Abstract
The functions, form and mechanical properties of cells are inextricably linked to their extracellular environment. Cells from solid tissues change fundamentally when, isolated from this environment, they are cultured on rigid two-dimensional substrata. These changes limit the significance of mechanical measurements on cells in two-dimensional culture and motivate the development of constructs with cells embedded in three-dimensional matrices that mimic the natural tissue. While measurements of cell mechanics are difficult in natural tissues, they have proven effective in engineered tissue constructs, especially constructs that emphasize specific cell types and their functions, e.g. engineered heart tissues. Tissue constructs developed as models of disease also have been useful as platforms for drug discovery. Underlying the use of tissue constructs as platforms for basic research and drug discovery is integration of multiscale biomaterials measurement and computational modelling to dissect the distinguishable mechanical responses separately of cells and extracellular matrix from measurements on tissue constructs and to quantify the effects of drug treatment on these responses. These methods and their application are the main subjects of this review.
Keywords: tissue constructs, tissue mechanics, cell mechanics, Zahalak model, homogenization, drug discovery
1. Introduction
From its earliest days [1] to the present [2], the field of tissue engineering has emphasized clinical applications to tissue regeneration and repair. In this review, we discuss the applications of tissue constructs towards other purposes: for basic research on tissue mechanics and development and for phenotypic screening to discover pharmaceuticals and other bioactive materials. Engineered tissues assembled from primary or cultured cells and extracellular matrix (ECM) materials provide simplified test beds in which to study the mechanical, structural and functional properties of constructs designed specifically for these purposes.
Dissecting the responses of cells and the ECM from experiments performed on even highly idealized tissue constructs requires integrated experimentation and modelling. Central to this integrated toolset are mechanical testing protocols to characterize tissue constructs, multiscale homogenization models to interpret these measurements in the context of contributions from cells and the ECM, and biochemical tools to perturb cells and subcellular structures.
As motivation for the study of cellular biophysics in three-dimensional culture, we begin with our perspective on key observations and challenges associated with two-dimensional measurements of cell mechanics. We then review the strengths and limitations of tissue constructs as systems for probing cell and ECM mechanics, and describe several integrated experimental and multiscale modelling approaches for quantifying these. We summarize several key observations of cell and ECM mechanics in tissue constructs, and conclude with perspectives on open challenges.
1.1. Perspective on cell mechanics
Cells in solid tissues such as muscle and epithelial cells and fibroblasts have different mechanical properties and functions than circulating cells such as erythrocytes and leucocytes. The former collectively define and mechanically stabilize the structure of organs such as the heart, kidney and lungs, and drive dynamic functions such as muscle contraction and wound healing. The latter operate as individuals transporting oxygen in the blood and defending against transformed cells and foreign and infectious agents. Different experimental methods have been developed to study the mechanical properties of these two classes of cells and different conceptual theoretical approaches have been applied to interpret them in structural terms [3,4].
Studies of the mechanical properties of single cells, e.g. by compression between two plates [5] or by using micropipettes [6,7] date back to the 1930s and 1950s, respectively. Micropipette aspiration, in particular, has been extensively used to study erythrocytes and leucocytes [8–11]. These studies clarified how erythrocytes with diameters of 7–8 µm could squeeze through capillaries with much smaller diameters by demonstrating that resistance of an erythrocyte to shear and bending was weak, whereas resistance to membrane area expansions was much stronger.
Another approach was by indentation using a device similar in principle to an atomic force microscope (AFM) [12,13]. This device was also used to measure the area expansion modulus of erythrocytes [14]. It was especially useful to investigate contractile functions and the role of myosin II in the capping process in lymphocytes and Dictyostelium amoebae [15,16] and in the contribution of cell stiffening to the retention of neutrophils in the pulmonary microcirculation during acute inflammatory processes [17–19]. More recently, AFM has also been used to study the mechanical properties of adherent cells in a variety of contexts [20].
The response to traction or compression of a single cell held between two plates has also provided interesting information about cellular viscoelasticity in different timescale ranges that were relevant for elastic and contractile responses [21]. A similar approach was used to determine the contributions of collagen, titin, microtubules and intermediate filaments to the passive tension of individual cardiac muscle cells [22].
These measurements of individual cells in a culture environment have provided valuable information about the mechanical properties of both circulating cells and isolated tissue cells. For the latter, however, their separation from their natural environment limits the significance of the measurements. The functions, form and mechanical properties of cells are inextricably linked to their extracellular environment [23–26]. Therefore, it is necessary to measure the mechanical properties and functions of tissue cells within a three-dimensional matrix that mimics their natural environment. This is difficult to do in natural tissues. Engineered tissues allow the construction of tissue models that emphasize specific cell types and their functions, e.g. engineered heart tissues (EHTs). Finally, it is also therefore necessary to develop methods of analysis to determine the distinct mechanical properties of the cells and matrix from measurements of the engineered tissue constructs. These methods and their application are the main subjects of this review.
1.2. Engineered tissue constructs
There are many advantages to using these designed and simplified constructs:
(1) Specification of composition. Tissue constructs can be assembled from cells of specified tissue types, either primary cells or tissue culture lines, e.g. fibroblasts, endothelial cells or skeletal, smooth or cardiac muscle. These cells can be conveniently modified genetically prior to tissue assembly, e.g. using standard procedures of transfection to insert specific genes to modify cell functions or to provide fluorescent markers of cellular structures. The initial composition of the ECM can also be specified although the cells eventually secrete their own ECM components.
(2) Simplified composition. One can begin with constructs that contain a single cell type and by varying the density and ECM components investigate the cell autonomous properties, cell–cell and cell–ECM interactions. One can increase the complexity of constructs, including cells of different types to investigate the effects of their interactions on the structure and mechanical properties of the engineered tissue.
(3) Mimicking developmental processes. Cells remodel tissue constructs by compressing the ECM in which they are embedded and by establishing multicellular structures, e.g. cardiac and skeletal muscle myofibrils. These processes serve as models of natural functions such as wound healing and muscle development.
(4) Readily study mechanics, structure and function. Tissue constructs are readily adapted to test mechanical functions such as the viscoelasticity and contractility that are important to the normal functions of the tissues they mimic. Similarly, mechanical tests are important to understand structural and mechanical changes that occur in response to pathological conditions such as malignant transformation and fibrosis.
(5) Readily manufactured in large quantities and with reproducible properties. Large numbers of tissue constructs can be assembled under identical conditions to provide reproducible systems with which to conduct multi-parametric tests. As will be illustrated below, miniaturization of constructs both conserves materials and also facilitates conducting multiple tests of structure and mechanical function.
(6) Long lifetimes. In contrast to some complex organ tissues, the corresponding tissue constructs can be maintained over long periods. For example, the classical Langendorf preparation of an excised heart undergoes a significant deterioration of function over a few hours [27]. In contrast, cardiac tissue constructs preserve stable contractile function over many days [28].
(7) Applicability to screening. As discussed below, the mechanical and structural properties of tissue constructs provide convenient and informative properties with which to test the effects of chemical activators or inhibitors in a parallel and relatively rapid format. The ability to test rapidly large numbers of essentially identical tissue constructs is useful both as a research tool and to identify activities of candidate pharmaceuticals.
Disadvantages of tissue constructs include:
(1) Differences between tissue constructs and the biological tissues they mimic. The simplification of the compositions of tissue constructs is a valuable feature for understanding the functions and properties of specified cells and their interactions with one another and the ECM. Nevertheless, functions in biological tissues that depend on interactions among cell types, e.g. nerve–muscle interactions or interactions that depend on paracrine communication of different cell types, will not be accessible in a construct unless specifically included in its design. For example, although the cardiac muscle cells and fibroblasts make up the majority of cells in the heart, endothelial cells secrete products that influence heart function and development. The behaviour of a construct containing only cardiomyocytes and fibroblasts may provide important information about normal and pathological properties of heart muscle, but will lack functions that depend on endothelial cells. Furthermore, there can be important structural differences between a tissue construct and the biological tissue it is meant to mimic. Tissue constructs are typically less well organized and with a lower cell density. These differences can lead to important functional differences that should be taken into account in the interpretation of studies of tissue function. An important and continuing goal for tissue engineering is to bring the structural and functional properties of engineered constructs into ever closer similarity with the biological tissues they are meant to mimic.
(2) Lack of support infrastructure. Cells of a number of different types may be required for normal development and function, e.g. paracrine signals that might be absent from a simplified construct model. Constructs containing cells with high rates of energy expenditure are limited by the rate of transport of nutrients and oxygen through the construct to cells within. For example, the density of cardiomyocytes within constructs is limited to values lower than in authentic heart muscle owing to the lack of a vascular system to deliver the required nutrients and oxygen. Considerable effort is now being devoted to providing heart and skeletal muscle tissue constructs with a vascular system [29–32].
1.3. Perspective on the mechanical properties of cells and extracellular matrix in tissues and tissue constructs
This review emphasizes the role of engineered tissue constructs as a platform firstly for basic research on the mechanical, structural and functional properties of tissue constructs and, by extension, of the biological tissues they mimic and secondly for phenotypic screening of the effects of chemical activators, chemical inhibitors, and genetic modifications on tissue functions. To understand the mechanical characteristics of tissue constructs requires information about a number of properties of the cells, the ECM and their interactions, which must be obtained experimentally and then interpreted with reference to the structural constituents of the cells and ECM [3].
(1) Mechanical properties of cells. The mechanical properties of cells depend mainly on the cytoskeleton, three systems of filaments that include the actin microfilaments, tubulin-based microtubules and the intermediate filament systems that are based on tissue-specific subunits such as vimentin (characteristic of mesenchymal or connective tissue cells), desmin (muscle cells), keratin (epithelial cells, skin), neurofilaments and nuclear lamins. The actin cytoskeleton together with several types of the motor protein myosin is principally responsible for developing contractile force, both in muscle and non-muscle cells. Microtubules are responsible for the segregation of chromosomes during the metaphase of mitosis and also serve as tracks for the transport of molecular cargo throughout the cell. The intermediate filaments appear to serve a primarily structural role protecting cells from excessive mechanical stress [33].
(2) Mechanical properties of the ECM. The structure of the ECM is dominated by protein fibrils composed of collagen and elastin that resist tension exerted on the tissue, charged glycoproteins (proteoglyclans) that resist tissue compression and glycoproteins such as fibronectin that connect protein receptors on cells (integrins) to the ECM fibres, e.g. collagen. ECM fibrils, both through their intrinsic properties and their interactions, strongly influence the viscoelastic properties of tissues. The rheological properties of collagen gels have been extensively studied [34–40]. An important subject for further study is to determine how cells remodel the ECM to increase its stiffness by compressing the ECM and cross-linking the collagen filaments [41–47].
(3) Cell–cell mechanical interactions [48]. Cells directly connect to one another via three kinds of structures: adherens junctions, tight junctions and gap junctions. Of these, adherens junctions and the related desmosomes are likely to have the most influence on the mechanical properties of tissues. Transmembrane proteins called cadherins link adjacent cells in adherens junctions. The extracellular domains of cadherin molecules on each of the juxtaposed membranes of two cells form Ca2+-sensitive (homotypic) bonds with each other. The cytoplasmic portion of the cadherin molecules links via catenin molecules to actin filaments within the cell. Adherens junctions anchor cells to one another allowing the cell network to sustain mechanical stress. The development of contractile force by actin–myosin interactions can impose a tension on cells linked by adherens junctions and, for example, contributes to the folding of epithelial sheets during embryonic development. Similarly, desmosomes link cells mechanically via cadherin family molecules (desmoglein and desmocollin) that anchor to intracellular intermediate filaments, specifically to keratin filaments in epithelial cells and desmin filaments in heart muscle cells. The main role of tight junctions is to form a selective permeability barrier in sheets of epithelial cells preventing both the intermixing of specific proteins on the apical and basolateral surfaces of the cells and the diffusion of molecules between the cells from one side of the sheet to the other. Gap junctions provide channels for ionic transport from one cell to another as discussed below under the heading of ‘Intercellular communication’.
(4) Cell–ECM interactions. Cells on two-dimensional substrata are anchored to the ECM by a family of transmembrane proteins called integrins. Evidence suggests that the picture might be more complicated in three dimensions [49–51], but integrins certainly play a role in both physiological [52] and pathophysiological [53] processes for cells in three dimensions. The extracellular domains of integrins bind to ECM filaments such as collagen; the intracellular domains bind to complexes of proteins, focal adhesions, that include vinculin, talin, α-actinin and others that, in turn, link to the actin cytoskeleton. Similarly, hemidesmosomes link the intermediate filament system in epithelial cells to the basal lamina, an extracellular layer of proteins, including type IV collagen and the glycoprotein laminin, secreted by the epithelial cells. Cells can sense the exertion of force exerted on focal adhesions and activate signalling molecules in response [54,55]. Another cell–ECM linkage system that is specific to cardiac and skeletal muscle is a multiprotein complex that includes the protein dystrophin. Dystrophin links both to the actin cytoskeleton and to the transmembrane protein dystroglycan that binds to laminin α2 in the ECM. This complex provides mechanical stability to muscle cells; its lack leads to muscle wasting diseases of which Duchenne muscular dystrophy is the best known [56].
(5) Intercellular communication. Gap junctions form channels between adjacent cells that allow the passage of ions and other small molecules between cells. The channels (connexons) are composed of protein molecules called connexins. Hence, gap junctions couple cells electrically and metabolically. For example, the action potential that initiates the contraction of the heart spreads from one heart muscle cell to the next via gap junctions. In addition to these mechanical and electrical interactions via direct cell–cell contact, cells communicate by emitting and receiving chemical messengers, i.e. paracrine interactions that include growth factors, hormones and other types of molecules.
(6) Nuclear transduction. Mechanical forces can be transmitted directly to the cell nucleus [57]. Nuclear distortion is sufficient, in some cases, to affect gene expression [58,59]. The nuclear lamina and lamins underlie the ability of the nucleus to receive mechanical signals [60]. Lamin A appears to be critical to the ability of a cell to sense its mechanical environment and differentiate appropriately [61]. Connections between the nucleus and the cytoskeleton occur through the ‘linker of nucleoskeleton and cytoskeleton’ complex [62]. The nucleus can directly sense and respond to mechanical forces through nesprin-1, which triggers a stiffening of the nucleus when stressed provided that an intact nuclear lamina exists; the effect is mediated by force-sensitive phosphorylation of the inner nuclear membrane protein emerin [63]. These phenomena have strong implications for the fate of cells migrating through constricted channels [64,65].
A comprehensive understanding of the mechanical properties and functions of tissues and engineered tissue constructs would require extensive information about each of these subjects, which is not yet available. Some examples of recent work on several of these subjects are discussed below.
2. Experimental systems for measuring the mechanical properties of tissue constructs
2.1. Single cell systems
An early demonstration that non-muscle cells exert contractile force was the ability of fibroblasts and other types of cells to wrinkle a thin, soft silicone rubber layer [66]. Using this method, it was shown that cells that migrate rapidly, leucocytes and nerve growth cones, exert weaker forces than slowly migrating cells such as fibroblasts. Hence, the tractions exerted by the latter could be important more in morphogenesis and tissue remodelling than in locomotion [67], a suggestion supported by later work discussed below. This approach has been made more quantitative by seeding cells atop beam-columns sufficiently compliant to deform in flexure in response to cellular tractions [68], and by tracking beads embedded in a two-dimensional substrate on which the cells are cultured [69,70] or in three dimensions in hydrogel matrices [71]. These measurements provide a quantitative dimension to long-standing studies of gel compression by fibroblasts that have been related to their function in wound healing and other morphogenetic processes [72–74].
2.2. Cell-populated microspheres
Extending beyond these idealized single cell assays requires integration of theoretical modelling with quantitative measurements performed upon a tissue construct. Theoretical and experimental studies of fibroblast-populated collagen microspheres (figure 1a) provide one approach to estimating cell traction forces in three-dimensional tissue constructs [41,75–78]. As fibroblasts within the microspheres constrict and remodel the collagen ECM, the spheres change diameter in a way that is predicted by theoretical models. Matching of model to experiment enables estimation of the time-dependent mechanical action of the fibroblasts.
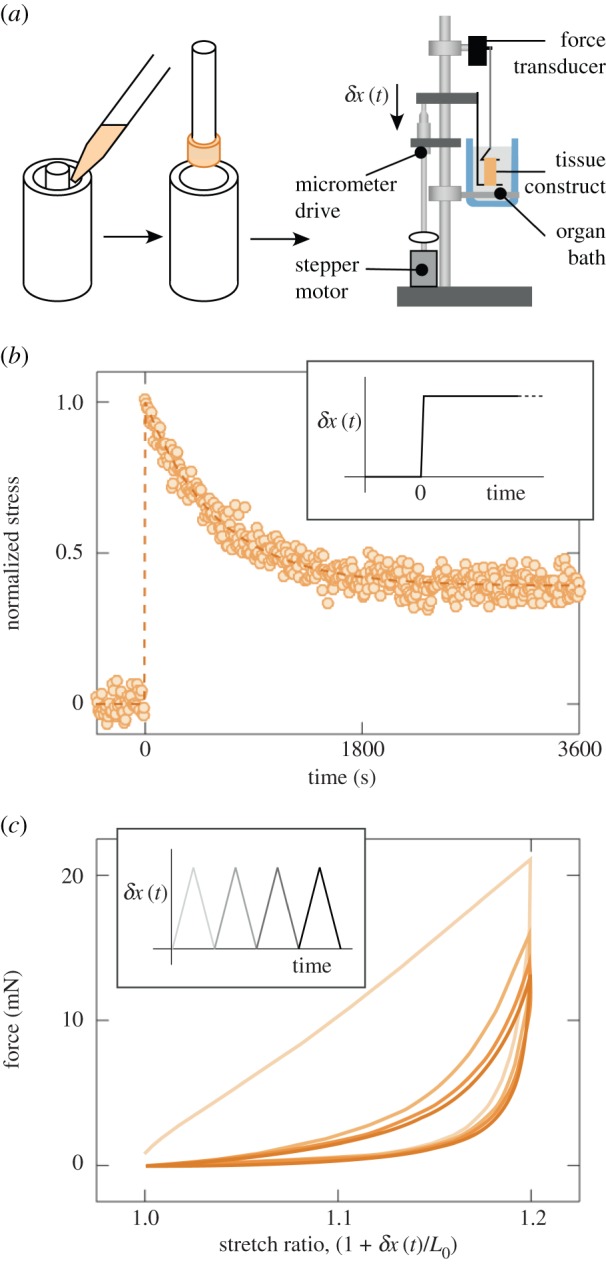
Figure 1.
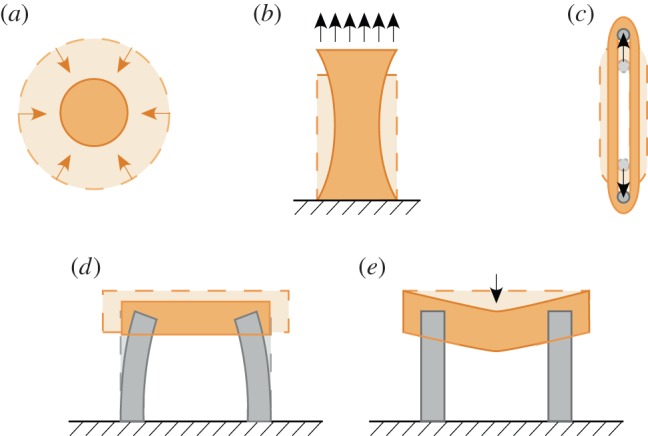
Experimental systems available for probing tissue construct mechanics. (a) The Barocas–Tranquillo cell-seeded collagen microsphere assay, in which contractile force exerted by cells can be estimated from the time-varying diameter of the collagen microsphere. (b) Uniaxial stretch of rectangular tissue constructs, which leads to a spatially varying strain field. (c) Uniaxial stretch of ring-shaped tissue constructs, which provides a more uniform strain field along the tensile flanks of the tissue construct. (d) Van den Bergh devices, which estimate tissue construct contractile force from the displacements of the flexible cantilevers. (e) Palpation devices, which estimate tissue construct mechanics from the resistance to a centrally applied downward force. (Online version in colour.)
2.3. Uniaxial tests on planar tissue constructs
Delvoye and co-workers directly measured the contractile force developed in rectangular fibroblast-containing collagen tissue constructs (figure 1b). One end of the construct was attached to a strain gauge; the other to a micrometer that could be used to stretch the gel to a desired tension level [79]. They demonstrated that the force exerted by the constructs increased with the number of cells and with application of fetal calf serum and decreased upon disruption of the actin cytoskeleton with cytochalasin B. Furthermore, the construct restored its original force value after an increase or decrease of applied tension, indicating the viscoelastic nature of the construct and possibly a homeostatic force regulation mechanism. Kolodney & Wysolmerski [80] obtained similar results using a similar approach in a comparison of forces generated in tissue constructs containing either fibroblasts or endothelial cells and provided greater detail on changes in the organization of the actin cytoskeleton. The same method was used to demonstrate the correlation between force generation and the phosphorylation of the regulatory light chain of myosin II in a fibroblast-containing tissue construct [81]. Hence, the regulation of force in non-muscle tissue constructs is similar to that in smooth muscle. An analogous approach was also used both to illustrate the increase in force over time as fibroblasts compressed a tissue construct [82] and to show that this force development differs for fibroblasts extracted from different tissues, e.g. skin versus tendon or articular cartilage [42]. The interpretation of measurements can be complicated by non-uniformity of tissue deformation at the ends of rectangular constructs where they are attached to strain gauges, force transducers or fixed anchorage points.
2.4. Ring-shaped tissue constructs
Stress concentrations associated with attachment pose a challenge to interpretation of the measurements described above. One way to reduce these potential complications is to use a ‘dog-bone’ configuration in which the construct is widened at it ends to minimize the effects of the attachments on the mechanical properties of the central parts of the construct. An example is provided by an interesting study of tensile mechanical properties of collagen gels by Roeder et al. [83].
A widespread approach to reducing effects of stress concentrations when measuring mechanical properties is to use ring-shaped tissue constructs formed in annular moulds [45] (figure 1c). A suspension of cells in a solution of collagen kept in liquid form at low temperature is placed in the annular space (typically 2–3 mm wide) between the inner surface of cylindrical mould and a central mandrel. When the mould is placed in an incubator at 37°C, the collagen gels and the cells remodel and compress the gelled collagen to form a tissue construct (typically reducing its volume to 10% of its original value within 24 h). Removal of the construct from the mandrel yields a loop than can be suspended between an isometric force transducer and a linear microstepping motor. The force transducer measures force generated by contraction of muscle or non-muscle cells. The transducer also measures forces developed when the tissue is stretched to yield the tissue stiffness at various magnitudes and rates of strain. Regions of compression exist at the points of contact between the construct and the loading bars, but the effects of these can be minimized by tailoring the loading bars [84]. These constructs have been used to study the distinct contributions of cells and ECM to the viscoelastic properties of engineered tissues as described below.
2.5. Flexural assays
Newly developed methods are available to measure contractile force and stiffness of tissue constructs rapidly in a 96-well plate format suitable for drug screening and for testing multiple parameters over wide ranges of conditions. One of these was adapted specifically for engineered skeletal muscle constructs although it is also applicable to other types of muscle [85]. In each well of a 96-well plate are two flexible posts around which the tissue construct forms as the collagen ECM is remodelled by the cells (figure 1d). Contraction of the construct bends the posts, which have a known (calibrated) bending force constant. The extent of bending is measured using an imaging system and the contractile force is determined from the degree of bending and the force constant. A miniaturized version of this method has recently been developed [86].
2.6. Palpation assays
An additional high-throughput technique for probing tissue construct mechanics also uses the ability of the cells to compress and remodel the collagen gel to form a horizontal membrane-like tissue construct stretched between two bars (1 mm diameter, separated by 4 mm) in each well in a 96-well plate [28] (figure 1e). Four or eight probes, each attached to an isometric force transducer, stretch the constructs simultaneously in a row of wells. This provides the force required to stretch the constructs a defined amount, which can be related to the corresponding increase in tension, a measure of both the stiffness and the contractile force generated in the constructs. In addition to increasing the rate of measurement, the small sizes of the constructs increase accessibility to nutrients and oxygen and also conserve precious materials.
3. Coupled chemomechanical experimentation and modelling to estimate the mechanical properties of tissue constructs
3.1. What do moduli of tissue constructs mean?
An elementary test of a tissue's mechanical properties is to measure the force increment, δF, required to stretch the tissue by an amount δx. However, for each of the methods shown in figure 1, δF is related to δx differently. We briefly summarize here what is meant by a material property such as a modulus, as opposed to a structural property such as an experiment-specific stiffness.
For a prismatic specimen stretched a sufficiently small amount δx, the incremental stiffness can be characterized through a spring constant, k, using Hooke's law, δF
=
kδx. However, the values of δF and therefore k depend not only on the material of the construct, but also on its cross-sectional area. To compare among tissue constructs, it is preferable to use parameters that characterize material properties independent of the size and shape of the measured specimens. A more general parameter is the nominal stress, σ, which provides the ratio of the current value of F to the reference (unstretched) cross-sectional area, A0, so that δσ
=
δF/A0. Complementary to stress is the engineering strain increment, 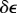 , which could be defined as δx/L0, where L0 is the unstretched reference length of the tissue construct.
, which could be defined as δx/L0, where L0 is the unstretched reference length of the tissue construct.
To account for deformations more complicated than simple uniaxial extension and specimen shapes more complex than those in a prismatic specimen loaded in uniaxial tension, a more detailed definition of stress is required involving a stress tensor σ. We focus on the symmetric engineering stress tensor, defined so that the vector of tractions acting within a specimen on an imaginary surface with normal n prior to stressing can be written as σn, and the component in stress in the direction t on that surface as 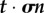 (cf. [87]). For example, the three components of the traction vector on a surface that parallels the xy plane (normal vector nz) are a normal stress
(cf. [87]). For example, the three components of the traction vector on a surface that parallels the xy plane (normal vector nz) are a normal stress 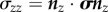 , which is a force per unit area acting across the defined surface in the z-direction; and two ‘shear’ stresses
, which is a force per unit area acting across the defined surface in the z-direction; and two ‘shear’ stresses 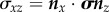 and
and 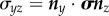 , acting across the defined surface perpendicular to the z-axis in the x- and y-directions, respectively.
, acting across the defined surface perpendicular to the z-axis in the x- and y-directions, respectively.
Similarly, deformations are often more complex than simple uniaxial extension, δx, and so require a more detailed definition of strain. Consider a material point in a specimen whose position in the undeformed body is defined by the vector X. After displacement by the vector u(X), the coordinates become x(X) = u(X) + X. The density of energy that a body stores when loaded relates to how these displacements vary over the body, quantified by the gradient 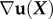 of the displacement field; here,
of the displacement field; here, 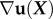 is calculated relative to the positions of material points before the body has deformed [88]. The simple extension of δx/L is the tensor of linearized strains,
is calculated relative to the positions of material points before the body has deformed [88]. The simple extension of δx/L is the tensor of linearized strains, 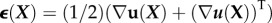 . The diagonal terms represent a rate of change of displacement per unit length in a particular direction; for example,
. The diagonal terms represent a rate of change of displacement per unit length in a particular direction; for example, 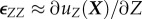 represents the gradient of the Z-component of displacement along the (undeformed) z-axis. In contrast, the off-diagonal terms, in the limit of small strains, correspond to a change of angle owing to shearing in the defined surface; for example,
represents the gradient of the Z-component of displacement along the (undeformed) z-axis. In contrast, the off-diagonal terms, in the limit of small strains, correspond to a change of angle owing to shearing in the defined surface; for example, 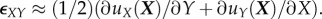 Note that many of the articles cited in this review use nonlinear strain measures such as the Green–Lagrange strain tensor, defined as
Note that many of the articles cited in this review use nonlinear strain measures such as the Green–Lagrange strain tensor, defined as 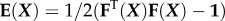 , where 1 is the identity tensor and
, where 1 is the identity tensor and 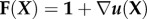 is the deformation gradient. However, in the ensuing discussion, we focus primarily on the limit of small strains.
is the deformation gradient. However, in the ensuing discussion, we focus primarily on the limit of small strains.
The object when performing a test on a tissue construct is typically to relate measurements and/or estimates of these stress and strain fields to obtain the constitutive relations between them. This is challenging because, first, the tissue constructs are, in general, nonlinear, meaning that the relationship between stress and strain is more complicated than simply 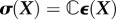 , in which
, in which 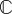 is a fourth-order stiffness tensor; second, tissue constructs are, in general, not isotropic, meaning that their resistance to stretch differs depending upon the direction in which they are stretched and that
is a fourth-order stiffness tensor; second, tissue constructs are, in general, not isotropic, meaning that their resistance to stretch differs depending upon the direction in which they are stretched and that  would thus be comprised of more than two ‘material’ constants; third, tissue constructs are inherently inhomogeneous, meaning that
would thus be comprised of more than two ‘material’ constants; third, tissue constructs are inherently inhomogeneous, meaning that 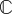 varies with position and fourth, tissue constructs are typically viscoelastic, meaning that their response to a stretching force depends on the time that has elapsed after the stretch. The strategy used to make estimates of constitutive relations is typically to apply very small increments of strain, in which linear viscoelasticity provides useful estimates of mechanical response, and to wait for sufficiently long intervals of time between these increments that the previous increments have little effect on the subsequent mechanical responses.
varies with position and fourth, tissue constructs are typically viscoelastic, meaning that their response to a stretching force depends on the time that has elapsed after the stretch. The strategy used to make estimates of constitutive relations is typically to apply very small increments of strain, in which linear viscoelasticity provides useful estimates of mechanical response, and to wait for sufficiently long intervals of time between these increments that the previous increments have little effect on the subsequent mechanical responses.
The behaviour of a linear viscoelastic material depends upon its relaxation function, μ(t), and the history of straining of the material. After an instantaneous stretch 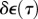 at time τ, the stress at a time t > τ is
at time τ, the stress at a time t > τ is 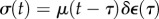 . To obtain the stress after a particular regimen of straining,
. To obtain the stress after a particular regimen of straining, 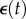 , that occurs over the time interval t0 to t (t > t0), we sum the responses from each infinitesimal time interval to obtain
, that occurs over the time interval t0 to t (t > t0), we sum the responses from each infinitesimal time interval to obtain
| 3.1 |
in which the desired material property is the relaxation function μ(t − τ). A number of ways exist to generalize and fit the constitutive law of equation (3.1) [40], including schemes for incorporating material nonlinearity [36,89–92] and for generalizing to multiaxial straining (cf. Schapery [93]; Lakes [94]); however, we note that much ambiguity exists on this issue, with central issues being the incorporation of elastic nonlinearity, the degrees to which relaxation depends upon strain and the interactions of strain components.
Relaxation functions for tissue constructs are close to linear for times that are sufficiently short or long [95], meaning that an asymptote in stress is usually reached after sufficient time elapses. The simplest analyses of the mechanical properties of tissue constructs involve analysis of the initial peak and the long-time asymptote of a step test such as that in figure 2. These are the focus of the following discussion. Although detailed anisotropic models for the mechanics of nonlinear collagenous tissues are becoming more advanced [75,97–101], we focus on isotropic approximations of cell, ECM and tissue construct moduli.
Figure 2.
(a) Simple uniaxial tests can be performed using the ring-shaped tissue constructs of figure 1c. (b) In response to a rapid, step-like stretch of a tissue construct followed by an isometric hold (inset), an initial rise in force is observed, followed by a relaxation. (c) In response to cyclic loading, substantial changes to hysteresis occur between the first and second loadings, but the tissue construct eventually approaches a steady state after repeated stretches (adapted from Wagenseil et al. [96]). (Online version in colour.)
3.2. Mechanical tests
Two kinds of measurements of mechanical properties are common. One is a step test in which the construct is rapidly stretched and then held at its new length. The rapid stretch causes a rapid increase of force. Then, as the construct is held constant in its stretched condition, the force decreases owing to viscoelastic relaxation over an interval determined by the characteristic relaxation times of the material (figure 2b). The parameters of greatest interest are the amplitude of force increase, the shape of the relaxation curve, which indicates the range of relaxation times that describe the material and the long-time asymptote. Ideally, for this kind of measurement 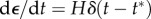 , where t* is the instant at which the stretch is applied, H scales the magnitude of the step stretch and δ(t) is Dirac's delta function. Hence, the stress σ(t) decays as
, where t* is the instant at which the stretch is applied, H scales the magnitude of the step stretch and δ(t) is Dirac's delta function. Hence, the stress σ(t) decays as 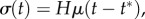 where t* > t0 and μ(t − t*) may display multiple relaxation modes.
where t* > t0 and μ(t − t*) may display multiple relaxation modes.
The other common test method is a ramp test (figure 2c). The construct is stretched at a steady rate 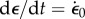 , so that over the interval from 0 to δt the construct is stretched
, so that over the interval from 0 to δt the construct is stretched  . After this loading phase, the construct is gradually unloaded, typically at the same rate, so that it is returned to its initial length,
. After this loading phase, the construct is gradually unloaded, typically at the same rate, so that it is returned to its initial length, 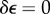 at t = 2δt. This yields a hysteresis curve; the force at any time during the loading phase is greater than the force at the same extent of stretch during unloading. The area between the two curves is represented by the energy dissipated, e.g. by viscous drag, during the cycle. The hysteresis area is sometimes normalized by dividing the area between the loading and unloading curves by the total area under the unloading curve. The hysteresis area, the peak force and the shape of the loading and unloading curves are the parameters of greatest interest for this type of measurement. A complication is that tissue constructs loaded in uniaxial tension cannot sustain compression, meaning that the compressive portion of the hysteresis curve can be truncated (figure 2c).
at t = 2δt. This yields a hysteresis curve; the force at any time during the loading phase is greater than the force at the same extent of stretch during unloading. The area between the two curves is represented by the energy dissipated, e.g. by viscous drag, during the cycle. The hysteresis area is sometimes normalized by dividing the area between the loading and unloading curves by the total area under the unloading curve. The hysteresis area, the peak force and the shape of the loading and unloading curves are the parameters of greatest interest for this type of measurement. A complication is that tissue constructs loaded in uniaxial tension cannot sustain compression, meaning that the compressive portion of the hysteresis curve can be truncated (figure 2c).
3.2.1. Preconditioning and the response to repeated loading
A systematic decrease of stiffness from one stretch measurement to the next has been observed in both natural tissues and in tissue constructs. This is particularly important in tests on tissue constructs, because a typical protocol, as described below, involves repeating tests on a single tissue construct to compare responses affected by a particular agonist or inhibitor. To eliminate the uncertainty caused by changes owing to repeated loading, it is customary to subject tissues to a number of ‘preconditioning’ loading/unloading cycles prior to measurement [89]. For natural tissues, it is typically sufficient to pre-stretch a tissue 3–15 times to ensure stable stiffness measurements thereafter [96]. For at least some engineered tissue constructs, however, more stretch cycles are required. During a series of many stretches, the stiffness of tissue constructs assembled from chicken embryo fibroblasts and collagen continues to diminish by successively smaller extents [96]. The hysteresis and peak force both drop by about 25% after the first stretch (figure 2c). The shape of the loading curve changes from nearly linear or with slight negative (concave-down) curvature on the first stretch to highly nonlinear with positive (concave-up) curvature on the second and all subsequent stretches. The hysteresis reaches a constant value of about 60% of its initial value by the 20th stretch, but the peak force continues to decline. After the seventh stretch, the peak force drops 3–4% and after 80 stretches the peak force declines by 0.3% per cycle [96]. If the construct is allowed to rest for several minutes after a stretch cycle, then the peak force and hysteresis increase. The longer the rest, the larger the increase. It seems likely that this effect exceeds what should be expected from viscoelastic relaxation and involves active cellular remodelling processes.
Overstretching a construct circumvents the need for large numbers of stretch cycles to precondition a tissue. For example, for a test carried to a maximum stretch of λmax, overstretching to 1.05 λmax for 10 cycles yields a steady-state peak force for subsequent stretches to λmax [96]. Despite these differences for both biological tissues and engineered constructs, the dependence of peak force and hysteresis on stretch rate is relatively weak. For both kinds of tissues, the peak force approximately doubles and the hysteresis increases 25% for an increase in stretch rate of two orders of magnitude [96].
The relaxation of stress after a step stretch occurs over a wide time range that can include very slow components [95]. Although variation of mechanical response from one loading cycle to the next can be attributed, in part, to incomplete viscoelastic relaxation, studies of biological tissues have shown that ‘strain softening’ is a major cause of the observed behaviour. Strain softening is a progressive decrease of stiffness and hysteresis as the tissue is stretched to greater levels of maximum stretch and has been attributed at least in part to disruption of links in the ECM [102,103], and to disruption of cytoskeletal structures in cells Nekouzadeh et al. [104] and Lee et al. [105]. A simple linear viscoelastic model (e.g. a generalized, three-element Maxwell model that includes both short and long viscoelastic relaxation times) can explain many features of tissue construct behaviour such as the increase of peak force with increase of stretch rate, the continuous drop of peak force over conditioning cycles, differences between first and second stretches being greater than between subsequent stretches, and varying degrees of recovery during rest intervals of varying duration. However, the generalized Maxwell model cannot predict strain softening or preconditioning by overstretch, or nonlinear force–stretch behaviour [96].
3.3. Integrated chemomechanical experimentation and modelling
To test mechanical and functional properties of a construct, one often makes a series of measurements under different media conditions. For example, one might measure the stiffness of a construct in an initial ‘baseline’ state using a ramp loading and unloading cycle, then again after activation of actin–myosin contraction, and then once more after disruption of the actin cytoskeleton. Although we do not present an exhaustive review of agents useful for mechanobiology, we list a few key examples here to highlight the nature of the approach and to introduce agents used in the ensuing examples.
At the level of whole cells, treatment with deoxycholate or Triton X100 eliminates cells without perturbing the mechanics of the ECM, enabling ECM mechanics to be estimated through the models described below [95,104]. A broad range of agonists and inhibitors of actomyosin contraction exist. Although fetal calf serum is an effective agonist, specific contractile pathways can be targeted. Blebbistatin can be used to inhibit myosin II activity [106,107]. Y-27632, the Rho-associated kinase inhibitor, can be used to specifically affect the rho/ROCK pathway [108], and ML-7 can inhibit myosin light chain kinase [109]. Calcium-dependent contraction can be modulated via thapsigargin, which releases internal stores of calcium, with BAPTA AM, which chelate intracellular calcium and with EGTA, which chelates calcium in the external medium [105,106,110,111].
More specific inhibitors can be applied to probe the roles of cytoskeletal filament networks on tissue construct mechanics. The actin cytoskeleton can be disrupted by drugs that affect polymerization dynamics. Cytochalasin D (CD), long used to probe the role of the actin cytoskeleton in cell stiffness [112,113] and contraction [80], serves as a means of disrupting the actin cytoskeleton [114]. The marine toxins latrunculin A and B serve a similar role [114–116]. Microtubule polymerization can be similarly disrupted by treatment with nocodazole, although this is complicated by interactions with phosphorylation of the myosin regulatory light chain [81]. Microtubules can also be stabilized by paclitaxel (taxol). Although intermediate filament systems do not appear to affect cell mechanics at physiological strain levels, the phosphatase inhibitor, calyculin A, can be used at high concentration to disrupt actin, microtubule and vimentin cytoskeletal networks [117–119].
In the following sections, we describe ways that coupled mechanical and biochemical experiments can be interpreted through integrated multiscale models to quantify changes to the structure and function of cells and ECM within tissue constructs.
4. Mechanical behaviour of tissue constructs
Three questions should be addressed at the outset of studies of tissue construct mechanics. First, how similar are the mechanical properties of the constructs to those of the biological tissues they are meant to mimic? Second, what is the structural basis of the measured mechanical properties? Third, how are the mechanical properties regulated? In the following sections, we summarize key results that shed light on these questions.
4.1. Tissue constructs replicate key behaviours of biological tissues
4.1.1. Stretch-dependent stiffening
The relationship between stress and stiffness has been studied extensively in ring-shaped fibroblast-populated matrices (FPMs) assembled from chicken embryo fibroblasts and type I collagen [45]. Using the device in figure 2, dynamic stiffness was measured by subjecting the FPM to a small (less than 0.5%) sinusoidal strain (0.5 Hz) that induced a corresponding sinusoidal variation of force. The dynamic stiffness was defined here as the amplitude of the force change divided by the strain amplitude. The dynamic stiffness was measured as the construct was subjected to a slow ramp loading/unloading cycle (approx. 0.01% s−1) that produced a force versus stretch hysteresis curve.
Exposure to calf serum activates actin–myosin contraction and so increases the force the FPMs exerted. Conversely, treatment by CD disrupts the actin cytoskeleton and so decreases the force. In contrast to a simple linear spring for which the stiffness is constant, the dynamic stiffness of an FPM is proportional to force as the force is changed not only by a ramp strain but also by treatment with calf serum or CD. Hence, dF/dx = αF, and so force is proportional to the exponential of strain (F ∼ exp(αx)). This relationship is also a characteristic property of many biological tissues [89,120] and so represents an important congruence between biological tissues and engineered tissue constructs.
The apparent viscoelasticity indicated by the hysteresis loop in ramp loading/unloading cycles suggests that force and stiffness should vary with the rate of strain. This dependence is, however, quite weak. The dynamic stiffness of calf serum-activated FPMs increases less than twofold over a 100-fold change in oscillation frequency [45]. For non-activated and CD-treated constructs that exert lower levels of force, the dependence on frequency is even weaker. This weak dependence of stiffness on strain rate resembles that seen for biological tissues [89]. Decomposition of the relaxation modulus into components that measure the elasticity and viscosity of the construct shows that the elastic resistance to stretch is greater than the viscous resistance and that the viscous resistance arises mostly from the cells [45].
4.1.2. Frank–Starling mechanism
Tissue constructs engineered to mimic heart tissue are an important tool for drug discovery and for testing cardiotoxicity [121]. The earliest of these tissue constructs were composed of chick embryo fibroblasts in rat tail (type I) collagen [122], and various forms of mechanical and electrical conditioning have been shown to improve the structure and contractile function of these and related cardiac tissue constructs [123–126].
Although the periodic contractile forces in these EHTs are far below those of normal myocardium, they appear to be reasonable mimics qualitatively. One important qualitative feature retained by EHTs is the Frank–Starling mechanism, characteristic of heart muscle. In the Frank–Starling response, pressure developed during heart contraction increases with increasing ventricular filling volume or applied pressure, enabling cardiac output to be adjusted in response to physiological demand. Dependence of contractile force on stretch in skeletal muscle (figure 3b) results from stretch-dependent variation of the degree of overlap of thick (myosin) and thin (actin) filaments (number of functioning myosin cross-bridges). The much steeper dependence of contractile force on change of tissue length observed in cardiac muscles [128] is ascribed to (i) a strain- or stress-dependent increase of release of calcium from sarcoplasmic reticulum, (ii) a change in calcium sensitivity of the contractile apparatus, and (iii) associated stretch-dependent changes to cross-bridge kinetics [129,130]. EHTs display a corresponding steep slope in the variation of twitch force with baseline force (figure 3) [127].
Figure 3.
(a) In tissue constructs containing chick embryo cardiomyocytes and chick myofibroblasts, both the periodic and baseline force vary nonlinearly with strain with during a ramp loading and unloading. (b) The relationship between developed tension and tissue construct length approximates the Frank–Starling law that is characteristic of heart muscle (adapted from Asnes et al. [127]). (Online version in colour.)
4.1.3. Modelling of disease
Tissue constructs can replicate key features of a range of pathologies. The aforementioned EHTs, when synthesized with a population of myofibroblasts, serve as a model of fibrotic cardiomyopathy, including loss of contractile function associated with overgrowth of myofibroblasts [131]. A broad range of cancers are replicated faithfully in tissue constructs that incorporate tumour cells [132]. One early exemplary system recapitulates breast cancer-like acini in vitro through a three-dimensional basement membrane culture assay [133]. Another exemplary system is the high-throughput Xu model for ovarian cancer, which captures the strong interdependence of fibroblasts and ovarian cancer cells in the formation of multicellular acini [134]. Cell migration in tissue constructs is fundamentally different from that observed in cells on two-dimensional substrata, including uniaxial trajectories, independence of motion on ECM ligand density, strong dependence on myosin II contractility and a positioning of the centrosome posterior to the nucleus [135]. Many of these features are central to the migration of cells, especially cancerous cells, in three-dimensional tissues [136,137]. Recent advances in controlling the pore size, composition and mechanics of the ECM enable more detailed replication and perturbation of the tumour microenvironment [138]. The ability of tissue constructs to replicate these elements of pathological tissues make them important tools for probing the roles of the ECM and mechanical environment on pathologies including a range of cancers [139].
4.2. Integrated modelling and experimentation can quantify the structural basis of mechanical function
The linkage between structure and mechanical function in tissue constructs is evident from even the simplest observations of their form and response. We begin with some fundamental observations, then proceed to discuss several multiscale modelling approaches that shed light on the structural basis of tissue construct mechanics.
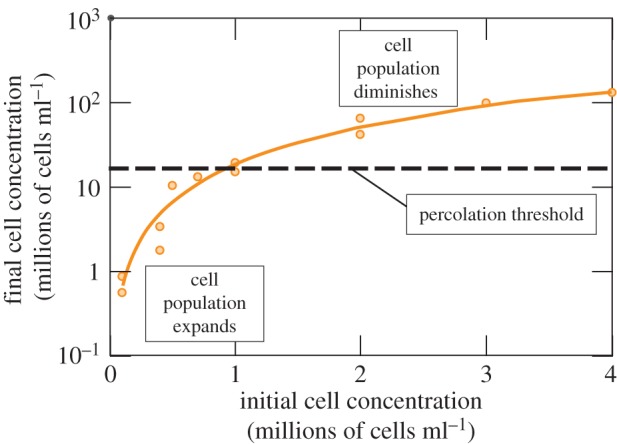
Geometric and morphological changes occur to FPMs as cells remodel collagen. An illustration involves FPMs synthesized with cell concentrations ranging from 105 to 106 cells ml−1, which showed increasing volume reduction over 2 days of culture with increasing initial cell concentration. For example, FPMs cultured with 106 cells ml−1 of collagen began with a concentration 10 times that of FPMs cultured with 105 cells ml−1 of collagen, but after 2 days had a concentration 41 times that achieved in the less dense FPMs [45]. This trend is evident as well in FPMs cultured over 3 days which showed that the degree to which cells reduce the volume of a tissue construct during remodelling increases with increasing number of cells per unit volume (figure 4) [95]. In this latter study, cells were further found to proliferate or die off to approach a concentration near the steric percolation threshold, the concentration of cells at which they form a continuous (steric) network. Cells in FPMs with the lowest starting cell concentrations proliferated, whereas those in the FPMs with the highest starting cell concentrations decreased in number.
Figure 4.
The cells in a tissue construct constrict and remodel the ECM over a 3 day incubation. Cell concentrations increase in all cases. The number of cells decreases or increases during incubation, so that the final cell concentration approaches the percolation threshold (adapted from Marquez et al. [140]). (Online version in colour.)
Simple mechanical tests on ring-shaped FPMs showed systematic variation of the cellular contribution to force for the constructs containing 0.4, 0.7 and 1.0 million cells; the constructs containing 100 000 cells, however, contributed less than predicted from the trend established by the higher concentrations. When normalized by the force per cross-sectional area of the cells in the construct, the hysteresis curves for the cellular component for the three higher concentrations nearly coincided, indicating that the force per cell was similar in these constructs, but the corresponding curve for the lowest concentration was substantially lower [45]. This reveals that cell–cell interactions for the three higher concentrations were similar and were different from those experienced at the lowest concentration. This may have been due to the establishment of an interconnected network at the higher concentrations with the cells interacting mechanically either directly via the formation of junctions (adherens or tight junctions) or linked through the collagen traction fibres [99,141]. In contrast, the cells at the lowest concentration appear to have been mechanically isolated, as supported by cross-sectional images devoid of cells; FPMs with the three higher cell concentrations had cells in all cross-sectional images. In contrast to the cellular contribution, the ECM contribution to the force normalized to the cross-sectional area of the ECM increases systematically as the cell concentration increases. This contribution exceeds that predicted by area changes associated with simple syneresis, indicating the contribution of factors such as ECM cross-linking, long-range ECM organization and synthesis of new ECM by cells [99,141,142].
4.2.1. Parallel decomposition
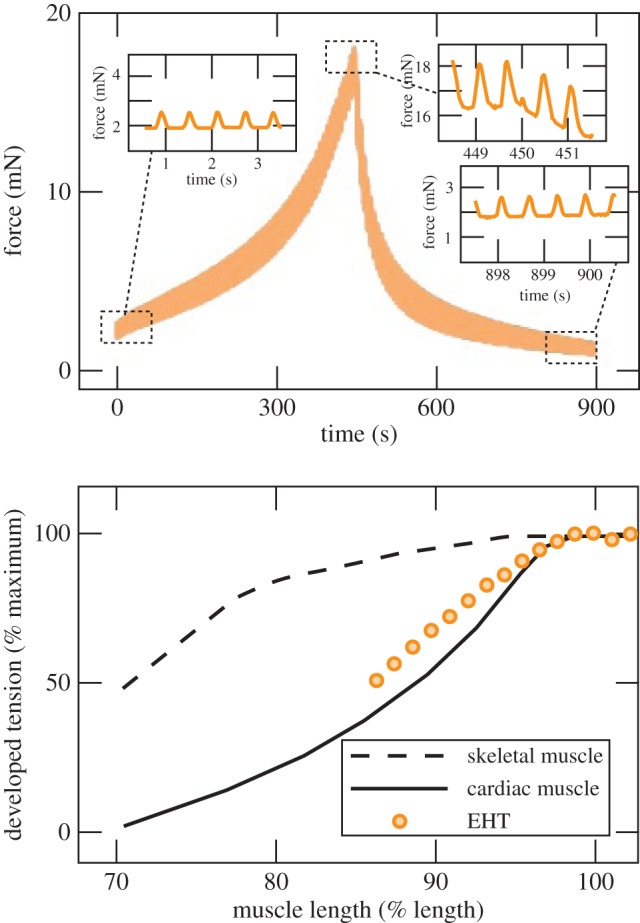
The most elementary question about the relationship between the structure of a tissue construct and its mechanical properties is, what are the relative magnitudes of the contributions of the cells and the ECM? The simplest approach involves assuming that these contributions add in parallel (an assumption discussed in detail below), so that the force, Ft, exerted by the untreated intact tissue construct as it contracts or as it is stretched is Ft = Fc + Fm, where Fc and Fm are the forces exerted by the cells and the ECM, respectively. Supposing further that treatment of the construct by CD effectively disconnects the contributions of cells, leaving only the contribution of the ECM, Fm can be measured during a ramp loading/unloading cycle of a CD-treated construct [45]. The difference between the response of the untreated tissue construct (solid line for an FPM, figure 5) a CD-treated construct (dashed line, figure 5) is then an estimate of the force exerted by the cells: Fc = Ft − Fm.
Figure 5.
Force versus strain for activated and CD-treated FPMs. The ECM contribution is approximated by the force versus strain curve of the CD-treated FPM. The difference between the two provides an estimate of the cell response (adapted from Wakatsuki et al. [45]). (Online version in colour.)
Even this simple decomposition provides much insight into the mechanical behaviour of cells and the ECM. Except at the lowest strains, the hysteresis curves for the ECM and the FPM are similar, highly nonlinear and with concave-up curvature, a pattern incompatible with linear viscoelasticity [45]. For the cellular component, the curves are more nearly linear with a relatively shallow slope, suggesting a response dominated by a small elastic response superimposed upon a constant contractile stress. Regions of negative curvature are evident in the regions of lowest and highest force, reflective of the strain-induced disruption of the actin cytoskeleton that later analyses confirmed [104,105,143]. At the lowest strains, the force exerted by the ECM is very small, so that the hysteresis curve for the intact construct also shows negative curvature. As the strain increases, the force exerted by the ECM surpasses that exerted by the cells and so dominates the hysteresis curve of the intact tissue. The stiffening of the ECM as strain increases is understood as a hallmark of collagen fibres being recruited differentially with increasing strain [100,144], and could serve to protect a tissue from overextension. Below we describe a molecular theory of strain stiffening that depends on the semi-flexible character of cytoskeletal or ECM filaments.
4.2.2. Multiscale cell-to-tissue modelling
The structural basis of the mechanical properties of tissue constructs can be considered at several levels: interconnected cellular networks, the systems of polymeric fibres that that make up the cytoskeleton (actin, microtubules, intermediate filaments) and the ECM (collagen, elastin, etc.) and finally the individual cytoskeletal and ECM filaments. Quantifying the contributions of these components from the experimental approaches described above requires modelling efforts more detailed than those described above, accounting for such features as the organization, volume fraction and contractility of cells; the nature of the ECM and the connectivity between cells, ECM and subcellular components. A broad range of active hydrogel models exist now, capable of predicting the polarization and function of tissue constructs [145–147]. We summarize here as an example the Zahalak model and its extensions.
A feature common to all of these models is consideration not of force but of stress. Ramp or step stretch measurements provide values of stiffness (change in force/change in length owing to stretch) and time course of the relaxation of force. These properties depend on the dimensions of the construct being tested, e.g. all other things being equal, the stiffness of a construct will increase with its thickness. It is, therefore, preferable to reduce the measurements of force to nominal stress and to represent the relaxation properties in terms of the relaxation modulus as described above. The Zahalak model [148] is a constitutive model of the tissue in which structural features of the cells and the ECM are represented as elastic and viscous elements. The moduli and the viscosities of these elements, derived by comparing the model to the experimental results, supply quantitative parameters with which to compare constructs and to predict dependence of stress on deformation history. The Zahalak model makes explicit the separate contributions of cells and the ECM. The model focuses on the limit of small strains (less than 10%).
For a tissue construct assembled from collagen and contractile cells such as myofibroblasts, the Zahalak model arises by combining in parallel the passive, viscoelastic contributions of ECM and the passive viscoelastic and active contributions of the myofibroblasts
| 4.1 |
where 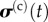 and
and 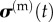 are the average stresses in the cells and ECM, respectively, and fc(t) is the volume fraction of cells.
are the average stresses in the cells and ECM, respectively, and fc(t) is the volume fraction of cells. 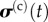 and
and 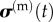 vary with time owing to passive viscoelastic effects, active cellular contraction and ECM remodelling, and fc(t) varies owing to cell proliferation or death and cell or ECM remodelling. Because all features of a living tissue construct vary with respect to time, we drop the notation indicating time variance in the ensuing discussion. We also note that individual mechanical tests are typically conducted over time increments for which changes to fc and effects of remodelling are negligible.
vary with time owing to passive viscoelastic effects, active cellular contraction and ECM remodelling, and fc(t) varies owing to cell proliferation or death and cell or ECM remodelling. Because all features of a living tissue construct vary with respect to time, we drop the notation indicating time variance in the ensuing discussion. We also note that individual mechanical tests are typically conducted over time increments for which changes to fc and effects of remodelling are negligible.
Zahalak estimated the cellular stress σ(c) by regarding cells as contractile rods with a specified distribution of orientations. In analogy to the Hill [149] model for skeletal muscle, a damped cellular contractile apparatus acts in parallel and in series with elastic springs having spring constants kp and ks, respectively (figure 6). The spring constant kp represents the static elasticity and ks the increased stiffness of activated cells. The damped contractile element exerts a force F0 that can be altered by agents that activate or inhibit actin–myosin contraction. The contractile element is in parallel with a damper of time constant τc and a viscosity ksτc. This yields a linear, first-order differential equation that relates the time variation of the force of the cell F, to the history of the length changes of the cell [148].
Figure 6.
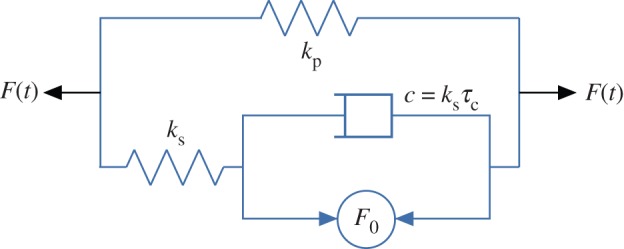
The linearized Hill model for skeletal muscle combines parallel and series springs with a damped active element. (Online version in colour.)
Zahalak calculated the contribution of the cells to the stress σ in the tissue construct by averaging contributions over the cellular orientation distribution. For the kth cell in the tissue construct, pointed in the direction of a unit vector n(k), this contribution is a tensor 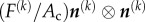 , where Ac is the cross-sectional area of a cell, and the tensor product
, where Ac is the cross-sectional area of a cell, and the tensor product  converts the scalar stress component
converts the scalar stress component 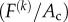 into a stress tensor. For example, for a cell pointed in a Cartesian X-direction, the matrix of the tensor would simply have a component
into a stress tensor. For example, for a cell pointed in a Cartesian X-direction, the matrix of the tensor would simply have a component 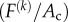 in the 11-position, and zeros everywhere else; for a cell aligned in an arbitrary direction relative to Cartesian axes, the matrix would have Cartesian components
in the 11-position, and zeros everywhere else; for a cell aligned in an arbitrary direction relative to Cartesian axes, the matrix would have Cartesian components 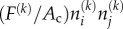 , where i and j adopt the numbers 1–3 to represent the X-, Y- and Z-directions. The cellular contribution to the tissue construct stress σ is found by integrating these contributions over the probability distribution of the orientation of the cells, P(n(k)), and scaling by the cellular volume fraction
, where i and j adopt the numbers 1–3 to represent the X-, Y- and Z-directions. The cellular contribution to the tissue construct stress σ is found by integrating these contributions over the probability distribution of the orientation of the cells, P(n(k)), and scaling by the cellular volume fraction
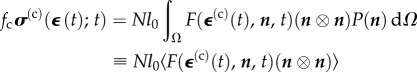 |
4.2 |
where Ω represents a sphere of unit area, 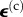 is the time history of the average strain in the cells, N is the number of cells per unit volume, l0 is the length of the cells, and the term Nl0 is equal to the cellular volume fraction divided by the cell cross-sectional area (
is the time history of the average strain in the cells, N is the number of cells per unit volume, l0 is the length of the cells, and the term Nl0 is equal to the cellular volume fraction divided by the cell cross-sectional area (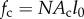 ). A broad range of imaging techniques exists to estimate P(n), N and l0 [150–152]. Then, the following differential equation can be written for the evolution of σ(c) as a function of the time history of
). A broad range of imaging techniques exists to estimate P(n), N and l0 [150–152]. Then, the following differential equation can be written for the evolution of σ(c) as a function of the time history of 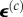 :
:
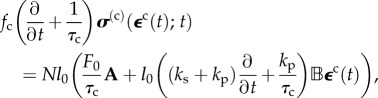 |
4.3 |
where the two ‘anisotropy tensors' have components 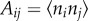 and
and 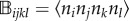 , in which i, j, k and l take the values of 1–3, and
, in which i, j, k and l take the values of 1–3, and  was defined in the second line of equation (4.2).
was defined in the second line of equation (4.2).
The relationship of the average strain field in the cells 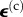 to the average strain field in a tissue construct
to the average strain field in a tissue construct 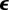 is a function of the mechanical properties and loading history of both the cells and the ECM. As an illustration suppose that cells are very stiff compared with the ECM and are present at very low concentration. As the construct is stretched, the cells experience little strain, and so the stiffness will be determined by the strain of the ECM. If, however, the cells form a continuous network from one end of the construct to the other, then the cells and ECM will stretch together and the stiffness of the construct will be dominated by the stiffness of the cell network. As suggested by this example the extent to which the cells and ECM stretch coordinately depends on the concentration and mutual interactions of the cells in the construct. A broad range of linear [153,154] and nonlinear [155,156] homogenization techniques exist for predicting this, although these are less accurate for high volume fractions of cells [157]. The approach of Marquez et al. [158] was to define a ‘strain factor,’ S, the ratio of the average strain of a cell along its axis to the average strain of the tissue construct resolved along the cell axis,
is a function of the mechanical properties and loading history of both the cells and the ECM. As an illustration suppose that cells are very stiff compared with the ECM and are present at very low concentration. As the construct is stretched, the cells experience little strain, and so the stiffness will be determined by the strain of the ECM. If, however, the cells form a continuous network from one end of the construct to the other, then the cells and ECM will stretch together and the stiffness of the construct will be dominated by the stiffness of the cell network. As suggested by this example the extent to which the cells and ECM stretch coordinately depends on the concentration and mutual interactions of the cells in the construct. A broad range of linear [153,154] and nonlinear [155,156] homogenization techniques exist for predicting this, although these are less accurate for high volume fractions of cells [157]. The approach of Marquez et al. [158] was to define a ‘strain factor,’ S, the ratio of the average strain of a cell along its axis to the average strain of the tissue construct resolved along the cell axis, 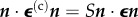 , where, in a two-dimensional elastic tissue,
, where, in a two-dimensional elastic tissue, 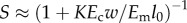 in which Ec and Em are the effective tangent moduli for the cell and ECM, respectively, and w and l0 are the width and lengths of the cells, respectively [159]; similar scaling laws exist for three-dimensional tissue constructs [158]. The Eshelby [160] solution for an isolated ellipsoidal linear elastic inclusion in a linear elastic ECM can be manipulated to yield K = 2. Monte Carlo simulations for randomly oriented two-dimensional ribbon-shaped cells yield K = 2.2. This approach provides a reasonable approximation for isolated cells with an aspect ratio of 10 : 1 or greater, independent of cellular anisotropy, provided that stress fibres are aligned with the long axis of the cell [161]. When the cells are at high concentration, their mutual interactions extend their effective lengths and disproportionately increase the modulus through their effect on the ECM. Hence, one can define an ‘effective’ cell length and modulus, leff and Eeff, from which one can construct an effective normalized cell stiffness
in which Ec and Em are the effective tangent moduli for the cell and ECM, respectively, and w and l0 are the width and lengths of the cells, respectively [159]; similar scaling laws exist for three-dimensional tissue constructs [158]. The Eshelby [160] solution for an isolated ellipsoidal linear elastic inclusion in a linear elastic ECM can be manipulated to yield K = 2. Monte Carlo simulations for randomly oriented two-dimensional ribbon-shaped cells yield K = 2.2. This approach provides a reasonable approximation for isolated cells with an aspect ratio of 10 : 1 or greater, independent of cellular anisotropy, provided that stress fibres are aligned with the long axis of the cell [161]. When the cells are at high concentration, their mutual interactions extend their effective lengths and disproportionately increase the modulus through their effect on the ECM. Hence, one can define an ‘effective’ cell length and modulus, leff and Eeff, from which one can construct an effective normalized cell stiffness 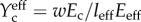 and from this the strain factor
and from this the strain factor 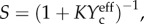 where K is a scaling factor. A key result is that S approaches 1 at cell volume fractions at or above the steric percolation threshold.
where K is a scaling factor. A key result is that S approaches 1 at cell volume fractions at or above the steric percolation threshold.
The contribution of the ECM in equation (4.1), 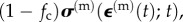 can be represented by any of a number of viscoelastic relations, commonly broken into volumetric and distortional relaxation moduli. A common choice for modelling a biological material is Fung's quasi-linear viscoelastic (QLV) model, which applies to materials that show elastic nonlinearity but that retain some features of linear viscoelasticity in their relaxation responses. Supposing that the ECM is an incompressible Fung QLV material, σ(m)(t) can be expressed in terms of the pressure and a convolution of an ‘elastic stress' and a relaxation function, μ(t). Note that the weighted volumetric averages of cellular and ECM strains must sum to the average strain in the tissue construct, so that
can be represented by any of a number of viscoelastic relations, commonly broken into volumetric and distortional relaxation moduli. A common choice for modelling a biological material is Fung's quasi-linear viscoelastic (QLV) model, which applies to materials that show elastic nonlinearity but that retain some features of linear viscoelasticity in their relaxation responses. Supposing that the ECM is an incompressible Fung QLV material, σ(m)(t) can be expressed in terms of the pressure and a convolution of an ‘elastic stress' and a relaxation function, μ(t). Note that the weighted volumetric averages of cellular and ECM strains must sum to the average strain in the tissue construct, so that 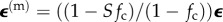 .
.
In a typical experimental measurement, one first determines the stress relaxation for the tissue construct σ11(t) in response to an uniaxial step stretch in the X-direction that results in a strain of magnitude 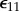 . Then, after disconnecting the cells from the ECM using CD or a detergent, one determines the relaxation function for the ECM μ(t) from a second step stretch of the same magnitude. The complicated equations that describe σ(t) in response to a prescribed strain can be reduced to simple forms for measurements with small uniaxial step strains
. Then, after disconnecting the cells from the ECM using CD or a detergent, one determines the relaxation function for the ECM μ(t) from a second step stretch of the same magnitude. The complicated equations that describe σ(t) in response to a prescribed strain can be reduced to simple forms for measurements with small uniaxial step strains 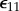 [148]. For a tissue construct with a volume fraction of cells fc that is near the percolation threshold, the relaxation function for the ECM can be approximated from the measured stress relaxation,
[148]. For a tissue construct with a volume fraction of cells fc that is near the percolation threshold, the relaxation function for the ECM can be approximated from the measured stress relaxation, 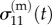 as
as 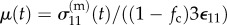 . To account for the cellular contribution to the stress, it is necessary to determine the cell anisotropy tensors from the specified distribution of cell orientations. Based on experimental observation of the tested tissue constructs, it is reasonable to approximate the orientation distribution as planar isotropic [45,148]. Then, one can determine spring constants and viscosity for the cells from the measured responses to a fast uniaxial step stretch from
. To account for the cellular contribution to the stress, it is necessary to determine the cell anisotropy tensors from the specified distribution of cell orientations. Based on experimental observation of the tested tissue constructs, it is reasonable to approximate the orientation distribution as planar isotropic [45,148]. Then, one can determine spring constants and viscosity for the cells from the measured responses to a fast uniaxial step stretch from
| 4.4 |
where τc = η/ks is the relaxation time for the cells [148]. From the long-time value of the ECM relaxation function μ∞, one can determine the contractile stress, σ0 (derived from the contractile force, F0) from the following
| 4.5 |
Here, 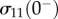 is the stress sustained by the tissue after it has reached a steady state prior to the step stretch. Experimental measurements for these myofibroblast-based tissues showed that the relative contribution of cells was greater at the lowest strains because of the exponential increase of the stiffness of the ECM with stretch compared with an approximately linear dependence of the stiffness of the cells. The force produced by a single myofibroblast was in the range 700–800 nN (σ0 ∼ 400 Pa), as expected, much larger than the value for individual leucocytes deduced from measurements of cortical tension by micropipette aspiration [9]. The moduli determined for the myofibroblasts in engineered constructs are comparable to those of smooth muscle cells, suggesting that the transformation of normal fibroblasts to the wound-healing (myofibroblast) phenotype is in the direction of smooth muscle cells as suggested by functional and structural criteria Tomasek et al. [162].
is the stress sustained by the tissue after it has reached a steady state prior to the step stretch. Experimental measurements for these myofibroblast-based tissues showed that the relative contribution of cells was greater at the lowest strains because of the exponential increase of the stiffness of the ECM with stretch compared with an approximately linear dependence of the stiffness of the cells. The force produced by a single myofibroblast was in the range 700–800 nN (σ0 ∼ 400 Pa), as expected, much larger than the value for individual leucocytes deduced from measurements of cortical tension by micropipette aspiration [9]. The moduli determined for the myofibroblasts in engineered constructs are comparable to those of smooth muscle cells, suggesting that the transformation of normal fibroblasts to the wound-healing (myofibroblast) phenotype is in the direction of smooth muscle cells as suggested by functional and structural criteria Tomasek et al. [162].
4.2.3. Polymer-based structural models can explain certain aspects of tissue construct mechanics
The mechanical properties of cells and their ECM determine the mechanical resilience of organs and tissues. A plausible starting point for analysing the mechanics of both cells and the ECM is their behaviour as polymer systems [3,163,164]. One might suppose that the viscoelastic properties of cells arise simply from the viscoelastic properties of the cytoskeletal filament systems. The time-dependent deformability of single cells [12,13], tissues [89] and tissue constructs [95], however, show strain stiffening, i.e. the slope of the force–deformation (stiffness) curve increases with increasing strain. This is incompatible with simple linear viscoelasticity. This nonlinear behaviour likely arises at least in part from the intrinsic stiffness of the cytoskeletal and ECM filaments.
The persistence length, lp, a conventional measure of the polymer's stiffness, is the average distance over which the axial direction of a polymer filament loses correlation with itself, i.e. undergoes random bending. The higher lp, the stiffer the filament. The relative stiffness of a polymer molecule can be simply judged by comparing lp with the total contour length of the polymer molecule, Lc. In cells and ECM, biological polymer filaments are typically neither very flexible with 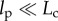 nor very rigid with
nor very rigid with 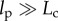 , but rather are in the semi-flexible regime in which lp and Lc are of similar magnitudes. The persistence lengths of cytoskeletal filaments vary over a fairly wide range, extending from microtubules, lp ≈ 5200 µm [165], to actin microfilaments, lp ≈ 20 µm [165,166] and to vimentin and desmin intermediate filaments, lp ≈ 1 µm [167–169]. Collagen fibrils are stiffer still with lp ≈ 1 cm [169–171]. In cross-linked networks, with the effective contour length being the distance between network nodes, the polymer molecules bend significantly between the nodes, but are not so flexible as to form random coils over this distance.
, but rather are in the semi-flexible regime in which lp and Lc are of similar magnitudes. The persistence lengths of cytoskeletal filaments vary over a fairly wide range, extending from microtubules, lp ≈ 5200 µm [165], to actin microfilaments, lp ≈ 20 µm [165,166] and to vimentin and desmin intermediate filaments, lp ≈ 1 µm [167–169]. Collagen fibrils are stiffer still with lp ≈ 1 cm [169–171]. In cross-linked networks, with the effective contour length being the distance between network nodes, the polymer molecules bend significantly between the nodes, but are not so flexible as to form random coils over this distance.
A molecular theory shows that networks of semi-flexible polymers, including cytoskeletal and extracellular filaments, conform to universal nonlinear stress–strain relationships at low-to-intermediate strains [172]. The theory develops from a relationship that relates the tension that elongates the end-to-end distance of a filament to its bending modulus [173]. The strain-stiffening behaviour results from the semi-flexible character of the polymer filaments; increasing strain of the network increases the stiffness of the filaments. The persistence length of the filaments determines the strain at which stiffening becomes important. Stiffer cytoskeletal filaments, e.g. F-actin or collagen, undergo strain stiffening at small strains (a few percent), whereas more flexible filaments such as vimentin stiffen only at much larger strains (approaching 100%) [172]. The maximum extent of strain stiffening depends on the compliance of the filaments and the strain at which the filaments rupture.
Although this molecular theory of the properties of passive semi-flexible filament networks helps to explain the strain stiffening of cells and tissues, it does not consider the important role of cell contractility in determining cell and ECM stiffness. Measurements by micro-indentation provide examples of the dependence on contractility of the stiffness of lymphocytes [15] and Dictyostelium amoebae [16], cells that are not in solid tissues. Cross-linking cell surface proteins initiate a process called ‘capping’ that includes a redistribution of the cross-linked membrane proteins to one region of the cell and a change of cell shape associated with an increase of myosin-dependent contractility [16]. The contraction increases cell stiffness by stretching the cytoskeletal cortex around the interior of the cell. The tensegrity model provides an explicit structural interpretation of this concept in the form of a dynamic balance between an actin–myosin cortical contractile force supported by compression-resistant cytoskeletal structures such as microtubules or, for tissue cells, by linkage to the ECM [174,175]. The role of microtubules as the principal structures that support cortical tension is still uncertain. The tensegrity model predicts that disruption of microtubules should increase the tension that cells exert on their substratum or, if in three-dimensional culture, to the ECM and this has been observed [81,176]. The disruption of microtubules in engineered fibroblast tissue constructs, however, also increased actin–myosin contractility, which seemed to account for most if not all of the increased cell stiffness [81]. Later work suggests that both increased contractility and load-shifting from microtubules could be responsible for the measured tension increase [55].
4.3. Regulation of mechanical properties
4.3.1. Elastic estimates
How do cells and the ECM affect one another's mechanical properties? In two dimensions, AFM indentation analyses of cells on synthetic gel substrates of varying stiffness revealed that the indentation stiffness was associated with an elastic modulus approximately equal to or slightly lower than those of their substrates for a range of soft gels, up to a saturating value of 20 kPa [177]. In three-dimensional FPMs, the opposite trend was observed for cells, and both cell and collagen ECM moduli change as a function of remodelling and cell density. These latter compared consecutive loadings of an FPM, with the second loading conducted after action of a detergent to lyse cells. We note that when cells are eliminated from tissue constructs by treatment with a detergent such as deoxycholate, the volumes previously occupied by the cells are then filled with fluid. The effect of this replacement will depend on the stiffness of the cells that have been removed. Hence, it may be necessary to take this effect into account to obtain an accurate estimate of the modulus of the ECM from measurements of the stiffness of a construct after detergent treatment. FPMs at 3 days of culture and myofibroblast concentrations less than 107 cells ml−1 showed an ECM modulus that was nearly indistinguishable from that measured on the CD-treated construct, whereas for FPMs containing approximately 14 × 107 cells ml−1 the difference between the two was about an order of magnitude [140]. Short-term (immediately after rapid loading) and long-term (after 3600 s of relaxation) elastic moduli increased by roughly four orders of magnitude as the cell concentration was increased from about 0.6 × 106 to about 150 × 106 cells ml−1 (figure 7). Estimated cell moduli dropped over the same range, with the cell and ECM long-term and short-term moduli intersecting at a final cell concentration above the steric percolation threshold [140]. These results indicate that interactions among cells at high concentration have a large effect on the mechanical properties of the construct.
Figure 7.
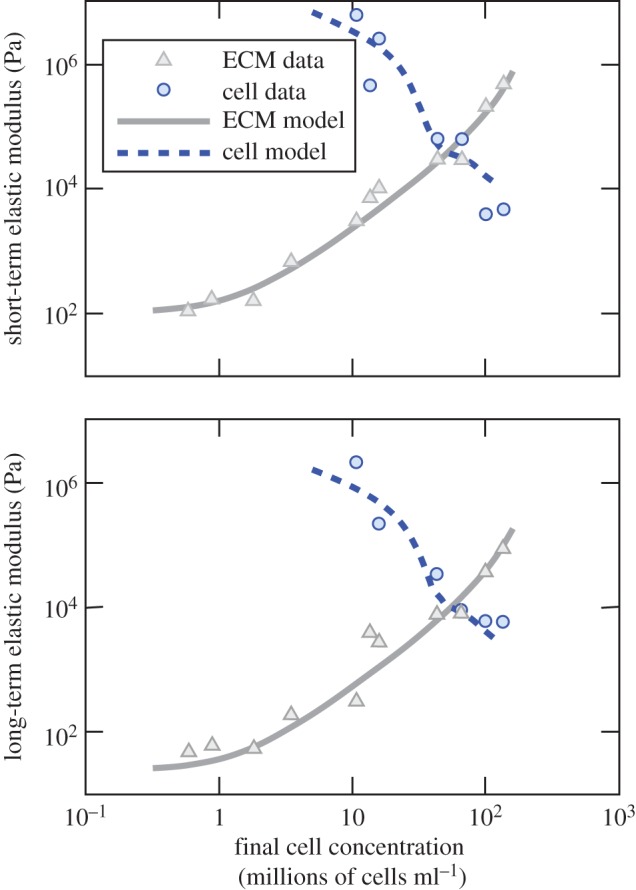
The modulus of ECM in a tissue construct increases with increasing cell concentration, and, contrary to cells cultured on two-dimensional substrata, the effective modulus of the cells decreases. The crossover occurs at a cell concentration near the percolation threshold for the cells. (Online version in colour.)
Do cells behave differently in two versus three dimensions? The comparison is difficult, because cell moduli estimated by both the AFM and FPM studies are effective moduli subject to artefacts. AFM probes cells on substrata, in a direction perpendicular to the plane of their stress fibres, and moduli must be estimated using the Hertz solution that is accurate only for indentation of homogeneous, isotropic media. However, interpretation of three-dimensional FPM data is also challenging, especially at low cell concentrations, because anisotropic ECM remodelling gives cells long-range interactions that effectively increase their apparent size [99,141] and because local collagen synthesis [142] can lead to inhomogeneity in the ECM. However, when taken together with the observation that cell populations seem to adapt to approach the percolation threshold, these results suggest that cells in three dimensions adapt themselves, their numbers and their ECM to match the moduli of cells and ECM.
4.3.2. Regulation of cellular form and function
Cells regulate their shapes and contractility by forming stress fibres and by establishing focal adhesions in response to the local topographical features and adhesive properties of their two-dimensional substrate or three-dimensional ECM environment. Laws governing how cells regulate their form and function are desirable from the perspectives of both fundamental biophysics and of drug screening to be able to detect subtle changes. Although models for cells within tissue constructs still require development and validation, we conclude by discussing one of these in detail to provide a sense of the state of the art. A groundbreaking advance presented elsewhere in this issue derives an expression for the free energy of a cell within an elastic ECM that can be applied to predict a broad range of these responses [178].
Here, we focus on the Deshpande model, another model that links dynamic biochemical processes to cell mechanical properties [179–184]. The Deshpande model was motivated by studies of cells adherent to patterned substrata or to an array of microneedles that illustrated how the shape and compliance of the adhesions sites influence the organization of the cytoskeleton and the focal adhesions [68,185]. The model addresses two interdependent remodelling processes—formation (and dissolution) of stress fibres and of focal adhesions—that are modulated by the mechanical stress (tensile forces) sustained by the cell. Earlier work has shown that stress fibres form when a cell is subjected to mechanical stress, e.g. under conditions of isometric tension [186,187], whereas relaxation of the tension caused disassembly of stress fibres.
An activation signal C(t) and the tension T acting on a stress fibre control the balance between the fibre's growth or disassembly. If the signal is initiated at time t0, its magnitude decays as 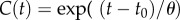 , where θ is the signal relaxation time. Suppose that the dimensionless parameter η (0 ≤ η ≤ 1) is the fraction of the maximum possible concentration of polymerized actin and phosphorylated myosin, i.e. activated actomyosin, under the current conditions in the cell. Then, the rate of stress fibre formation is proportional to 1 − η (the remaining available unpolymerized actin and myosin) and the activation signal C(t). The rate of stress fibre disassembly is proportional to η and (1 − T/T0), where T0 =η
Tmax is the isometric tension for the current value of η, and Tmax is the tension at maximum activation (η = 1). Hence,
, where θ is the signal relaxation time. Suppose that the dimensionless parameter η (0 ≤ η ≤ 1) is the fraction of the maximum possible concentration of polymerized actin and phosphorylated myosin, i.e. activated actomyosin, under the current conditions in the cell. Then, the rate of stress fibre formation is proportional to 1 − η (the remaining available unpolymerized actin and myosin) and the activation signal C(t). The rate of stress fibre disassembly is proportional to η and (1 − T/T0), where T0 =η
Tmax is the isometric tension for the current value of η, and Tmax is the tension at maximum activation (η = 1). Hence,
| 4.6 |
where kf and kb are dimensionless rate constants for the assembly and disassembly of the stress fibres. The rate of stress fibre disassembly is maximal when T = 0, and there is no disassembly under conditions of isometric tension. There is a graded rate of disassembly at intermediate levels of tension [179]. One gains some insight into the model by examining the steady-state value of η: 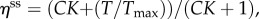 where
where 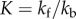 is in effect an equilibrium constant for stress fibre assembly. Suppose also that C is maintained at a steady-state activation level Css. As expected, ηss approaches 1 when CssK is large compared to unity and to T/Tmax. When CssK is small compared to unity and to T/Tmax, then ηss approaches T/Tmax, meaning that the steady state level of stress fibre assembly is proportional to T. This model accounts for several observed phenomena: the increase in cell-generated tension with increase of substrate stiffness, the relationship between the structural polarization of the cell and its shape and pattern of attachment to the substratum, and the high concentration of stress fibres at points of substrate attachment [179,180].
is in effect an equilibrium constant for stress fibre assembly. Suppose also that C is maintained at a steady-state activation level Css. As expected, ηss approaches 1 when CssK is large compared to unity and to T/Tmax. When CssK is small compared to unity and to T/Tmax, then ηss approaches T/Tmax, meaning that the steady state level of stress fibre assembly is proportional to T. This model accounts for several observed phenomena: the increase in cell-generated tension with increase of substrate stiffness, the relationship between the structural polarization of the cell and its shape and pattern of attachment to the substratum, and the high concentration of stress fibres at points of substrate attachment [179,180].
The model for focal adhesion formation supposes that integrin molecules can be in either a high- or a low-affinity form for binding to ECM ligands [181,183]. The molecules in the low-affinity state are supposed to be in a bent confirmation and can be converted to a straightened high-affinity state by mechanical tension. Furthermore, the low-affinity molecules are mobile in the cell membrane, whereas high-affinity molecules are immobilized by their connection to the ECM. The equilibrium balance between integrin molecules in low- and high-affinity states is controlled by the tension exerted on the cell membrane either externally or by cell contractile forces. When an activation signal initiates cell contractile force, the formation of focal adhesions follows a sequence of steps: (i) the contractile force develops tension at sites of connection between the high-affinity integrin molecules and the ECM (typically at the cell edges); (ii) the increased tension converts more integrin molecules from the low-to-high affinity state increasing the size of the focal adhesion; and (iii) the concentration of low-affinity integrin molecules is replenished by diffusion from other parts of the cell membrane. Hence, as the traction force increases, the size of the focal adhesion increases correspondingly as expected from experimental observations [186,187]. The model also is consistent with observations that focal adhesions concentrate at the edges of the cell and that focal adhesions disassemble when contractility is inhibited [188].
This model has been successful in predicting the regulation of cell form and function for cells patterned on two-dimensional substrata, including for complex three-dimensional loadings of these cells [189–192]. The validation of this for cells in three-dimensional tissue constructs remains an ongoing focus of research efforts [4].
5. Concluding remarks
The basic toolbox for the dissection of tissue constructs by chemical treatment and integrated multiscale modelling and experiment has uncovered a broad range of important fundamental behaviours of cells and ECM in three-dimensional culture. Models of tissue constructs such as the Zahalak model and its modifications enable dissection of tissue construct mechanical responses into cellular and ECM components. Models of ECM and cell regulation such as the Shenoy and Deshpande models constitute important advances in our ability to dissect cellular biophysics in three dimensions and will serve to assess how drugs affect this biophysics. Validating these models in tissue constructs is an important direction for future efforts, and identifying situations in which their predictions fail will certainly uncover new and interesting biophysics. Key areas of uncertainty are the structure and mechanical properties of the ECM of the pericellular region, and how cells choose to remodel themselves and these local- and tissue-level environments to reach desired set points. More complicated tissue construct models are becoming available, and new predictive models for how features such as vasculature, heterogeneous cell types, and developing or diseased cells are needed to fully harness their potential though integrated multiscale modelling and experiment.
Competing interests
We declare we have no competing interests.
Funding
This work was supported in part by the NIH through grant no. R01 HL109505, and jointly by the NSF and NIH through multiscale modelling grant no. U01EB016422.
References
- 1.Nerem R. 1992. Tissue engineering in the USA. Med. Biol. Eng. Comput. 30, CE8–CE12. ( 10.1007/BF02446171) [DOI] [PubMed] [Google Scholar]
- 2.Lanza R, Langer R, Vacanti J (eds). 2014. Principles of tissue engineering, 4th edn Amsterdam, the Netherlands: Elsevier. [Google Scholar]
- 3.Elson EL. 1988. Cellular mechanics as an indicator of cytoskeletal structure and function. Annu. Rev. Biophys. Biophys. Chem. 17, 397–430. ( 10.1146/annurev.bb.17.060188.002145) [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez ML, McGarry PJ, Sniadecki NJ. 2013. Review on cell mechanics: experimental and modeling approaches. Appl. Mech. Rev. 65, 060801 ( 10.1115/1.4025355) [DOI] [Google Scholar]
- 5.Cole KS. 1932. Surface forces of the arbacia egg. J. Cell Comp. Physiol. 1, 1–9. ( 10.1002/jcp.1030010102) [DOI] [Google Scholar]
- 6.Mitchison J, Swann M. 1954. The mechanical properties of the cell surface. I. The cell elastimeter. J. Exp. Biol. 31, 443–460. [Google Scholar]
- 7.Mitchison J, Swann M. 1954. The mechanical properties of the cell surface. II. The unfertilized sea-urchin egg. J. Exp. Biol. 31, 461–472. [Google Scholar]
- 8.Evans EA, Skalak R.1980. Mechanics and thermodynamics of biomembranes. Boca Raton, FL: CRC Press.
- 9.Evans E, Yeung A. 1989. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys. J. 56, 151 ( 10.1016/S0006-3495(89)82660-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skalak R, Chien S, Schmid-Schönbein G. 1984. Viscoelastic deformation of white cells: theory and analysis. Kroc Found. Ser. 16, 3. [PubMed] [Google Scholar]
- 11.Elgsaeter A, Stokke BT, Mikkelsen A, Branton D. 1986. The molecular basis of erythrocyte shape. Science 234, 1217–1223. ( 10.1126/science.3775380) [DOI] [PubMed] [Google Scholar]
- 12.Petersen NO, McConnaughey WB, Elson EL. 1982. Dependence of locally measured cellular deformability on position on the cell, temperature, and cytochalasin B. Proc. Natl Acad. Sci. USA 79, 5327–5331. ( 10.1073/pnas.79.17.5327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahalak G, McConnaughey W, Elson E. 1990. Determination of cellular mechanical properties by cell poking, with an application to leukocytes. J. Biomech. Eng. 112, 283–294. ( 10.1115/1.2891186) [DOI] [PubMed] [Google Scholar]
- 14.Daily B, Elson EL, Zahalak GI. 1984. Cell poking. Determination of the elastic area compressibility modulus of the erythrocyte membrane. Biophys. J. 45, 671 ( 10.1016/S0006-3495(84)84209-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasternak C, Elson EL. 1985. Lymphocyte mechanical response triggered by cross-linking surface receptors. J. Cell Biol. 100, 860–872. ( 10.1083/jcb.100.3.860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasternak C, Spudich JA, Elson EL. 1989. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature 341, 549–551. ( 10.1038/341549a0) [DOI] [PubMed] [Google Scholar]
- 17.Worthen GS, Schwab BR, Elson EL, Downey GP. 1989. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science 245, 183–186. ( 10.1126/science.2749255) [DOI] [PubMed] [Google Scholar]
- 18.Downey GP, Doherty DE, Schwab B, Elson E, Henson P, Worthen G. 1990. Retention of leukocytes in capillaries: role of cell size and deformability. J. Appl. Physiol. 69, 1767–1778. [DOI] [PubMed] [Google Scholar]
- 19.Downey GP, Elson EL, Schwab B, Erzurum SC, Young SK, Worthen GS. 1991. Biophysical properties and microfilament assembly in neutrophils: modulation by cyclic amp. J. Cell Biol. 114, 1179–1190. ( 10.1083/jcb.114.6.1179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radmacher M. 2002. Measuring the elastic properties of living cells by the atomic force microscope. Methods Cell Biol. 68, 67–90. ( 10.1016/S0091-679X(02)68005-7) [DOI] [PubMed] [Google Scholar]
- 21.Thoumine O, Nerem RM, Girard FR. 1995. Oscillatory shear stress and hydrostatic pressure modulate cell-matrix attachment proteins in cultured endothelial cells. In Vitro Cell Dev. Biol. Anim. 31, 45–54. ( 10.1007/BF02631337) [DOI] [PubMed] [Google Scholar]
- 22.Granzier HL, Irving TC. 1995. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys. J. 68, 1027–1044. ( 10.1016/S0006-3495(95)80278-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cukierman E, Pankov R, Yamada KM. 2002. Cell interactions with three-dimensional matrices. Curr. Opin. Cell Biol. 14, 633–640. ( 10.1016/S0955-0674(02)00364-2) [DOI] [PubMed] [Google Scholar]
- 24.Nelson CM, Bissell MJ. 2006. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 22, 287 ( 10.1146/annurev.cellbio.22.010305.104315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paszek MJ, et al. 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254. ( 10.1016/j.ccr.2005.08.010) [DOI] [PubMed] [Google Scholar]
- 26.Yamada KM, Cukierman E. 2007. Modeling tissue morphogenesis and cancer in 3D. Cell 130, 601–610. ( 10.1016/j.cell.2007.08.006) [DOI] [PubMed] [Google Scholar]
- 27.Sutherland FJ, Hearse DJ. 2000. The isolated blood and perfusion fluid perfused heart. Pharmacol. Res. 41, 613–627. ( 10.1006/phrs.1999.0653) [DOI] [PubMed] [Google Scholar]
- 28.Marquez JP, Legant W, Lam V, Cayemberg A, Elson E, Wakatsuki T. 2009. High-throughput measurements of hydrogel tissue construct mechanics. Tissue Eng. C Methods 15, 181–190. ( 10.1089/ten.tec.2008.0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CC, George SC. 2008. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng. A 15, 1363–1371. ( 10.1089/ten.tea.2008.0314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whisler JA, Chen MB, Kamm RD. 2012. Control of perfusable microvascular network morphology using a multiculture microfluidic system. Tissue Eng. C Methods 20, 543–552. ( 10.1089/ten.tec.2013.0370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moya ML, Hsu Y-H, Lee AP, Hughes CC, George SC. 2013. In vitro perfused human capillary networks. Tissue Eng. C Methods 19, 730–737. ( 10.1089/ten.tec.2012.0430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morin KT, Tranquillo RT. 2013. In vitro models of angiogenesis and vasculogenesis in fibrin gel. Exp. Cell Res. 319, 2409–2417. ( 10.1016/j.yexcr.2013.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janmey PA, Euteneuer U, Traub P, Schliwa M. 1991. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J. Cell Biol. 113, 155–160. ( 10.1083/jcb.113.1.155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nestler FHM, Hvidt S, Ferry JD, Veis A. 1983. Flexibility of collagen determined from dilute solution viscoelastic measurements. Biopolymers 22, 1747–1758. ( 10.1002/bip.360220710) [DOI] [PubMed] [Google Scholar]
- 35.Knapp DM, Barocas VH, Moon AG, Yoo K, Petzold LR, Tranquillo RT. 1997. Rheology of reconstituted type I collagen gel in confined compression. J. Rheol. (1978-present) 41, 971–993. ( 10.1122/1.550817) [DOI] [Google Scholar]
- 36.Pryse KM, Nekouzadeh A, Genin GM, Elson EL, Zahalak GI. 2003. Incremental mechanics of collagen gels: new experiments and a new viscoelastic model. Ann. Biomed. Eng. 31, 1287–1296. ( 10.1114/1.1615571) [DOI] [PubMed] [Google Scholar]
- 37.Forgacs G, Newman SA, Hinner B, Maier CW, Sackmann E. 2003. Assembly of collagen matrices as a phase transition revealed by structural and rheologic studies. Biophys. J. 84, 1272–1280. ( 10.1016/S0006-3495(03)74942-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y-L, Leone LM, Kaufman LJ. 2009. Elastic moduli of collagen gels can be predicted from two-dimensional confocal microscopy. Biophys. J. 97, 2051–2060. ( 10.1016/j.bpj.2009.07.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shayegan M, Forde NR. 2013. Microrheological characterization of collagen systems: from molecular solutions to fibrillar gels. PLoS ONE 8, e70590 ( 10.1371/journal.pone.0070590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babaei B, Davarian A, Pryse KM, Elson EL, Genin GM. 2015. Efficient and optimized identification of generalized Maxwell viscoelastic relaxation spectra. J. Mech. Behav. Biomed. Mater. 55, 32–41. ( 10.1016/j.jmbbm.2015.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barocas VH, Moon AG, Tranquillo RT. 1995. The fibroblast-populated collagen microsphere assay of cell traction force. Part 2. Measurement of the cell traction parameter. J. Biomech. Eng. 117, 161–170. ( 10.1115/1.2795998) [DOI] [PubMed] [Google Scholar]
- 42.Eastwood M, Porter R, Khan U, McGrouther G, Brown R. 1996. Quantitative analysis of collagen gel contractile forces generated by dermal fibroblasts and the relationship to cell morphology. J. Cell Physiol. 166, 33–42. () [DOI] [PubMed] [Google Scholar]
- 43.Huang D, Chang TR, Aggarwal A, Lee RC, Ehrlich HP. 1993. Mechanisms and dynamics of mechanical strengthening in ligament-equivalent fibroblast-populated collagen matrices. Ann. Biomed. Eng. 21, 289–305. ( 10.1007/BF02368184) [DOI] [PubMed] [Google Scholar]
- 44.Girton T, Oegema T, Grassl E, Isenberg B, Tranquillo R. 2000. Mechanisms of stiffening and strengthening in media-equivalents fabricated using glycation. J. Biomech. Eng. 122, 216–223. ( 10.1115/1.429652) [DOI] [PubMed] [Google Scholar]
- 45.Wakatsuki T, Kolodney MS, Zahalak GI, Elson EL. 2000. Cell mechanics studied by a reconstituted model tissue. Biophys. J. 79, 2353–2368. ( 10.1016/S0006-3495(00)76481-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gyoneva L, Hovell CB, Pewowaruk RJ, Dorfman KD, Segal Y, Barocas VH. 2016. Cell–matrix interaction during strain-dependent remodelling of simulated collagen networks. Interface Focus 6, 20150069 ( 10.1098/rsfs.2015.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Susilo ME, Paten JA, Sander EA, Nguyen TD, Ruberti JW. 2016. Collagen network strengthening following cyclic tensile loading. Interface Focus 6, 20150088 ( 10.1098/rsfs.2015.0088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. 2002. Molecular biology of the cell, 5th edn New York, NY: Garland Science. [Google Scholar]
- 49.Mecham RP, Heuser J. 1990. Three-dimensional organization of extracellular matrix in elastic cartilage as viewed by quick freeze, deep etch electron microscopy. Connect Tissue Res. 24, 83–93. ( 10.3109/03008209009152425) [DOI] [PubMed] [Google Scholar]
- 50.Fraley SI, Feng Y, Krishnamurthy R, Kim D-H, Celedon A, Longmore GD, Wirtz D. 2010. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat. Cell Biol. 12, 598–604. ( 10.1038/ncb2062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kubow KE, Horwitz AR. 2011. Reducing background fluorescence reveals adhesions in 3D matrices. Nat. Cell Biol. 13, 3–5. ( 10.1038/ncb0111-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geiger B, Spatz JP, Bershadsky AD. 2009. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21–33. ( 10.1038/nrm2593) [DOI] [PubMed] [Google Scholar]
- 53.Castelló-Cros R, Khan DR, Simons J, Valianou M, Cukierman E. 2009. Staged stromal extracellular 3D matrices differentially regulate breast cancer cell responses through PI3K and beta1-integrins. BMC Cancer 9, 94 ( 10.1186/1471-2407-9-94) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S, Butler P, Wang Y, Hu Y, Han DC, Usami S, Guan J-L, Chien S. 2002. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc. Natl Acad. Sci. USA 99, 3546–3551. ( 10.1073/pnas.052018099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang N, Naruse K, Stamenovic D, Fredberg JJ, Mijailovich SM, Tolic-Nørrelykke IM, Polte T, Mannix R, Ingber DE. 2001. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl Acad. Sci. USA 98, 7765–7770. ( 10.1073/pnas.141199598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell KP. 1995. Three muscular dystrophies: loss of cytoskeleton–extracellular matrix linkage. Cell 80, 675–679. ( 10.1016/0092-8674(95)90344-5) [DOI] [PubMed] [Google Scholar]
- 57.Maniotis AJ, Chen CS, Ingber DE. 1997. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl Acad. Sci. USA 94, 849–854. ( 10.1073/pnas.94.3.849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang N, Tytell JD, Ingber DE. 2009. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75–82. ( 10.1038/nrm2594) [DOI] [PubMed] [Google Scholar]
- 59.Isermann P, Lammerding J. 2013. Nuclear mechanics and mechanotransduction in health and disease. Curr. Biol. 23, R1113–R1121. ( 10.1016/j.cub.2013.11.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. 2004. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Invest. 113, 370 ( 10.1172/JCI200419670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swift J, et al. 2013. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 ( 10.1126/science.1240104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guilluy C, Burridge K. 2015. Nuclear mechanotransduction: forcing the nucleus to respond. Nucleus 6, 19–22. ( 10.1080/19491034.2014.1001705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, Burridge K. 2014. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 16, 376–381. ( 10.1038/ncb2927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedl P, Wolf K, Lammerding J. 2011. Nuclear mechanics during cell migration. Curr. Opin. Cell Biol. 23, 55–64. ( 10.1016/j.ceb.2010.10.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harada T, et al. 2014. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J. Cell Biol. 204, 669–682. ( 10.1083/jcb.201308029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris AK, Wild P, Stopak D. 1980. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science 208, 177–179. ( 10.1126/science.6987736) [DOI] [PubMed] [Google Scholar]
- 67.Harris AK, Stopak D, Wild P. 1981. Fibroblast traction as a mechanism for collagen morphogenesis. Nature 290, 249–251. ( 10.1038/290249a0) [DOI] [PubMed] [Google Scholar]
- 68.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. 2003. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl Acad. Sci. USA 100, 1484–1489. ( 10.1073/pnas.0235407100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dembo M, Oliver T, Ishihara A, Jacobson K. 1996. Imaging the traction stresses exerted by locomoting cells with the elastic substratum method. Biophys. J. 70, 2008 ( 10.1016/S0006-3495(96)79767-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee J, Leonard M, Oliver T, Ishihara A, Jacobson K. 1994. Traction forces generated by locomoting keratocytes. J. Cell Biol. 127, 1957–1964. ( 10.1083/jcb.127.6.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Legant WR, Miller JS, Blakely BL, Cohen DM, Genin GM, Chen CS. 2010. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat. Methods 7, 969–971. ( 10.1038/nmeth.1531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bell E, Ivarsson B, Merrill C. 1979. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl Acad. Sci. USA 76, 1274–1278. ( 10.1073/pnas.76.3.1274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grinnell F. 1994. Mini-review on the cellular mechanisms of disease fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 124, 401–404. ( 10.1083/jcb.124.4.401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grinnell F. 2003. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 13, 264–269. ( 10.1016/S0962-8924(03)00057-6) [DOI] [PubMed] [Google Scholar]
- 75.Barocas VH, Tranquillo RT. 1997. An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J. Biomech. Eng. 119, 137–145. ( 10.1115/1.2796072) [DOI] [PubMed] [Google Scholar]
- 76.Barocas V, Tranquillo R. 1997. A finite element solution for the anisotropic biphasic theory of tissue-equivalent mechanics: the effect of contact guidance on isometric cell traction measurement. J. Biomech. Eng. 119, 261–268. ( 10.1115/1.2796090) [DOI] [PubMed] [Google Scholar]
- 77.Moon AG, Tranquillo RT. 1993. Fibroblast-populated collagen microsphere assay of cell traction force: part 1. Continuum model. AIChE J. 39, 163–177. ( 10.1002/aic.690390116) [DOI] [PubMed] [Google Scholar]
- 78.Knapp DM, Tower TT, Tranquillo RT, Barocas VH. 1999. Estimation of cell traction and migration in an isometric cell traction assay. AIChE J. 45, 2628–2640. ( 10.1002/aic.690451219) [DOI] [Google Scholar]
- 79.Delvoye P, Wiliquet P, Levêque J-L, Nusgens BV, Lapière CM. 1991. Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. J. Invest. Dermatol. 97, 898–902. ( 10.1111/1523-1747.ep12491651) [DOI] [PubMed] [Google Scholar]
- 80.Kolodney MS, Wysolmerski RB. 1992. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J. Cell Biol. 117, 73–82. ( 10.1083/jcb.117.1.73) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kolodney M, Elson E. 1993. Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. J. Biol. Chem. 268, 23 850–23 855. [PubMed] [Google Scholar]
- 82.Eastwood M, McGrouther DA, Brown RA. 1994. A culture force monitor for measurement of contraction forces generated in human dermal fibroblast cultures: evidence for cell–matrix mechanical signalling. Biochim. Biophys. Acta Gen. Subjects 1201, 186–192. ( 10.1016/0304-4165(94)90040-X) [DOI] [PubMed] [Google Scholar]
- 83.Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL. 2002. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J. Biomech. Eng. 124, 214–222. ( 10.1115/1.1449904) [DOI] [PubMed] [Google Scholar]
- 84.Thomopoulos S, Das R, Birman V, Smith L, Ku K, Elson EL, Pryse KM, Marquez JP, Genin GM. 2011. Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression. Tissue Eng. A 17, 1039–1053. ( 10.1089/ten.tea.2009.0499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vandenburgh H, Shansky J, Benesch-Lee F, Barbata V, Reid J, Thorrez L, Valentini R, Crawford G. 2008. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 37, 438–447. ( 10.1002/mus.20931) [DOI] [PubMed] [Google Scholar]
- 86.Legant WR, Pathak A, Yang MT, Deshpande VS, McMeeking RM, Chen CS. 2009. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proc. Natl Acad. Sci. USA 106, 10 097–10 102. ( 10.1073/pnas.0900174106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bower AF. 2009. Applied mechanics of solids. Boca Raton, FL: CRC press. [Google Scholar]
- 88.Gurtin ME, Fried E, Anand L. 2010. The mechanics and thermodynamics of continua. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 89.Fung Y-C. 1993. Biomechanics: mechanical properties of living tissues. New York, NY: Springer. [Google Scholar]
- 90.Nekouzadeh A, Pryse KM, Elson EL, Genin GM. 2007. A simplified approach to quasi-linear viscoelastic modeling. J. Biomech. 40, 3070–3078. ( 10.1016/j.jbiomech.2007.03.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Babaei B, Abramowitch SD, Elson EL, Thomopoulos S, Genin GM. 2015. A discrete spectral analysis for determining quasi-linear viscoelastic properties of biological materials. J. R. Soc. Interface 12, 20150707 ( 10.1098/rsif.2015.0707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wineman A. 2009. Nonlinear viscoelastic solids: a review. Math. Mech. Solids 14, 300–366. ( 10.1177/1081286509103660) [DOI] [Google Scholar]
- 93.Schapery R. 2000. Nonlinear viscoelastic solids. Int. J. Solids Struct. 37, 359–366. ( 10.1016/S0020-7683(99)00099-2) [DOI] [Google Scholar]
- 94.Lakes RS. 1998. Viscoelastic solids, vol. 9 Boca Raton, FL: CRC Press. [Google Scholar]
- 95.Marquez JP, Genin GM, Pryse KM, Elson EL. 2006. Cellular and matrix contributions to tissue construct stiffness increase with cellular concentration. Ann. Biomed. Eng. 34, 1475–1482. ( 10.1007/s10439-006-9160-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wagenseil JE, Wakatsuki T, Okamoto RJ, Zahalak GI, Elson EL. 2003. One-dimensional viscoelastic behavior of fibroblast populated collagen matrices. J. Biomech. Eng. 125, 719–725. ( 10.1115/1.1614818) [DOI] [PubMed] [Google Scholar]
- 97.Nguyen T, Jones R, Boyce B. 2008. A nonlinear anisotropic viscoelastic model for the tensile behavior of the corneal stroma. J. Biomech. Eng. 130, 041020 ( 10.1115/1.2947399) [DOI] [PubMed] [Google Scholar]
- 98.Nair A, Baker BM, Trappmann B, Chen CS, Shenoy VB.2014. Remodeling of fibrous extracellular matrices by contractile cells: predictions from discrete fiber network simulations. ( http://arxiv.org/abs/1409.6216)
- 99.Abhilash A, Baker BM, Trappmann B, Chen CS, Shenoy VB. 2014. Remodeling of fibrous extracellular matrices by contractile cells: predictions from discrete fiber network simulations. Biophys. J. 107, 1829–1840. ( 10.1016/j.bpj.2014.08.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sacks MS. 2003. Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. J. Biomech. Eng. 125, 280–287. ( 10.1115/1.1544508) [DOI] [PubMed] [Google Scholar]
- 101.Sacks MS, Zhang W, Wognum S. 2016. A novel fibre-ensemble level constitutive model for exogenous cross-linked collagenous tissues. Interface Focus 6, 20150090 ( 10.1098/rsfs.2015.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Emery J, Omens J, McCulloch A. 1997. Strain softening in rat left ventricular myocardium. J. Biomech. Eng. 119, 6–12. ( 10.1115/1.2796067) [DOI] [PubMed] [Google Scholar]
- 103.Gregersen H, Emery J, McCulloch A. 1998. History-dependent mechanical behavior of guinea-pig small intestine. Ann. Biomed. Eng. 26, 850–858. ( 10.1114/1.109) [DOI] [PubMed] [Google Scholar]
- 104.Nekouzadeh A, Pryse KM, Elson EL, Genin GM. 2008. Stretch-activated force shedding, force recovery, and cytoskeletal remodeling in contractile fibroblasts. J. Biomech. 41, 2964–2971. ( 10.1016/j.jbiomech.2008.07.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee S-L, et al. 2012. Physically-induced cytoskeleton remodeling of cells in three-dimensional culture. PLoS ONE 7, e45512 ( 10.1371/journal.pone.0045512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wakatsuki T, Wysolmerski RB, Elson EL. 2003. Mechanics of cell spreading: role of myosin II. J. Cell Sci. 116, 1617–1625. ( 10.1242/jcs.00340) [DOI] [PubMed] [Google Scholar]
- 107.Limouze J, Straight AF, Mitchison T, Sellers JR. 2004. Specificity of blebbistatin, an inhibitor of myosin II. J. Muscle Res. Cell Motil. 25, 337–341. ( 10.1007/s10974-004-6060-7) [DOI] [PubMed] [Google Scholar]
- 108.Riento K, Ridley AJ. 2003. Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 4, 446–456. ( 10.1038/nrm1128) [DOI] [PubMed] [Google Scholar]
- 109.Qiu H, et al. 2010. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ. Res. 107, 615–619. ( 10.1161/CIRCRESAHA.110.221846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thastrup O, Cullen PJ, Drøbak B, Hanley MR, Dawson AP. 1990. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl Acad. Sci. USA 87, 2466–2470. ( 10.1073/pnas.87.7.2466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Emmert DA, Fee JA, Goeckeler ZM, Grojean JM, Wakatsuki T, Elson EL, Herring BP, Gallagher J, Wysolmerski RB. 2004. Rho-kinase-mediated Ca2+-independent contraction in rat embryo fibroblasts. Am. J. Physiol. Cell Physiol. 286, C8–C21. ( 10.1152/ajpcell.00428.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Elson EL, Pasternak C, Daily B, Young J-I, McConnaughey WB. 1984. Cross-linking surface immunoglobulin increases the stiffness of lymphocytes. Mol. Immunol. 21, 1253–1257. ( 10.1016/0161-5890(84)90018-X) [DOI] [PubMed] [Google Scholar]
- 113.Wang N, Butler JP, Ingber DE. 1993. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127. ( 10.1126/science.7684161) [DOI] [PubMed] [Google Scholar]
- 114.Wakatsuki T, Schwab B, Thompson NC, Elson EL. 2001. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J. Cell Sci. 114, 1025–1036. [DOI] [PubMed] [Google Scholar]
- 115.Coué M, Brenner SL, Spector I, Korn ED. 1987. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 213, 316–318. ( 10.1016/0014-5793(87)81513-2) [DOI] [PubMed] [Google Scholar]
- 116.Spector I, Shochet NR, Blasberger D, Kashman Y. 1989. Latrunculins—novel marine macrolides that disrupt microfilament organization and affect cell growth. I. Comparison with cytochalasin D. Cell Motil. Cytoskeleton 13, 127–144. ( 10.1002/cm.970130302) [DOI] [PubMed] [Google Scholar]
- 117.Chartier L, Rankin LL, Allen RE, Kato Y, Fusetani N, Karaki H, Watabe S, Hartshorne DJ. 1991. Calyculin-A increases the level of protein phosphorylation and changes the shape of 3T3 fibroblasts. Cell Motil. Cytoskeleton 18, 26–40. ( 10.1002/cm.970180104) [DOI] [PubMed] [Google Scholar]
- 118.Eriksson JE, Brautigan DL, Vallee R, Olmsted J, Fujiki H, Goldman RD. 1992. Cytoskeletal integrity in interphase cells requires protein phosphatase activity. Proc. Natl Acad. Sci. USA 89, 11 093–11 097. ( 10.1073/pnas.89.22.11093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hirano K, Chartier L, Taylor RG, Allen RE, Fusetani N, Karaki H, Hartshorne DJ. 1992. Changes in the cytoskeleton of 3T3 fibroblasts induced by the phosphatase inhibitor, calyculin-A. J. Muscle Res. Cell Motil. 13, 341–353. ( 10.1007/BF01766462) [DOI] [PubMed] [Google Scholar]
- 120.Kenedi RM, Gibson T, Daly CH. 1965. Biomechanics and related bioengineering topics, chapter: bio-engineering studies of the human skin II. Oxford, UK: Pergamon Press. [Google Scholar]
- 121.Wakatsuki T, Fee J, Elson E. 2004. Phenotypic screening for pharmaceuticals using tissue constructs. Curr. Pharm. Biotechnol. 5, 181–189. ( 10.2174/1389201043376940) [DOI] [PubMed] [Google Scholar]
- 122.Eschenhagen T, et al. 1997. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 11, 683–694. [DOI] [PubMed] [Google Scholar]
- 123.Maidhof R, Tandon N, Lee EJ, Luo J, Duan Y, Yeager K, Konofagou E, Vunjak-Novakovic G. 2012. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J. Tissue Eng. Regen. Med. 6, e12–e23. ( 10.1002/term.525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sondergaard CS, Mathews G, Wang L, Jeffreys A, Sahota A, Wood M, Ripplinger CM, Si M-S. 2012. Contractile and electrophysiologic characterization of optimized self-organizing engineered heart tissue. Ann. Thorac. Surg. 94, 1241–1249. ( 10.1016/j.athoracsur.2012.04.098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. 2011. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 109, 47–59. ( 10.1161/CIRCRESAHA.110.237206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hirt MN, Hansen A, Eschenhagen T. 2014. Cardiac tissue engineering state of the art. Circ. Res. 114, 354–367. ( 10.1161/CIRCRESAHA.114.300522) [DOI] [PubMed] [Google Scholar]
- 127.Asnes CF, Marquez JP, Elson EL, Wakatsuki T. 2006. Reconstitution of the Frank-Starling mechanism in engineered heart tissues. Biophys. J. 91, 1800–1810. ( 10.1529/biophysj.105.065961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Allen D, Kentish J. 1985. The cellular basis of the length–tension relation in cardiac muscle. J. Mol. Cell Cardiol. 17, 821–840. ( 10.1016/S0022-2828(85)80097-3) [DOI] [PubMed] [Google Scholar]
- 129.Fuchs F, Smith SH. 2001. Calcium, cross-bridges, and the Frank-Starling relationship. Physiology 16, 5–10. [DOI] [PubMed] [Google Scholar]
- 130.Milani-Nejad N, et al. 2015. The Frank-Starling mechanism involves deceleration of cross-bridge kinetics and is preserved in failing human right ventricular myocardium. Am. J. Physiol. Heart Circul. Physiol. ( 10.1152/ajpheart.00685.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Genin GM, Abney TM, Wakatsuki T, Elson EL. 2011. Cell-cell interactions and the mechanics of cells and tissues observed in bioartificial tissue constructs. In Mechanobiology of cell–cell and cell–matrix interactions, pp. 75–103. Springer. [Google Scholar]
- 132.Nyga A, Cheema U, Loizidou M. 2011. 3D tumour models: novel in vitro approaches to cancer studies. J. Cell Commun. Signal. 5, 239–248. ( 10.1007/s12079-011-0132-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weaver V, Fischer A, Peterson O, Bissell M. 1996. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem. Cell Biol. 74, 833–851. ( 10.1139/o96-089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu F, Celli J, Rizvi I, Moon S, Hasan T, Demirci U. 2011. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol. J. 6, 204–212. ( 10.1002/biot.201000340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Doyle AD, Wang FW, Matsumoto K, Yamada KM. 2009. One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 184, 481–490. ( 10.1083/jcb.200810041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Friedl P, Zänker KS, Bröcker E-B. 1998. Cell migration strategies in 3-D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function. Microsc. Res. Tech. 43, 369–378. () [DOI] [PubMed] [Google Scholar]
- 137.Friedl P, Wolf K. 2003. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer 3, 362–374. ( 10.1038/nrc1075) [DOI] [PubMed] [Google Scholar]
- 138.Cassereau L, Miroshnikova YA, Ou G, Lakins J, Weaver VM. 2015. A 3D tension bioreactor platform to study the interplay between ECM stiffness and tumor phenotype. J. Biotechnol. 193, 66–69. ( 10.1016/j.jbiotec.2014.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu P, Weaver VM, Werb Z. 2012. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406. ( 10.1083/jcb.201102147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marquez JP, Elson EL, Genin GM. 2010. Whole cell mechanics of contractile fibroblasts: relations between effective cellular and extracellular matrix moduli. Phil. Trans. R. Soc. A 368, 635–654. ( 10.1098/rsta.2009.0240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang H, Abhilash A, Chen CS, Wells RG, Shenoy VB. 2014. Long-range force transmission in fibrous matrices enabled by tension-driven alignment of fibers. Biophys. J. 107, 2592–2603. ( 10.1016/j.bpj.2014.09.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rowe RA, Pryse KM, Asnes CF, Elson EL, Genin GM. 2015. Collective matrix remodeling by isolated cells: Unionizing home improvement do-it-yourselfers. Biophys. J. 108, 2611–2612. ( 10.1016/j.bpj.2015.04.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Elson E, Genin G. 2013. The role of mechanics in actin stress fiber kinetics. Exp. Cell Res. 319, 2490–2500. ( 10.1016/j.yexcr.2013.06.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Raz E, Lanir Y. 2009. Recruitment viscoelasticity of the tendon. J. Biomech. Eng. 131, 111008 ( 10.1115/1.3212107) [DOI] [PubMed] [Google Scholar]
- 145.Zemel A, Bischofs I, Safran S. 2006. Active elasticity of gels with contractile cells. Phys. Rev. Lett. 97, 128103 ( 10.1103/PhysRevLett.97.128103) [DOI] [PubMed] [Google Scholar]
- 146.De R, Safran SA. 2008. Dynamical theory of active cellular response to external stress. Phys. Rev. E 78, 031923 ( 10.1103/PhysRevE.78.031923) [DOI] [PubMed] [Google Scholar]
- 147.Wang H, Svoronos AA, Boudou T, Sakar MS, Schell JY, Morgan JR, Chen CS, Shenoy VB. 2013. Necking and failure of constrained 3D microtissues induced by cellular tension. Proc. Natl Acad. Sci. USA 110, 20 923–20 928. ( 10.1073/pnas.1313662110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zahalak GI, Wagenseil JE, Wakatsuki T, Elson EL. 2000. A cell-based constitutive relation for bio-artificial tissues. Biophys. J. 79, 2369–2381. ( 10.1016/S0006-3495(00)76482-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hill A. 1938. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. B 126, 136–195. ( 10.1098/rspb.1938.0050) [DOI] [Google Scholar]
- 150.Marquez JP. 2006. Fourier analysis and automated measurement of cell and fiber angular orientation distributions. Int. J. Solids Struct. 43, 6413–6423. ( 10.1016/j.ijsolstr.2005.11.003) [DOI] [Google Scholar]
- 151.Nekouzadeh A, Genin GM. 2011. Quantification of fibre polymerization through fourier space image analysis. Proc. R. Soc. A 46, 2310–2329. ( 10.1098/rspa.2010.0623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Neu CP, Genin GM (eds). 2014. Handbook of imaging in biological mechanics. Boca Raton, FL: CRC Press. [Google Scholar]
- 153.Milton GW. 2002. The theory of composites, vol. 6 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 154.Genin GM, Birman V. 2009. Micromechanics and structural response of functionally graded, particulate-matrix, fiber-reinforced composites. Int. J. Solids Struct. 46, 2136–2150. ( 10.1016/j.ijsolstr.2008.08.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Castañeda PP. 1996. Exact second-order estimates for the effective mechanical properties of nonlinear composite materials. J. Mech. Phys. Solids 44, 827–862. ( 10.1016/0022-5096(96)00015-4) [DOI] [Google Scholar]
- 156.Castanèda PP, Suquet P. 1998. Nonlinear composites. Adv. Appl. Mech. 34, 171–302. ( 10.1016/S0065-2156(08)70321-1) [DOI] [Google Scholar]
- 157.Saadat F, Birman V, Thomopoulos S, Genin GM. 2015. Effective elastic properties of a composite containing multiple types of anisotropic ellipsoidal inclusions, with the application to the attachment of tendon to bone. J. Mech. Phys. Solids 82, 367–377. ( 10.1016/j.jmps.2015.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Marquez JP, Genin GM, Zahalak GI, Elson EL. 2005. The relationship between cell and tissue strain in three-dimensional bio-artificial tissues. Biophys. J. 88, 778–789. ( 10.1529/biophysj.104.041947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marquez JP, Genin GM, Zahalak GI, Elson EL. 2005. Thin bioartificial tissues in plane stress: the relationship between cell and tissue strain, and an improved constitutive model. Biophys. J. 88, 765–777. ( 10.1529/biophysj.104.040808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eshelby JD. 1957. The determination of the elastic field of an ellipsoidal inclusion, and related problems. Proc. R. Soc. Lond. A 241, 376–396. ( 10.1098/rspa.1957.0133) [DOI] [Google Scholar]
- 161.Marquez JP, Genin GM, Elson EL. 2006. On the application of strain factors for approximation of the contribution of anisotropic cells to the mechanics of a tissue construct. J. Biomech. 39, 2145–2151. ( 10.1016/j.jbiomech.2005.06.010) [DOI] [PubMed] [Google Scholar]
- 162.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. 2002. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349–363. ( 10.1038/nrm809) [DOI] [PubMed] [Google Scholar]
- 163.Satcher RL Jr, Dewey CF Jr. 1996. Theoretical estimates of mechanical properties of the endothelial cell cytoskeleton. Biophys. J. 71, 109 ( 10.1016/S0006-3495(96)79206-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wen Q, Janmey PA. 2011. Polymer physics of the cytoskeleton. Curr. Opin. Solid State Mater. Sci. 15, 177–182. ( 10.1016/j.cossms.2011.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gittes F, Mickey B, Nettleton J, Howard J. 1993. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 120, 923–934. ( 10.1083/jcb.120.4.923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ott A, Magnasco M, Simon A, Libchaber A. 1993. Measurement of the persistence length of polymerized actin using fluorescence microscopy. Phys. Rev. E 48, R1642 ( 10.1103/PhysRevE.48.R1642) [DOI] [PubMed] [Google Scholar]
- 167.Mücke N, Kreplak L, Kirmse R, Wedig T, Herrmann H, Aebi U, Langowski J. 2004. Assessing the flexibility of intermediate filaments by atomic force microscopy. J. Mol. Biol. 335, 1241–1250. ( 10.1016/j.jmb.2003.11.038) [DOI] [PubMed] [Google Scholar]
- 168.Schopferer M, Bär H, Hochstein B, Sharma S, Mücke N, Herrmann H, Willenbacher N. 2009. Desmin and vimentin intermediate filament networks: their viscoelastic properties investigated by mechanical rheometry. J. Mol. Biol. 388, 133–143. ( 10.1016/j.jmb.2009.03.005) [DOI] [PubMed] [Google Scholar]
- 169.Winheim S, Hieb AR, Silbermann M, Surmann E-M, Wedig T, Herrmann H, Langowski J, Mücke N. 2011. Deconstructing the late phase of vimentin assembly by total internal reflection fluorescence microscopy (TIRFM). PLoS ONE 6, e192022 ( 10.1371/journal.pone.0019202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stein AM, Vader DA, Weitz DA, Sander LM. 2011. The micromechanics of three-dimensional collagen-I gels. Complexity 16, 22–28. ( 10.1002/cplx.20332) [DOI] [Google Scholar]
- 171.Yang L, van der Werf KO, Koopman BF, Subramaniam V, Bennink ML, Dijkstra PJ, Feijen J. 2007. Micromechanical bending of single collagen fibrils using atomic force microscopy. J. Biomed. Mater. Res. A 82, 160–168. ( 10.1002/jbm.a.31127) [DOI] [PubMed] [Google Scholar]
- 172.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. 2005. Nonlinear elasticity in biological gels. Nature 435, 191–194. ( 10.1038/nature03521) [DOI] [PubMed] [Google Scholar]
- 173.MacKintosh F, Käs J, Janmey P. 1995. Elasticity of semiflexible biopolymer networks. Phys. Rev. Lett. 75, 4425 ( 10.1103/PhysRevLett.75.4425) [DOI] [PubMed] [Google Scholar]
- 174.Ingber DE, Madri JA, Jamieson JD. 1981. Role of basal lamina in neoplastic disorganization of tissue architecture. Proc. Natl Acad. Sci. USA 78, 3901–3905. ( 10.1073/pnas.78.6.3901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ingber DE. 1997. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 59, 575–599. ( 10.1146/annurev.physiol.59.1.575) [DOI] [PubMed] [Google Scholar]
- 176.Danowski B. 1989. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J. Cell Sci. 93, 255–266. [DOI] [PubMed] [Google Scholar]
- 177.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. 2007. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 93, 4453–4461. ( 10.1529/biophysj.106.101386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shenoy VB, Wang H, Wang X. 2016. A chemo-mechanical free energy-based approach to model durotaxis and extracellular stiffness-dependent contraction and polarization of cells. Interface Focus 6, 20150067 ( 10.1098/rsfs.2015.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Deshpande VS, McMeeking RM, Evans AG. 2006. A bio-chemo-mechanical model for cell contractility. Proc. Natl Acad. Sci. USA 103, 14 015–14 020. ( 10.1073/pnas.0605837103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Deshpande VS, McMeeking RM, Evans AG. 2007. A model for the contractility of the cytoskeleton including the effects of stress-fibre formation and dissociation. Proc. R. Soc. A 463, 787–815. ( 10.1098/rspa.2006.1793) [DOI] [Google Scholar]
- 181.Deshpande VS, Mrksich M, McMeeking RM, Evans AG. 2008. A biomechanical model for coupling cell contractility with focal adhesion formation. J. Mech. Phys. Solids 56, 1484–1510. ( 10.1016/j.jmps.2007.08.006) [DOI] [Google Scholar]
- 182.McGarry J, Fu J, Yang M, Chen C, McMeeking R, Evans A, Deshpande V. 2009. Simulation of the contractile response of cells on an array of micro-posts. Phil. Trans. R. Soc. A 367, 3477–3497. ( 10.1098/rsta.2009.0097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pathak A, Deshpande VS, McMeeking RM, Evans AG. 2008. The simulation of stress fibre and focal adhesion development in cells on patterned substrates. J. R. Soc. Interface 5, 507–524. ( 10.1098/rsif.2007.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wei Z, Deshpande VS, McMeeking RM, Evans AG. 2008. Analysis and interpretation of stress fiber organization in cells subject to cyclic stretch. J. Biomech. Eng. 130, 031009 ( 10.1115/1.2907745) [DOI] [PubMed] [Google Scholar]
- 185.Parker KK, et al. 2002. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 16, 1195–1204. ( 10.1096/fj.02-0038com) [DOI] [PubMed] [Google Scholar]
- 186.Burridge K, Chrzanowska-Wodnicka M. 1996. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12, 463–519. ( 10.1146/annurev.cellbio.12.1.463) [DOI] [PubMed] [Google Scholar]
- 187.Mochitate K, Pawelek P, Grinnell F. 1991. Stress relaxation of contracted collagen gels: disruption of actin filament bundles, release of cell surface fibronectin, and down-regulation of DNA and protein synthesis. Exp. Cell Res. 193, 198–207. ( 10.1016/0014-4827(91)90556-A) [DOI] [PubMed] [Google Scholar]
- 188.Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. 2003. Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 307, 355–361. ( 10.1016/S0006-291X(03)01165-3) [DOI] [PubMed] [Google Scholar]
- 189.Ofek G, Dowling EP, Raphael RM, McGarry JP, Athanasiou KA. 2010. Biomechanics of single chondrocytes under direct shear. Biomech. Model. Mechanobiol. 9, 153–162. ( 10.1007/s10237-009-0166-1) [DOI] [PubMed] [Google Scholar]
- 190.McGarry J. 2009. Characterization of cell mechanical properties by computational modeling of parallel plate compression. Ann. Biomed. Eng. 37, 2317–2325. ( 10.1007/s10439-009-9772-4) [DOI] [PubMed] [Google Scholar]
- 191.Ronan W, Deshpande VS, McMeeking RM, McGarry JP. 2014. Cellular contractility and substrate elasticity: a numerical investigation of the actin cytoskeleton and cell adhesion. Biomech. Model. Mechanobiol. 13, 417–435. ( 10.1007/s10237-013-0506-z) [DOI] [PubMed] [Google Scholar]
- 192.Ronan W, Deshpande VS, McMeeking RM, McGarry JP. 2012. Numerical investigation of the active role of the actin cytoskeleton in the compression resistance of cells. J. Mech. Behav. Biomed. Mater. 14, 143–157. ( 10.1016/j.jmbbm.2012.05.016) [DOI] [PubMed] [Google Scholar]


