Abstract
Sirtuins (Sirt1–Sirt7) comprise a family of nicotinamide adenine dinucleotide (NAD+)-dependent enzymes. While deacetylation reflects their main task, some of them have deacylase, adenosine diphosphate-ribosylase, demalonylase, glutarylase, and desuccinylase properties. Activated upon caloric restriction and exercise, they control critical cellular processes in the nucleus, cytoplasm, and mitochondria to maintain metabolic homeostasis, reduce cellular damage and dampen inflammation—all of which serve to protect against a variety of age-related diseases, including cardiovascular pathologies. This review focuses on the cardiovascular effects of Sirt1, Sirt3, Sirt6, and Sirt7. Most is known about Sirt1. This deacetylase protects from endothelial dysfunction, atherothrombosis, diet-induced obesity, type 2 diabetes, liver steatosis, and myocardial infarction. Sirt3 provides beneficial effects in the context of left ventricular hypertrophy, cardiomyopathy, oxidative stress, metabolic homeostasis, and dyslipidaemia. Sirt6 is implicated in ameliorating dyslipidaemia, cellular senescence, and left ventricular hypertrophy. Sirt7 plays a role in lipid metabolism and cardiomyopathies. Most of these data were derived from experimental findings in genetically modified mice, where NFκB, Pcsk9, low-density lipoprotein-receptor, PPARγ, superoxide dismutase 2, poly[adenosine diphosphate-ribose] polymerase 1, and endothelial nitric oxide synthase were identified among others as crucial molecular targets and/or partners of sirtuins. Of note, there is translational evidence for a role of sirtuins in patients with endothelial dysfunction, type 1 or type 2 diabetes and longevity. Given the availability of specific Sirt1 activators or pan-sirtuin activators that boost levels of the sirtuin cofactor NAD+, we anticipate that this field will move quickly from bench to bedside.
Keywords: Sirtuins, Cardiovascular, Translational, Metabolism, Aging
Sirtuins: mediators of caloric restriction
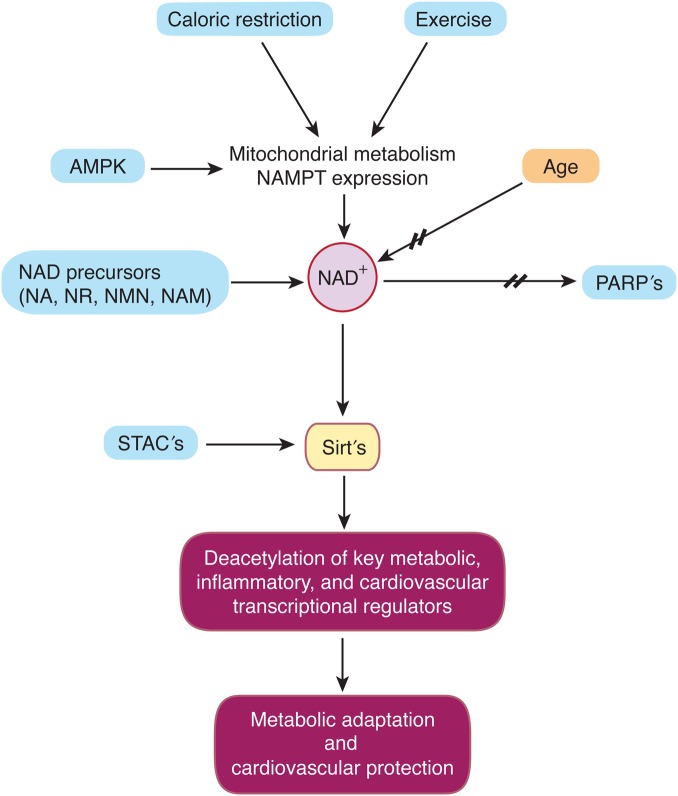
Silent Information Regulator 2 (SIR2) proteins—Sirtuins—are a family of histone deacetylases (HDACs) that catalyse deacetylation of both histone and non-histone lysine residues. Beyond deacetylase and deacylase activity, some sirtuins exert adenosine diphosphate (ADP)-ribosylase, demalonylase, desuccinylase, or glutarylase properties. Post-transcriptional modification of a wide range of protein targets of the seven mammalian orthologs (Sirt1–7) has been described in diverse settings. Their requirement for nicotinamide adenine dinucleotide (NAD+) distinguishes sirtuins from other HDAC classes and defines them as class III HDACs. This need of NAD+ for their enzymatic activity closely links sirtuins to the cellular energy status, increasing their activity at times of low energy availability such as caloric restriction and exercise (Figure 1).1–4
Figure 1.
Specific and unspecific sirtuin activation. Caloric restriction, exercise, and increased activity of 5′-adenosine monophosphate activated protein kinase drive mitochondrial metabolism and expression of nicotinamide phosphoribosyltransferase. Consecutive synthesis of nicotinamide adenine diphosphate activates sirtuins. Another route of sirtuin activation is increased nicotinamide adenine diphosphate biosynthesis via supplementation of nicotinamide adenine diphosphate precursors such as nicotinic acid, nicotinamide, nicotinamide mononucleotide, and nicotinamide riboside or through inhibition of nicotinamide adenine dinucleotide-consuming activities, such as poly-adenosine diphosphate-ribose-polymerases or CD38. Sirt1-activating compounds may mimic the effects of Sirt1. Stimulation of Sirt1 activity by these distinct pathways improves the capacity of the cells/organism for metabolic adaptation and/or cardiovascular protection.
To date, caloric restriction is the most robust intervention that has been reproducibly shown to prolong life span and maintain health in both invertebrates and vertebrates, including mammals.5,6 The first association between sirtuins and longevity in budding yeast 16 years ago7 sparked efforts in numerous laboratories to unravel the mechanisms underlying sirtuin-mediated prolongation of life span and elucidate their potential to postpone the onset of age-related diseases. In this context, the seven mammalian sirtuin orthologs have been studied in diverse disease models, including insulin resistance and diabetes, inflammation, neurodegenerative disease, cancer, and more recently, in cardiovascular pathologies such as cardiac hypertrophy, heart failure, endothelial dysfunction, and atherosclerosis.
This review focuses on the recent advances of sirtuin research in experimental cardiovascular disease and discusses their potential clinical applications (Table 1).
Table 1.
Cardiovascular and metabolic effects of sirtuins and pan-sirtuin activators; genetic and drug interventions: gain- and loss-of-function in animals and humans
| Sirtuin | Model | Key findings: mediators | Reference |
|---|---|---|---|
| Experimental | |||
| Sirt1 | Sirt1 LOF: ↑ angiogenesis; endothelial cells | Potente et al.25 | |
| Sirt1 | ApoE−/− | Sirt1 EC GOF: ↓ atherosclerosis | Zhang et al.12 |
| Sirt1 | ApoE−/− | Sirt1 LOF: ↑ atherosclerosis; foam cells; Lox1, NFkB | Stein et al.13 |
| Sirt1 | ApoE−/− | Sirt1 LOF: ↑ endothelial activation (VCAM-1), NFkB | Stein et al.14 |
| Sirt1 | WT | Sirt1 LOF: ↑ thrombosis; endothelial cells, NFkB | Breitenstein et al.20 |
| Sirt1 | LDLR−/− | Sirt1 GOF: ↑ atherosclerosis; SREB in liver | Qiang et al.23 |
| Sirt1 | ApoE−/− | Sirt1 GOF in VSMC: ↓ atherosclerosis | Gorenne et al.16 |
| Sirt1 | ApoE−/− | STAC (SRT3025): ↓ atherosclerosis, PCSK9, LDLR | Miranda et al.22 |
| Sirt3 | Sirt3 GOF: ↓ LVH | Sundaresan et al.39 | |
| Sirt3 | ApoE−/− | Sirt3 LOF: = atherosclerosis, ↓metabolic flexibility | Winnik et al.37 |
| Sirt3 | TAC | Sirt3 LOF: ↑LVH with fibrosis | Hafner et al.40 |
| Sirt6 | Sirt6 LOF: accelerated aging—hypoglycaemia | Mostoslavsky et al.60 | |
| Sirt6 LOF: ↑ LVH; Sirt6 GOF: ↓ LVH |
Sundaresan et al.50 | ||
| Sirt6 Hep LOF: ↑ PCSK9, plasma LDL-chol, Sirt6 Hep GOF: ↓ PCSK9, plasma LDL-chol |
Tao et al.55 | ||
| Sirt7 | Sirt7 LOF: ↑ CMP, liver steatosis, TG | Ryu et al.66 | |
| Sirt7 LOF: ↑ LVH, inflammatory CMP, fibrosis | Vakhrusheva et al.97 | ||
| PanSirt | |||
| NAD+ | Aging | NAD+ booster in old mice: restores mitochondrial function and metabolic abnormalities | Gomes et al.83 |
| NR | DIO | NAD+ booster in DIO: improves metabolic abnormalities | Canto et al.88 |
| NR | Aging | NAD+ booster in old mice: restores mitochondrial function | Mouchiroud et al.84 |
| Exercise | Aging | Exercise in old rats: Increases NAD+, NAMPT, Sirt1, Sirt6 | Koltai et al.86 |
| Clinical | |||
| Sirt1 | Adults | Sirt1 SNPs: ↓ energy expenditure in offspring of T2D pts | Lagouge et al.19 |
| Sirt1 | ACS | Monocytes in ACS pts: ↓ Sirt1 expression than in healthy | Breitenstein et al.70 |
| Sirt1 | Adults | Carotid atherosclerosis: ↓ Sirt1 expression | Gorenne et al.16 |
| Sirt1 | Adults | Smokers: STAC (SRT2104): ↓ endothelial dysfct, LDL-C | Venkatasubramanian et al.82 |
| Sirt6 | Adults | Failing hearts: ↓ Sirt6 expression | Sundaresan et al.50 |
Experimental and human data showing cardiovascular effects of sirtuin modulation; LOF, loss-of-function; GOF, gain-of-function; DIO, diet-induced obesity; SRT3025, SRT2104: STAC, Sirt1-activating compound; CMP, cardiomyopathy; LVH, left ventricular hypertrophy; ACS, acute coronary syndromes; NAD+, nicotinamide adenine dinucleotide; NAMPT, nicotinamide phosphoribosyltransferase; NR, nicotinamide riboside; T2D, type 2 diabetes; pts, patients.
Sirtuins: emerging roles in cardiovascular and metabolic diseases
Atherosclerosis: a chronic immunometabolic disease
Atherosclerosis with its sequelae myocardial infarction or stroke comprises the major cause for morbidity and mortality in the Western world.8 At the cellular and molecular level, atherosclerosis results from a complex interplay between modified low density lipoproteins (LDL), activated endothelial cells, monocyte-derived macrophages, T cells, and the vessel wall: Oxidative stress in endothelial cells, endothelial dysfunction, and subsequent recruitment of macrophages into the sub-endothelial space are major steps in early atherogenesis. In response to para- and autocrine inflammatory mediators such as tumour necrosis factor α and different interleukins, invading monocytes transdifferentiate into macrophages, proliferate, and ingest abundantly present modified LDL-cholesterol (LDL-C). Thereby, sub-endothelial macrophages turn into foam cells that promote plaque formation and subsequent plaque rupture.9 Plasma cholesterol and in particular its LDL subfraction are central players for both the initiation and progression of atherosclerosis.10
It is intriguing that many risk factors for cardiovascular disease such as age, hypercholesterolaemia, obesity, and type 2 diabetes are conditions in which the activation of the sirtuins has been shown to exert protective effects in experimental models.
Sirt1 in cardiovascular disease
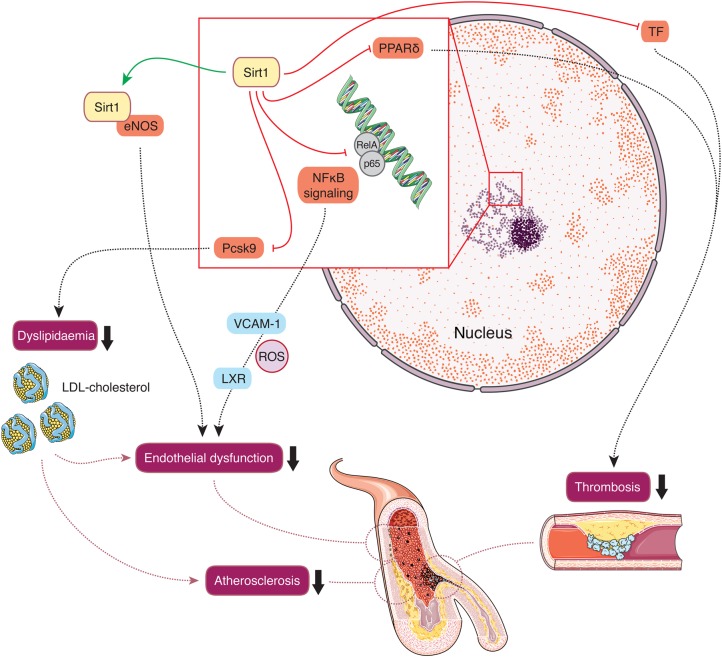
Sirt1 is found in the nucleus and cytoplasm (Figure 2). Among all seven sirtuin isoforms, most is known about Sirt1. First evidence of a connection between Sirt1 and endothelial cells was that Sirt1 activates endothelial nitric oxide synthase (eNOS).11 Later on, studies in genetically engineered mouse models have demonstrated that Sirt1 exerts atheroprotective effects by activating eNOS or by diminishing NFκB activity in endothelial cells and macrophages.12–14 Moreover, pharmacological Sirt1 activation protected endothelial cells from senescence induced by disturbed flow.15 Another report assigned Sirt1 in vascular smooth muscle cells a protective role against DNA damage, medial degeneration, and atherosclerosis.16 These reports place macrophages, endothelial, and vascular smooth muscle cells at centre stage for Sirt1-mediated atheroprotection.17
Figure 2.
Cardiovascular effects of Sirt1. Within the nucleus Sirt1 interacts with diverse transcription factors, inhibiting NFκB signalling, and consecutive pro-inflammatory cytokine expression, e.g. vascular cell adhesion molecule 1 as well as expression of the reverse cholesterol transporter LXR. Moreover, Sirt1 reduces plasma Pcsk9 levels, thereby increasing hepatic low-density lipoprotein-cholesterol receptor density and thus decreasing plasma low-density lipoprotein-cholesterol levels. Along with activation of endothelial nitric oxide synthase, these effects improve endothelial dysfunction and decrease atherosclerosis. In addition, Sirt1 deacetylates NFκB and inhibits tissue factor activity and thereby slows arterial thrombus formation. Sirt1-mediated tissue factor inhibition may further follow activation of peroxisome proliferator-activated receptor delta and Cox2-derived prostaglandin synthesis.
Sirt1 also has wide-ranging effects on metabolic homeostasis, mainly through its role as a master regulator of mitochondrial integrity.18,19 Sirt1 activation hence results in improved glucose tolerance and lipid homeostasis and reduced inflammatory tone, which all are also atheroprotective.1,2
Pharmacological Sirt1 inhibition has been reported to increase thrombosis by inhibiting tissue factor activation via NFκB.20 Similarly, cyclooxygenase-2-derived prostacyclin and PPARδ activation were found to decrease arterial thrombus formation by suppressing tissue factor in a sirtuin-1-dependent manner.21 Thus, activation of Sirt1 protects from arterial thrombosis. In the context of atherosclerosis, pharmacological Sirt1 activation lowered plasma LDL-C levels by inhibiting proprotein convertase subtilisin/kexin 9 (Pcsk9) secretion, thereby increasing hepatic LDL-receptor (LDL-R) availability and consecutive LDL-C clearing.22 Conversely, absence of LDL-R abolished the atheroprotective effects of pharmacological Sirt1 activation.22 These experiments explained the controversial finding that genetic Sirt1 overexpression increased atherosclerosis in LDL-R knockout mice.23 Moreover, Sirt1 was reported to promote angiogenesis via inhibition of endothelial notch signaling.24,25
Sirt1 also provides cardiac protection: Sirt1-deficient mice exhibit increased injury in response to ischaemia-reperfusion, whereas injury was decreased in Sirt1 transgenic mice.26 Moreover, Sirt1 was shown to protect from catecholamine-induced cardiomyopathy in mice.27
Sirt3 in cardiovascular disease
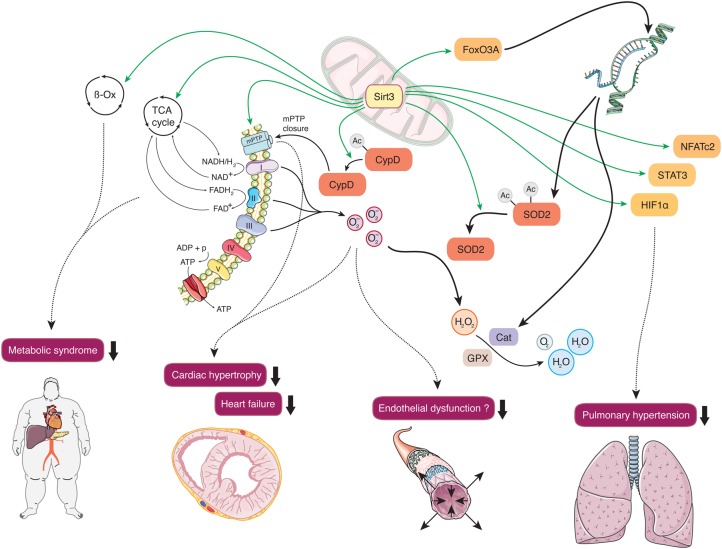
Together with Sirt4 and Sirt5, Sirt3 is located in the mitochondria (Figure 3). Sirt3 regulates global mitochondrial lysine acetylation,28 boosting antioxidant defense, and preserving mitochondrial function.29–32 Mitochondrial dysfunction plays a central role in a number of cardiovascular diseases, ranging from hypertrophic and dilated cardiomyopathy, heart failure, and pulmonary hypertension to endothelial dysfunction in early atherogenesis.33–35
Figure 3.
Cardiovascular effects of Sirt3. Mitochondrial Sirt3 drives the tricarboxylic acid cycle, β-oxidation, and oxidative phosphorylation, thus maintaining metabolic homeostasis and preventing the development of risk factors associated with the metabolic syndrome. Deacetylation and consecutive activation of superoxide dismutase 2 mediates antioxidative protection, diminishes cardiac hypertrophy, and may improve endothelial dysfunction. Activation of the transcription factors NFATc2, STAT3, and HIF1α prevent the development of pulmonary hypertension.
The protective effects of caloric restriction on oxidative stress depend on the presence of Sirt3, which increased the ratio of glutathione to glutathione disulphide.36 Similarly, deletion of Sirt3 was associated with increased malondialdehyde levels in LDL-R knockout mice, yet without affecting atherogenesis.37 Concordantly, overexpression of Sirt3 decreased cellular levels of reactive oxygen species (ROS), an effect that was brought about by Sirt3-mediated deacetylation and activation of superoxide dismutase 2 (SOD2), the mitochondrial isoform of this group of major ROS detoxifying enzymes.36,38 Importantly, excessive ROS, subsequent mitochondrial DNA damage, and progressive respiratory chain dysfunction activate several signalling pathways underlying endothelial dysfunction and vascular inflammation in atherogenesis.33 Sirt3-dependent transcriptional induction of SOD2 and catalase was shown to prevent cardiac hypertrophy by deacetylation of the transcription factor forkhead box O3a (Foxo3a).39 Moreover, Sirt3-deficient mice were more susceptible to age-dependent and trans aortic constriction-induced left ventricular hypertrophy via activation of the mitochondrial permeability transition pore.40 Sirt3 was also reported to prevent stress-induced mitochondrial apoptosis of mammalian cardiomyocytes and to protect endothelial mitochondria from oxidative damage.31,41
Interestingly, Sirt3 knockout mice develop spontaneous pulmonary hypertension.42 Moreover, mitochondria isolated from pulmonary artery smooth muscle cells of Sirt3-deficient mice displayed a reduced oxygen consumption rate compared with controls. Concordantly, Sirt3 deficiency in both murine and human pulmonary artery smooth muscle cells was associated with an induction of the three transcription factors HIF1α, STAT3, and NFATc2, all known to be essential in the development of pulmonary arterial hypertension.42
Sirt3 has been shown to orchestrate mitochondrial metabolism by driving not only oxidative phosphorylation29,43,44 but also the tricarboxylic acid cycle,32 and β-oxidation.45 In this context, Sirt3 deficiency accelerated the development of the metabolic syndrome in mice, a cluster of risk factors for cardiovascular diseases.37,46
Sirt6 in cardiovascular disease
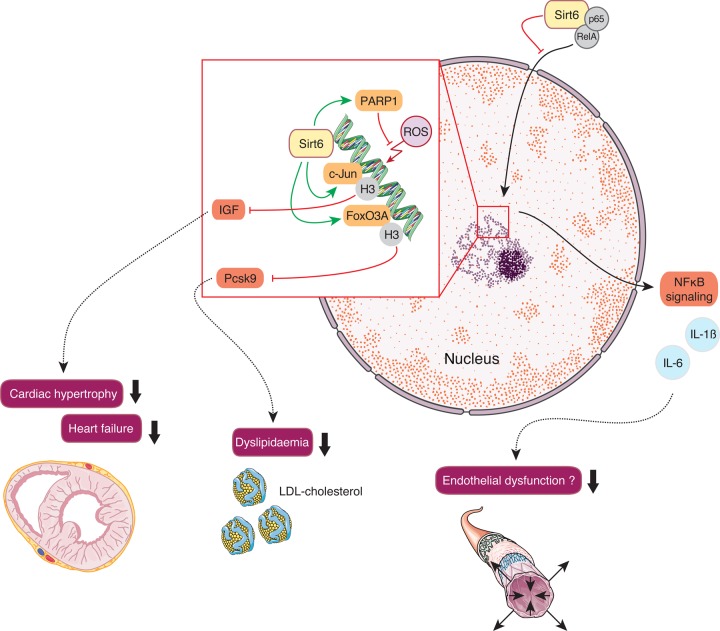
Sirt6 is a nuclear chromatin-associated deacylase (Figure 4). Recent reports provided mechanistic insight on the ADP-ribosyltransferase substrate-specific deacylase activity of both acetyl and long-chain fatty-acyl groups.47 Sirt6 plays a role in cardiovascular disease including cardiac hypertrophy, heart failure, myocardial hypoxic damage, and metabolism.48–50 Sirt6 has gained attention for its role in human telomere and genome stabilization,51 gene expression and DNA repair,52 glucose and fat homeostasis,53–55 and inflammation.56,57
Figure 4.
Cardiovascular effects of Sirt6. Nuclear Sirt6-induced inhibition of insulin-like growth factor signalling prevents cardiac hypertrophy. Moreover, sirtuin 6-mediated reduction of plasma Pcsk9 levels increases hepatic low-density lipoprotein-receptor density and decreases plasma low-density lipoprotein-cholesterol levels. Poly[adenosine diphosphate-ribose] polymerase 1 activation halts reactive oxygen species-mediated DNA damage. Moreover, physical interaction of Sirt6 with the NFκB subunits RelA and p65 prevents their translocation to the nucleus and inhibits pro-inflammatory NFκB-signalling, thereby potentially protecting from endothelial dysfunction.
Sirt6 contributes to chromosomal stability by promoting double-strand break repair by homologous recombination (HR).58 DNA double-strand breaks are a hallmark of genomic instability in aging tissues. Notably, overexpression of Sirt6 in pre-senescent cells stimulated HR.58 This support in HR can be partially attributed to a physical interaction and consecutive stimulation of Sirt6 with poly[ADP ribose] polymerase 1, thereby preventing the premature aging phenotype of Sirt6 knockout mice.59,60 Concomitantly, male Sirt6 transgenic mice revealed an increased life span compared with wild-type controls.54 Interestingly, the heart is among the organs with the highest Sirt6 expression, suggesting a role for Sirt6-mediated protection from myocardial senescence.61
Indeed, Sirt6 was identified as a negative endogenous regulator of myocardial IGF-Akt signaling, a pathway that upon constitutive activation eventually leads to cardiac hypertrophy. Sirt6 deficiency enhanced H3K9 acetylation, facilitating binding of the stress-responsive transcription factor c-Jun. Subsequent boosting of myocardial IGF signalling resulted in cardiac hypertrophy and heart failure.50 Along this line, nicotinamide mononucleotide adenylyltransferase, a vital enzyme in NAD biogenesis, prevented angiotensin II-induced cardiac hypertrophy.48 Moreover, cardiomyocytes from Sirt6 transgenic mice were protected from prolonged hypoxia ex vivo, an effect that was in part attributed to increased Sirt6-mediated deacetylation and inactivation of RelA (p65), a subunit of NFκB.49
By the transcriptional repression of NFκB-dependent targets, Sirt6 plays an important role in stress response, thereby protecting against inflammatory and degenerative diseases.56,57 Importantly, this interaction with NFκB-signalling may contribute to premature senescence and early lethality upon Sirt6 deficiency.62,63 Vascular inflammation is a key regulatory process in atherogenesis.64 Thus, Sirt6 deficiency in cultured endothelial cells increased expression of pro-inflammatory cytokines such as interleukin 1β along with an increased transcriptional activity of NFκB.65 Intriguingly, hepatic Sirt6 was also shown to suppress transcription of Pcsk9, thereby preventing hepatic LDL-R degradation and consecutively reducing plasma LDL-C levels in mice.55 Increased levels of LDL-C in concert with enhanced pro-inflammatory cytokines trigger endothelial activation and launch the vicious immunometabolic cycle of atherogenesis.
Sirt7 in cardiovascular disease
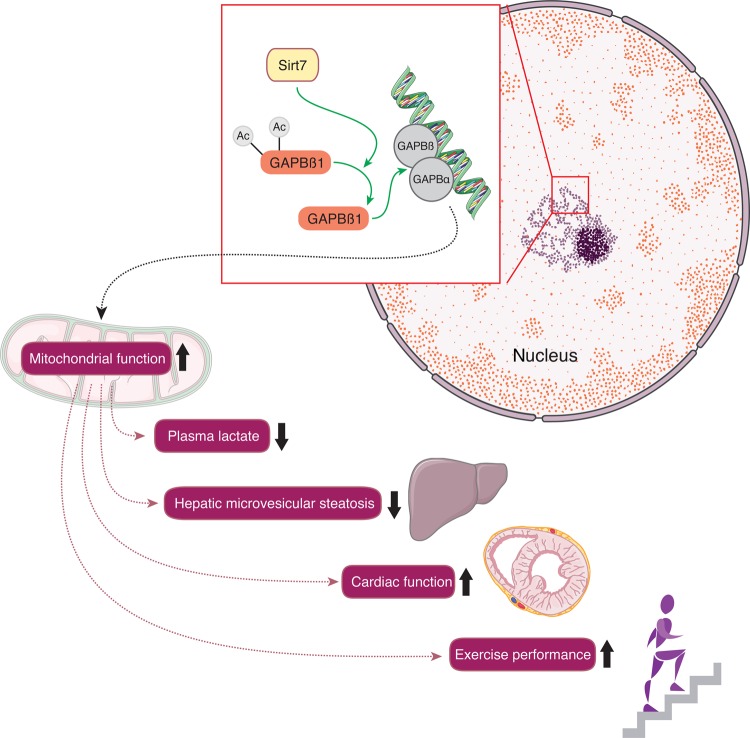
Data from two different laboratories, using two independently generated mouse lines have also linked Sirt7 with cardiomyopathy, the main cause of death of Sirt7-deficient mice (Figure 5).66,67 In fact, Sirt7 deficiency in mice induces multi-systemic mitochondrial dysfunction, which is reflected by increased blood lactate levels, reduced exercise performance due to cardiac dysfunction, hepatic micro-vesicular steatosis, and age-related hearing loss.66,68 This link between Sirt7 and mitochondrial function can be translated to humans, where Sirt7 overexpression rescues the mitochondrial functional defect in fibroblasts of patients with a mutation in NADH dehydrogenase [ubiquinone] iron-sulphur protein 1. These wide-ranging effects of Sirt7 on mitochondrial homeostasis are the consequence of the deacetylation of distinct lysine residues located in the hetero- and homodimerization domains of GA-binding protein (GABP)β1, a master regulator of nuclear-encoded mitochondrial genes. Sirt7-mediated deacetylation of GABPβ1 facilitates complex formation with GABPα and the transcriptional activation of the GABPα/GABPβ heterotetramer. These data suggest that Sirt7 is a dynamic nuclear regulator of mitochondrial function through its impact on GABPβ1. The role of Sirt7 in endothelial function and atherothrombosis remains unknown.
Figure 5.
Cardiovascular effects of Sirt7. Nuclear Sirt7 deacetylates distinct lysine residues located in the hetero- and homodimerization domains of GA-binding protein (GABP)β1, a master regulator of nuclear-encoded mitochondrial genes. Along these lines, Sirt7 improves mitochondrial function in numerous tissues including cardiac and skeletal muscle where it protects from cardiomyopathy, lowers lactate levels, and improves exercise performance, respectively. Moreover, Sirt7 protects from hepatic micro-vesicular steatosis.
Sirtuins: a role in human cardiovascular disease
Data on sirtuins in human cardiovascular diseases are scarce. Of note, reports about associations have to be differentiated from findings related to potential causal effects.
Most studies report lower sirtuin expression levels in contexts of cardiovascular disease compared with healthy controls. However, none of them tracked specific sirtuin activity. First, data were derived from patients with insulin resistance: Low Sirt1 expression in insulin-sensitive tissues correlated with impaired stimulation of energy expenditure by insulin. Thus, these findings associated impaired regulation of mitochondrial function with insulin resistance in humans.69 Additional reports came from patients with atherothrombosis: Sirt1 expression levels were found to be lower in human atherosclerotic carotid arteries compared with non-diseased arteries.16 Sirt1 expression levels were also lower in monocytes of patients with acute coronary syndromes compared with healthy controls.70 In parallel, Sirt6 expression and inflammatory activity in diabetic atherosclerotic plaques increased upon incretin treatment and was associated with a more stable plaque phenotype.71
In contrast to associative reports, genetic studies imply a more stringent cause–effect relationship. A report in Finnish subjects demonstrated a direct involvement of Sirt1 in modulating energy expenditure and metabolic homeostasis: Three of five single-nucleotide polymorphisms (SNPs) were significantly associated with whole body energy expenditure as evaluated either during fasting or during a hyperinsulinemic clamp.19 Another study showed that a point mutation of Sirt1 was associated with human type 1 diabetes and ulcerative colitis; expression of this mutation in insulin-producing cells resulted in overproduction of nitric oxide and pro-inflammatory cytokines.72 Yet, no association was observed between five known SNPs of the Sirt1 gene with longevity using an extensive DNA data bank of 1573 long-lived individuals with matched younger controls.73 Of note, many other studies reporting SNPs confer limitations such as low patient numbers or insufficient adjustments in control groups.
Moreover, Sirt3 deficiency was associated with pulmonary arterial hypertension and the metabolic syndrome both in mice and humans.42,46 Sirt6 levels were decreased in human failing hearts50; in addition, genetic variants of Sirt6 and uncoupling protein 5 were associated with carotid atherosclerotic plaque burden.74 To date, there are no reports about genetic variants of Sirt7 and human disease.
Pharmacological modulation of sirtuin activity
Two reports that resveratrol protected mice against diet-induced obesity and insulin resistance with Sirt1 as a crucial mediator created considerable attention.18,19 These effects of resveratrol supplementation, mimicking caloric restriction, could later on be translated into humans.75 Subsequently, more specific Sirt1-activating compounds (STACs) applied in experimental diet-induced obesity mimicked the protective effects of caloric restriction, showing mitochondrial activation, and subsequent prevention of obesity along with an improvement of the diabetic phenotype.76,77 Moreover, STAC improved survival and healthspan in obese mice.78
However, the specificity of these initial STAC was called into question: A non-physiological fluorescent substrate that was used for the Sirt1 activity assays was shown to lead to artefactual results.79 Nevertheless, the substrate specificity of STAC was consecutively underlined: Mutation of a single amino acid in Sirt1, Glu230 located in a structured N-terminal domain was critical for Sirt1 activation.80 Given the conservation of Glu230 down to model organisms (drosophila), the presence of an endogenous activator appears logical, which however remains to be proven. Furthermore, the most recent pharmacological STAC have been used with beneficial effects in mice for atheroprotection and extension of lifespan,22,81 as well as in healthy smokers for improving endothelial dysfunction and lowering LDL-C.82
While Sirt1-specific activators may ultimately prove operative,22,80 there is increasing evidence that pan-sirtuin activators might be more effective.83–85 The metabolite NAD+ is an essential co-substrate for the activity of all sirtuins. Its levels decline in response to high-fat diet, DNA damage, and aging.84 Reports on sirtuin-dependent beneficial effects of increasing NAD+ levels on metabolic homeostasis suggest that this strategy provides a novel and promising concept for cardiovascular protection.
NAD+ levels are maintained by balancing its biosynthesis/salvage and breakdown (Figure 1). NAD+ content can be boosted by exercise,4,86 inhibition of NAD+-consuming enzymes [sirtuins, poly-ADP-ribose-polymerases (PARPs),87 and cyclic ADP-ribose synthases], and administration of NAD+ precursors, such as nicotinic acid, nicotinamide, nicotinamide mononucleotide,83 and nicotinamide riboside.84,88 NAD+ precursor compounds as well as PARP and CD38 inhibitors have been used in several long-term mouse studies confirming wide-ranging health benefits on the metabolic, immune, and nervous systems.89 Raising NAD+ levels may improve cardiac function in the context of ischaemia-reperfusion,90 but may be damaging in the setting of acute myocardial infarction.91 Thus, the net effects of increasing NAD+ levels are likely to be dose- and time-dependent; such a biphasic response is well known from nitric oxide. The effects of raising NAD+ levels long term in the context of chronic cardiovascular disease are likely to be beneficial. Yet, this has to be formally tested.
Sirtuins in cardiovascular diseases: conclusions and perspectives
The beneficial effects of sirtuins on inflammation, lipid metabolism, and numerous areas of cardiovascular disease are well documented at the pre-clinical level. The prognostic value of deranged pathways of inflammation and lipids for human cardiovascular disease is established.92,93 Multi-omics approaches will enlarge our instrumentarium of diagnostic and prognostic biomarkers in cardiovascular diseases.94 Moreover, large databases for linking genotypes to cardiovascular phenotypes and prognosis are available.95 Yet, the tracking of specific sirtuin activity remains a challenge.96 Some genetic reports imply a cause–effect relationship of Sirt1 SNPs with metabolic homeostasis. Yet, conclusive genetic analyses from large databases (GWAS) about the role of Sirt1 SNPs are still pending. At the experimental level, both specific Sirt1 activators and pan-sirtuin-activating compounds are in development and hold great promise for future applications in treating and preventing cardiovascular and metabolic diseases.
Funding
This work was funded by the Swiss National Science Foundation (J.A., C.M.M.), the Ecole Polytechnique Fédérale de Lausanne (J.A.), the NIH/NIA (D.A.S., J.A.), the Paul Glenn Foundation for Medical Research (D.A.S.), the University Research Priority Program Integrative Human Physiology at the University of Zurich (C.M.M.), Matching Funds by the University of Zurich (S.W., C.M.M.), Sirtris, a GlaxoSmithKline company (C.M.M.), Hartmann-Müller Foundation (C.M.M.), and the Zurich, Heart House—Foundation for Cardiovascular Research, Switzerland. Funding to pay the Open Access publication charges for this article was provided by the Foundation for Cardiovascular Research, Zurich, Switzerland.
Conflict of interest: J.A. is a scientific founder of and consultant to MitoBridge, D.A.S. is a founder and consultant to Metrobiotech and consultant to GlaxoSmithKline.
References
- 1.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 2012;13:225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res 2012;110:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guarente L, Franklin H. Epstein lecture: sirtuins, aging, and medicine. N Engl J Med 2011;364:2235–2244. [DOI] [PubMed] [Google Scholar]
- 4.White AT, Schenk S. NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab 2012;303:E308–E321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth GS, Ingram DK, Lane MA. Caloric restriction in primates and relevance to humans. Ann N Y Acad Sci 2001;928:305–315. [DOI] [PubMed] [Google Scholar]
- 6.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009;325:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 1999;13:2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Association for Cardiovascular P, Rehabilitation Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D, Guidelines ESCCfP, Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 9.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med 2002;8:1211–1217. [DOI] [PubMed] [Google Scholar]
- 11.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005;310:314–317. [DOI] [PubMed] [Google Scholar]
- 12.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res 2008;80:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein S, Lohmann C, Schafer N, Hofmann J, Rohrer L, Besler C, Rothgiesser KM, Becher B, Hottiger MO, Boren J, McBurney MW, Landmesser U, Luscher TF, Matter CM. SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. Eur Heart J 2010;31:2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein S, Schafer N, Breitenstein A, Besler C, Winnik S, Lohmann C, Heinrich K, Brokopp CE, Handschin C, Landmesser U, Tanner FC, Luscher TF, Matter CM. SIRT1 reduces endothelial activation without affecting vascular function in ApoE-/- mice. Aging (Albany NY) 2010;2:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warboys CM, de Luca A, Amini N, Luong L, Duckles H, Hsiao S, White A, Biswas S, Khamis R, Chong CK, Cheung WM, Sherwin SJ, Bennett MR, Gil J, Mason JC, Haskard DO, Evans PC. Disturbed flow promotes endothelial senescence via a p53-dependent pathway. Arterioscler Thromb Vasc Biol 2014;34:985–995. [DOI] [PubMed] [Google Scholar]
- 16.Gorenne I, Kumar S, Gray K, Figg N, Yu H, Mercer J, Bennett M. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation 2013;127:386–396. [DOI] [PubMed] [Google Scholar]
- 17.Winnik S, Stein S, Matter CM. SIRT1 – an anti-inflammatory pathway at the crossroads between metabolic disease and atherosclerosis. Curr Vasc Pharmacol 2012;10:1–3. [DOI] [PubMed] [Google Scholar]
- 18.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006;444:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006;127:1109–1122. [DOI] [PubMed] [Google Scholar]
- 20.Breitenstein A, Stein S, Holy EW, Camici GG, Lohmann C, Akhmedov A, Spescha R, Elliott PJ, Westphal CH, Matter CM, Luscher TF, Tanner FC. Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells. Cardiovasc Res 2011;89:464–472. [DOI] [PubMed] [Google Scholar]
- 21.Barbieri SS, Amadio P, Gianellini S, Tarantino E, Zacchi E, Veglia F, Howe LR, Weksler BB, Mussoni L, Tremoli E. Cyclooxygenase-2-derived prostacyclin regulates arterial thrombus formation by suppressing tissue factor in a sirtuin-1-dependent-manner. Circulation 2012;126:1373–1384. [DOI] [PubMed] [Google Scholar]
- 22.Miranda MX, van Tits LJ, Lohmann C, Arsiwala T, Winnik S, Tailleux A, Stein S, Gomes AP, Suri V, Ellis JL, Lutz TA, Hottiger MO, Sinclair DA, Auwerx J, Schoonjans K, Staels B, Luscher TF, Matter CM. The Sirt1 activator SRT3025 provides atheroprotection in Apoe-/- mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur Heart J 2015;36:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiang L, Lin HV, Kim-Muller JY, Welch CL, Gu W, Accili D. Proatherogenic abnormalities of lipid metabolism in SirT1 transgenic mice are mediated through Creb deacetylation. Cell Metab 2011;14:758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guarani V, Deflorian G, Franco CA, Kruger M, Phng LK, Bentley K, Toussaint L, Dequiedt F, Mostoslavsky R, Schmidt MH, Zimmermann B, Brandes RP, Mione M, Westphal CH, Braun T, Zeiher AM, Gerhardt H, Dimmeler S, Potente M. Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Nature 2011;473:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 2007;21:2644–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 2010;122:2170–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai L, Yan L, Gao S, Hu CL, Ge H, Davidow A, Park M, Bravo C, Iwatsubo K, Ishikawa Y, Auwerx J, Sinclair DA, Vatner SF, Vatner DE. Type 5 adenylyl cyclase increases oxidative stress by transcriptional regulation of manganese superoxide dismutase via the SIRT1/FoxO3a pathway. Circulation 2013;127:1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr, Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 2007;27:8807–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Nam M, Fan W, Akie TE, Hoaglin DC, Gao G, Keaney JF, Jr, Cooper MP. Nutrient sensing by the mitochondrial transcription machinery dictates oxidative phosphorylation. J Clin Invest 2014;124:768–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010;143:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tseng AH, Shieh SS, Wang DL. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med 2013;63:222–234. [DOI] [PubMed] [Google Scholar]
- 32.Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem 2012;287:14078–14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 2005;25:29–38. [DOI] [PubMed] [Google Scholar]
- 34.Wallace DC. Mitochondrial defects in cardiomyopathy and neuromuscular disease. Am Heart J 2000;139(2 Pt 3):S70–S85. [DOI] [PubMed] [Google Scholar]
- 35.Wallace DC. Mitochondrial diseases in man and mouse. Science 1999;283:1482–1488. [DOI] [PubMed] [Google Scholar]
- 36.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 2010;12:662–667. [DOI] [PubMed] [Google Scholar]
- 37.Winnik S, Gaul DS, Preitner F, Lohmann C, Weber J, Miranda MX, Liu Y, van Tits LJ, Mateos JM, Brokopp CE, Auwerx J, Thorens B, Luscher TF, Matter CM. Deletion of Sirt3 does not affect atherosclerosis but accelerates weight gain and impairs rapid metabolic adaptation in LDL receptor knockout mice: implications for cardiovascular risk factor development. Basic Res Cardiol 2014;109:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 2010;40:893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 2009;119:2758–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 2008;28:6384–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulin R, Dromparis P, Sutendra G, Gurtu V, Zervopoulos S, Bowers L, Haromy A, Webster L, Provencher S, Bonnet S, Michelakis ED. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab 2014;20:827–839. [DOI] [PubMed] [Google Scholar]
- 43.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 2008;105:14447–14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahman M, Nirala NK, Singh A, Zhu LJ, Taguchi K, Bamba T, Fukusaki E, Shaw LM, Lambright DG, Acharya JK, Acharya UR. Drosophila Sirt2/mammalian SIRT3 deacetylates ATP synthase beta and regulates complex V activity. J Cell Biol 2014;206:289–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010;464:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV, Jr, Kahn CR, Verdin E. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell 2011;44:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kugel S, Mostoslavsky R. Chromatin and beyond: the multitasking roles for SIRT6. Trends Biochem Sci 2014;39:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai Y, Yu SS, Chen SR, Pi RB, Gao S, Li H, Ye JT, Liu PQ. Nmnat2 protects cardiomyocytes from hypertrophy via activation of SIRT6. FEBS Lett 2012;586:866–874. [DOI] [PubMed] [Google Scholar]
- 49.Maksin-Matveev A, Kanfi Y, Hochhauser E, Isak A, Cohen HY, Shainberg A. Sirtuin 6 protects the heart from hypoxic damage. Exp Cell Res 2015;330:81–90. [DOI] [PubMed] [Google Scholar]
- 50.Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S, Parekh V, Pillai VB, Ravindra PV, Gupta M, Jeevanandam V, Cunningham JM, Deng CX, Lombard DB, Mostoslavsky R, Gupta MP. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med 2012;18:1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, Chang HY, Bohr VA, Ried T, Gozani O, Chua KF. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 2008;452:492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCord RA, Michishita E, Hong T, Berber E, Boxer LD, Kusumoto R, Guan S, Shi X, Gozani O, Burlingame AL, Bohr VA, Chua KF. SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging (Albany NY) 2009;1:109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 2010;140:280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanfi Y, Peshti V, Gil R, Naiman S, Nahum L, Levin E, Kronfeld-Schor N, Cohen HY. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell 2010;9:162–173. [DOI] [PubMed] [Google Scholar]
- 55.Tao R, Xiong X, DePinho RA, Deng CX, Dong XC. FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. J Biol Chem 2013;288:29252–29259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 2009;136:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawahara TL, Rapicavoli NA, Wu AR, Qu K, Quake SR, Chang HY. Dynamic chromatin localization of Sirt6 shapes stress- and aging-related transcriptional networks. PLoS Genet 2011;7:e1002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mao Z, Tian X, Van Meter M, Ke Z, Gorbunova V, Seluanov A. Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proc Natl Acad Sci USA 2012;109:11800–11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science 2011;332:1443–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 2006;124:315–329. [DOI] [PubMed] [Google Scholar]
- 61.Pereira CV, Lebiedzinska M, Wieckowski MR, Oliveira PJ. Regulation and protection of mitochondrial physiology by sirtuins. Mitochondrion 2012;12:66–76. [DOI] [PubMed] [Google Scholar]
- 62.Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev 2007;21:3244–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beauharnois JM, Bolivar BE, Welch JT. Sirtuin 6: a review of biological effects and potential therapeutic properties. Mol Biosyst 2013;9:1789–1806. [DOI] [PubMed] [Google Scholar]
- 64.Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lappas M. Anti-inflammatory properties of sirtuin 6 in human umbilical vein endothelial cells. Mediators Inflamm 2012;2012:597514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ryu D, Jo YS, Lo Sasso G, Stein S, Zhang H, Perino A, Lee JU, Zeviani M, Romand R, Hottiger MO, Schoonjans K, Auwerx J. A SIRT7-dependent acetylation switch of GABPbeta1 controls mitochondrial function. Cell Metab 2014;20:856–869. [DOI] [PubMed] [Google Scholar]
- 67.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res 2008;102:703–710. [DOI] [PubMed] [Google Scholar]
- 68.Yoshizawa T, Karim MF, Sato Y, Senokuchi T, Miyata K, Fukuda T, Go C, Tasaki M, Uchimura K, Kadomatsu T, Tian Z, Smolka C, Sawa T, Takeya M, Tomizawa K, Ando Y, Araki E, Akaike T, Braun T, Oike Y, Bober E, Yamagata K. SIRT7 controls hepatic lipid metabolism by regulating the ubiquitin-proteasome pathway. Cell Metab 2014;19:712–721. [DOI] [PubMed] [Google Scholar]
- 69.Rutanen J, Yaluri N, Modi S, Pihlajamaki J, Vanttinen M, Itkonen P, Kainulainen S, Yamamoto H, Lagouge M, Sinclair DA, Elliott P, Westphal C, Auwerx J, Laakso M. SIRT1 mRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes 2010;59:829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breitenstein A, Wyss CA, Spescha RD, Franzeck FC, Hof D, Riwanto M, Hasun M, Akhmedov A, von Eckardstein A, Maier W, Landmesser U, Luscher TF, Camici GG. Peripheral blood monocyte Sirt1 expression is reduced in patients with coronary artery disease. PLoS ONE 2013;8:e53106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balestrieri ML, Rizzo MR, Barbieri M, Paolisso P, D'Onofrio N, Giovane A, Siniscalchi M, Minicucci F, Sardu C, D'Andrea D, Mauro C, Ferraraccio F, Servillo L, Chirico F, Caiazzo P, Paolisso G, Marfella R. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of incretin treatment. Diabetes 2015;64:1395–1406. [DOI] [PubMed] [Google Scholar]
- 72.Biason-Lauber A, Boni-Schnetzler M, Hubbard BP, Bouzakri K, Brunner A, Cavelti-Weder C, Keller C, Meyer-Boni M, Meier DT, Brorsson C, Timper K, Leibowitz G, Patrignani A, Bruggmann R, Boily G, Zulewski H, Geier A, Cermak JM, Elliott P, Ellis JL, Westphal C, Knobel U, Eloranta JJ, Kerr-Conte J, Pattou F, Konrad D, Matter CM, Fontana A, Rogler G, Schlapbach R, Regairaz C, Carballido JM, Glaser B, McBurney MW, Pociot F, Sinclair DA, Donath MY. Identification of a SIRT1 mutation in a family with type 1 diabetes. Cell Metab 2013;17:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flachsbart F, Croucher PJ, Nikolaus S, Hampe J, Cordes C, Schreiber S, Nebel A. Sirtuin 1 (SIRT1) sequence variation is not associated with exceptional human longevity. Exp Gerontol 2006;41:98–102. [DOI] [PubMed] [Google Scholar]
- 74.Dong C, Della-Morte D, Wang L, Cabral D, Beecham A, McClendon MS, Luca CC, Blanton SH, Sacco RL, Rundek T. Association of the sirtuin and mitochondrial uncoupling protein genes with carotid plaque. PLoS ONE 2011;6:e27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 2011;14:612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007;450:712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 2008;8:347–358. [DOI] [PubMed] [Google Scholar]
- 78.Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martin-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R. SRT1720 improves survival and healthspan of obese mice. Sci Rep 2011;1:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 2010;285:8340–8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA, E SY. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 2013;339:1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mercken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, Scheibye-Knudsen M, Palacios HH, Licata JJ, Zhang Y, Becker KG, Khraiwesh H, Gonzalez-Reyes JA, Villalba JM, Baur JA, Elliott P, Westphal C, Vlasuk GP, Ellis JL, Sinclair DA, Bernier M, de Cabo R. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 2014;13:787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Venkatasubramanian S, Noh RM, Daga S, Langrish JP, Joshi NV, Mills NL, Hoffmann E, Jacobson EW, Vlasuk GP, Waterhouse BR, Lang NN, Newby DE. Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J Am Heart Assoc 2013;2:e000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013;155:1624–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J. The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 2013;154:430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Houtkooper RH, Auwerx J. Exploring the therapeutic space around NAD+. J Cell Biol 2012;199:205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koltai E, Szabo Z, Atalay M, Boldogh I, Naito H, Goto S, Nyakas C, Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev 2010;131:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 2011;13:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 2012;15:838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.von Lukowicz T, Hassa PO, Lohmann C, Boren J, Braunersreuther V, Mach F, Odermatt B, Gersbach M, Camici GG, Stahli BE, Tanner FC, Hottiger MO, Luscher TF, Matter CM. PARP1 is required for adhesion molecule expression in atherogenesis. Cardiovasc Res 2008;78:158–166. [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE 2014;9:e98972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Montecucco F, Bauer I, Braunersreuther V, Bruzzone S, Akhmedov A, Luscher TF, Speer T, Poggi A, Mannino E, Pelli G, Galan K, Bertolotto M, Lenglet S, Garuti A, Montessuit C, Lerch R, Pellieux C, Vuilleumier N, Dallegri F, Mage J, Sebastian C, Mostoslavsky R, Gayet-Ageron A, Patrone F, Mach F, Nencioni A. Inhibition of nicotinamide phosphoribosyltransferase reduces neutrophil-mediated injury in myocardial infarction. Antioxid Redox Signal 2013;18:630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chasman DI, Giulianini F, MacFadyen J, Barratt BJ, Nyberg F, Ridker PM. Genetic determinants of statin-induced low-density lipoprotein cholesterol reduction: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Circ Cardiovasc Genet 2012;5:257–264. [DOI] [PubMed] [Google Scholar]
- 93.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 94.Hoefer IE, Steffens S, Ala-Korpela M, Bäck M, Badimon L, Bochaton-Piallat ML, Boulanger C, Caliguri G, Dimmeler S, Egido J, Evans PC, Guzik T, Kwak BR, Landmesser U, Mayr M, Monaco C, Pasterkamp G, Tuñón J, Weber C, On behalf of the ESC Working Group Atherosclerosis and Vascular Biology. Novel methodologies for biomarker discovery in atherosclerosis Circulating biomarkers in atherosclerosis. Eur Heart J 2015;36:2635–2642. [DOI] [PubMed] [Google Scholar]
- 95.Ridker PM, Chasman DI, Zee RY, Parker A, Rose L, Cook NR, Buring JE, Women's Genome Health Study Working G. Rationale, design, and methodology of the Women's Genome Health Study: a genome-wide association study of more than 25,000 initially healthy American women. Clin Chem 2008;54:249–255. [DOI] [PubMed] [Google Scholar]
- 96.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov 2012;11:443–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vakhrusheva O, Braeuer D, Liu Z, Braun T, Bober E. Sirt7-dependent inhibition of cell growth and proliferation might be instrumental to mediate tissue integrity during aging. J Physiol Pharmacol 2008;59(Suppl. 9):201–212. [PubMed] [Google Scholar]