Abstract
Introduction
Multiple sclerosis (MS) typically requires life-long management with disease-modifying therapies (DMTs). Many DMTs require regular self-injection, and can be associated with injection site reactions, pain, and needle/injection phobia—but these can be addressed by improvements in autoinjector design. The aim of this study was to investigate patient satisfaction and preference for BETACONNECT™ (Bayer Pharma AG), a novel interferon beta-1b autoinjector.
Methods
Patients in Germany performing self-injections using BETACONNECT took part in the study. Data were collected through an online 15-min structured survey. Participants rated their experience with BETACONNECT on a 6-point scale and those satisfied with BETACONNECT were asked to describe the reason using a free-text box.
Results
One-hundred and eighteen patients with MS completed the survey. Ninety percent preferred BETACONNECT to their previous injection method (only 4% previously used manual injections, so most had previously used other autoinjectors). Ninety-two percent were very confident/confident in their ability to perform an injection using BETACONNECT. The most common free-text responses to “Why are you satisfied with the BETACONNECT™ autoinjector?” were ease of use (46%), less irritation/pain at the injection site (33%), and smoother injections (24%). Features considered most useful were automated injections (98%), adjustable injection speed (98%), and adjustable injection depth (98%). Ninety-seven percent thought it was easy to know when an injection was complete and 95% agreed/strongly agreed it was easy to learn to use the autoinjector. Seventy-three percent agreed that the quietness and effortlessness of the BETACONNECT reduced their level of injection anxiety, 92% that its size and shape makes it easy to handle during injections, and 67% that it decreases injection site pain. Eighty percent of those using the reminder function thought they were less likely to miss an injection.
Conclusion
Patients with MS self-injecting interferon beta-1b expressed a high level of satisfaction and preference for BETACONNECT. Thus, BETACONNECT represents a valid option to improve patients’ overall injection experience.
Funding
Bayer HealthCare Pharmaceuticals.
Electronic supplementary material
The online version of this article (doi:10.1007/s40120-015-0036-y) contains supplementary material, which is available to authorized users.
Keywords: Beta-interferons, Disease-modifying therapies, Electronic autoinjector, Patient survey, Relapsing–remitting multiple sclerosis
Introduction
Multiple sclerosis (MS) is a chronic and debilitating autoimmune disease affecting the central nervous system, with a worldwide prevalence of approximately 33 cases per 100,000 individuals [1]. Typically, the first manifestation of MS occurs when patients are in their 20 or 30 s, and it is normal to live with MS for about 30 or 40 years [2, 3]. Medication for MS usually includes long-term management with disease-modifying therapies (DMTs) such as interferon beta-1b, which can alter the pathological immune responses associated with MS, prevent relapses, and slow disease progression [4].
Other than the development of new treatments, improving the management of MS can focus on a number of factors, including the delivery systems of existing drug therapies. Many of the currently approved DMTs require patients to perform regular self-injections, which can be associated with injection site reactions, pain, and needle phobia or other injection anxieties. However, the use and ongoing development of autoinjectors have the potential to reduce the incidence of injection site reactions [5, 6], reduce needle phobia and patients’ anxiety about self-administered injections [7], and increase adherence [8]. This is supported by new autoinjector designs that could make the injection process easier, particularly for patients with visual, dexterity or cognitive problems (for example, by incorporating electronic reminders to overcome forgetfulness) [5, 6, 9].
A variety of injection devices for many DMTs have been developed and evaluated with respect to patient satisfaction [10–15]. However, the new fully electronic BETACONNECT™ (Bayer Pharma AG) autoinjector evaluated in the current study encompasses many advances in autoinjector design and technology as outlined in the following section, which may further enhance patient comfort, satisfaction and adherence, and is hoped to further improve management of MS. Thus, the aim of the current study was to investigate patient satisfaction and preference for this new interferon beta-1b autoinjector. We hypothesized that patients would be more satisfied with the BETACONNECT autoinjector in comparison with other methods they used previously to inject interferon beta-1b. We also aimed to investigate the reasons underlying patient satisfaction.
Methods
BETACONNECT™ Autoinjector
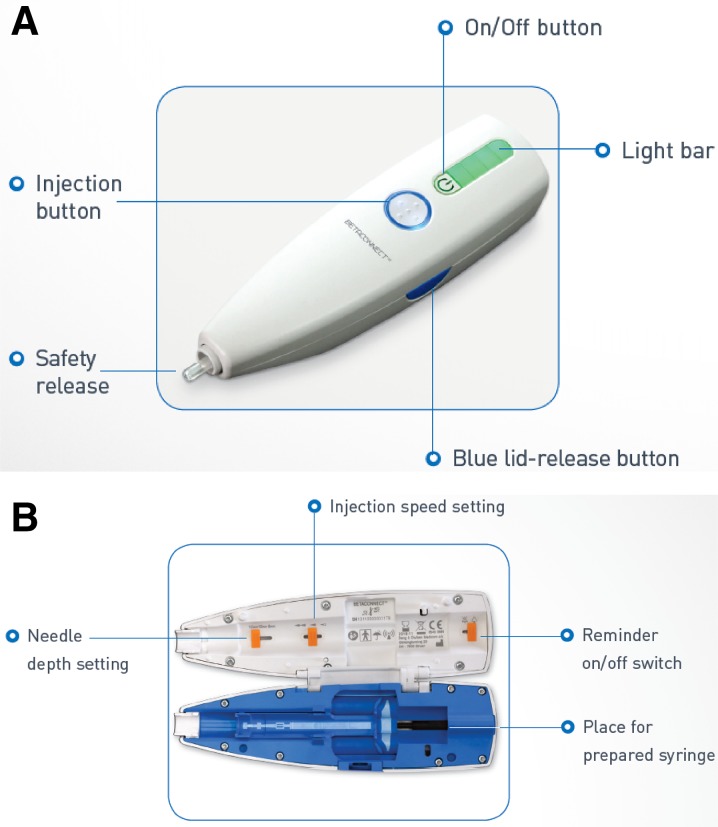
The BETACONNECT autoinjector (Fig. 1) is fully electronic and offers many improvements over older mechanical devices used for the delivery of other MS therapies. It has an intuitive user interface and ergonomic design making it easy to handle and allowing one-handed injections. It features adjustable injection speed and depth to allow individual injection settings, and a low-force safety release to ensure the device is positioned correctly at the time of the injection. The BETACONNECT autoinjector’s electronic injection is nearly silent and features four-phase injection technology: (1) automatic needle insertion; (2) delivery of the medication; (3) dwell time, when the needle remains in the skin momentarily, reducing the risk of an injection site reactions; and (4) automatic needle retraction. The autoinjector then provides both optical and audible signals of injection completion. It also features audible and visible indicators of battery status and a dose reminder function. Finally, this novel device automatically records data such as injection date and time, injection depth, injection speed, and injection volume as patients perform injections. It also offers the possibility to share data with healthcare providers via an app called myBETAapp™ (Bayer Pharma AG) and the Navigator, a dashboard monitoring application, to enhance communication between patients and healthcare professionals. Table 1 shows some of the key characteristics of the BETACONNECT autoinjector [12, 16–21].
Fig. 1.
The BETACONNECT™ electronic autoinjector. a The ergonomic design makes the device easy to handle and allows one-handed injections, with the needle hidden during the entire injection process, and audible and visible indicators of battery status, safety release, and dose reminder functions. b The syringe for interferon beta-1b release is placed within the autoinjector, and the interior features injection setting controls
Table 1.
| Feature | Main rationale/benefit |
|---|---|
| Ergonomic design | Allows ease of handling and one-handed operation: useful for patients with reduced manual dexterity (about 79% of patients with MS) [16] or short “thumb reach” (particularly a problem for some women) [17] |
| Intuitive user interface | Simplicity and ease of use are considered important features by patients with MS [12]. May be helpful as about 65% of patients with MS have cognitive impairment which can accrue in all MS sub-types [18] |
| Safety release | Important owing to reduced manual dexterity in the majority of patients with MS (as noted above) |
| Automatic needle insertion/retraction and needle hidden at all times | Avoidance of needle phobia and reduce the risk of inadvertent needle-stick injury [19] |
| End-of-dose indicators (visible and audible) | Considered important by patients with MS [12] |
| Adjustable injection speed and depth | Regarded as important features, and lack of control of injection process including speed and depth given as a reason for not using an injection device [12] |
| Injection reminder | Forgetting to take medications for MS is the most common reason for non-adherence to MS drug therapy [20, 21] |
MS multiple sclerosis
Participants
Patients using BETACONNECT in Germany were invited to participate in the survey, through either invitations sent by mail or invitation cards distributed by BETAPLUS® nurses (Bayer-sponsored nurses who train patients with MS and help them to manage their disease). Participation required use of BETACONNECT for at least 2 weeks, use of interferon beta-1b for at least 6 months, and performance of self-injections the majority of the time. All patients were aged 18 years or older and had been diagnosed with MS more than 6 months ago.
Survey Instruments and Data Collection
Data were collected through an online 15-min structured survey. Participants rated the usefulness of features offered by BETACONNECT on a 6-point scale which ranged from 1 (very useless) to 6 (very useful), with results presented as the percentage of those who rated each feature “useful” or “very useful” (rating of 5 or 6). Respondents were able to skip questions for features they were unaware of and/or did not have enough experience with to provide a response. Participants also rated BETACONNECT on its intuitiveness, ease of use, and its effect on their injection experience. Ratings were provided on a 6-point scale ranging from 1 (strongly disagree) to 6 (strongly agree), with results presented as the percentage of those who “agreed” or “strongly agreed” (rating of 5 or 6). Participants satisfied with BETACONNECT were asked to describe the reason why they are satisfied with the autoinjector in an open-ended free-text box. The open-ended free-text box allowed participants to describe their feelings about the device completely unaided, reducing the potential influence of the survey design. The answers provided were coded by frequency that they were mentioned. Patients were also asked to rate the ease of performing injections (rating from very difficult to very easy) and how confident they were in performing an injection with the BETACONNECT (rating from very unconfident to very confident). The survey was created and managed by an independent market research company, and funded by Bayer HealthCare Pharmaceuticals, Inc., Whippany, NJ, USA. Respondents were offered a gift card valued at €10 for their participation.
All procedures followed were in accordance with the 1964 Declaration of Helsinki and its subsequent revisions. Informed consent was obtained from all patients included in the study. This article describes a non-interventional study, and so does not involve any new studies of human or animal subjects performed by any of the authors.
Results
Demographic Characteristics
A total of 118 respondents with MS agreed to participate, and completed the survey. The demographic characteristics of survey participants are shown in Table 2. Nearly three-quarters (72%) of respondents were female, average age was 47.2 years, and the majority (69%) had been using the autoinjector for more than 1 month. Before using BETACONNECT, the majority of participants had been administering interferon beta-1b using a different autoinjector (BETACOMFORT® 56%, BETAJECT COMFORT® 39%, BETAJECT LITE® 14%; all Bayer Pharma AG) or manual injection with a syringe (4%).
Table 2.
Demographic characteristics of survey participants (n = 118), including previous injection method(s)
| Characteristic | Number (%) |
|---|---|
| Females | 85 (72%) |
| Age | |
| <36 years | 13 (11%) |
| 36–45 years | 18 (15%) |
| 46–55 years | 35 (30%) |
| 56–65 years | 24 (20%) |
| ≥60 years | 1 (1%) |
| Previous injection method(s)a | |
| BETACOMFORT® | 66 (56%) |
| BETAJECT® Comfort | 46 (39%) |
| BETAJECT® Lite | 16 (14%) |
| Manual, no autoinjector | 5 (4%) |
| No answer | 4 (3%) |
| Length of time using BETACONNECT™ | |
| <1 month | 37 (31%) |
| >1 month | 81 (69%) |
| No answer | 0 (0%) |
aMultiple selections were allowed to cover any previous injection method the patient had used, thus these percentages total more than 100%
Overall Perceptions of BETACONNECT™
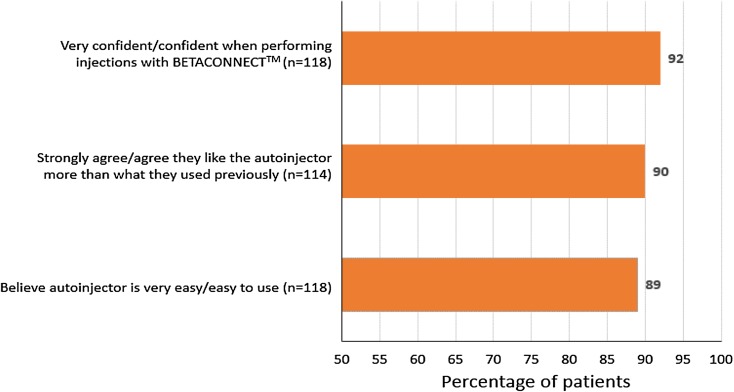
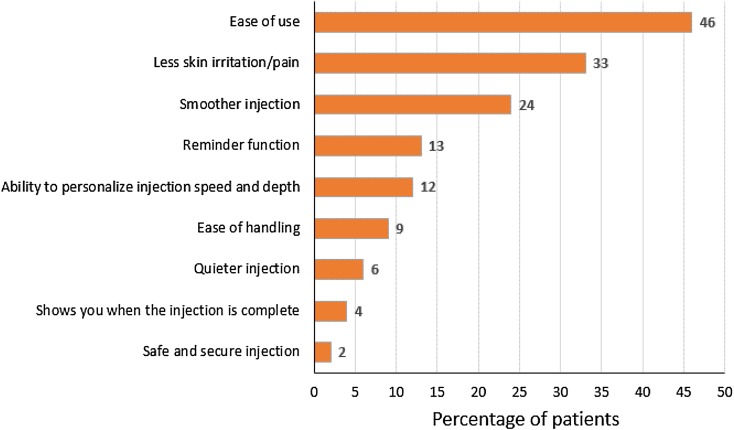
The overall ratings for the autoinjector were very positive, with 90% of respondents stating that they preferred BETACONNECT to their previous injection method and 92% reporting that they were very confident or confident in their ability to perform an injection using the BETACONNECT autoinjector (Fig. 2). A total of 85 patients who were satisfied or very satisfied with the BETACONNECT device, responded to the open-ended question “Why are you satisfied with the BETACONNECT™ autoinjector?” The most common reasons given were its ease of use (46%), less irritation/pain at the injection site (33%), and smoother injections (24%; Fig. 3). Patients also appreciated the reminder function (13%), ability to personalize the injection speed and depth (12%), ease of handling (9%), and quieter injections (6%).
Fig. 2.
Overall impressions of the BETACONNECT™ autoinjector. Base: total respondents, excluding those who answered “don’t know”
Fig. 3.
Primary reasons for satisfaction with BETACONNECT™ (unaided responses to open-ended questions, n = 85). Base: respondents satisfied with BETACONNECT™ and who answered: “Why are you satisfied with the BETACONNECT™ autoinjector?”
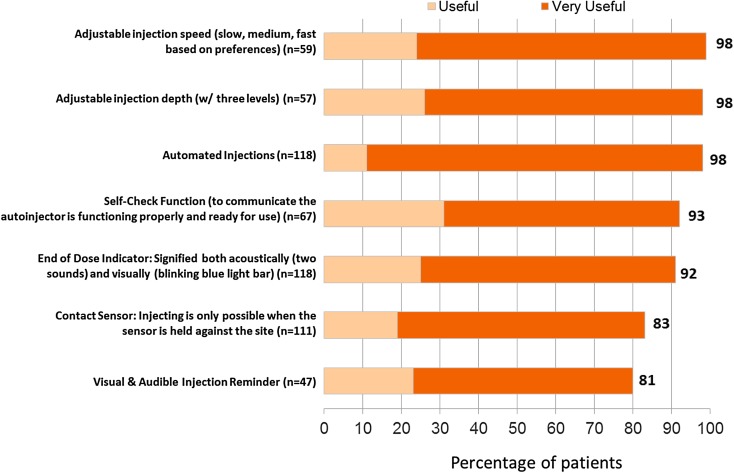
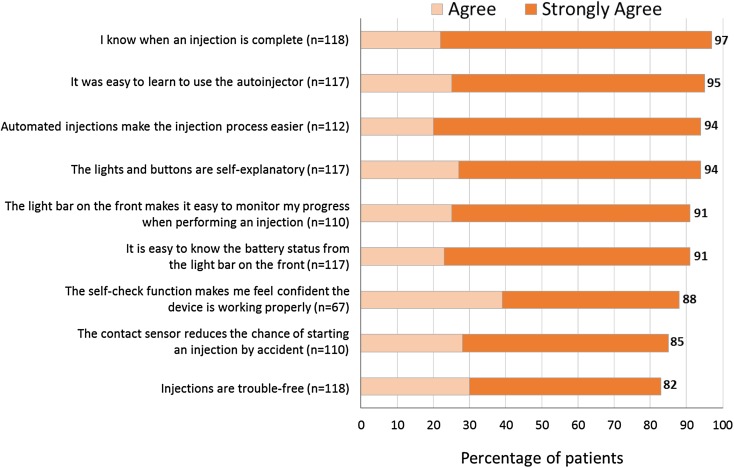
The features of BETACONNECT that were rated as most useful (i.e., rated “useful” or “highly useful”) are shown in Fig. 4. Features considered as most useful (by 98% of survey responders in each instance) were automated injections, adjustable injection speed, and adjustable injection depth. Features that help guide the injection process such as the self-check function (which communicates that the autoinjector is functioning and ready for use) and the end-of-dose indicator (signified acoustically and visually) were also rated as “useful” or “very useful” by more than 9 in 10 respondents using these features.
Fig. 4.
Usefulness of BETACONNECT™ features. Base: respondents aware of/using feature and who did not answer “don’t know”
Ease of Use
Responses related to the intuitiveness and ease of use of the autoinjector were also favorable: 97% thought it was easy to know when an injection was complete and 95% agreed/strongly agreed it was easy to learn to use the autoinjector (Fig. 5). Likewise, 94% agreed/strongly agreed that the lights and buttons are self-explanatory, and 91% that the light bar makes it easy to monitor the progress of an injection. Among those aware of the contact sensor (n = 110), 85% agreed/strongly agreed it reduces the chance of starting an injection by accident.
Fig. 5.
Intuitiveness and ease of use of the autoinjector. Base: respondents aware of/using feature and who did not answer “don’t know”
Effect of BETACONNECT on the Patient Experience
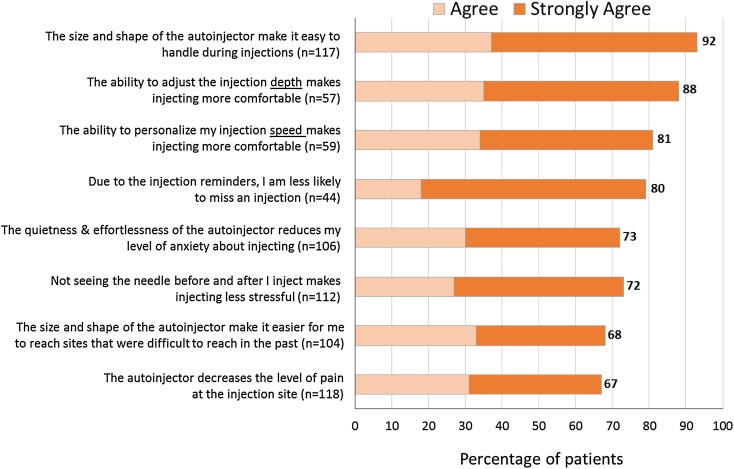
Participants using the various features of BETACONNECT largely agreed they had a beneficial effect on their injection experience (Fig. 6). Among those personalizing their injection depth (n = 57) and speed (n = 59), 88% and 81%, respectively, thought that these features make injecting more comfortable. Moreover, 73% agreed that the quietness and effortlessness of the BETACONNECT autoinjector reduced their level of anxiety about injecting. BETACONNECT also received positive ratings regarding its ease of handling: 92% agreed that the size and shape of BETACONNECT makes it easy to handle during injections and 68% thought that BETACONNECT makes it easier to reach injection sites. Moreover, 67% agreed that the autoinjector decreases the level of injection site pain. These results also suggest that BETACONNECT may help improve compliance, as 80% of those using the reminder function thought they were less likely to miss an injection.
Fig. 6.
Effect of BETACONNECT™ on the patient experience. Base: respondents aware of/using feature and who did not answer “don’t know”
Discussion
Interferon beta-1b is an injectable DMT with an established and favorable long-term safety and efficacy profile for patients with MS [22–24]. In addition, administration of DMTs using an autoinjector can reduce the number of adverse events such as injection site reactions, increase adherence to treatment, and improve patients’ quality of life [6, 8, 25]. Whilst it is known that the use of an autoinjector is generally superior to manual injection for administering DMTs [6], ongoing improvements in autoinjector design represent additional opportunities to improve interferon beta-1b therapy. Two of the problems associated with most mechanical autoinjectors are the noise of operation and that the needle is not hidden after the injection process [19]. These issues may contribute to needle/injection phobia, and in fact a survey of patients with MS reported that among those using mechanical autoinjectors, most users (54%) were unsatisfied or only moderately satisfied with their device [14]. As such, there is considerable scope to improve patients’ injection experience, with many of these needs being met by the development of autoinjectors such as BETACONNECT. BETACONNECT features a fully electronic silent injection process and a needle that is hidden before, during, and after injection. Nevertheless, it is particularly important to measure patient satisfaction with new autoinjectors, such as BETACONNECT, as increased treatment satisfaction is associated with better adherence, as shown by numerous studies covering a wide range of medical conditions and treatments, including MS [21, 26]. For patients with MS, satisfaction with treatment (odds ratio [OR] 1.54; 95% CI 1.20–1.98; P = 0.0007) and ease of injection (OR 1.47; 95% CI 1.15–1.87; P = 0.002) were independent predictors of DMT adherence [21].
A patient satisfaction survey has recently been reported by Weller et al. [27] and forms the precursor to the current report. Weller et al. [27] surveyed the use of the BETACONNECT autoinjector in 1365 patients using a 13-question structured paper survey to gauge overall satisfaction with the device and the relative importance of its features. They found that 88% felt that the autoinjector was good or very good, 92% that it was helpful or very helpful, and 89% of respondents would most likely/probably recommend its use [27]. The current survey was conducted to further validate patient satisfaction with the BETACONNECT autoinjector and also to gain a much deeper understanding of the autoinjector’s effect on patients’ injection experience. Though only 118 patients completed the current survey, it was longer (lasting 15 min), and more questions were answered and in greater depth, for example, using an open-ended question on reasons for satisfaction with BETACONNECT. It is known that quantitative patient satisfaction surveys benefit by supplementation with open response fields that allow patients to add free-text comments [28]. Free-text comments allow patients to think freely and communicate their opinions openly, and so may reflect something closer to an accurate patient experience than a reliance on structured questions and answers alone. Potential weaknesses of the current study are that it was performed in an uncontrolled setting and without a longitudinal follow-up, and had a relatively small sample size.
The overall results from the current survey show a preference for BETACONNECT over the previous device or method of injection and suggest that using the BETACONNECT autoinjector may increase satisfaction with treatment among patients on interferon beta-1b therapy. General impressions of using the BETACONNECT injector in the current survey were extremely favorable. These results are also in line with another study where “overall convenience” was cited by patients with MS as the most common benefit of using another autoinjector [29], whereas in other surveys better control of needle depth and injection speed was the most common response given as a desirable feature of autoinjectors for MS therapies [12, 14]. It is interesting to note that less skin irritation/pain and smoother injection were the second and third most common reasons given to be satisfied with BETACONNECT. This may reflect new BETACONNECT features such as its four-phase injection technology to help reduce the risk of an injection site reaction and its smooth fully electronic (rather than mechanical) operation.
Conclusions
Results from this study confirm the positive impact of the new BETACONNECT autoinjector and the relevance of its features in reducing injection site pain, promoting comfort, smooth injections, and ease of use, including improved ability to reach injection sites. As such, BETACONNECT represents a valid option for people that require interferon beta-1b injections for the improvement of their overall injection experience. More studies are currently underway to evaluate BETACONNECT with regard to patient adherence and persistence.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study and the article processing charges for this publication were funded by Bayer HealthCare Pharmaceuticals. The results of this survey were presented at the annual meeting of the Consortium of Multiple Sclerosis Centers, May 27–30, 2015, Indianapolis, IN, USA. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. We would like to thank the BETAPLUS nurses in Germany for distributing the survey invitation cards, and their ongoing work supporting patients with MS. We also thank Thomas Schreiner, Ivonne Weller, and Julika Vogelreuter, Bayer Vital GmbH, Leverkusen, for their ongoing support and important contributions to the study. Valuable consultation was provided throughout the project by Dr Gustavo Suarez (Bayer HealthCare Pharmaceuticals, Whippany, NJ, USA) and Dr Martina Sintzel (mcs medical communication services, Küsnacht, Switzerland). We are also grateful for medical writing services provided by Dr Richard Clark (freelance medical writer, Dunchurch, Warwickshire, UK). Support for this assistance was funded by Bayer HealthCare Pharmaceuticals.
Disclosures
Professor Ziemssen has received personal compensation for participating on advisory boards, trial steering committees and data and safety monitoring committees, as well as for scientific talks and project support from: Bayer HealthCare, Biogen Idec, Elan, Genzyme, Merck Serono, Novartis, Roche, Sanofi-Aventis, Synthon and Teva. Amy Perrin Ross has acted as a consultant/Advisory Board Member for Bayer Healthcare. Lauren Sylvester is an employee of Bayer HealthCare Pharmaceuticals, Whippany, NJ, USA. Mark Rametta is an employee of Bayer HealthCare Pharmaceuticals.
Compliance with ethics guidelines
All procedures followed were in accordance with the 1964 Declaration of Helsinki and its subsequent revisions. Informed consent was obtained from all patients included in the study. This article describes a non-interventional study, and so does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Multiple Sclerosis International Foundation (MSIF). Atlas of MS 2013: mapping multiple sclerosis around the world. http://www.msif.org.
- 2.Degenhardt A, Ramagopalan SV, Scalfari A, Ebers GC. Clinical prognostic factors in multiple sclerosis: a natural history review. Nat Rev Neurol. 2009;5(12):672–682. doi: 10.1038/nrneurol.2009.178. [DOI] [PubMed] [Google Scholar]
- 3.Weinshenker BG, Bass B, Rice GPA, et al. The natural history of multiple sclerosis: a geographically based study. 1. Clinical course and disability. Brain. 1989;112(1):133–146. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- 4.Plosker GL. Interferon-β-1b: a review of its use in multiple sclerosis. CNS Drugs. 2011;25(1):67–88. doi: 10.2165/11206430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Mikol D, Lopez-Bresnahan M, Taraskiewicz S, et al. A randomized, multicentre, open-label, parallel-group trial of the tolerability of interferon beta-1a (Rebif) administered by autoinjection or manual injection in relapsing-remitting multiple sclerosis. Mult Scler. 2005;11(5):585–591. doi: 10.1191/1352458505ms1197oa. [DOI] [PubMed] [Google Scholar]
- 6.Brochet B, Lemaire G, Beddiaf A, et al. Reduction of injection site reactions in multiple sclerosis (MS) patients newly started on interferon beta 1b therapy with two different devices. Rev Neurol (Paris). 2006;162(6–7):735–740. doi: 10.1016/S0035-3787(06)75071-8. [DOI] [PubMed] [Google Scholar]
- 7.Lugaresi A, Rottoli MR, Patti F. Fostering adherence to injectable disease-modifying therapies in multiple sclerosis. Expert Rev Neurother. 2014;14(9):1029–1042. doi: 10.1586/14737175.2014.945523. [DOI] [PubMed] [Google Scholar]
- 8.Pozzilli C, Schweikert B, Ecari U, et al. Supportive strategies to improve adherence to IFN beta-1b in multiple sclerosis—results of the BetaPlus observational cohort study. J Neurol Sci. 2011;307:120–126. doi: 10.1016/j.jns.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Lugaresi A. Addressing the need for increased adherence to multiple sclerosis therapy: can delivery technology enhance patient motivation? Expert Opin Drug Deliv. 2009;6(9):995–1002. doi: 10.1517/17425240903134769. [DOI] [PubMed] [Google Scholar]
- 10.Phillips JT, Fox E, Grainger W, Tuccillo D, Liu S, Deykin A. An open-label, multicenter study to evaluate the safe and effective use of the single-use autoinjector with an Avonex® prefilled syringe in multiple sclerosis subjects. BMC Neurol. 2011;11:126. doi: 10.1186/1471-2377-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devonshire V, Arbizu T, Borre B, et al. Patient-rated suitability of a novel electronic device for self-injection of subcutaneous interferon beta-1a in relapsing multiple sclerosis: an international, single-arm, multicentre, Phase IIIb study. BMC Neurol. 2010;10:28. doi: 10.1186/1471-2377-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayas A, Japp G, Fulda U, Kallmann BA. Injection devices in MS therapy: survey on neurologists, MS-nurses and patients. Nervenheilkunde. 2010;29:57–62. [Google Scholar]
- 13.de Sa J, Urbano G, Reis L. Assessment of new application system in Portuguese patients with relapsing-remitting multiple sclerosis. Curr Med Res Opin. 2010;26(9):2237–2242. doi: 10.1185/03007995.2010.508688. [DOI] [PubMed] [Google Scholar]
- 14.Verdun di Cantogno E, Russell S, Snow T. Understanding and meeting injection device needs in multiple sclerosis: a survey of patient attitudes and practices. Patient Prefer Adherence. 2011;5:173–180. [DOI] [PMC free article] [PubMed]
- 15.D’Arcy C, Thomas D, Stoneman D, Parkes L. Patient assessment of an electronic device for subcutaneous self-injection of interferon beta-1a for multiple sclerosis: an observational study in the UK and Ireland. Patient Prefer Adherence. 2012;6:55–61. doi: 10.2147/PPA.S26250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson S, Ytterberg C, Claesson IM, et al. High concurrent presence of disability in multiple sclerosis. Associations with perceived health. J Neurol. 2007;254:767–773. doi: 10.1007/s00415-006-0431-5. [DOI] [PubMed] [Google Scholar]
- 17.Valentine V, Kruger DF. Considerations in insulin delivery device selection. Diabetes Technol Ther. 2010;12(Suppl 1):S98–S100. doi: 10.1089/dia.2010.0007. [DOI] [PubMed] [Google Scholar]
- 18.Patti F. Cognitive impairment in multiple sclerosis. Mult Scler. 2009;15:2–8. doi: 10.1177/1352458508096684. [DOI] [PubMed] [Google Scholar]
- 19.Lugaresi A. RebiSmart™ (version 1.5) device for multiple sclerosis treatment delivery and adherence. Expert Opin Drug Deliv. 2013;10(2):273–283. doi: 10.1517/17425247.2013.746311. [DOI] [PubMed] [Google Scholar]
- 20.Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol. 2009;256(4):568–576. doi: 10.1007/s00415-009-0096-y. [DOI] [PubMed] [Google Scholar]
- 21.Devonshire V, Lapierre Y, Macdonell R, et al. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18(1):69–77. doi: 10.1111/j.1468-1331.2010.03110.x. [DOI] [PubMed] [Google Scholar]
- 22.Ebers GC, Traboulsee A, Li D, et al. Analysis of clinical outcomes according to original treatment groups 16 years after the pivotal IFNB-1b trial. J Neurol Neurosurg Psychiatry. 2010;81(8):907–912. doi: 10.1136/jnnp.2009.204123. [DOI] [PubMed] [Google Scholar]
- 23.Edan G, Kappos L, Montalban X, et al. Long-term impact of interferon beta-1b in patients with CIS: 8-year follow-up of BENEFIT. J Neurol Neurosurg Psychiatry. 2014;85(11):1183–1189. doi: 10.1136/jnnp-2013-306222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reder AT, Oger JF, Kappos L, O’Connor P, Rametta M. Short-term and long-term safety and tolerability of interferon β-1b in multiple sclerosis. Mult Scler Relat Disord. 2014;3(3):294–302. doi: 10.1016/j.msard.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Pozzilli C, Schweikert B, Ecari U, Oentrich W, Bugge JP. Quality of life and depression in multiple sclerosis patients: longitudinal results of the BetaPlus study. J Neurol. 2012;259(11):2319–2328. doi: 10.1007/s00415-012-6492-8. [DOI] [PubMed] [Google Scholar]
- 26.Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48. doi: 10.2147/PPA.S24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weller I, Saake A, Schreiner T, Vogelreuter J, Petroff N. Patient satisfaction with the BETACONNECT™ autoinjector for interferon beta-1b. Patient Prefer Adherence. 2015;9:951–959. doi: 10.2147/PPA.S85917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riiskjær E, Ammentorp J, Kofoed PE. The value of open-ended questions in surveys on patient experience: number of comments and perceived usefulness from a hospital perspective. Int J Qual Health Care. 2012;24(5):509–516. doi: 10.1093/intqhc/mzs039. [DOI] [PubMed] [Google Scholar]
- 29.Singer B, Wray S, Miller T, et al. Patient-rated ease of use and functional reliability of an electronic autoinjector for self-injection of subcutaneous interferon beta-1a for relapsing multiple sclerosis. Mult Scler Relat Disord. 2012;1(2):87–94. doi: 10.1016/j.msard.2011.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.