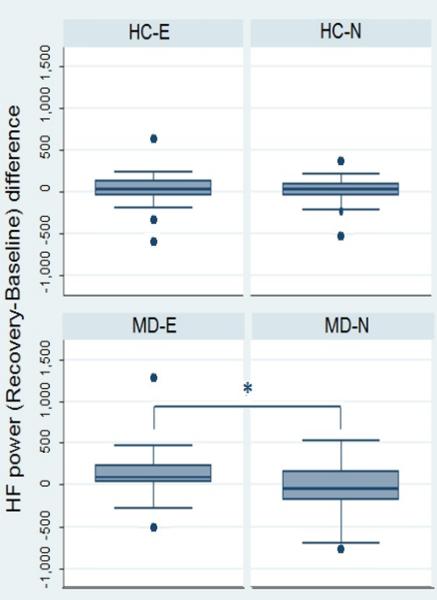
No significant differences between groups were observed for any of the standard HRV features during each phase of the emotional task (table 2). Figure 1 shows the resulting instantaneous HF power time series estimated from each one of the 4 experimental groups (MD-N, MD-E, HC-N, HC-E). These examples show an instantaneous average of the HF power along the whole experimental protocol timeline evidencing a steady increase in this feature during the audiovisual stimulus which is more pronounced and remains elevated for a longer time in subjects exposed to the emotional version of the story. This was confirmed in the analyses of the reactivity scores, which showed a significant higher increase of HF power in MD subjects in the emotional group compared to those on the neutral version of the test during the arousing segment of the audiovisual stimulus (figure 2) and the recovery phase (figure 3). These differences, however, were not significant between HC subgroups.
Table 2.
Results of Point Process spectral analyses during the emotional elicitation test
| Baseline | Story | Recovery | |
|---|---|---|---|
| RR (ms) | |||
| HC (N) | 756.8 ± 167.1 | 761.6 ± 178.4 | 787.5 ± 140.8 |
| HC (E) | 779.8 ± 143.4 | 809.8 ± 223.3 | 821.8 ± 177.2 |
| MD (N) | 785.8 ± 139.2 | 799.6 ± 122.2 | 801.9 ± 161.9 |
| MD (E) | 761.0 ± 127.3 | 802.4 ± 195.6 | 788.3 ± 127.0 |
| RR SD (ms) | |||
| HC (N) | 497.4 ± 590.1 | 325.8 ± 825.0 | 609.1 ± 620.9 |
| HC (E) | 898.7 ± 949.8 | 607.4 ± 1243.4 | 922.0 ± 668.4 |
| MD (N) | 625.5 ± 1038.8 | 539.9 ± 961.3 | 706.9 ± 948.1 |
| MD (E) | 478.1 ± 633.1 | 442.9 ± 657.3 | 672.8 ± 664.0 |
| LF power (ms2) | |||
| HC (N) | 571.2 ± 914.0 | 574.3 ± 700.82 | 760.8 ± 470.2 |
| HC (E) | 822.6 ± 1530.0 | 697.6 ± 1214.7 | 1097.7 ± 1889.4 |
| MD (N) | 715.2 ± 1248.8 | 822.2 ± 1039.5 | 1137.5 ± 1108.6 |
| MD (E) | 773.0 ± 898.6 | 728.4 ± 1419.5 | 1036.6 ± 1647.5 |
| LF/HF | |||
| HC (N) | 1.83 ± 2.62 | 2.22 ± 3.90 | 2.64 ± 3.13 |
| HC (E) | 2.17 ± 1.84 | 1.14 ± 1.70 | 2.18 ± 1.53 |
| MD (N) | 1.99 ± 2.66 | 2.10 ± 2.51 | 2.04 ± 4.51 |
| MD (E) | 3.10 ± 4.53 | 2.21 ± 1.81 | 2.49 ± 2.70 |
RR, RR interval; RR-SD, RR interval-standard deviation; LF, low frequency; HC, healthy control; MD, major depressive subject; N, neutral version; E, emotional version.
Figure 1.

Instantaneous HF power time series estimated for each one of the subgroups along the experimental protocol. HC, healthy controls; MD, depressed subjects.
Figure 2.
Reactivity scores for HF power during the emotional/neutral segment of the story compared with baseline. HC-E, healthy controls - emotional version; HC-N, healthy controls – neutral version; MD-E, depressed subjects – emotional version; MD-N, depressed subjects – neutral version. *p=0.01.
Figure 3.
Reactivity scores for HF power during the recovery phase of the test compared with baseline. HC-E, healthy controls - emotional version; HC-N, healthy controls – neutral version; MD-E, depressed subjects – emotional version; MD-N, depressed subjects – neutral version. *p=0.03.
Recall session results
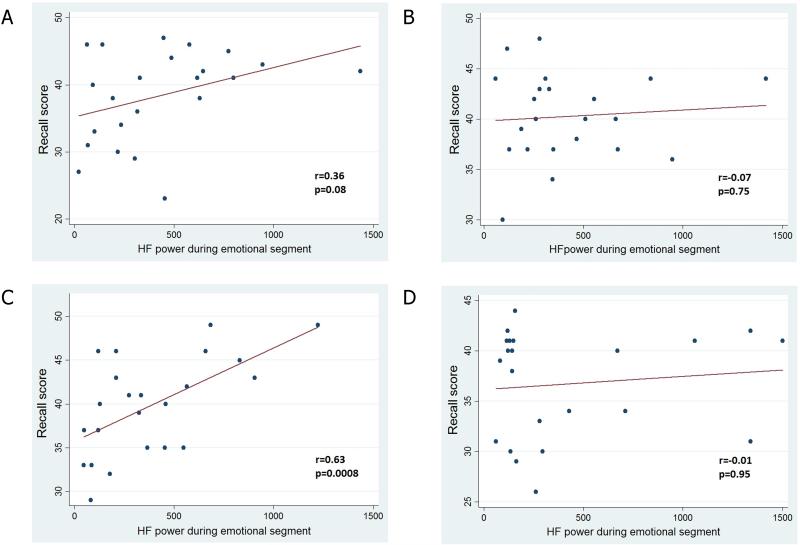
The analyses of the scores obtained in the recall session showed a significant main effect of the version of the story (F 1,92=6.17, p=0.01), with no significant main group effect (F1,92=0.31, p=0.58) or interaction group × version (F 1,92=0.49, p=0.48). Post-hoc analyses showed a higher recall score in subjects with MD (40.3±6.0 vs 36.6±5.4, p=0.03) exposed to the emotional version compared to those who observed the neutral version, with no significant differences in the HC group (40.1±4.1 vs 38.0±1.3, p=0.2). A correlation analysis showed a significant relationship between the HF power during the emotional segment of the story with the recall score of the memory task in depressed subjects, without any significant correlations in the other groups (Figure 4). The results of a multivariate analysis revealed that the HF power during the exposure to the emotional stimulus was independently associated with the score of the memory task in depressed subjects, after adjusting for age, gender and educational level (table 3).
Figure 4.
Correlation analyses between HF power during the emotional test and the recall score by subgroup. A, healthy controls- emotional version; B, healthy controls – neutral version; C, depressed subjects – emotional version; D, depressed subjects – neutral version.
Table 3.
Results of the linear regression analysis for the recall session score in depressed subjects exposed to the emotional version of the story
| Coef | t | p value | 95% CI | |
|---|---|---|---|---|
| HF power (ms2) | 0.003 | 2.96 | 0.008 | 0.0009; 0.005 |
| Age (years) | 0.02 | 0.09 | 0.931 | −0.52; 0.57 |
| Gender (male) | −2.43 | −1.00 | 0.332 | −7.55; 2.68 |
| Education (years) | 0.66 | 1.12 | 0.276 | −0.57; 1.89 |
HF, high frequency power.
DISCUSSION
The main finding of the study indicates that MD patients present an increased vagal tone in response to emotional stimuli with negative valence, which directly correlates with an enhanced declarative memory. These results suggest an important participation of the parasympathetic nervous system in the encoding of emotionally arousing material and the reported mood congruent memory bias observed in major depression. One strength of our study was the tight control of factors known to influence vagal activity. Thus, MD subjects with cardiovascular risk factors, comorbid psychiatric disorders or receiving psychotropic medications were excluded.
In this study, point-process modeling was chosen to perform reliable estimates of time-varying parasympathetic activity which was quantified through instantaneous HF power. Indeed, the experimental protocol foresaw a narrow time-window (30 seconds) characterizing the participants’ elicitation (neutral or emotional). Traditional parametric or nonparametric methods applied to heartbeat dynamics would have not been able to perform a reliable HF power estimation in such a short observation in time (Barbieri et al., 2005). Conversely, the point-process modeling is able to track the autonomic activity on the cardiovascular control in an instantaneous fashion (Citi et al., 2012). Several measures, which are defined in the time and frequency domains, were also derived from the time-varying point-process analysis of heartbeat dynamics. Namely, in the time domain we estimated the mean RR and its standard deviation, whereas in the frequency domain we estimated the LF power, the HF power, and the LF/HF ratio. As described in the experimental results section, coherent and significant results were obtained through HF estimates exclusively. Possible reasons of this finding can be related to the high specificity characterizing the dynamics of the time domain measures, especially true for the mean RR, and the unreliability of the LF power of being an index of sympathetic cardiac tone (Reyes del Paso et al., 2013). As a direct consequence, the LF/HF ratio would be unreliable as well.
Our results of a significant increase in HF value during the emotional task are consistent with some reports of autonomic reactivity to aversive audiovisual stimuli. Basic emotions have shown to be associated with distinct patterns of cardiorespiratory activity. For instance, some studies have found that the elicitation of disgust in relation to mutilation, injury and blood scenes, as the ones presented in our study, is characterized by a pattern of heart rate deceleration and increased vagal activation (Sokhadze, 2007; Shenhav and Mendes, 2014). Sokhadze (2007) in a study involving 29 undergraduate college students exposed to a visual stimulation with IAPS (International Affective Picture System) slides with mutilated bodies, reported an increase of HF power (0.1 ± 0.13 ms2 , p< 0.01) and decrease of LF/HF ratio ( −0.32 ± 0.42, p=0.018) compared to baseline. In another study, Shenhav and Mendes (2014) exposed 95 healthy participants to short film clips showing painful bodily injures such as visible leg fractures and found a significant heart rate deceleration and higher HF reactivity when compared to measurements obtained in response to neutral scenes. The authors concluded that the elicitation of disgust by painful-injury imagery is characterized by increased parasympathetic regulation of cardiac activity, which supports our results obtained in response to the emotional version of the story showing a child requiring surgical reattachment of his feet after a car accident. On the other hand, studies evaluating the effects of sadness elicitation have revealed two broad physiological patterns (Kreibig, 2010), an activating response characterized by increased cardiovascular sympathetic tone, reported in studies using personalized recall or directed facial action, and a deactivating response, characterized by sympathetic withdrawal, most commonly reported during tasks using standardized imagery, film material and audio excerpts. Our results are concordant with the reported second class of physiological response, given that we used standardized audiovisual material non-based on personal experiences to elicit sadness.
Depressed subjects exposed to the emotional version of the task were able to recall more elements of the story compared to those in the neutral group, being consistent with the theory of a cognitive bias in depression involving the preferential encoding and recall of negative over positive or neutral material (Matt et al., 1992). In addition, we observed a stronger relationship between vagal activation in MD subjects during the emotional task and the ability to recall elements from the story three days later, which was not significant in HC. These results point out to the participation of the parasympathetic nervous system in the modulation of mnemonic processes for negative material in major depression. One mechanism by which emotional material may have been better remembered could have been through enhanced attentional encoding during the task; this is reflected in the increased HF power observed in depressed subjects during the emotional segment of the story compared to those in the neutral version group. Furthermore, the persistence of an increased parasympathetic activation during the recovery session in depressed subjects in the emotional version group could also reflect difficulties in disengaging from this material once it captures initial attention (Gotlib and Joormann, 2010).
Separate neuroimaging studies have evidenced the participation of similar brain areas in the regulation of cardiac autonomic tone and encoding of emotional stimuli (Critchley et al., 2013). Several studies have consistently found heightened amygdala, insula and anterior cingulate cortex activation, regions that are crucial for the processing of emotional information, in clinically depressed patients relative to non-depressed subjects during the passive viewing of negatively valenced material (Lee et al., 2007; Mitterschiffthaler et al., 2008; Peluso et al., 2009), suggesting that biases in encoding of negative information in depression may be associated with functional abnormalities in these brain areas. In addition, sustained amygdala activation has been also associated with increased self-reported rumination, a key factor in the maintenance of depressed mood (Siegle et al., 2002). Interestingly, these areas have also been involved in the central modulation of parasympathetic function in response to emotion (Beissner et al., 2013).
According to Porges's Polivagal Theory (Porges, 1995; Porges, 2003), cardiac vagal reactivity is an important substrate for behavioral regulation in response to changing environmental demands. In this theory, the vagus nerve represents an integrated neural system that communicates in a bidirectional manner between the heart and the brain and could be involved in the regulation of mood and cognitive function (Porges, 2007). Approximately 80% of the vagal fibers are afferent and provide information regarding visceral state. This information is integrated in the nucleus tractus solitarius (NTS), which connects with higher brain regions known to modulate affect, such as the periaqueductal gray, amygdala, hypothalamus, insula, orbitofrontal cortex and anterior cingulate cortex (Bennarroch, 1993; Porges, 2007). Thus afferent feedback from the heart to the brain through the vagus and NTS could play a regulatory role in emotional response. In addition, neural pathways originating in these cortical areas project to NTS and medulary efferent (premotor) parasympathetic nuclei, including the nucleus ambiguus, regulating cardiac pacemaker activity via myelinated vagal pathways (Porges, 2007). On this context, we speculate that a decreased suppression of amygdala and insular cortex in major depression results in dysregulation of cardiac parasympathetic responses to negatively valenced stimuli. This altered peripheral response in turn increases afferent feedback to NTS and cortex, potentiating the arousal effect of emotional information, and subsequently enhancing memory encoding and facilitation of memory recall.
LIMITATIONS
It should be noted that our results could only be interpreted as responses to elicitation of specific negative emotions (e.g. sadness, disgust), the relationship between HF changes and encoding/recall of positive stimuli or other negative emotions might be quite different from what we observed, and should be examined separately. A second limitation is that, although the memory test used in the study was originally designed to administer the recall test two weeks after the presentation of the audiovisual paradigm (Cahill and McGaugh, 1995), we decreased this time gap to only three days because of the importance of evaluating the subjects' performance before receiving a significant dose of any antidepressant medication. This could have reduced the potential of the test in differentiating the two experimental conditions, but it does not affect the significance of the observed effects.
CONCLUSIONS
In conclusion, our results demonstrate the participation of the parasympathetic nervous system in the mood-congruent cognitive bias observed in major depression. These findings are clinically relevant because it could constitute the mechanism by which depressed patients maintain maladaptive patterns of negative information processing that trigger and sustain depressed mood. Future research should use functional neuroimaging to further clarify our understanding of the specific brain circuitry involved in the relationship between cardiac vagal modulation and the encoding of emotionally arousal material in depressed subjects.
Highlights.
We measured autonomic reactivity to negative emotions in depressed subjects
A point-process model was used to determine variations in dynamic autonomic tone
We determined the relationship between autonomic indices and long-term memory
Parasympathetic activity increased during the emotionally arousing stimuli
Vagal reactivity indices were associated with long-term memory performance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: ANS, autonomic nervous system; ECG, electrocardiogram; HC, healthy control; HF, high frequency; HRV, heart rate variability; KS, Kolmogorov-Smirnov; LF, low frequency; MAD, median absolute deviation; MD, major depression.
REFERENCES
- 1.Holland AC, Kensinger EA. Emotion and Autobiographical Memory. Phys. Life Rev. 2010;7:88–131. doi: 10.1016/j.plrev.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matt GE, Vázquez C, Campbell WK. Mood-congruent recall of affectively toned stimuli: A meta-analytic review. Clin. Psychol. Rev. 1992;12:227–255. [Google Scholar]
- 3.Critchley HD, Eccles J, Garfinkel SN. Interaction between cognition, emotion, and the autonomic nervous system. Handb. Clin. Neurol. 2013;117:59–77. doi: 10.1016/B978-0-444-53491-0.00006-7. [DOI] [PubMed] [Google Scholar]
- 4.García-Gómez RG, López-Jaramillo P, Tomaz C. [The role played by the autonomic nervous system in the relation between depression and cardiovascular disease]. Rev. Neurol. 2007;44:225–233. [PubMed] [Google Scholar]
- 5.Tylee A, Gandhi P. The importance of somatic symptoms in depression in primary care. Prim. Care Companion J. Clin. Psychiatry. 2005;7:167–176. doi: 10.4088/pcc.v07n0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol. Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Garcia RG, Zarruk JG, Guzman JC, Barrera C, Pinzon A, Trillos E, Lopez- Jaramillo P, Morillo CA, Maior RS, Diaz-Quijano FA, Tomaz C. Sex differences in cardiac autonomic function of depressed young adults. Biol. Psychol. 2012;90:179–185. doi: 10.1016/j.biopsycho.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 9.Barbieri R, Matten EC, Alabi AA, Brown EN. A point-process model of human heartbeat intervals: new definitions of heart rate and heart rate variability. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H424–435. doi: 10.1152/ajpheart.00482.2003. [DOI] [PubMed] [Google Scholar]
- 10.Valenza G, Citi L, Gentili C, Lanata A, Scilingo EP, Barbieri R. Characterization of depressive States in bipolar patients using wearable textile technology and instantaneous heart rate variability assessment. IEEE J. Biomed. Health Inform. 2015;19:263–274. doi: 10.1109/JBHI.2014.2307584. [DOI] [PubMed] [Google Scholar]
- 11.Valenza G, Citi L, Gentili C, Lanatá A, Scilingo EP, Barbieri R. Point-process nonlinear autonomic assessment of depressive states in bipolar patients. Methods Inf. Med. 2014;53:296–302. doi: 10.3414/ME13-02-0036. [DOI] [PubMed] [Google Scholar]
- 12.Valenza G, Garcia RG, Citi L, Scilingo EP, Tomaz CA, Barbieri R. Nonlinear digital signal processing in mental health: characterization of major depression using instantaneous entropy measures of heartbeat dynamics. Front. Physiol. 2015;6:74. doi: 10.3389/fphys.2015.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zung WW. A self-rating depression scale. Arch. Gen. Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 14.Campo-Arias A, Diaz-Martinez LA, Rueda-Jaimes G, Cadena L, del P, Hernandez NL. Validation of Zung's Self-rating Depression Scale among the Colombian general population. Soc. Behav. Personal. 2006;34:87–94. [Google Scholar]
- 15.Oliveira S.B. de, Garrido LMM, Cotes CAC, Sarmiento ELP, Tomaz CAB. Evaluación de la memoria declarativa asociada con contenido emocional en una muestra colombiana. Rev. Latinoam. Psicol. 2004;36:229–242. [Google Scholar]
- 16.Cahill L, McGaugh JL. A novel demonstration of enhanced memory associated with emotional arousal. Conscious Cogn. 1995;4:410–421. doi: 10.1006/ccog.1995.1048. [DOI] [PubMed] [Google Scholar]
- 17.Citi L, Brown EN, Barbieri R. A real-time automated point-process method for the detection and correction of erroneous and ectopic heartbeats. IEEE Trans. Biomed. Eng. 2012;59:2828–2837. doi: 10.1109/TBME.2012.2211356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbieri R, Brown EN. Analysis of heartbeat dynamics by point process adaptive filtering. IEEE Trans. Biomed. Eng. 2006;53:4–12. doi: 10.1109/tbme.2005.859779. [DOI] [PubMed] [Google Scholar]
- 19.Reyes del Paso GA, Langewitz W, Mulder LJM, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50:477–487. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- 20.Shenhav A, Mendes WB. Aiming for the stomach and hitting the heart: dissociable triggers and sources for disgust reactions. Emotion. 2014;14:301–309. doi: 10.1037/a0034644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokhadze EM. Effects of music on the recovery of autonomic and electrocortical activity after stress induced by aversive visual stimuli. Appl. Psychophysiol. Biofeedback. 2007;32:31–50. doi: 10.1007/s10484-007-9033-y. [DOI] [PubMed] [Google Scholar]
- 22.Kreibig SD. Autonomic nervous system activity in emotion: a review. Biol. Psychol. 2010;84:394–421. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu. Rev. Clin. Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peluso MAM, Glahn DC, Matsuo K, Monkul ES, Najt P, Zamarripa F, Li J, Lancaster JL, Fox PT, Gao JH, Soares JC. Amygdala hyperactivation in untreated depressed individuals. Psychiatry Res. 2009;173:158–161. doi: 10.1016/j.pscychresns.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitterschiffthaler MT, Williams SCR, Walsh ND, Cleare AJ, Donaldson C, Scott J, Fu CH. Neural basis of the emotional Stroop interference effect in major depression. Psychol. Med. 2008;38:247–256. doi: 10.1017/S0033291707001523. [DOI] [PubMed] [Google Scholar]
- 26.Lee BT, Whi Cho Seong, Soo Khang Hyung, Lee BC, Choi IG, Lyoo IK, Ham BJ. The neural substrates of affective processing toward positive and negative affective pictures in patients with major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:1487–1492. doi: 10.1016/j.pnpbp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol. Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 28.Beissner F, Meissner K, Bär KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 30.Porges SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiol. Behav. 2003;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 31.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 32.Porges SW. The Polyvagal Perspective. Biol. Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]