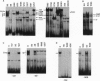
Abstract
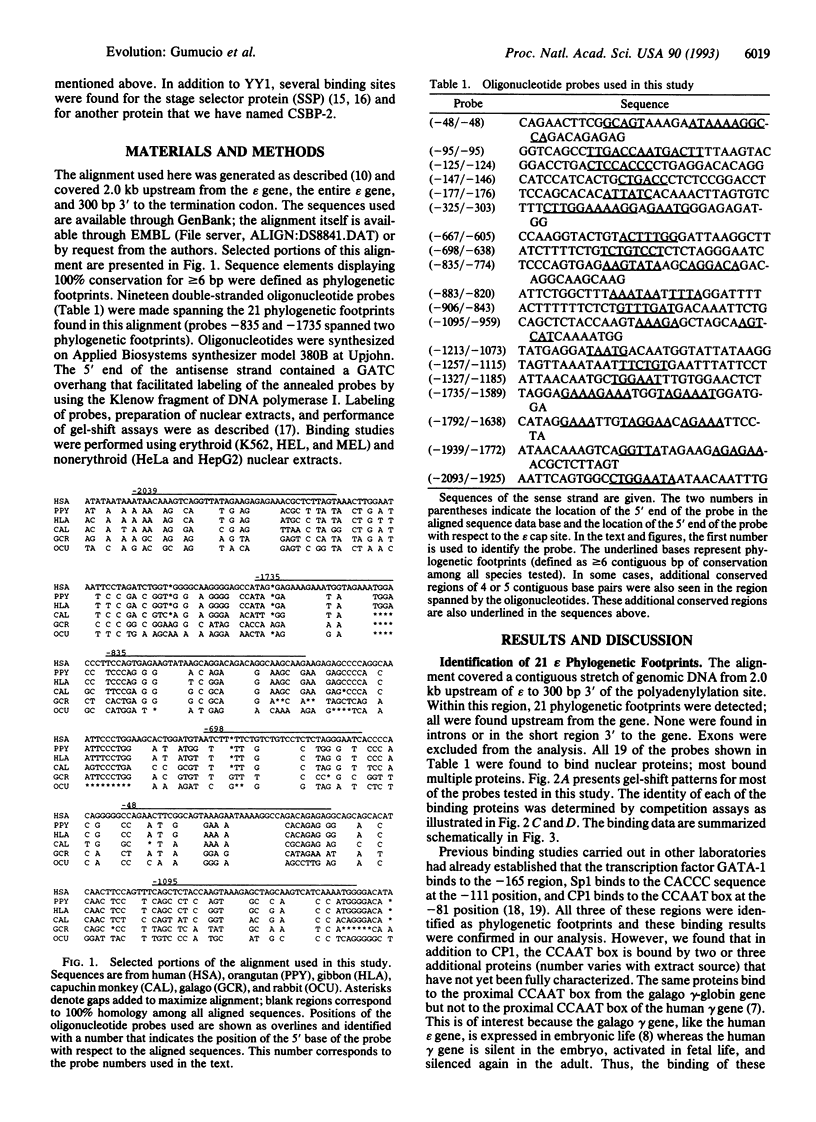
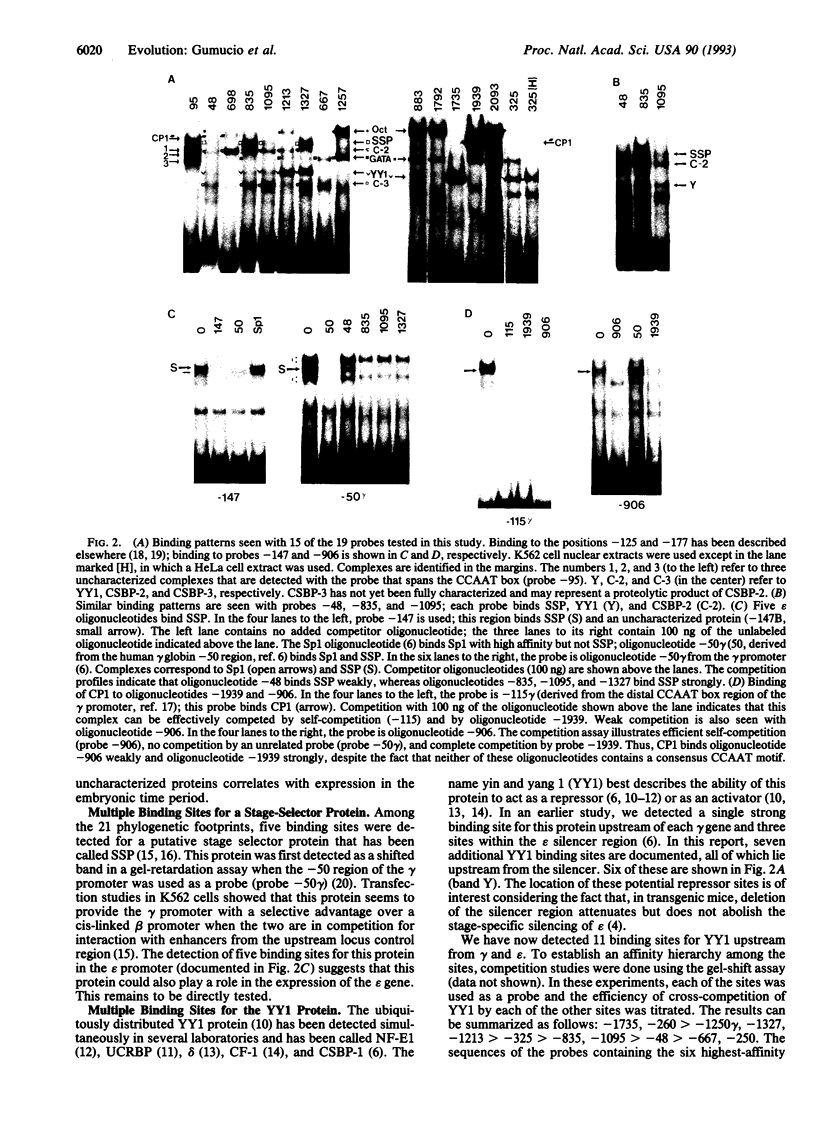
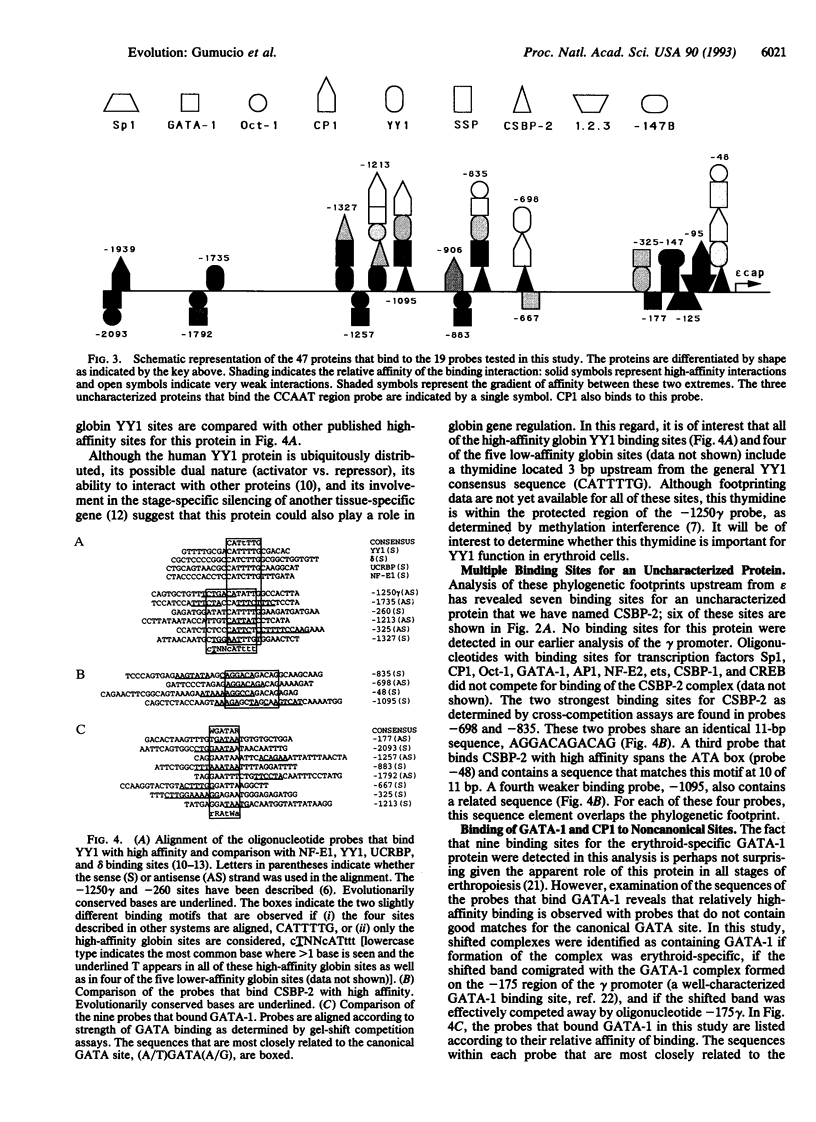
The human epsilon-globin gene undergoes dramatic changes in transcriptional activity during development, but the molecular factors that control its high expression in the embryo and its complete repression at 6-8 weeks of gestation are unknown. Although a putative silencer has been identified, the action of this silencer appears to be necessary but not sufficient for complete repression of epsilon gene expression, suggesting that multiple control elements may be required. Phylogenetic footprinting is a strategy that uses evolution to aid in the elucidation of these multiple control points. The strategy is based on the observation that the characteristic developmental expression pattern of the epsilon gene is conserved in all placental mammals. By aligning epsilon genomic sequences (from -2.0 kb upstream to the epsilon polyadenylylation signal), conserved sequence elements that are likely binding sites for trans factors can be identified against the background of neutral DNA. Twenty-one such conserved elements (phylogenetic footprints) were found upstream of the epsilon gene. Oligonucleotides spanning these conserved elements were used in a gel-shift assay to reveal 47 nuclear binding sites. Among these were 8 binding sites for YY1 (yin and yang 1), a protein with dual (activator or repressor) activity; 5 binding sites for the putative stage selector protein, SSP; and 7 binding sites for an as yet unidentified protein. The large number of high-affinity interactions detected in this analysis further supports the notion that the epsilon gene is regulated by multiple redundant elements.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey W. J., Slightom J. L., Goodman M. Rejection of the "flying primate" hypothesis by phylogenetic evidence from the epsilon-globin gene. Science. 1992 Apr 3;256(5053):86–89. doi: 10.1126/science.1301735. [DOI] [PubMed] [Google Scholar]
- Cao S. X., Gutman P. D., Dave H. P., Schechter A. N. Identification of a transcriptional silencer in the 5'-flanking region of the human epsilon-globin gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5306–5309. doi: 10.1073/pnas.86.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. R., Becker K. G., Ennist D. L., Gleason S. L., Driggers P. H., Levi B. Z., Appella E., Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992 Jan;12(1):38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q. H., Stern J., Dean A. Transcriptional role of a conserved GATA-1 site in the human epsilon-globin gene promoter. Mol Cell Biol. 1991 May;11(5):2558–2566. doi: 10.1128/mcb.11.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M., Koop B. F., Czelusniak J., Weiss M. L. The eta-globin gene. Its long evolutionary history in the beta-globin gene family of mammals. J Mol Biol. 1984 Dec 25;180(4):803–823. doi: 10.1016/0022-2836(84)90258-4. [DOI] [PubMed] [Google Scholar]
- Gumucio D. L., Heilstedt-Williamson H., Gray T. A., Tarlé S. A., Shelton D. A., Tagle D. A., Slightom J. L., Goodman M., Collins F. S. Phylogenetic footprinting reveals a nuclear protein which binds to silencer sequences in the human gamma and epsilon globin genes. Mol Cell Biol. 1992 Nov;12(11):4919–4929. doi: 10.1128/mcb.12.11.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio D. L., Rood K. L., Blanchard-McQuate K. L., Gray T. A., Saulino A., Collins F. S. Interaction of Sp1 with the human gamma globin promoter: binding and transactivation of normal and mutant promoters. Blood. 1991 Oct 1;78(7):1853–1863. [PubMed] [Google Scholar]
- Gumucio D. L., Rood K. L., Gray T. A., Riordan M. F., Sartor C. I., Collins F. S. Nuclear proteins that bind the human gamma-globin gene promoter: alterations in binding produced by point mutations associated with hereditary persistence of fetal hemoglobin. Mol Cell Biol. 1988 Dec;8(12):5310–5322. doi: 10.1128/mcb.8.12.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N., Kelley D. E., Perry R. P. Delta, a transcription factor that binds to downstream elements in several polymerase II promoters, is a functionally versatile zinc finger protein. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9799–9803. doi: 10.1073/pnas.88.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane S. M., Ney P. A., Vanin E. F., Gumucio D. L., Nienhuis A. W. Identification of a stage selector element in the human gamma-globin gene promoter that fosters preferential interaction with the 5' HS2 enhancer when in competition with the beta-promoter. EMBO J. 1992 Aug;11(8):2961–2969. doi: 10.1002/j.1460-2075.1992.tb05366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. I., Tsai S. F., Orkin S. H. Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature. 1989 Mar 30;338(6214):435–438. doi: 10.1038/338435a0. [DOI] [PubMed] [Google Scholar]
- Park K., Atchison M. L. Isolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3' enhancer and the immunoglobulin heavy-chain mu E1 site. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Raich N., Enver T., Nakamoto B., Josephson B., Papayannopoulou T., Stamatoyannopoulos G. Autonomous developmental control of human embryonic globin gene switching in transgenic mice. Science. 1990 Nov 23;250(4984):1147–1149. doi: 10.1126/science.2251502. [DOI] [PubMed] [Google Scholar]
- Raich N., Papayannopoulou T., Stamatoyannopoulos G., Enver T. Demonstration of a human epsilon-globin gene silencer with studies in transgenic mice. Blood. 1992 Feb 15;79(4):861–864. [PubMed] [Google Scholar]
- Riggs K. J., Merrell K. T., Wilson G., Calame K. Common factor 1 is a transcriptional activator which binds in the c-myc promoter, the skeletal alpha-actin promoter, and the immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1991 Mar;11(3):1765–1769. doi: 10.1128/mcb.11.3.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Shih D. M., Wall R. J., Shapiro S. G. Developmentally regulated and erythroid-specific expression of the human embryonic beta-globin gene in transgenic mice. Nucleic Acids Res. 1990 Sep 25;18(18):5465–5472. doi: 10.1093/nar/18.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagle D. A., Koop B. F., Goodman M., Slightom J. L., Hess D. L., Jones R. T. Embryonic epsilon and gamma globin genes of a prosimian primate (Galago crassicaudatus). Nucleotide and amino acid sequences, developmental regulation and phylogenetic footprints. J Mol Biol. 1988 Sep 20;203(2):439–455. doi: 10.1016/0022-2836(88)90011-3. [DOI] [PubMed] [Google Scholar]
- Yu C. Y., Motamed K., Chen J., Bailey A. D., Shen C. K. The CACC box upstream of human embryonic epsilon globin gene binds Sp1 and is a functional promoter element in vitro and in vivo. J Biol Chem. 1991 May 15;266(14):8907–8915. [PubMed] [Google Scholar]