Abstract
A long-standing goal for biologists has been to understand how female preferences operate in systems where males have evolved numerous sexually selected traits. Jumping spiders of the Maratus genus are exceptionally sexually dimorphic in appearance and signalling behaviour. Presumably, strong sexual selection by females has played an important role in the evolution of complex signals displayed by males of this group; however, this has not yet been demonstrated. In fact, despite apparent widespread examples of sexual selection in nature, empirical evidence is relatively sparse, especially for species employing multiple modalities for intersexual communication. In order to elucidate whether female preference can explain the evolution of multi-modal signalling traits, we ran a series of mating trials using Maratus volans. We used video recordings and laser vibrometry to characterize, quantify and examine which male courtship traits predict various metrics of mating success. We found evidence for strong sexual selection on males in this system, with success contingent upon a combination of visual and vibratory displays. Additionally, independently produced, yet correlated suites of multi-modal male signals are linked to other aspects of female peacock spider behaviour. Lastly, our data provide some support for both the redundant signal and multiple messages hypotheses for the evolution of multi-modal signalling.
Keywords: animal communication, multi-modal signals, sexual selection, female preference, multiple messages hypothesis, redundant signal hypothesis
1. Introduction
Decades of research exploring the effect of female preference has established that this mode of selection can lead to exaggerated traits [1–4]. Despite this, we still have a relatively poor understanding of if/how female preferences have shaped the more extreme examples of sexual ornamentation seen in the animal kingdom, specifically those characterized by an elaboration of a whole suite of signals. For example, birds of paradise (family: Paradisaeidae) are considered one of the most extravagant groups in this regard, exhibiting vocal signalling, extreme variation in coloration and intricate dances that accompany both [5,6]. Although the ostentatious traits and behaviours exhibited by this and analogous systems are often attributed to sexual selection, empirical support for this idea is lacking. Moreover, studies that identify particular aspects of multi-faceted signals important for mating success are scarce.
While it is clear that selection acts on several traits simultaneously [7–11], previous research has primarily examined individual traits in isolation or focused on species employing simple signals for mate attraction (i.e. bird colour patches, cricket calls, etc.). Thus, a potentially biased impression that females assess males based on single traits exists in the literature. Additionally, prevailing theoretical work, which predicts the evolution of female preferences for one informative signal, not multiple indicators of quality [7,12–14] (but see [15]), has reinforced an emphasis on simple trait-choice relationships.
Australian peacock spiders of the Maratus genus (family: Salticidae) truly serve as excellent organisms to study complex signal evolution within a multi-dimensional framework. During courtship, male peacock spiders wave ornamented abdominal flaps and elongated third legs to nearby females [16–18]. In conjunction with visual displays, males also use vibratory signals [17] for intersexual communication. Preliminary data suggest that this group may contain as many as 40 different species [16] varying widely in habitat, distribution, morphology and behaviour. The main objective of our research was to examine male courtship displays and female behaviour of one species, Maratus volans, in order to pinpoint if/which male traits or trait combinations predict mating success and to better understand the nature of the selective pressures acting on male peacock spiders. We expect that there will be multiple traits of importance to females, all of which will be positively correlated with various metrics of mating success, such as copulation, shorter latency to mate, longer mating duration and egg laying. We also anticipate other aspects of female behaviour (in particular, orientation and aggression towards males) will be linked, positively and negatively, respectively, with the same traits that are important for mating success.
As peacock spiders are diverse in vibrational and visual signalling traits, with repertoires rivaling those of the better-known birds of paradise, this group provides a unique system for evaluating current theories of complex signal evolution. At present, there are several non-mutually exclusive hypotheses to explain the evolution of multi-modal signalling based on: (i) the quality, type and/or amount of information conveyed in complex displays; (ii) the efficacy with which these signals are transferred in response to varying biotic and abiotic factors; and (iii) considerations of potential interactions between signals and how integration of signal elements could affect signal production or reception. Another goal of this study was to explore evidence for two of the main hypotheses related to adaptive preferences for informative signals [7,10]. The first, the multiple messages hypothesis, proposes that each component of multi-modal signals will be informative to females in a different way. By contrast, the redundant signal hypothesis proposes that different multi-modal signal components will independently reflect the same information, providing back-up for intrinsic signalling error. To investigate support for either of these hypotheses, we examined patterns of correlation between different courtship traits. One simple prediction of the redundant signal hypothesis is that elements of multi-modal signals are expected to have tight covariance [10]. Conversely, the multiple messages hypothesis [10,19–21] predicts independence between each signal component, and thus we do not expect distinct signal elements to covary.
Given that complex multi-modal signals are used by many animals, not just peacock spiders [22,23], major objectives for behavioural ecologists and evolutionary biologists are to elucidate both the preferences for, and function of, these signals. Such insight will not only inform what we know about the evolution of extremely exaggerated traits, but also why some species seem to use simpler modes of communication. One benefit of our study is that it examines how authentic integrated multi-modal signal structure affects mating success, rather than focusing on single traits in isolation or manipulated traits at the extreme ends of naturally occurring variation. Another advantage of this work is that we were able to measure mating success at multiple stages (i.e. latency to mate, copulation, mating duration and egg laying) and correlate these data with various courtship traits. Both aspects of this research contribute to a more complete and realistic picture of how female preferences are driving mate choice, and in turn guiding both male and female behaviour.
2. Material and methods
(a). Sampling
Juvenile M. volans specimens were collected around Sydney, New South Wales from 5 August to 29 November 2011. Live spiders were brought back to the laboratory, where they were housed individually with leaf litter from their environment and kept on a 14 L : 10 D cycle. Spiders were fed a diet of fruit flies (Drosophila melanogaster) and crickets (Acheta domestica).
(b). First mating trials
Mating trials between mature males and females were conducted from 28 October to 12 December 2011, between the hours of 09.00 and 16.00. Weights of all individuals were recorded prior to trials. For each trial (n = 64), a unique male and female, both virgin, were paired and all interactions were recorded using a Canon EOS Kiss X4 with a 100 mm macro lens. The camera was stationary and positioned directly above the arena. Concurrently, vibratory courtship was captured using a laser vibrometer (Polytec PDV100) and recorded onto a digital recorder (Sound Devices 744 T, 48.1 kHz sampling rate).
Courtship recordings were conducted on an arena of nylon fabric stretched over a circular frame (diameter: approx. 10 cm). This fabric was used as it has previously been shown to pass male signalling frequencies with minimal distortion [24]. Several pieces of reflective tape (approx. 1 mm²) were stuck to the surface to serve as measurement points for the vibrometer. Transparency sheets were fastened to the frame to create a 10 cm tall cylinder around the arena, which prevented spiders from escaping during trials. We used a tungsten halogen light (800 W bulb) to provide broad-spectrum illumination (3200 K). Laboratory temperature was monitored using an ibutton (Maxim DS1923), and averaged 27°C, which is well within the natural temperature range experienced in the wild.
Our set-up allowed males and females to move about freely in the arena, and thus interactions would be more similar to those in the wild. Males were given 15 min to court and attempt a mating. After this point, if a female was not paying attention to a male, or was being aggressive towards him, the trial was terminated. If the female was still watching the male's courtship display at 15 min, we allowed him to continue courting until the female (i) turned away, (ii) became aggressive or (iii) copulated with the male. We cleaned the arenas with 75% ethanol between use to remove any chemical cues.
(c). Second mating trials
To assess re-mating rates, all females that mated in the trials above (n = 16), as well as six additional females that mated during preliminary trials, were tested with a second male (total n = 22). For each trial, we paired a novel male with a previously mated female 2 days after her initial mating. All interactions were measured and recorded in the same manner as the first mating trials.
(d). Visual display analysis
We first constructed ethograms for male and female behaviours (electronic supplementary material, table S1). We next used JWatcher Video [25] to score each trial. We used proportions of time spent engaged in each behaviour, rather than durations, because trials varied considerably depending on a male's success. We also calculated the rate of third leg movement in the ‘third-leg-wave’ display, which directly corresponded to fan movement in the ‘fan-dance’ display as well [17], using an average of three distinct samples of each behaviour randomly selected from the beginning, middle and end of male courtship. A male's proximity to a female was scored using four categorical ranges measured in terms of the focal female's body length (approx. 4 mm): (i) 0–5, (ii) 6–10, (iii) 11–15 or (iv) more than 15 body lengths. Lastly, for females, we tallied all occurrences of aggressive events towards males.
(e). Vibrational signal analysis
We imported the vibrometry recordings into Sony Soundforge Pro (v. 10.0e) for various signal analyses. To quantify vibratory signals, we first randomly selected continuous sequences of ‘rumble-rumps’ (Rb-Rus), which are the primary vibratory signal given by males early on and throughout the majority of the display [17]. For this study, we sampled three sequences across displays and, when possible, chose sequences consisting of at least five Rb-Rus. Rumble-rumps separated by another type of male display were not considered part of the same sequence. For each sequence, we calculated the mean Rb-Ru duration, as well as the mean number and rate of Rus within each Rb-Ru. Overall mean durations of Rb-Rus were calculated from the mean duration of three Rb-Ru within the sequence, averaged across three different sequences.
Using JWatcher we calculated the proportion of time males were producing bouts of Rb-Rus by summing these bouts and dividing them by the total trial time. We also calculated the amount of silence taken up by gaps between Rb-Rus. This allowed us to calculate a ‘signal-to-silence’ ratio, which we defined as the mean proportion of time males were actively producing vibrations during sequences. We calculated signal-to-silence ratios by multiplying the number of Rb-Ru signals produced in each sequence by the mean Rb-Ru signal duration within that sequence, and dividing the product by the total sequence duration. Again, when possible, these values were averaged across the three sequences taken for a given male.
Only successful males made it to the final courtship stage, the pre-mount display [17], during which males produce two other types of vibratory signals: ‘crunch-rolls’ (Cr-Rolls) and ‘grind-revs’ (Gr-Revs). For these males, we also measured the number of Cr-Rolls produced at the beginning of the display, duration of the Cr-Roll sequence and mean duration of Cr-Rolls, duration of the first and second distinct phases of Gr-Rev production, as well as number and duration of individual Gr-Revs in the first Gr-Rev phase. We also measured the duration of the pre-mount display.
Additionally, we examined the dominant (peak) frequency and bandwidth (10 dB above and below peak frequency) for each of the signals produced using custom written Matlab scripts (Mathworks Inc., v 2013b). For Rb-Rus, we averaged peak frequency and bandwidth for nine different signals (three signals were taken from each of the three sampled Rb-Ru sequences). As males produce only a single sequence of Cr-Rolls and Gr-Revs, the peak frequency and bandwidth for these signals are not means, but instead were measured from a single sample. Finally, males that successfully mate with a female produce vibrations similar to Gr-Revs continuously throughout copulation. Although we did not examine the duration of the signal (as it is closely linked to copulation duration), we did measure the peak frequency and bandwidth for a sample of these vibrations.
(f). Statistical analyses
All statistical analyses were performed using the software JMP (v. 11.1.1, SAS Institute Inc., 2013) and G*Power (v. 3.1.9.2) [26].
(i). Male behaviours/traits that predict mating success
Two principal component (PC) analyses were performed using the correlation matrix approach to standardize data and the varimax rotation method to simplify the interpretation [27,28]. Components were extracted using a scree test and variables were considered to have high loadings if they had a value of greater than or equal to 0.5 or less than or equal to −0.5.
The first PC analysis (PCA) included all male traits and behaviours, as well as the various vibrational signal components (table 1). We used a generalized linear model (GLM) with a binomial distribution and logit link function to analyse how male mating success was related to factor scores of the retained PCs. Trial date was included as a random effect.
Table 1.
PCs with varimax rotation: includes all males (n = 64), loadings with values of greater than or equal to 0.5 or less than or equal to −0.5 are bolded (prop., proportion; dur., duration; freq., frequency).
| male behaviour/trait | comp. A1 | comp. A2 | comp. A3 | comp. A4 | comp. A5 | comp. A6 | comp. A7 | comp. A8 | comp. A9 | comp. A10 | behaviour groupings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| fan-dance (prop.) | 0.947 | 0.055 | 0.032 | 0.053 | 0.014 | −0.002 | 0.111 | 0.197 | 0.124 | 0.135 | visual effort |
| third-leg-wave (prop.) | 0.576 | 0.056 | −0.012 | 0.040 | 0.068 | −0.020 | 0.092 | 0.150 | 0.033 | 0.791 | |
| side-step (prop.) | 0.950 | −0.027 | 0.037 | 0.044 | −0.072 | 0.033 | 0.113 | 0.081 | 0.190 | 0.144 | |
| leg-wave rate | 0.195 | 0.113 | 0.042 | 0.107 | −0.102 | −0.115 | 0.935 | 0.060 | 0.176 | 0.061 | visual vigour |
| vibrate (prop.) | 0.289 | 0.094 | −0.043 | 0.174 | 0.021 | −0.055 | 0.187 | 0.008 | 0.914 | 0.024 | vibrational effort |
| signal-to-silence (prop.) | 0.262 | 0.149 | 0.153 | −0.026 | 0.108 | −0.053 | 0.061 | 0.926 | 0.008 | 0.099 | vibrational vigour |
| mean no. of Rus | 0.017 | 0.801 | 0.549 | −0.041 | −0.015 | 0.011 | 0.100 | 0.144 | 0.067 | −0.007 | vibrational qualities early (Rb-Rus) |
| mean Rb-Ru dur. | 0.050 | −0.058 | 0.961 | −0.162 | −0.068 | 0.099 | 0.024 | 0.119 | −0.050 | −0.005 | |
| mean rate of Rus | 0.022 | 0.939 | −0.260 | 0.125 | 0.065 | −0.010 | 0.063 | 0.069 | 0.057 | 0.047 | |
| mean peak freq. | 0.076 | 0.082 | −0.164 | 0.961 | −0.053 | 0.011 | 0.099 | −0.023 | 0.151 | 0.025 | |
| mean bandwidth | 0.022 | −0.001 | 0.089 | 0.009 | −0.010 | 0.989 | −0.097 | −0.044 | −0.044 | −0.011 | |
| male weight | −0.033 | 0.044 | −0.063 | −0.048 | 0.987 | −0.010 | −0.087 | 0.090 | 0.016 | 0.037 | size |
| variance | 2.332 | 1.585 | 1.360 | 1.019 | 1.018 | 1.009 | 0.987 | 0.979 | 0.955 | 0.683 | |
| % variance | 19.430 | 13.212 | 11.336 | 8.491 | 8.481 | 8.406 | 8.228 | 8.157 | 7.959 | 5.694 | |
| cumulative % variance | 19.430 | 32.642 | 43.979 | 52.470 | 60.950 | 69.356 | 77.584 | 85.741 | 93.700 | 99.394 |
In order to examine if/how male traits affected latency to mate, copulation duration and success in egg laying, we ran a second PCA for the subset of males that successfully copulated (n = 16). This PCA contained the original explanatory values as well as signals produced by males during the pre-mount display (table 2). We used two separate GLMs, each with a normal probability distribution and identity function, to look at latency to mate and copulation duration. We used a third GLM with a binomial distribution and logit link function to examine female egg laying. For these three tests, we used Firth bias-adjusted estimates to correct for small sample sizes (n = 16).
Table 2.
PCs with varimax rotation: includes only the subset of successful males (n = 16), loadings with values of greater than or equal to 0.5 or less than or equal to −0.5 are bolded (prop., proportion; dur., duration; freq., frequency; cop., copulation).
| male behaviour/trait | comp. B1 | comp. B2 | comp. B3 | comp. B4 | comp. B5 | comp. B6 | comp. B7 | comp. B8 | comp. B9 | comp. B10 | comp. B11 | comp. B12 | behaviour groupings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fan-dance (prop.) | 0.936 | −0.023 | −0.036 | −0.249 | 0.134 | −0.023 | −0.078 | −0.101 | 0.042 | −0.002 | −0.117 | 0.062 | visual effort |
| third-leg-wave (prop.) | 0.681 | 0.086 | −0.262 | −0.488 | 0.109 | −0.083 | −0.140 | −0.103 | −0.035 | −0.328 | −0.069 | −0.233 | |
| side-step (prop.) | 0.926 | 0.138 | −0.022 | −0.227 | 0.039 | −0.021 | 0.018 | 0.056 | −0.231 | 0.063 | 0.066 | −0.011 | |
| leg-wave rate | −0.024 | 0.110 | −0.609 | −0.005 | 0.293 | −0.242 | 0.220 | 0.102 | 0.401 | −0.044 | −0.485 | 0.106 | visual vigour |
| vibrate (prop.) | −0.917 | 0.274 | −0.237 | 0.098 | 0.018 | −0.102 | 0.016 | 0.029 | 0.018 | 0.001 | −0.042 | 0.050 | vibrational effort |
| signal-to-silence (prop.) | 0.350 | −0.398 | −0.100 | −0.130 | 0.416 | 0.029 | −0.474 | −0.380 | 0.261 | −0.190 | −0.131 | −0.110 | vibrational vigour |
| mean no. of Rus | 0.015 | 0.350 | −0.036 | 0.067 | 0.737 | −0.159 | −0.040 | −0.041 | 0.518 | −0.112 | −0.037 | −0.022 | vibrational qualities early (Rb-Rus) |
| mean Rb-Ru dur. | 0.148 | 0.273 | −0.069 | 0.022 | 0.874 | −0.034 | −0.071 | −0.051 | −0.273 | −0.212 | −0.055 | 0.052 | |
| mean rate of Rus | −0.146 | 0.180 | 0.043 | −0.156 | −0.080 | −0.075 | −0.143 | −0.137 | 0.917 | 0.107 | −0.003 | −0.019 | |
| mean Rb-Ru peak freq. | −0.086 | −0.096 | −0.069 | 0.079 | −0.286 | 0.250 | −0.063 | −0.156 | 0.095 | 0.851 | 0.229 | −0.012 | |
| mean bandwidth | −0.104 | 0.103 | 0.905 | −0.054 | −0.048 | 0.042 | 0.057 | 0.073 | −0.111 | 0.028 | −0.338 | 0.119 | |
| pre-mount dur. | −0.362 | 0.437 | 0.017 | 0.090 | −0.094 | −0.268 | −0.184 | 0.099 | −0.033 | 0.136 | −0.099 | 0.715 | vibrational qualities late (Cr-Rolls and Gr-Revs) |
| no. of Cr-Rolls | −0.368 | −0.067 | 0.015 | 0.860 | −0.057 | 0.123 | −0.128 | 0.014 | −0.042 | 0.007 | −0.196 | −0.111 | |
| Cr-Roll sequence dur. | −0.394 | 0.000 | 0.093 | 0.875 | 0.181 | −0.025 | −0.026 | −0.069 | −0.108 | 0.062 | −0.093 | 0.012 | |
| mean Cr-Roll dur. | −0.155 | −0.063 | 0.166 | 0.226 | 0.269 | −0.097 | 0.674 | 0.526 | −0.017 | −0.063 | 0.015 | −0.039 | |
| no. of Gr-Revs, phase I | 0.127 | 0.915 | 0.056 | −0.061 | 0.239 | −0.044 | −0.034 | −0.102 | 0.169 | −0.146 | 0.003 | 0.064 | |
| Gr-Rev dur., phase I | −0.180 | 0.917 | −0.091 | −0.039 | 0.136 | −0.135 | −0.030 | −0.146 | 0.107 | 0.126 | −0.095 | 0.122 | |
| mean Gr-Rev dur., phase I | −0.014 | 0.380 | −0.331 | 0.067 | 0.254 | −0.626 | 0.078 | 0.452 | −0.058 | −0.227 | −0.113 | 0.010 | |
| Gr-Rev dur., phase II | −0.166 | 0.359 | 0.260 | −0.196 | −0.444 | −0.411 | −0.270 | −0.113 | 0.246 | 0.450 | 0.074 | 0.099 | |
| peak freq.: Cr-Rolls | −0.039 | −0.050 | 0.139 | 0.154 | −0.054 | 0.933 | 0.009 | −0.020 | −0.093 | 0.159 | −0.067 | −0.162 | |
| bandwidth: Cr-Rolls | 0.176 | 0.163 | 0.092 | 0.191 | −0.075 | 0.272 | −0.163 | 0.361 | −0.092 | 0.644 | −0.479 | −0.024 | |
| peak freq.: Gr-Revs, phase I | 0.155 | −0.211 | 0.076 | −0.283 | 0.101 | 0.534 | −0.173 | 0.413 | −0.447 | 0.299 | −0.008 | −0.185 | |
| bandwidth: Gr-Revs, phase I | −0.078 | −0.251 | −0.116 | −0.102 | −0.129 | −0.040 | −0.051 | 0.888 | −0.126 | −0.033 | −0.140 | 0.123 | |
| peak freq.: Gr-Revs, phase II | 0.244 | −0.079 | 0.820 | 0.254 | 0.036 | 0.107 | 0.224 | −0.145 | 0.237 | 0.041 | 0.098 | −0.174 | |
| bandwidth: Gr-Revs, phase II | 0.003 | −0.043 | 0.067 | −0.284 | −0.142 | −0.044 | 0.889 | −0.167 | −0.127 | −0.072 | 0.129 | −0.068 | |
| peak freq.: cop. vibrations | −0.039 | −0.110 | 0.594 | 0.141 | −0.061 | 0.275 | 0.572 | −0.080 | 0.134 | −0.264 | −0.029 | −0.278 | |
| bandwidth: cop. vibrations | −0.010 | −0.034 | −0.127 | −0.172 | −0.059 | −0.035 | 0.125 | −0.080 | −0.006 | 0.096 | 0.944 | −0.047 | |
| male weight | 0.319 | 0.047 | −0.276 | −0.473 | 0.183 | −0.238 | −0.084 | 0.132 | 0.048 | −0.231 | −0.060 | 0.644 | size |
| variance | 3.971 | 2.831 | 2.733 | 2.599 | 2.241 | 2.230 | 2.169 | 1.942 | 1.935 | 1.930 | 1.710 | 1.261 | |
| % variance | 14.182 | 10.112 | 9.760 | 9.280 | 8.003 | 7.963 | 7.746 | 6.934 | 6.911 | 6.893 | 6.107 | 4.504 | |
| cumulative % variance | 14.182 | 24.294 | 34.054 | 43.334 | 51.337 | 59.300 | 67.046 | 73.980 | 80.892 | 87.785 | 93.891 | 98.395 |
Finally, we ran two-sided, unpaired t-tests (assuming unequal variances) to examine whether male orientation towards a female, total courtship effort, proximity of time spent at different distances from the female and male movement patterns (motion towards or away from the female) were different between successful and unsuccessful males. Using the subset of males that successfully mated, we also ran linear regressions and two-sided, unpaired t-tests (assuming unequal variances) to investigate whether these additional male behaviours were related to mating latency, copulation duration or egg-laying behaviour.
(ii). Female behaviour
We used a logistic regression and a one-tailed Fisher's exact test to determine whether greater female orientation or aggression, respectively, were correlated with mating. Then, to further examine whether greater female orientation, aggression (number of attacks) or the presence of female abdomen wiggling (another behaviour we observed from some females; see the electronic supplementary material, table S1) were related to the same male qualities that predict mating success, we ran GLMs using the original explanatory variables.
In order to see whether a greater number of mated females were aggressive towards males than either receptive (those that went on to mate) or unreceptive (those that did not mate) virgin females, we used a one-tailed Fisher's exact test. We also tested whether mated females performed more aggressive attacks or paid less attention towards males than either category of virgin females using a one-way ANOVA. A one-way ANOVA was also used to test for abdomen wiggling (electronic supplementary material, table S1) behavioural differences among receptive and unreceptive virgins as well as previously mated females. For each ANOVA, female ID was included as a random effect, and we used Tukey's HSD to determine which means were unequal between groups.
3. Results
We conducted 64 mating trials with virgin females, of which 16 (25%) ended with a male successfully copulating with a female. We also conducted 22 mating trials with mated females that were each presented with a second male; none of these females re-mated.
(a). Male behaviours/traits that predict mating success
Of the 10 PC scores included in our first analysis (table 1), PC A1, A8 and A9 strongly predicted copulation (electronic supplementary material, table S2; GLM: χ2 = 36.08, p < 0.0001). PC A1 had positive loadings for fan-dancing, side-stepping and third-leg-waving, suggesting these visual displays are important for male mating success. As these behaviours cluster together in the sense that they all measure the proportion of time that visual displays are performed, A1 was labelled ‘visual effort’. PC A8 only had a single trait load positively, the signal-to-silence ratio, our metric to quantify differences in tempo (i.e. ‘vibrational vigour’). PC A9 had a positive loading for the total proportion of time males spent vibrating, suggesting that ‘vibrational effort’ is important to females. Neither female age nor trial date had an effect on male mating success and were thus subsequently dropped from all final models reported here.
For the males that successfully mated, PC B1, B4, B6 and B11 significantly (table 2) predicted the latency to copulation (electronic supplementary material, table S3; GLM: χ2 = 21.09, p < 0.05). Because the power to detect differences in latency to mate was low (power = 0.067, effect size = 0.182), our results reflect Firth bias-adjusted estimates for small sample sizes; this is also true for our tests on copulation duration (power = 0.067, effect size = 0.282) and egg-laying behaviour (power = 0.062, effect size = 0.131) summarized below. Similar to PC A1, PC B1 had positive loadings for visual effort (fan-dancing, side-stepping and third-leg-waving), but negative loading for vibrational effort. This is the opposite pattern seen for the role of vibratory effort on mating success, but was largely driven by the fact that visual and vibratory displays are performed asynchronously. Consequently, for successful males that spent a majority of their time courting, engagement in one behaviour diminished time available for the other. PCs B4, B6 and B11 were all related to specific vibrational qualities of crunch-rolls and grind-revs, which are the late-stage vibrations produced during the pre-mount display. Essentially, shorter latencies to mate were correlated with more Cr-Rolls, increased Cr-Roll duration, shorter Gr-Rev duration and higher Cr-Roll peak frequency.
Five PC scores (B1, B2, B5, B10 and B12) from table 2 predicted copulation duration (GLM: χ2 = 40.80, p < 0.0001; electronic supplementary material, table S4). Again, PC B1 significantly predicted success, indicating that visual effort was important, and negatively related to vibrational effort. Both PCs B5 and B10 had loadings for vibrational characteristics of early-stage vibrations, and in the case of B10 also late-stage vibrations. Copulation duration was positively correlated with a greater number of rumps, increased Rb-Ru duration, higher Rb-Ru peak frequency, larger Cr-Roll bandwidth, a greater number of Gr-Revs and a longer Gr-Rev duration in phase 1, as well as a longer pre-mount duration. Finally, PC B12 also had positive loading for male weight, suggesting that heavier males were more successful. No PC scores significantly explained whether females successfully laid eggs (GLM: χ2 = 11.460, p = 0.4899), although copulation duration and egg-laying behaviour were significantly positively related (unpaired t-test: t9.88 = 2.02, p = 0.04).
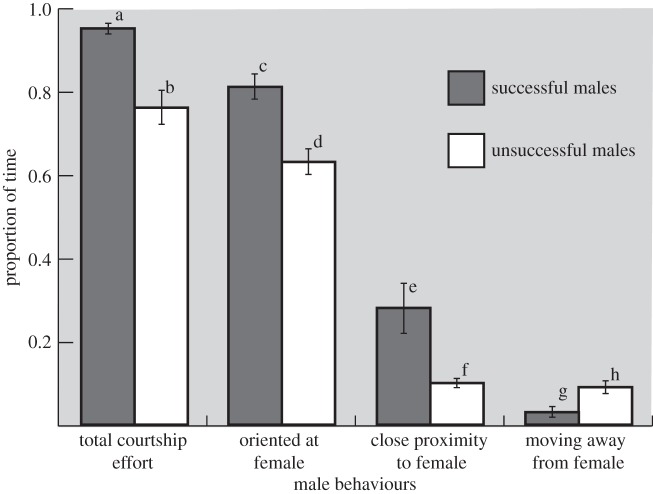
In terms of other male behaviours, total courtship effort (the proportion of time males were engaged in any display type) was greater for successful males (figure 1; unpaired t-test: t57.16 = −4.91, p < 0.0001). Successful males also spent more time oriented at females during mating trials (figure 1; unpaired t-test: t46.82 = 4.10, p = 0.0002), and more time in the closest category of proximity, less than or equal to five female body lengths, (figure 1; unpaired t-test: t16.64 = 2.72, p = 0.01). Lastly, unsuccessful males spent a higher proportion of time moving away from females as compared with those that were successful (figure 1; unpaired t-test: t46.12 = −2.87, p = 0.01).
Figure 1.
Proportion of time successful and unsuccessful males were engaged in various courtship behaviours.
Neither latency to mate nor copulation duration was related to any of the following male behaviours: proportion of time oriented, total courtship effort, proportion of time spent in any of the different distances categories from the female and male movement patterns (motion towards or away from the female). There was also no difference in total male courtship effort towards females that went on to lay eggs versus those that did not.
(b). Female behaviour
For virgin females, greater orientation to males was positively correlated with mating (r2 = 0.185, χ2 = 13.33, p = 0.0003). Visual effort (PC A1) and male weight (PC A5) in table 1 significantly predicted greater female orientation (electronic supplementary material, table S5; GLM: χ2 = 18.90, p = 0.04). Additionally, females spent a greater proportion of time oriented towards males that spent a greater proportion of time in the closest proximity category (r2 = 0.12, F1,62 = 7.30, p = 0.009).
Unlike female orientation, female aggression was expressed more by unreceptive females (Fisher's: p = 0.02); only 1 out of 16 (6.3%) females that mated ever attacked the male first, whereas 17 out of 48 (35.4%) females that did not mate attacked their paired male at least once as he courted. In the GLM examining which male behaviours correlated with greater female aggression, only two PC scores were significant and negatively correlated with female aggression: PCs A7 and A9, which had positive loadings for leg-waving rates and vibrational effort, respectively (electronic supplementary material, table S6; GLM: χ2 = 18.74, p = 0.044), were significant and negatively correlated with female aggression. Males also spent less time oriented (unpaired t-test: t41.10 = −4.10, p = 0.0002) to more aggressive females and their total courtship effort towards these females was lower (unpaired t-test: t24.01 = −3.34, p < 0.0027).
Females' abdomen wiggling behaviour was similar to aggression in that unreceptive females were more likely to perform the display (Fisher's: p = 0.04); we never saw this behaviour from virgin females that went on to mate, whereas 10 out of 48 (20.8%) unreceptive virgin females abdomen-wiggled. In the GLM examining whether abdomen wiggling was associated with any male traits, we found that visual effort (PC A1) and vibrational effort (PC A9) were positively correlated with abdomen wiggling, similar to mating success. Additionally, both PC A3 and PC A6 had positive loadings on specific vibrational characteristics related to Ru-Rbs, and were negatively (A3) and positively (A6) correlated with female abdomen wiggling (electronic supplementary material, table S7; GLM: χ2 = 23.57, p = 0.009).
Lastly, we found that previously mated and virgin females differed in their response to male displays during our trials. When looking at female orientation across mated and both receptive and unreceptive virgin females, we found significant differences across the three groups (figure 2; F2,84 = 7.22, p = 0.0015). Receptive virgin females spent a greater proportion of time oriented towards males than unreceptive virgin or mated females. However, compared with virgin females, mated females were much more aggressive; during trials, 20 out of 22 (90.9%) mated females attacked the male at least once, compared with 35.4% of unreceptive virgin females (Fisher's: p < 0.0001) and 6.3% of receptive virgin females (Fisher's: p < 0.0001). Additionally, the number of aggressive attacks differed significantly across these same three groups (figure 2; F2,84 = 37.582, p = 0.0001), with mated females performing significantly more attacks on males than either receptive or unreceptive virgins. Lastly, a greater number of mated females (14/22, 63.6%) performed abdomen wiggling displays compared with receptive (20.8%; Fisher's: p < 0.0001) and unreceptive virgins (0%; Fisher's: p = 0.0007). The proportion of time that females spent abdomen wiggling differed significantly across the three groups (figure 2; F2,84 = 8.46, p = 0.0005), with mated females spending a significantly higher proportion of time abdomen wiggling than both receptive and unreceptive virgins.
Figure 2.
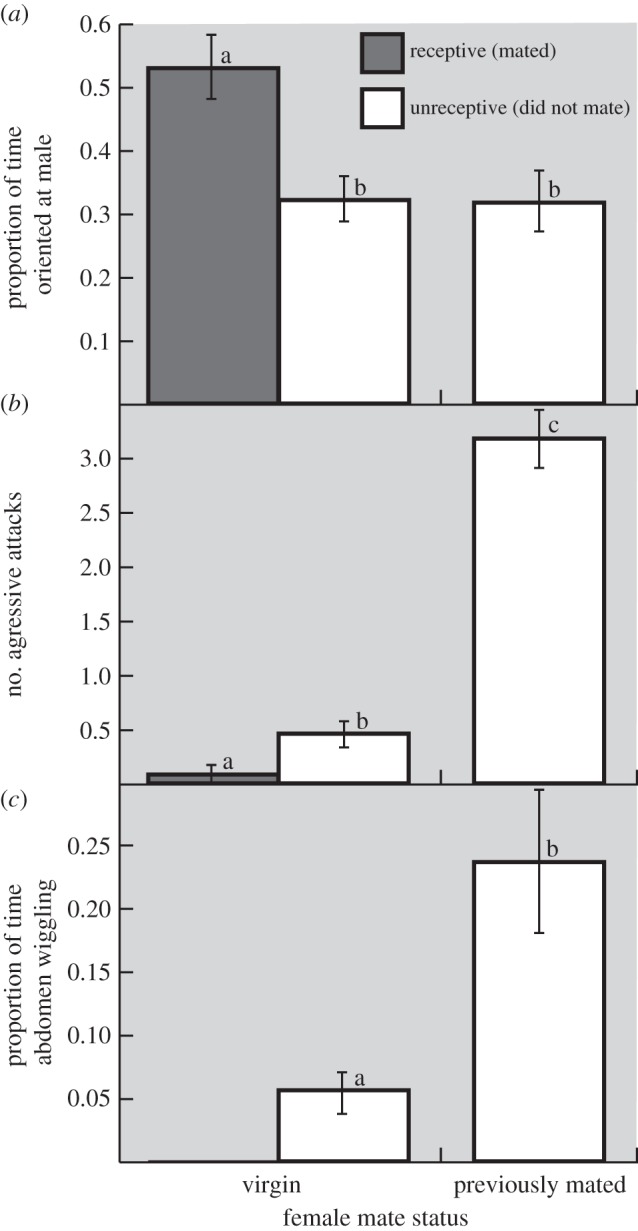
Female (a) orientation, (b) aggression and (c) abdomen wiggling towards males based on mate status (virgin versus previously mated).
4. Discussion
One of the greatest challenges in mating behaviour studies is to elucidate which male traits are important to female mating decisions, especially when complex displays spanning many modalities are involved. The main objective of this research was to explore both visual motion and vibratory courtship traits of one species of peacock spider, M. volans, to better understand multi-dimensional female preferences in this system. We found that M. volans males use a combination of visual and vibratory signalling, and our data indicate that each modality is important for mating success (table 3). Females were more likely to mate with males that put forth more visual effort, those that spent the largest proportion of time engaged in these displays. The production of vibrational signals (specifically, the proportion of time males spent vibrating and the vigour with which they signalled) was also linked, although less strongly, with mating success. For females that mated, increased visual courtship effort by males was also strongly correlated with reduced latency to mate, and increased copulation durations. Although we found no relationship between visual courtship effort and egg production, copulation duration and egg laying were highly positively correlated.
Table 3.
A summary: various aspects of male courtship significantly affect female mating and associated behaviours. Positive and negative correlations between male traits and female behaviours are denoted with a ‘+’ and ‘−‘ sign, respectively. The first column shows behavioural groupings of male traits according to clusters that were revealed by the PC analysis in table 1.
| mating success | mating latency | copulation duration | female orientation | female aggression | female abdomen wiggling | |
|---|---|---|---|---|---|---|
| visual effort | + | − | + | + | + | |
| visual vigour | − | |||||
| vibrational effort | + | + | − | − | ||
| vibrational vigour | + | + | ||||
| size | + | − |
The finding that visual effort explained more than twice the variance in male mating success than either vibrational effort or vigour suggests that, in M. volans, visual signalling modalities are more important for success (e.g. [29]). Overall, though, females prefer males that excelled at multiple aspects of their performance (total courtship effort). Successful males were also more persistent, continuously moving towards the female to stay in close proximity and maintain constant visual contact with her (figure 1). These results support previous research that courtship effort and/or motor performance may be better indicators of male quality than individual trait elements [30–32]. Alternatively, it may be the male's ability to keep a female interested that matters most and greater courtship effort across modalities prevents habituation to male displays.
For peacock spiders, female orientation was especially informative as a metric of female preference. Many of the same characteristics that predicted male mating success also predicted the attention males garnered from females. Females demonstrated aggressive behaviours when unreceptive, particularly when males made less vibratory effort and performed leg waving at lower rates. The other female behaviour we scored, abdomen wiggling, was exclusively performed by females that did not mate, thus we think this female display is an anti-receptivity signal to males, akin to that of other taxa [33–35]. A female may benefit from deterring unworthy mates from continuing their efforts as displaying males are potentially much more conspicuous to predators, drawing unwanted attention her way [36]. This form of feedback is perhaps also important to males, in that they may tailor their behaviour to better avoid costs associated with courting an unreceptive female [37,38]; specifically, wasted time, lost energy and, in some cases, death.
Aspects of both major hypotheses for the evolution of multi-modal signalling are consistent with our results. As we found many suites of correlated traits across males, our study provides at least partial evidence for the redundant signal hypothesis. Some of these correlations were unsurprising because certain male displays are often produced in conjunction (i.e. third-leg-waving, fan-dancing and side-stepping), albeit using different independent morphological structures. Beyond these correlations, though, there appear to be several other traits with tight covariance across modalities (i.e. vibrational effort with visual vigour). On the other hand, even though many of the visual and vibrational traits we looked at were highly correlated, when separated into disjoint sets using varimax rotation, traits primarily segregated by modality and each modality independently predicted mating success. The independence of each modality may suggest that each offers unique information about distinct aspects of male quality, as predicted by the multiple messages hypothesis [10,19–21]. Further support for this hypothesis comes from the fact that individual vibrational signalling qualities affected each stage of the mating process (copulation, latency, duration, egg laying) in a different way.
For this study, we were unable to measure the complex abdominal fan ornamentation of M. volans males (i.e. size, shape, reflectance). This is because the small size of the fans and complex colour patches precluded the use of a traditional spectrophotometer. Future work using a hyperspectral camera [39] will avoid these problems and will allow us to (i) investigate how variation in colour traits affects female preferences, (ii) explore patterns of correlation between colour and other aspects of courtship, and (iii) examine if ornamentation patterns act as independent signals or if they serve predominantly as amplifiers for other elements of courtship displays, as observed in the more simply ornamented Schizocosa wolf spiders [40]. In peacock spiders, males are much more likely to perform vibratory displays when females are not looking at them [17], suggesting that vibrations may serve to capture a female's attention, and direct her focus towards other more salient visual signals.
Our research demonstrates that, in peacock spiders, sexual selection in the form of female preference acts on complex groupings of correlated and non-correlated suites of male traits. At present, our data best support theoretical models that predict the optimal coding strategy for receivers is a combination of redundancy and multiple messages, as this allows for robust yet efficient processing of complex information [41]. In this study, low mating rates and no evidence for multiple mating in M. volans suggest that selection on males of this group is strong. Many Maratus species are found in sympatry and robustness may be especially vital for these spiders, or in other systems where mating errors are likely to come at a high cost. There is already at least some evidence from insects that multi-modal signals facilitate more quick and reliable decision-making [42–44]. In a mate selection context, these types of benefits may outweigh potential costs associated with having multiple signals [7,45]. However, experimental manipulations of male signals and work on male quality are still needed in order to more accurately evaluate and distinguish between existing hypotheses related to the evolution of complex displays. Across taxa, surprisingly few studies exist quantifying trait combinations that predict mating success in semi-natural contexts. We advocate for an increase in these types of studies in order to better place empirical manipulations of signalling behaviour into their proper context and to help address hypotheses on signal evolution and function.
Supplementary Material
Acknowledgements
We would like to thank Erica Bree Rosenblum and the rest of the Rosenblum lab for very helpful comments on the manuscript. We would also like to thank Rob Brooks for generously providing laboratory space. Additionally, we would like to thank Jürgen Otto for specimen location information.
Ethics
All research was conducted under appropriate permits for collecting spiders.
Data accessibility
Data are deposited in the Dryad repository (http://dx.doi.org/10.5061/dryad.9gr00).
Authors' contributions
M.B.G. conceived of the study, conducted all field and lab work, and data analysis, as well as wrote the manuscript. D.O.E. and M.M.K. participated in the design of the study, contributed necessary equipment and helped edit the manuscript. A research grant to M.M.K. primarily funded this work. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
We are grateful to the funding agencies that supported this research: The Hermon Slade Foundation (Project Grant to M.M.K.), Animal Behavior Society (Student Research Grant to M.B.G.), Sigma Xi, U. C. Berkeley Chapter (Student Research Grant to M.B.G.), as well as the NSF's East Asian Pacific Summer Institutes and Graduate Research Fellowship Programs (M.B.G.).
References
- 1.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Masta SE, Maddison WP. 2002. Sexual selection driving diversification in jumping spiders. Proc. Natl Acad. Sci. USA 99, 4442–4447. ( 10.1073/pnas.072493099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman SW, Patricelli GL, Borgia G. 2004. Variable female preferences drive complex male displays. Nature 428, 742–745. ( 10.1038/nature02419) [DOI] [PubMed] [Google Scholar]
- 4.Ritchie M. 2007. Sexual selection and speciation. Annu. Rev. Ecol. Evol. Syst. 38, 79–102. ( 10.1146/annurev.ecolsys.38.091206.095733) [DOI] [Google Scholar]
- 5.Scholes E. 2008. Evolution of the courtship phenotype in the birds of paradise genus Parotia (Aves: Paradisaedae): homology, phylogeny, and modularity. Biol. J. Linn. Soc. 94, 491–504. ( 10.1111/j.1095-8312.2008.01012.x) [DOI] [Google Scholar]
- 6.Irestedt M, Jønsson KA, Fjeldså J, Christidis L, Ericson PGP. 2009. An unexpectedly long history of sexual selection in birds-of-paradise. BMC Evol. Biol. 9, 235 ( 10.1186/1471-2148-9-235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Partan SR, Marler P. 2005. Issues in the classification of multimodal communication signals. Amer. Nat. 166, 231–245. ( 10.1086/431246) [DOI] [PubMed] [Google Scholar]
- 8.Blows M, Brooks R, Kraft P. 2003. Exploring complex fitness surfaces: multiple ornamentation and polymorphism in male guppies. Evolution 57, 1622–1630. ( 10.1111/j.0014-3820.2003.tb00369.x) [DOI] [PubMed] [Google Scholar]
- 9.Guilford T, Dawkins MS. 1993. Receiver psychology and the design of animal signals. Trends Neurosci. 16, 430–436. ( 10.1016/0166-2236(93)90068-W) [DOI] [PubMed] [Google Scholar]
- 10.Hebets EA, Papaj DR. 2005. Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214. ( 10.1007/s00265-004-0865-7) [DOI] [Google Scholar]
- 11.Hunt J, Breuker CJ, Sadowski JA, Moore AJ. 2009. Male-male competition, female mate choice and their interaction: determining total sexual selection. J. Evol. Biol. 22, 13–26. ( 10.1111/j.1420-9101.2008.01633.x) [DOI] [PubMed] [Google Scholar]
- 12.Pomiankowski A, Iwasa Y. 1993. Evolution of multiple sexual preferences by Fisher runaway process of sexual selection. Proc. R. Soc. Lond. B 253, 173–181. ( 10.1098/rspb.1993.0099) [DOI] [Google Scholar]
- 13.Iwasa Y, Pomiankowski A. 1994. The evolution of mate preferences for multiple sexual ornaments. Evolution 48, 853–867. ( 10.2307/2410492) [DOI] [PubMed] [Google Scholar]
- 14.Pomiankowski A, Iwasa Y. 1998. Runaway ornament diversity caused by Fisherian sexual selection. Proc. Natl Acad. Sci. USA 95, 5106–5111. ( 10.1073/pnas.95.9.5106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuijper B, Pen I, Weissing FJ. 2012. A guide to sexual selection theory. Annu. Rev. Ecol. Evol. Syst. 43, 287–311. ( 10.1146/annurev-ecolsys-110411-160245) [DOI] [Google Scholar]
- 16.Girard MB, Endler JA. 2014. Peacock spiders. Curr. Biol. 24, R588–R590. ( 10.1016/j.cub.2014.05.026) [DOI] [PubMed] [Google Scholar]
- 17.Girard MB, Kasumovic MM, Elias DO. 2011. Multi-modal courtship in the peacock spider, Maratus volans (O.P.-Cambridge, 1874). PLoS ONE 6, e25390 ( 10.1371/journal.pone.0025390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhl G, Elias DO. 2011. Communication. In Spider behavior: flexibility and versatility (ed. Herberstein ME.), pp. 127–190. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 19.Moller AP, Pomiankowski A. 1993. Why have birds got multiple sexual ornaments? Behav. Ecol. 32, 167–176. ( 10.1007/bf00173774) [DOI] [Google Scholar]
- 20.Johnstone RA. 1996. Multiple displays in animal communication: ‘backup signals’ and ‘multiple messages’. Phil. Trans. R. Soc. Lond. B 351, 329–338. ( 10.1098/rstb.1996.0026) [DOI] [Google Scholar]
- 21.Jacob S, Rieucau G, Heeb P. 2011. Multimodal begging signals reflect independent indices of nestling condition in European starlings. Behav. Ecol. 22, 1249–1255. ( 10.1093/beheco/arr121) [DOI] [Google Scholar]
- 22.Vallin A, Jakobsson S, Lind J, Wiklund C. 2005. Prey survival by predator intimidation: an experimental study of peacock butterfly defence against blue tits. Proc. R. Soc. B 272, 1203–1207. ( 10.1098/rspb.2004.3034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narins PM, Grabul DS, Soma KK, Gaucher P, Ho W. 2005. Cross-modal integration in a dart-poison frog. Proc. Natl Acad. Sci. USA 102, 2426–2429. ( 10.1073/pnas.0406407102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elias DO, Mason AC. 2014. The role of wave and substrate heterogeneity in vibratory communication: practical issues in studying the effect of vibratory environments in communication. In Studying vibrational communication (eds Cocroft RB, Gogala M, Hill PSM, Wessel A), pp. 215–247. Berlin, Germany: Springer. [Google Scholar]
- 25.Blumstein DT, Daniel JC, Evans CS. 2010. JWatcher software. See http://www.jwatcher.ucla.edu/.
- 26.Faul F, Erdfelder E, Lang AG, Buchner A. 2007. G*POWER 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. ( 10.3758/BF03193146) [DOI] [PubMed] [Google Scholar]
- 27.Kaiser HF. 1958. The varimax criterion for analytic rotation in factor analysis. Psychometrika 23, 187–200. ( 10.1007/bf02289233) [DOI] [Google Scholar]
- 28.McGarigal K, Cushman S, Stafford S. 2000. Multivariate statistics for wildlife and ecology research. New York, NY: Springer. [Google Scholar]
- 29.Papke RS, Kemp DJ, Rutowski RL. 2007. Multimodal signalling: structural ultraviolet reflectance predicts male mating success better than pheromones in the butterfly Colias eurytheme L. (Pieridae). Anim. Behav. 73, 47–54. ( 10.1016/j.anbehav.2006.07.004) [DOI] [Google Scholar]
- 30.Shamble PS, Wilger DJ, Swoboda KA, Hebets EA. 2009. Courtship effort is a better predictor of mating success than ornamentation for male wolf spiders. Behav. Ecol. 20, 1242–1251. ( 10.1093/beheco/arp116) [DOI] [Google Scholar]
- 31.Byers J, Hebets EA, Podos J. 2010. Female mate choice based upon male motor performance. Anim. Behav. 79, 771–778. ( 10.1016/j.anbehav.2010.01.009) [DOI] [Google Scholar]
- 32.Barske J, Schlinger BA, Wikelski M, Fusani L. 2011. Female choice for male motor skills. Proc. R. Soc. B 278, 3523–3528. ( 10.1098/rspb.2011.0382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowe L. 1992. Convenience polyandry in a water strider: foraging conflicts and female control of copulation frequency and guarding duration. Anim. Behav. 44, 189–202. ( 10.1016/0003-3472(92)90025-5) [DOI] [Google Scholar]
- 34.Blanckenhorn WU, Muhlhauser C, Morf C, Reusch T, Reuter M. 2000. Female choice, female reluctance to mate and sexual selection on body size in the dung fly Sepsis cynipsea. Ethology 95, 466–482. [Google Scholar]
- 35.Schnell AK, Smith CL, Hanlon RT, Harcourt RT. 2015. Female receptivity, mating history, and familiarity influence the mating behavior of cuttlefish. Behav. Ecol. Sociobiol. 69, 283–292. [Google Scholar]
- 36.Su KFY, Li D. 2006. Female-biased predation risk and its different effect on male and female courtship behaviour of jumping spiders. Anim. Behav. 71, 531–537. ( 10.1016/j.anbehav.2005.04.024) [DOI] [Google Scholar]
- 37.Linley JR, Hinds MJ. 1975. Quantity of the male ejaculate influenced by female unreceptivity in the fly, Culicoides melleus. J. Insect Physiol. 21, 281–285. ( 10.1016/0022-1910(75)90023-2) [DOI] [PubMed] [Google Scholar]
- 38.Baena ML, Eberhard WG. 2007. Appearances deceive: female ‘resistance’ in a sepsid fly is not a test of male ability to hold on. Ethol. Ecol. Evol. 19, 27–50. ( 10.1080/08927014.2007.9522579) [DOI] [Google Scholar]
- 39.Garcia JE, Girard MB, Kasumovic M, Petersen P, Wilksch PA, Dyer AG. 2015. Differentiating biological colours with few and many sensors: spectral reconstruction with RGB and hyperspectral cameras. PLoS ONE 10, e0125817 ( 10.1371/journal.pone.0125817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hebets EA. 2005. Attention-altering signal interactions in the multimodal courtship display of the wolf spider Schizocosa uetzi. Behav. Ecol. 16, 75–82. ( 10.1093/beheco/arh133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ay N, Flack J, Krakauer D. 2007. Robustness and complexity co-constructed in multimodal signalling networks. Phil. Trans. R. Soc. B 362, 441–447. ( 10.1098/rstb.2006.1971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Doorn GS, Weissing FJ. 2004. The evolution of female preferences for multiple indicators of quality. Amer. Nat. 164, 173–186. ( 10.1086/422203) [DOI] [PubMed] [Google Scholar]
- 43.Kulahci IG, Dornhaus A, Papaj DR. 2008. Multimodal signals enhance decision making in foraging bumble bees. Proc. R. Soc. B 275, 797–802. ( 10.1098/rspb.2007.1176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balkenius A, Dacke M. 2013. Learning of multi-modal stimuli in hawkmoths. PLoS ONE 8, e71137 ( 10.1371/journal.pone.0071137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts JA, Taylor PW, Uetz GW. 2007. Consequences of complex signaling: predator detection of multimodal cues. Behav. Ecol. 18, 236–240. ( 10.1093/beheco/arl079) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are deposited in the Dryad repository (http://dx.doi.org/10.5061/dryad.9gr00).