Abstract
Targeted drug delivery has been the major topic in drug formulation and delivery. As nanomedicine emerges to create nano scale therapeutics and diagnostics, it is still essential to embed targeting capability to these novel systems to make them useful. Here we discuss various targeting approaches for delivery of therapeutic and diagnostic nano materials in view of search for more universal methods to target diseased tissues. Many diseases are accompanied with hypoxia and acidosis. Coating nanoparticles with pH Low Insertion Peptides (pHLIPs) increases efficiency of targeting acidic diseased tissues. It has been showing promising results to create future nanotheranostics for cancer and other diseases which are dominating in the present world.
Keywords: pHLIP, nanomedicine, passive targeting, active targeting, tissue acidity, environmental targeting
1. Introduction
For a long time people have been working on developing therapeutic agents so that they can tune pharmacological and pharmacokinetic properties to treat diseases and get desired results. In their continuous quest for food and survival, our ancestors must have experienced the effects of natural sources of pharmacologically active chemical substances produced by organic and inorganic materials such as plants, fungi, insects, animal excreta, reptiles and mineral ores for better or for worse. They must have learnt to extract active ingredients from natural resources using crude methods and enhanced to use as pain killers or to heal wounds or to treat all types of diseases known to them. Ebers papyrus, dated back to ~ 1500 BC provides a detailed description of medical treatments used by ancient Egyptians (Jones, 2011; Shadlen, 2011). In early as 7 century AD, metal, mineral and herbal based particles called Bhashma has been used in Ayurvedic medicine in Indian sub-continent. Modern analysis showed that these formulations contained Fe2 O3, FeS2, CuS and SiO2 and also particle sizes were regulated in the range of 1-2 μm (Mohaptra and Jha, 2010; Pal et al., 2014). Undoubtedly, these ancient knowledge had laid the foundation for modern drug formulation and delivery and made a huge breakthrough in this field as the chemical analysis became first available to us in 19th century (Ansari and Farha Islam, 2012 ).
While the conventional drugs are still being widely used, the innovation of therapeutic nanoparticles has been radically changing the future of drug formulation and delivery (Cai and Chen, 2007; Davis, 2008; Gao et al., 2005; Heath et al., 1980; Shi et al., 2010; Zhang et al., 2007). Nanoparticles are becoming more popular due to their unique tunable physicochemical properties. They have shown promising results in delivery of variety of molecules improving the therapeutic index of drugs by enhancing their efficacy and/or increasing their tolerability in the body. Nano-carries could also improve the bioavailability of water-insoluble drugs, carry large payloads, protect the therapeutic agents from physiological barriers, as well as enable the development of novel classes of bioactive macromolecules (Swami et al., 2012).
2. Targeted delivery of nanoparticles
Almost a century ago, Paul Ehrlich introduced the concept of targeted drug delivery. It was considered as a hypothetical ‘magic bullet’ as an entity consisting of two components — the first one should recognize and bind the target, while the second should provide a therapeutic action in this target. Currently, the concept of ‘magic bullet’ includes a coordinated behavior of three components – drug, targeting moiety and pharmaceutical carrier (Torchilin, 2000). Nanoparticles can be designed to have all three properties of the revised version of Ehrlich's “magic bullet”, and they could be used as therapeutics and/or diagnostics. When designing the nano-drugs it is essential to understand the target region. Target regions could be whole organs (heart, lung, brain, liver and etc), tissues (muscle), cells (nerve, dendrite and etc), disease specific structures (tumor cells) or cellular components. The efficacy of the therapeutics, effectiveness of the diagnostics, safety, affordability and access will measure the final success of nanoparticles in medicine in regard to its applied value to the patients.
3. Three major ways of delivery of nanoparticles
Nanoparticle drug delivery systems use the characteristics of disease tissues to selectively target their payloads, either by passive, active or physical targeting (Egusquiaguirre et al., 2012; Petros and DeSimone, 2010).
3.1 Passive targeting
When nanoparticles localize into specific organs or site of disease via biological mechanisms, such as RES (reticuloendothelial system) or EPR (enhanced permeability and retention) effects, they are known as ‘passive targeting agents’ (Shilo et al., 2012).
RES also called macrophage system or mononuclear phagocyte system, is a class of cells which are part of the body's defense mechanisms. If nanoparticles are not protected against RES, they will end up in liver, spleen or lymph nodes very soon. Even though this seems to be a disadvantage, RES mechanism can be successfully used for mapping liver (Aviv et al., 2009; Hainfeld et al., 2014; Kim et al., 2007; Kojima et al., 2010; Rabin et al., 2006; Shilo et al., 2012), spleen (Boote et al., 2010; Hainfeld et al., 2014; Oh et al., 2011; Rabin et al., 2006; Sun et al., 2009; Xiao et al., 2010) and lymph nodes (Aviv et al., 2009; Oh et al., 2011; Rabin et al., 2006). The information gain from mapping of lymph nodes gives vital indications for cancer staging and metastatic potential of tumor, which could prevent unnecessary dissection surgery. The EPR effect is very common for most of the solid tumors and has been exploiting as a passive mechanism to deliver therapeutic agents. As tumor grows its architecture of vasculature become quite abnormal, showing lack of lymphatic drainage and leaky blood vessels. This allows the long circulating nanoparticles to accumulate in tumor site overtime at higher levels compared to other organs (Acharya and Sahoo, 2011; Greish, 2007; Huang et al., 2012; Khalid et al., 2006; Li and Szoka, 2007; Maruyama, 2011; Rasmussen et al., 2010; Torchilin, 2010; Wang et al., 2012a).
3.2 Active Targeting
Active targeting uses peripherally conjugated specific targeting moieties for enhanced delivery of nanoparticles. The targeting moieties are various ligands including antibodies, peptides, aptamers or small molecules that possess high affinity toward unique molecular signatures found in diseased tissue to achieve active targeting (Byrne et al., 2008). Three general categories of active targeting methods are i) angiogenesis-associated targeting, ii) uncontrolled cell proliferation targeting and iii) tumor cell targeting.
Chemical stimulation for angiogenesis is caused by variety of proangiogenic factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). They are the key ingredients of this very complex biological mechanism which is essential for life. Pathological angiogenesis or abnormally rapid proliferation of blood vessels is common for growth of solid tumors. Therefore the targeting of angiogenesis has become a focus for cancer therapeutics (Chung and Ferrara, 2011; Folkman, 2002; Goth et al., 2003; Hicklin and Ellis, 2005; Jain, 2002; John and Tuszynski, 2001; Khalid et al., 2006; Seaman et al., 2007). Thus, the growth factors are attacked by inhibitors of angiogenesis to regulate the tumor progression (Folkman, 1996; Mousa, 2000).
Another significant target for cancer cells are the cell proliferation markers. These markers are not unique to cancer cells but they are overexpressed in certain cancer cells. The human epidermal receptors (HER), transferrin receptors and folate receptors are widely being employed. Actively targeting nanoparticles have been using the monoclonal antibodies to target overexpressed cell proliferation receptors (Byrne et al., 2008; Gullotti and Yeo, 2009; Mamot et al., 2003; Qian et al., 2002; Sudimack and Lee, 2000).
According to the American Cancer Society statistics, in US, it is estimated that more than 1.6 million new cases of cancer will be diagnosed and more than 0.5 million cancer deaths will occur in 2014. The four most common types of cancers that will be diagnosed in 2014 in the United States are breast in women and prostate in men, lung, and colorectal. Therefore targeting of specific tumor cells is becoming another popular area. FDA has already approved several monoclonal antibodies for the treatment of specific types of cancers. Trastuzumab, a humanized monoclonal antibody against HER-2 which is overexpressed in human breast cancer, for the treatment of HER-2-positive metastatic breast cancer was introduced in 1998. Panitumumab, a human antibody against EGFR produced in transgenic mice (XenoMouse), for the treatment of EGFR-positive colorectal cancer was approved in 2006. Avastin, a humanized antibody against VEGF, for the treatment of metastatic colorectal cancer was introduced in 2004. Cetuximab, a chimeric antibody directed to EGFR (HER-1) for the treatment of advanced colorectal cancer appeared in 2004. Avastin® (Genentech, South San Francisco, CA) is a recombinant humanized anti-VEGF monoclonal antibody for the treatment of non-small cell lung cancer, metastatic colorectal cancer and metastatic breast cancer were approved (Byrne et al., 2008).
The major advantage of the active targeting over the passive targeting is a selective delivery of nanoparticles to the specific tumors/pathogenic tissues, which remain in the site of disease for an extended period of time, thereby increasing the local accumulation of the nanoparticles in the sites of interest. (Baldini et al., 1997; Gonzalez-Angulo et al., 2007; Kaufman, 2006; Meacham and Morrison, 2013). However, the heterogeneity and adaptability of cancers are difficult to overcome, which makes it problematic to create a common cure based on active targeting. Therefore, it is important to identify a universal hallmark for majority of cancers or consider use of external physical stimuli as a targeting strategy.
3.3 Physical Targeting
In many cases, pathological area differs from normal tissues in certain physical properties, such as temperature, lack of oxygen and pH. These natural properties are common for majority of cancers independent of origin, and could be exploited as targeting approaches. The principle behind use of nanoparticles for physical targeting is that a stimulus, which may be applied externally or originate within the pathological site, induces either a physical change in the structure of the nanoparticle itself, thereby causing the eradication of the target or modulating the rate at which an embedded drug is released. pH, lack of oxygen, temperature, ultrasound, electromagnetic radiation and mechanical forces serve as stimuli for physical targeting.
3.3.1 Magnetic-sensitive systems
Use of magnetic sensitive nanoparticles was first proposed by Widder, Senyi and their colleagues in late 1970s (Senyei et al., 1978; Widder et al., 1978). These nanoparticles are primarily fabricated in such way that therapeutic agents are attached to, or encapsulated within, a magnetic core or shell by giving capability of functionalizing their surfaces. Once functionalized, the nanoparticles are injected into the bloodstream, often using a catheter to position the injection site near the target. Powerful rare earth magnets are focused over the target site to apply magnetic fields with high-gradient and the forces affecting the particles. So, the field permits nanoparticles to be captured and released at the target. Clinical trials done by Koda et al. to deliver doxorubicin hydrochloride showed great deal of success for hepatocellular carcinoma (Koda et al., 2002). This method may be effective for targets close to the body's surface, but as the magnetic field strength falls off rapidly with distance, sites deeper within the body become more difficult to target (McBain et al., 2008). Several groups recently proposed a way to overcome this hurdle by implanting magnets near the target site, within the body (Kubo et al., 2000; Yellen et al., 2005). However, this method is invasive and not systematic, which apply significant restrictions for clinical use. Moreover this method would not target metastases, which make cancer a lethal desease.
Key advantages of magnetic NPs are they can be (i) visualized (super paramagnetic NPs are used in MRI);(Andreas et al., 2012; Thorek et al., 2006) (ii) guided or held in place by means of a magnetic field; (iii) heated in a magnetic field to trigger drug release or to produce hyperthermia/ablation of tissue; (iv) targeted specific locations in the body; (v) reduced the quantity of drug needed to attain a particular concentration in the vicinity of the target; (vi) reduced of the concentration of the drug at non target sites minimizing severe side effects and (vii) used to perform magnetic guided surgeries for surgically impossible tumors either due to too hemorrhagic or localized in tissues with high risk of healthy tissue injury.
Despite of these advantages they also inherit limitations. MNPs accumulate throughout the cross section from the external source to the depth marking the effective field limit which is a drawback. Magnetic agglomeration at the absence of magnetic field is tackled by superparamagnetic NPs. Creation of smaller NPs, is essential to achieve super-paramagnetism. As the size becomes smaller, particles’ response to the magnetic field is remarkably reduced. As a result of that it makes difficult to direct particles and keep them in the proximity of the target while withstanding the drag of blood flow.
3.3.2 Ultrasound-sensitive systems
When nanoparticles are used to target solid tumors, homogeneous distribution of most of them in tumor is always questionable. Although nanoparticles can extravasate from the blood to the extracellular matrix, they do not always travel far away from the blood vessels. Thus, only a small population of cancer cells located close to the blood vessels will be exposed to the cytotoxic drugs thus minimizing it's therapeutic index. As nanoparticles stay for longer periods of time, they could target and bring harm to normal tissues. Researchers have explored ways to use ultrasound to enhance the cellular uptake of nanoparticles in the target sites by increasing the permeability of the capillary walls, pushing them through the extracellular matrix, enhancing the release of the drug from the nanoparticles and improving the cellular uptake.
Escoffre and his colleagues showed on 2D and 3D in vitro models that the focused-ultrasound not only helps nanoparticles to penetrate into the interior of the tumor, it also helps to release drugs to the intercellular space without altering chemical properties (Dalecki, 2004; Escoffre et al., 2013; Hagtvet et al., 2011; Pitt et al., 2004b). The use of phenomenon of cavitation, which is the formation of bubbles in a medium exposed to intense focused ultrasound to disrupt the targeted tissue, is another application of this kind. Microbubble-based drug-delivery vehicles can flow through the vasculature into the ultrasound focal zone within the tumor region and they can release payloads upon the rupture. These bubbles can be also used as imaging contrast agents to improve diagnosis and detection of cancer (Dijkmans et al., 2004; Ferrara et al., 2007; Ibsen et al., 2013; Lindner, 2002; Unger et al., 2001).
Non-invasiveness, the absence of ionizing radiations, and the facile regulation of tissue penetration depth by tuning frequency, duty cycles and time of exposure can be identified as the main advantages of using ultrasound sensitive systems (Mura et al., 2013). Ultrasound can trigger release of drugs from various nanocarriers by destabilization. Ultrasound triggered delivery systems has the ability to release drugs into cytosol as result of pore formation in the cell membrane. Therefore they can bypass degenerative endocytotic pathway which is especially useful in DNA transfection (Pitt et al., 2004a). Low frequency ultrasounds can be used to promote the delivery of some liposomal based drugs through the skin to inhibit progression of melanocytic lesions(Tran et al., 2008). However, the increased vessel permeability through ultrasound can also impact negatively by promoting metastatic dissemination. Possible metastatic dissemination can be minimized by using NPs containing agents, which are able to efficiently interact with ultrasonic waves and decrease the threshold frequencies of ultrasound waves. Effectiveness of microbubbles based drug delivery for tissue targeting may be limited by their short lifespan and absence of extravasation. The development of perfluorocarbon (PFC) nanoemulsions and PFC droplets functionalized with aptamers, which can be converted into microbubbles under therapeutic ultrasounds have significantly improved cellular uptake and/or release of drugs in tumor sites(Rapoport et al., 2009).
3.3.3 Temperature-sensitive systems
Above we gave examples of external stimuli applied to diseased area to enhance delivery of nanoparticles and/or release of imaging/therapeutic moieties. Another approach relays on exploration of natural properties or microenvironment in diseased tissue. It is observed that certain types of malignant cancer (bladder, prostate and etc) (Stefanadis et al., 2001) and some other disease conditions show difference in local temperature compared to normal tissues. Understanding of the differences of local temperature in pathological sites paved the path to temperature-sensitive delivery systems. Long circulating, thermo-sensitive nanocarriers can be fabricated and used to exploit micro-environmental temperature differences in diseased sites. For example, in 1978, Yatvin et al. first time suggested a method to use temperature-sensitive liposomes to target mild hyperthermia in disease sites. Active or passive targeting methods have to be first employed to accumulate thermo-sensitive nanocarriers in targeted sites. As the nanocarriers accumulate in target sites, carried drugs are released in response to the micro-environmental temperature in more controlled manner.
In general, the thermo-sensitive polymers have the ability to swell and de-swell, depending on temperature. The two basic types of thermo-sensitive materials are: i) positive temperature-sensitive hydrogels that are swollen and hydrated at higher temperatures and contract on cooling below the upper critical solution temperature (UCST) and ii) negative temperature-sensitive hydrogels that are swollen at lower temperatures and contract on heating above the lower critical solution temperature (LCST). Water solubility of the system decreases as temperature increases. The latest type of system dominates the literature on drug release applications (Ta and Porter, 2013). Upon the introduction of UCST/LCST polymers to nanocarrier systems, they gained the ability to actively respond to the temperature changes in the microenvironment. As they respond to the environmental temperature these systems remarkably change their features such as conformation, solubility and hydrophilic/hydrophobic balance to release therapeutic agents in those sites (Fitzpatrick et al., 2012).
Injectable thermo-sensitive hydrogel systems are also considered as potential thermo-sensitive nano-carries owe to their number of advantages, including simplicity of drug formulation, protective environment for drugs, prolonged and localized drug delivery, and ease of application. Chitosan and related derivatives, poly(N-isopropylacrylamide)-based (PNIPAAM) copolymers, poly(ethylene oxide)/poly(propylene oxide) (PEO/PPO) copolymers and its derivatives, and poly(ethylene glycol)/ biodegradable polyester copolymers are widely used by researchers to construct injectable thermo-sensitive hydrogels (Gong et al., 2013; Lindner et al., 2004; McCoy et al., 2010; Stefanadis et al., 2001; Yatvin et al., 1978).
DPPC/DSPC are the most common lipids used in making thermo sensitive liposomes. With the right lipid composition temperature- dependent fusion of liposomes can be achieved at mild hyperthermia region (39 °C- 42 °C) enhancing increased level of drug available at the diseased site (Ta and Porter, 2013). Early in vivo work done over a range of tumor models, including various carcinomas, sarcomas, and lymph node metastases, and over a range of drugs, including adriamycin, methotrexate, bleomycin and cisplatin showed the benefits of temperature-sensitive liposomes when use with mild hyperthermia (Iga et al., 1991; Maekawa et al., 1987; Nishimura et al., 1990; Tacker and Anderson, 1982; Weinstein et al., 1979; Yatvin et al., 1981; Zou et al., 1990). Temperature sensitive nano-systems offer numerous advantages such as elimination of invasive surgical procedures, the ability to bypass physiological barriers, and allowing drugs to reach hard-to-access sites in the body, ability to stealth the thermo sensitive systems against immune system and environmental degradation and ability to combine this with induced hyperthermia. Owe to extensive studies done in these systems over past three decades that gives the capability for wide variety of choices to design nanocarriers of desired architectures. Limitations of thermo-sensitive nanocarriers can be overcome by combining them with additional stimuli-responsive materials.
3.3.4 Targeting hypoxia
Hypoxia (low oxygen concentrations) plays a vital role in many tumors by contributing to chemoresistance, radioresistance, angiogenesis, vasculogenesis, invasiveness, resistance to cell death, altered metabolism and genomic instability. The hypoxic regions often lie surrounding areas of necrosis in solid tumors (Brahimi-Horn et al., 2007; Brown and Wilson, 2004; Wilson and Hay, 2011). Hypoxia-inducible factor (HIF), which is a transcriptional complex, acts as the hypoxia sensor in a cell. Overexpression of HIF-α, the regulatory subunit of HIF is a measure of increased vascular density, severity of tumor grade, failure of conventional treatment and prognosis. Therefore HIF has become an attractive, direct and indirect therapeutic target in recent years (Carroll and Ashcroft, 2006; Harris, 2002; Poon et al., 2009). Thambi et al. reported about self-assembled nanoparticles called hypoxia-responsive nanoparticles, which can selectively release hydrophobic agents under hypoxic conditions (Thambi et al., 2014). In vivo studies showed selective accumulation of nanoparticles at the hypoxic regions in tumors compared to normal cells.
Development of hypoxia targeting nanoparticles opens new paths to overcome drug delivery impediments mentioned earlier. For instance, the brain which demands high amount of oxygen is vulnerable to hypoxia as a result of irregularity of the blood flow induced by either cardiac failure, traumatic brain injury, brain cancer or subcortical vascular disease. Neuronal dysfunction and cell death in major neurodegenerative diseases also have direct and indirect links to hypoxia. Since HIF involved in virtually all aspects of the response to hypoxia, HIF based nanocarriers have the potential advantage of simultaneously regulating the expression of a large number of genes thus treatment of diseases. However, for HIF related therapies it is vital to discriminate between various HIF isoforms (Freeman, 2005; Rapisarda and Melillo, 2012). Another potential advantage of hypoxia targeting NPs is the ability to treat inaccessible brain tumors, which cannot be achieved through conventional surgeries.
3.3.5 Targeting acidity
It's well known that the cancer cells undergo glycolysis even in the presence of oxygen which has very significant importance to cancer cells survival and proliferation. As a result, high glycolytic activity and production of carbonic and lactic acids, which are intensively pumped out cells to keep intracellular pH near neutral, are common characteristics of solid tumors. It leads to extracellular acidification of tumor microenvironment, which promotes cancer invasiveness and aggressiveness (Gatenby and Gillies, 2008; LaMonte et al., 2013; Mahoney et al., 2003; Wojtkowiak et al., 2011). This acidic microenvironment is common even for several other diseased tissues, such as ischemia, inflammation, arthritis and atherosclerosis. Thus, extracellular acidity might serve as a general marker for detecting and targeting of diseased tissue.
pH-sensitive nanoparticle systems are the one of the well-studied delivery systems. These systems contain pH sensitive polymers, lipids or peptides such as pHLIPs (pH Low Insertion Peptides). Key principle of making pH sensitive fusogenic liposomes is to identify a lipid which is stable at physiological pH but destabilized upon acidification following cellular internalization, thereby, promoting the release of their contents into the cytosol. First generation of such liposomes are designed using the cone shape of phosphatidylethanolamine (PE) lipids (Karanth and Murthy, 2007). Cytoplasmic delivery of membrane-impermeable therapeutic agents has been improved by the DOPE-based pH sensitive liposomes (Chu et al., 1990; Lee et al., 1998; Subbarao et al., 1987). The layer-by-layer (LbL) nanoparticle assembling technique is developed to prepare pH-sensitive nanoparticles. They contain a pH-sensitive outer stealth layer which allows targeting and retaining of nanoparticles in acidic tumor regions (Poon et al., 2011). pH sensitive systems can be also achieved by the self-assembled surfactants. They are mostly known as niosomes and analogues to liposomes. For example, Di Marzio and colleagues reported that the use of non-ionic surfactants polysorbate 20 and polysorbate 21 together with cholesterol can form highly stable pH sensitive nano-vesicles which have the potential to deliver both hydrophilic and hydrophobic therapeutic agents to target sites (Di Marzio et al., 2011). pH sensitive systems also show great success for delivering drugs through oral drug administration due to vast environmental pH differences in gastrointestinal system (Makhlof et al., 2009, 2011; Sonaje et al., 2010). The development of highly pH sensitive NPs to target acidity helps not only for treatments but also for diagnosis of wide array of carcinomas and many other pathological diseases.
4. pHLIP-technology
The pHLIP technology, which was introduced several years ago, has been showing success in targeted drug delivery for cancer and other pathogenic conditions. pHLIP, being a membrane peptide, has affinity to cellular membranes and it targets extracellular acidity. In contrast to other pH-sensitive systems, it “senses” acidity at the surface of cancer cells, where pH is the lowest due to the reversed pH gradient compared to cells in healthy tissue (Chiche et al., 2010).
4.1. Molecular mechanism of pHLIP action
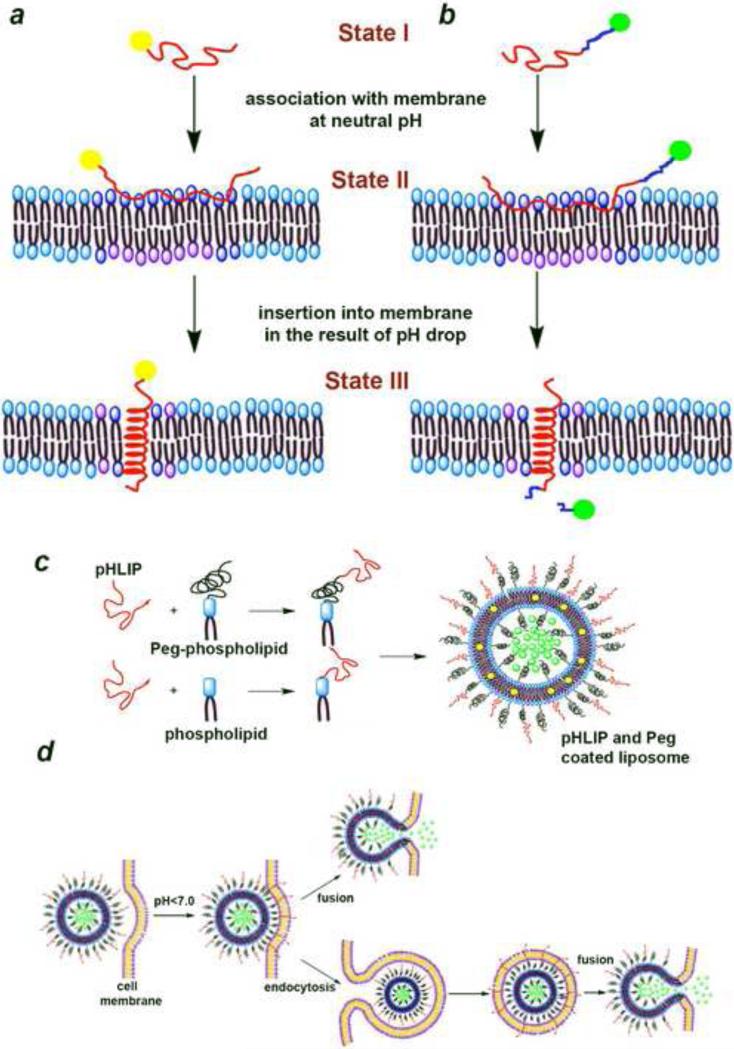
The original pHLIP composed of 36 amino acids is an isolated C-helix of bacteriorhodopsin. The water-soluble pHLIP peptide has been observed to behave differently at different pHs when it is in the vicinity of the lipid bilayer. The pHLIP binds to lipid bilayer surfaces as an unstructured monomer above pH 7, and it spontaneously inserts as an α-helix across lipid bilayers at low pH, with a pKapp of 6 (Hunt et al., 1997). The insertion of pHLIP into membrane, is reversible and unidirectional, in most cases the C-terminus is translocated across the membrane whereas the N-terminus stays outside of the membrane (Reshetnyak et al., 2008; Reshetnyak et al., 2007). The opposite occurs for reverse sequence (Weerakkody et al., 2013). The insertion mechanism is based on the protonation of Asp/Glu residues located in the membrane inserting parts of the pHLIP (Andreev et al., 2014; Barrera et al., 2011; Hunt et al., 1997; Karabadzhak et al., 2012). The thermodynamics and kinetics of pHLIPs membrane-associated folding and insertion has been extensively investigated. Peptide's three major forms (in solution, attached to, and inserted across lipid bilayers ) are monomeric at peptide concentration less than ~ 7 μM (Reshetnyak et al., 2007). The process of insertion and folding (helix formation) is fast and varies from milliseconds to minute depending on peptide sequence (Andreev et al., 2010; Karabadzhak et al., 2012). The energy of peptide's association with bilayer is about 6-7 kcal/mol (Reshetnyak et al., 2008). As pHLIP inserts into the membrane and folds it releases additional energy (about 2 kcal/mol) (Reshetnyak et al., 2008), which could be utilized to tether cargo molecules to the surface of cancer cells (Fig 1a), or move cell impermeable cargo molecules across the membrane into a cell (Fig. 1b). It has been shown tumor targeting of fluorescent, PET and SPECT probes attached to the N-terminus of pHLIP (Cruz-Monserrate et al., 2014; Daumar et al., 2012; Macholl et al., 2012; Reshetnyak et al., 2011; Vavere et al., 2009; Viola-Villegas et al., 2014). As biological membrane remains the main barrier for cytoplasmic drug delivery it was demonstrated that the energy of membrane-associated folding of pHLIP can be used to successfully deliver into cells polar and membrane-impermeable molecules including cyclic peptides, toxins, peptide nucleic acids (PNAs) linked to pHLIP's C-terminus via cleavable links (An et al., 2010; Moshnikova et al., 2013; Reshetnyak et al., 2006; Thévenin et al., 2009; Wijesinghe et al., 2011). Over a decade the original pHLIP peptide has given birth to more than 20 pHLIP variants and available to use as probing and delivery agent (Weerakkody et al., 2013). The ability of pHLIP to probe extracellular acidic environment, which is associated with tumors and many other pathological conditions has opened new avenues for future drug delivery.
Fig.1.
Different applications of pHLIP for targeting of acidic tissues. Tethering of cargo molecules to the surface of cells (a). Cytoplasmic delivery of cargo molecules and it's release by break of cleavable link (shown in blue) (b). Assembly of multifunctional pHLIP-coated liposomes containing polar (green) and hydrophobic (yellow) payloads (c). Schematic presentation of interactions of lipid bilayer of the pHLIP-coated multifunctional liposomes with plasma membrane of a cell (presentation of liposome in the endosome is schematic and not in a scale) (d).
4.2. pHLIP based nanomedicine
Roughly a half a century ago, a British biophysicist and a medical scientist, Alec Bangham made a remarkable observation that phospholipids in aqueous systems can form closed bilayered structures and named them Bangasomes. Today we call them liposomes. After Bangham and his collaborators established the existence of liposomes, the liposomes have moved a long journey from being just another exotic entity of biophysical and colloidal science research and became a pharmaceutical carrier of choice for various real-world applications, as we outlines in previous sections. As a result of that, now liposomal drugs has approved for clinical applications or they are undergoing clinical evaluation (Torchilin, 2005).
When pHLIP is introduced to the conventional liposomes as a coating moiety (Fig. 1c), it allows to enhance the therapeutic value of the liposomes. It was shown that the presence of pHLIP on the surface of PEGylated-liposomes enhanced membrane fusion and lipid exchange in a pH dependent fashion, leading to increase of cellular uptake and payload release (Fig. 1d), and inhibition of cell proliferation by liposomes containing ceramide (Yao et al., 2013b). For liposomal drug production and storage the controlling of their size becomes a major issue. When liposomes are in storage they could fuse with each other and give a poly-disperse liposomal mixture and diminish the desirable properties as nano-carriers. The same study (Yao et al., 2013a) reveals that liposomes containing just 5% of pHLIP lipid composition minimize inter-liposomal fusion and make them stable for several days if stored at neutral pH, since pHLIP brings overall negative charge to the surface of liposomes at neutral and high pHs. At the same time, drop of a pH leads to the protonation of Asp/Glu residues, reduces overall negative charge at the surface of liposomes and enhances interaction of pHLIP-coated liposomes with cellular membranes. It was studied the interaction between pHLIP-coated liposomes and cells as a function of incubation time of 15 min vs. 60 min and it was shown existence of different pathways of liposome-cell interaction. Predominantly direct liposomal fusion with plasma membrane occurs during short incubation period; while during long incubation period, both fusion and cellular internalization through endocytosis (most probably, macro pinocytosis) could happen. Since pegylation helps to prevent opsonization, it promotes longer circulation of liposomes in blood, and enhances probability for liposomes to interact with cell membrane in a pH dependent manner. Thus, the pH-sensitive, “fusogenic” pHLIP-coated liposomes could be used to selectively deliver various diagnostic and therapeutic agents to acidic diseased cells.
Emmetiere et al. (Emmetiere et al., 2013) (Emmetiere et al., 2013) (Emmetiere et al., 2013) introduced dual-delivery approach using pHLIP to tether liposomes to cancer cells in tumor (Emmetiere et al., 2013). First, pHLIP was conjugated to the tetrazine (Tz), which is one of the bioorthogonally reactive small molecules to form pHLIP-Tz conjugate. pHLIP-Tz was injected into mice to selectively label the surface of cancer cells. Then, bioorthogonally reactive trans-cyclooctenes coated liposomes containing 18F PET isotope were given as a second injection. The long circulating radiolabeled liposomes were accumulated in tumor sites mainly via EPR effect, followed the click reaction between tetrazine-pHLIP and trans-cyclooctenes in liposome coat resulting in covalent conjugation and tethering of liposomes to cancer cells. This in vivo click reaction allows achieving high signal/noise ratio, higher accumulation of radiolabeled liposomes in tumor site, low nonspecific binding and reduced toxicity to kidneys and bone marrow.
According to the World Health Organization statistics the ischemic heart diseases are the top leading cause of death, affecting millions of men and women worldwide. Several techniques for passive and active imaging and as well as drug delivery to the diseased cardiac tissue have been developed during past few years (Galagudza et al., 2010; Scott et al., 2008), which including different types of nanoparticles for drug delivery such as liposomes, drug–polymer conjugates, polymeric micelles, dendrimers, nanoshells, and nucleic acid-based carriers (Torchilin, 2005; Verma et al., 2005; Wang et al., 2008). A study was done to evaluate ability of pHLIP-coated liposomes to target ischemic myocardium using two murine ischemia models: regional ischemia induced by coronary artery occlusion and global low-flow ischemia in isolated hearts (Sosunov et al., 2013). In both models, two candidates from the pHLIP family (WT and Var7) were chosen along with pH insensitive kVar7 (K-pHLIP), which has Lys instead of key Asp/Glu residues and cannot insert into membrane at any pH (Weerakkody et al., 2013). It was shown that pHLIP-coated liposomes bind to ischemic regions but not to normal myocardium while kVar7 and liposomes coated just with PEG (no pHLIP) do not show any targeting (Sosunov et al., 2013). The study ensures that pHLIP-coated liposomes have the ability to target ischemic regions with therapeutic agents as well as to mark disease regions to assist pHLIP-fluorescent-aided cardiac surgeries.
Another novel approach of pHLIP-coated-liposomes is the delivery of nano-pores to induce apoptosis in cancer cells (Wijesinghe et al., 2013). Proper ion balance between intracellular and extracellular media is crucial for normal cell functioning. Any alterations in the conductance of membranes for ions will lead to cell death. To change cellular ion ballance the pore-forming gramicidin A (10 mol%) was delivered to cellular membrane using pHLIP-coated DOPC liposomes. Liposomes were stable for more than a month in 4 °C. Hydrophobic gramicidin A monomers were introduced into pHLIP-coated-liposomes, where it makes beta-helices and forms transmembrane porse with diameter of 4-5 Å that can transfer monovalent cations through the membrane at rate of 107 cations per second (Hladky and Haydon, 1972). As we outlined above, when pHLIP-coated liposomes target cancer cells, they either fuse with the plasma membrane or are up-taken by endocytosis and then fuse with lysosomal membranes. It leads to delivery of gramicidin A pores (nano-pores) to cellular membranes of cancer cells. These nano-pores acidify the intracellular space and eliminate the vital Na+/K+ ion balance. This method not only opens pathways to treat acidic solid tumors, but also give opportunity to deliver various membrane peptides and proteins to the cells, widening the applications in biotechnology and nano-medicine.
Nano-gold particles have also caught attention in pHLIP-nanotechnology. Beneficial properties of gold in medicine has been considered for centuries (Wang et al., 2012b). And gold has been used in modern science as a contrast agent in electron microscopy. There are well-established methods to obtain uniform stable gold nano-structures in the range of few nanometers up to couple hundred of nanometers (Papasani et al., 2012). Tuning size and shape of nano-gold structures allows to obtain unique physical properties, which can be very useful for multiple applications across both therapeutics and diagnostics (Brullot et al., 2012). Functionalized nano-gold particles are valuable for wide variety of nanomedicinal uses and now they are undergoing through evaluations of toxicity (Papasani et al., 2012), stability (Gao et al., 2012), pharmacokinetics (Simpson et al., 2013), cellular trafficking (Gu et al., 2012; Lin et al., 2012; Sadauskas et al., 2009; Wang et al., 2013), efficacy for gene regulation (Sharma et al., 2011) and drug delivery (Gu et al., 2012), and use in photo-thermal therapies (Letfullin et al., 2011; Raoof et al., 2012).
Lan et al. showed that nano-gold particles which are 1.4 nm size in diameter conjugated to N-terminus of pHLIP can successfully target cancer cells at low pH (Yao et al., 2013a). In vivo studies done using mice-tumor models demonstrated high tumor uptake in both intravenous and intra-tumoral administration compared to non-functionalized nano-gold particles. The beauty of pHLIP-nano gold technology is in its capability of providing the specific targeting, enhancing local concentration in tumor mass in homogeneous way while remaining in cells for an extended period (several days), by allowing applications of radiation therapy and imaging.
Davies and colleagues employed pHLIP to deliver luminescent europium coated nanoparticles into platelets (Davies et al., 2012). The 13 nm gold nanoparticles were co-coated with a europium luminescent, EuL and the pHLIP to give pHLIP.EuL.Au. Human platelets are vulnerable to transfection or microinjection. But with this method authors could deliver nanoparticles which have roughly 640 lanthanide probes per particle. The result shows that the internalization of nanoparticles into the platelets happened only at low pH and not at normal pH. The significance of this research is that pHLIP can translocate multimodal nanoparticles in a pH dependent manner.
Non-viral vectors are also a hot topic in gene therapy due to their advantages over viral vectors such as simplicity of use, ease of large-scale production and lack of specific immune response (Niidome and Huang, 2002). Recently it was reported a novel use of pHLIP to deliver pDNA to tumor cells (Han et al., 2013a). DGL-PEG-pHLIP were made by conjugating the surface of dendrigraft poly-L-lysines (DGLs, generation 3 with 123 amino groups per molecule) to the N-terminus of pHLIP followed by electrostatic interactions between negatively charged pDNA and positively charged DGL head group of DGL-PEG-pHLIP to create DGL-PEG-pHLIP/pDNA nanoparticles. Results of in vitro studies showed that higher cellular uptake of nanoparticles occurs at low pH (pH 6.0) compared to normal pH (pH 7.4) and nanoparticles enter cells mainly by adsorptive mediated endocytosis. The in vivo studies also followed the same pattern by showing high tumor uptake of DGL-PEG-pHLIP/pDNA nanoparticles. It was clear that pHLIP enhanced the pH-controlled localization of the nanoparticles in tumors.
Zhao and colleagues (2013) show that pHLIP peptide not only targets acidic tumor micro environment but also it can release nanoparticles in a controlled manner to the intracellular space. They chose mesoporous silica nanoparticles (MSN), in particular MCN-41, to load with doxorubicin. MSN has the attributes of high homogeneous porosity, inertness, biocompatibility, high payload capacity and easy surface functionalization capability. The MSN particles were attached to the C-terminus of pHLIP by disulfide bond. At low pH (< 6.5), doxorubicin-loaded pHLIPss-MSN rapidly inserted into the cell membrane and translocated MSN into cytoplasm. The disulfide bond was cleaved in cytoplasm to release doxorubicin.
Table 1 summarizes the current pHLIP applications in nanotechnology we discussed above.
Table 1.
Current pHLIP applications in nanotechnology
| pHLIP nanotechnology | How it works | Administration | Authors |
|---|---|---|---|
| PEGylated-liposomes containing ceramide | Increase ceramide levels in cancer tissues promote cell death and tumor inhibition | In-vitro and In-vivo | (Yao et al., 2013c) |
| pHLIP-Tz conjugates and trans-cyclooctenes coated liposomes containing 18F PET isotopes | Tetrazine(Tz) labeled cancer cells are targeted by trans-cyclooctenes coated liposomes to radio label the tumor site | In-vivo | (Emmetiere et al., 2013) |
| WT and Var7 coated liposomes | pHLIP coated liposomes binds to ischemic regions and assist pHLIP-fluorescent -aided cardiac surgeries | In-vivo | (Sosunov et al., 2013) |
| pHLIP liposomal nano-pores | Hydrophobic gA is delivered to cancer cells to form channels in cell membrane to destroy ion balance and induce apoptosis | In-vitro | (Wijesinghe et al., 2013) |
| pHLIP nano-gold particles | Homogeneous accumulation of gold in tumor sites allow radiation therapy and imaging | In-vivo | (Yao et al., 2013a) |
| Eu coated pHLIP nanoparticles | At low pH, more lanthanide probes can be internalized to human platelets breaking the targeting barrier | In-vitro | (Davies et al., 2012) |
| pDNA nanoparticles | High uptake of DGL-PEG-pHLIP/pDNA in tumors | In-vivo, In-vitro | (Han et al., 2013b) |
| dox MSNs | Dox are loaded to porous silica nanoparticles and deliver to cancer cells using pHLIP | In-vitro | (Zhao et al., 2013) |
5. Conclusion
There are several challenges for targeted nano drug delivery systems to overcome. Still most of these drug systems undergo in vitro and in vivo testing using animal models. Therefore their relevancy to the real patients has to be evaluated extensively. Each nano drug platform is distinctive and need to be assessed experimentally as new system, which is strenuous. Nanoparticles stability, size uniformity, controlled drug release rate, sterile preparations in large scale and manufacturing cost have to be addressed in order to make them available to the market. But with recent scientific advances, next ten years it is expected to see large number of targeted drug delivery systems based on nanoparticles in the market. pHLIP nanotechnology has been showing its potential capability to address most of the challenges mentioned above. pHLIP technology used alone or combined with other approaches might lead to new formulations translatable to clinics.
Highlights.
Nanomaterials are used to target both imaging and therapeutic agents
Targeting a tumor environment might better address issue of tumor heterogeneity
pHLIP senses acidity on the surface of cells
pHLIP targets nanomaterials to acidic diseased tissue
pHLIP promotes cytoplasmic delivery of nanomaterial's payloads
Acknowledgments
The work was supported by the NIH grants CA133890, CA174413 and GM073857 to OAA and YKR.
Abbreviations
- bFGF
basic fibroblast growth factor
- EGFR
epidermal growth factor receptor
- DGLs
dendrigraft poly-L-lysines
- DPPC
1,2-dihexadecanoyl-sn-glycero-3-phosphocholine
- DSPC
1,2-dioctadecanoyl-sn-glycero-3-phosphocholine
- EuL
europium luminescent
- HER
human epidermal receptors
- HIF
Hypoxia-inducible factor
- LbL
Layer-by-Layer
- MSP
mesoporous silica nanoparticles
- PEO/PPO
poly(ethylene oxide)/poly(propylene oxide)
- PET
positron emission tomography
- pHLIP
pH Low Insertion Peptide
- SPECT
single photon emission computed tomography
- Tz
tetrazine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Advanced drug delivery reviews. 2011;63:170–183. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- An M, Wijesinghe D, Andreev OA, Reshetnyak YK, Engelman DM. pH-(low)-insertion-peptide (pHLIP) translocation of membrane impermeable phalloidin toxin inhibits cancer cell proliferation. Proceedings of the National Academy of Sciences. 2010;107:20246–20250. doi: 10.1073/pnas.1014403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreas K, Georgieva R, Ladwig M, Mueller S, Notter M, Sittinger M, Ringe J. Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials. 2012;33:4515–4525. doi: 10.1016/j.biomaterials.2012.02.064. [DOI] [PubMed] [Google Scholar]
- Andreev OA, Engelman DM, Reshetnyak YK. Targeting diseased tissues by pHLIP insertion at low cell surface pH. Frontiers in physiology. 2014;5 doi: 10.3389/fphys.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari S, Farha Islam M. Influence of nanotechnology on herbal drugs: A Review. Journal of advanced pharmaceutical technology & research. 2012;3:142. doi: 10.4103/2231-4040.101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H, Bartling S, Kieslling F, Margel S. Radiopaque iodinated copolymeric nanoparticles for X-ray imaging applications. Biomaterials. 2009;30:5610–5616. doi: 10.1016/j.biomaterials.2009.06.038. [DOI] [PubMed] [Google Scholar]
- Baldini M, Healey E, Recht M, Strauss M, Gary M, DeCamp M, Jr, Malcolm M, Swanson M, Scott J, Liptay M. Patterns of failure after trimodality therapy for malignant pleural mesothelioma. The Annals of thoracic surgery. 1997;63:334–338. doi: 10.1016/s0003-4975(96)01228-3. [DOI] [PubMed] [Google Scholar]
- Barrera FN, Weerakkody D, Anderson M, Andreev OA, Reshetnyak YK, Engelman DM. Roles of carboxyl groups in the transmembrane insertion of peptides. J Mol Biol. 2011;413:359–371. doi: 10.1016/j.jmb.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boote E, Fent G, Kattumuri V, Casteel S, Katti K, Chanda N, Kannan R, Katti K, Churchill R. Gold nanoparticle contrast in a phantom and juvenile swine: models for molecular imaging of human organs using x-ray computed tomography. Academic radiology. 2010;17:410–417. doi: 10.1016/j.acra.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahimi-Horn MC, Chiche J, Pouysségur J. Hypoxia and cancer. J Mol Med. 2007;85:1301–1307. doi: 10.1007/s00109-007-0281-3. [DOI] [PubMed] [Google Scholar]
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nature Reviews Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- Brullot W, Valev VK, Verbiest T. Magnetic-plasmonic nanoparticles for the life sciences: calculated optical properties of hybrid structures. Nanomedicine : nanotechnology, biology, and medicine. 2012;8:559–568. doi: 10.1016/j.nano.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Advanced drug delivery reviews. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1α versus HIF-2α in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer research. 2006;66:6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- Chiche J, Brahimi-Horn MC, Pouyssegur J. Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med. 2010;14:771–794. doi: 10.1111/j.1582-4934.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C-J, Dijkstra J, Lai M-Z, Hong K, Szoka FC. Efficiency of cytoplasmic delivery by pH-sensitive liposomes to cells in culture. Pharmaceutical research. 1990;7:824–834. doi: 10.1023/a:1015908831507. [DOI] [PubMed] [Google Scholar]
- Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annual review of cell and developmental biology. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- Cruz-Monserrate Z, Roland CL, Deng D, Arumugam T, Moshnikova A, Andreev OA, Reshetnyak YK, Logsdon CD. Targeting pancreatic ductal adenocarcinoma acidic microenvironment. Sci Rep. 2014;4:4410. doi: 10.1038/srep04410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalecki D. Mechanical bioeffects of ultrasound. Annu. Rev. Biomed. Eng. 2004;6:229–248. doi: 10.1146/annurev.bioeng.6.040803.140126. [DOI] [PubMed] [Google Scholar]
- Daumar P, Wanger-Baumann CA, Pillarsetty N, Fabrizio L, Carlin SD, Andreev OA, Reshetnyak YK, Lewis JS. Efficient (18)F-Labeling of Large 37-Amino-Acid pHLIP Peptide Analogues and Their Biological Evaluation. Bioconjug Chem. 2012;23:1557–1566. doi: 10.1021/bc3000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Lewis DJ, Watson SP, Thomas SG, Pikramenou Z. pH-controlled delivery of luminescent europium coated nanoparticles into platelets. Proceedings of the National Academy of Sciences. 2012;109:1862–1867. doi: 10.1073/pnas.1112132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature reviews Drug discovery. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- Di Marzio L, Marianecci C, Petrone M, Rinaldi F, Carafa M. Novel pH-sensitive non-ionic surfactant vesicles: comparison between Tween 21 and Tween 20. Colloids and Surfaces B: Biointerfaces. 2011;82:18–24. doi: 10.1016/j.colsurfb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Dijkmans PA, Juffermans LJM, Musters RJP, van Wamel A, ten Cate FJ, van Gilst W, Visser CA, de Jong N, Kamp O. Microbubbles and ultrasound: from diagnosis to therapy. European Journal of Echocardiography. 2004;5:245–246. doi: 10.1016/j.euje.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Egusquiaguirre SP, Igartua M, Hernández RM, Pedraz JL. Nanoparticle delivery systems for cancer therapy: advances in clinical and preclinical research. Clinical and Translational Oncology. 2012;14:83–93. doi: 10.1007/s12094-012-0766-6. [DOI] [PubMed] [Google Scholar]
- Emmetiere F, Irwin C, Viola-Villegas NT, Longo V, Cheal SM, Zanzonico P, Pillarsetty N, Weber WA, Lewis JS, Reiner T. (18)F-labeled-bioorthogonal liposomes for in vivo targeting. Bioconjug Chem. 2013;24:1784–1789. doi: 10.1021/bc400322h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoffre J, Novell A, de Smet M, Bouakaz A. Focused ultrasound mediated drug delivery from temperature-sensitive liposomes: in-vitro characterization and validation. Physics in medicine and biology. 2013;58:8135. doi: 10.1088/0031-9155/58/22/8135. [DOI] [PubMed] [Google Scholar]
- Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Biomedical Engineering. 2007;9 doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SD, Fitzpatrick LE, Thakur A, Mazumder MAJ, Sheardown H. Temperature-sensitive polymers for drug delivery. 2012 doi: 10.1586/erd.12.24. [DOI] [PubMed] [Google Scholar]
- Folkman J. Fighting cancer by attacking its blood supply. Scientific American. 1996;275:150–156. doi: 10.1038/scientificamerican0996-150. [DOI] [PubMed] [Google Scholar]
- Folkman J. Seminars in oncology. Elsevier; 2002. Role of angiogenesis in tumor growth and metastasis. pp. 15–18. [DOI] [PubMed] [Google Scholar]
- Freeman R. Targeting Hypoxia-Inducible Factor (HIF) as a Therapeutic Strategy for CNS Disorders. Current Drug Target -CNS & Neurological Disorders. 2005;4:85–92. doi: 10.2174/1568007053005154. [DOI] [PubMed] [Google Scholar]
- Galagudza MM, Korolev DV, Sonin DL, Postnov VN, Papayan GV, Uskov IS, Belozertseva AV, Shlyakhto EV. Targeted drug delivery into reversibly injured myocardium with silica nanoparticles: surface functionalization, natural biodistribution, and acute toxicity. International journal of nanomedicine. 2010;5:231. doi: 10.2147/ijn.s8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Huang X, Liu H, Zan F, Ren J. Colloidal stability of gold nanoparticles modified with thiol compounds: bioconjugation and application in cancer cell imaging. Langmuir. 2012;28:4464–4471. doi: 10.1021/la204289k. [DOI] [PubMed] [Google Scholar]
- Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. < i> In vivo</i> molecular and cellular imaging with quantum dots. Current Opinion in Biotechnology. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nature Reviews Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- Gong C, Qi T, Wei X, Qu Y, Wu Q, Luo F, Qian Z. Thermosensitive polymeric hydrogels as drug delivery systems. Current medicinal chemistry. 2013;20:79–94. [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Breast Cancer Chemosensitivity. Springer; 2007. Overview of resistance to systemic therapy in patients with breast cancer. pp. 1–22. [DOI] [PubMed] [Google Scholar]
- Goth MI, Hubina E, Raptis S, Nagy GM, Tóth BE. Physiological and pathological angiogenesis in the endocrine system. Microscopy research and technique. 2003;60:98–106. doi: 10.1002/jemt.10248. [DOI] [PubMed] [Google Scholar]
- Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: A royal gate for targeted anticancer nanomedicines. Journal of Drug Targeting. 2007;15:457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- Gu Y-J, Cheng J, Man CW-Y, Wong W-T, Cheng SH. Gold-doxorubicin nanoconjugates for overcoming multidrug resistance. Nanomedicine : nanotechnology, biology, and medicine. 2012;8:204–211. doi: 10.1016/j.nano.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Gullotti E, Yeo Y. Extracellularly Activated Nanocarriers: A New Paradigm of Tumor Targeted Drug Delivery. Molecular pharmaceutics. 2009;6:1041–1051. doi: 10.1021/mp900090z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagtvet E, Evjen TJ, Olsen DR, Fossheim SL, Nilssen EA. Ultrasound enhanced antitumor activity of liposomal doxorubicin in mice. Journal of Drug Targeting. 2011;19:701–708. doi: 10.3109/1061186X.2010.551401. [DOI] [PubMed] [Google Scholar]
- Hainfeld J, Slatkin D, Focella T, Smilowitz H. Gold nanoparticles: a new X-ray contrast agent. 2014 doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- Han L, Ma H, Guo Y, Kuang Y, He X, Jiang C. pH-Controlled Delivery of Nanoparticles into Tumor Cells. Adv Healthc Mater. 2013a doi: 10.1002/adhm.201300013. [DOI] [PubMed] [Google Scholar]
- Han L, Ma H, Guo Y, Kuang Y, He X, Jiang C. pH-Controlled Delivery of Nanoparticles into Tumor Cells. Advanced healthcare materials. 2013b;2:1435–1439. doi: 10.1002/adhm.201300013. [DOI] [PubMed] [Google Scholar]
- Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nature Reviews Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Heath TD, Fraley RT, Papahdjopoulos D. Antibody targeting of liposomes: cell specificity obtained by conjugation of F (ab′) 2 to vesicle surface. Science. 1980;210:539–541. doi: 10.1126/science.7423203. [DOI] [PubMed] [Google Scholar]
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. Journal of Clinical Oncology. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Hladky SB, Haydon DA. Ion transfer across lipid membranes in the presence of gramicidin A: I. Studies of the unit conductance channel. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1972;274:294–312. doi: 10.1016/0005-2736(72)90178-2. [DOI] [PubMed] [Google Scholar]
- Huang K, Ma H, Liu J, Huo S, Kumar A, Wei T, Zhang X, Jin S, Gan Y, Wang PC, He S, Zhang X, Liang X-J. Size-Dependent Localization and Penetration of Ultrasmall Gold Nanoparticles in Cancer Cells, Multicellular Spheroids, and Tumors in Vivo. ACS Nano. 2012;6:4483–4493. doi: 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JF, Rath P, Rothschild KJ, Engelman DM. Spontaneous, pH-dependent membrane insertion of a transbilayer α-helix. Biochemistry. 1997;36:15177–15192. doi: 10.1021/bi970147b. [DOI] [PubMed] [Google Scholar]
- Ibsen S, Schutt CE, Esener S. Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug design, development and therapy. 2013;7:375. doi: 10.2147/DDDT.S31564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iga K, Hamaguchi N, Igari Y, Ogawa Y, Gotoh K, Ootsu K, Toguchi H, Shimamoto T. Enhanced antitumor activity in mice after administration of thermosensitive liposome encapsulating cisplatin with hyperthermia. Journal of Pharmacology and Experimental Therapeutics. 1991;257:1203–1207. [PubMed] [Google Scholar]
- Jain RK. Tumor angiogenesis and accessibility: Role of vascular endothelial growth factor. Seminars in Oncology. 2002;29:3–9. doi: 10.1053/sonc.2002.37265. [DOI] [PubMed] [Google Scholar]
- John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol. Oncol. Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- Jones AW. Early drug discovery and the rise of pharmaceutical chemistry. Drug testing and analysis. 2011;3:337–344. doi: 10.1002/dta.301. [DOI] [PubMed] [Google Scholar]
- Karabadzhak AG, Weerakkody D, Wijesinghe D, Thakur MS, Engelman DM, Andreev OA, Markin VS, Reshetnyak YK. Modulation of the pHLIP transmembrane helix insertion pathway. Biophys J. 2012;102:1846–1855. doi: 10.1016/j.bpj.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanth H, Murthy R. pH-Sensitive liposomes-principle and application in cancer therapy. Journal of pharmacy and pharmacology. 2007;59:469–483. doi: 10.1211/jpp.59.4.0001. [DOI] [PubMed] [Google Scholar]
- Kaufman D. Challenges in the treatment of bladder cancer. Annals of Oncology. 2006;17:v106–v112. doi: 10.1093/annonc/mdj963. [DOI] [PubMed] [Google Scholar]
- Khalid M, Simard P, Hoarau D, Dragomir A, Leroux J-C. Long Circulating Poly(Ethylene Glycol)-Decorated Lipid Nanocapsules Deliver Docetaxel to Solid Tumors. Pharmaceutical Research. 2006;23:752–758. doi: 10.1007/s11095-006-9662-5. [DOI] [PubMed] [Google Scholar]
- Kim D, Park S, Lee JH, Jeong YY, Jon S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. Journal of the American Chemical Society. 2007;129:7661–7665. doi: 10.1021/ja071471p. [DOI] [PubMed] [Google Scholar]
- Koda J, Venook A, Walser E, Goodwin S. European Journal of Cancer. PERGAMON-ELSEVIER SCIENCE LTD; THE BOULEVARD, LANGFORD LANE, KIDLINGTON, OXFORD OX5 1GB, ENGLAND: 2002. A multicenter, phase I/II trial of hepatic intra-arterial delivery of doxorubicin hydrochloride adsorbed to magnetic targeted carriers in patients with hepatocellular carcinoma. pp. S18–S18. [Google Scholar]
- Kojima C, Umeda Y, Ogawa M, Harada A, Magata Y, Kono K. X-ray computed tomography contrast agents prepared by seeded growth of gold nanoparticles in PEGylated dendrimer. Nanotechnology. 2010;21:245104. doi: 10.1088/0957-4484/21/24/245104. [DOI] [PubMed] [Google Scholar]
- Kubo T, Sugita T, Shimose S, Nitta Y, Ikuta Y, Murakami T. Targeted delivery of anticancer drugs with intravenously administered magnetic liposomes in osteosarcoma-bearing hamsters. International journal of oncology. 2000;17:309–324. doi: 10.3892/ijo.17.2.309. [DOI] [PubMed] [Google Scholar]
- LaMonte G, Tang X, Chen JL-Y, Wu J, Ding C-KC, Keenan MM, Sangokoya C, Kung H-N, Ilkayeva O, Boros LG. Acidosis induces reprogramming of cellular metabolism to mitigate oxidative stress. Cancer & metabolism. 2013;1:23. doi: 10.1186/2049-3002-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Wang S, Turk M, Low P. The effects of pH and intraliposomal buffer strength on the rate of liposome content release and intracellular drug delivery. Bioscience reports. 1998;18:69–78. doi: 10.1023/a:1020132226113. [DOI] [PubMed] [Google Scholar]
- Letfullin RR, Iversen CB, George TF. Modeling nanophotothermal therapy: kinetics of thermal ablation of healthy and cancerous cell organelles and gold nanoparticles. Nanomedicine : nanotechnology, biology, and medicine. 2011;7:137–145. doi: 10.1016/j.nano.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Li W, Szoka F., Jr. Lipid-based Nanoparticles for Nucleic Acid Delivery. Pharmaceutical Research. 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- Lin IC, Liang M, Liu T-Y, Monteiro MJ, Toth I. Cellular transport pathways of polymer coated gold nanoparticles. Nanomedicine : nanotechnology, biology, and medicine. 2012;8:8–11. doi: 10.1016/j.nano.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Lindner JR. Evolving applications for contrast ultrasound. The American Journal of Cardiology. 2002;90:72–80. doi: 10.1016/s0002-9149(02)02951-x. [DOI] [PubMed] [Google Scholar]
- Lindner LH, Eichhorn ME, Eibl H, Teichert N, Schmitt-Sody M, Issels RD, Dellian M. Novel temperature-sensitive liposomes with prolonged circulation time. Clinical Cancer Research. 2004;10:2168–2178. doi: 10.1158/1078-0432.ccr-03-0035. [DOI] [PubMed] [Google Scholar]
- Macholl S, Morrison MS, Iveson P, Arbo BE, Andreev OA, Reshetnyak YK, Engelman DM, Johannesen E. In vivo pH imaging with (99m)Tc-pHLIP. Mol Imaging Biol. 2012;14:725–734. doi: 10.1007/s11307-012-0549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa S, Sugimachi K, Kitamura M. Selective treatment of metastatic lymph nodes with combination of local hyperthermia and temperature-sensitive liposomes containing bleomycin. Cancer treatment reports. 1987;71:1053–1059. [PubMed] [Google Scholar]
- Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics: I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochemical pharmacology. 2003;66:1207–1218. doi: 10.1016/s0006-2952(03)00467-2. [DOI] [PubMed] [Google Scholar]
- Makhlof A, Tozuka Y, Takeuchi H. pH-Sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. European Journal of Pharmaceutics and Biopharmaceutics. 2009;72:1–8. doi: 10.1016/j.ejpb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Makhlof A, Tozuka Y, Takeuchi H. Design and evaluation of novel pH-sensitive chitosan nanoparticles for oral insulin delivery. European Journal of Pharmaceutical Sciences. 2011;42:445–451. doi: 10.1016/j.ejps.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Mamot C, Drummond DC, Greiser U, Hong K, Kirpotin DB, Marks JD, Park JW. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR-and EGFRvIII-overexpressing tumor cells. Cancer research. 2003;63:3154–3161. [PubMed] [Google Scholar]
- Maruyama K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Advanced drug delivery reviews. 2011;63:161–169. doi: 10.1016/j.addr.2010.09.003. [DOI] [PubMed] [Google Scholar]
- McBain SC, Yiu HH, Dobson J. Magnetic nanoparticles for gene and drug delivery. International journal of nanomedicine. 2008;3:169. doi: 10.2147/ijn.s1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy CP, Brady C, Cowley JF, McGlinchey SM, McGoldrick N, Kinnear DJ, Andrews GP, Jones DS. Triggered drug delivery from biomaterials. Expert opinion on drug delivery. 2010;7:605–616. doi: 10.1517/17425241003677731. [DOI] [PubMed] [Google Scholar]
- Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohaptra S, Jha C. Physicochemical characterization of Ayurvedic bhasma (Swarna makshika bhasma): An approach to standardization. International journal of Ayurveda research. 2010;1:82. doi: 10.4103/0974-7788.64409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshnikova A, Moshnikova V, Andreev OA, Reshetnyak YK. Antiproliferative effect of pHLIP-amanitin. Biochemistry. 2013;52:1171–1178. doi: 10.1021/bi301647y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa SA. Angiogenesis Inhibitors and Stimulators: Potential Therapeutic Implications. 2000 [Google Scholar]
- Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene therapy. 2002;9:1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Ono K, Hiraoka M, Masunaga S.-i., Jo S, Shibamoto Y, Sasai K, Abe M, Iga K, Ogawa Y. Treatment of murine SCC VII tumors with localized hyperthermia and temperature-sensitive liposomes containing cisplatin. Radiation research. 1990;122:161–167. [PubMed] [Google Scholar]
- Oh MH, Lee N, Kim H, Park SP, Piao Y, Lee J, Jun SW, Moon WK, Choi SH, Hyeon T. Large-scale synthesis of bioinert tantalum oxide nanoparticles for X-ray computed tomography imaging and bimodal image-guided sentinel lymph node mapping. Journal of the American Chemical Society. 2011;133:5508–5515. doi: 10.1021/ja200120k. [DOI] [PubMed] [Google Scholar]
- Pal D, Sahu CK, Haldar A. Bhasma: The ancient Indian nanomedicine. Journal of advanced pharmaceutical technology & research. 2014;5:4. doi: 10.4103/2231-4040.126980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasani MR, Wang G, Hill RA. Gold nanoparticles: the importance of physiological principles to devise strategies for targeted drug delivery. Nanomedicine : nanotechnology, biology, and medicine. 2012;8:804–814. doi: 10.1016/j.nano.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nature Reviews Drug Discovery. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery-a general review. Expert opinion on drug delivery. 2004a;1:37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery – a general review. Expert Opinion on Drug Delivery. 2004b;1:37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon E, Harris AL, Ashcroft M. Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert reviews in molecular medicine. 2009;11:e26. doi: 10.1017/S1462399409001173. [DOI] [PubMed] [Google Scholar]
- Poon Z, Chang D, Zhao X, Hammond PT. Layer-by-layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumor hypoxia. ACS nano. 2011;5:4284–4292. doi: 10.1021/nn200876f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacological reviews. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- Rabin O, Perez JM, Grimm J, Wojtkiewicz G, Weissleder R. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nature materials. 2006;5:118–122. doi: 10.1038/nmat1571. [DOI] [PubMed] [Google Scholar]
- Raoof M, Corr SJ, Kaluarachchi WD, Massey KL, Briggs K, Zhu C, Cheney MA, Wilson LJ, Curley SA. Stability of antibody-conjugated gold nanoparticles in the endolysosomal nanoenvironment: implications for noninvasive radiofrequency-based cancer therapy. Nanomedicine : nanotechnology, biology, and medicine. 2012;8:1096–1105. doi: 10.1016/j.nano.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapisarda A, Melillo G. Overcoming disappointing results with antiangiogenic therapy by targeting hypoxia. Nature Reviews Clinical Oncology. 2012;9:378–390. doi: 10.1038/nrclinonc.2012.64. [DOI] [PubMed] [Google Scholar]
- Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam K-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. Journal of Controlled Release. 2009;138:268–276. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JW, Martinez E, Louka P, Wingett DG. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opinion on Drug Delivery. 2010;7:1063–1077. doi: 10.1517/17425247.2010.502560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshetnyak Y, Yao L, Zheng S, Kuznetsov S, Engelman D, Andreev O. Measuring Tumor Aggressiveness and Targeting Metastatic Lesions with Fluorescent pHLIP. Mol Imaging Biol. 2011;13:1146–1156. doi: 10.1007/s11307-010-0457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshetnyak YK, Andreev OA, Lehnert U, Engelman DM. Translocation of molecules into cells by pH-dependent insertion of a transmembrane helix. Proceedings of the National Academy of Sciences. 2006;103:6460–6465. doi: 10.1073/pnas.0601463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshetnyak YK, Andreev OA, Segala M, Markin VS, Engelman DM. Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane. Proceedings of the National Academy of Sciences. 2008;105:15340–15345. doi: 10.1073/pnas.0804746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshetnyak YK, Segala M, Andreev OA, Engelman DM. A monomeric membrane peptide that lives in three worlds: in solution, attached to, and inserted across lipid bilayers. Biophysical journal. 2007;93:2363–2372. doi: 10.1529/biophysj.107.109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadauskas E, Danscher G, Stoltenberg M, Vogel U, Larsen A, Wallin H. Protracted elimination of gold nanoparticles from mouse liver. Nanomedicine : nanotechnology, biology, and medicine. 2009;5:162–169. doi: 10.1016/j.nano.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Scott RC, Crabbe D, Krynska B, Ansari R, Kiani MF. Aiming for the heart: targeted delivery of drugs to diseased cardiac tissue. 2008 doi: 10.1517/17425247.5.4.459. [DOI] [PubMed] [Google Scholar]
- Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St. Croix B. Genes that Distinguish Physiological and Pathological Angiogenesis. Cancer Cell. 2007;11:539–554. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyei A, Widder K, Czerlinski G. Magnetic guidance of drug-carrying microspheres. Journal of Applied Physics. 1978;49:3578–3583. [Google Scholar]
- Shadlen KC. early drug discovery and the rise of pharmaceutical chemistry. 2011 doi: 10.1002/dta.301. [DOI] [PubMed] [Google Scholar]
- Sharma A, Tandon A, Tovey JCK, Gupta R, Robertson JD, Fortune JA, Klibanov AM, Cowden JW, Rieger FG, Mohan RR. Polyethylenimine-conjugated gold nanoparticles: Gene transfer potential and low toxicity in the cornea. Nanomedicine : nanotechnology, biology, and medicine. 2011;7:505–513. doi: 10.1016/j.nano.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano letters. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo M, Reuveni T, Motiei M, Popovtzer R. Nanoparticles as computed tomography contrast agents: current status and future perspectives. Nanomedicine : nanotechnology, biology, and medicine. 2012;7:257–269. doi: 10.2217/nnm.11.190. [DOI] [PubMed] [Google Scholar]
- Simpson CA, Salleng KJ, Cliffel DE, Feldheim DL. In vivo toxicity, biodistribution, and clearance of glutathione-coated gold nanoparticles. Nanomedicine : nanotechnology, biology, and medicine. 2013;9:257–263. doi: 10.1016/j.nano.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Sonaje K, Lin KJ, Wang JJ, Mi FL, Chen CT, Juang JH, Sung HW. Self-assembled pH-sensitive nanoparticles: a platform for oral delivery of protein drugs. Advanced Functional Materials. 2010;20:3695–3700. [Google Scholar]
- Sosunov EA, Anyukhovsky EP, Sosunov AA, Moshnikova A, Wijesinghe D, Engelman DM, Reshetnyak YK, Andreev OA. pH (low) insertion peptide (pHLIP) targets ischemic myocardium. Proceedings of the National Academy of Sciences. 2013;110:82–86. doi: 10.1073/pnas.1220038110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanadis C, Chrysochoou C, Markou D, Petraki K, Panagiotakos D, Fasoulakis C, Kyriakidis A, Papadimitriou C, Toutouzas P. Increased temperature of malignant urinary bladder tumors in vivo: the application of a new method based on a catheter technique. Journal of clinical oncology. 2001;19:676–681. doi: 10.1200/JCO.2001.19.3.676. [DOI] [PubMed] [Google Scholar]
- Subbarao NK, Parente RA, Szoka FC, Jr, Nadasdi L, Pongracz K. The pH-dependent bilayer destabilization by an amphipathic peptide. Biochemistry. 1987;26:2964–2972. doi: 10.1021/bi00385a002. [DOI] [PubMed] [Google Scholar]
- Sudimack J, Lee RJ. Targeted drug delivery via the folate receptor. Advanced drug delivery reviews. 2000;41:147–162. doi: 10.1016/s0169-409x(99)00062-9. [DOI] [PubMed] [Google Scholar]
- Sun IC, Eun DK, Na JH, Lee S, Kim IJ, Youn IC, Ko CY, Kim HS, Lim D, Choi K. Heparin-Coated Gold Nanoparticles for Liver-Specific CT Imaging. Chemistry-A European Journal. 2009;15:13341–13347. doi: 10.1002/chem.200902344. [DOI] [PubMed] [Google Scholar]
- Swami A, Shi J, Gadde S, Votruba AR, Kolishetti N, Farokhzad OC. Nanoparticles for targeted and temporally controlled drug delivery. Multifunctional Nanoparticles for Drug Delivery Applications. 2012:9–29. [Google Scholar]
- Ta T, Porter TM. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. Journal of Controlled Release. 2013;169:112–125. doi: 10.1016/j.jconrel.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacker J, Anderson R. Delivery of antitumor drug to bladder cancer by use of phase transition liposomes and hyperthermia. The Journal of urology. 1982;127:1211–1214. doi: 10.1016/s0022-5347(17)54299-8. [DOI] [PubMed] [Google Scholar]
- Thambi T, Deepagan VG, Yoon HY, Han HS, Kim S-H, Son S, Jo D-G, Ahn C-H, Suh YD, Kim K, Chan Kwon I, Lee DS, Park JH. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials. 2014;35:1735–1743. doi: 10.1016/j.biomaterials.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Thévenin D, An M, Engelman DM. pHLIP-mediated translocation of membrane-impermeable molecules into cells. Chemistry & biology. 2009;16:754–762. doi: 10.1016/j.chembiol.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorek DJ, Chen A, Czupryna J, Tsourkas A. Superparamagnetic Iron Oxide Nanoparticle Probes for Molecular Imaging. Ann Biomed Eng. 2006;34:23–38. doi: 10.1007/s10439-005-9002-7. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Drug targeting. European Journal of Pharmaceutical Sciences. 2000;11:S81–S91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nature reviews Drug discovery. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Drug delivery. Springer; 2010. Passive and active drug targeting: drug delivery to tumors as an example. pp. 3–53. [DOI] [PubMed] [Google Scholar]
- Tran MA, Gowda R, Sharma A, Park E-J, Adair J, Kester M, Smith NB, Robertson GP. Targeting V600EB-Raf and Akt3 using nanoliposomal-small interfering RNA inhibits cutaneous melanocytic lesion development. Cancer research. 2008;68:7638–7649. doi: 10.1158/0008-5472.CAN-07-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger EC, Hersh E, Vannan M, Matsunaga TO, McCreery T. Local drug and gene delivery through microbubbles. Progress in cardiovascular diseases. 2001;44:45–54. doi: 10.1053/pcad.2001.26443. [DOI] [PubMed] [Google Scholar]
- Vavere AL, Biddlecombe GB, Spees WM, Garbow JR, Wijesinghe D, Andreev OA, Engelman DM, Reshetnyak YK, Lewis JS. A novel technology for the imaging of acidic prostate tumors by positron emission tomography. Cancer Res. 2009;69:4510–4516. doi: 10.1158/0008-5472.CAN-08-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma DD, Levchenko TS, Bernstein EA, Torchilin VP. ATP-loaded liposomes effectively protect mechanical functions of the myocardium from global ischemia in an isolated rat heart model. Journal of Controlled Release. 2005;108:460–471. doi: 10.1016/j.jconrel.2005.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola-Villegas NT, Carlin SD, Ackerstaff E, Sevak KK, Divilov V, Serganova I, Kruchevsky N, Anderson M, Blasberg RG, Andreev OA, Engelman DM, Koutcher JA, Reshetnyak YK, Lewis JS. Understanding the pharmacological properties of a metabolic PET tracer in prostate cancer. Proc Natl Acad Sci U S A. 2014;111:7254–7259. doi: 10.1073/pnas.1405240111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AZ, Gu F, Zhang L, Chan JM, Radovic-Moreno A, Shaikh MR, Farokhzad OC. Biofunctionalized targeted nanoparticles for therapeutic applications. 2008 doi: 10.1517/14712598.8.8.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AZ, Langer R, Farokhzad OC. Nanoparticle Delivery of Cancer Drugs. Annual Review of Medicine. 2012a;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- Wang G, Norton AS, Pokharel D, Song Y, Hill RA. KDEL peptide gold nanoconstructs: promising nanoplatforms for drug delivery. Nanomedicine : nanotechnology, biology, and medicine. 2013;9:366–374. doi: 10.1016/j.nano.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Wang G, Papasani MR, Cheguru P, Hrdlicka PJ, Hill RA. Gold-peptide nanoconjugate cellular uptake is modulated by serum proteins. Nanomedicine : nanotechnology, biology, and medicine. 2012b;8:822–832. doi: 10.1016/j.nano.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Weerakkody D, Moshnikova A, Thakur MS, Moshnikova V, Daniels J, Engelman DM, Andreev OA, Reshetnyak YK. Family of pH (low) insertion peptides for tumor targeting. Proceedings of the National Academy of Sciences. 2013;110:5834–5839. doi: 10.1073/pnas.1303708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J, Magin R, Yatvin M, Zaharko D. Liposomes and local hyperthermia: selective delivery of methotrexate to heated tumors. Science. 1979;204:188–191. doi: 10.1126/science.432641. [DOI] [PubMed] [Google Scholar]
- Widder KJ, Senyei AE, Scarpelli DG. Magnetic microspheres: a model system for site specific drug delivery in vivo. Experimental Biology and Medicine. 1978;158:141–146. doi: 10.3181/00379727-158-40158. [DOI] [PubMed] [Google Scholar]
- Wijesinghe D, Arachchige MC, Lu A, Reshetnyak YK, Andreev OA. pH dependent transfer of nano-pores into membrane of cancer cells to induce apoptosis. Scientific reports. 2013;3 doi: 10.1038/srep03560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesinghe D, Engelman DM, Andreev OA, Reshetnyak YK. Tuning a polar molecule for selective cytoplasmic delivery by a pH (Low) insertion peptide. Biochemistry. 2011;50:10215–10222. doi: 10.1021/bi2009773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nature Reviews Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Molecular pharmaceutics. 2011;8:2032–2038. doi: 10.1021/mp200292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M, Nyagilo J, Arora V, Kulkarni P, Xu D, Sun X, Davé DP. Gold nanotags for combined multi-colored Raman spectroscopy and x-ray computed tomography. Nanotechnology. 2010;21:035101. doi: 10.1088/0957-4484/21/3/035101. [DOI] [PubMed] [Google Scholar]
- Yao L, Daniels J, Moshnikova A, Kuznetsov S, Ahmed A, Engelman DM, Reshetnyak YK, Andreev OA. pHLIP peptide targets nanogold particles to tumors. Proceedings of the National Academy of Sciences. 2013a;110:465–470. doi: 10.1073/pnas.1219665110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Daniels J, Wijesinghe D, Andreev OA, Reshetnyak YK. pHLIP(R)-mediated delivery of PEGylated liposomes to cancer cells. J Control Release. 2013b;167:228–237. doi: 10.1016/j.jconrel.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Daniels J, Wijesinghe D, Andreev OA, Reshetnyak YK. pHLIP®-mediated delivery of PEGylated liposomes to cancer cells. Journal of Controlled Release. 2013c;167:228–237. doi: 10.1016/j.jconrel.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatvin M, Mühlensiepen H, Porschen W, Weinstein J, Feinendegen L. Selective delivery of liposome-associated cis-dichlorodiammineplatinum (II) by heat and its influence on tumor drug uptake and growth. Cancer research. 1981;41:1602–1607. [PubMed] [Google Scholar]
- Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- Yellen BB, Forbes ZG, Halverson DS, Fridman G, Barbee KA, Chorny M, Levy R, Friedman G. Targeted drug delivery to magnetic implants for therapeutic applications. Journal of Magnetism and Magnetic Materials. 2005;293:647–654. [Google Scholar]
- Zhang L, Gu F, Chan J, Wang A, Langer R, Farokhzad O. Nanoparticles in medicine: therapeutic applications and developments. Clinical Pharmacology & Therapeutics. 2007;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Meng H, Wang N, Donovan MJ, Fu T, You M, Chen Z, Zhang X, Tan W. A Controlled-Release Nanocarrier with Extracellular pH Value Driven Tumor Targeting and Translocation for Drug Delivery. Angewandte Chemie International Edition. 2013;52:7487–7491. doi: 10.1002/anie.201302557. [DOI] [PubMed] [Google Scholar]
- Zou Y, Ueno M, Yamagishi M, Horikoshi I, Yamashita I, Tazawa K, Gu X. Targeting behavior of hepatic artery injected temperature sensitive liposomal adriamycin on tumor-bearing rats. Selective cancer therapeutics. 1990;6:119–127. doi: 10.1089/sct.1990.6.119. [DOI] [PubMed] [Google Scholar]