Significance
By utilizing patient-specific induced pluripotent stem cells (iPSCs) of fibrodysplasia ossificans progressiva (FOP) and gene-corrected (rescued) FOP-iPSCs, we discovered a novel mechanism in ectopic bone formation: The disease-causing mutation endows ACVR1 with the ability to transmit the signal of an unexpected ligand, Activin-A. We believe this is a milestone study for FOP research and provides a novel platform for searching therapeutic targets of this intractable disease.
Keywords: iPSC, fibrodysplasia ossificans progressiva, heterotopic ossification, BMP, TGF
Abstract
Fibrodysplasia ossificans progressiva (FOP) is a rare genetic disease characterized by extraskeletal bone formation through endochondral ossification. FOP patients harbor point mutations in ACVR1 (also known as ALK2), a type I receptor for bone morphogenetic protein (BMP). Two mechanisms of mutated ACVR1 (FOP-ACVR1) have been proposed: ligand-independent constitutive activity and ligand-dependent hyperactivity in BMP signaling. Here, by using FOP patient-derived induced pluripotent stem cells (FOP-iPSCs), we report a third mechanism, where FOP-ACVR1 abnormally transduces BMP signaling in response to Activin-A, a molecule that normally transduces TGF-β signaling but not BMP signaling. Activin-A enhanced the chondrogenesis of induced mesenchymal stromal cells derived from FOP-iPSCs (FOP-iMSCs) via aberrant activation of BMP signaling in addition to the normal activation of TGF-β signaling in vitro, and induced endochondral ossification of FOP-iMSCs in vivo. These results uncover a novel mechanism of extraskeletal bone formation in FOP and provide a potential new therapeutic strategy for FOP.
Heterotopic ossification (HO) is defined as bone formation in soft tissue where bone normally does not exist. It can be the result of surgical operations, trauma, or genetic conditions, one of which is fibrodysplasia ossificans progressiva (FOP). FOP is a rare genetic disease characterized by extraskeletal bone formation through endochondral ossification (1–6). The responsive mutation for classic FOP is 617G > A (R206H) in the intracellular glycine- and serine-rich (GS) domain (7) of ACVR1 (also known as ALK2), a type I receptor for bone morphogenetic protein (BMP) (8–10). ACVR1 mutations in atypical FOP patients have been found also in other amino acids of the GS domain or protein kinase domain (11, 12). Regardless of the mutation site, mutated ACVR1 (FOP-ACVR1) has been shown to activate BMP signaling without exogenous BMP ligands (constitutive activity) and transmit much stronger BMP signaling after ligand stimulation (hyperactivity) (12–25).
To reveal the molecular nature of how FOP-ACVR1 activates BMP signaling, cells overexpressing FOP-ACVR1 (12–20), mouse embryonic fibroblasts derived from Alk2R206H/+ mice (21, 22), and cells from FOP patients, such as stem cells from human exfoliated deciduous teeth (23), FOP patient-derived induced pluripotent stem cells (FOP-iPSCs) (24, 25) and induced mesenchymal stromal cells (iMSCs) from FOP-iPSCs (FOP-iMSCs) (26) have been used as models. Among these cells, Alk2R206H/+ mouse embryonic fibroblasts and FOP-iMSCs are preferred because of their accessibility and expression level of FOP-ACVR1 using an endogenous promoter. In these cells, however, the constitutive activity and hyperactivity is not strong (within twofold normal levels) (22, 26). In addition, despite the essential role of BMP signaling in development (27–31), the pre- and postnatal development and growth of FOP patients are almost normal, and HO is induced in FOP patients after physical trauma and inflammatory response postnatally, not at birth (1–6). These observations led us to hypothesize that FOP-ACVR1 abnormally responds to noncanonical BMP ligands induced by trauma or inflammation.
Here we show that FOP-ACVR1 transduced BMP signaling in response to Activin-A, a molecule that normally transduces TGF-β signaling (10, 32–34) and contributes to inflammatory responses (35, 36). Our in vitro and in vivo data indicate that activation of TGF-β and aberrant BMP signaling by Activin-A in FOP-cells is one cause of HO in FOP. These results suggest a possible application of anti–Activin-A reagents as a new therapeutic tool for FOP.
Results
Activin-A Abnormally Transduced BMP Signaling in FOP-iMSCs, but Not in resFOP-iMSCs.
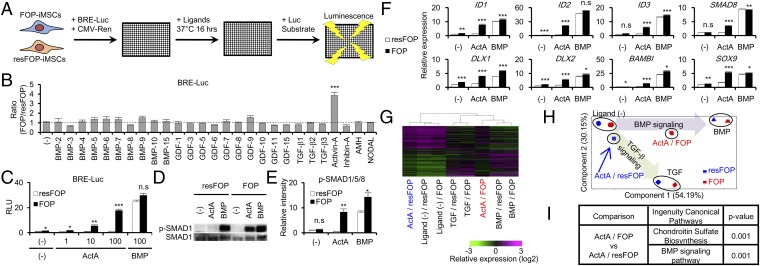
To screen noncanonical BMP ligands that activate BMP signaling through FOP-ACVR1 but not through WT-ACVR1, we focused our attention on FOP-iMSCs from FOP patient-derived iPSCs as test cells and mutation-rescued FOP-iMSCs (resFOP-iMSCs) as genetically matched control cells (26). A BMP-specific luciferase reporter construct (BRE-Luc) was transfected into both FOP-iMSCs and resFOP-iMSCs, and detection of luminescence was made 16 h after ligand stimulation (Fig. 1A). Consistent with previous reports (14, 18), several BMP ligands, such as BMP-6 and BMP-7, induced higher luminescence in FOP-iMSCs than resFOP-iMSCs, but at less than 1.4-fold (Fig. 1B and SI Appendix, Fig. S1). Interestingly, Activin-A treatment significantly increased the luciferase activity in FOP-iMSCs, but not in resFOP-iMSCs (Fig. 1 B and C and SI Appendix, Fig. S1). This result was confirmed in another rescue clone and another patient-derived FOP- and resFOP-iMSCs (SI Appendix, Fig. S2). The phosphorylation of SMAD1/5/8, cytoplasmic BMP signaling transducers, and the expression of downstream genes of BMP signaling were also induced specifically in FOP-iMSCs (Fig. 1 D–F). Global gene-expression profiling revealed that Activin-A treatment substantially transduced BMP-like signaling in FOP-iMSCs, but not in resFOP-iMSCs (Fig. 1 G–I). These results indicated that Activin-A abnormally transduced BMP signaling in FOP-iMSCs.
Fig. 1.
Activin-A abnormally transduced BMP signaling in FOP-iMSCs. (A) Scheme of FOP-ACVR1 specific ligand screening. (B) Activin-A caused the highest increase in BRE-Luc activity (FOP/resFOP) among TGF-β superfamily ligands tested. (C) Activin-A increased BRE-Luc activity in FOP-iMSCs, but not in resFOP-iMSCs. (D) Representative image of Western blot analysis. Activin-A induced phosphorylation of SMAD1/5/8 (p-SMAD1) in FOP-iMSCs, but not in resFOP-iMSCs. After 6-h serum starvation, FOP- and resFOP-iMSCs were treated with ligands for 1 h. (E) Quantification of relative p-SMAD1/5/8 phosphorylation levels corrected by total SMAD1/5/8. (F) Higher expression levels of BMP target genes in FOP-iMSCs stimulated with Activin-A in microarray analysis. (G–I) Global gene expression analysis showed Activin-A transduced BMP signaling in FOP-iMSCs. Hierarchical clustering analysis (G) and a PCA plot (H) of FOP- and resFOP-iMSCs using differentially expressed gene sets. (I) Ingenuity pathway analysis using genes differentially expressed between FOP- and resFOP-iMSC treated with Activin-A. Results are the mean ± SE. n = 3–4 (BRE-Luc assay) and n = 3 (Western blot and microarray analysis). n.s., no significant difference; *P < 0.05; **P < 0.01; ***P < 0.001 by Dunnett’s multiple comparisons t test compared with the no ligand treatment control (B) and by Student’s t test compared with resFOP-iMSCs treated with the same ligands (C, E, and F). ActA, 100 ng/mL Activin-A; BMP, 100 ng/mL BMP-7; TGF, 10 ng/mL TGF-β3 (D–I).
Molecular Mechanisms of Abnormal BMP Signaling Evoked by Activin-A.
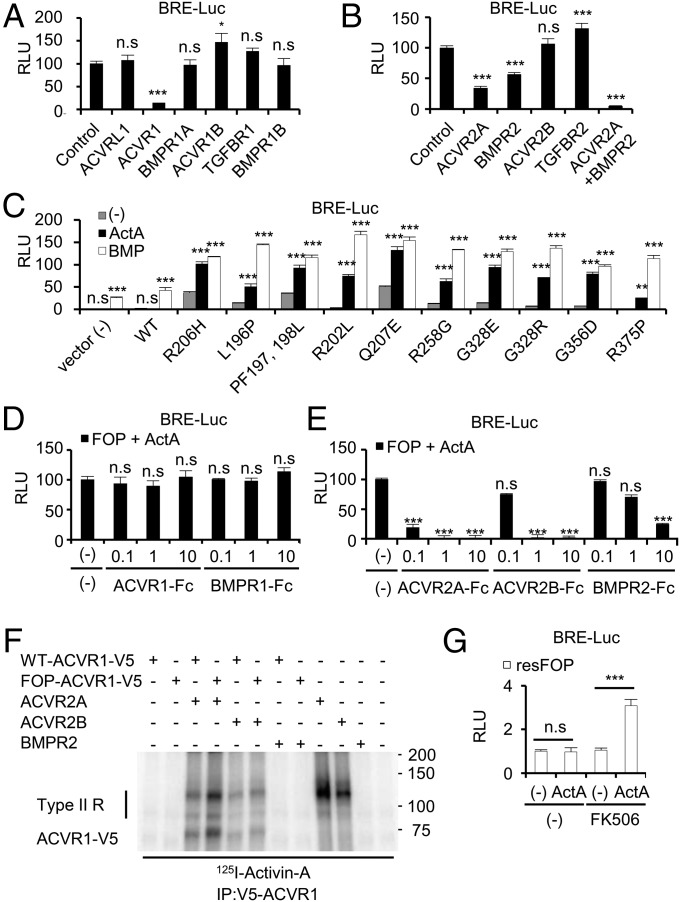
Next, to check the necessity and sufficiency of FOP-ACVR1 on BMP signaling, loss-of-function and gain-of-function studies were performed. Treatment of siRNAs specific for type I receptors in FOP-iMSCs revealed a critical requirement of FOP-ACVR1 in Activin-A–dependent BMP signaling (Fig. 2A; knockdown efficiencies are shown in SI Appendix, Fig. S3). Treatment of siRNAs specific for type II receptors showed the involvement of both ACVR2A and BMPR2 in this abnormal activation (Fig. 2B and SI Appendix, Fig. S3). Conversely, overexpression of the mutant ACVR1 found in FOP patients conferred Activin-A responsiveness in U2OS cells (Fig. 2C). This neofunction of FOP-ACVR1 was also confirmed in HEK293 and HepG2 cells (SI Appendix, Fig. S4). These results indicated that Activin-A activates abnormal BMP signaling through FOP-ACVR1.
Fig. 2.
Molecular mechanisms of abnormal BMP signaling evoked by Activin-A. (A and B) Activin-A transduced FOP-ACVR1-mediated BMP signaling through ACVR2A and BMPR2. FOP-iMSCs transiently transfected with BRE-Luc, CMV-Renilla, and siRNAs specific for type I receptors (A) or type II receptors (B) were stimulated with Activin-A for 16 h. Note, neither ACVR1C nor AMHR2 were expressed in FOP-iMSCs. (C) Other FOP mutant receptors also transduced BMP signaling by Activin-A stimulation. U2OS cells transiently transfected with BRE-Luc, CMV-Renilla, and FOP mutant receptors were stimulated with 20 ng/mL Activin-A or 10 ng/mL BMP-7 for 16 h. (D and E) Activin-A strongly bound to the extracellular region of ACVR2A, 2B and weakly to BMPR2, but not to ACVR1. (F) Binding of 125I-Activin-A to LentiX293T transfected with hACVR1-V5, SNAP-hACVR2A, SNAP-hACVR2B, or hBMPR2. Cells were affinity labeled with 125I-Activin-A and cross-linked by disuccinimidyl suberate. Type II R, Type II receptors. (G) resFOP-iMSCs acquired Activin-A responsiveness by FK506 treatment. resFOP-iMSCs transiently transfected with BRE-Luc and CMV-Renilla were treated with 1 μM FK506 or Activin-A for 16 h. n.s., no significant difference; *P < 0.05; **P < 0.01; ***P < 0.001 by Dunnett’s multiple comparisons t test compared with the control siRNA transfected-FOP-iMSCs (A and B), to the no ligand treatment controls transfected with the same receptors (C), or to the no Fc-fusion receptors treatment control (D and E), and by Student’s t test (G). Results are the mean ± SE. n = 4–8.
Because Activin-A normally transduces TGF-β–SMAD2/3 signaling (10, 32–34), the phosphorylation of SMAD2/3 and activation of a TGF-β–responsive luciferase reporter construct (CAGA-Luc) were analyzed. The levels of phosphorylation and activation in FOP-iMSCs were similar to those in resFOP-iMSCs (SI Appendix, Fig. S5). Knockdown experiments revealed the involvement of ACVR1B and ACVR2A in this signaling (SI Appendix, Fig. S6). These results indicated that Activin-A transduces TGF-β–SMAD2/3 signaling through ACVR1B/ACVR2A in FOP-iMSCs.
To dissect the molecular mechanism of how FOP-ACVR1 transduces abnormal BMP signaling, we assessed to which receptors Activin-A was potentially bound. Treatment of the soluble extracellular region of FOP-ACVR1 (ACVR1-Fc; same as WT-ACVR1) did not affect the Activin-A–dependent activation of BRE-Luc in FOP-iMSCs (Fig. 2D), whereas treatment of ACVR2A-Fc and ACVR2B-Fc strongly and BMPR2-Fc weakly decreased the activity (Fig. 2E). Because knockdown experiments indicated signal transduction of Activin-A on BMP signaling through FOP-ACVR1, these results suggested that Activin-A is indirectly bound to FOP-ACVR1. Next, we checked whether the binding affinity of FOP-ACVR1 to Activin-A with or without type II receptors is altered. Cross-linking experiments revealed that the binding affinity was slightly enhanced when either ACVR2A or ACVR2B was coexpressed (Fig. 2F). FOP mutations are found in the intracellular region of ACVR1 around the regulatory GS domain and protein kinase domain, and thought to destabilize the inactive state of ACVR1 through the binding of inhibitory protein FKBP12 (12, 15, 17, 37). Thus, we checked whether treatment of FK506, an inhibitor of FKBP12, conferred Activin-A–dependent activation of BMP signaling in resFOP-iMSCs. As expected, treatment of FK506 rendered the responsiveness of Activin-A in resFOP-iMSCs (Fig. 2G), although FK506 enhanced the constitutive activity in FOP-iMSCs (SI Appendix, Fig. S7). Taken together, the abnormal reactivity of FOP-ACVR1 to Activin-A could be caused, at least partially, by differential affinity for Activin-A and the dysregulation of inhibitory mechanisms. However, further investigation is required for more detailed understanding of the aberrant activation of BMP signaling by Activin-A.
Enhanced Chondrogenesis of FOP-iMSCs via BMP and TGF-β Signaling by Activin-A Stimulation.
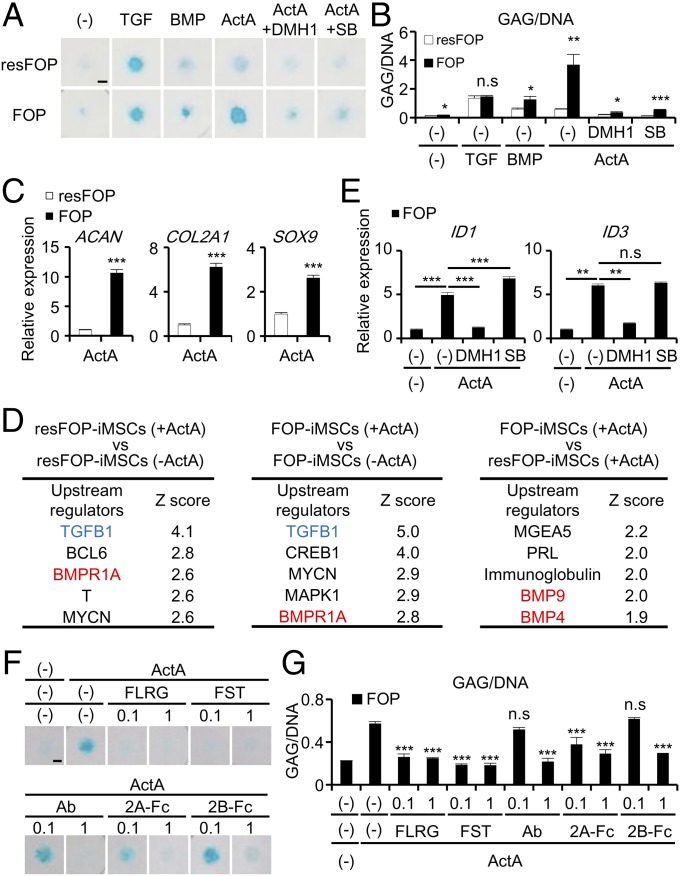
Because HO occurs through endochondral ossification in FOP patients (1–6) and pathway analysis of FOP-iMSCs revealed that Activin-A induces chondrogenic pathways in FOP-iMSCs (Fig. 1I), the impact of Activin-A on chondrogenesis was assessed. After treatment of chondrogenic basal medium with TGF-β3 for 7 d, we found the glycosaminoglycan (GAG) production/DNA ratio (GAG/DNA) in 2D micromass of FOP-iMSCs was comparable to that of resFOP-iMSCs (Fig. 3 A and B). Treatment of BMP-7 induced slightly higher GAG/DNA in FOP-iMSCs compared with resFOP-iMSCs, consistent with the idea that cells expressing FOP-ACVR1 have higher sensitivity for BMP ligands. These results also indicated that both TGF-β and BMP signaling play critical roles for chondrogenesis in the 2D micromass assay of both FOP-iMSCs and resFOP-iMSCs. In sharp contrast, treatment of Activin-A induced significantly higher GAG/DNA in FOP-iMSCs compared with resFOP-iMSCs. We also found Activin-A treatment increased the expression of chondrogenic markers (ACAN, COL2A1, and SOX9) in FOP-iMSCs (Fig. 3C), indicating that Activin-A treatment is sufficient to induce enhanced chondrogenesis in these cells. To verify the accuracy of our FOP-iMSCs model, we performed a 2D-chonodrogenic assay with retinoic acid receptor-γ agonists (CD437 and R667) (38, 39) and confirmed reduction of GAG/DNA in a concentration-dependent manner (SI Appendix, Fig. S8).
Fig. 3.
Enhanced chondrogenesis of 2D chondrogenic micromass of FOP-iMSCs by Activin-A stimulation, which was suppressed by Activin-A inhibitors. (A–G) Two-dimensional chondrogenic micromass assay of FOP- and resFOP-iMSCs at day 7. (A) Representative images of Alcian blue staining. (Scale bar, 200 μm.) (B) Enhanced GAG/DNA in the micromass of FOP-iMSCs cultured with Activin-A, and which was inhibited by 1 μM DMH1 or 1 μM SB431542 (SB) treatment. TGF, 1 ng/mL TGF-β3. (C) Higher expression levels of early chondrogenic markers (ACAN, COL2A1, and SOX9) in the micromass of FOP-iMSCs cultured with Activin-A. (D) Upstream analysis using genes up- or down-regulated at least twofold after chondrogenic differentiation with or without Activin-A. (E) DMH1 (1 μM), but not SB (1 μM) inhibit the expression of BMP downstream target genes 16 h after stimulation by Activin-A. (F and G) Activin-A-triggered enhanced chondrogenesis of FOP-iMSCs was inhibited by several Activin-A inhibitors. Results are the mean ± SE. n = 4 (B, C, G), n = 3 (E), and n = 1 (D). n.s., no significant difference; *P < 0.05; **P < 0.01; ***P < 0.001 by Student’s t test compared with resFOP treated with the same ligands with or without the same compounds (B and C) and by Dunnett’s multiple comparisons t test compared with Activin-A-treated FOP-iMSCs (E) or Activin-A-treated micromass without Activin-A inhibitors (G).
To gain molecular insights underlying the enhanced chondrogenesis, unbiased transcriptome analysis of FOP-iMSCs and resFOP-iMSC with or without Activin-A treatment was performed. We identified two BMP signaling components, BMP4 and BMP9, as upstream regulators in FOP-iMSCs (Fig. 3D, Right), consistent with the fact that Activin-A abnormally transduces BMP signaling in FOP-iMSCs. This analysis also identified TGF-β1 and BMPR1A as upstream regulators in FOP-iMSCs and resFOP-iMSCs treated with Activin-A (Fig. 3D, Left and Center), indicating that BMP signaling as well as TGF-β signaling were activated not only in FOP-iMSCs, but also resFOP-iMSCs during chondrogenesis, even though short-term administration of Activin-A did not induce BMP-SMAD1/5/8 signaling in resFOP-iMSCs (Fig. 1 C–F).
Because our data indicated that both BMP and TGF-β signaling were activated in Activin-A–treated FOP-iMSCs during chondrogenesis (Fig. 3D, Center), a specific inhibitor of either BMP signaling (DMH1) or TGF-β signaling (SB431542) was administrated to discriminate the involvement of these two signaling pathways in the observed enhanced chondrogenesis. Treatment of DMH1 diminished enhanced GAG/DNA in FOP-iMSCs (Fig. 3 A and B), consistent with Activin-A abnormally transducing BMP signaling in FOP-iMSCs. Intriguingly, treatment of SB431542 also abrogated enhanced GAG/DNA in FOP-iMSC, but did not decrease the level of two downstream BMP signaling targets, ID1 and ID3 (Fig. 3E), suggesting that the abrogation was not caused by a side effect of SB431542 on BMP signaling. Taken together, these results strongly suggest that the enhanced chondrogenesis in FOP-iMSCs is caused by the dual activation of BMP and TGF-β signaling via the administration of Activin-A.
In addition to chemical cytoplasmic inhibitors, administration of extracellular Acitivin-A inhibitors, such as Follistatin-related gene protein, Follistatin, anti–Activin-A Ab, ACVR2A-Fc (2A-Fc), and ACVR2B-Fc (2B-Fc), also significantly suppressed the Activin-A dependent enhancement of chondrogenesis (Fig. 3 F and G). These results indicated that Activin-A inhibitors have the potential to become new therapeutic agents.
Enhanced Calcification of FOP-3DCI Pellets in Vivo.
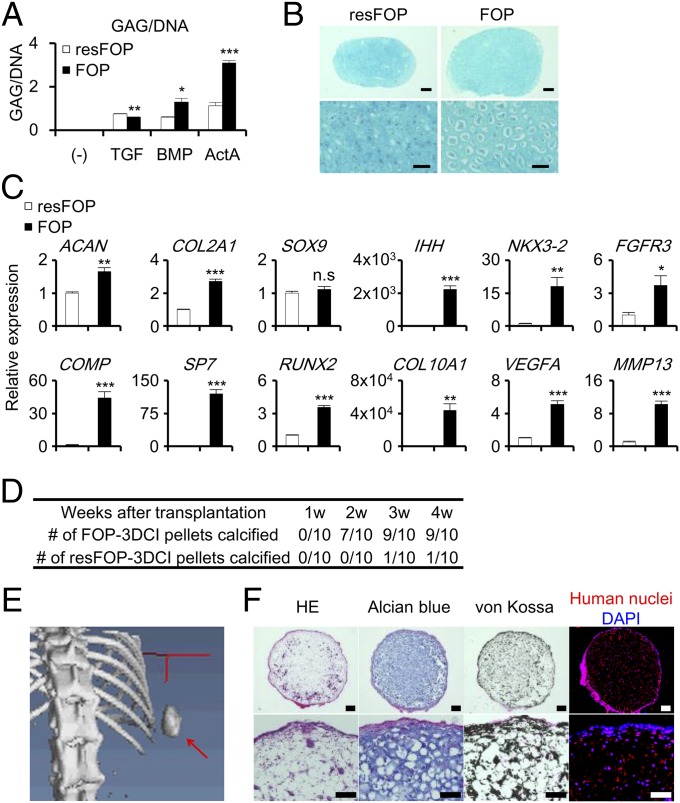
Although the 2D micromass assay is suitable for the verification of exogenous factors, the 3D chondrogenic induction (3DCI) pellet assay enables the analysis of more mature chondrocytes in vitro and also allows the transplantation of the pellets in vivo. After culture in chondrogenic basal medium with TGF-β3, BMP-7, or Activin-A for 17 d, GAG/DNA of 3DCI pellets from FOP-iMSCs (FOP-3DCI pellets) were observed as comparable, slightly higher, and markedly higher than those from resFOP-iMSCs (resFOP-3DCI pellets), respectively (Fig. 4A), consistent with the results from the 2D micromass culture (Fig. 3 A and B). Histological analyses revealed that the FOP-3DCI pellets cultured with Activin-A contained more mature chondrocytes than did resFOP-3DCI pellets (Fig. 4B). Quantitative PCR analysis revealed that markers for mature chondrocytes (40), such as COL10A1, VEGFA, RUNX2, and MMP13, were induced stronger in FOP-3DCI pellets than in resFOP-3DCI pellets (Fig. 4C and SI Appendix, Fig. S9). In addition, we observed that FK506 treatment enhanced chondrogenesis in resFOP-3DCI pellets treated with Activin-A (SI Appendix, Fig. S10). These results indicated that Activin-A treatment enhanced chondrogenic differentiation in FOP-3DCI pellets in vitro.
Fig. 4.
Enhanced chondrogenesis of 3DCI pellets of FOP-iMSCs by Activin-A stimulation, which spontaneously calcified in vivo. (A) GAG/DNA of 3DCI pellet from FOP- and resFOP-iMSCs cultured with Activin-A (ActA), BMP-7 (BMP), or TGF-β3 (TGF) at day 17. (B and C) 3DCI pellet assay from FOP- and resFOP-iMSCs cultured with Activin-A at day 21. (B) Alcian blue staining of FOP- and resFOP-3DCI pellets. [Scale bars, 200 μm (Upper); 50 μm (Lower).] (C) Higher expression levels of late chondrogenic markers were seen in the FOP-3DCI pellets. (D–F) FOP-3DCI pellets spontaneously calcified in vivo. FOP- or resFOP-3DCI pellets cultured for 21 d with Activin-A were subcutaneously transplanted in NOD/ShiJic-scid Jcl (NOD/SCID) mice. Ten mice were transplanted with both FOP-3DCI (right side) and resFOP-3DCI pellets (left side) for 28 d. (D) Number of FOP- or resFOP-3DCI pellets calcified in vivo assessed by X-ray imaging. (E) A μCT image shows a calcified FOP-3DCI pellet (red arrow). (F) Histological analysis of transplanted FOP-3DCI pellets. H&E, Alcian blue staining (sulfated polysaccharides), von Kossa staining (calcium), and anti-human nuclei staining are shown. [Scale bars, 200 μm (Upper); 100 μm (Lower).] Results are the mean ± SE. n = 3 (A and C). n.s., no significant difference; *P < 0.05; **P < 0.01; ***P < 0.001 by Student’s t test compared with resFOP treated with the same ligands (A and C).
Chondrogenesis is a critical step in endochondral ossification through which ectopic bones are formed in FOP patients. To further characterize the FOP-3DCI pellets, we subcutaneously transplanted the pellets into the backs of immunodeficient mice and observed whether calcification without stimulus occurred. Before transplantation, no calcification was observed in 3DCI pellets (SI Appendix, Fig. S11). Four weeks after transplantation, X-ray photos showed a dense radiopaque mass in 9 of 10 mice transplanted with FOP-3DCI pellets, but only 1 in 10 mice transplanted with resFOP-3DCI pellets (Fig. 4D and SI Appendix, Fig. S12A). Microcomputed tomography (µCT) images showed multiple calcified nodules in the entire mass (Fig. 4E and SI Appendix, Fig. S12B). Histological analyses revealed enlarged chondrocytes surrounded by a calcified matrix (Fig. 4F), which closely resembled the calcified zone in growth plates. Contribution of transplanted cells to the central cartilaginous zone was confirmed by immunostaining with anti-human nuclei antibody (HNA), whereas HNA-positive and -negative cells were detected in the surface calcified zone, indicating the contribution of both transplanted human cells and host mouse cells. Because 3DCI pellets were no longer exposed to exogenous Activin-A after transplantation, these results indicated that FOP-3DCI pellets spontaneously proceeded to the last step of differentiation of growth plate chondrocytes in vivo.
Activin-A Induces Endochondral Ossification of FOP-iMSCs in Vivo.
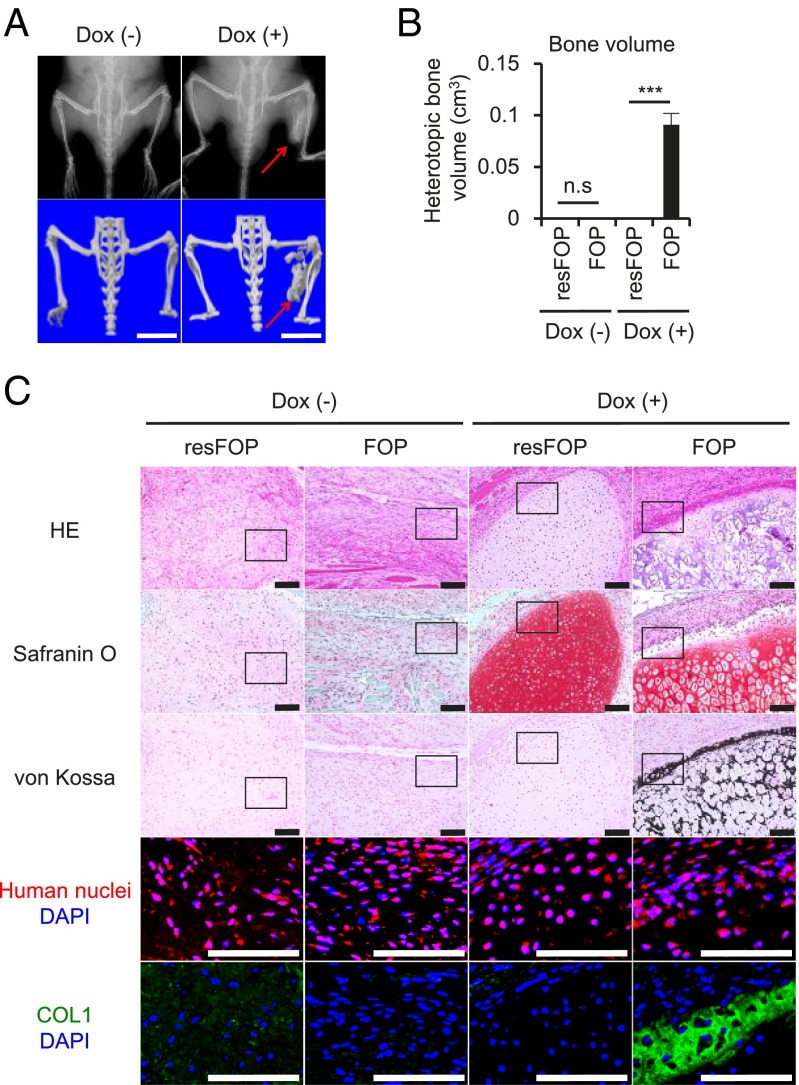
Finally, to investigate whether Activin-A can induce heterotopic endochondral ossification of FOP-iMSCs in vivo, FOP- and resFOP-iMSCs were transplanted into the gastrocnemius muscle of NOD/SCID mice with C3H10T1/2 harboring Doxycycline (Dox)-inducible Activin-A. Six weeks after transplantation, only the combination of FOP-iMSCs and Activin-A expression induced HO in the injected site (Fig. 5 A and B and SI Appendix, Fig. S13). Histological analyses revealed that without Dox, transplanted iMSCs contributed to fibrous tissue in the muscle (Fig. 5C) [Dox (−) groups]. After Dox induction, resFOP-iMSCs contributed to GAG-rich mature chondrocytes, whereas no calcification was observed (Fig. 5C) [Dox (+), resFOP]. In the Dox (+) FOP group, cartilaginous tissue resembling hypertrophic and calcified chondrocytes was observed, consistent with the result of FOP-3DCI pellets (Fig. 4 B and F). Furthermore, we observed calcified tissue neighboring the cartilaginous site, where expression of COL1, a marker of bone formation, was found (Fig. 5C) [Dox (+), FOP]. Finally, cartilaginous and calcified cells were both HNA+. Taken together, these data indicate Activin-A induces heterotopic endochondral ossification in FOP in vivo.
Fig. 5.
Transplanted FOP-iMSCs were ossified in vivo by Activin-A stimulation. (A–C) FOP- (right leg) and resFOP-iMSCs (left leg) were transplanted into the gastrocnemius muscle of NOD/SCID mice with Dox-inducible Activin-A expressing C3H10T1/2. Transplanted cells were analyzed 6 wk after transplantation. (A) X-ray and μCT images. Red arrows show FOP-iMSCs derived bone. (Scale bars, 10 mm.) (B) Heterotopic bone volume (cm3) of each group. Results are the mean ± SE. n = 3. n.s., no significant difference; ***P < 0.001 by Student’s t test compared with resFOP transplanted tissue. (C) Histological analysis of FOP- and resFOP-iMSCs derived tissue. HE, Safranin O, von Kossa, anti-human nuclei staining, and anti-COL1 staining are shown. (Scale bars, 100 μm.)
Discussion
Taking advantage of MSCs derived from patient-derived iPSCs, we here present a novel in vivo model of FOP to evaluate the role and mechanism of action of Activin-A in HO. Only FOP-iMSCs cotransplanted with Activin-A–expressing C3H10T1/2 cells in NOD/SCID mice showed bone and cartilage formation (Fig. 5), clearly demonstrating the contribution of Activin-A to endochondral ossification of FOP-cells in vivo. Although Activin-A also induced extracellular matrix-rich cartilage in resFOP-iMSCs, hypertrophic chondrocytes were found in FOP-iMSCs but not in resFOP-iMSCs, indicating that FOP-ACVR1 with Activin-A accelerated the terminal differentiation of chondrocytes. Intriguingly, FOP-3DCI pellets were calcified in vivo without exogenous ligand stimulation, suggesting that Activin-A is not essential for the late steps of HO. Indeed, administration of Activin-A with transplants (Activin-A soaked with Gelfoam) did not accelerate the calcification of FOP-3DCI pellets according to X-ray observations (SI Appendix, Fig. S14). HO in FOP patients can be divided into two phases, inflammation and destruction of connective tissues (phase 1) and bone formation (phase 2) (5). The latter can be further subdivided into three stages: fibroproliferation and angiogenesis (2A), chondrogenesis (2B), and osteogenesis (2C). Our data indicated that Activin-A plays a critical role in stage 2B, but neither in stage 2A, because DNA content did not increase in FOP-iMSCs after Activin-A treatment (SI Appendix, Fig. S15), nor in stage 2C, as the in vivo treatment of Activin-A of FOP-3DCI pellets did not enhance calcification. We expect that, similar to the findings of stage 2B in the present work, our FOP-iPSCs can be used to study the signaling mechanisms that contribute to phase 1 and stages 2A and 2C.
Most recently, it was reported that neutralizing antibody against Activin-A suppresses HO in R206H-ACVR1 knock-in mouse by Hatsell et al. (41). This finding supports our study, which suggests Activin-A is a crucial trigger for HO in both FOP model mice and FOP patients, and modulating Activin-A/FOP-ACVR1 signaling is a promising drug target for FOP. In the Hatsel et al. report, however, FK506 did not endow Activin-A responsiveness in WT-ACVR1 overexpressing cells, whereas we show that FK506 conferred Activin-A–dependent activation of BMP signaling in resFOP-iMSCs and enhanced 3D chondrogenesis (Fig. 2G and SI Appendix, Fig. S10). This discrepancy might be because of the different concentrations of FK506 tested.
The current prevailing concept of the FOP pathology is that missense mutations endow ACVR1 with constitutive activity or hyperactivity after ACVR1 binds to BMP. In the present report, we demonstrated a novel third mechanism, where FOP-ACVR1 transduces BMP signaling in response to Activin-A. In FOP-iMSCs, Activin-A transduced both TGF-β and BMP signaling through ACVR1B and FOP-ACVR1, respectively. This conclusion was supported by unbiased transcriptome analyses, which suggested that during chondrogenesis, Activin-A stimulation induced the dual activation of BMP and TGF-β signaling in FOP-iMSCs. Consistently, we found administration of either SB431542 or DMH1, specific inhibitors of TGF-β and BMP, respectively, abrogated the enhanced chondrogenesis in FOP-iMSCs. Based on these observations, we propose that enhanced chondrogenesis in FOP-iMSCs by Activin-A treatment is a result of abnormal activation of BMP signaling along with normal TGF-β signaling. More intriguingly, this neofunction could disrupt tissue homeostasis by dysregulating BMP signaling intensity. This intensity is stabilized via transcriptional negative feedback loops (33). For example, GREM1 is known to be a downstream gene of BMP signaling, and its protein functions as a BMP ligand antagonist (32, 33, 42). Consistent with our findings, Activin-A stimulation in FOP-iMSCs induced stronger expression of GREM1 than that in resFOP-iMSCs (SI Appendix, Fig. S16). Importantly, GREM1 does not antagonize Activin-A signaling (42). These results suggest that Activin-A–stimulated BMP signaling in FOP-iMSCs is outside the negative feedback regulation loops network. Therefore, aberrant induction and escaping from negative feedback regulation should be hallmarks of BMP signaling in FOP, which stimulates the formation of ectopic bones. Understanding how canonical ligands and noncanonical ligands, as demonstrated in this report, are involved in the activation of BMP signaling in the clinical situation, remains an important issue awaiting future clarification.
Materials and Methods
Full experimental procedures and associated references are available in SI Appendix, SI Materials and Methods.
Cell Culture.
The induction and maintenance of induced neural crest cells (iNCCs) and iMSCs derived from iPSC were previously described (43). FOP-iPSCs used in this study [FOP-iPSCs from patient 1 and 2, previously described as vFOP4-1 and vFOP5-22 (25), respectively] harbor the R206H heterozygous mutation in ACVR1, and gene-corrected resFOP-iPSCs were generated by BAC-based homologous recombination (26). All experiments shown in Figs. 1–5 were performed using FOP-iPSCs from patient 1 and resFOP-iPSCs (cl1) (26).
FOP-ACVR1 Specific Ligand Screening.
FOP- and resFOP-iMSCs transiently transfected with BRE-Luc and CMV-Renilla were seeded into 384-well plates and treated with TGF-β superfamily ligands. After 16-h incubation, relative luciferase units (RLU) were measured. In Fig. 1B, the highest concentrations tested in SI Appendix, Fig. S1 are shown.
Two-Dimensional Chondrogenic Induction.
iMSCs (1.5 × 105) were suspended in 5 µL of chondrogenic basal medium and subsequently transferred to fibronectin-coated 24-well plates (BD Biosciences). After 1 h, a total of 1 mL of the chondrogenic basal medium supplemented with several ligands or inhibitors was added. Micromass cultures were maintained at 37 °C under 5% (vol/vol) CO2 for 7 d.
Three-Dimensional Chondrogenic Induction.
iMSCs (2.5 × 105) were suspended in chondrogenic basal medium supplemented with 100 ng/mL Activin-A, 100 ng/mL BMP-7, or 10 ng/mL TGF-β3, and subsequently transferred to PrimeSurface 96U (Sumitomo Bakelite) (Fig. 4A) or 15-mL tubes (Corning). Cells were centrifuged to form pellets and maintained at 37 °C under 5% (vol/vol) CO2. The culture medium was changed every 2–3 d.
In Vivo Calcification of 3DCI Pellets.
The 3DCI pellets cultured with 100 ng/mL Activin-A for 21 d in vitro were wrapped in 0.5 cm × 1 cm Gelfoam (Pfizer) and transplanted beneath the dorsal skin of immunodeficient NOD/SCID mice (CLEA Japan) (44). Four weeks later, transplanted 3DCI pellets were harvested and analyzed.
iMSCs Transplantation with Activin-A–Producing Cells.
FOP- (right leg) and resFOP-iMSCs (left leg) (4 × 106, respectively) were transplanted into the gastrocnemius muscle of NOD/SCID mice with C3H-DoxOn-hINHBA (5 × 105), which can achieve continuous exposure of Activin-A on transplanted iMSCs in vivo by administration of Dox. Six weeks after transplantation, transplanted cells were harvested and analyzed.
Study Approval.
All experimental protocols dealing with human subjects were approved by the Ethics Committee of the Department of Medicine and Graduate School of Medicine, Kyoto University. Written informed consent was provided by each donor. All animal experiments were approved by the institutional animal committee of Kyoto University.
Supplementary Material
Acknowledgments
We thank Dr. S. Kawai for preparing patients’ samples; Ms. Y. Tezuka for the microarray experiments; Dr. H. Sakurai for kind advices about immunostaining; Dr. K. Woltjen for providing the PB-TAC-ERN vector; Dr. H. Matsushita and the Center for Anatomical, Pathological, and Forensic Medical Researches, Kyoto University Graduate School of Medicine, for preparing microscope slides; Dr. A. Ikeda for invaluable comments and discussion; Drs. C. Alev and P. Karagiannis for reading the manuscript; Dr. S. Yamanaka for supproting/initiating fibrodysplasia ossificans progressiva research; and members of the J.T. and M.I. laboratories for their support during this study. This work was supported by Grants-in-aid for Scientific Research from The Japan Society for the Promotion of Science (#23791636, #25293320); the Leading Project for Realization of Regenerative Medicine from MEXT; in part by the Program for Intractable Diseases Research utilizing Disease-specific iPS cells from The Japan Science and Technology Agency and iPS Cell Research Fund (to M.I. and J.T.); and A-STEP, Adaptable and Seamless Technology Transfer Program through target-driven R&D, Exploratory Research (M.I.).
Footnotes
Conflict of interest statement: K. Hino, K. Horigome, and H.E. are employees of Sumitomo Dainippon Pharma Co., Ltd; and M.I. and J.T. are supported by a research fund from Sumitomo Dainippon Pharma Co., Ltd.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession nos. GSE62783 and GSE69459)
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510540112/-/DCSupplemental.
References
- 1.Kaplan F, et al. The phenotype of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3(3-4):183–188. [Google Scholar]
- 2.Shore E, Feldman G, Xu M, Kaplan F. The genetics of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3(3-4):201–204. [Google Scholar]
- 3.Kaplan FS, Groppe J, Pignolo RJ, Shore EM. Morphogen receptor genes and metamorphogenes: Skeleton keys to metamorphosis. Ann N Y Acad Sci. 2007;1116:113–133. doi: 10.1196/annals.1402.039. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan FS, et al. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22(1):191–205. doi: 10.1016/j.berh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6(9):518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan FS, Chakkalakal SA, Shore EM. Fibrodysplasia ossificans progressiva: Mechanisms and models of skeletal metamorphosis. Dis Model Mech. 2012;5(6):756–762. doi: 10.1242/dmm.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shore EM, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 8.Urist MR. Bone: Formation by autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 9.Wozney JM, et al. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 10.Mueller TD, Nickel J. Promiscuity and specificity in BMP receptor activation. FEBS Lett. 2012;586(14):1846–1859. doi: 10.1016/j.febslet.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan FS, et al. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30(3):379–390. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaikuad A, et al. Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem. 2012;287(44):36990–36998. doi: 10.1074/jbc.M112.365932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda T, et al. A unique mutation of ALK2, G356D, found in a patient with fibrodysplasia ossificans progressiva is a moderately activated BMP type I receptor. Biochem Biophys Res Commun. 2008;377(3):905–909. doi: 10.1016/j.bbrc.2008.10.093. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda T, et al. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem. 2009;284(11):7149–7156. doi: 10.1074/jbc.M801681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Q, et al. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009;119(11):3462–3472. doi: 10.1172/JCI37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song GA, et al. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285(29):22542–22553. doi: 10.1074/jbc.M109.094557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dinther M, et al. ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Miner Res. 2010;25(6):1208–1215. doi: 10.1359/jbmr.091110. [DOI] [PubMed] [Google Scholar]
- 18.Ohte S, et al. A novel mutation of ALK2, L196P, found in the most benign case of fibrodysplasia ossificans progressiva activates BMP-specific intracellular signaling equivalent to a typical mutation, R206H. Biochem Biophys Res Commun. 2011;407(1):213–218. doi: 10.1016/j.bbrc.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Le VQ, Wharton KA. Hyperactive BMP signaling induced by ALK2(R206H) requires type II receptor function in a Drosophila model for classic fibrodysplasia ossificans progressiva. Dev Dyn. 2012;241(1):200–214. doi: 10.1002/dvdy.22779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagarova J, et al. Constitutively active ALK2 receptor mutants require type II receptor cooperation. Mol Cell Biol. 2013;33(12):2413–2424. doi: 10.1128/MCB.01595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakkalakal SA, et al. An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res. 2012;27(8):1746–1756. doi: 10.1002/jbmr.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Culbert AL, et al. Alk2 regulates early chondrogenic fate in fibrodysplasia ossificans progressiva heterotopic endochondral ossification. Stem Cells. 2014;32(5):1289–1300. doi: 10.1002/stem.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billings PC, et al. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP) J Bone Miner Res. 2008;23(3):305–313. doi: 10.1359/JBMR.071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamasaki M, et al. Pathogenic mutation of ALK2 inhibits induced pluripotent stem cell reprogramming and maintenance: Mechanisms of reprogramming and strategy for drug identification. Stem Cells. 2012;30(11):2437–2449. doi: 10.1002/stem.1221. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto Y, et al. Induced pluripotent stem cells from patients with human fibrodysplasia ossificans progressiva show increased mineralization and cartilage formation. Orphanet J Rare Dis. 2013;8:190. doi: 10.1186/1750-1172-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto Y, et al. New protocol to optimize iPS cells for genome analysis of fibrodysplasia ossificans progressiva. Stem Cells. 2015;33(6):1730–1742. doi: 10.1002/stem.1981. [DOI] [PubMed] [Google Scholar]
- 27.Hogan BL. Bone morphogenetic proteins: Multifunctional regulators of vertebrate development. Genes Dev. 1996;10(13):1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 28.Gu Z, et al. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999;126(11):2551–2561. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- 29.Mishina Y, Crombie R, Bradley A, Behringer RR. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol. 1999;213(2):314–326. doi: 10.1006/dbio.1999.9378. [DOI] [PubMed] [Google Scholar]
- 30.Massagué J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103(2):295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 31.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147(1):35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 32.Piek E, Heldin CH, Ten Dijke P. Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J. 1999;13(15):2105–2124. [PubMed] [Google Scholar]
- 33.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24(2):218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 34.Renlund N, O’Neill FH, Zhang L, Sidis Y, Teixeira J. Activin receptor-like kinase-2 inhibits activin signaling by blocking the binding of Activin to its type II receptor. J Endocrinol. 2007;195(1):95–103. doi: 10.1677/JOE-07-0281. [DOI] [PubMed] [Google Scholar]
- 35.Phillips DJ, de Kretser DM, Hedger MP. Activin and related proteins in inflammation: Not just interested bystanders. Cytokine Growth Factor Rev. 2009;20(2):153–164. doi: 10.1016/j.cytogfr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Antsiferova M, Werner S. The bright and the dark sides of Activin in wound healing and cancer. J Cell Sci. 2012;125(Pt 17):3929–3937. doi: 10.1242/jcs.094789. [DOI] [PubMed] [Google Scholar]
- 37.Groppe JC, Wu J, Shore EM, Kaplan FS. In vitro analyses of the dysregulated R206H ALK2 kinase-FKBP12 interaction associated with heterotopic ossification in FOP. Cells Tissues Organs. 2011;194(2-4):291–295. doi: 10.1159/000324230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin B, et al. Selective synthetic ligands for human nuclear retinoic acid receptors. Skin Pharmacol. 1992;5(1):57–65. doi: 10.1159/000211018. [DOI] [PubMed] [Google Scholar]
- 39.Shimono K, et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-γ agonists. Nat Med. 2011;17(4):454–460. doi: 10.1038/nm.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuscik MJ, Hilton MJ, Zhang X, Chen D, O’Keefe RJ. Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest. 2008;118(2):429–438. doi: 10.1172/JCI34174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatsell SJ, et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to Activin A. Sci Transl Med. 2015;7(303):303ra137. doi: 10.1126/scitranslmed.aac4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell. 1998;1(5):673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- 43.Fukuta M, et al. Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS One. 2014;9(12):e112291. doi: 10.1371/journal.pone.0112291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoyama K, et al. Enhanced chondrogenesis of induced pluripotent stem cells from patients with neonatal-onset multisystem inflammatory disease occurs via the caspase 1-independent cAMP/protein kinase A/CREB pathway. Arthritis Rheumatol. 2015;67(1):302–314. doi: 10.1002/art.38912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.