Abstract
Genetic studies have identified a glutamate-ammonia ligase gene (GLUL) polymorphism associated with cardiovascular disease morbidity and mortality among people with type 2 diabetes (T2D). We sought to determine whether GLUL rs10911021 is associated prospectively with adjudicated cardiovascular composite end points among overweight/obese individuals with T2D and whether a lifestyle intervention resulting in weight loss could diminish this association. Look AHEAD is a randomized, controlled trial to determine the effects of intensive lifestyle intervention (ILI), including weight loss and physical activity, relative to diabetes support and education, on cardiovascular outcomes. Look AHEAD participants included in this report were 3,845 overweight/obese individuals with T2D who provided consent for genetic analyses. Over a median of 9.6 years of follow-up, the risk (C) allele for GLUL rs10911021 was significantly associated with the primary composite end point of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina among individuals with no history of cardiovascular disease (CVD) at baseline using additive genetic models (hazard ratio 1.17 [95% CI 1.01–1.36]; P = 0.032). Results appeared more consistent in recessive models and among individuals with no known history of CVD at baseline; ILI did not alter these associations. These results extend the association of GLUL rs10911021 to incident CVD morbidity and mortality in the setting of T2D.
Introduction
Both environmental and genetic factors are well established to contribute to the pathogenesis of cardiovascular disease (CVD), a leading cause of death in persons with diabetes (1). A genome-wide association study identified rs10911021, a single nucelotide polymorphism (SNP) upstream of the glutamate-ammonia ligase gene (GLUL), as being specifically associated with CVD in the setting of type 2 diabetes (T2D) (2) and all-cause mortality (3). This finding was novel because rs10911021 was not associated with the risk of developing T2D, insulin resistance, or glucose intolerance (2).
The Look AHEAD (Action for Health in Diabetes) Study is a multicenter trial that tested whether a randomly assigned intensive lifestyle intervention (ILI) designed to bring about weight loss and increased fitness would reduce the incidence of CVD compared with diabetes support and education (DSE) that did not have weight-loss or physical activity goals. Participants in the ILI arm lost significantly greater amounts of weight, and showed greater improvement in fitness and indices of diabetes control compared with participants in the DSE arm after 1 year (4). Despite this, after a median of 9.6 years of follow-up, Look AHEAD participants in the two study arms showed similar rates of cardiovascular morbidity and mortality (5). In the study here, we sought to independently replicate the association of the C allele at GLUL rs10911021 with incident CVD in the setting of T2D by determining whether GLUL rs10911021 predicted the primary and secondary study CVD end points in Look AHEAD and, if so, whether the effect of rs10911021 on CVD outcomes was diminished by a randomized lifestyle intervention.
Research Design and Methods
Study Cohort
The design and methods of Look AHEAD have previously been reported (6), as have the baseline characteristics of the randomized cohort (7). Briefly, 5,145 ethnically diverse overweight and obese subjects with T2D, aged 45–76 years, were randomized to either the ILI or DSE arm. Of the 5,125 Look AHEAD participants, we previously derived an unrelated subset of 4,016 participants based on genotyping using the Cardio-MetaboChip (8). The final analysis was based on 3,845 participants (genotyping of the GLUL rs10911021 SNP using a TaqMan assay on a 7900HT [Life Sciences] was not successful in 171 participants). Randomization occurred from August 2001 through April 2004. Data contributing to the current analyses were collected through 14 September 2012 when the ILI was stopped because of lack of effect on the incidence of major CVD events (5). The median follow-up was 9.6 years (interquartile range 8.9–10.3), and <4% of all patients randomly assigned to a study group were lost to follow-up. Look AHEAD was approved by local institutional review boards, including genetic analyses. All participants provided informed consent.
Measures
CVD history at baseline was defined by self-report of a prior history of heart attack, stroke, bypass surgery, stent placement, angioplasty, carotid endarterectomy, angioplasty of lower-extremity artery, aortic aneurysm repair, or heart failure or congestive heart failure. During annual visits and telephone calls every 6 months, staff members who were unaware of study group assignments queried participants about all medical events and hospitalizations. Hospital and other records were reviewed for potential cardiovascular events, with adjudication according to standard criteria by a central review committee who were unaware of study group assignments. The Look AHEAD primary composite CVD outcome was cardiovascular death or the first occurrence of nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina (5). The three prespecified composite secondary outcomes included 1) cardiovascular death or first occurrence of nonfatal myocardial infarction or nonfatal stroke; 2) all-cause mortality or first occurrence of myocardial infarction, stroke, or hospitalization for angina; and 3) all-cause mortality or first occurrence of myocardial infarction, stroke, hospitalization for angina, coronary artery bypass grafting, percutaneous coronary intervention, hospitalization for heart failure, or peripheral vascular disease.
Statistical Modeling
The relationship between the GLUL rs10911021 and CVD outcomes was estimated using conditional Cox proportional hazards regression models assuming a gamma frailty model to account for dependence between participants at the same clinical site (9). Note that with conditional models, as opposed to marginal population-averaged models, effects are interpreted as a comparison between two subjects at the same clinical site. We present results for rs10911021 assuming an additive effect of the risk (C) allele to provide the most direct replication of the prior literature (2), as well as a recessive effect (CC vs. CT/TT), as the latter provided a better model fit (lower Akaike information criterion). All models were evaluated with and without an interaction term between the GLUL rs10911021 and intervention arm (as well as main effects for each) and began with adjustments for age, sex, history of CVD, and genetic ancestry (top three multidimensional scaling vectors estimated from existing Cardio-MetaboChip data [10]). Unless otherwise indicated, all analyses were performed using the R Statistical Computing Environment.
Results
Demographic characteristics of the genetic subsample at baseline are included in Table 1. Consistent with the parent cohort, no differences in demographic characteristics, history of CVD, duration of diabetes, HbA1c, cardiovascular risk factors, or medication use were observed at baseline across ILI and DSE. There was no evidence of deviation from Hardy-Weinberg equilibrium for rs10911021 (P > 0.35 in participants of self-reported non-Hispanic white, African American, and a combined group of Hispanic and American Indian ancestries). No differences in GLUL rs10911021 genotype frequencies were observed between ILI and DSE. The GLUL rs10911021 C allele was not associated with risk factors for CVD including LDL, systolic and diastolic blood pressure, triglycerides, HDL, and HbA1c (Supplementary Table 1).
Table 1.
Baseline characteristics of Look AHEAD participants included in genetic substudy
| ILI (N = 1,967) | DSE (N = 1,967) | P | |
|---|---|---|---|
| rs10911021 genotype | 0.107 | ||
| TT | 315 (16.0) | 291 (15.5) | |
| CT | 968 (49.2) | 873 (46.5) | |
| CC | 684 (34.8) | 714 (38.0) | |
| Age, years | 58.9 ± 6.8 | 59.1 ± 6.9 | 0.281 |
| Female sex | 1,144 (58.2) | 1,064 (56.7) | 0.363 |
| Self-reported race/ethnicity | 0.508 | ||
| Non-Hispanic white | 1,372 (69.8) | 1,343 (71.5) | |
| African American | 298 (15.1) | 279 (14.9) | |
| Hispanic | 221 (11.2) | 180 (9.6) | |
| American Indian | 40 (2.0) | 37 (2.0) | |
| Other | 36 (1.8) | 39 (2.1) | |
| Ancestral groupa | 0.241 | ||
| Non-Hispanic white | 1,351 (68.7) | 1,330 (70.8) | |
| African American | 318 (16.2) | 297 (15.8) | |
| Hispanic/American Indian | 298 (15.1) | 251 (13.4) | |
| History of CVD | 290 (14.7) | 260 (13.8) | 0.454 |
| Use of diabetes medications | 1,724 (87.6) | 1,636 (87.1) | 0.654 |
| Use of insulin | 368 (18.7) | 356 (19.0) | 0.877 |
| Use of hypertension medications | 1,498 (76.2) | 1,383 (73.8) | 0.088 |
| Use of lipid-lowering medications | 1,016 (51.7) | 1,005 (53.5) | 0.275 |
| Aspirin use | 0.639 | ||
| Never | 865 (44.0) | 827 (44.0) | |
| Sometimes | 184 (9.4) | 191 (10.2) | |
| Every day | 913 (46.4) | 852 (45.4) | |
| Unknown | 5 (0.3) | 8 (0.4) | |
| Current smoking | 92 (4.7) | 74 (4.0) | 0.303 |
| Duration of diabetes,years | 5 (2–10) | 5 (2–10) | 0.669 |
| Weight, kg | 101.6 ± 19.5 | 102.1 ± 18.7 | 0.400 |
| BMI, kg/m2 | 36.1 ± 6.0 | 36.1 ± 5.8 | 0.644 |
| Waist circumference, cm | 114.4 ± 14.2 | 114.8 ± 13.7 | 0.449 |
| HbA1c, % | 7.2 ± 1.1 | 7.3 ± 1.2 | 0.654 |
| Blood pressure, mmHg | |||
| Systolic | 128.9 ± 17.4 | 129.8 ± 17.2 | 0.103 |
| Diastolic | 70.0 ± 9.4 | 70.4 ± 9.6 | 0.285 |
| LDL, mg/dL | 111.8 ± 32.2 | 112.0 ± 32.7 | 0.862 |
| HDL, mg/dL | 43.0 ± 11.6 | 43.2 ± 11.7 | 0.651 |
| Triglycerides, mg/dL | 157 (109.5–224) | 154 (108–221) | 0.279 |
Data are n (%), mean ± SD, or median (interquartile range). P values for continuous measures are based on ANOVA or Kruskal-Wallis tests as appropriate. For categorical measures, P values are based on χ2 or Fisher exact tests as appropriate.
aGenetically defined based on estimated admixture proportions (see research design and methods).
Event rates of the primary and secondary composite outcomes in this genetic substudy of Look AHEAD are presented in Supplementary Table 2. Prospective associations of the GLUL rs10911021 variant with the primary composite outcomes of Look AHEAD are presented in Table 2. When analyzed using an additive model, the risk (C) allele for rs10911021 as described by Qi et al. (2) was significantly associated with CVD outcomes in participants who did not have CVD at baseline (hazard ratio [HR] 1.17 [95% CI 1.01–1.36]; P = 0.032), with a somewhat weaker but similar effect in all participants regardless of CVD history (P = 0.069) and in the subsample of non-Hispanic white participants only.
Table 2.
Association of rs10911021 with the primary composite CVD outcome in the Look AHEAD Study
| N | Interaction with intervention arm |
No interaction with intervention arm |
||||
|---|---|---|---|---|---|---|
| DSE: HR (95% CI)a | ILI: HR (95% CI)a | Pinteraction | HR (95% CI)a | P | ||
| Additive modela | ||||||
| All participantsb | 3,845 | 1.10 (0.94–1.29) | 1.13 (0.96–1.33) | 0.834 | 1.11 (0.99–1.25) | 0.069 |
| All participants without history of CVDc | 3,295 | 1.14 (0.93–1.38) | 1.22 (0.99–1.51) | 0.624 | 1.17 (1.01–1.36) | 0.032 |
| Non-Hispanic white participantsd | 2,681 | 1.13 (0.94–1.36) | 1.09 (0.90–1.33) | 0.804 | 1.11 (0.97–1.27) | 0.121 |
| Non-Hispanic white participants without history of CVDe | 2,264 | 1.09 (0.90–1.31) | 1.07 (0.88–1.30) | 0.882 | 1.08 (0.94–1.23) | 0.279 |
| Recessive modelf | ||||||
| All participantsb | 3,845 | 1.17 (0.94–1.46) | 1.25 (0.99–1.56) | 0.704 | 1.21 (1.03–1.42) | 0.020 |
| All participants without history of CVDc | 3,295 | 1.26 (0.96–1.65) | 1.39 (1.04–1.86) | 0.616 | 1.32 (1.08–1.61) | 0.006 |
| Non-Hispanic white participantsd | 2,681 | 1.18 (0.92–1.51) | 1.25 (0.96–1.63) | 0.755 | 1.21 (1.01–1.45) | 0.037 |
| Non-Hispanic white participants without history of CVDe | 2,264 | 1.32 (0.97–1.79) | 1.33 (0.94–1.89) | 0.973 | 1.33 (1.05–1.67) | 0.016 |
Primary composite CVD outcome includes death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina.
aHR per each additional copy of the risk C allele for rs10911021.
bAdjusted for age, sex, top three multidimensional scaling axes, intervention arm (no interaction model), history of CVD, and clinic (gamma frailty).
cSame as adjustments in b without adjustment for history of CVD.
dAdjusted for age, sex, intervention arm (no interaction model), history of CVD, and clinic (gamma frailty).
eSame as adjustments in d without adjustment for history of CVD.
fHR for CC homozygotes vs. TT or CT genotypes.
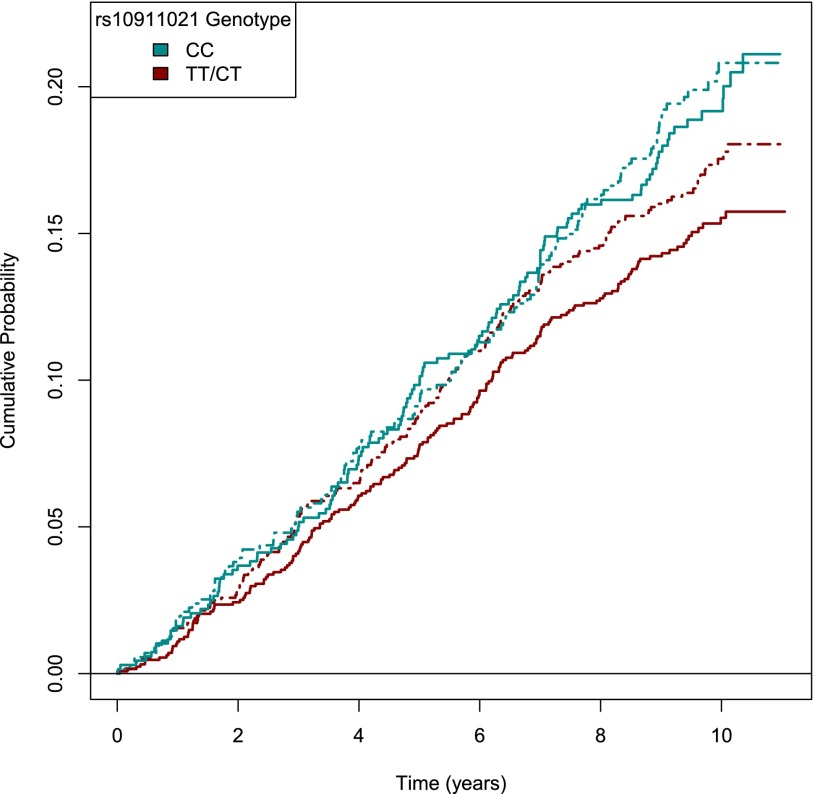
Stronger results were observed in recessive models. Across all participants, the GLUL rs10911021 CC genotype was significantly associated with the primary composite end point of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina (HR per risk allele 1.21 [95% CI 1.03–1.42]) (Fig. 1). Consistent effects were observed among the largest racial/ethnic group, non-Hispanic white participants (HR 1.04 [95% CI 1.02–1.07]). Similar to the additive models presented in Supplementary Table 3, results were most robust among individuals without a history of CVD at baseline (full sample with CVD history: HR 1.32 [95% CI 1.08–1.61]) in which the level of significance surpassed correction for multiple hypothesis testing. In no case did the magnitude of genetic association differ across the ILI and DSE intervention arms for the primary outcome in the additive models (interaction P > 0.61).
Figure 1.
Primary composite outcome by rs10911021 genotype and lifestyle intervention. The incidence of the primary outcome (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina) over a 9.6-year median follow-up by rs10911021 genotype and randomized study intervention arm. Solid lines denote participants randomized to ILI, and dashed lines indicate randomization to DSE.
Table 3 presents prespecified secondary CVD outcomes analyzed using recessive genetic models only. A significant association was found between the GLUL rs10911021 polymorphism and secondary outcome 2, which was comprised of death from any cause or first occurrence of nonfatal myocardial infarction or nonfatal stroke in the full sample (HR 1.20 [95% CI 1.04–1.38]), non-Hispanic white participants (HR 1.18 [95% CI 1.00–1.39]), and individuals with no known history of CVD at baseline (full sample: HR 1.26 [95% CI 1.06–1.50]; non-Hispanic white subsample: HR 1.24 [95% CI 1.02–1.52]).
Table 3.
Association of rs10911021 using recessive model with secondary composite CVD outcomes in the Look AHEAD Study
| DSE: HR (95% CI)a | ILI: HR (95% CI)a | Pinteraction | HR (95% CI)a | P | |
|---|---|---|---|---|---|
| Secondary outcome 1 | |||||
| All participantsb | 1.02 (0.78–1.33) | 1.31 (1.00–1.74) | 0.194 | 1.15 (0.95–1.40) | 0.158 |
| All participants without Hx of CVDc | 1.08 (0.78–1.50) | 1.47 (1.03–2.10) | 0.215 | 1.24 (0.97–1.59) | 0.084 |
| Non-Hispanic white participantsd | 1.00 (0.73–1.35) | 1.38 (1.00–1.92) | 0.152 | 1.16 (0.93–1.45) | 0.200 |
| Non-Hispanic white participants without Hx of CVDe | 1.13 (0.77–1.65) | 1.44 (0.92–2.24) | 0.412 | 1.25 (0.93–1.67) | 0.134 |
| Secondary outcome 2 | |||||
| All participantsb | 1.13 (0.92–1.38) | 1.27 (1.04–1.56) | 0.400 | 1.20 (1.04–1.38) | 0.014 |
| All participants without Hx of CVDc | 1.21 (0.95–1.54) | 1.32 (1.02–1.70) | 0.626 | 1.26 (1.06–1.50) | 0.009 |
| Non-Hispanic white participantsd | 1.10 (0.87–1.37) | 1.28 (1.01–1.62) | 0.357 | 1.18 (1.00–1.39) | 0.050 |
| Non-Hispanic white participants without Hx of CVDe | 1.20 (0.91–1.57) | 1.30 (0.96–1.75) | 0.696 | 1.24 (1.02–1.52) | 0.035 |
| Secondary outcome 3 | |||||
| All participantsb | 1.01 (0.83–1.21) | 1.24 (1.03–1.50) | 0.121 | 1.12 (0.98–1.28) | 0.109 |
| All participants without Hx of CVDc | 1.11 (0.89–1.40) | 1.24 (0.98–1.56) | 0.528 | 1.17 (1.00–1.38) | 0.057 |
| Non-Hispanic white participantsd | 0.98 (0.79–1.22) | 1.21 (0.98–1.51) | 0.174 | 1.09 (0.93–1.27) | 0.281 |
| Non-Hispanic white participants without Hx of CVDe | 1.14 (0.88–1.48) | 1.20 (0.91–1.57) | 0.800 | 1.17 (0.96–1.41) | 0.113 |
Secondary outcome 1 includes death from cardiovascular causes or first occurrence of nonfatal myocardial infarction or nonfatal stroke. Secondary outcome 2 includes death from any cause or first occurrence of nonfatal myocardial infarction or nonfatal stroke. Secondary outcome 3 includes death from any cause or first occurrence of nonfatal myocardial infarction, nonfatal stroke, hospitalization for angina, coronary artery bypass surgery, percutaneous coronary intervention, hospitalization for heart failure, carotid endarterectomy, or peripheral vascular disease.
aHR for rs10911021 CC homozygotes vs. TT or CT genotypes.
bAdjusted for age, sex, top three multidimensional scaling axes, intervention arm (no interaction model), history of CVD (Hx of CVD), and clinic (gamma frailty).
cSame as adjustment in b without adjustment for history of CVD.
dAdjusted for age, sex, intervention arm (no interaction model), history of CVD, and clinic (gamma frailty).
eSame as adjustment in d without adjustment for history of CVD.
Discussion
There are two key findings of this report. First, our replication of the association of the C allele at GLUL rs10911021 with incident CVD in Look AHEAD individuals without a prior history of CVD model should reduce any concern about false discovery or “winners curse” in the two reports to date (2,3). The demonstration that GLUL rs10911021 was associated with incident and not prevalent coronary artery disease present at baseline further emphasizes that the risk associated with GLUL rs10911021 occurs in the context of established T2D. It is important to note that the adjudicated composite CVD outcomes in Look AHEAD include stroke, which was not part of the end point used by Qi et al. (2). The consistency of our findings across the primary and one of the secondary end points that include fatal and nonfatal myocardial infarction suggests that the results are comparable. Our findings should motivate further study of rs10911021 in stroke risk in the setting of diabetes. The lack of association of GLUL rs10911021 with traditional risk factors of CVD, including LDL, HDL, triglycerides, and blood pressure, suggests that the CVD risk may be through a novel mechanism. Previously, the association of rs10911021 with CVD was shown to be weakened by adjustment for serum pyroglutamic-to-glutamic acid ratio (2) consistent with genetic effects on GLUL triggering abnormal glutamate/glutamic acid metabolism leading to CVD in the setting of T2D. Chronic glutamate treatment has a cytotoxic effect on pancreatic β-cells (11) that may promote β-cell failure. Biochemical and basic science studies are required to further define the mechanism by which rs10911021 and its associated abnormalities of glutamic acid metabolism may uniquely contribute to CVD in the setting of T2D.
Second, the behavioral intervention did not conclusively alter the association of GLUL rs10911021 with the primary and secondary end points of Look AHEAD despite demonstrated efficacy of the intervention at 1, 4, and 10 years in producing weight loss (4,5). None of the SNP × treatment arm interactions were statistically significant. In some respects, a lack of treatment interaction of GLUL rs10911021 with CVD may be understandable because the ILI had no overall effect on the primary or secondary CVD outcomes (7). The association of the SNP with coronary artery disease was identified in multiple cohorts of people with T2D and who likely had a wide range of behavioral and fitness practices (12). In addition, the lifestyle intervention was not designed to influence dietary glutamine content, which may be important because a separate randomized trial of a glutamine supplement observed beneficial effects on obesity, systolic blood pressure, and glucose control (13).
This study has strengths and limitations. This ancillary study is derived from the largest randomized, controlled trial of behavioral weight loss, including ∼3,850 individuals who gave genetic consent, randomly assigned to a control group or an intensive lifestyle intervention focusing on weight-loss and physical activity promotion that reduced weight and improved fitness relative to the control group. The subject group of Look AHEAD is further comprised exclusively of overweight or obese individuals with T2D and is similar in size to the composite of all three independent cohorts studied by Qi et al. (2). CVD morbidity and mortality were prospectively adjudicated by treatment-blind reviewers over a median of 9.6 years of follow-up with excellent retention rates. Limitations include a relatively small number of CVD events that, although sufficient to detect genetic effects across treatment arms, may have precluded detection of SNP × treatment arm interaction. Moreover, although our findings from Look AHEAD may apply to a growing population of individuals with T2D, our findings are not generalizable to population without diabetes.
Overall, our findings build upon prior research by demonstrating prospective association of GLUL rs10911021 with incident cardiovascular morbidity and mortality over a median of 9.6 years of follow-up among individuals at high risk for CVD owing to being overweight or obese and having T2D. This prospective association was not conclusively altered by lifestyle intervention promoting weight loss and physical activity.
Article Information
Acknowledgments. The investigators thank the Look AHEAD Study participants and the investigative staff at each clinical site for contributions to the success of the trial. A complete listing of the Look AHEAD investigators/personnel can be found in the Supplementary Data online.
Funding. This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (DK-57136).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. G.S.H. and J.M.M. proposed the ancillary study. N.M.P. performed the data analysis. G.S.H., J.M.M., N.M.P., C.E.L., W.C.K., K.C.J., and L.E.W. wrote the manuscript. Please see the Supplementary Data online for a complete list of the authors recognized for the contribution of funding, conception and design of the Look AHEAD Study, acquisition of data, and data interpretation. All authors reviewed and edited the manuscript. All authors gave final approval of the version to be published. G.S.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
Appendix
The Look AHEAD Research Group. Gordon S. Huggins (Chair), MCRI Center for Translational Genomics, Tufts Medical Center and Tufts University School of Medicine, Boston, MA; Robert I. Berkowitz, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA; George L. Blackburn, Center for the Study of Nutrition Medicine, Department of Surgery, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; George A. Bray, Department of Clinical Obesity, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA; Lawrence Cheskin, The Global Obesity Prevention Center at Johns Hopkins, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD; Jeanne M. Clark, Department of Medicine and Epidemiology, The Johns Hopkins University, Baltimore, MD; Mace Coday, Department of Preventive Medicine, Memphis East Clinic, Memphis, TN; Mark A. Espeland, Department of Biostatistical Sciences, Wake Forest School of Medicine, Winston-Salem, NC; Mary Evans, Department of Digestive Diseases and Nutrition, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD; John P. Foreyt, Department of Medicine, Baylor College of Medicine, Houston, TX; Stephen Glasser, Department of Medicine/Division of Preventive Medicine, University of Alabama at Birmingham, Birmingham, AL; Frank L. Greenway, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA; Edward W. Gregg, Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA; Robert L. Hanson, Diabetes Epidemiology and Clinical Research Section, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ; Helen P. Hazuda, Department of Medicine, Division of Nephrology, University of Texas Health Science Center at San Antonio, San Antonio, TX; James O. Hill, Anschutz Health and Wellness Center, University of Colorado, Aurora, CO; Edward S. Horton, Joslin Diabetes Center and Department of Medicine, Harvard Medical School, Boston, MA; John M. Jakicic, University of Pittsburgh, Department of Health and Physical Activity, Pittsburgh, PA; Robert W. Jeffery, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN; Karen C. Johnson, Department of Preventive Medicine, The University of Tennessee Health Science Center, Memphis, TN; Steven E. Kahn, Division of Metabolism, Endocrinology and Nutrition, Department of Medicine, VA Puget Sound Health Care System and University of Washington, Seattle, WA; Dalane W. Kitzman, Cardiology Department, Wake Forest University Health Sciences, Winston-Salem, NC; William C. Knowler, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ; Cora E. Lewis, Division of Preventive Medicine, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL; Jeanne M. McCaffery, Department of Psychiatry and Human Behavior, The Miriam Hospital/Alpert School of Medicine at Brown University, Providence, RI; Sara A. Michaels, Northern Navajo Medical Center, Shiprock, NM; Maria G. Montez, Department of Medicine, Division of Nephrology, University of Texas Health Science Center at San Antonio, San Antonio, TX; Anne Murillo, VA Puget Sound Health Care System and University of Washington, Seattle, WA; David M. Nathan, Diabetes Unit, Department of Medicine, Massachusetts General Hospital Diabetes Center and Harvard Medical School, Boston, MA; Nicholas M. Pajewski, Department of Biostatistical Sciences, Wake Forest School of Medicine, Winston-Salem, NC; Jennifer Patricio, New York Obesity Research Center, St. Luke's-Roosevelt Hospital Center, New York, NY; Anne Peters, Edward R. Roybal Comprehensive Health Center, Los Angeles, CA; Xavier Pi-Sunyer, New York Obesity Research Center, St. Luke's-Roosevelt Hospital Center, New York, NY; Henry Pownall, Department of Cardiology, Methodist Hospital Research Institute, Houston, TX; Bruce Redmon, Department of Endocrinology/Medicine, University of Minnesota, Minneapolis, MN; Judy Regensteiner, Center for Women's Health Research, University of Colorado Health Sciences Center, Denver, CO; Helmut O. Steinberg, Division of Endocrinology, Diabetes and Metabolism, The University of Tennessee Health Science Center, Memphis, TN; Thomas A. Wadden, Department of Psychiatry, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA; Lynne E. Wagenknecht, Department of Public Health Services, Wake Forest School of Medicine, Winston-Salem, NC; Jacqueline Wesche-Thobaben, Physical Activity and Weight Management Research Center, University of Pittsburgh, Pittsburgh, PA; and Rena R. Wing, Department of Psychiatry and Human Behavior, The Miriam Hospital/Alpert School of Medicine at Brown University, Providence, RI.
Footnotes
Clinical trial reg. nos. NCT00017953 and NCT01270763, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-0890/-/DC1.
A full listing of the Look AHEAD Research Group can be found in the appendix, and a listing of the Look AHEAD Research Group investigators/personnel can be found in the Supplementary Data online.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi L, Qi Q, Prudente S, et al. Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 diabetes. JAMA 2013;310:821–828 [DOI] [PMC free article] [PubMed]
- 3.Prudente S, Shah H, Bailetti D, et al. Genetic variant at the GLUL locus predicts all-cause mortality in patients with type 2 diabetes. Diabetes 2015;64:2658–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Look Ahead Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007;30:1374–1383 [DOI] [PMC free article] [PubMed]
- 5.Look Ahead Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed]
- 6.Ryan DH, Espeland MA, Foster GD, et al.; Look AHEAD Research Group . Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628 [DOI] [PubMed] [Google Scholar]
- 7.Look Ahead Research Group, Bray G, Gregg E, Haffner S, et al. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res 2006;3:202–215 [DOI] [PMC free article] [PubMed]
- 8.Look Ahead Research Group. Prospective association of a genetic risk score and lifestyle intervention with cardiovascular morbidity and mortality among individuals with type 2 diabetes: the Look AHEAD randomised controlled trial. Diabetologia 2015;58:1803–1813 [DOI] [PMC free article] [PubMed]
- 9.Glidden DV, Vittinghoff E. Modelling clustered survival data from multicentre clinical trials. Stat Med 2004;23:369–388 [DOI] [PubMed] [Google Scholar]
- 10.Voight BF, Kang HM, Ding J, et al. The MetaboChip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet 2012;8:e1002793 [DOI] [PMC free article] [PubMed]
- 11.Di Cairano ES, Davalli AM, Perego L, et al. The glial glutamate transporter 1 (GLT1) is expressed by pancreatic beta-cells and prevents glutamate-induced beta-cell death. J Biol Chem 2011;286:14007–14018 [DOI] [PMC free article] [PubMed]
- 12.CARDIoGRAMplusC4D Consortium, Deloukas P, Kanoni S, Willenborg C, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet 2013;45:25–33 [DOI] [PMC free article] [PubMed]
- 13.Mansour A, Mohajeri-Tehrani MR, Qorbani M, Heshmat R, Larijani B, Hosseini S. Effect of glutamine supplementation on cardiovascular risk factors in patients with type 2 diabetes. Nutrition 2015;31:119–126 [DOI] [PubMed]