Abstract
The ventral pallidum (VP) plays a critical role in the processing and execution of motivated behaviors. Yet this brain region is often overlooked in published discussions of the neurobiology of mental health (e.g., addiction, depression). This contributes to a gap in understanding the neurobiological mechanisms of psychiatric disorders. This review is presented to help bridge the gap by providing a resource for current knowledge of VP anatomy, projection patterns and subregional circuits, and how this organization relates to the function of VP neurons and ultimately behavior. For example, ventromedial (VPvm) and dorsolateral (VPdl) VP subregions receive projections from nucleus accumbens shell and core, respectively. Inhibitory GABAergic neurons of the VPvm project to mediodorsal thalamus, lateral hypothalamus, and ventral tegmental area, and this VP subregion helps discriminate the appropriate conditions to acquire natural rewards or drugs of abuse, consume preferred foods, and perform working memory tasks. GABAergic neurons of the VPdl project to subthalamic nucleus and substantia nigra pars reticulata, and this VP subregion is modulated by, and is necessary for, drug-seeking behavior. Additional circuits arise from nonGABAergic neuronal phenotypes that are likely to excite rather than inhibit their targets. These subregional and neuronal phenotypic circuits place the VP in a unique position to process motivationally-relevant stimuli and coherent adaptive behaviors.
Keywords: limbic system, striatopallidum, nucleus accumbens, ventral tegmental area, GABA, dopamine, glutamate, opiate, motivation, reward, addiction, depression
1.0. Introduction
More than four decades ago, the ventral pallidum (VP) was delineated from the subcommissural part of the substantia innominata by Heimer and colleagues (Heimer, 1972; Heimer and Wilson, 1975; Switzer et al., 1982; Heimer et al., 1982). In early discussions, Mogenson et al., (1980) proposed that the VP integrated limbic/emotionally salient signals from the nucleus accumbens (Acb) to brain motor systems. Swerdlow and Koob (1987) furthered this hypothesis with studies showing how the Acb to VP projection links the mesoaccumbal dopamine system to motor circuitry. At the time, dopamine was already well-known to be involved in reward-motivated behavior (Wise, 1980). Soon after, it was revealed that the VP is innervated by dopamine inputs from the midbrain and that dopamine directly alters VP neuronal firing (Napier and Potter, 1989). As early as 1991, Napier and colleagues (1991a) put forth the concept that in addition to integrating various inputs from Acb, the VP incorporates reward-related signals carried by midbrain dopaminergic neurons. This concept was quickly expanded to encompass the idea that dopamine transmission within the VP regulates a collection of behaviors, including locomotion and cognition (Napier 1992c). Building on the role of VP dopamine, and Mogenson's original concepts involving the VP in brain circuits that direct “motivation to action” (Mogenson et al., 1980), it was subsequently proposed that the VP forms part of a “final common pathway” for drug-seeking behavior (Kalivas and Volkow, 2005) and for reward processing in general (Smith et al., 2009). These concepts served as modern-day assessments of the ventral striatopallidal system. As our understanding of this system has grown, the importance of subregional circuits involving the ventromedial VP (VPvm) and dorsolateral VP (VPdl) with the Acb shell (AcbSh) and Acb core (AcbC) has become apparent. Furthermore, although considered a largely inhibitory structure, a substantial proportion of neurons residing in VP express vesicular glutamate transporter 2 (VGluT2) mRNA (Hur and Zaborszky, 2005), indicating subpopulations of VP neurons have the capacity for glutamatergic neurotransmission. In addition, the cholinergic neurons residing within VP receive GABAergic input from the Acb (Zaborszky and Cullinan, 1992), make local connections within VP as well as extrinsic projections to the prefrontal cortex and the basolateral amygdala. Therefore, the goal of this review is to provide a new conceptual framework for the VP that incorporates current understanding of its subregional afferents, efferents and neuronal function and the roles for its subregions and neuronal phenotypes in behavior.
We put forth that the contribution of VP towards a variety of motivated behaviors is dependent upon the participation of GABAergic neurons belonging to individual VP subregions, as well as from nonGABAergic neurons, which affect discrete neuronal circuits. GABAergic VPvm neurons, with AcbSh afferents and thalamocortical, dopaminergic, and hypothalamic targets, are involved in discriminating the stimulus conditions of reward/drug acquisition, consumption, and working memory. NonGABAergic VP neurons, with dopaminergic and cortical targets, provide excitatory signals that likely oppose the VPvm-mediated signals. GABAergic VPdl neurons innervated by AcbC neurons and projecting to motor-related structures including subthalamic nucleus (STN) and substantia nigra pars reticulata (SNr), are involved in mediating reward motivated behavior (e.g., drug-seeking responses). These circuits adapt to repeated exposure to reward-related stimuli (e.g., repeated drug use), and these adaptations alter the integrative capacity of the VP which can lead to deficits in the output of motivation and reward. Thus, understanding the subregional neuroanatomy of the VP, and its related circuits, will broaden our understanding on the underpinnings of such behavioral dysfunctions.
2.0 Neuroanatomy
2.1. Boundaries of the ventral pallidum and its subregional compartmentation
Pallidal brain structures are linked to basal ganglia circuitries. In the basal ganglia, pallidal structures include the globus pallidus (GP), the rodent homolog of the external pallidal segment in higher species, and the entopeduncular nucleus (EPN), the rodent homolog of the internal pallidal segment. The VP occupies the rostral, subcommissural part of the area historically known as the substantia innominata, a major component of the ventral striatopallidal system that is ventral to the anterior commissure, and together with the ventral striatum belongs to the ventral striatopallidal system (Heimer, 1972; Heimer and Wilson, 1975; Haber et al. 1983; Heimer et al. 1997).
Outside of the VP, groups of cells and fibers in the caudal (sublenticular) substantia innominata that bridge the centromedial amygdala to the bed nucleus of stria terminalis were named the ‘extended amygdala’ (Alheid, 2003; de Olmos and Heimer, 1999). The more or less continuous collection of large, corticopetal neurons, consisting of primarily cholinergic and GABAergic neurons, stretching from the diagonal band area in the rostral forebrain to the level of the caudal part of the globus pallidus, is called the basal nucleus of Meynert in the clinical literature (Zaborszky et al., 2008; 2012, 2015a,b). The neurons of the basal nucleus of Meynert (basal forebrain magnocellular complex) intermingle with neurons of the ventral striatopallidal system and the extended amygdala.
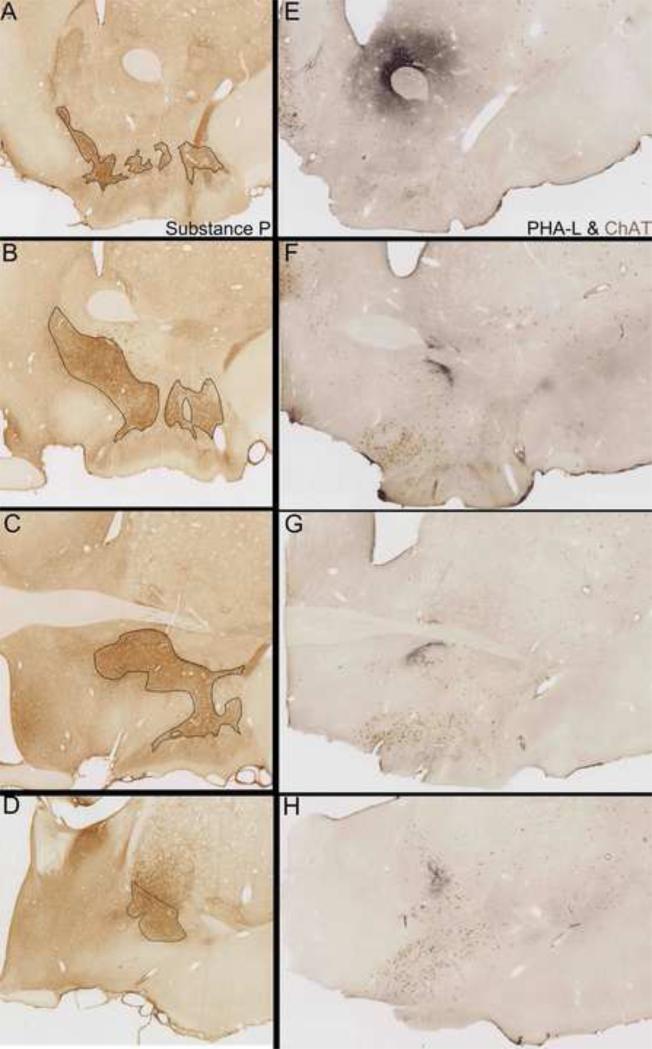
In rodents, the boundaries of the VP are defined by “wooly fiber”-like elements that originate from the Acb and express substance P-immunoreactivity (IR) and enkephalin-IR (Haber and Nauta 1983; Hill and Switzer, 1984; Groenewegen and Russchen, 1984; Zahm and Heimer, 1990; Heimer et al., 1991, 1997; see Figure 1A-D for four anteroposterior planes of VP). Substance P-IR is more strongly expressed and selective for VP than enkephalin-IR because enkephalin-IR is also observed in neighboring structures, such as bed nucleus of the stria terminalis (Haber and Nauta, 1983). Unfortunately, current brain atlases have not utilized these markers to delineate the VP boundaries.
Figure 1. Delineation of the ventral pallidum and topographic input from nucleus accumbens core to the dorsolateral ventral pallidum subregion.
A-D. Four anteroposterior planes of the VP, defined by the presence of substance P-IR (black outline). A. Plane of the “finger-like” rostral VP subregion. B-D. Planes of the VP that contain the ventromedial, dorsolateral, and ventrolateral VP subregions (shown in Figure 2). In the caudal extreme of the VP (D), substance P-IR is also observed in the more dorsally located globus pallidus (D). E-H. Four anteroposterior planes showing labeling of the anterograde tracer phaseolus vulgaris leucoagglutinin (black label) at the injection site within the AcbC (E) and efferent fibers within the dorsolateral VP (F-H). Note labeled cells at the injection site are concentrated in the heavily stained area, slight enhancement of background labeling due to local edema. Labeling in panels G-H are from a slightly medially-shifted AcbC injection compared to labeling from case in panels E-F. Brown labeling indicates neurons immunolabeled for choline acetyl transferase (ChAT; a marker for cholinergic elements). Material from Dr. Zaborszky.
The boundaries of the VP in the primate are more difficult to delineate. We follow the same convention used for the rodent, as described by Haber and colleagues (1990). Accordingly, the primate VP is a crescent-shaped structure ventral to the anterior commissure expressing both enkephalin-IR and substance P-IR wooly fibers. The VP in primates has common features of both the external and internal segments of the globus pallidus; the external, enkephalin rich component of the VP lies ventral and adjacent to the anterior commissure. The internal, substance P component of the ventral pallidum, lies as a ventral and rostral extension of the internal segment of the globus pallidus, often interdigitating with finger-like processes of the ventral striatum. Delineation of primate VP also has come from tracing studies (Hreib et al., 1988; Russchen et al., 1985; Haber et al., 1990), which are consistent with the rodent (sections 3.0 and 4.0). For the remainder of the review, we will refer to studies within the rodent VP unless explicitly stated otherwise.
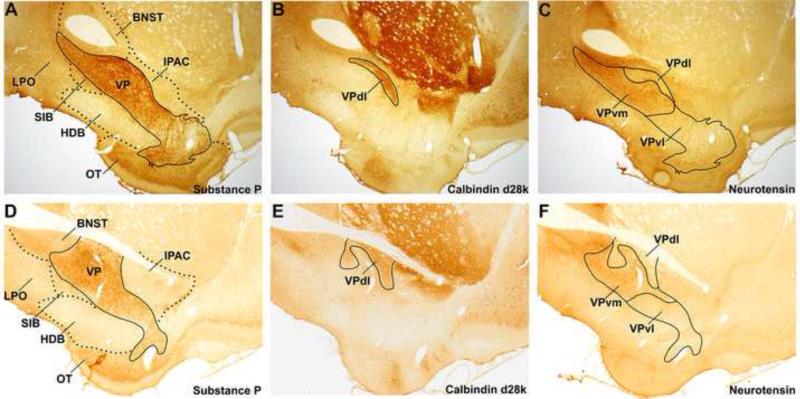
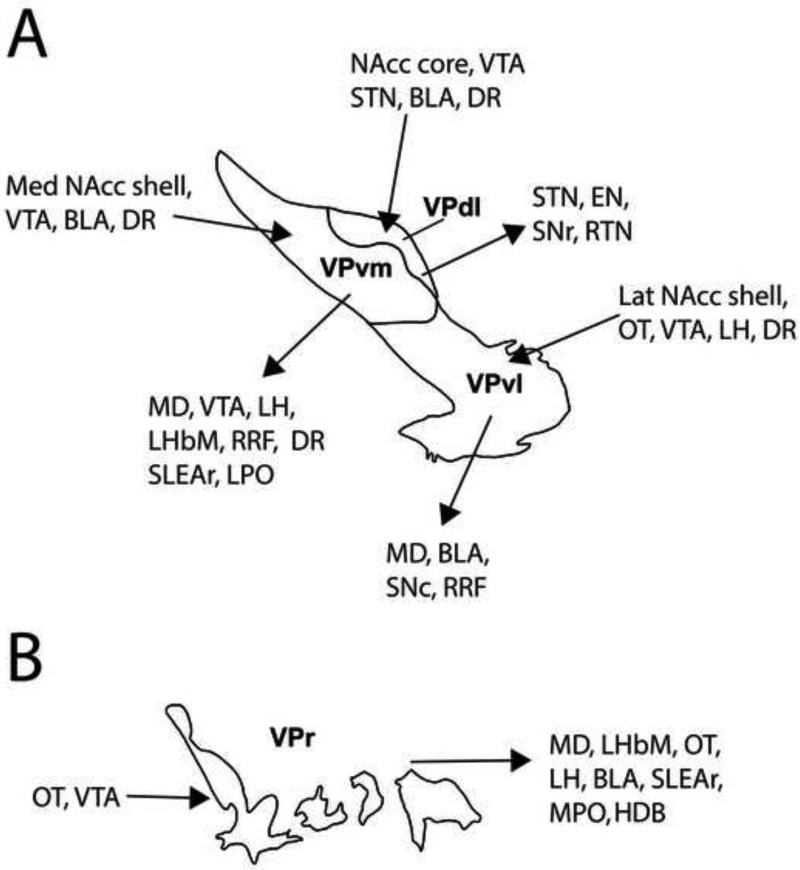
Several “neurochemically distinct” subregions of the VP have been delineated, all of which exhibit substance P-IR and enkephalin-IR wooly fibers (Figure 2A,D). The largest VP subregion, VPvm, receives projections from AcbSh, and exhibits fibers with neurotensin-IR but not fibers with calbindin-d28k-IR (Figure 2B-C, E-F; Zahm and Heimer, 1988, 1990; Zahm 1989; Zahm et al., 1996; Geisler and Zahm, 2006a). Conversely, the crescent-shaped VPdl receives projections from AcbC (Figure 1E-H) and exhibits fibers with calbindin-d28k but not fibers with neurotensin-IR (Figure 2B-C, E-F; Zahm et al., 1996; Riedel et al., 2002; Tripathi et al., 2010, 2013). The ventrolateral VP subregion (VPvl), exhibits little to no neurotensin-IR or calbindin-d28k-IR (2B-C, E-F). The rostral VP subregion (VPr, following the convention of Mengual and colleagues (Tripathi et al., 2010, 2013), best appreciated in sagittal sections, is described as finger-like extensions dorsal to the olfactory tubercle and ventral to Acb that lack neurotensin-IR and calbindin d28k-IR (Figure 1A; Heimer, 1978; Haber and Nauta, 1983; Zaborszky et al., 1986; Tripathi et al., 2010, 2013). The afferent/efferent connections of the VP subregions will be further delineated in sections 3.0 and 4.0.
Figure 2. Subregions of the ventral pallidum.
A,D. Two anteroposterior levels of the VP, defined by the presence of substance P-IR, at approximately +0.36 mm (A) and −0.12 mm (D). B-C. Sections proximal to tissue in panel D showing calbindin d28k-IR (B) and neurotensin-IR (C). E-F. Sections proximal to tissue in panel D showing calbindin d28k-IR (E) and neurotensin-IR (F). The VPdl subregion exhibits fibers with calbindin-d28k-IR but not neurotensin-IR. The VPvm subregion exhibits fibers with neurotensin-IR but not calbindin-d28k-IR, and the VPr and VPvl subregions do not express calbindin-d28k-IR or neurotensin-IR. This compartmentation of VP is observed across the anteroposterior extent of VP, except in the VPr (Zahm and Heimer, 1988, 1990; Zahm 1989; Zahm et al., 1996; Riedel et al., 2002; Tripathi et al., 2010, 2013). All sections are 30 μm thick. Neighboring locations to the VP are demarcated by dotted lines. Material from Dr. Root (Morales laboratory, NIDA).
2.2. Neuronal morphology, phenotypes, and functional subpopulations
VP neurons typically exhibit oval-, fusiform-, or triangle-shaped somata measuring 15-30 μm in diameter (Young 1984; Záborszky et al., 1986; Pang et al., 1998) with two to four thick, long, sparsely ramified smooth dendrites emerging from the cell body, covered by axon terminals (Heimer and Wilson, 1975; Young 1984; Zahm et al., 1985; Záborszky et al., 1986). A subset of VP neurons rostral to the crossing of the anterior commissure (both VPvm and VPdl) exhibits spiny dendrites (Kupchik and Kalivas, 2013).
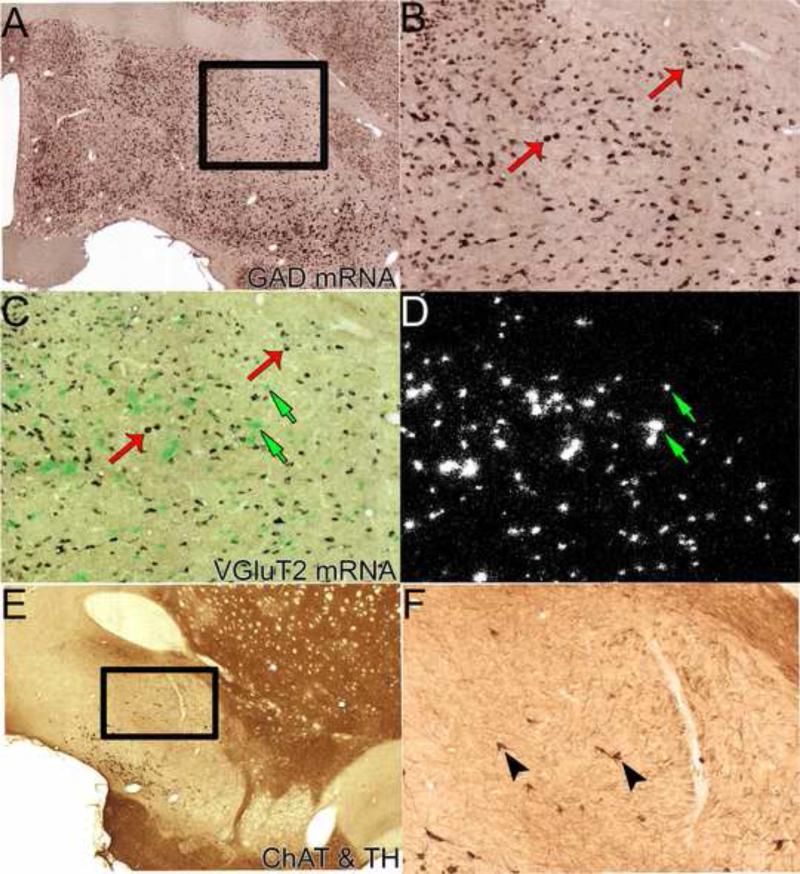
VP GABAergic neurons that express GAD65 and/or GAD67 mRNA are the major neuronal population in every VP subregion (Figure 3A-B). VP neurons also express calretinin, calbindin, parvalbumin, neuropeptide Y, or somatostatin (Zaborszky et al., 2012), although it remains for future studies to identify their colocalization with GAD or VGluT2. GABAergic neurons are covered extensively by GABAergic boutons, mostly from the Acb or local connections (Zahm et al., 1985, Figure 4). GABAergic neurons in the VP receive both GABAergic and nonGABAergic input from ventral striatal areas (Zaborszky and Heimer, 1986). Within GAD-IR terminals, symmetrical synapses are established on both perikarya and dendrites, typical of inhibitory neurotransmission.
Figure 3. Neuronal phenotypes of the ventral pallidum.
A-B. GABAergic neurons. Double in situ hybridization; the purple labeled neurons display digoxigenin-labeling for GAD 65 and GAD 67 mRNAs. Box in A showing VP regions displayed in B, C, and D. B. Higher resolution photograph of GAD mRNA neurons. Note abundance of GABAergic neurons, two examples shown by red thin arrows. C-D. Glutamatergic neurons. The same section as B further processed with radioactive in situ hybridization for VGluT2 mRNA under brightfield (C) and darkfield (D) illumination. Clusters of green grains (C) or white grains (D) indicate VGluT2 mRNA neurons. Note abundance of glutamatergic neurons, uniquely localized within VPvm. Examples of VGluT2-expressing neurons indicated by green small arrows. E-F. Immunohistochemistry for tyrosine hydroxylase (TH; a marker for noradrenergic/dopaminergic elements) and choline acetyl transferase (ChAT; a marker for cholinergic elements). Boxed in region in the low power photomicrograph (E) is the region shown in the high power photomicrograph (F). The VP is delineated by fewer TH-fibers than neighboring structures (i.e., bed nucleus of the stria terminalis, interstitial nucleus of the anterior commissure, striatum, and tubercle). ChAT-labeled soma (brown diaminobenzadine reaction); two examples of cholinergic neurons are indicated by black arrow heads. Material from Dr. Root (Morales laboratory, NIDA).
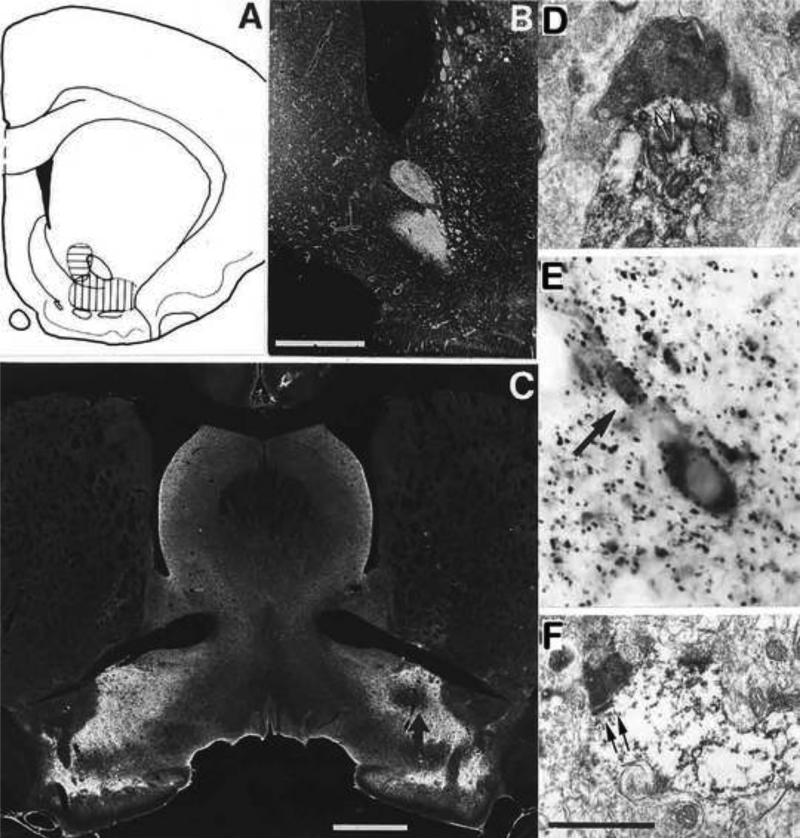
Figure 4. Accumbens neurons establish inhibitory synapses onto VP GABA neurons.
A-C. Accumbal to ventral pallidal projections determined by lesion studies. A. Electrolytic lesion locations in the AcbC (horizontal hatching) and AcbSh (vertical hatching). B. Degenerating terminals in VP after AcbC lesion using a silver-impregnation method (Gallyas et al., 1980). C. Loss of GAD-IR (arrow) in VP after lesion of the AcbSh. D-F. Electron microscopy evidence of a GABAergic AcbSh and AcbC projection to GABAergic VP neurons. D. A large degenerating bouton establishing a symmetric synapse with a GAD-positive dendrite in the VP after lesion of the AcbC. E. A GABAergic cell and dendrite ensheathed by GAD-expressing terminals in the VP. F. Small degenerating bouton contacting a GAD-expressing dendrite after lesion of the AcbSh. Arrows in D and F point to the postsynaptic membrane. Scale bars in B,C: 1 mm, F: 1 μm (also refers to D). Material from Dr. Zaborszky.
The vesicular transporters for glutamate are differentially expressed by VP glutamatergic neurons. Most VP glutamatergic neurons express VGluT2 mRNA (Figure 3C-D; Hur and Zaborszky, 2005; Geisler et al., 2005, 2007), while a small population expresses VGluT3 mRNA (Poulin et al., 2006), and none express VGluT1 mRNA (Hur et al., 2009). The number, electrophysiological properties and morphological characteristics of VP glutamatergic neurons are unknown, although some of the VGluT3-expressing cells are cholinergic (Poulin et al., 2006). While not empirically determined, it appears that the VPvm is dense in VGluT2-expressing neurons, while VPdl and VPvl subregions contain only few such cells (Hur and Zaborszky, 2005).
Cholinergic neurons residing within VP typically have large (~30 μm in diameter) multipolar somata with four to seven thick tapering dendrites (Bengtson and Osborne, 1999) (Figure 3E-F). Axons of cholinergic neurons give rise to abundant local collaterals (Duque et al., 2007) and project to the cerebral cortex and the amygdala (Groenewegen and Russchen 1984; Carlsen et al., 1985: Záborszky et al., 1986, 1992, 2005, 2012). Cholinergic neurons are found in each VP subregion, but most are within VPvm, and their total numbers are small (Gritti et al., 1993; Záborszky et al., 1999). Cholinergic neurons residing in the VP receive dopaminergic, noradrenergic, adrenergic, GABAergic, and glutamatergic inputs (for review, see Zaborszky et al., 2012) and receive topographically organized input from the Acb (Záborszky and Cullinan, 1992). Outputs from cholinergic neurons residing in the VP project to the prefrontal cortex (Gritti et al., 1997, 1999; Zaborszky et al., 2012; Zaborszky, unpublished observations), the basolateral amygdala (Carlsen et al., 1985), and also establish local synapses onto VP GABAergic neurons (Záborszky et al., 1986). In light of recent studies showing that dorsal pallidal cholinergic cells are integrated in basal ganglia circuitry (Saunders et al., 2015a,b), it will be important to conduct similar cell-type specific studies to determine if this is the case with VP cholinergic neurons as well.
Numerous attempts have been made to sort VP neurons electrophysiologically into different categories based on action potential characteristics, responses to pharmacological agents, anatomical location and phenotypes. Emerging evidence points to functionally relevant neuronal subpopulations within the VP. Whether recording from awake behaving rodent or nonhuman primate preparations, in vivo anesthetized preparations, or in vitro preparations in rodents (Mitchell et al., 1987; Wilson and Rolls, 1990; Yang and Mogenson, 1989; Napier and Potter, 1989; Napier et al., 1991a,b; Chrobak and Napier, 1993; Lavin and Grace, 1996; Pang et al., 1998; Turner, Mignon, Napier, 2002; Heidenreich et al., 2004; Tindell et al., 2005; Root et al., 2010, 2013; Avila and Lin, 2014a), it has long been known that VP neurons exhibit great variability in their basal firing rates and spiking patterns. While firing rate or pattern do not tightly co-vary with particular action potential characteristics (e.g., Turner et al., 2002), correlations are reported for electrophysiological characteristics that are subthreshold to spiking, as well as to morphology and transmitter phenotypes (Lavin and Grace, 1996; Pang et al., 1998; Kupchik and Kalivas, 2013). For example, “Type l” neurons of Pang and colleagues (Pang et al., 1988) and “Type B” neurons of Lavin and Grace (1996), both recorded in vivo, approximated the noncholinergic neuron characteristics obtained during in vitro recordings (Bengtson and Osborne, 2000). Type I neurons exhibit few or no axon branches near the soma suggesting these cells were projection neurons (Pang et al., 1998). Type B neurons of Lavin and Grace (1996) encompassed 27% of the recorded cells and these exhibited a ramp-like depolarization preceding spike discharges with prominent afterhyperpolarizations in a 1.3 ms waveform. “Type II” neurons described by Pang et al. (1998) approximate the characteristics of “Type A” VP neurons described by Lavín and Grace (1996). Type A neurons were the most common cell (53%) in the latter study, and these neurons exhibited no afterhyperpolarizations in their long duration (2.8 ms) waveforms. Type II neurons of Pang et al. (1998) were determined to be noncholinergic, and these neurons exhibited extensive axonal arborizations that did not extend past their dendritic arbor, suggesting they were interneurons, though extra-VP termination cannot be ruled out. In either case, the results of Pang and colleagues (1998) indicate that Type II neurons, and not type I neurons, affect local VP processing.
Using awake behaving recordings and analyzing firing patterns across discrete behavioral events from neurons in several basal forebrain regions (e.g., substantia innominata, medial and lateral parts of the horizontal diagonal band, ventral globus pallidus, caudal VP, etc), Avila and Lin (2014a) observed three general types of neurons that also differed in electrophysiological characteristics (e.g., baseline firing rate, inter-spike intervals, waveform complexity). Most neurons were categorized as Type I (46%), which belong to a group of motivational salience-encoding neurons observed throughout all basal forebrain regions (Lin and Nicolelis, 2008; Avila and Lin, 2014b). The Type I neurons of Avila and Lin (2014a) are highly sensitive to cues predicting the start of a reward trial as well as conditioned stimuli and the reward itself, especially when predicted rewards are comparatively robust. The motivational salience signaling of Type I neurons is correlated with faster decision speed (Avila and Lin, 2014b) and a short latency frontal cortex potential (Nguyen and Lin, 2014). Together, Type I neurons were interpreted as non-cholinergic corticopetal basal forebrain neurons (Avila and Lin, 2014a). In contrast, Type II (14%) and type III (16%) neurons do not change firing rates following cue-presentation but are modulated during discrete behaviors involved in obtaining rewards (e.g., fixation as a response requirement to obtain rewards, behaviors related to approaching the reward (fixation port exit, reward port entry), and consumption (onset of licking)). The Type II and Type III neurons of Avila and Lin (2014a) are separated by their increasing or decreasing firing rate changes during fixation and movement events and were interpreted as belonging to the VP.
Using in vitro slice methods, Kupchik and Kalivas (2013) reported a neuronal subtype located in VP subfields rostral to the crossing of the anterior commissure that exhibits electrophysiological characteristics similar to ventral striatal and extended amygdala neurons. This neuronal subtype exhibits a more hyperpolarized membrane potential with no spontaneous action potentials, and is comparatively more sensitive to glutamatergic influences than other VP neurons. The richness of the morphological and functional characteristics of VP neurons point to heterogeneity of the structure and to the diversity of processes that these neurons likely integrate. These concepts are explored in subsequent sections of this review.
3.0. Afferent inputs and changes in firing rates induced by these inputs
In the following subsections we review the afferent connections to the VP subregions and responsiveness of VP neurons to these inputs (Figure 5). While most of the afferent (and efferent) projection patterns of VP subregions are well delineated, few studies have considered whether or not neurons belonging to distinct VP subregions exhibit differential sensitivity to various afferent-associated transmitters. As such evaluations are critical to understanding the functional circuits in which the VP participates, these studies are highlighted.
Figure 5. General overview of afferents and efferents of the ventral pallidum.
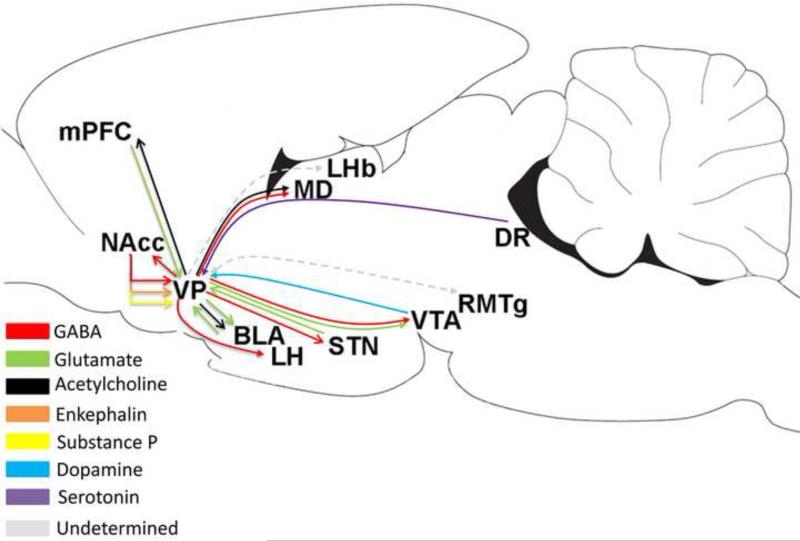
Nonsubregional illustration of the major transmitter phenotype and associated brain structures of the projections. Supporting literature includes: VP/mPFC (medial prefrontal cortex) - Carlsen et al. 1985; Hur and Zaborszky, 2005. VP/Acb Haber and Nauta, 1983; Zahm et al. 1985; Churchill et al. 1990; Kalivas et al. 1993; Groenewegen and Russchen 1984; Chrobak and Napier, 1993; Napier et al. 1995. VP/BLA - Fuller et al. 1987; Carlsen et al. 1985; Poulin et al. 2006; Maslowski-Cobuzzi and Napier, 1994; Mitrovic and Napier, 1998. VP/STN - Bevan et al. 1997; Ricardo et al. 1980; Turner et al., 2001, 2008. VP/LH - Bevan et al. 1997. VP/DR - Semba et al. 1988. VP/VTA – Maslowski-Cobuzzi and Napier, 1994; Mitrovic and Napier, 2002; Klitenick et al. 1992; Geisler et al. 2005, 2007; Kalivas et al. 1993. VP/RMTg - Jhou et al. 2009; Taylor et al. 2014. VP/LHb - Groenwegen et al. 1993. VP/RTN - Young et al. 1984; O'Donnell et al. 1997. VP/MD - Young et al. 1984; Mariotti et al. 2001; MD/mPFC Pirot et al. 1994. The VP projection to LHb and RMTg projection to VP have not explicitly tested a GABAergic phenotype. Sagital outline modified from Paxinos and Watson (2007).
3.1.1. Inputs from the nucleus accumbens: GABA
The largest input to VP is from the Acb. This input has been detailed by lesion degeneration (Williams et al., 1977; Haber and Nauta, 1983; Zahm and Heimer, 1987), anterograde and retrograde tracer methods (Swanson and Cowan 1975; Powell and Leman 1976; Conrad and Pfaff 1976a; Williams et al., 1977; Troiano and Siegel 1978a; Nauta et al., 1978; Mogenson et al., 1983; Haber and Nauta, 1983; Groenewegen and Russchen 1984; Lu et al., 1988; Churchill et al., 1990; Maurice et al., 1997, 1998; Zahm and Heimer 1990; Heimer et al., 1991; Záborszky and Cullinan 1992; Usuda et al., 1998) and with electrophysiological approaches (Chrobak and Napier, 1993). The Acb projection typically exits the Acb caudally (Tripathi et al., 2010) via the medial forebrain bundle (Conrad and Pfaff 1976a; Troiano and Siegel, 1978a) but on occasion can extend rostrally before hooking caudally towards VP (Chang and Kitai, 1985). Surprisingly, definitive evidence that Acb neurons establish synapses onto GABAergic VP neurons is lacking in the literature. Using lesion degeneration and immunoelectron microscopy, we reveal here that AcbSh and AcbC neurons establish symmetric synapses (characteristic of inhibitory GABAergic neurotransmission) onto GAD-IR VP neuron dendrites (Figure 4).
Acb projections to VP are topographically organized (Figure 6). The medial AcbSh projection is contained within the neurotensin-immunoreactive VPvm subregion (Zahm and Heimer, 1988; Zahm, 1989; Zahm and Heimer, 1990; Heimer et al., 1991; Zahm and Brog, 1992). Neurotensin-IR within the VPvm depends on the integrity of the Acb, as lesions of this structure reduce neurotensin-IR in VPvm (Geisler and Zahm, 2006a). Anterograde tracers injected in AcbSh produce an abundance of labeled terminals within the VPvm, which continue as a broad column through the rostrocaudal extent of VPvm and into the sublenticular regions beyond VP, such as extended amygdala and lateral hypothalamus (LH) (Zahm and Heimer 1990; Heimer et al., 1991, 1997). The lateral AcbSh as well as the lateral olfactory tubercle innervates the VPvl, which is devoid of neurotensin-IR (Heimer et al., 1987; Heimer et al., 1991; Groenewegen et al., 1993). Consistent with the innervation topography of neurotensin-containing inputs to the VPvm, but not VPvl, neurotensin reduces VP firing rates in two-thirds of VPvm neurons, but has no effect on VPvl neurons (Michaud et al., 2000). The AcbC projection is contained within the calbindin-d28k- immunopositive VPdl subregion (Zahm and Brog 1992; Zahm et al., 1996; Tripathi et al., 2010). These anatomical observations are supported by functional characterizations of the AcbC to VP projection topography, wherein electrical activation of the AcbC evoked short-latency responses consistent with monosynaptic inputs in 74% of the accumbal-sensitive neurons within the VPdl, but only 43% of responding neurons recorded from the VPvm (Chrobak and Napier, 1993). Single-axon tracings have shown that a minority of core neurons that project to the VPdl collateralize within the lateral VPvm (Tripathi et al., 2010), suggesting that the accumbal-sensitive neurons in lateral parts of the VPvm received axon collaterals from core neurons that targeted VPdl. Finally, a sparse Acb projection, but large projection from the olfactory tubercle, terminates within the “finger-like” VPr (Zahm and Heimer, 1987; Tripathi et al., 2010).
Figure 6. Subregional afferent and efferent connections of the ventral pallidum.
A. Known afferent and efferent projections of the VPvm, VPdl, and VPvl subregions. Subregions are illustrated to represent projections from any anteroposterior location within these VP subregions. Anatomical studies have also demonstrated that the BLA projects to, and receives projections from, cholinergic neurons that reside in all VP subregions (Gritti et al., 1993; Poulin et al., 2006; Mascagni and McDonald, 2009; Záborszky et al., 1986, 1999, 2012), but electrophysiological studies consistent with monosynaptic afferents suggest a wider influence on VP neuronal populations (Maslowski-Cobuzzi and Napier, 1994; Mitrovic and Napier, 1998). Glutamatergic neuron distribution for VP subregions has yet to be validated, but appear to be located largely within the VPvm, and thus far have been shown to project to the VTA (Geisler et al. 2007). VTA and DR appear to project to all VP subregions (Maslowski-Cobuzzi and Napier, 1994; Mitrovic and Napier, 2002; Klitenick et al. 1992; Semba et al. 1988). B. Known afferent and efferent projections of the VPr subregion.
Early anatomical and functional studies on Acb to VP projections documented the involvement of GABA in these inputs (Walass and Fonnum, 1979; Jones and Mogenson, 1980; Zaborszky et al., 1986; Chrobak and Napier, 1993). The number of GABAergic synapses on VP neurons has been estimated to be greater than 80% (Zahm et al., 1985), most likely reflecting Acb and local GABAergic connections. Neurochemical studies have shown that the VP contains high concentrations of extracellular GABA (Bourdelais and Kalivas, 1990, 1992; Xi and Stein, 2000; Lawrence et al., 2003; Tang et al. 2005; Li et al., 2009; Wydra et al., 2013) and intense immunoreactivity for the GABA synthesizing enzyme, GAD (Oertel et al., 1984; Mitrovic et al., 1999). AcbSh and AcbC projections synapse onto both GABAergic and cholinergic VP cells (Grove et al., 1986; Záborszky et al., 1991; Záborszky and Cullinan, 1992). Cholinergic neurons receive prominent GABAergic inputs to their cell bodies and proximal dendrites (Zaborszky, 1989), and those from the Acb establish symmetric synapses, characteristic for inhibitory terminals (Zaborszky and Cullinan, 1992).
VP exhibits intense immunoreactivity for ionotropic GABA-A receptors (Zilles et al., 1991; Henderson, 1995; Hartig et al., 1995) and low immunoreactivity for metabotropic GABA-B receptors (Margeta-Mitrovic et al., 1999). Consistent with high expression levels of ionotropic GABAergic receptors, early studies in anesthetized rats verified that local applications of GABA dramatically decrease firing in nearly all neurons tested (e.g., Jones and Mogenson, 1980; Lamour et al., 1986; Napier et al., 1991b; Chrobak and Napier, 1993). Local application of the GABA-A receptor antagonist bicuculline increases firing in most tested VP neurons (Yang and Mogenson, 1985; Chrobak and Napier, 1993; Turner et al., 2001) presumably by displacing endogenously released GABA. This tonic GABAergic inhibition involves Acb inputs, as intra-Acb infusions of the local anesthetic, procaine, can robustly increase VP firing (Napier 1992), and applications of GABA-A antagonists onto VP neurons nullify the suppression in firing rate that occurs with electrical stimulation of the Acb (Chrobak and Napier, 1993).
3.1.2. Inputs from the nucleus accumbens: GABA co-localized with peptides
GABAergic fibers within VP are often co-localized with enkephalin, dynorphin or substance P (Reiner and Anderson, 1990; Zahm, 1985, 1989). Enkephalin and GABA are typically observed in boutons that make symmetrical synapses on VP somata and proximal dendrites (Zahm et al., 1985; Bolam et al., 1986), indicative of a strong inhibitory transmission unto VP neurons.
Enkephalin and dynorphin are natural ligands of the mu and kappa opioid receptors. All three major types of opioid receptors are identified within the VP (Lahti et al., 1989; Moskowitz and Goodman, 1984; Pilapil et al., 1987), with mu receptors having the highest levels (Lahti et al., 1989; Moskowitz and Goodman, 1984). The functional pharmacology of VP responses to opioids is complex, and includes the modulation of several other VP transmitters (for review, see Napier and Mitrovic, 1999). Local in vivo application of agonists pharmacologically verified to be specific for their respective subtype have revealed that approximately 50-70% of VP neurons exhibit sensitivity to one type of opioid receptor agonist (Napier et al., 1992a; Chrobak and Napier, 1993; Mitrovic and Napier, 1996; Johnson and Napier 1997; Mitrovic and Napier, 1998). In a study that directly compared mu, kappa and delta opioid agonists (Mitrovic and Napier, 1995), agonists for mu and kappa receptors predominantly decreased VP firing (52% and 41% of tested neurons, respectively) while delta receptor agonists had slightly more decreases than increases (24% vs. 13%). As a significant portion of VP neurons did not respond to any opioid agonist (e.g., 61 out of 191 neurons tested, Mitrovic and Napier, 1995) these electrophysiological observations concur with anatomical observations by Zahm et al., (1985) that some VP neurons do not receive inputs from opioid-containing fibers.
VP neurons exhibit moderate levels of tachykinin receptors (Danks et al., 1986; Shults et al., 1984; Rothman et al., 1984), and it has been verified that cholinergic neurons that reside within VP are included in those VP cells that express substance P receptors (Chen et al., 2001). Substance P or the metabolically stable substance P analog, DiMeC7 (pGlu5,MePhe8,MeGly9)-substance P5) increases firing of approximately 40-50% of tested VP neurons (Mitrovic and Napier, 1996, 1998; Napier et al., 1995) and substance P antagonists block increases in firing rate induced by Acb stimulation (Mitrovic and Napier, 1998). Suggesting that the cholinergic neurons residing within VP may be engaged, cultured cholinergic neurons from the basal forebrain show significant depolarization and spike facilitation to bath applied substance P, and these effects are related to the ability of the tachykinin to suppress inwardly rectifying potassium channels (for review, see Nakajima et al., 1991).
3.1.3. Inputs from the nucleus accumbens: Integration of firing
A wealth of information regarding the functional consequences of activating Acb-VP pathways, and how the major transmitter systems interact at the level of postsynaptic VP neurons, was provided by early electrophysiological studies. Electrical stimulation of the rat Acb can evoke short latency (2-6 ms) inhibition of VP spiking (Chrobak and Napier, 1993; Mitrovic and Napier, 1998), which likely reflects endogenously released amino acids acting on pallidal ionotropic receptors. However, accumbal-evoked VP responding often exhibits comparatively longer latency (>7 ms) (Mogenson, Swanson, Wu, 1983; Chrobak and Napier, 1993; Lavín and Grace, 1996; Mitrovic and Napier, 1998), indicative of metabotropic receptor activation and/or integration of substance P, enkephalin, and GABA influences. Indeed, most in vivo studies show that VP neuronal firing exhibits both increases and decreases in response to Acb stimulation (Mogenson, Swanson and Wu, 1983; Chrobak and Napier, 1993; Mitrovic and Napier, 1998) (but see Lavín and Grace (1996)). Moreover, Acb evoked VP responses are antagonized by microiontophoretically applied antagonists of GABA-A, opioid (Chrobak and Napier, 1993) and substance P (Mitrovic and Napier, 1998) receptors.
The concept that VP neurons can integrate various accumbal influences fits with anatomical and electrophysiological descriptions of the Acb to VP projections (Groenewegen and Russchen, 1984; Bolam et al., 1986; Heimer et al. 1991; Zahm and Heimer, 1990, 1993; Záborszky and Cullinan 1992; Chrobak and Napier, 1993; Napier et al., 1995; Mitrovic and Napier, 1996; Johnson and Napier 1997; Mitrovic and Napier, 1998; Pickel et al., 2012). One third of VP neurons sampled in vivo were observed to be sensitive to both the mu agonist DAMGO and substance P (Mitrovic and Napier, 1996). In these neurons, DAMGO antagonized substance P-evoked increases in firing and conversely substance P antagonized decreases in firing rates induced by DAMGO (Mitrovic and Napier, 1996). Local (microiontophoretic) application of another mu opioid agonist, morphine, was shown to reduce Acb-evoked VP inhibition (Chrobak and Napier, 1993) as well as the inhibitory effects of GABA on VP activity, and this latter effect occurred at local concentrations that were not sufficient to directly alter firing (Johnson and Napier, 1997) consistent with a modulatory role for mu receptor activation (for review, see Napier and Mitrovic, 1999).
Neurotransmitters released into the VP upon Acb activation also modulate input influences from non-Acb afferents. For example, the amygdala provides a glutamatergic input to the VP (described below), and in spite of the ability of substance P to increase spontaneous firing rates, the neuropeptide attenuates amygdala-evoked increases in VP firing rate, without altering firing rate increases caused by iontophoretically-applied glutamate (Mitrovic and Napier, 1998). This profile suggests that substance P acts presynaptically to reduce glutamate release, an effect that is bypassed by exogenous glutamate. In contrast, DAMGO potentiates VP firing increases induced by both amygdala stimulation and glutamate iontophoresis (Mitrovic and Napier, 1998), consistent with the idea that mu receptors can act both pre- and post-synaptically to modify the excitatory effects of glutamate. These functional studies concur with the anatomical observations that mu opioid receptors are located on both presynaptic and postsynaptic elements in the Acb-VP pathway (Olive et al., 1997). Similar functional analysis was used to show that mu opioid receptors can presynaptically regulate endogenous dopamine at the level of the VP (Mitrovic and Napier, 2002). Thus, both substance P and opioid neuropeptides released from Acb to VP projections are positioned to regulate the influences of several VP afferent systems, including Acb GABAergic, amygdala glutamatergic and midbrain dopaminergic inputs. Indeed, intra-VP activation of mu opioid receptors presynaptically reduces the release of VP dopamine subsequent to VTA activation (Mitrovic and Napier, 2002). This suggests that significant integration of a diversity of inputs occurs at the level of VP neurons, a concept that deviates from the classic basal ganglia model where VP is simply inhibited upon Acb activation (Alexander et al., 1986).
3.2. Dopaminergic inputs
Early predictions that the VP is an important dopaminoreceptive brain region (Napier et al., 1991a) are validated by numerous laboratories using a variety of techniques. The dopaminergic inputs are topographically oriented with the lateral VTA (parabrachial pigmented nucleus) projecting to VPr, VPvm, VPdl, and VPvl and the midline VTA projecting to medial parts of VP (Zahm and Heimer, 1988; Klitenick et al., 1992; Groenewegen et al., 1993; Del-Fava et al., 2007; Taylor et al., 2014). VP afferents arising from the substantia nigra (pars compacta or pars reticulata) are sparse (Beckstead et al., 1979; Prensa and Parent, 2001). Dopaminergic projections from the VTA or SN synapse onto multiple neuronal types in the VP including parvalbumin-immunoreactive, nonparvalbumin-immunoreactive, and cholinergic neurons (Zaborszky, 1989; Gaykema and Záborszky 1996, 1997a,b). Indirect evidence for dopaminergic regulation of forebrain cholinergic function is lent by the robust increase (>200%) in acetylcholine turnover (i.e., hemicholinium binding) seen in terminal regions for these cholinergic neurons (the prefrontal cortex and amygdala) in rats after 6-hydroxydopamine-induced lesions of the dopaminergic inputs (Muma et al., 2001).
The dopaminergic projection to VP is not dense (Beckstead et al., 1979; Klitenick et al., 1992); relatively few fibers in VP exhibit tyrosine hydroxylase-IR (Figure 3E; Seifert et al., 1998; Prensa and Parent, 2001) and the concentration of dopamine and its metabolites is low (Napier and Potter, 1989; Muma et al., 2001). Nonetheless, intra-VTA infusion of the glutamate receptor agonists NMDA and AMPA clearly increases extracellular dopamine levels in the VP (Kretschmer et al., 2000) and electrical activation of the VTA/medial SNc results in profound, dopamine-mediated effects on VP neuronal function (Maslowski-Cobuzzi and Napier 1994; Mitrovic and Napier, 2002). For example, stimulation of the VTA/medial SNc produces short latency changes in firing rate of almost 90% of recorded VP neurons, and of these, roughly 60% show decreases and 30% show increases in firing rate (Maslowski-Cobuzzi and Napier 1994; Mitrovic and Napier, 2002). This multifunctional response profile is recapitulated by locally (microiontophoretically) applied dopamine (Napier and Potter, 1989; Napier et al., 1991; Johnson and Napier 1997a; Mitrovic and Napier, 2002) and likely reflects both direct and indirect effects. Local application of dopamine antagonists attenuate the ability of VTA/medial SNc stimulation to alter VP neuronal firing, consistent with the conclusion that VTA/medial SNc activation releases dopamine from terminals within the VP (Maslowski-Cobuzzi and Napier 1994; Mitrovic and Napier, 2002).
D1, D2, and D3 receptors are localized within the VP (Contreras et al., 1987; Beckstead et al, 1988; Richfield et al., 1989; Mansour et al., 1990; Tziortzi et al., 2010) and application of agonists for these receptors directly onto VP neuronal elements is sufficient to alter VP firing (Napier and Maslowski-Cobuzzi, 1994). Systemic administration of the non-selective DA agonist, apomorphine alters VP firing by activating both D1 and D2 receptors (Maslowski and Napier, 1991a; Napier et al., 1991), and systemic administration of agonists with a preference for activating D1 receptors (Maslowski and Napier, 1991b; Heidenreich et al., 1995) or D2 receptors (Maslowski and Napier, 1991b) all alter VP firing in doses that also alter behavior in rats. Locally (microiontophoretically) applied DA agonists result in response profiles that differ from those observed with systemic administration of the drugs (e.g., Napier and Maslowski-Cobuzzi, 1994; Heidenreich et al., 2004 versus Napier and Maslowski 1991b; Heidenreich et al., 1995, respectively). These differences reflect the ability of systemically administered agonists to influence brain regions that subsequently input the recorded VP neurons, whereas microiontophoretically applied agonists only activate receptors within a restricted, local milieu of the recorded VP neurons.
The particular neuronal elements on which the various dopaminergic receptors are located are becoming apparent, and it is clear that both presynaptic and postsynaptic locales are involved. Ultrastructural immunocytochemical analysis shows that a few of the D2-receptor labeled axons in the VP contained tyrosine hydroxylase, suggesting only a small proportion of dopamine terminals exhibit D2 autoreceptors (Mengual and Pickel, 2002). This is consistent with in vivo microdialysis findings which show some, but minimal, D2 autoreceptor regulation of extracellular dopamine in the VP (Melendez et al., 2005). D2 autoreceptors are physiologically relevant as in vivo studies show that up to 43% of the VP neurons responding to VTA/medial SNc stimulation with a monosynaptic profile are antagonized by local applications of D2 receptor blockers (Maslowski and Napier, 1994). There is no evidence that dopaminergic neurons express D1 receptors; thus, it is unlikely that D1 receptors are located on dopaminergic terminals within the VP. Consistent with this assumption, the ability of D1 agonists to alter VP neuronal activity is not diminished by removal of endogenous dopamine (Heidenreich et al., 2004). Interestingly, however, intra-VP application of SCH-23390, a D1-like receptor antagonist, increases extracellular levels of dopamine up to approximately 600% of baseline, suggesting that D1 receptors in the VP are substantially involved in regulating extracellular dopamine (Melendez et al., 2005). D1 receptor mRNA has been found in the amygdala, a major glutamatergic input to the VP (Fremeau et al., 1991), and D1–mediated VP firing is under the control of amygdala inputs (Napier, 1992b), suggesting that the excitatory input to the VP from the amygdala may be regulated by tonic D1 receptor inhibition. Therefore, it is possible that intra-VP SCH-23390 may increase excitatory (potentially amygdaloid) transmission on dopaminergic terminals, which would enhance terminal dopamine release.
In situ hybridization studies demonstrate both D1 and D2 receptor mRNA in GABAergic projections from the Acb to the VP (Lu et al., 1997, 1998), and while these studies have not been verified with assessments of receptor protein, they do suggest that some dopaminergic receptors in the VP may be located on presynaptic GABAergic terminals. DA does modulate the inhibitory effects of GABA at the level of the VP (Johnson and Napier, 1997a). However, responding by VP neurons to D1 or D2 receptor-preferring agonists is maintained following pharmacologic inactivation of the Acb (Napier 1992b), functionally demonstrating the presence of some dopamine receptors that are downstream to Acb GABAergic inputs.
3.3. Glutamatergic inputs
VP receives comparatively less glutamatergic input than does the Acb; however, locally applied glutamate robustly increases firing of almost all recorded VP neurons (Napier et al., 1991, 1995; Mitrovic and Napier, 1998; McDaid et al., 2005, 2006). Glutamate can be consistently measured within the VP using in vivo microdialysis (Chapman and See, 1996; Kretschmer et al., 2000; Kemppainen et al., 2010). Although significant concentrations of extracellular glutamate are observed in VP dialysates, the bulk of the measured concentrations are insensitive to local application of potassium chloride (Kemppainen et al., 2010). This is consistent with glutamate microdialysis studies performed in the striatum, Acb, and PFC, each showing that basal extracellular glutamate is derived primarily from non-synaptic (glial) origins (Baker et al., 2002, 2003; Melendez et al., 2005).
Glutamate acts on ionotropic and metabotropic receptors. NMDA and AMPA receptors are ionotropic receptors and evaluations of mRNA, protein, and ligand binding reveal the presence of NMDA and AMPA receptors in the VP (Page and Everitt, 1995). AMPA receptors are hetero-oligomers, or tetramers, composed of GluR protein subunits 1-4. Immunoblotting and immunohistochemistry have determined that GluR1 and GluR2 subunits are located within the VP (Martin et al., 1993; Mickiewicz and Napier, 2011; Herrold et al., 2013). GluR1 is selectively expressed within the majority noncholinergic neurons of the VP (Martin et al., 1993). VP neurons are sensitive to NMDA or AMPA, and most (82%) are sensitive to both (Turner et al., 2001). Intra-VP infusions of low to moderate doses of NMDA (0.23 or 0.45 μg) increases Fos-like immunoreactivity within the VP as much as five fold (Turner et al., 2008). Metabotropic glutamate receptor subunit proteins are also observed within the VP (Shigemoto et al., 1992; Herrold et al., 2011, 2013). The neuronal consequences of activating these receptors have not yet been determined for the VP.
A major source of glutamatergic inputs to the VP is from the medial STN. These projections target the VPdl and appears to extend into the dorsolateral portions of VPvm (Ricardo et al., 1980; Groenewegen and Berendse 1990; Turner et al., 2001), primarily terminating on distal dendrites (Záborszky et al., 1991). Most VP firing rates are altered by STN stimulation, with 69% of neurons exhibiting excitation and 31% exhibiting inhibition (Turner et al., 2001). Since the excitatory latency is roughly half (5 ms) of the inhibitory latency (10.8 ms), the excitatory and inhibitory effects of STN stimulation likely arose from monosynaptic glutamate and polysynaptic lateral inhibition routes, respectively.
VP receives light projections from infralimbic but not prelimbic cortex (Sesack et al., 1989; Takagishi and Chiba 1991; Vertes, 2004). The cortical projection to VP is glutamatergic, typically synapsing onto dendritic spines or dendritic shafts of noncholinergic, parvalbumin-immunoreactive neurons (Záborszky et al., 1997; Gaykema and Zaborszky, 1997). Although direct cortical projections to VP are significantly less substantial than other afferents, these projections are sufficient to modulate VP activity. For example, medial prefrontal cortex lesions decrease VP firing rates and reduce the occurrence of particular subtypes of VP neuron by 70% (i.e., the electrophysiologically-classified Type B cells; Lavín and Grace, 1998). These outcomes may reflect a loss of direct excitatory prefrontal inputs; however, indirect effects from mPFC-Acb-VP or mPFC-amygdala-VP pathways cannot be excluded.
Anatomical evaluations show that the amygdala projects to cholinergic neurons that reside within the VP (Záborszky et al., 1984, 1986; Carlsen et al., 1985; Poulin et al., 2006). Functional evaluations of the amygdala-VP projection shows that short latency evoked responses (likely monosynaptic) are seen in 54-98% of recorded VP neurons (Maslowski-Cobuzzi and Napier 1994; Mitrovic and Napier, 1998), suggesting that other VP neuronal phenotypes are also innervated by the amygdala. Although the amygdala projection is predominantly glutamatergic (Fuller et al., 1987), amygdala stimulation produces both short latency inhibition and excitation in separate populations of VP neurons; with excitation observed more in the medial than lateral VP (Yim and Mogenson 1983; Maslowski-Cobuzzi and Napier 1994; Mitrovic and Napier, 1998). The short latency excitation is antagonized by local applications of glutamatergic ionotropic receptor antagonists (Mitrovic and Napier, 1998), modulated by dopamine from projections arising in the VTA/medial SNc (Napier 1992b; Maslowski-Cobuzzi and Napier, 1994), and modulated by substance P and opioid projections from Acb (Mitrovic and Napier, 1998). A GABAergic projection to the basal forebrain, including the VP, originating from somatostatin-containing GABAergic neurons of the amygdala has been described (McDonald et al., 2012), suggesting this separate population of amygdala neurons provides an inhibitory influence onto VP.
Recent reports describe ascending glutamatergic and GABAergic projections that arise within the VTA (Hnasko et al., 2012; Taylor et al. 2014; Root et al., 2014a,b). Glutamatergic VTA neurons have a heterogeneous molecular composition (Yamaguchi et al., 2011; Li et al., 2013; Morales and Root, 2014) and additional anatomical evaluations will be necessary to identify the glutamatergic VTA phenotypes innervating VP.
3.4. Serotonergic inputs
Serotonergic systems have long been viewed as playing a crucial role in forebrain function. VP exhibits serotonin transporter-IR (Sur et al., 1996) and the dorsal raphe (DR) projects to VP (Semba et al., 1988; Jones and Cuello, 1989; Vertes, 1991; Hermann et al., 1996). Post mortem VP tissue contains high concentrations of 5-HT and its metabolites (Napier and Potter, 1989), and 5-HT is detected in vivo using microdialysis in rats (Sizemore et al., 2000). Although the morphological location is not well-characterized, high densities of several 5-HT receptor subtypes have been detected in the VP (Appel et al., 1990; Waeber et al. 1996; Sari et al. 1999; Chen and Lawrence, 2000; Murrough et al. 2011). The few functional evaluations of VP 5-HT that have been conducted thus far point to complex and potentially phenotype–selective effects. Intravenous administration of 5-HT1A (but not 5-HT1B) agonists alters firing of two-thirds of the recorded VP neurons, with increases and decreases in activity equally observed (Heidenreich and Napier, 2000). Intravenous administration of a 5-HT2A/2C agonist alters firing in 92% of the recorded VP neurons, with rate increases occurring in 58% of the responding cells (Napier and Istre, 2008). An in vitro electrophysiological study of VP slices from neonatal rats revealed that 5-HT depolarizes noncholinergic neurons and hyperpolarizes cholinergic neurons (Bengtson et al., 2004). Future studies on the potential for specific receptor subtypes to be expressed in a neuronal phenotypic manner would shed important new light on the consequences of VP 5-HT transmission. Serotonin-containing axons apparently do not enter into synaptic connections with VP cholinergic neurons based on electron microscopic studies (Hajszan and Zaborszky, 2000).
3.5 Comparing ventral and dorsal striatopallidal systems: possible direct and indirect circuits
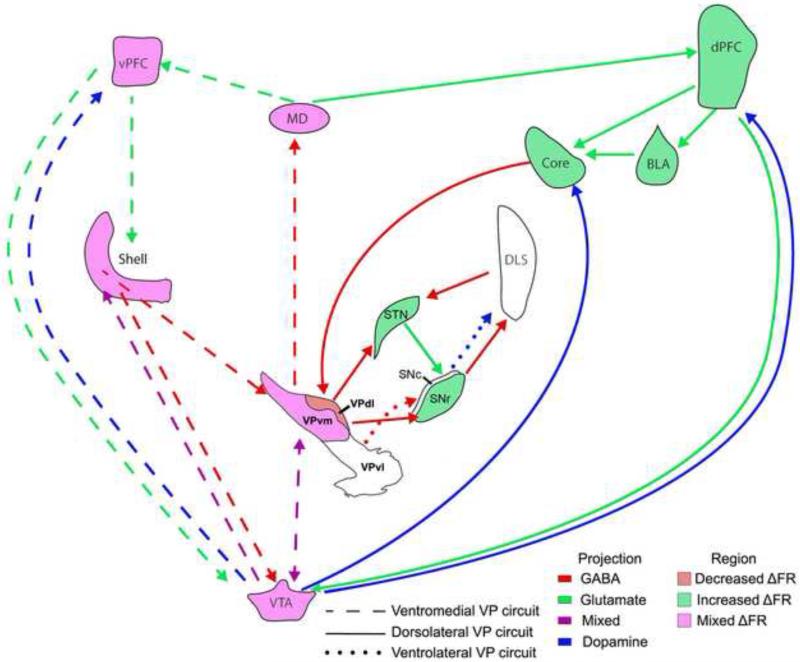
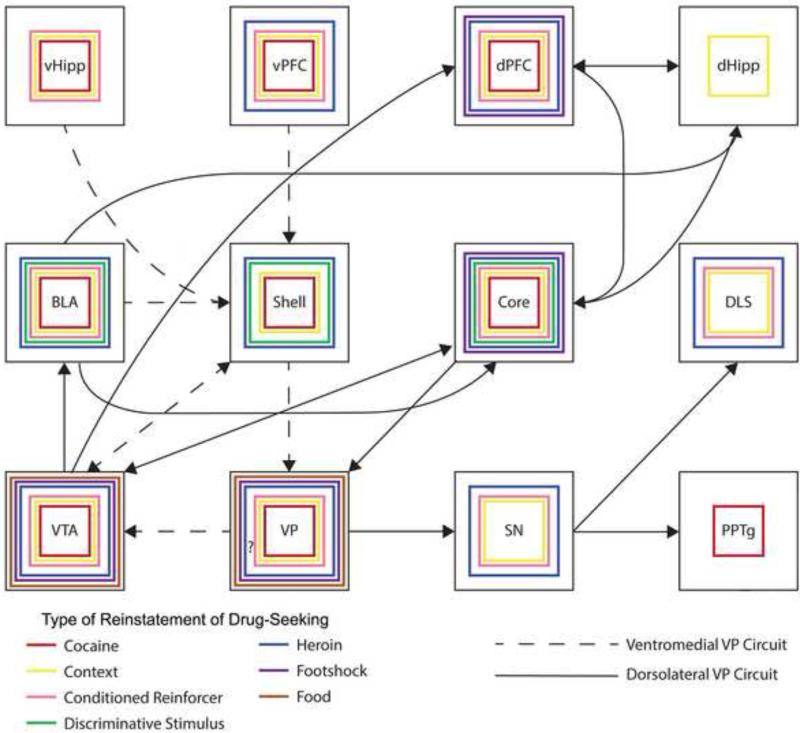
Within the basal ganglia, interspersed medium spiny neurons of the dorsal striatum form a “direct” pathway to the internal GP/SNr or an “indirect” pathway consisting of the striatum-external GP-STN-internal GP/SNr. Whether or not the ventral striatum/Acb is also organized into ‘direct and indirect pathways’ is not clear. On the basis of connectivity, Sesack and Grace (2010) suggested two circuits: AcbC-SNr-mediodorsal thalamus (MD) direct and AcbC-VPdl-STN-SNr-MD indirect pathways, as well as AcbSh-VTA-MD direct and AcbSh-VPvm-VTA-MD indirect pathways. Direct and indirect activation was proposed to activate or inhibit motor plans related to goal-directed behavior, respectively. Alternatively, on the basis of single axon tracings Tripathi et al. (2010, 2013) suggested two direct pathways, AcbSh-VPvm-MD and AcbC-VPr-MD, and an indirect AcbC-VPdl-STN-VPvm-MD pathway. Thus, there appears to be several possible direct or indirect pathways involving VP, which may play a role in the involvement of the VP in a wide array of motivated behaviors (Section 5.0).
In the dorsal striatum, direct pathway neurons express D1 receptors and preprotachykinin mRNA (substance P) whereas neurons in the indirect pathway express D2 receptors and preproenkephalin mRNA. At least in rats (discussed below), distinctions of direct vs. indirect pathways based on Acb D1/D2 expression or opioid/tachykinin expression are not as compelling as they are for the dorsal striatum. For example, of AcbSh neurons projecting to VP, between 44.32% (Lu et al., 1998) and 75.52% (Lu et al., 1997) express D1 and 33.71% express D2 receptor mRNA (Lu et al., 1988). Of AcbC neurons projecting to VP, 31% express D1 and 45% express D2 receptor mRNA. Thus, significant numbers of Acb neurons that express D1 or D2 receptor mRNA project to VP. Results are similar with respect to substance P and enkephalin expressing neurons. Of AcbSh neurons projecting to VP, 39% express preprotachykinin mRNA (substance P) and 36% express preproenkephalin (Lu et al., 1998). Of AcbC neurons projecting to VP, 33% express preprotachykinin mRNA (substance P) and 55% express preproenkephalin (Lu et al., 1998). Thus, similar to dopamine receptor expression, significant numbers of Acb neurons that express substance P or enkephalin project to VP. These results suggest that an indirect pathway from Acb to VP cannot be determined by methods used to delineate the dorsal striatum indirect pathway.
With respect to a ventral striatal direct pathway, D1 receptors are largely expressed on AcbSh neurons projecting to VTA (76%) and D2 receptors are rarely expressed in these neurons (2%; Lu et al., 1998). Furthermore, preprotachykin mRNA is often observed in AcbSh neurons projecting to the VTA (61%) whereas preproenkephalin mRNA is mostly absent from AcbC neurons projecting to VTA (4%; Lu et al., 1998). Recent studies have found differences between D1/substance P or D2/enkephalin expressing Acb neurons with regard to drug abuse (MacAskill et al., 2012; Yawata et al., 2012; Bock et al., 2013). Thus, it is possible that indirect D1/D2 or substance P/enkephalin expressing neurons involve different circuits (e.g., Kupchik et al., 2014) and effects on behavior, but these circuits are not wholly congruent with the dorsal striatum pathways.
4.0 Outputs and loops
There is a rich literature that demonstrates the wide array of brain regions which are linked to the VP. In the following subsections we review the efferent connections of VP subregions and neuronal phenotypes (Figures 5-6).
4.1 Thalamus
4.1.1 Mediodorsal thalamus
One major target of VPvm is the MD (Haber et al., 1985; Zahm and Heimer, 1990; Groenewegen et al., 1993; Kalivas et al., 1993; Zahm et al., 1996; Churchill et al., 1996; Heimer et al., 1997; O'Donnell et al., 1997; Tripathi et al., 2013), and ultrastructural visualization of this projection has identified large terminals that synapse primarily on dendritic shafts (Kuroda and Price 1991). VPr and VPvl also project to the MD (Young et al., 1984; Zahm and Heimer, 1987; Groenewegen et al., 1993; Tripathi et al., 2013) but the VPdl projection to MD is significantly less than that from other VP subregions (Zahm et al., 1996; O'Donnell et al., 1997). The VP projection to MD is predominantly GABAergic and partly cholinergic (Haber et al., 1985; Young et al., 1984; Kuroda and Price 1991; Ray et al., 1992; Mariotti et al., 2001). It is of great interest to ascertain the functions of these two VP-MD projections.
The MD response to electrical stimulation of the VP is predominantly inhibitory, with 83% of recorded cells decreasing, and 13% of cells increasing firing rates within 1-4 ms of VP activation (Vives and Mogenson, 1985; Mogenson et al.1987; Lavín and Grace, 1994, 1998). However, VP-evoked inhibition followed by a rebound excitation is also reported for 76% of recorded MD neurons (Mariotti et al., 2001). These findings are consistent with circuit-related direct and indirect responses.
VPvm efferents terminate onto MD neurons that project to the prefrontal cortex (Vives and Mogenson, 1985; Lavín and Grace, 1996; O'Donnell et al., 1997). The part of MD that receives VP inputs projects strongly to cortical areas that in turn project to AcbC (Zahm and Brog, 1992; Zahm et al., 1996; Zahm, 1999). Therefore, VPvm may be capable of altering AcbC-VPdl processing through a serial, laterally spiraling circuit.
4.1.2 Reticular nucleus of the thalamus
The reticular thalamus contains GABAergic neurons that provide topographic innervation to all thalamic nuclei (Houser et al., 1980). The VP projects to the rostral reticular thalamus (Jourdain et al., 1989; Cornwall et al., 1990; Groenewegen et al., 1993; O'Donnell et al., 1997; Tripathi et al., 2013). VP stimulation evokes IPSPs in roughly 73% of reticular thalamus cells at 2.7 ms latency (Lavín and Grace, 1994). The reticular thalamus projects GABAergic inputs into MD (Ray et al., 1992). The presence of this projection helps explain the small percentage of MD neurons that are excited by VP stimulation, as inhibition of the reticular thalamus disinhibits MD neurons (Mogenson et al., 1987).
4.1.3. Other thalamic targets
The VP has weak projections to several thalamic nuclei, including ventromedial nucleus, nucleus reuniens, paraventricular, intralaminar central medial and paracentral nuclei (Groenewegen et al., 1999). Most of these projections originate within the VPvm (Tripathi et al., 2013). A large VP projection to the paraventricular thalamus has been reported to arise from the anterior VP (Chen and Su, 1990), but thorough examination of paraventricular afferents demonstrated this is likely not to be the case (Li and Kirouac, 2012).
4.2. Lateral habenula (epithalamus)
The VPvm and VPr project to the medial part of the lateral habenula (LHb; Troiano and Siegel 1978b; Ray et al., 1992; Groenewegen et al., 1993; Zahm et al., 1996; Tripathi et al., 2013), and 28% of VPvm and 36% of VPr neurons that project to the thalamus collateralize within the LHb (Tripathi et al., 2013). The mesopontine rostromedial tegmental nucleus (RMTg), a GABAergic structure that is characterized by LHb input, has reciprocal connections with the VP (Jhou et al., 2009a). Thus, the VP is linked with the RMTg directly and polysynaptically through the lateral habenula. Currently no studies have examined the influence of the VP on the RMTg, or vice versa. Examination of these pathways will be of interest given that the RMTg projects to VTA, SNc, DR, and pedunculopontine tegmental nucleus (PPTg) (Jhou et al., 2009a; Lavezzi et al., 2012) and such a circuit would have the potential to influence a wide variety of motivated behaviors.
4.3. Dopaminergic mesencephalon
A major output of VP subregions and individual neuronal phenotypes is directed topographically towards the dopaminergic mesencephalon. GABAergic (Kalivas et al., 1993), neurotensinergic (Zahm et al., 2001; Geisler and Zahm, 2006b), and glutamatergic (VGluT2; Geisler et al., 2008) VP neurons project to VTA. The VPvm projects predominantly to the VTA and retrorubral field while the VPdl largely projects to the SNr (Haber et al., 1985; Zahm 1989; Kalivas et al., 1993; Groenewegen et al., 1993; Zahm et al., 1996; Geisler and Zahm, 2005; Colussi-Mas et al., 2007), but also collateralizes within the most lateral portions of VTA (Tripathi et al., 2013). VPvl projects to SNc and retrorubral field, but not VTA (Groenewegen et al., 1993; Oertel and Mugnaini, 1984; Bevan et al., 1996). VP neurons robustly inhibit VTA dopamine and nondopamine neurons via GABA release (Hjelmstaad et al., 2013) and this action can reduce the number of VTA neurons that are in an active/firing state (Floresco et al., 2003).
The projection of VP to the dopaminergic mesencephalon is ideally positioned to alter the efficacy of the limbic/cognitive/motor serial spiral loop circuit from AcbSh to putamen (Haber et al., 2000). This is most readily appreciated by VPvm projections to the lateral portions of VTA (lateral parabrachial pigmented nucleus and lateral paranigral nucleus), and these regions of the VTA topographically send dopaminergic projections to Acb shell and core (Haber et al., 2000). Furthermore, as noted above and by Zahm et al., (2011), outputs from the lateral portions of VP (e.g., VPdl and VPvl), exhibit a termination pattern that spreads laterally from the VTA to the SNc, and these midbrain regions send dopaminergic projections to the dorsal striatum (Haber et al., 2000). VP neurons establish synapses with both VTA and SNc dopaminergic neurons (Uchida et al., 2012; Ogawa et al., 2014). Taken together, the subregional VP projections to VTA and SNc likely represent different mechanisms by which the VP may affect both ventral and dorsal striatum.
4.4. Lateral hypothalamus, subthalamic nucleus, and entopeduncular nucleus
A major target of the VP subregions is the LH (Groenewegen et al., 1993; Tripathi et al., 2013). The projection displays a mediolateral topography whereby VPr, VPvm, and VPdl axons target the most lateral, central, and medial portions of LH, respectively (Tripathi et al., 2013).
The VPdl sends GABAergic projections to the dorsomedial STN (Haber et al., 1985; Zahm, 1989; Groenewegen and Berendse 1990; Groenewegen et al., 1993; Bell et al., 1995; Zahm et al., 1996; Bevan et al., 1997), consistent with inhibition observed in this structure in response to electrical VP stimulation (Maurice et al., 1997, 1998). In rats, intra-VP injections of 45 μg/0.5 μl NMDA or 50 ng/0.5 μl bicuculline increases Fos-IR in the dorsomedial STN, while Fos activation is not seen in the lateral STN (Turner et al., 2008). Given that VP outputs are largely GABAergic, the increased activity in this VP projection target may reflect indirectly mediated disinhibitory effects of VP activation, but if so, it is noteworthy that the polysynaptic pathways follow the direct projections of VP outputs. For example, the medial STN, which receives inputs from VPdl is activated, but the lateral STN which receives inputs from GP is not altered by VP stimulation. This observation is particularly interesting as STN dendrites are oriented from medial to lateral zones of VP and GP projections, receiving synapses from these structures along their dendrites (Bevan et al., 1997); therefore, some integration of GP and VP influences would be expected to occur. Furthermore, the prelimbic and medial orbital cortices project to this STN region, indicating expanded integration within the dorsomedial STN (Kolomiets et al., 2001). Further examination of pallidal to STN systems is needed to more clearly define these relationships.
The VPdl also sends a light GABAergic projection to the EPN (Zahm et al., 1996; Maurice et al., 1997; Bevan et al., 1997) and intra-VP injections of NMDA or bicuculline increase Fos-IR in the EPN (Turner et al. 2008). These VP to EPN projections are largely separate from GP projections, but exhibit some overlap (Bevan et al., 1997). Yet, EPN dendrites are not organized in a similar manner as STN neurons, and receive comparatively less VP input than the STN, suggesting more topographic influence from the GP. Nevertheless, intra-VP NMDA robustly increases Fos-IR in the EPN as well as its ontogenetically linked region, the SNr (Turner et al., 2008), and both are targets associated with projections from the VPdl.
A single-axon tracing study showed that the VP targets the rostral sublenticular extended amygdala and the area of the horizontal limb of the diagonal band and these projections arise from the VPvm and VPr (Tripathi et al., 2013). Given that cholinergic, GABAergic, and glutamatergic neurons reside within these areas, future investigations will be necessary to determine the type of postsynaptic neuron that receives VPvm and VPr projections.
4.5. Nucleus accumbens
Similar to the dorsal pallidostriatal projection (Staines et al., 1981), the VP projects back to its major striatal afferent source, the Acb, but the projection pattern is different for the two pallidal regions. GP neurons selectively innervate parvalbumin-immunoreactive GABAergic interneurons within the dorsal striatum (Bevan et al., 1998). The VP projection to Acb is less specific than GP, and consists of thin branching axons with numerous varicosities suggesting en passant synapses onto several Acb dendrites (Haber et al., 1985). In fact, the ventral pallidoaccumbal projection exhibits approximately equal percentages of VPdl and VPvm neurons projecting to either the AcbSh or AcbC (Tripathi et al., 2013; for subregionally nonspecific pallidoaccumbal projections, see also Heimer et al., 1991; Brog et al., 1993; Groenewegen et al., 1993; Spooren et al., 1996). The VPr exhibits a larger percentage of neurons that project to the olfactory tubercle than other striatal subregions (Tripathi et al., 2013). Orthodromic Acb activation from electrical VP stimulation occurs with roughly 7 ms latency and about half of these orthodromically activated neurons respond to hippocampal fimbria stimulation (Hakan et al., 1992; Yang and Mogenson, 1985). In contrast to striatopallidal cells, both dorsal and ventral pallidostriatal neurons exhibit delta, but not mu opioid receptor expression (Olive et al., 1997). Some parvalbumin-immunoreactive VP neurons project to the Acb (Kuo and Chang, 1992) suggesting the pallidostriatal projection is GABAergic.
4.6. Amygdala and prefrontal cortex
The VP projects to the basolateral amygdala (BLA) (Conrad and Pfaff 1976b; Troiano and Siegel 1978b; Haber et al., 1985; Carlsen et al., 1985; Mascagni and McDonald, 2009). Roughly 75% of VP neurons that project to the BLA are from cholinergic neurons that reside within the VP (Carlsen et al., 1985; Záborszky et al., 1986). Tracer injections in BLA and cortical regions demonstrate scant double-labeling (Záborszky et al., 1986), suggesting cholinergic neurons within the VP send individual, noncollateralized projections. Only 30% of cholinergic neurons that reside within the VP co-express VGluT3 mRNA; within the subset of these cholinergic neurons that also project to the BLA, nearly all (92%) express VGluT3 mRNA (Poulin et al., 2006). Intra-VP injections of mu, kappa, and delta opiate agonists significantly reduce acetylcholine turnover in the amygdala, suggesting that cholinergic neurons within the VP that project to the amygdala are regulated by opioid receptors (Table 1).
Table 1.
Effects of intra-VP injections of opioids on hemicholinium binding in VP cholinergic terminal regions.
| Treatment (nmoles/0.5μl/side) | Frontal Cortex | Amygdala |
|---|---|---|
| Vehicle | 12.7±1.2 | 111.9±8.4 |
| DAMGO (0.03) | 13.4±2.2 | 88.8±8.7 |
| DAMGO (1.0) | 11.1±0.9 | 83.2±14.2 |
| DAMGO (33) | 8.5±0.9* | 68.3±5.4* |
| CTOP (0.01) + DAMGO (33) | 8.8±0.6* | 107.8±20.2 |
| U50488H (10) | 10.4±2.1 | 89.4±23.3 |
| U50488H (33) | 10.3±1.9 | 65.0±10.0* |
| nBNT (0.1) + U50488H (33) | 9.9±0.9 | 132.3±19.3 |
| DPDPE (10) | 12.1±1.3 | 68.3±5.4* |
| Naltrindole10mg/kg,ip + DPDPE (10) | 9.3±1.6 | 105.5±16.9 |
Bilateral intra-VP injections were accomplished in awake male Sprague-Dawley rats via injectors inserted into chronically embedded cannula following published methods (e.g., see Napier, 1992). Injections were infused as 0.1μl/min. The rats were killed 30 min after infusion, brain regions were harvested and fast frozen, and hemicholinium binding was conducted by Dr. Linda Gorman, as previously published (Muma et al., 2001). Data are in fmoles hemicholinium/mg protein. Sample size varied from 6-12. When given alone, the tested antagonists did not alter binding (data not shown), but were effective in blocking the agonist-induced decreases, with the exception of DAMGO effects on the cortex.
p=0.05 vs. respective vehicle controls. DAMGO, mu receptor agonist; CTOP mu receptor antagonist; U50488H, kappa agonist; nBNT (norbinaltorphamine) kappa antagonist; DPDPE, delta agonist; Nalttrindole), delta antagonist. Intra-VP antagonists were given immediately before intra-VP agonists, with the excpetion of naltrindol, which was administered intraperitoneally, 30 min prior to intra-VP DPDPE. Data from Dr. Napier.
Some cholinergic neurons that reside within the VP directly innervate the cortical mantle (Rye et al., 1984; Jourdain et al., 1989), including frontal (Woolf et al., 1983), prefrontal (Funahashi 1983), medial prefrontal (Gritti et al., 1997, 1999 (Zaborszky et al., 2012; Zaborszky, unpublished observations), and the entorhinal (Manns et al., 2001) cortex.
Cortical excitability is regulated by local cholinergic receptors (Pirch et al., 1992; Hars et al., 1993) and pallidocortical projections that involve the cholinergic neurons that reside in the VP (Rigdon and Pirch, 1984; Pirch et al., 1985; Rigdon and Pirch, 1986). Intra-VP injections of NMDA increase Fos-like staining in the frontal cortex (Turner et al., 2008) and intra-VP injections of the GABA-A agonist, muscimol suppresses firing of cortical neurons (Rigdon and Pirch, 1984; Pirch et al., 1991). In contrast, cortically projecting cholinergic neurons within the VP are not regulated by intra-VP kappa or delta opiate agonists; mu activation does reduce acetylcholine turnover in the frontal cortex (but notably, this is not blocked by a mu antagonist) (Table 1). The lack of cortical regulation by at least kappa and delta opioid receptors in the VP differs from that obtained for VP influences on acetylcholine turnover in the amygdala (Table 1) suggesting that the two termination sites may be regulated by separate cholinergic systems emanating from within and around the VP. Collectively, these findings suggest that pallidocortical cholinergic neurons that reside within the VP express ionotropic glutamatergic and GABAergic receptors, and perhaps mu opioid receptors.
4.7 Projections to the brain stem
The VP sparsely projects to the PPTg and midbrain extrapyramidal area (Swanson et al. 1984; Haber et al., 1985; Grove 1988; Semba and Fibiger, 1992; Steininger et al., 1992; Tripathi et al., 2013; for review, see Heimer et al., 1997; though see Groenewegen et al., 1993). Albeit sparse, there is a topography in the PPTg projections, wherein axons arising from the VPvm and VPr, but not the VPdl, exhibit collaterals within PPTg (Tripathi et al., 2013). This input, combined with more robust inputs from other nearby regions (e.g., extended amygdala, preoptic-hypothalamic continuum) (Steininger et al. 1992; Semba and Fibiger, 1992) provide influence on PPTg function. The VP may also indirectly influence the PPTg, as the VPdl projects to the SN, and EPN, which in turn project to PPTg and midbrain extrapyramidal area (Haber et al. 1985; Steininger et al. 1992; Semba and Fibiger, 1992). Further examination is necessary to determine the differential extent of PPTg versus midbrain extrapyramidal targeting of VP neurons.
VP has sparse projections to the raphe nuclei (Conrad and Pfaff 1976b; Peyron et al., 1998) and locus coeruleus (Groenewegen et al., 1993), thereby potentially affecting serotonergic and noradrenergic neurotransmitter systems. Little is known regarding the subregional distribution (although it appears that the projection arises from the VPvm), physiology, receptors, or behavioral function of these VP projections. Recent studies have revleaed that VP neurons synapse onto dorsal raphe 5-HT neurons, and to a lesser extent, onto median raphe 5-HT neurons (Dorocic et al., 2014; Ogawa et al., 2014).
5.0. VP influences on behavior
A wealth of information is emerging regarding the roles of VP in behavior and in recent years, subregional dissection of these roles has begun. In the following sections, we overview VP-regulated behaviors, and propose functional roles for the two major VP subregions, VPvm and VPdl. The roles of VPr and VPvl require future investigation. In considering the role of a brain structure in behavior, it is important to be mindful that this may reflect a modulatory function of behaviors that are engendered by other structures or by the circuit in which the VP is embedded. It is also worth considering the possibility that the VP may serve as a generator of particular behaviors, which subsequently may or may not be modified by downstream structures. As such nuances largely remain unclear for the VP, here we attempt to provide an overview of known behavioral readouts that may involve the VP, regardless of the particular role that the VP has in the orchestration of a behavior per se.
Of the many methods that examined VP function, most utilized microinjection approaches. Due to the multitude of basal forebrain circuits and neuronal phenotypes, we suggest that future studies using microinjection methods include site injection controls (i.e., evaluating the effects of injecting test compounds into neighboring sites; e.g., Napier and Chrobak, 1992; Robertson and Jian 1995; Gong et al., 1999; Johnson and Napier, 2000; Chrobak and Napier, 2002; Zahm et al., 2014). In addition, our laboratories have found that high doses, large infusion volumes, and faster infusion rates have the potential to confound “inactivation” interpretations due to nonspecific effects in adjacent nuclei. Furthermore, vehicle treatments (saline or artificial cerebrospinal fluid) injected into VP even as slow as 0.1 μl/min for a total volume of only 0.25 μg are sufficient to produce a persistent deficits in radial arm maze performance (Chrobak and Napier, 2002). While motor (e.g., Johnson and Napier, 2000; Skoubis and Maidment, 2003) and place conditioning behaviors (e.g., Nikolaus et al., 1999; Skoubis and Maidment, 2003; Zarrindast et al., 2007) are not altered by intra-VP injections of a variety of treatment vehicles, the observation that radial arm maze performance deficits can occur suggests that at least some VP neurons are sensitive to fluid perturbation and/or that some behavioral readouts are more sensitive to such perturbations.
5.1. Motor behavior
The VP regulates a wide repertoire of motor behavior, including those that are not under conscious control (e.g., startle reflexes), those that are related to volitional actions, those reflecting learning and memory, and those motivated by reward. These point to the likelihood that VP subregions and neuronal subpopulations within them are relatively specialized for different behaviors, and that highly interactive circuits are involved. Numerous transmitter systems, and interactions among these systems, are involved in VP-regulated motor function (Table 2). Examples include the following: Injections of the GABA-A receptor antagonists within VP results in sniffing, gnawing, tongue protrusion, and chewing behaviors in rats (Zahm et al., 2014) and cats (Cools et al., 1989; Spooren et al., 1989). A similar pattern of oro-facial dyskinesia-like behavior also occurs following intra-VP injections of dopaminergic agonists in cats (Spooren et al., 1991) and in rats (Table 3). Unilateral injection of the mu receptor agonist DAMGO (Hoffman et al., 1991; Napier, 1992a) or injections of the GABAergic agonist muscimol (Kitamura et al., 2001) engender dose-dependent contraversive circling behavior. Intra-VP dopamine also produces a robust activation of motor behavior, the magnitude of which is greater than that obtained with similar injections into the dorsal striatum (Napier and Chrobak, 1992). Intra-VP injections of D1- or D2 receptor antagonists (SCH23390 and sulpiride, respectively), block the locomotor effects of subsequent intra-VP injections of mu opioid receptor agonist, DAMGO (Napier, 1992), demonstrating an opioidergic and dopaminergic interaction within VP.
Table 2.
VP manipulations and motoric effects.
| Reference | VP Manipulation | System | Effects | Task | Result |
|---|---|---|---|---|---|
| Kitamura et al., 2001 | Muscimol | GABA | GABA-A Agonist | Contraversive pivoting induced by AcbSh SKF 38393/quinpi role | Blocked |
| Kitamura et al., 2001 | Bicuculline | GABA | GABA-A Antagonist | Contraversive pivoting induced by AcbSh SKF 38393/quinpi role | Blocked |
| Kitamura et al., 2001 | Muscimol | GABA | GABA-A Agonist | Contraversive turning | Increase |
| Napier 1992 | DADL | Opioid | Multiple | Contraversive turning | Increase |
| Napier 1992 | DAMGO | Opioid | Mu agonist | Contraversive turning | Increase |
| Hoffman et al., 1991 | DAMGO | Opioid | Mu agonist | Contraversive turning | Increase |
| Hoffman et al., 1991 | DPDPE | Opioid | Delta agonist | Contraversive turning | Increase |
| Hoffman et al., 1991 | U50,488H | Opioid | Kappa agonist | Contraversive turning | No effect |
| Napier 1992 | DAMGO + SCH 23390 | Opioid + Dopamine | Mu agonist + D2 Antagonist | Contraversive turning | Attenuated |
| Napier 1992 | DAMGO + Sulpiride | Opioid + Dopamine | Mu agonist + D2 Antagonist | Contraversive turning | Attenuated |
| Kitamura et al., 2001 | Bicuculline | GABA | GABA-A Antagonist | Contraversive turning induced by AcbSh carbachol | Attenuated |
| Kitamura et al., 2001 | Muscimol | GABA | GABA-A Agonist | Contraversive turning induced by AcbSh carbachol | Blocked |
| Gong et al., 1996 | Cocaine | DA | Multiple | Locomotion | Decrease |
| Gong et al., 1996 | Amphetamine | DA | Multiple | Locomotion | Increase |
| Gong et al., 1996 | Dopamine | DA | Multiple | Locomotion | Increase |
| Alesdatter and Kalivas 1993 | SCH 23390 | Dopamine | D1 antagonist | Locomotion | Decrease |
| Alesdatter and Kalivas 1993 | Raclopride | Dopamine | D2 antagonist | Locomotion | No effect |
| Austin and Kalivas 1991 | Haloperidol | Dopamine | D2 Antagonist | Locomotion | No effect |
| Austin and Kalivas 1991 | Fluphenazine | Dopamine | D1/D2 antagonist | Locomotion | No effect |
| Fletcher et al., 1998 | Amphetamine | Dopamine | Multiple | Locomotion | Increase |
| Gong et al., 1999 | SKF 38393 | Dopamine | D1 Agonist | Locomotion | Mixed |
| Gong et al., 1999 | Quinpirole | Dopamine | D2 Agonist | Locomotion | Decrease |
| Hooks and Kalivas 1994 | Dopamine | Dopamine | Dopamine | Locomotion | Increase |
| Klitenick et al., 1992 | Dopamine | Dopamine | Multiple | Locomotion | Increase |
| Johnson et al., 1996 | Dopamine + VTA baclofen | Dopamine | Multiple | Locomotion | No effect |
| Austin and Kalivas 1991 | Haloperidol+picrotoxin | Dopamine + GABA | D2 Antagonist + GABA-A Antagonist | Locomotion | Attenuated |
| Austin and Kalivas 1991 | Fluphenazine+picrotoxin | Dopamine + GABA | D1/D2 antagonist + GABAA Antagonist | Locomotion | Attenuated |
| Alesdatter and Kalivas 1993 | Fluphenazine+DA MGO | Dopamine + Opioid | D1/D2 antagonist + Mu agonist | Locomotion | Attenuated |
| Alesdatter and Kalivas 1993 | SCH 23390+DAMGO | Dopamine + Opioid | D1 antagonist + Mu agonist | Locomotion | Attenuated |
| Alesdatter and Kalivas 1993 | Raclopride+DAMGO | Dopamine + Opioid | D2 antagonist + Mu agonist | Locomotion | No effect |
| Austin and Kalivas 1991 | DAMGO+haloperidol | Dopamine + Opioid | D2 antagonist + Mu agonist | Locomotion | Attenuated |
| Austin and Kalivas 1991 | Fluphenazine+DAMGO | Dopamine + Opioid | D1/D2 antagonist + Mu agonist | Locomotion | Attenuated |
| Austin and Kalivas 1989 | Picrotoxin | GABA | GABA-A Antagonist | Locomotion | Increase |
| Austin and Kalivas 1989 | Bicuculline | GABA | GABA-A Antagonist | Locomotion | Increase |
| Austin and Kalivas 1989 | Phaclofen | GABA | GABA-B Antagonist | Locomotion | No effect |
| Austin and Kalivas 1989 | Muscimol | GABA | GABA-A Agonist | Locomotion | Decrease |
| Austin and Kalivas 1989 | Muscimol+Picrotoxin | GABA | GABA-A Agonist + GABA-A Antagonist | Locomotion | Blocked |
| Austin and Kalivas 1990 | Bicuculline | GABA | GABA-A Antagonist | Locomotion | Increase |
| Austin and Kalivas 1990 | Picrotoxin | GABA | GABA-A Antagonist | Locomotion | Increase |
| Austin and Kalivas 1990 | Phaclofen | GABA | GABA-B Antagonist | Locomotion | No effect |
| Austin and Kalivas 1991 | Picrotoxin | GABA | GABA-A Antagonist | Locomotion | Increase |
| Churchill et al., 1998 | Muscimol | GABA | GABA-A Agonist | Locomotion | Increase |
| Churchill et al., 1998 | Muscimol + Acb DAMGO | GABA | GABA-A Agonist | Locomotion | Blocked |
| Churchill et al., 1998 | Muscimol + Acb DA | GABA | GABA-A Agonist | Locomotion | Blocked |
| Fletcher et al., 1998 | Picrotoxin | GABA | GABA-A Antagonist | Locomotion | Increase |
| Gong et al., 1997 | Picrotoxin | GABA | GABA-A Antagonist | Locomotion | Increase |
| Kalivas et al., 1991 | Picrotoxin | GABA | GABA-A Antagonist | Locomotion | Increase |
| Klitenick et al., 1992 | Muscimol | GABA | GABA-A Agonist | Locomotion | Increase |
| Lawrence et al., 2003 | Picrotoxin | GABA | GABA-A Antagonist | Locomotion | Increase |
| Patel and Slater 1988 | Picrotoxin | GABA | GABA-A Antagonist | Locomotion | Increase |
| Patel and Slater 1988 | Bicuculline | GABA | GABA-A Antagonist | Locomotion | Increase |
| Smith et al., 2005 | Bicuculline | GABA | GABA-A Antagonist | Locomotion | Increase |
| Swerdlow and Koob 1987 | Picrotoxin | GABA | GABA-A Antagonist | Locomotion | Increase |
| Swerdlow and Koob 1987 | Picrotoxin + mediodorsal thalamus lesion | GABA | GABA-A Antagonist | Locomotion | Blocked |
| Patel and Slater 1988 | Muscimol + Acb ADTN (DA analog) | GABA | GABA-A Agonist | Locomotion | Decrease |
| Patel and Slater 1988 | Baclofen + Acb ADTN (DA analog) | GABA | GABA-B Agonist | Locomotion | Decrease |
| Patel and Slater 1988 | Isoguvacine + Acb ADTN (DA analog) | GABA | GABA-A Agonist | Locomotion | Decrease |
| Patel and Slater 1988 | Picrotoxin + Acb ADTN (DA analog) | GABA | GABA-A Antagonist | Locomotion | No effect |
| Patel and Slater 1988 | Bicuculline + Acb ADTN (DA analog) | GABA | GABA-A Antagonist | Locomotion | No effect |
| Wallace and Uretsky 1991 | Muscimol + Acb AMPA | GABA | GABA-A Agonist | Locomotion | Blocked |
| Wallace and Uretsky 1991 | Muscimol + Acb amphetamine | GABA | GABA-A Agonist | Locomotion | Blocked |
| Wallace and Uretsky 1991 | Muscimol + Acb kainic acid | GABA | GABA-A Agonist | Locomotion | Blocked |
| Wallace and Uretsky 1991 | Muscimol + Acb NMDA | GABA | GABA-A Agonist | Locomotion | Blocked |
| Wallace and Uretsky 1991 | Muscimol + Acb picrotoxin | GABA | GABA-A Agonist | Locomotion | Blocked |
| Klitenick et al., 1992 | Muscimol+Dopamine | GABA + Dopamine | GABA-A Agonist + multiple | Locomotion | Increase |
| Wallace and Uretsky 1991 | Picrotoxin + DNQX | GABA + Glutamate | GABA-A Antagonist + AMPA antagnoist | Locomotion | Attenuated |
| Austin and Kalivas 1989 | Muscimol+DAMGO | GABA + Opioid | GABA-A Agonist + Mu agonist | Locomotion | Blocked |
| Churchill and Kalivas 1999 | AMPA | Glutamate | AMPA | Locomotion | Increase |
| Churchill and Kalivas 1999 | AMPA + mediodorsal thalamus procaine | Glutamate | AMPA | Locomotion | No effect |
| Churchill and Kalivas 1999 | AMPA + midbrain extrapyramidal region procaine | Glutamate | AMPA | Locomotion | Blocked |
| Churchill et al., 1998 | AMPA | Glutamate | AMPA | Locomotion | Increase |
| Churchill et al., 1998 | AMPA + VTA Baclofen | Glutamate | AMPA | Locomotion | Blocked |
| Gong et al., 1997 | AMPA | Glutamate | AMPA | Locomotion | Increase |
| Hooks and Kalivas 1994 | AMPA | Glutamate | AMPA agonist | Locomotion | Increase |
| Li et al., 2009 | AMN082 | Glutamate | R7 agonist | Locomotion | No effect |
| Wallace and Uretsky 1991 | AMPA | Glutamate | AMPA agonist | Locomotion | Increase |
| Wallace and Uretsky 1991 | kainate | Glutamate | AMPA agonist | Locomotion | Increase |
| Wallace and Uretsky 1991 | NMDA | Glutamate | NMDA agonist | Locomotion | Increase |
| Wallace and Uretsky 1991 | DNQX + Acb amphetamine | Glutamate | AMPA Antagonist | Locomotion | No effect |
| Wallace and Uretsky 1991 | DNQX + Acb AMPA | Glutamate | AMPA Antagonist | Locomotion | No effect |
| Wallace and Uretsky 1991 | GAMS + Acb amphetamine | Glutamate | Antagonist | Locomotion | No effect |
| Wallace and Uretsky 1991 | GAMS + Acb AMPA | Glutamate | Antagonist | Locomotion | No effect |
| Wallace and Uretsky 1991 | DAA + Acb amphetamine | Glutamate | NMDA antagonist | Locomotion | No effect |
| Wallace and Uretsky 1991 | DAA + Acb AMPA | Glutamate | NMDA antagonist | Locomotion | No effect |
| Johnson et al., 1996 | AMPA + VTA Baclofen | Glutamate | AMPA agonist | Locomotion | Blocked |
| Wallace and Uretsky 1991 | AMPA + muscimol | Glutamate + GABA | AMPA agonist + GABA agonist | Locomotion | Blocked |
| De Leonibus et al., 2001 | Lesion | NA | NA | Locomotion | Increase |
| Johnson et al., 1996 | Lesion | NA | NA | Locomotion | Decrease |
| De Leonibus et al., 2001 | Lesion + Acb MK801 | NA | NA | Locomotion | Blocked |
| Johnson et al., 1996 | DAMGO + VTA baclofen | Opiod | Mu agonist | Locomotion | Blocked |
| Kemppainen et al., 2012 | DAMGO | Opioid | Mu agonist | Locomotion | Increase |
| Alesdatter and Kalivas 1993 | DAMGO | Opioid | Mu agonist | Locomotion | Increase |
| Austin and Kalivas 1989 | DAMGO | Opioid | Mu agonist | Locomotion | Increase |
| Austin and Kalivas 1989 | DPEN | Opioid | Delta agonist | Locomotion | Increase |
| Austin and Kalivas 1989 | Naloxone | Opioid | Multiple | Locomotion | No effect |
| Austin and Kalivas 1990 | DAMGO | Opioid | Mu agonist | Locomotion | Increase |
| Austin and Kalivas 1990 | DPDPE | Opioid | Delta agonist | Locomotion | Increase |
| Austin and Kalivas 1991 | DAMGO | Opioid | Mu agonist | Locomotion | Increase |
| Churchill and Kalivas 1999 | DAMGO | Opioid | Mu agonist | Locomotion | Increase |
| Churchill and Kalivas 1999 | DAMGO with Mediodorsal thalams procaine | Opioid | Mu agonist | Locomotion | Blocked |
| Churchill and Kalivas 1999 | DAMGO + midbrain extrapyramidal region procaine | Opioid | Mu agonist | Locomotion | Blocked |
| Churchill et al., 1998 | DAMGO | Opioid | Mu agonist | Locomotion | Increase |
| Churchill et al., 1998 | DAMGO + VTA Baclofen | Opioid | Mu agonist | Locomotion | Blocked |
| Hoffman et al., 1991 | DAMGO | Opioid | Mu agonist | Locomotion | Increase |
| Hoffman et al., 1991 | DPDPE | Opioid | Delta agonist | Locomotion | No effect |
| Hoffman et al., 1991 | U50,488H | Opioid | Kappa agonist | Locomotion | No effect |
| Kalivas et al., 2001 | AMPA | Glutamate | AMPA receptor Agonist | Locomotion | Increase |
| Kalivas et al., 2001 | VP AMPA & MD GABA-B saclofen | Glutamate | AMPA receptor Agonist | Locomotion | No effect |
| Kalivas et al., 2001 | DAMGO | Opioid | Mu agonist | Locomotion | Increase |
| Kalivas et al., 2001 | VP DAMGO & MD GABA-B saclofen | Opioid | Mu agonist | Locomotion | Blocked |
| Kalivas et al., 1991 | DAMGO | Opioid | Mu agonist | Locomotion | Increase |
| Skoubis and Maidment 2003 | Naloxone | Opioid | Multiple | Locomotion | Decrease |
| Skoubis and Maidment 2003 | CTOP | Opioid | Mu agonist | Locomotion | Increase |
| Smith et al., 2005 | DAMGO | Opioid | Mu agonist | Locomotion | Increase |
| Austin and Kalivas 1990 | DAMGO+muscimol | Opioid + GABA | Mu agonist + GABA-A Agonist | Locomotion | Attenuated |
| Gong et al., 1996 | Procaine | Sodium | Antagonist | Locomotion | Decrease |
| Napier et al., 1995 | DiMeC7 | Substance P | Agonist | Locomotion | Increase |
| Gong et al., 1997 | 6-OHDA | DA/NE | NA | Locomotion induced by cocaine | Decrease |
| McFarland and Kalivas 2001 | Fluphenazine | Dopamine | D1/D2 antagonist | Locomotion induced by cocaine | Decrease |
| McFarland and Kalivas 2001 | Baclofen/Muscimol | GABA | GABA-A/B Agonist | Locomotion induced by cocaine | No effect |
| Skoubis and Maidment 2003 | Naloxone | Opioid | Multiple | Locomotion induced by cocaine | No effect |
| Tang et al., 2005 | CTAP | Opioid | Mu Antag | Locomotion induced by cocaine | No effect |
| Kretschmer 2000 | Lesion | NA | NA | Locomotion induced by dizocilpine | No effect |
| Hooks and Kalivas 1995 | Muscimol | GABA | GABA-A Agonist | Locomotion induced by novelty | Decrease |
| Hooks and Kalivas 1994 | DAMGO | Opioid | Mu agonist | Locomotion induced by novelty | Increase |
| Uchida et al., 2005 | Shell SKF82958/quinpirole + VP muscimol | Dopamine | Multiple | Repetitive jaw movements | Blocked |
| Uchida et al., 2005 | Shell SKF82958/quinpirole + VP bicuculline | Dopamine | Multiple | Repetitive jaw movements | Blocked |
| Uchida et al., 2005 | Bicuculline | GABA | GABA-A Antagonist | Repetitive jaw movements | No effect |
| Kretschmer 2000 | Lesion | NA | NA | Stereotyped sniffing induced by dizocilpine | No effect |
| Shimura et al., 2006 | Bicuculline | GABA | GABA-A Antagonist | Rearing | Increase |
| Shimura et al., 2006 | Muscimol | GABA | GABA-A Agonist | Rearing | Decrease |
| Shimura et al., 2006 | Bicuculline | GABA | GABA-A Antagonist | Horizontal Movements | Increase |
| Shimura et al., 2006 | Muscimol | GABA | GABA-A Agonist | Horizontal Movements | Decrease |
Manipulation refers to an intra-VP injection or VP lesion, unless stated otherwise.
Table 3.
Intra-VP injections of dopamine and dopamine agonists produced oral dyskinesia that is comparable to that seen with intraperitoneal amphetamine.
| Treatment (intra-VP, μg/0.5μl/side) | Dyskinesia Score |
|---|---|
| Vehicle | 9±2.0 |
| Amphetamine 1mg/kg ip | 24±5.8** |
| Dopamine (10) | 49±11.4** |
| Quinpirole (13.5)# | 19±3.5** |
| SKF82958 (21.7)# | 34±9.8** |
| SCH23390 0.1mg/kg ip + SKF82958 (21.7)# | 7±3.3 |
| SCH23390 (0.1) + SKF82958 (21.7)# | 16±4.4 |
An oral dyskinesia count was assigned if the animal engaged in any one of the following: chewing, teeth-grinding, licking/tongue protrusions or yawing. The oral behaviors were quantified for one min, every five min, for a total of 13 assessment periods. Intra-VP injections are described in Table 1 legend. Intraperitoneal (ip) amphetamine served as the positive control.
equal molar to 10 μg dopamine. ANOVA with post doc Dunnets
* P<0.05
VP-regulated motor behavior is influenced by Acb projections. Evidence for the influence of Acb on VP-mediated locomotion includes the following: Intra-VP substance P, a neuropeptide released from Acb projections to VP, increases locomotion (Napier et al., 1995). Injection of the mu opioid receptor agonist DAMGO, a receptor activated by enkephalinergic projections from Acb neurons, or injection of GABA-A receptor antagonists increase locomotion (Austin and Kalivas, 1990; Napier 1992a) and simultaneous VP injection of the GABA-A agonist muscimol reduces this effect (Austin and Kalivas, 1990). Intra-Acb-DAMGO, dopamine, AMPA, amphetamine, kainic acid, NMDA, and picrotoxin induced locomotion is blocked by VP muscimol (Wallace and Uretsky, 1991; Churchill et al., 1998; Patel and Slater, 1988). VP lesions decrease locomotion induced by intra-Acb MK801 (a NMDA antagonist) (De Leonibus et al., 2001), though it is not clear why intra-Acb NMDA and MK801 both increase locomotion (see also Ikemoto and Bonci (2014) for a similar discrepancy between intra-Acb NMDA antagonists and optogenetic stimulation of Acb glutamatergic afferents with reward).
Transmitter systems within the VP that are not contained in Acb efferents are also capable of altering motor function. For example, the raphe provides serotonergic inputs to the VP (see section 3.4) and intra-VP injections of the 5-HT2C agonist, MK212 suppresses motor activity (Graves et al., 2013) at doses (1 and 6.6 ng/0.5 μl) that are subthreshold to those needed to alter motor function when injected into the Acb (Filip and Cunningham, 2002). Glutamatergic inputs also arise from non-Acb structures and intra-VP injections of glutamatergic agonists enhance motor output (Churchill and Kalivas, 1999). Likewise, there is a direct dopaminergic input to the VP, and intra-VP injections of dopamine (Napier and Chrobak, 1992), and D1 or D2 agonists (Gong et al., 1999; Napier and Rehman, 1992) alter motor activity. Thus, while most VP inputs are from Acb, VP-mediated motor behavior appears to include non-Acb sources as well.
VP-mediated motor behavior involves the VPvm. Cools and colleagues (Kitamura et al., 2001; Uchida et al., 2005) proposed that AcbSh-mediated motor effects are transmitted through the VPvm. AcbSh injections of carbachol, a nonselective acetylcholine receptor agonist, or a combined dopamine D1/D2 receptor agonist, produce locomotor effects or repetitive jaw movements which are blocked by VP injection of muscimol (Kitamura et al., 2001). In turn, several regions that are targets of VPvm projections play a role in locomotion. Locomotion induced by intra-VP DAMGO or AMPA is blocked by intra-VTA baclofen administration (Johnson et al., 1996; Churchill et al., 1998), illustrating an extension of the results from Cools and colleagues wherein the locomotor effects of AcbSh stimulation at least partially involve a AcbSh–VPvm–VTA pathway. It is also possible that the AcbSh-VPvm-MD pathway influences locomotion, though mixed results have been reported. Whereas injection of DAMGO into MD produces locomotion (Klitenick and Kalivas, 1994), injection of the sodium channel blocker procaine into MD does not alter spontaneous locomotion (Mogenson et al., 1989; Churchill and Kalivas, 1999) or locomotion induced by VP picrotoxin (Mogenson and Wu, 1988) or VP AMPA (Churchill and Kalivas, 1993). However, intra-MD procaine (Churchill and Kalivas, 1993) or the GABA-B antagonist saclofen (Kalivas et al., 2001) can block locomotion induced by intra-VP DAMGO.
The projection targets of VPdl neurons are also implicated in locomotion. For example, STN and SNr, recipients of VPdl projections, strongly influence PPTg and midbrain extrapyramidal area (Saper and Loewy, 1982; Haber et al., 1985; Rye et al., 1987; Semba and Fibiger, 1992; Steininger et al., 1992), and procaine injections into the midbrain extrapyramidal area blocks locomotion induced by intra-VP AMPA or DAMGO (Churchill and Kalivas, 1999). These outcomes raise the possibility that some locomotor effects are elicited by VPdl-STN or VPdl-SNr pathways that affect midbrain extrapyramidal areas. Taken together, VP-mediated motor behavior likely involves both the VPvm and VPdl subregions.
5.2 Consummatory behaviors
We define consummatory behaviors as those directly involved in mastication, including natural reward consumption and taste reactivity (Table 4).
Table 4.
VP manipulations and consummatory effects.
| Reference | VP Manipulation | System | Effects | Task | Result |
|---|---|---|---|---|---|
| Inui et al., 2007 | Bicuculline | GABA | GABA-A Antagonist | Consumption | Increase |
| Stratford et al., 1999 | Bicuculline | GABA | GABA-A Antagonist | Consumption | Increase |
| Stratford and Wirtshafter, 2012 | Lesion | NA | NA | Consumption induced by shell muscimol | Attenuated |
| Stratford and Wirtshafter, 2013 | Bicuculline | GABA | GABA-A Antagonist | Consumption | Increase |
| Stratford and Wirtshafter, 2013 | Bicuculline | GABA | GABA-A Antagonist | Consumption after LH lesion | Attenuated |
| Covelo et al. 2014 | Bicuculline | GABA | GABA-A Antagonist | Consumption of fat | Increase |
| Taha et al., 2009 | Muscimol | GABA | GABA-A Agonist | Consumption | Decrease |
| Smith et al., 2005 | Bicuculline | GABA | GABA-A Antagonist | Consumption | Increase |
| McAlonen et al., 1993 | Lesion | NA | NA | Consumption | No effect |
| Smith et al., 2005 | DAMGO | Opioid | Mu agonist | Consumption | Mixed |
| Smith et al., 2007 | DAMGO | Opioid | Mu agonist | Consumption | Increase |
| Harvey et al., 2002 | 3-PBC | GABA | A1 Mixed | Saccharin Consumption | Decrease |
| Shimura et al., 2006 | Muscimol | GABA | A Agonist | Consumption of Preferred saccharin | Decrease |
| Shimura et al., 2006 | Bicuculline | GABA | GABA-A Antagonist | Consumption of Preferred saccharin | Increase |
| Shimura et al., 2006 | CNQX | Glutamate | AMPA Antagonist | Consumption of Preferred saccharin | Decrease |
| Shimura et al., 2006 | DAMGO | Opioid | Mu agonist | Consumption of Preferred saccharin | Mixed |
| Shimura et al., 2006 | SCH-23390 | Dopamine | D2 Antagonist | Consumption of Preferred saccharin | Decrease |
| Shimura et al., 2006 | Sulpiride | Dopamine | D2 agonist | Consumption of Preferred saccharin | No effect |
| Shimura et al., 2006 | Muscimol | GABA | GABA-A Agonist | Consumption of Nonpreferred quinine | Decrease |
| Shimura et al., 2006 | Bicuculline | GABA | GABA-A Antagonist | Consumption of Nonpreferred quinine | Decrease |
| Shimura et al., 2006 | CNQX | Glutamate | AMPA Antagonist | Consumption of Nonpreferred quinine | No effect |
| Shimura et al., 2006 | DAMGO | Opioid | Mu agonist | Consumption of Nonpreferred quinine | Decrease |
| Shimura et al., 2006 | SCH-23390 | Dopamine | D2 Antagonist | Consumption of Nonpreferred quinine | No effect |
| Shimura et al., 2006 | Sulpiride | Dopamine | D2 agonist | Consumption of Nonpreferred quinine | No effect |
| Shimura et al., 2006 | Muscimol | GABA | GABA-A Agonist | Consumption of Water | Decrease |
| Shimura et al., 2006 | Bicuculline | GABA | GABA-A Antagonist | Consumption of Water | No effect |
| Shimura et al., 2006 | CNQX | Glutamate | AMPA Antagonist | Consumption of Water | No effect |
| Shimura et al., 2006 | DAMGO | Opioid | Mu agonist | Consumption of Water | Decrease |
| Shimura et al., 2006 | SCH-23390 | Dopamine | D2 Antagonist | Consumption of Water | No effect |
| Shimura et al., 2006 | Sulpiride | Dopamine | D2 agonist | Consumption of Water | No effect |
| Farrar et al., 2008 | Muscimol | GABA | GABA-A Agonist | Consumption during food self-administration | Mixed |
| Farrar et al., 2008 | Muscimol | GABA | GABA-A Agonist | Consumption of preferred substance | Mixed |
| Taha et al., 2009 | Muscimol | GABA | GABA-A Agonist | Consumption induced by Acb DAMGO | Blocked |
| Taha et al., 2009 | Lesion | NA | NA | Consumption induced by Acb DAMGO | No effect |
| Smith et al., 2007 | VP Naloxone & Acb DAMGO | Opioid | Antagonist | Consumption induced by Acb DAMGO | No effect |
| Smith et al., 2007 | VP DAMGO & Acb naloxone | Opioid | Mu agonist | Consumption induced by VP DAMGO | Decrease |
| Johnson et al., 1996b | Lesion | NA | NA | Responses during food self-administration | Decrease |
| Fletcher et al., 1998 | Amphetamine | Dopamine | Multiple | Responses during food self-administration with conditioned reinforcer | Increase |
| Fletcher et al., 1998 | Picrotoxin | GABA | GABA-A Antagonist | Food self-administration with conditioned reinforcer | Decrease |
| Smith et al., 2005 | Bicuculline | GABA | GABA-A Antagonist | Food carrying | Increase |
| Smith et al., 2005 | DAMGO | Opioid | Mu agonist | Food carrying | No effect |
| Inui et al., 2007 | Bicuculline | GABA | GABA-A Antagonist | Hedonic taste reactivity | Increase |
| Smith et al., 2005 | Bicuculline | GABA | GABA-A Antagonist | Hedonic taste reactivity | No effect |
| Smith et al., 2005 | DAMGO | Opioid | Mu agonist | Hedonic taste reactivity | Mixed |
| Smith et al., 2007 | DAMGO | Opioid | Mu agonist | Hedonic taste reactivity | Increase |
| Shimura et al., 2006 | Muscimol | GABA | GABA-A Agonist | Hedonic taste reactivity | Decrease |
| Shimura et al., 2006 | Bicuculline | GABA | GABA-A Antagonist | Hedonic taste reactivity | No effect |
| Shimura et al., 2006 | CNQX | Glutamate | AMPA Antagonist | Hedonic taste reactivity | No effect |
| Shimura et al., 2006 | DAMGO | Opioid | Mu agonist | Hedonic taste reactivity | No effect |
| Shimura et al., 2006 | SCH-23390 | Dopamine | D2 Antagonist | Hedonic taste reactivity | No effect |
| Inui et al., 2007 | Bicuculline | GABA | GABA-A Antagonist | Aversive taste reactivity | Decrease |
| Shimura et al., 2006 | Muscimol | GABA | GABA-A Antagonist | Aversive taste reactivity | Increase |
| Shimura et al., 2006 | Bicuculline | GABA | GABA-A Antagonist | Aversive taste reactivity | No effect |
| Shimura et al., 2006 | CNQX | Glutamate | AMPA Antagonist | Aversive taste reactivity | No effect |
| Shimura et al., 2006 | DAMGO | Opioid | Mu agonist | Aversive taste reactivity | No effect |
| Shimura et al., 2006 | SCH-23390 | Dopamine | D2 Antagonist | Aversive taste reactivity | No effect |
| Smith et al., 2007 | VP Naloxone & Acb DAMGO | Opioid | Antagonist | Taste reactivity induced by Acb DAMGO | Blocked |
| Smith et al., 2007 | VP DAMGO & Acb naloxone | Opioid | Antagonist | Taste reactivity induced by VP DAMGO | Blocked |
| Inui et al., 2007 | Bicuculline | GABA | GABA-A Antagonist | Taste aversion | Blocked |
Manipulation refers to an intra-VP injections or VP lesion, unless stated otherwise.
5.2.1 Consumption
Activation of GABA-A receptors in VP decreases food intake. For example, intra-VP injection of the GABA-A receptor agonist muscimol reduces consumption (Shimura et al., 2006; Taha et al., 2009) and conversely intra-VP injection of the GABA-A receptor antagonist bicuculline increases food intake (Stratford et al., 1999; Smith and Berridge, 2005; Inui et al., 2007). Interestingly, rats will selectively increase consumption of fat following intra-VP bicuculline injection, suggesting VP may play a strong role in fat intake (Covelo et al. 2014). Activation of opioid receptors in VP also increases consumption. Intra-VP DAMGO increases consumption (Smith and Berridge, 2005), and there may be a temporal component to this process as intra-VP DAMGO decreases consumption up to one hour following injection but increases consumption two hours following injection (Shimura et al., 2006). Delta antagonists injected in VP also increase consumption (Inui and Shimura, 2014).
The AcbSh is capable of influencing consummatory behavior through the VPvm as well as other output pathways, such as the projection to LH and nucleus tractus solitarius (Will et al., 2003; Stratford and Kelly, 1999; Stratford et al., 1997, 1999; Taha et al., 2009). For instance, on the one hand intra-VP muscimol blocks food intake induced by intra-AcbSh DAMGO (Taha et al., 2009). On the other hand, AcbSh injection of DAMGO is still capable of engendering consumption in VP-lesioned rats, suggesting that AcbSh and VP have common convergent projections can individually alter feeding (Taha et al., 2009). This convergent region likely does not involve the MD since lesions to this structure do not affect consumption (McAlonen et al., 1993). The most likely regions of convergence are the LH and VTA, to which both VPvm and AcbSh project (Haber et al., 1985, 2000; Zahm 1989; Kalivas et al., 1993; Groenewegen et al., 1993; Zahm et al., 1996; Sano and Yokoi, 2007) and are involved in consumption (Noel and Wise, 1993, 1995; Hamilton and Bozarth, 1988; Jenck et al., 1987; Mucha and Iverson, 1986; Segall and Margules, 1989; Kim et al., 2009; Echo et al., 2002; Khaimova et al., 2004; Stratford and Kelly, 1999; Will et al., 2003; Turenius et al., 2009; Stratford and Wirtshafter, 2012, 2013). Furthermore, disconnection of VTA and LH antagonizes consumption (Jenck et al., 1986) supporting the idea that both structures are components of AcbSh and VPvm consumption circuitry.
5.2.2 Cue-induced feeding
Cue-induced feeding involves the BLA, as lesions of this structure block the ability of a cue previously paired with meal interruption (Galarce et al., 2010), or food (Holland et al., 2002; Holland and Gallagher, 2003) to increase consumption. The BLA projection to AcbSh is critical for the expression of outcome specific Pavlovian Instrumental Transfer (PIT) (Shiflett and Balleine, 2010). This cue-induced feeding paradigm links Pavlovian cues associated with a specific food with responding on a lever that previously delivered that same food.
AcbSh, but not AcbC lesions, decrease outcome-specific PIT (Corbit et al., 2001). VP-projecting AcbSh neurons exhibit elevated fos-IR during PIT compared to lever pressing or conditioned stimulus controls (Leung and Balleine, 2013). Contralateral disconnection of AcbSh and VP blocks outcome specific PIT (Leung and Balleine, 2013). Given that AcbSh specifically projects to VPvm, it is likely that a BLA-AcbSh-VPvm circuit is critical for outcome specific PIT. Furthermore, it likely that the VPvm projection to MD plays a significant role in mediating this type of PIT (Balleine et al., 2014).
5.2.3 Taste reactivity and food preference
The VP is involved in regulating consumption and taste reactions in response to preferred foods. For instance, intra-VP bicuculline or SCH-23390 injections increased consumption of a preferred saccharine solution but had no effect on water or quinine (Shimura et al., 2006). Consistent with VP activity being associated with preferred tastants, Tindell and colleagues (2006) found that single VP neurons were sensitive to positive (“liking”), but not negative (“disliking”), orofacial taste reactions. An additional report by this group found increased firing rates in response to a conditioned stimulus that predicted salt infusion when under salt-deprived conditions (Tindell et al., 2009), demonstrating that VP neurons are also sensitive to cues related to hedonic taste reactions. Whether VP subregions exhibit differential changes in firing rate during hedonic facial reactions or cues is not known. However, a rostral-caudal gradient in hedonic taste reactivity induced by AcbSh and VP DAMGO injections has been observed (Smith and Berridge, 2005, 2007). A portion of the caudal, sublenticular parts of VP that have been interpreted as a transition zone involving the lateral preoptic area and extended amygdala (Zahm et al., 2013), has been referred as a “hedonic hotspot” (Smith and Berridge, 2005, 2007).
The VP appears capable of regulating negative taste reactions as well. Extracellular VP GABA concentrations are increased following presentation of a saccharine cue that predicts nausea, but not when a saccharine cue predicts saline, or when a quinine cue predicts nausea or saline (Inui et al., 2009). Thus, these scientists hypothesized that VP GABA is involved in mediating perceived shifts from palatability to predicted illness. In support of this interpretation, intra-VP muscimol increases negative taste reactivity in response to saccharine, which normally induces positive taste reactions (Shimura et al., 2006). Conversely, in rats that have learned that a saccharine cue predicts nausea, bicuculline injection in VP prior to sampling the cue results in increased positive taste responses and lower aversive taste responses (Inui et al., 2007; see Yamamoto, 2007 for review). Given that intra-BLA glutamate enhances conditioned taste aversion (Ferreira et al., 2005) and BLA lesions disrupt taste aversion learning (Borsini and Rolls, 1984; Rollins et al., 2001), a BLA-AcbSh-VPvm pathway could potentially mediate some aspects of taste reactivity, such as palatability and learning.
VPdl may be involved in the self-administration of preferred foods, though VPdl has received less attention than VPvm. Intra-VP muscimol decreases the effort emitted by rats for a preferred food while not altering the response-capacity or the original food preferences of the animal (Farrar et al., 2008). This effect may be due to the AcbC influence on the VPdl because disconnection of unilateral AcbC and contralateral VP impaired performance when access to food required ten responses but not when food access required one response (Mingote et al., 2008). However, the VPdl was not explicitly disconnected alone in this experiment, and the influence of the AcbSh and VPvm on food preference performance was not examined.
5.3. Maternal behavior
The integrity of the VTA is critical for maternal behavior (Numan and Smith, 1984; Numan et al., 2009) and dopaminergic VTA projections to AcbSh are involved in a circuit related to these behaviors. AcbSh dopamine concentrations increase prior to the onse of maternal behavior (pup licking/grooming) and the magnitude and duration of these behaviors significantly correlates with extracellular AcbSh dopamine concentrations (Champagne et al., 2004). Intra-AcbSh of D1 antagonists, but not intra-VP injections, decrease pup retrievals even though rats increase the number of “hovers” over the pups (Numan et al., 2005a). Increasing GABAergic tone by injections of muscimol within VP, but not AcbSh, decreases pup retrievals, increases the latency to sniff pups, and decreases nursing duration of dams (Numan et al., 2005b). It is likely that maternal behavior involves a VTA dopamine – AcbSh GABA – VP pathway (Table 5).
Table 5.
VP manipulations and cognitive, maternal, rewarding (selfstimulation), and aversive effects.
| Reference | VP Manipulation | System | Effects | Task | Result |
|---|---|---|---|---|---|
| Numan et al., 2005a | SCH 23390 | Dopamine | D1 Antagonist | Maternal Behavior (Pup retrieval) | No effect |
| Numan et al., 2005b | Muscimol | GABA | GABA-A Agonist | Maternal Behavior (Pup retrieval + Nursing duration) | Decrease |
| Numan et al., 2005b | VP muscimol (contralateral) + MPOA lesion (ipsilateral) | GABA | GABA-A Agonist | Maternal Behavior (Pup retrieval + Nursing duration) | Decrease |
| Swerdlow et al., 1990 | Picrotoxin | GABA | GABA-A Antagonist | Pre-pulse inhibition | Decrease |
| Swerdlow et al., 1990 | VP Muscimol + Acb DA | GABA | GABA-A Agonist | Disruption of Pre-pulse inhibition by Acb DA | Blocked |
| Kodsi and Swerdlow 1995a | Medial VP Picrotoxin | GABA | GABA-A Antagonist | Pre-pulse inhibition | Decrease |
| Kodsi and Swerdlow 1995a | Central and lateral VP Picrotoxin | GABA | GABA-A Antagonist | Pre-pulse inhibition | No effect |
| Kodsi and Swerdlow 1995a | 2-OH-saclofen | GABA | GABA-B Antagonist | Pre-pulse inhibition | No effect |
| Kodsi and Swedlow 1995b | Picrotoxin | GABA | GABA-A Antagonist | Pre-pulse inhibition | Decrease |
| Swerdlow et al., 1990 | Picrotoxin | GABA | Antagonist | Pre-pulse inhibition | Decrease |
| Kretschmer and Koch 1998 | Lesion | NA | NA | Pre-pulse inhibition | No effect |
| Sipes and Geyer 1997 | DOI | Serotonin | 5-HT2A agonist | Pre-pulse inhibition | Decrease |
| Sipes and Geyer 1997 | MDL 100,907 | Serotonin | 5-HT2A antagonist | Pre-pulse inhibition | Increase |
| Ferry et al., 2000 | Lesion | NA | NA | Reversal learning | Decrease |
| Zhang et al., 2005 | Muscimol | GABA | GABA-A Agonist | Working memory task performance (lever) | Decrease |
| Zhang et al., 2005 | AMPA | Glutamate | AMPA agonist | Working memory task performance (lever) | Decrease |
| Zhang et al., 2005 | Lesion | NA | NA | Working memory task performance (lever) | Decrease |
| Zhang et al., 2005 | DAMGO | Opioid | Mu Agonist | Working memory task performance (lever) | Decrease |
| Kalivas et al., 2001 | AMPA | Glutamate | AMPA agonist | Working memory task performance (maze) | Decrease |
| Kalivas et al., 2001 | DAMGO | Opioid | Mu Agonist | Working memory task performance (maze) | Decrease |
| Kalivas et al., 2001 | VP DAMGO & MD GABA GABA-B saclofen | Opioid | Mu Agonist | Working memory task performance (maze) impairment | Blocked |
| Chrobak and Napier 2002 | Saline or aCSF | NA | NA | Working memory task performance (maze) | Decrease |
| Floresco et al., 1999 | Lidocaine | Sodium | Antagonist | Working memory task performance (maze) | Decrease |
| Huston et al., 1987 | Lesion | NA | NA | Brain Stimulation Reward | Decrease |
| Johnson and Stellar 1994b | Lesion | NA | NA | Brain Stimulation Reward | No effect |
| Johnson et al., 1993 | DAMGO | Opioid | Mu agonist | Brain Stimulation Reward | Mixed |
| Johnson and Stellar 1994a | DPDPE | Opioid | Delta agonist | Brain Stimulation Reward | Decrease |
| Waraczynski and Demco 2006 | Lidocaine | Sodium | Antagonist | Threshold of self-stimulation of Medial Forebrain Bundle | No effect |
| Ollman et al., 2014 | Neurotensin | Neurotensin | Agonist | Conditioned place preference | Established |
| Panagis and Spyraki 1996 | VP self-stimulation | Dopamine | Multiple | Threshold of self-stimulation and Systemic cocaine | Decrease |
| Panagis and Spyraki 1996 | VP self-stimulation | Dopamine | D3 agonist | Threshold of self-stimulation and Systemic 7-OH-DPAT | Decrease |
| Panagis and Spyraki 1996 | VP self-stimulation | Dopamine | D2 Antagonist | Threshold of self-stimulation and Systemic haloperidol | Increase |
| Panagis and Spyraki 1996 | VP self-stimulation | Dopamine | D1 Antagonist | Threshold of self-stimulation and Systemic SCH 23390 | Increase |
| Panagis and Spyraki 1996 | VP self-stimulation | Dopamine | D1 Antagonist | Threshold of self-stimulation and Systemic raclopride | Increase |
| Panagis and Spyraki 1996 | VP self-stimulation | Dopamine | D2 Antagonist | Threshold of self-stimulation and Systemic Sulpiride | Increase |
| Panagis and Kastellakis 2002 | VP self-stimulation | GABA | GABA-A Agonist | Threshold of self-stimulation and VTA muscimol | Increase |
| Panagis and Kastellakis 2002 | VP self-stimulation | GABA | GABA-B Agonist | Threshold of self-stimulation and VTA baclofen | Increase |
| Panagis and Kastellakis 2002 | VP self-stimulation | Glutamate | NMDA agonist | Threshold of self-stimulation and VTA NMDA | No effect |
| Panagis and Kastellakis 2002 | VP self-stimulation | Glutamate | AMPA agonist | Threshold of self-stimulation and VTA AMPA | No effect |
| Panagis et al., 1998 | VP self-stimulation | Opioid | Multiple | Threshold of self-stimulation and VTA morphine | Decrease |
| Smith et al., 2005 | Bicuculline | GABA | GABA-A Antagonist | Digging | Increase |
| Smith et al., 2005 | DAMGO | Opioid | Mu agonist | Digging | No effect |
| Mingote et al., 2008 | Muscimol | GABA | GABA-A Agonist | Effort task impairment induced by Acb Adenosine A2A agonist | Blocked |
| Smith et al., 2005 | Bicuculline | GABA | GABA-A Antagonist | Forepaw “defensive” treading | Increase |
| Smith et al., 2005 | DAMGO | Opioid | Mu agonist | Forepaw “defensive” treading | Increase |
| Lawrence et al., 2003 | Picrotoxin | GABA | GABA-A Antagonist | Latent Inhibition | No effect |
| Lawrence et al., 2003 | Muscimol | GABA | GABA-A Agonist | Latent Inhibition | No effect |
Manipulation refers to an intra-VP injection or VP lesion, unless stated otherwise.
Preoptic circuits also interact with the VP to regulate maternal behavior. Disconnection of the VP (via unilateral injections of muscimol) and medial preoptic area (via contralateral lesions), a region that is highly responsive to maternity-related hormones and cues (see Numan and Stolzenberg, 2009 for review), reduces pup retrievals and nursing duration (Numan et al., 2005b). Maternal behavior that involves the medial preoptic area and VP likely engage the VTA because both medial preoptic area and VPvm project to VTA (Geisler and Zahm, 2005) and unilateral medial preoptic lesions coupled with contralateral VTA lesions disrupt maternal behavior (Numan et al., 2009). These outcomes led Numan and colleagues (Numan, 2006; Numan and Stolzenberg, 2009) to hypothesize that pup-related stimuli and hormonal information converge within the medial preoptic area, which projects to the VTA and increases dopamine within AcbSh. In turn, AcbSh reduces its GABAergic influence on the VP to elicit maternal responsiveness. It is inferred that the VPvm, which receives AcbSh projections, is highly involved in maternal behavior.
5.4 Behaviors related to cognition
5.4.1. Sensorimotor Gating
Sensorimotor gating refers to the suppression of sensory, motor, or cognitive events that may interfere with focused attention and sequential information processing. The acoustic startle reflex induced by an unexpected loud sound can be greatly reduced if preceded by a weak auditory warning sound (referred to as prepulse inhibition). Thus, prepulse inhibition (PPI) of an acoustic startle is used as an operational measure of sensorimotor gating and a reduction in PPI is associated with deficits in focus, motivation and cognition.
PPI of the startle reflex is mediated at or below the pons, and the reflex regulated by ascending dopaminergic projections and descending corticostriatopallidal systems. Accordingly, intra-VP injections of GABA-A antagonists disrupt PPI in rats (Swerdlow et al., 1990; Kodsi and Swerdlow, 1995a,b). Other transmitter systems converge on this descending system at the level of the VP to modulate PPI, e.g., intra-VP injections of the 5-HT2A agonist, DOI, disrupts PPI (Sipes and Geyer, 1997) (Table 5).
The AcbSh, VP, and MD play a role in PPI (Swerdlow et al., 1990; Kodsi and Swerdlow, 1995a,b;1997), suggesting the involvement of a serial AcbSh-VPvm-MD pathway. Another pathway, involving the VPvm or VPr neurons that project to the PPTg (Tripathi et al., 2013), may potentially play a role in PPI because lesions of, or muscimol injections into the PPTg reduce PPI (Kodsi and Swerdlow, 1997). STN lesions do not alter PPI (Kodsi and Swerdlow, 1997), and PPI is more sensitive to intra-VP injections of the GABA-A antagonist picrotoxin within more medially-centered sites compared with more laterally-centered sites (Kodsi and Swerdlow, 1995a). Given that the VPdl is the location of STN-projecting VP neurons, the VPdl-STN pathway is unlikely to participate in PPI.
5.4.2 Working memory and associative learning
Task performance involving working memory can be disrupted by manipulations of Acb, VP, mPFC, and orbitofrontal cortex (Table 5; Ferry et al., 2000). Lidocaine injections into either the Acb or the VP immediately prior to testing in radial arm maze tasks significantly increases performance errors (injections during training have no effect; Seamans and Phillips, 1994; Floresco et al., 1999). Intra-VP DAMGO or AMPA impairs working memory, which is reduced by MD GABA blockade (Kalivas et al., 2001); therefore, an Acb-VPvm-MD-cortical pathway maybe necessary for optimal performance in working memory tasks. However, it is important to note that intra-VP vehicle injections can disrupt working memory performance (Chrobak and Napier, 2002); therefore, appropriate controls need to be rigorously applied to these studies. VP involvement in cognitive function can also be inferred by studies of structures that input the VP. For example, the STN is involved in mnemonic processes (Baunez et al., 2011), and it will be of great interest for future evaluations to ascertain the role of the STN-VP-STN circuit, and in particular the VPdl-STN pathway, in cognitive function.
Early assessments of cortically evoked electrophysiological events related to reinforcement-mediated learning implicated a role for the VP in associative learning processes. Using scalp electroencephalographic (EEG) recordings of human frontal cortical regions, Walter and colleagues (1964) discovered that a long (1 to 2 sec) descending potential developed as an association was made between a conditioned stimulus (CS+) and a conditioned response or reinforcer. Early studies described this evoked EEG event as a ‘contingent negative variation’ (CNV) (Walter et al., 1964). CNV occurs in the time between the CS+ and the response, develops with learning of an operant task, and declines with extinction (Walter et al., 1964). The development of the CNV is thought to reflect learning and its magnitude provides a reliable means to ascertain the degree of association or the salience of a stimulus that was previously paired with a reinforcer. Pirch and Barnes (1972) described a negative slow (or steady) potential (SP) recorded from rat cortex during the 1-2 sec interval between a CS+ and the delivery of a reward. Similar to the human CNV, shifts in rat SP reflects learning of the association between the CS+ and a reward, and the SP also shows extinction upon removal of the reward. These SP characteristics are independent of the CS modality (tone, light or subthreshold brain stimulation) and type of reinforcer (food, rewarding brain stimulations, footshock) (Rucker et al., 1986; Pirch, 1993), and clearly involve dopaminergic transmission (Pirch et al., 1981; Pirch, Napier and Corbus, 1981; Pirch and Corbus, 1983). The SPs correlate with event-related changes in single neuron firing in both the frontal cortex (Pirch and Peterson, 1981; Pirch et al., 1985) as well as in the VP (Rigdon and Pirch, 1986; note: only a caudal portion of VP was tested, which was termed the substantia innominata in this report). Event-related cortical SPs and single unit responses are regulated by cortical cholinergic receptors (Pirch et al., 1992; Hars et al., 1993), and their development is largely dependent upon intact projections from the basal forebrain cholinergic neurons, including those that reside in the VP (Rigdon and Pirch, 1984; Pirch et al., 1985; Rigdon and Pirch, 1986). Moreover, intra-VP microinjection of the GABA-A agonist, muscimol produces a profound suppression of cortical unit responses to conditioned stimuli (Rigdon and Pirch, 1984; Pirch et al., 1991) whereas basal forebrain stimulation (Hars et al., 1993) and microiontophoretic application of acetylcholine onto the cortical neurons (Pirch et al., 1991) enhances conditioned stimulus-evoked responses in the cortex. These historical studies help explain more recent work showing that cholinergic transmission in the frontal cortex is critical for the development of cue detection (see Parikh et al., 2007). The operational requirements for an organism to link cues with important stimuli and to translate significant stimuli into appropriate motor responding are critical for survival. It appears that the VP, including cholinergic corticopetal neurons that reside within this region, are neuroanatomical substrates that influence these functions.
5.5. VP supports brain self-stimulation behavior
Historically, one of the first approaches towards examining brain reward mechanisms involved studies using the intracranial self-stimulation procedure in which animals will respond on a lever to self-deliver an electrical stimulus directly to specific parts of their brains (Olds and Milner, 1954). It has been reported that the entire VP supports self-stimulation at low frequency thresholds (Panagis et al., 1995; Burgdorf et al., 2007) and this behavior induces c-fos-IR in medial prefrontal cortex, Acb, lateral hypothalamus, and VTA (Panagis et al., 1997). Dopamine appears to be involved in VP self-stimulation because systemic cocaine or 7-OH-DPAT (a D2/D3 agonist) decreases threshold frequencies, whereas haloperidol, raclopride, and sulpiride (D2 antagonists) or SCH23390 (a D1 antagonist) increases threshold frequencies to maintain VP self-stimulation (Table 5; Panagis and Spyraki, 1996). Furthermore, intra-VTA muscimol or baclofen increase (Panagis and Kastellakis, 2002) and morphine decrease (Panagis et al., 1998) VP frequency thresholds, suggesting that the VP projection to VTA, which arises predominantly from the VPvm, participates in brain stimulation reward.
AcbSh 6-OHDA-induced lesions have no effect on VP self-stimulation thresholds while VP lesions or intra-VP lidocaine injections decrease medial forebrain bundle self-stimulation (Huston et al., 1987; Waraczynski and Demco, 2006) without affecting the locus of rise or threshold (Johnson and Stellar, 1994a,b; Waraczynski and Demco, 2006). These data indicate that the VP–VTA pathway, which is downstream from the AcbSh, is sufficient to support self-stimulation behavior. Anterior-posterior VP differences exist in mediating self-stimulation behavior. Caudal VP injections of DAMGO increase self-stimulation of the LH while more rostral VP injections decrease hypothalamic self-stimulation (Johnson et al., 1993). However, because the ascending and descending fibers of the medial forebrain bundle pass through the VPvl, actions of locally administered drugs or electrical stimulation could be confused with VP cellular actions.
5.6 Aversion
VP has a role in mediating the effects of aversive stimuli as well as predicting aversive outcomes (Table 5): Intra-VP injections of naloxone or the mu opioid antagonist CTOP is sufficient for the formation of a conditioned place aversion (Skoubis and Maidment, 2003). In rats that developed anxiety and despair-like behavior, intra-VP glutamate antagonists restore reduced VTA dopamine neuron activity to levels observed in nondepressed rats, perhaps through a BLA-VP-VTA circuit (Chang and Grace, 2014). Complex stereotyped behaviors such as “defensive” forepaw treading are induced by DAMGO or bicuculline injections throughout the VP, even though these drugs can also increase “liking” orofacial reactions and consumption within the posterior regions of VP (Smith et al., 2005). D1 receptor agonists injected in VP enhance inhibitory avoidance learning (Peczely et al. 2014).
The RMTg is critical for aversive processing and this region is linked with the VP through a VPvm – lateral habenula – RMTg pathway (Zahm et al., 1996; Jhou et al., 2009a; Goncalves et al., 2012). In rats and primates, RMTg and lateral habenula neurons decrease firing in response to reward-related cues but increase firing following reward omission (Matsumoto and Hikosaka, 2007; Jhou et al., 2009b; Hong and Hikosaka, 2008). The decreased firing of midbrain dopaminergic neurons that follow unexpected negative events (reward omission), is mediated by the lateral habenula – RMTg – midbrain dopamine serial pathway (Hong et al. 2011). Whether and how the VP plays a role in reward-prediction through its connections with lateral habenula, VTA, and/or RMTg is largely unexplored (Hong and Hikosaka, 2013). Primate VP neurons were not found to exhibit reward-prediction error signals (Tachibana and Hikosaka, 2012). Nevertheless, the circuits of the VPvm suggest complex manipulation of VTA neurons. In one examination (section 6.2.2), a small population of rat VP neurons were observed to exhibit differential firing patterns following cocaine reward versus no cocaine reward, suggesting some neurons may distinguish reward receipt from errors (Root et al., 2013).
6.0. Drugs of abuse; Influences on VP function and behavior
Neuroscience has come to view drug and alcohol addiction as a chronically relapsing disorder with an impaired ability to inhibit drug-seeking behavior (Kalivas, 2009; Koob and Volkow, 2010). This impairment could arise, in part, from alterations in VP function. It is therefore expected that if abused drugs act within the VP, there may be changes in motivated behavior as well as the processing of cue salience and/or mnemonic events. To discuss these possibilities, this section will overview the effects that abused drugs have on VP function as well as the subregionally-specific consequences that these alterations have on behaviors associated with substance use disorders.
6.1. VP consequences of exposure to abused drugs
6.1.1 Cocaine
6.1.1.1 Sensitivity
Individual VP neurons are comparatively more sensitive to intravenously injected cocaine than Acb or VTA neurons (Johnson and Napier, 1996; Henry and White, 1992; White, 1990). Based on this observation, Johnson and Napier (1996) posed that the behavioral consequences of low dose cocaine may largely reflect VP function, a concept that will be further explored in the below discussions. Consistent with this possibility, pharmacological inactivation of the Acb does not alter the number of VP neurons that respond to intravenous cocaine, nor change the capacity of cocaine to inhibit VP firing; however, excitatory VP effects of cocaine were enhanced with Acb inactivation (Johnson and Napier, 1996). This latter outcome may reflect a disinhibitory effect, as cocaine can reduce AcbC to VP GABA transmission (Torregrossa et al., 2008). Thus, while VP neuronal responding to systemic cocaine can be independent of the Acb, the Acb can regulate the magnitude of some VP cocaine-mediated firing patterns. To explore this interpretation, and the VP integration of other inputs, the Napier laboratory evaluated whether cocaine can modulate the effects of glutamate and GABA on VP firing. This was accomplished by using local (microiontophoretic) applications of cocaine in anesthetized rats wherein cocaine ejections that were too low to alter spontaneous firing were sufficient to modulate the excitatory effects of glutamate in 85% of the neurons tested, and the inhibitory effects of GABA in 50% of the neurons tested. These actions of cocaine mirror the ability of microiontophoretically applied dopamine to modulate VP glutamate and GABA (Johnson and Napier, 1997). Thus, the high sensitivity of VP neurons to cocaine likely relates to the ability of cocaine to elevate VP dopamine (overviewed below), and thus activate VP dopamine receptors, as well as to modulate GABA and glutamate transmission at the level of VP neurons.
The sensitivity of VP neurons to cocaine may provide a neurophysiological substrate for maintaining psychostimulant self-administration. Intravenous cocaine alters the firing rate in over 80% of VP neurons tested (Johnson and Napier, 1996). During cocaine self-administration, most VP neurons exhibit a ‘progressive reversal’ slow phasic firing pattern which consists of a post-cocaine change in firing rate followed by a reversal of this change as the drug level decays over time (Root et al., 2012). In the Acb, the slow phasic progressive reversal firing pattern is correlated with the time between cocaine infusions (Peoples and West, 1996), is independent of locomotion (Peoples et al., 1998), and in both the Acb and VP, is highly correlated with cocaine concentrations that wax and wane over several hours of a self-administration binge (Nicola and Deadwyler, 2000; Root et al., 2012). Given that drug level is the prepotent stimulus regulating psychostimulant self-administration (Norman and Tsibulsky, 2006; Pickens and Thompson, 1968; Root et al., 2011), West and colleagues proposed that the slow phasic progressive reversal pattern within the Acb (Peoples and West, 1996) and VP (Root et al., 2012) constitutes a mechanism that transduces fluctuating cocaine levels into neural signals that influence continued drug self-administration.
Significant numbers of VP neurons that project to VTA exhibit c-fos-IR in response to self-administration of cocaine (Geisler et al., 2008), and because the VPvm is the primary VP nucleus projecting to VTA, a VPvm-VTA pathway may be involved in maintaining cocaine self-administration. The region medial to VP, the lateral preoptic area (LPO), is also sensitive to fluctuating self-administered cocaine-levels. In fact, this preoptic area is comparatively more sensitive to fluctuating cocaine-levels during the maintenance phase of cocaine self-administration than VP (Barker et al., 2014). Similar to VP, the LPO receives input from Acb and projects to VTA (Usuda et al., 1998; Kowski et al., 2008), and therefore the LPO likely contributes to maintaining cocaine self-administration behavior (Barker et al., 2014a). Further research will be necessary to determine the unique contributions of VP and LPO towards the maintenance of cocaine self-administration. In both cases, because positive and negative emotional states also correlate with rising and falling self-administered cocaine levels (Barker et al., 2010, 2014b), it will also be interesting to determine if affective state can be discerned from VP or LPO firing patterns during self-administration.
6.1.1.2 Neurochemistry and adaptation
Cocaine dose-dependently increases extracellular dopamine concentrations in VP following non-contingent systemic (Gong et al., 1997a) and local (Gong et al., 1997a, 1998) administration. Extracellular concentrations of dopamine and 5-HT are also increased in VP during cocaine self-administration (Sizemore et al., 2001). The capacity of cocaine to alter VP neurotransmission appears to reflect aspects of motivation, as different changes in dopamine and glutamate turnover were observed following 30 days of cocaine self-administration but not after yoked exposure alone (Smith et al., 2003). Cocaine experience also alters basal glutamate and GABA extracellular concentrations in the VP during abstinence (Wydra et al. 2013). These outcomes may reflect adaptations that are related to the protracted cocaine treatment. Studies by Napier, Chafer and Shippenberg (Napier et al., 2001) of rats taken three days after five once daily injections of 20 mg/kg cocaine indicated a decrease in basal uptake and release with no change in dopamine levels in the VP. These latter studies also revealed that increases in VP dopamine caused by an acute challenge of cocaine are reduced in cocaine-experienced rats. These outcomes add to the emerging evidence (as further discussed below) of the dose, treatment duration and context of the cocaine experience all may influence the maladaptive nature of transmitter turnover within the VP.
Chronic exposure to cocaine results in a complex set of neuronal adaptations within the VP. Basal/spontaneous firing rates (in anesthetized rats) are reduced by >30% three days after five once-daily (non-contingent) injections of 15 mg/kg cocaine (a treatment protocol that induces behavioral sensitization; McDaid et al., 2005). This cocaine treatment protocol also reduces the ability of exogenously applied GABA to inhibit firing and promotes the ability of glutamate to increase firing (McDaid et al., 2005). These cocaine-mediated adaptations likely involve VP dopamine because the behaviorally sensitizing protocol for cocaine treatment enhances the ability of dopamine and the D1 receptor agonist SKF38393 to alter VP neuronal activity (Table 6) as well as the ability of dopamine to modulate amino acid transmission in the VP (Johnson and Napier, 1997). These observations suggest that after the development of cocaine sensitization, VP neurons become hyper-responsive to the rate-altering effects of dopamine and amino acid transmitters contained in VP afferents. Thus, chronic cocaine results in a reduced ability of GABAergic inputs to blunt an enhanced glutamatergic drive, and given that these effects are superimposed on a reduced background firing, the summated consequences would serve to promote glutamatergic and dopaminergic influences on VP function. The role of D1 receptors in these adaptations may be particularly relevant, as receptors in the D2 family are not altered by even robust chronic cocaine treatments (20 mg/kg/day for 14 days; Stanwood et al., 2000).
Table 6.
Comparisons of ejection current-response relationships for microiontophoretically applied dopamine and D1 agonists on VP neuronal firing between cocaine naïve and cocaine exposed rats.
| Cocaine Naïve | Cocaine Exposed | ||||||
|---|---|---|---|---|---|---|---|
| lontophoresed treatment | Response Category | Threshold2 (nA) | Ecur50 (nA) | Emax (% BL)3 | Threshold2 (nA) | Ecur50 (nA) | Emax (% BL)3 |
| Dopamine | Increase | 26±4 | 36±4 | +93±17 | 10±3* | 29±5 | +101±18 |
| Dopamine | Decrease | 32±4 | 38±4 | -58±5 | 15±4* | 39±7 | -73±4* |
| D1 Agonists1 | Decrease | 33±9 | 44±10 | -41±4 | 13±3* | 36±7 | -86±4* |
Both SKF38393 and SKF82958 were tested. The data did not differ for the two agonists and were pooled.
Threshold, the nA of current that produced a change in firing of 20% from baseline.
BL, pretreatment baseline firing, standardized as 100%. Protocols follow those used in McDaid et al., 2005. In brief, cocaine was given once daily as 15mg/kg (intraperitoneal) for five days. Three days later (a time when this treatment paradigm is sufficient to evoke sensitized motor responding to an acute cocaine challenge), the rats were prepared for in vivo electrophysiological evaluations of individual VP neurons. Microiontophoresis was used to locally apply dopamine and the D1 agonists, SKF38392 and SKF82958. Recordings conducted by Dr. Pat Johnson. Chronic exposure to cocaine decreased the threshold for both the firing rate increases and decreases to DA, and responding to the D1 agonists. Repeated cocaine administration also increased the magnitude of the rate decreases induced by DA and the D1 agonists (Emax). Potency (indicated by the iontophoretic current that produced 50% of the Emax, designated as Ecur50) for DA or the D1 agonists was not changed by chronic cocaine.
p<0.05, compared to cocaine naïve. Data from Dr. Napier.
The complexities of adaptations involving VP afferents are also observed after cocaine self-administration (Kupchik et al., 2014). A tonic reduction in GABA transmission is associated with elevated VP enkephalin levels, likely reflecting the reduced ability of VP mu receptor activation to presynaptically inhibit GABA release and mu receptor dependent long-term depression of GABA transmission is eliminated. Given that many Acb efferents to VP are GABAergic and enkephalinergic, these results suggest that this Acb neuronal subtype is altered by a history of cocaine self-administration.
6.1.2 Amphetamine, methamphetamine, and 3,4-methylenedioxy-methamphetamine (MDMA)
In vivo studies illustrate that VP neurons are readily engaged by low doses of amphetamines. VP neurons exhibit dose-related changes in firing to intravenously administered methamphetamine, with an ED50 of about 0.6 mg/kg (McDaid et al., 2007; Napier and Istre, 2008). A single 5 mg/kg intraperitoneal injection of amphetamine activates Fos in numerous VP neurons, many of which project to the VTA (Colussi-Mas et al., 2007).
Changes in VP are observed following repeated administration of abused amphetamines. With five, once-daily subcutaneous (non-contingent) injections of 2.5 mg/kg methamphetamine, the maximal excitatory effect (Emax) of acute methamphetamine is enhanced by 150% following three days of abstinence (Napier and Istre, 2008), whereas after 30 days, Emax is reduced to half of that obtained from methamphetamine-naïve rats (McDaid et al., 2007). Moderate doses of MDMA (5 and 10 mg/kg) produce a dose-related increase the number of Fos-immunoreactive neurons in the VP, and the MDMA-induced effect is enhanced two days after five days of repeated injections (Colussi-Mas and Schenk, 2008). We interpret these effects as VP neurons adapting to repeated exposure of amphetamines, the nature of which reflects the dosing protocol and the post-treatment withdrawal time. This interpretation also follows changes in VP signal transduction/gene transcription that are associated with chronic exposure to psychostimulants. For example, protein levels of the activated (phosphorylated) cAMP response element binding protein (pCREB; a constitutively expressed transcriptional regulator) are decreased in the Acb and the VP fourteen days after five once-daily injections of methamphetamine, a treatment/time paradigm that produces motor sensitization (McDaid et al., 2006). This finding is consistent with reports that increased Acb pCREB/CREB is associated with reductions in cocaine-induced motor sensitization (Sakai et al., 2002) and reward-motivated behaviors (Carlezon et al., 1998). Basal ΔFosB (a long-lasting transcription factor) is elevated in the Acb and VP following three days of abstinence from repeated methamphetamine (McDaid et al., 2006), consistent with ability of overexpression of ΔFosB in the Acb to enhance cocaine-induced motor behavior (Kelz et al.,1999). However, only in the VP did ΔFosB levels remain elevated following fourteen days of abstinence, which correlated with persistent methamphetamine-induced motor sensitization (McDaid et al., 2006). Amphetamine-induced CPP also is associated with c-Fos activity in the VP, AcbSh, and AcbC, as well as synaptogenesis in the VP and AcbSh (but not AcbC) (Rademacher et al., 2006).
Amphetamine-induced changes in monoaminergic systems are not widely studied for the VP. Three days after five once-daily injections of 2.5 mg/kg methamphetamine in rats, 5-HT2A/2C receptors are functionally upregulated in the VP (Napier and Istre, 2008). The effects of amphetamines on VP glutamate and GABA systems have only involved indices of receptor trafficking. For instance, a single low dose (1 mg/kg) of methamphetamine does not alter surface expression dynamics of AMPA or mGluR5 receptors in the VP (Herrold et al., 2013), nor is surface expression of mGlu5 or GABA-B receptors changed five days after three every other day treatments of 1 mg/kg methamphetamine, even though methamphetamine-induced CPP was observed (Herrold et al., 2011). However, the portion of mGluR5 receptors trafficked to the cell surface is enhanced fourteen days after three once-daily injection of 1 mg/kg methamphetamine, which is a time frame when methamphetamine induces expression of an mGluR5-dependent motor sensitization (Herrold et al., 2013).
Chronic exposure to amphetamines also alters VP processing of natural rewards and their predictors. VP neurons in animals sensitized to, or under the influence of amphetamine exhibit larger changes in firing rate following reward proximal cues, as well as following natural reward presentation, compared with nonsensitized animals (Tindell et al., 2005).
6.1.3 Alcohol
In alcohol-preferring rats, extracellular dopamine concentrations are increased during anticipation, self-administration, and following self-administration of ethanol, but not during similar periods for water or sucrose (Melendez et al., 2004). The enhanced dopamine concentrations likely reflects activity of the VTA because VTA dopamine neurons projecting to the VP are stimulated by local administration of ethanol, which is mediated at least in part by 5-HT3 receptor subtypes (Ding et al., 2011). The VP input may arise from more caudal parts of the VTA as injection of dopamine receptor antagonists into VP disrupts intracranial ethanol self-administration within the posterior VTA. Inhibiting VP D2 receptors significantly increases ethanol intake in alcohol-preferring rats (Melendez et al., 2005), suggesting that activating D2 receptors in the VP limits ethanol intake.
VP GABA and opioids are capable of bidirectionally controlling ethanol consumption. GABA-A or GABA-B receptor agonists decrease ethanol intake whereas the GABA-A antagonist bicuculline increases ethanol consumption (Kemppainen et al., 2012a). Of the GABA-A receptors within the VP, the GABA-A1 isoform clearly plays a role in regulating ethanol self-administration (Harvey et al., 2002; June et al., 2003). Intra-VP injection of the mu opioid agonists DAMGO or morphine dose-dependently decreases ethanol intake and conversely, blocking mu receptors with CTOP increases ethanol intake (Kemppainen et al., 2012b). Simultaneous VP administration of DAMGO and bicuculline blocks induced ethanol intake (Kemppainen et al., 2012a).
6.1.4 Opioids
Repeated VP injections of mu opioid agonists DAMGO or morphine are sufficient to induce behavioral sensitization (Zarrindast et al., 2007; Mickiewicz et al., 2009; Rokosik et al., 2013), revealing that activation of mu opioid receptors is sufficient for the induction of the behavior. Showing that these receptors are also necessary, behavioral sensitization induced by repeated systemic injections of morphine is blocked by intra-VP injections of the mu opioid antagonist CTOP (Johnson and Napier, 2000; Mickiewicz et al., 2008). Moreover, VP ionotropic glutamatergic receptors are necessary for the maintenance of behavioral sensitization previously induced by systemic injections of morphine, as maintenance is blocked by VP injections of the glutamate antagonists, CNQX and AP-5 (Dallimore et al., 2006). Glutamatergic adaptations to chronic exposure to opiates include an increase in VP mGlu5 receptor surface expression (Herrold et al., 2013). VP GABA is another critical contributor to the behavioral effects of chronic opioids. Self-administration of heroin decreases extracellular GABA concentrations in the VP (Caillé and Parsons 2004), and elevated extracellular VP GABA concentrations decrease heroin self-administration (Xi and Stein, 2000).
6.1.5 Stimulant interactions with opioids at the level of the VP
As many abused drugs alter the function of VP, part of the maladaptations that occur with chronic exposure to one abused drug may influence the function of another. For example, opioid receptor expression is increased within VP as a consequence of chronic cocaine exposure (Hammer, 1989) and the capacity of morphine to alter VP neuronal activity is enhanced in cocaine-sensitized rats (McDaid et al., 2005). Likewise, in post-mortem cocaine users, VP dynorphin concentration is increased by over 300% from controls (Frankel et al., 2008).
6.2 Self-administration
6.2.1 Circuits
VP is part of several circuits that are necessary for drug self-administration (Table 7; Figure 7). In addition to the VP, regions included in these these circuits are the ventral tegmental area, medial prefrontal cortex, AcbC and AcbSh, VP, BLA, and MD (McGregor and Roberts, 1993, 1995; Weissenborn et al., 1997, 1998; Fabbricatore et al., 2010; Di Ciano 2008; Bari and Pierce, 2005; Di Ciano and Everitt 2004; You et al., 2007; Xi and Stein, 2002; Roberts and Koob, 1982; Kantak et al., 2002; Yun and Fields, 2003; Carelli et al., 2003; Roberts et al., 1980; Pettit et al., 1984; Schenk et al., 1991; Sun and Rebec, 2005; Hubner and Koob, 1990; Li et al. 2009; Goeders and Smith, 1983). The VPvm is positioned to affect most of the above brain regions but VPdl is also highly likely to play key roles in drug self-administration.
Table 7.
VP manipulations and effects related to drugs of abuse.
| Reference | VP Manipulation | System | Effects | Task | Result |
|---|---|---|---|---|---|
| Dallimore et al., 2006 | CNQX and AP-5 | Glutamate | Multiple | Behavioral sensitization with morphine | Blocked |
| Kalivas et al., 1991 | DAMGO | Opioid | Mu agonist | Behavioral sensitization with morphine | Expressed |
| Rokosik et al., 2013 | DAMGO | Opioid | Mu agonist | Behavioral sensitization with morphine | Expressed |
| Johnson and Napier 2000 | CTOP | Opioid | Mu | Behavioral sensitization with morphine | Blocked |
| Mickiewicz et al., 2008 | CTOP | Opioid | Mu | Behavioral sensitization with morphine | Blocked |
| Mickiewicz et al., 2009 | Morphine | Opioid | Multiple | Behavioral sensitization with morphine | Expressed |
| Zarrindast et al., 2007 | Morphine | Opioid | Multiple | Behavioral sensitization with morphine | Expressed |
| Chen et al., 2001 | MK801 | Glutamate | NMDA antagonist | Behavioral sensitization with amphetamine | Blocked |
| Skoubis and Maidment 2003 | Naloxone | Opioid | Multiple antagonist | Conditioned Place Aversion | Formed |
| Skoubis and Maidment 2003 | CTOP | Opioid | Mu antagonist | Conditioned Place Aversion | Formed |
| Gong et al., 1996 | Cocaine | DA | Multiple | Conditioned Place Preference | Formed |
| Gong et al., 1996 | Amphetamine | DA | Multiple | Conditioned Place Preference | Formed |
| Ikemoto 2003 | Cocaine | Dopamine | Multiple | Conditioned Place Preference | No effect |
| Gong et al., 1997 | Picrotoxin | GABA | Antagonist | Conditioned Place Preference | No effect |
| Gong et al., 1997 | AMPA | Glutamate | AMPA | Conditioned Place Preference | No effect |
| Gong et al., 1996 | Procaine | Sodium | Antagonist | Conditioned Place Preference | No effect |
| Gong et al., 1997 | 6-OHDA | DA/NE | NA | Conditioned place preference (cocaine) | Decrease |
| Skoubis and Maidment 2003 | Naloxone | Opioid | Multiple | Conditioned place preference (cocaine) | Attenuated |
| Dallimore et al., 2006 | CNQX and AP-5 | Glutamate | Multiple | Conditioned place preference (morphine) | Blocked |
| McAlonen et al., 1993 | Lesion | NA | NA | Conditioned place preference (sucrose) | Attenuated |
| Ikemoto 2003 | Cocaine | Dopamine | Multiple | Intracranial self-adminsitration | No effect |
| Torregrossa and Kalivas 2008 | NT8-13 | Neurotensin | Agonist | Reinstatement of cocaine-seeking maintained by conditioned reinforcer | Attenuated |
| McFarland and Kalivas 2001 | Fluphenazine | Dopamine | D1/D2 antagonist | Reinstatement of cocaine-seeking triggered by cocaine | No effect |
| McFarland and Kalivas 2001 | Baclofen/Muscimol | GABA | GABA-A/B Agonist | Reinstatement of cocaine-seeking triggered by cocaine | Decrease |
| Tang et al., 2005 | Cocaine | Multiple | Multiple | Reinstatement of cocaine-seeking triggered by cocaine | Increase |
| Tang et al., 2005 | Cocaine+Morphine | Multiple | Multiple | Reinstatement of cocaine-seeking triggered by cocaine | Mixed |
| Torregrossa and Kalivas 2008 | NT8-13 | Neurotensin | Agonist | Reinstatement of cocaine-seeking triggered by cocaine | Increase |
| Torregrossa and Kalivas 2008 | SR142948 | Neurotensin | Antagonist | Reinstatement of cocaine-seeking triggered by cocaine | No effect |
| Tang et al., 2005 | CTAP | Opioid | Mu Antag | Reinstatement of cocaine-seeking triggered by cocaine | Decrease |
| Tang et al., 2005 | Morphine | Opioid | Multiple | Reinstatement of cocaine-seeking triggered by cocaine | Increase |
| Li et al., 2009 | AMN082 | Glutamate | mGluR7 Agonist | Reinstatement of cocaine-seeking triggered by cocaine | Decrease |
| Stefanik et al. 2013 | Optogenetic inhibition of Core projection to VPdl | GABA | NA | Reinstatement of cocaine-seeking triggered by cocaine+cues | Decrease |
| McFarland et al., 2004 | Baclofen/Muscimol | GABA | GABA-A/B Agonist | Reinstatement of cocaine-seeking triggered by footshock | Decrease |
| Tang et al., 2005 | CTAP | Opioid | Mu Antagonist | Reinstatement of cocaine-seeking triggered by morphine | Blocked |
| Tang et al., 2005 | CTAP | Opioid | Mu Antagonist | Reinstatement of cocaine-seeking triggered by morphine | Blocked |
| Perry and McNally, 2013 | CTAP | Opioid | Mu Antagonist | Reinstatement of alcohol-seeking triggered by context | Blocked |
| Perry and McNally, 2013 | CTAP | Opioid | Mu Antagonist | Reinstatement of alcohol-seeking triggered by alcohol | Blocked |
| McFarland and Kalivas 2001 | Baclofen/Muscimol | GABA | GABA-A/B Agonist | Reinstatement of food-seeking triggered by food | Decrease |
| McFarland and Kalivas 2001 | Unilateral VP Baclofen/Muscimol & Contralateral mPFC baclofen/muscimol | GABA | GABA-A/B Agonist | Reinstatement of cocaine-seeking triggered by cocaine | Decrease |
| Tang et al., 2005 | CTAP | Opioid | Mu Antagonist | Reinstatement of food-seeking triggered by food | No effect |
| Rogers et al., 2008 | Baclofen/Muscimol | GABA | GABA-A/B Agonist | Reinstatement of heroin-seeking maintained by conditioned reinforcer | Blocked |
| Rodgers et al., 2008 | Baclofen/Muscimol | GABA | GABA-A/B Agonist | Reinstatement of heroin-seeking maintained by conditioned reinforcer | Blocked |
| Rogers et al., 2008 | Baclofen/Muscimol | GABA | GABA-A/B Agonist | Reinstatement of heroin-seeking triggered by heroin | Blocked |
| Rodgers et al., 2008 | Baclofen/Muscimol | GABA | GABA-A/B Agonist | Reinstatement of heroin-seeking triggered by heroin | Blocked |
| Li et al., 2009 | AMN082 | Glutamate | R7 agonist | Self-administration of cocaine | Decrease |
| Robledo and Koob 1993 | Lesion | NA | NA | Self-administration of cocaine | Decrease |
| Robledo and Koob 1993 | Lesion | NA | NA | Cocaine self-administration breaking point (progressive ratio) | No effect |
| Torregrossa and Kalivas 2008 | NT8-13 | Neurotensin | Agonist | Self-administration of cocaine | No effect |
| Torregrossa and Kalivas 2008 | SR142948 | Neurotensin | Antagonist | Self-administration of cocaine | No effect |
| Li et al., 2009 | AMN082 | Glutamate | R7 Agonist | Self-administration of cocaine breakpoint | Decrease |
| Robledo and Koob 1993 | Lesion | NA | NA | Self-administration of cocaine breakpoint | No effect |
| Torregrossa and Kalivas 2008 | NT8-13 | Neurotensin | Agonist | Self-administration of cocaine extinction | No effect |
| Torregrossa and Kalivas 2008 | SR142948 | Neurotensin | Antagonist | Self-administration of cocaine extinction | No effect |
| Harvey et al., 2002 | 3-PBC | GABA | GABA-A1 Mixed | Self-administration of cocaine ethanol | Decrease |
| June et al., 2003 | BCCt | GABA | GABA-A1 Mixed | Self-administration of ethanol | Decrease |
| Harvey et al., 2002 | 3-PBC | GABA | GABA-A1 Mixed | Self-administration of saccharine | Increase |
| Melendez et al. 2005 | Sulpiride | Dopamine | D2 antagonist | Ethanol intake | Increase |
| Kemppainen et al., 2012a | Muscimol | GABA | GABA-A Agonist | Ethanol intake | Decrease |
| Kemppainen et al., 2012a | Bicuculline | GABA | GABA-A Antagonist | Ethanol intake | Increase |
| Kemppainen et al., 2012a | Bicuculline + DAMGO | GABA + Opioid | Multiple | Ethanol intake | Blocked |
| Kemppainen et al., 2012a | Baclofen | GABA | GABA-B Agonist | Ethanol intake | Decrease |
| Kemppainen et al., 2012a | Saclofen | GABA | GABA-B Antagonist | Ethanol intake | No effect |
| Kemppainen et al., 2012b | DAMGO | Opioid | Mu agonist | Ethanol intake | Decrease |
| Kemppainen et al., 2012b | Morphine | Opioid | Agonist | Ethanol intake | Decrease |
| Kemppainen et al., 2012b | CTOP | Opioid | Mu antagonist | Ethanol intake | Increase |
Manipulation refers to an intra-VP injection or VP lesion, unless stated otherwise.
Figure 7. Proposed changes in firing rate during responses for drugs of abuse involving the VP subregions.
Neurons of the AcbC exhibit increased firing rates during responses for cocaine (Ghitza et al. 2004), which are likely the result of dPFC and BLA activation during the same behavior (Chang et al., 1997, 2000; Carelli et al., 2003). Activation of AcbC leads to decreased firing rates of VPdl neurons during the response (Root et al., 2013) and likely disinhibits its target structures SNr and STN (Lardeux et al., 2013; Fan et al., 2012). While AcbSh neurons are less sensitive to the response compared with AcbC neurons, AcbSh neurons exhibiting changes in firing rate during the response are heterogeneous (Ghitza et al., 2004). Such heterogeneity is found within the VPvm subregion (Root et al., 2013) and is likely to continue within its target regions VTA and MD. The involvement of VPvl and its circuits (SNc and DLS; dotted lines with arrows) is unclear (white color). Dashed lines with arrows represent circuits involving VPvm. Solid lines with arrows represent circuits involving VPdl. Purple shaded structure indicates heterogeneous (increased or decreased) change in firing rate during cocaine self-administering responses. Red shaded structure and green shaded structure indicates decreased and increases change in firing rate during cocaine self-administering responses. The illustrated subregions are proposed to represent subregions throughout the anteroposterior extent of the VP.
VPdl and VPvl are positioned to affect the nigrostriatal system (Figure 7). Dopaminergic antagonism of the substantia nigra (SN) (Quinlan et al., 2004) or dorsolateral striatum (Vanderschuren et al., 2005; Belin and Everitt, 2008) reduces cocaine self-administration suggesting the dopaminergic projection SN to dorsolateral striatum is involved in this behavior. Given that the VPvl projects to SNc, this VP subregion may play a role in modulating the nigrostriatal involvement in cocaine self-administration. STN lesions alter cocaine self-administration behavior, including cocaine breakpoints (Baunez et al., 2005; Uslaner et al., 2009; Rouaud et al., 2010). Both the AcbC and VPdl project to STN, suggesting the AcbC-STN and VPdl-STN pathways may also manipulate nigrostriatal involvement in cocaine self-administration.
6.2.2. Associations between VP neuronal activity and drug-motivated behaviors
6.2.2.1 Behavior-related firing patterns
Subsets of VP neurons exhibit “rapid phasic” changes in firing rate surrounding cocaine-reinforced responses (Root et al., 2010), supporting a VP involvement in drug-seeking behavior. Root and colleagues (2013) extended their initial investigation of VP firing patterns by investigating 1) what behaviors VP neurons are sensitive to during cocaine self-administration and 2) whether differences exist in the changes in firing rate between neurons of the VPdl and VPvm subregions, as delineated by substance P-IR, calbindin d28k-IR, and neurotensin-IR. A number of critical insights were made into VP subregional function.
Of responsive VP neurons, changes in firing rate occurred when animals were approaching to obtain cocaine, responding to acquire cocaine, retreating away after responding, or any combination of these behaviors (Root et al., 2013; Figure 8). Similar VP firing patterns were observed between the initial investigation using a lever-press response requirement, and a subsequent investigation using a vertical head movement response requirement, suggesting VP neurons acquire responsivity to drug-seeking behaviors, despite different motor demands to acquire cocaine. Ventral striatopallidal processing has been hypothesized to involve the “initiation” of actions (Mogenson et al., 1980) and if so, firing patterns might be expected to precede the onset of approaching to obtain cocaine. However, it was identified that VP firing patterns were coincident with approach, response, and retreat behaviors (Figure 8A,B), and did not occur preceding the onset of approach. Moreover, VP behavioral firing patterns during cocaine self-administration differed according to the particular subregion in which the VP neuron was recorded from. Root and colleagues (2013) interpreted these results as neurons within discrete VP subregions playing different roles in the process of self-administering cocaine.
Figure 8. VP subregions are differentially associated with drug-seeking behaviors during cocaine self-administration.
A. Top shows illustration of behaviors used to analyze changes in firing rate recorded from single VP neurons during discrete behaviors involved in self-administering cocaine. Approach occurred when the animal initially moved towards a set of photocells used as a response device (left), response occurred when the animal emitted a learned long distance vertical head movement (middle), and retreat occurred when the animal moved away from the photocells; right). Bottom shows an example approach-related firing pattern of a VPvm neuron. For A, B, F, and G, green dot is the onset of the approach and time zero is the offset of approach (left), blue dot is the onset of response and time zero is offset of response (middle), and red dot is offset of retreat while time zero is onset of retreat (right). B. An example response-related firing pattern of a VPvm neuron. C. VPdl neurons exhibit a significantly greater correlation between directional changes in firing rate during approach and response compared to VPvm neurons. Directional change in firing rate refers to change from baseline according to a B/(A+B) formula, where ‘B’ is firing rate during movement (e.g., approach or response), and ‘A’ is firing rare during baseline. D. Neurons classified as approach-response and approach-response-retreat were observed significantly more often within VPdl compared to VPvm, χ2(1) = 4.03, p = 0.04. E. Neurons classified as retreat-only and response-retreat were observed significantly more often within VPvm compared to VPdl, χ2(1) = 5.96, p = 0.01. F. Example approach-response firing pattern of VPdl neuron. G. Example approach-response-retreat firing pattern of VPdl neuron. * p < 0.05; *** p < 0.001. Data from Dr. Root while in the West laboratory (Rutgers), published in Root et al., 2013.
6.2.2.2 Differential roles of VP subregions
VPdl neurons exhibit significantly greater changes in firing rate than VPvm neurons during the drug-seeking approach as well as during the drug-taking response. When single VPdl neurons change in firing rate during the approach, these changes were highly likely to continue in the same direction (increase or decrease from baseline), with similar or greater magnitude through the response (Figure 8C-F). The stronger, sustained firing rate changes of VPdl neurons during approach and response implicate VPdl in the processing of drug-seeking and drug-taking as a singular behavior. A significant population of VPdl neurons continues to change its firing rate through the entire sequence of acquiring cocaine, approach-response-retreat (Figure 8G). In other words, VPdl sends a signal related to pursuing cocaine and the reaction to obtain cocaine.
The pursue and react signals of VPdl neurons are similar to proposed function of AcbC neurons in biasing an organism to “go to it” (the reward) (Floresco, 2014). VPdl neurons exhibit a greater change in firing rate during the approach and response for cocaine than VPvm neurons, and likewise, AcbC neurons exhibit a greater change in firing rate than medial AcbSh neurons during the same behaviors (Ghitza et al., 2004; Hollander and Carelli, 2005; Fabbricatore et al., 2010). Furthermore, during the response, AcbC neurons are significantly more excited than AcbSh neurons (Ghitza et al., 2004) and VPdl neurons are significantly more inhibited than VPvm neurons (Root et al., 2013), suggesting that excited AcbC neurons inhibit VPdl neurons during the response for cocaine (Figure 7). Inhibition of VPdl neurons is likely to disinhibit the VPdl targets STN and SNr during drug-taking movements. Putative GABA neurons recorded in the medial SNr, a termination site of VPdl (Zahm 1989; Zahm et al., 1996), exhibit robust changes in firing rate during responses (Fan et al., 2012). Taken together, the AcbC-VPdl circuit is an important regulator of drug seeking and taking behaviors, possibly through the SNr (Figure 7; further discussed in Section 6.3.2).
VPvm neurons are typically heterogeneous with respect to behavioral firing patterns and firing direction (increase or decrease from baseline). VPvm neurons have a significantly weaker approach-response firing change relationship than VPdl neurons (Figure 8C). However, VPvm exhibits some behavior-related firing patterns significantly more often than VPdl. These firing patterns occur during retreat alone and response-retreat (Figure 8E). Firing patterns involving the retreat may reflect processing of the outcome of responding, which likely is a function of the part of VP that receives inputs from AcbSh (e.g., the VPvm; Leung and Balleine, 2013). An outcome-processing component of the VPvm may reflect the proposed function of AcbSh neurons in biasing an organism to “stay on task” (Floresco, 2014).
The heterogeneous firing patterns of VPvm neurons implicates VPvm in facilitating its targets (e.g., mesocortical structures) with information related to the sequence of individual behaviors (approach, response, retreat), and consequences of actions (retreat, response-retreat) involved in predicting the attainment of cocaine. This is supported by the finding that approach or response-related firing patterns within the mPFC typically occur after approach or response-related firing patterns occur within the Acb (Chang et al., 2002). Our results together with those of Chang and colleagues suggest that mPFC behavioral firing patterns are facilitated by VPvm-MD-mPFC or VPvm-VTA-mPFC circuits (Figure 7). This may facilitate prefrontal processing of response-outcome relationships in order to guide future behavior. In conclusion, the VPvm and VPdl subregions appear to process distinct aspects of cocaine self-administration. Processing within both VP subregions is likely necessary to produce coherent self-administration behavior, with the VPvm signaling the conditions involved in attaining cocaine and the VPdl involved in the actions that obtain cocaine.
6.3. Drug-seeking behaviors
6.3.1 Cue reactivity
VP participates in the conditioning of stimulus-drug associations. CPP is formed following intra-VP dopamine, cocaine, or amphetamine injections (Gong et al., 1996; though see Ikemoto 2003). Several transmitters are important for this process. Cocaine-induced CPP can be attenuated by VP mu opioid receptor antagonism, which creates conditioned place aversion (Skoubis and Maidment, 2003), or by 6-OHDA-induced lesions of VP (Gong et al., 1997b). Further, morphine-induced CPP can be blocked by intra-VP injections of the AMPA and NMDA glutamate receptor antagonists CNQX and AP-5 (Dallimore et al., 2006). Dopamine within the Acb and VP are also involved in psychostimulant-induced CPP, and dopamine concentrations within both regions correlate with CPP to cocaine conditioned place preference (Sellings and Clarke, 2003; Gong et al., 1997b).
Drug-induced CPP also results in plasticity within VP. Repeated pairings of amphetamine and a unique context which produced CPP in rats results in increases in VP synaptophysin (a marker for synaptogenesis) as well as increases in tyrosine kinase B receptor protein (a receptor for BDNF, a neurotrophic factor that mediates synaptic plasticity) (Rademacher et al., 2006). These outcomes were not obtained when an association was not made between amphetamine treatments and a particular context (referred to as unpaired controls). As the amphetamine treatments were the same for these two conditions, it appears that ‘learning’ was the unique factor that contributed to VP synaptic plasticity.
6.3.2 Reinstatement
The VP is part of several circuits necessary for reinstatement of extinguished drug-seeking behavior (Figure 9). Injections of cocaine or high doses of morphine into VP alone are sufficient to reinstate extinguished drug-seeking responses (Tang et al., 2005). Reinstatement of extinguished seeking behavior is disrupted by intra-VP microinjections triggered by the following stimuli: conditioned reinforcers (commonly referred as “cue-induced”; Rodgers et al., 2008; Torregrossa and Kalivas, 2008), heroin (Rodgers et al., 2008), cocaine (McFarland et al., 2001; Torregrossa and Kalivas, 2008; Li et al., 2009; Tang et al., 2005), alcohol (Perry and McNally, 2013), footshock (McFarland et al., 2004), context (Perry and McNally, 2013), and food (McFarland and Kalivas, 2001) (Figure 9). Similar to self-administration behavior, cocaine-induced reinstatement decreases VP extracellular concentrations of GABA, which are blocked (along with reinstatement) by intra-VP injections of mu opioid receptor agonists (Tang et al., 2005) or mGluR7 agonists (Li et al., 2009).
Figure 9. Proposed reinstatement circuits involving the VP subregions.
Theoretical schema of brain regions and circuits that involve VP subregions and their participation in different types of reinstatement of drug-seeking behavior. Presence of colored squares indicates region is necessary for the type of reinstatement. Question mark in VP indicates that a test has not yet been performed. Absence of colored squares in all other regions indicates either test not yet performed or region not involved. Dashed lines represent circuits involving VPvm. Solid lines represent circuits involving VPdl. Supporting literature includes the following: Ventral hippocampus (vHipp) - Lasseter et al. 2010; Sun and Rebec 2003; vPFC – Peters et al., 2008; Rodgers et al. 2008; Bossert et al. 2011; LaLumiere et al., 2012; Dorsal prefrontal cortex (dPFC) - McFarland and Kalivas 2001, 2004; Rodgers et al. 2008; Fuchs et al. 2005; Capriles et al. 2003; Dorsal hippocampus (dHipp) - Fuchs et al. 2005; dPFC/dHipp - Fuchs et al. 2007; BLA - Kantak et al. 2002; Feltenstein and See 2007; Alleweireldt et al. 2006; Fuchs et al. 2005; You and Fields, 2003; Ledford et al. 2003; McLaughlin and See, 2003; Hayes et al. 2003; Fuchs and See 2002; Kruzich and See 2001; Grimm and See, 2000; Stefanik and Kalivas, 2013. BLA/dHipp - Fuchs et al. 2007; AcbSh – Peters et al., 2008; Rodgers et al. 2008; Bossert et al. 2007; BLA/AcbC – Stefanik and Kalivas, 2013; dPFC – Stefanik and Kalivas, 2013; BLA/AcbC – Stefanik and Kalivas, 2013; dPFC/AcbC – based on Kalivas and McFarland, 2001; vPFC/AcbSh – Peters et al., 2008; Bossert et al. 2012; LaLumiere et al., 2012; AcbC - Rodgers et al. 2008; McFarland and Kalivas 2001, 2004; Bossert et al. 2007; Xie et al., 2012; Fuchs et al., 2008; AcbC/VPdl – Stefanik et al., 2013; DLS - Fuchs et al. 2006; See et al. 2007; Rodgers et al. 2008; Bossert et al. 2009; SN/DLS – based on Bossert et al., 2009; VTA - McFarland and Kalivas 2001, 2004; Sun et al. 2005; Di Ciano and Everitt 2004; Wang et al. 2009; You et al. 2007; Schmidt et al. 2009; VP/VTA - Mahler et al. 2014; VP - Tang et al. 2005; Rodgers et al. 2008; McFarland and Kalivas 2001, 2004; Li et al. 2009; Torregrossa and Kalivas 2008; Lu et al. 2012; Mahler et al. 2012; Perry and McNally, 2013; VTA/dPFC – based on McFarland and Kalivas, 2001; McFarland et al., 2004; Capriles et al., 2003; SN - Sun et al. 2007; Rodgers et al. 2008; PPTg - Schmidt et al. 2009; VTA/AcbSh - LaLumiere et al., 2012; Bossert et al., 2007; VTA/BLA - LaLumiere et al., 2012; VTA/AcbC – based on Bossert et al., 2007.
The AcbC-VPdl circuit plays a role in drug-seeking behavior. AcbC is required for most reinstatement protocols and is most closely linked with dorsomedial PFC and dorsal hippocampal systems, both of which also have critical roles in reinstatement (Figure 9). Given that the VPdl projects to the SNr, which is also important for reinstatement (Rodgers et al., 2008), as is the SNr target, the dorsolateral striatum (Fuchs et al., 2006; See et al., 2007; Rodgers et al., 2008; Bossert et al., 2009), the VPdl likely has a role in reinstatement behaviors. AcbC neurons projecting to the VP exhibit elevated fos-IR during reinstatement (Perry and McNally, 2013). During the cocaine-seeking response, AcbC neurons exhibit elevated firing rates (Ghitza et al., 2004; Fabbricatore et al., 2010), which decrease VPdl firing rates during the same response behavior (Root et al., 2013). Preventing VPdl neurons from decreasing their firing rates during the response through optogenetic inhibition of AcbC-VPdl terminals blocks cocaine-plus-cue-primed reinstatement (Stefanik et al., 2013). Optical inhibition of the VPdl-AcbC and AcbC-SN pathways had no effect on this type of reinstatement, demonstrate the necessity of the AcbC-VPdl pathway for drug-seeking behavior.
The AcbSh-VPvm circuit is also likely to play a role in drug-seeking behavior. AcbSh pathways involving the ventromedial PFC and ventral hippocampal systems are involved in several reinstatement types (Figure 9). Given that the AcbSh discriminates cocaine-related cues from neutral cues during reinstatement (Ghitza et al., 2003), VPvm may play a role in cued reinstatement, but has not been investigated. Intra-VP injection of the neurotensin agonist NT8-13 potentiates cocaine-induced reinstatement and attenuates cue-reinforced reinstatement (Torregrossa and Kalivas, 2008). Because neurotensin-IR partially defines VPvm, these data raise the possibility that VPvm is involved in at least cocaine-induced reinstatement.
It has recently been established that the VP-VTA pathway is an important circuit involved in reinstatement. Of VP neurons that project to VTA (e.g., from the VPvm), significantly more exhibit c-fos-IR following conditioned reinforcer reinstatement than under extinction conditions alone, following neutral cue exposure, or following novel cue exposure (Mahler and Aston-Jones, 2012). Furthermore, the percent of fos-immunoreactive VP neurons projecting to VTA predicts the number of cocaine-seeking lever presses during reinstatement. These data are consistent with the notion that VPvm facilitates information related to the events or conditions that predict cocaine self-infusions (section 6.2.2).
Differential projection targets of VPvm neurons have the potential to control different drug-seeking behaviors. Alterations of VTA neurochemistry decrease most reinstatement methods (McFarland and Kalivas, 2001, 2004; Sun et al., 2005; Di Ciano and Everitt, 2004; Wang et al., 2005, 2009; You et al., 2007; Schmidt et al., 2009; Mahler et al., 2012; Lu et al., 2012; Figure 9). Disruption of the VP-VTA pathway arising from the anterior half of VP, but not from the posterior half of VP, blocks conditioned reinforcer reinstatement (Mahler et al. 2014). In addition, disruption of the VP-VTA pathway arising from the posterior half of VP, but not from the anterior half of VP, blocks cocaine-induced reinstatement (Mahler et al. 2014). While MD baclofen/muscimol injections have no effect on cocaine or footshock induced reinstatement (McFarland and Kalivas, 2001; McFarland et al., 2004), MD and VP lesions are reported to reduce sucrose-mediated CPP (McAlonan et al., 1993).
6.4 Beyond mice and rats
6.4.1 Prairie voles and zebra finches
While not covered in the present review, VP has a long evolutionary history (Smeets et al., 2000; O'Connell and Hofmann, 2011, 2012; Ganz et al., 2012). Similar to rats, subregional differences exist in VP function of other species. Research into the underlying neural mechanisms for monogamy using Microtus ochrogaster voles has revealed the VP as a critical nucleus for monogamous pair bonding. In this model, viral upregulation of vasopressin V1a receptors within and surrounding VP enhances pair bonding (Pitkow et al., 2001). Furthermore, VP V1a receptor antagonism decreases partner contact time compared with stranger contact time (partner preference) (Lim and Young, 2004). Critically, mRNA expression of the V1a receptor is localized to the VPvm (Lim et al., 2004), indicating that the circuits of the VPvm selectively influence pair bonding.
Research into the underlying neural mechanisms for zebra finch song learning have revealed that single VP neurons decrease firing rates most robustly in response to the bird's own unique song (Gale and Perkel, 2010). Such decreases may disinhibit VTA/SN neurons (Gale and Perkel, 2010). Future research is needed to identify the subregional VP contributions towards birdsong processing.
6.4.2 The role of the VP in human normal behaviors and brain disorders
As preclinical characterization of the VP is emerging, the role of this region in emotional processing, motivated behaviors, and the psychiatric consequence of its dysregulation in humans is gaining attention (for review, see Napier and Mickiewicz, 2010). Several clinical reports point to VP involvement in disorders of motivation and impulsivity. Functional magnetic resonance imaging (fMRI) during forced choice tasks that require controlling desire for immediate rewards show reductions in task-induced activation of the VP in male participants (Diekhof et al., 2012). Single-photon emission computed tomography of Parkinson disease patients with pathological gambling show enhanced resting state activity (regional cerebral blood flow) in the VP of these individuals compared to non-gambling patients (Cilia et al., 2008). fMRI can be used to assess rapid responses of the brain to “unseen” reward cues, or cues that are recognized outside of our awareness. Presentation of unseen cues for natural and drug-related rewards (Childress et al., 2008), or monetary rewards (Pessiglione et al., 2007), results in rapid activation of the VP prior to conscious recognition. Similar responses also predict the positive affect evoked by the same stimuli when presented in a visible manner (Childress et al., 2008). These findings are substantiated by fMRI reports of visually perceived foods showing increased VP activity that correlats with the degree of food ‘pleasantness’ (Rudenga et al., 2010; Simmons et al., 2014). Underscoring the role of the VP in dysregulated food reward processes is the positive correlation of positron emission tomography (PET) for 5HT4 receptor density in this region with body mass index indicative of obesity in humans (Haahr et al., 2012). PET for mu opioid receptors indicate that increases in negative affect ratings by healthy human volunteers, which are associated with sustained sadness (Zubieta et al., 2003), or sustained muscle pain (Zubieta et al., 2002), correlate with deactivation of these receptors in the VP. Underscoring the involvement of the VP in substance use disorders are PET observations that dopamine D3 receptors are upregulated in human methamphetamine polydrug users, which is in stark contrast to other forebrain regions wherein D2 receptors are down-regulated (Boileau et al., 2012). Suggesting VP involvement in cocaine addiction, an endogenous opioid, dynorphin, is strikingly upregulated (i.e., by 346%) in the VP of post mortem brains from human cocaine abusers (Frankel et al., 2008). In summary, the VP is not yet widely studied in humans and caution is needed in interpreting human imaging studies as the VP is often not clearly resolved (Zaborszky et al., 2015b). Nonetheless, current reports of the human brain largely concur with the conclusions drawn from research in laboratory animal models, and are consistent with predictions based on the anatomical connections of the VP. The role of this region as an integrator of sensory, emotional and cognitive information with appropriate motoric responses is becoming clear, and the association of pallidal dysfunction in human brain disorders is also becoming increasingly apparent.
7.0 Concluding remarks – differential information flow across VP subregions and neuronal phenotypes
The VP is necessary for a variety of behaviors. Some are adaptive, such as those involving seeking and consuming food or ensuring the health and safety of offspring. Other behaviors are pathological, such as the self-administration of abused drugs. In this review, we put forth the notion that VP regulates these diverse behaviors via unique channels of information processing from GABAergic neurons belonging to individual subregions and from nonGABAergic neuronal phenotypes.
The VPdl, which receives projections from the AcbC and targets the STN and SNr, is intimately connected with the extrapyramidal motor system. Though untested for other behaviors, VPdl is necessary for the reinstatement of drug-seeking behavior (Stefanik et al., 2013). Evidence suggests that VPdl is involved in processing various reward-motivated behaviors. VPdl neurons exhibit significantly larger changes in firing rate than VPvm neurons during approaches toward, and responses on, a cocaine-reinforced device (Root et al., 2013). Further, VPdl approach-related changes in firing rate largely continue through the completion of the response (Root et al., 2013), suggesting VPdl signals are related to the pursuit and attainment of rewards, similar to a “go to it” function of the AcbC (Floresco, 2014). Taken together, the VPdl subregion and its projections to STN and SNr constitute one mechanism by which various motivated behaviors may be mediated.
The VPvm receives projections from the AcbSh and targets the MD, VTA, LH, and lateral habenula. The broad targets of this subregion suggest that the VPvm is involved in a variety of situations that involve cognition, reward prediction, and feeding. VPvm neurons exhibit heterogeneous firing patterns including approach-only, response-only, and approach-response, and exhibit significantly more retreat-only and response-retreat patterns than do VPdl neurons (Root et al., 2013). These data suggest that VPvm neurons have a role in processing the sequence of behaviors (Root et al., 2013) or conditions (Mahler and Aston-Jones, 2012; Leung and Balleine, 2013; Mahler et al. 2014) that predict reward, similar to a “stay on task” function of the AcbSh (Floresco, 2014). Such processing may enable higher order structures (e.g., MD and mPFC) to regulate complex situations such as reversal learning (Ferry et al., 2000). The VPvm also regulates food consumption, likely through its LH and/or VTA projections. Taken together, the VPvm subregion and its diverse targets constitute another mechanism by which various cognitive or feeding-related behaviors may be processed.
The less understood “finger-like” VPr subregion appears to be more VPvm-like than VPdl-like. The VPr receives inputs from the olfactory tubercle rather than a substantial Acb input (Zahm and Heimer, 1987; Tripathi et al., 2010), but has some similar targets to VPvm, such as MD, LHb, and rostral sublenticular extended amygdala (Tripathi et al., 2013). VPr neurons also collateralize within basal forebrain areas that are populated with cholinergic neurons. The collateralization patterns of VPr neurons suggest that this subregion might exhibit differential function from other VP subregions. Identification of the postsynaptic basal forebrain neuronal phenotypes (i.e., cholinergic, glutamatergic, GABAergic) that VPr neurons synapse onto will provide further insight into VPr function.
The nonGABAergic subpopulations of VP neurons also appear to be more VPvm-like than VPdl-like. Similar to VPvm GABAergic neurons, glutamatergic neurons expressing VGluT2 mRNA project to at least the VTA (Geisler et al., 2007) and therefore may influence reward-related circuits. Though it remains unclear how cholinergic neurons are integrated into the VP circuit, these neurons likely to play a role in ventral striatopallidocortical processing by their receipt of accumbal projections (Zaborszky and Cullinan, 1992), establishment of local synapses in VP (Záborszky et al., 1986), and their targeting of mPFC neurons (Hur and Zaborszky, 2005), which also is the target of MD neurons receiving GABAergic VPvm input. Moreover, cholinergic neurons, including the subpopulation that co-expresses markers of glutamate transmission, provide a significant input to the amygdala (Carlsen et al., 1985; Záborszky et al., 1986; Poulin et al., 2006). Some cholinergic neurons of the VP also express vesicular GABA transporter and Gad2 (Saunders et al., 2015a,b), thus diverse subpopulations of cholinergic neurons provide additional neurochemical channels of information processing within VP circuits. Further subregional-based heterogeneity likely exists in pathways arising from discrete ventral striatopallidal cell types (e.g., D1, D2, or D3 receptor-expressing Acb neurons) and from the various types of VP neurons.
The underscoring of VP subregions and neuronal phenotypes in this review is not unlike the emphasis made by Heimer and colleagues (1997) when they discussed the importance of the AcbSh/AcbC divide, as well as VP/extended amygdala divide, towards opening “new and promising avenues for discussions of the neural basis of neuropsychiatric disorders and drug abuse”. Each VP subregion and neuronal phenotype likely processes distinct roles in facilitating motivated behaviors via unique modulation of their respective systems. At the behavioral output level, each unique channel of information processing integrates towards a coherent goal-directed behavior. For instance, the initial processing of the appropriate conditions in which to approach a reward may be carried out through the VPvm while the physical acts of approach and response involve the VPdl.
Finally, given the VP's role in behavior related to food or drug self-administration, responsiveness to drug cues in human subjects, and adaptations from chronic exposure to drugs of abuse, VP is a likely candidate for therapeutic intervention. Further research into the functionality of VP subregions, neuronal phenotypes, and neurotransmitter signaling capacities will undoubtedly advance neuroscientific insights into the neural bases of behavior, cognition, and psychiatric disorders.
Highlights.
VP contains several GABAergic subregions with distinct neuronal circuits
Additional circuits arise from nonGABAergic (e.g., glutamatergic) neuronal phenotypes
Dorsolateral VP neurons are sensitive to and necessary for drug-seeking responses
Ventromedial VP neurons discriminate conditions of reward acquisition and consumption
VP is an information integrator capable of dysregulation induced by drugs of abuse
Acknowledgements
This review and the included research were supported by the USPHSGs NS23945 (LZ), DA05255 (TCN) and DA015760 (TCN), and the Intramural Research Program at the National Institute on Drug Abuse (DHR). Research support was also provided by NRSAs to trainees in TCN's laboratory including: F32 DA05651 to P Johnson, F30 MH45180 to MS Turner, F31 DA019763 to F Shen, DA019783 to AL Mickiewicz, DA023306 to AA Herrold, DA021475 to RM Voigt, DA024923 to SM Graves and DA0331231 to SE Tedford. Gratitude is extended to each of these trainees for their contribution to VP-related research that is overviewed here. The authors thank Dr. Mark O. West for valuable discussions and mentorship to DHR under DA026252 (DHR) and DA006886 (MOW). The funders had no role in the decision to publish or preparation of the manuscript. The authors thank Dr. Marisela Morales for providing material prepared with in situ hybridization and immunohistochemical techniques.
Abbreviation List
- BLA
basolateral amygdala
- BM
basal nucleus of Meynert
- BNST
bed nucleus of the stria terminalis
- ChAT
choline acetyltransferase
- DLS
dorsolateral striatum
- dHipp
dorsal hippocampus
- dPFC
dorsal prefrontal cortex
- DR
dorsal raphe
- EPN
entopeduncular nucleus
- GABA
γ-amino butyric acid
- GAD
glutamic acid decarboxylase
- GP
globus pallidus
- GPi
internal globus pallidus/entopeduncular nucleus
- HDB
horizontal diagonal band
- Lat
lateral
- LH
lateral hypothalamus
- LHb
lateral habenula
- LHbM
medial part of lateral habenula
- LHbL
lateral part of lateral habenula
- LPO
lateral preoptic area
- IPAC
interstitial nucleus of the anterior commissure
- IR
immunoreactivity
- Med
medial
- MD
mediodorsal thalamus
- mPFC
medial prefrontal cortex
- MPO
medial preoptic nucleus
- Acb
nucleus accumbens
- AcbSh
nucleus accumbens shell
- AcbC
nucleus accumbens core
- NTS
nucleus tractus solitarius
- OT
olfactory tubercle
- PPTg
peduncopontine tegmental nucleus
- PPTg-MEA
pedunculopontine tegmental nucleus – mesencephalic extrapyramidal area
- RMTg
mesopontine rostromedial tegmental nucleus
- RRF
retrorubral field
- RTN
reticular thalamic nucleus
- SLEAR
rostral sublenticular extended amygdala
- SIB
basal part of substantia innominata
- SN
substantia nigra
- SNc
substantia nigra pars compacta
- SNr
substantia nigra pars reticulata
- STN
subthalamic nucleus
- TH
tyrosine hydroxylase
- VGluT
vesicular glutamate transporter
- vHipp
ventral hippocampus
- VP
ventral pallidum
- VPdl
dorsolateral ventral pallidum
- VPvm
ventromedial ventral pallidum
- VPr
rostral VP
- VPvl
ventrolateral VP
- vPFC
ventral prefrontal cortex
- VL/VMT
ventrolateral/ventromedial thalamus
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no financial interests to be disclosed.
References
- Alesdatter JE, Kalivas PW. Inhibition of mu opioid-induced motor activity in the ventral pallidum by D1 receptor blockade. Behav Pharmacol. 1993;4(6):645–651. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann NY Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27(1):1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31(2):363–74. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Anagnostakis Y, Spyraki C. Effect of morphine applied by intrapallidal microdialysis on the release of dopamine in the nucleus accumbens. Brain Res Bull. 1994;34(3):275–282. doi: 10.1016/0361-9230(94)90064-7. 1994. [DOI] [PubMed] [Google Scholar]
- Appel NM, Mitchell WM, Garlick RK, Glennon RA, Teitler M, De Souza EB. Autoradiographic characterization of (+-)-1-(2,5-dimethoxy-4-[125I] iodophenyl)-2-aminopropane ([125I]DOI) binding to 5-HT2 and 5-HT1c receptors in rat brain. J Pharmacol Exp Ther. 1990;255(2):843–57. [PubMed] [Google Scholar]
- Austin MC, Kalivas PW. Blockade of enkephalinergic and GABAergic mediated locomotion in the nucleus accumbens by muscimol in the ventral pallidum. Jpn J Pharmacol. 1989;50(4):487–90. doi: 10.1254/jjp.50.487. [DOI] [PubMed] [Google Scholar]
- Austin MC, Kalivas PW. Enkephalinergic and GABAergic modulation of motor activity in the ventral pallidum. J Pharmacol Exp Ther. 1990;252(3):1370–1377. [PubMed] [Google Scholar]
- Austin MC, Kalivas PW. Dopaminergic involvement in locomotion elicited from the ventral pallidum/substantia innominata. Brain Res. 1991;542(1):123–31. doi: 10.1016/0006-8993(91)91005-l. [DOI] [PubMed] [Google Scholar]
- Avila I, Lin S-C. Distinct neuronal populations in the basal forebrain encode motivational salience and movement. Frontiers in Behavioral Neuroscience. 2014a;8(421):1–12. doi: 10.3389/fnbeh.2014.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila I, Lin S-C. Motivational salience signal in the basal forebrain is coupled with faster and more precise decision speed. PLoS Biol. 2014b;12:e1001811. doi: 10.1371/journal.pbio.1001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22(20):9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Striano BM, Coffey KC, Root DH, Pawlak AP, Kim OA, Kulik J, Fabbricatore AT, West MO. Sensitivity to self-administered cocaine within the lateral preoptic-rostral lateral hypothalamic continuum. Brain Struct Funct. 2014a doi: 10.1007/s00429-014-0736-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Simmons SJ, Servilio LC, Bercovicz D, Ma S, Root DH, Pawlak AP, West MO. Ultrasonic vocalizations: evidence for an affective opponent process during cocaine self-administration. Psychopharmacology (Berl) 2014b;231(5):909–918. doi: 10.1007/s00213-013-3309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Ma S, Jha S, Megehee L, Pawlak AP, West MO. Dose-dependent differences in short ultrasonic vocalizations emitted by rats during cocaine self-administration. Psychopharmacology (Berl) 2010;211(4):435–42. doi: 10.1007/s00213-010-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Morris RW, Leung BK. Thalamocortical integration of instrumental learning and performance and their disintegration in addiction. Brain Res. 2014 doi: 10.1016/j.brainres.2014.12.023. in press. [DOI] [PubMed] [Google Scholar]
- Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135(3):959–68. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and 'natural' rewards. Nat Neurosci. 2005;8(4):484–9. doi: 10.1038/nn1429. [DOI] [PubMed] [Google Scholar]
- Baunez C, Yelnik J, Mallet L. Six questions on the subthalamic nucleus: lessons from animal models and from stimulated patients. Neuroscience. 2011;198:193–204. doi: 10.1016/j.neuroscience.2011.09.059. [DOI] [PubMed] [Google Scholar]
- Bechara A, van der Kooy D. The tegmental pedunculopontine nucleus: a brain-stem output of the limbic system critical for the conditioned place preferences produced by morphine and amphetamine. J Neurosci. 1989;9(10):3400–9. doi: 10.1523/JNEUROSCI.09-10-03400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJH. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Research. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Wooten GF, Trugman JM. Distribution of D1 and D2 dopamine receptors in the basal ganglia of the cat determined by quantitative autoradiography. J Comp Neurol. 1988;268(1):131–45. doi: 10.1002/cne.902680113. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57(3):432–41. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bell K, Churchill L, Kalivas PW. GABAergic projection from the ventral pallidum and globus pallidus to the subthalamic nucleus. Synapse. 1995;20(1):10–18. doi: 10.1002/syn.890200103. [DOI] [PubMed] [Google Scholar]
- Bengtson CP, Lee DJ, Osborne PB. Opposing electrophysiological actions of 5-HT on noncholinergic and cholinergic neurons in the rat ventral pallidum in vitro. J Neurophysiol. 2004;92(1):433–443. doi: 10.1152/jn.00543.2003. [DOI] [PubMed] [Google Scholar]
- Bengtson CP, Osborne PB. Electrophysiological properties of anatomically identified ventral pallidal neurons in rat brain slices. Ann N Y Acad Sci. 1999;877:691–694. doi: 10.1111/j.1749-6632.1999.tb09303.x. [DOI] [PubMed] [Google Scholar]
- Bengtson CP, Osborne PB. Electrophysiological properties of cholinergic and noncholinergic neurons in the ventral pallidal region of the nucleus basalis in rat brain slices. J Neurophysiol. 2000;83(5):2649–2660. doi: 10.1152/jn.2000.83.5.2649. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience. 2006;137(2):699–706. doi: 10.1016/j.neuroscience.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. J Neurosci. 1998;18(22):9438–9452. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Clarke NP, Bolam JP. Synaptic integration of functionally diverse pallidal information in the entopeduncular nucleus and subthalamic nucleus in the rat. The Journal of Neuroscience. 1997;17:308–324. doi: 10.1523/JNEUROSCI.17-01-00308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Smith AD, Bolam JP. The substantia nigra as a site of synaptic integration of functionally diverse information arising from the ventral pallidum and the globus pallidus in the rat. Neuroscience. 1996;75(1):5–12. doi: 10.1016/0306-4522(96)00377-6. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno-and hybridization histochemical characterization. J Comp Neurol. 1992;319(2):218–45. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16(5):632–8. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau Isabelle, Payer Doris, Houle Sylvain, Behzadi Arian, Rusjan Pablo M., Tong Junchao, Wilkins Diana, Selby Peter, George Tony P., Zack Martin, Furukawa Yoshiaki, McCluskey Tina, Wilson Alan A., Kish Stephen J. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: A postitron emission tomography study. The Journal of Neuroscience. 2012;32(4):1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Ingham CA, Izzo PN, Levey AI, Rye DB, Smith AD, Wainer BH. Substance P-containing terminals in synaptic contact with cholinergic neurons in the neostriatum and basal forebrain: a double immunocytochemical study in the rat. Brain Research. 1986;397:279–289. doi: 10.1016/0006-8993(86)90629-3. [DOI] [PubMed] [Google Scholar]
- Borsini F, Rolls ET. Role of noradrenaline and serotonin in the basolateral region of the amygdala in food preferences and learned taste aversions in the rat. Physiol Behav. 1984;33(1):37–43. doi: 10.1016/0031-9384(84)90010-6. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27(46):12655–63. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y. Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2009;206(1):51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14(4):420–2. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdelais A, Kalivas PW. Amphetamine lowers extracellular GABA concentration in the ventral pallidum. Brain Res. 1990;516(1):132–6. doi: 10.1016/0006-8993(90)90907-s. [DOI] [PubMed] [Google Scholar]
- Bourdelais AJ, Kalivas PW. Modulation of extracellular gamma-aminobutyric acid in the ventral pallidum using in vivo microdialysis. J Neurochem. 1992;58(6):2311–20. doi: 10.1111/j.1471-4159.1992.tb10979.x. [DOI] [PubMed] [Google Scholar]
- Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, Veh RW. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168(2):463–76. doi: 10.1016/j.neuroscience.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp Neurol. 1992;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O. Lateral habenula neurons signal errors in the prediction of reward information. Nat Neurosci. 2011;14(9):1209–16. doi: 10.1038/nn.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hong S, Hikosaka O. A pallidus habenula-dopamine pathway signals inferred stimulus values. J Neurophysiol. 2010;104(2):1068–76. doi: 10.1152/jn.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182(2):274–283. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Caillé S, Parsons LH. Intravenous heroin self-administration decreases GABA efflux in the ventral pallidum: an in vivo microdialysis study in rats. 2004;20(2):593–6. doi: 10.1111/j.1460-9568.2004.03497.x. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress-and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168(1-2):66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Williams JG, Hollander JA. Basolateral amygdala neurons encode cocaine self-administration and cocaine-associated cues. J Neurosci. 2003;23(23):8204–11. doi: 10.1523/JNEUROSCI.23-23-08204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. 1994;14(12):7735–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen J, Záborszky L, Heimer L. Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: a combined retrograde fluorescent and immunohistochemical study. The Journal of Comparative Neurology. 1985;234:155–167. doi: 10.1002/cne.902340203. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24(17):4113–23. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HT, Kitai ST. Projection neurons of the nucleus accumbens: an intracellular labeling study. Brain Res. 1985;347:112–116. doi: 10.1016/0006-8993(85)90894-7. [DOI] [PubMed] [Google Scholar]
- Chang HT. Noradrenergic innervation of the substantia innominata: a light and electron microscopic analysis of dopamine beta-hydroxylase immunoreactive elements in the rat. Exp Neurol. 1989;104(2):101–12. doi: 10.1016/s0014-4886(89)80002-0. [DOI] [PubMed] [Google Scholar]
- Chang HT, Kuo H. Calcitonin gene-related peptide (CGRP) in the rat substantia innominata and globus pallidus: a light and electron microscopic immunocytochemical study. Brain Res. 1989;495(1):167–72. doi: 10.1016/0006-8993(89)91232-8. [DOI] [PubMed] [Google Scholar]
- Chang CH, Grace AA. Amygdala-Ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.09.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MA, See RE. Differential effects of unique profile antipsychotic drugs on extracellular amino acids in the ventral pallidum and globus pallidus of rats. J Pharmacol Exp Ther. 1996;277(3):1586–94. [PubMed] [Google Scholar]
- Chen F, Lawrence AJ. 5-HT transporter sites and 5-HT1A and 5-HT3 receptors in Fawn-Hooded rats: a quantitative autoradiography study. Alcohol Clin Exp Res. 2000;24(7):1093–102. [PubMed] [Google Scholar]
- Chen J-C, Liang K-W, Huang Y-K, Liang C-S, Chiang Y-C. Significance of glutamate and dopamine neurons in the ventral pallidum in the expression of behavioral sensitization to amphetamine. Life Sciences. 2000;68:973–983. doi: 10.1016/s0024-3205(00)00995-4. [DOI] [PubMed] [Google Scholar]
- Chen L-W, Wei L-C, Liu H-L, Qiu Y, Chan Y-S. Cholinergic neurons expressing substance P receptor (NK 1) in the basal forebrain of the rat: a double immunocytochemical study. Brain Res. 2001;904:161–166. doi: 10.1016/s0006-8993(01)02460-x. [DOI] [PubMed] [Google Scholar]
- Chen S, Su HS. Afferent connections of the thalamic paraventricular and parataenial nuclei in the rat--a retrograde tracing study with iontophoretic application of Fluoro-Gold. Brain Res. 1990;522(1):1–6. doi: 10.1016/0006-8993(90)91570-7. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, et al. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One. 2008;3(1):e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Napier TC. Basal forebrain infusions impair delayed-non-match-to-sample radial arm maze performance. Pharmacol Biochem Behav. 2002;72(1-2):209–212. doi: 10.1016/s0091-3057(01)00752-3. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Napier TC. Opioid and GABA modulation of accumbens-evoked ventral pallidal activity. J Neural Transm. 1993;93:123–143. doi: 10.1007/BF01245342. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Napier TC, Hanin I, Walsh TJ. The pharmacology of basal forebrain involvement in cognition. In: The Basal Forebrain: Anatomy to Function. In: Napier TC, Kalivas PW, Hanin I, editors. Advances in Experimental Medicine and Biology. Vol. 295. Plenum Press; New York, NY.: 1991. pp. 383–98. [DOI] [PubMed] [Google Scholar]
- Churchill L, Klitenick MA, Kalivas PW. Dopamine depletion reorganizes projections from the nucleus accumbens and ventral pallidum that mediate opioid-induced motor activity. J Neurosci. 1994;18(19):8074–85. doi: 10.1523/JNEUROSCI.18-19-08074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill L, Klitenick MA, Kalivas PW. Dopamine depletion reorganizes projections from the nucleus accumbens and ventral pallidum that mediate opioid-induced motor activity. J Neurosci. 1998;18(19):8074–8085. doi: 10.1523/JNEUROSCI.18-19-08074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill L, Kalivas PW. The involvement of the mediodorsal nucleus of the thalamus and the midbrain extrapyramidal area in locomotion elicited from the ventral pallidum. Behav Brain Res. 1999;104(1-2):63–71. doi: 10.1016/s0166-4328(99)00051-0. [DOI] [PubMed] [Google Scholar]
- Churchill L, Dilts RP, Kalivas PW. Changes in gamma-aminobutyric acid, mu-opioid and neurotensin receptors in the accumbens-pallidal projection after discrete quinolinic acid lesions in the nucleus accumbens. Brain Res. 1990;511(1):41–54. doi: 10.1016/0006-8993(90)90223-x. [DOI] [PubMed] [Google Scholar]
- Churchill L, Zahm DS, Kalivas PW. The mediodorsal nucleus of the thalamus in rats--I. forebrain gabaergic innervation. Neuroscience. 1996;70(1):83–102. doi: 10.1016/0306-4522(95)00351-i. [DOI] [PubMed] [Google Scholar]
- Cilia R, Siri C, Marotta G, Isaias IU, De GD, Canesi M, et al. Functional abnormalities underlying pathological gambling in Parkinson disease. Arch Neurol. 2008;65:1604–1611. doi: 10.1001/archneur.65.12.1604. [DOI] [PubMed] [Google Scholar]
- Colussi-Mas J, Geisler S, Zimmer L, Zahm DS, Bérod A. Activation of afferents to the ventral tegmental area in response to acute amphetamine: a double-labelling study. Eur J Neurosci. 2007;26(4):1011–1025. doi: 10.1111/j.1460-9568.2007.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad LC, Pfaff DW. Autoradiographic tracing of nucleus accumbens efferents in the rat. Brain Res. 1976a;113(3):589–596. doi: 10.1016/0006-8993(76)90060-3. [DOI] [PubMed] [Google Scholar]
- Conrad LC, Pfaff DW. Efferents from medial basal forebrain and hypothalamus in the rat. I. An autoradiographic study of the medial preoptic area. J Comp Neurol. 1976b;169(2):185–219. doi: 10.1002/cne.901690205. [DOI] [PubMed] [Google Scholar]
- Contreras PC, Quirion R, Gehlert DR, Contreras ML, O'Donohue TL. Autoradiographic distribution of non-dopaminergic binding sites labeled by [3H]haloperidol in rat brain. Neurosci Lett. 1987;75(2):133–40. doi: 10.1016/0304-3940(87)90286-2. [DOI] [PubMed] [Google Scholar]
- Cools AR, Spooren W, Bezemer R, Cuypers E, Jaspers R, Groenewegen H. Anatomically distinct output channels of the caudate nucleus and orofacial dyskinesia: critical role of the subcomissural part of the globus pallidus in oral dyskinesia. Neuroscience. 1989;33(3):535–542. doi: 10.1016/0306-4522(89)90405-3. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21(9):3251–60. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. Projections to the rostral reticular thalamic nucleus in the rat. Exp Brain Res. 1990;80(1):157–171. doi: 10.1007/BF00228857. 1990. [DOI] [PubMed] [Google Scholar]
- Covelo IR, Patel ZI, Luviano JA, Stratford TR, Wirtshafter D. Manipulation of GABA in the ventral pallidum, but not the nucleus accumbens, induces intense, preferential, fat consumption in rats. Behav Brain Res. 2014;270:316–325. doi: 10.1016/j.bbr.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallimore JE, Mickiewicz AL, Napier TC. Intra-ventral pallidal glutamate antagonists block expression of morphine-induced place preference. Behav Neurosci. 2006;120(5):1103–14. doi: 10.1037/0735-7044.120.5.1103. [DOI] [PubMed] [Google Scholar]
- Danks JA, Rothman RB, Cascieri MA, Chicchi GG, Liang T, Herkenham M. A comparative autoradiographic study of the distributions of substance P and eledoisin binding sites in rat brain. Brain Res. 1986;385(2):273–81. doi: 10.1016/0006-8993(86)91073-5. [DOI] [PubMed] [Google Scholar]
- De Leon KR, Todtenkopf MS, Stellar JR. An examination of glutamate decarboxylase65 immunoreactive puncta with respect to rat ventral pallidum neurons after repeated cocaine administration. Neuroscience Letters. 2000;284:69–72. doi: 10.1016/s0304-3940(00)00973-3. [DOI] [PubMed] [Google Scholar]
- De Leonibus E, Mele A, Oliverio A, Pert A. Locomotor activity induced by the non-competetive N-Methyl-D-Aspartate antagonist, MK-801: Role of nucleus accumbens efferent pathways. Neuroscience. 2001;104(1):105–116. doi: 10.1016/s0306-4522(01)00047-1. [DOI] [PubMed] [Google Scholar]
- de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75(2):134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Del-Fava F, Hasue RH, Ferreira GP, Shammah-Lagnado JS. Efferent connections of the rostral linear nucleus of the ventral tegmental area in the rat. Neuroscience. 2007;145:1059–1076. doi: 10.1016/j.neuroscience.2006.12.039. [DOI] [PubMed] [Google Scholar]
- Di Ciano P. Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behav Neurosci. 2008;122(1):129–39. doi: 10.1037/0735-7044.122.1.129. [DOI] [PubMed] [Google Scholar]
- Di Ciano P. Distinct contributions of dopamine receptors in the nucleus accumbens core or shell to established cocaine reinforcement under a second-order schedule. Eur Neuropsychopharmacol. 2008;18(12):888–96. doi: 10.1016/j.euroneuro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24(32):7167–73. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof Esther K., Keil Maria, Obst Katrin U., Henseler Ilona, Dechent Peter, Falkai Peter, Gruber Oliver. A function neuroimaging study assessing gender difference in the neural mechanism underlying the ability to resiste impulsive desires. Brain Research. 2012;1473:63–77. doi: 10.1016/j.brainres.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Ingraham CM, Rodd ZA, McBride WJ. The reinforcing effects of ethanol within the posterior ventral tegmental area depend on dopamine neurotransmission to forebrain cortico-limbic systems. Addict Biol. 2014 doi: 10.1111/adb.12138. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hauser SR, Toalston JE, Bell RL, McBride WJ, Rodd ZA. Synergistic self-administration of ethanol and cocaine directly into the posterior ventral tegmental area: involvement of serotonin-3 receptors. J Pharmacol Exp Ther. 2012;340(1):202–9. doi: 10.1124/jpet.111.187245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30(1):218–29. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echo JA, Lamonte N, Ackerman TF, Bodnar RJ. Alterations in food intake elicited by GABA and opioid agonists and antagonists administered into the ventral tegmental area region of rats. Physiol Behav. 2002;76(1):107–16. doi: 10.1016/s0031-9384(02)00690-x. [DOI] [PubMed] [Google Scholar]
- Fabbricatore AT, Ghitza UE, Prokopenko VF, West MO. Electrophysiological evidence of mediolateral functional dichotomy in the rat accumbens during cocaine self-administration: tonic firing patterns. Eur J Neurosci. 2009 Dec. 2009;30(12):2387–400. doi: 10.1111/j.1460-9568.2009.07033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbricatore AT, Ghitza UE, Prokopenko VF, West MO. Electrophysiological evidence of mediolateral functional dichotomy in the rat nucleus accumbens during cocaine self-administration II: phasic firing patterns. Eur J Neurosci. 2010;31(9):1671–82. doi: 10.1111/j.1460-9568.2010.07230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–87. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fan D, Rossi MA, Yin HH. Mechanisms of action selection and timing in substantia nigra neurons. J Neurosci. 2012;32:5534–5548. doi: 10.1523/JNEUROSCI.5924-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar AM, Font L, Pereira M, Mingote S, Bunce JG, Chrobak JJ, Salamone JD. Forebrain circuitry involved in effort-related choice: Injections of the GABAA agonist muscimol into ventral pallidum alter response allocation in food-seeking behavior. Neuroscience. 2008;152(2):321–330. doi: 10.1016/j.neuroscience.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol Learn Mem. 2007;88(4):435–44. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry AT, Lu XC, Price JL. Effects of excitotoxic lesions in the ventral striatopallidal--thalamocortical pathway on odor reversal learning: inability to extinguish an incorrect response. Exp Brain Res. 2000;131(3):320–35. doi: 10.1007/s002219900240. [DOI] [PubMed] [Google Scholar]
- Filip M, Cunningham KA. Serotonin 5-HT(2C) receptors in nucleus accumbens regulate expression of the hyperlocomotive and discriminative stimulus effects of cocaine. Pharmacol Biochem Behav. 2002;71(4):745–56. doi: 10.1016/s0091-3057(01)00741-9. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM, Sabijan MS, DeSousa NJ. Injections of D-amphetamine into the ventral pallidum increase locomotor activity and responding for conditioned reward: a comparison with injections into the nucleus accumbens. Brain Res. 1998;805(1-2):29–40. doi: 10.1016/s0006-8993(98)00633-7. [DOI] [PubMed] [Google Scholar]
- Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Ann Rev Psych. 2014;66:20, 1–20, 28. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Braaksma DN, Phillips AG. Involvement of the ventral pallidum in working memory tasks with or without a delay. Ann N Y Acad Sci. 1999;877:711–7116. doi: 10.1111/j.1749-6632.1999.tb09308.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nature Neuroscience. 2003;6(9):968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Frascella J, Potenza MN, Brown LL, Childress AR. Shared brain vulnerabilities open the way for nonsubstance addictions: Carving addiction at a new joint? Ann. N.Y. Acad. Sci. 2010;1187:294–315. doi: 10.1111/j.1749-6632.2009.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Striatal and ventral pallidum dynorphin concentrations are markedly increased in human chronic cocaine users. Neuropharmacology. 2008;55(1):41–46. doi: 10.1016/j.neuropharm.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol. 2002;446(2):151–165. doi: 10.1002/cne.10191. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119(1):19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus-and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160(4):425–33. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26(13):3584–8. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200(4):545–56. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006;23(10):2809–13. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26(13):3584–8. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30(2):296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamergic/aspartergic afferents to the rat ventral striatopallidal region. J Comp Neurol. 1987;258(3):317–338. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- Funahashi S. Responses of monkey prefrontal neurons during a visual tracking task reinforced by substantia innominata self-stimulation. Brain Res. 1983;276:267–276. doi: 10.1016/0006-8993(83)90734-5. [DOI] [PubMed] [Google Scholar]
- Galarce EM, McDannald MA, Holland PC. The basolateral amygdala mediates the effects of cues associated with meal interruption on feeding behavior. Brain Res. 2010;1350:112–122. doi: 10.1016/j.brainres.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Perkel DJ. A basal ganglia pathway drives selective auditory responses in songbird dopaminergic neurons via disinhibition. J Neurosci. 2010;30(3):1027–1037. doi: 10.1523/JNEUROSCI.3585-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Person AL, Perkel DJ. A novel basal ganglia pathway forms a loop linking a vocal learning circuit with its dopaminergic input. J Comp Neurol. 2008;508(5):824–39. doi: 10.1002/cne.21700. [DOI] [PubMed] [Google Scholar]
- Gallyas F, Wolff JR, Bottcher H, Zaborszky L. A reliable method for demonstrating axonal degeneration shortly after axotomy. Stain Technol. 1980;55:291–297. doi: 10.3109/10520298009067257. [DOI] [PubMed] [Google Scholar]
- Ganz J, Kaslin J, Freudenreich D, Machate A, Geffarth M, Brand M. Subdivisions of the adult zebrafish subpallium by molecular marker analysis. J Comp Neurol. 2012;520(3):633–55. doi: 10.1002/cne.22757. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Záborszky L. Direct catecholaminergic-cholinergic interactions in the basal forebrain. II. Substantia nigra-ventral tegmental area projections to cholinergic neurons. J Comp Neurol. 1996;374(4):555–577. doi: 10.1002/(SICI)1096-9861(19961028)374:4<555::AID-CNE6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Záborszky L. Parvalbumin-containing neurons in the basal forebrain receive direct input from the substantia nigra-ventral tegmental area. Brain Res. 1997 Jan 30. 1997;747(1):173–179. doi: 10.1016/s0006-8993(96)01309-1. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27(21):5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Marinelli M, Degarmo B, Becker ML, Freiman AJ, Beales M, Meredith GE, Zahm DS. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacology. 2008;33(11):2688–2700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490(3):270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. On the retention of neurotensin in the ventral tegmental area (VTA) despite destruction of the main neurotensinergic afferents of the VTA—Implications for the organization of forebrain projections to the VTA. Brain Res. 2006a;1087:87–104. doi: 10.1016/j.brainres.2006.02.108. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Neurotensin afferents of the ventral tegmental area in the rat: [1] re-examination of their origins and [2] responses to acute psychostimulant and antipsychotic drug administration. Eur J Neurosci. 2006b;24:116–134. doi: 10.1111/j.1460-9568.2006.04928.x. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. The Journal of Neuroscience. 2003;23(19):7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko VF, West MO. Differences between accumbens core and shell neurons exhibiting phasic firing patterns related to drug-seeking behavior during a discriminative stimulus task. J Neurophysiol. 2004;92:1608–1614. doi: 10.1152/jn.00268.2004. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221(4612):773–5. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Gonçalves L, Sego C, Metzger M. Differential projections from the lateral habenula to the rostromedial tegmental nucleus and ventral tegmental area in the rat. J Comp Neurol. 2012;520(6):1278–300. doi: 10.1002/cne.22787. [DOI] [PubMed] [Google Scholar]
- Gong W, Justice JB, Jr, Neill D. Dissociation of locomotor and conditioned place preference responses following manipulation of GABA A and AMPA receptors in ventral pallidum. Prog Neuropsychopharmacol Biol Psychiatry. 1997a;21(5):839–852. doi: 10.1016/s0278-5846(97)00084-5. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill D, Justice JB., Jr Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Res. 1996;707(1):64–74. doi: 10.1016/0006-8993(95)01222-2. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill D, Justice JB., Jr 6-Hydroxydopamine lesion of ventral pallidum blocks acquisition of place preference conditioning to cocaine. Brain Res. 1997b;754(1-2):103–112. doi: 10.1016/s0006-8993(97)00059-0. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Justice JB., Jr GABAergic modulation of ventral pallidal dopamine release studied by in vivo microdialysis in the freely moving rat. Synapse. 1998;29(4):406–12. doi: 10.1002/(SICI)1098-2396(199808)29:4<406::AID-SYN12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Lynn M, Justice JB., Jr Dopamine D1/D2 agonists injected into nucleus accumbens and ventral pallidum differentially affect locomotor activity depending on site Neuroscience. 1999;93(4):1349–1358. doi: 10.1016/s0306-4522(99)00235-3. [DOI] [PubMed] [Google Scholar]
- Graves SM, Viskniskki AA, Cunningham KA, Napier TC. Serotonin(2C) receptors in the ventral pallidum regulate motor function in rats. Neuroreport. 2013;24(11):605–8. doi: 10.1097/WNR.0b013e3283630af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22(5):473–9. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383(2):163–177. [PubMed] [Google Scholar]
- Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458(1):11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mariotti M, Mancia M. Limbic and brainstem afferents to thalamic mediodorsal nucleus: a horseradish peroxidase study. Neuroscience Letters. 1987;76:345–350. doi: 10.1016/0304-3940(87)90427-7. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Jones BE. Codistribution of GABA-with acetylcholine-synthesizing neurons in the basal forebrain of the rat. J. Comp. Neurol. 1993;329:438–457. doi: 10.1002/cne.903290403. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57(1):113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW. Connections of the subthalamic nucleus with ventral striatopallidal parts of the basal ganglia in the rat. J Comp Neurol. 1990;294(4):607–622. doi: 10.1002/cne.902940408. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Graaf YG, Smeets WJAJ. Integration and segregation of limbic cortico-striatal loops at the thalamic level: an experimental tracing study in rats. Journal of Chemical Neuroanatomy. 1999;16:167–185. doi: 10.1016/s0891-0618(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223(3):347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- Grove EA, Domesick VB, Nauta WJ. Light microscopic evidence of striatal input to intrapallidal neurons of cholinergic cell group Ch4 in the rat: a study employing the anterograde tracer Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res. 1986;367(1-2):379–384. doi: 10.1016/0006-8993(86)91623-9. [DOI] [PubMed] [Google Scholar]
- Grove EA. Efferent connections of the substantia innominata in the rat. J Comp Neurol. 1988;277(3):347–64. doi: 10.1002/cne.902770303. [DOI] [PubMed] [Google Scholar]
- Haahr ME, Rasmussen PM, Madsen a,b K, Marner a,b L, Ratner a,b C, Gillings N, Baaré WFC, Knudsen GM. Obesity is associated with serotonin 4 receptor availabity in the brain reward circuitry. NeuroImage. 2012;61:884–888. doi: 10.1016/j.neuroimage.2012.03.050. [DOI] [PubMed] [Google Scholar]
- Haber S, Elde R. Correlation between Met-enkephalin and substance P immunoreactivity in the primate globus pallidus. Neuroscience. 1981;6(7):1291–1297. doi: 10.1016/0306-4522(81)90188-3. [DOI] [PubMed] [Google Scholar]
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol. 1985;235(3):322–35. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- Haber SN, Nauta WJ. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience. 1983;9(2):245–260. doi: 10.1016/0306-4522(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Haber SN, Wolfe DP, Groenewegen HJ. The relationship between ventral striatal efferent fibers and the distribution of peptide-positive woolly fibers in the forebrain of the rhesus monkey. Neuroscience. 1990;39:323–338. doi: 10.1016/0306-4522(90)90271-5. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Zaborszky L. Serotonin: From the Molecule to the Clinic. A Serotonin Club/ Brain Research Bulletin Conference. Elsevier Science; 2000. Serotoninergic innervation of basal forebrain neurons in the rat. p. 97. Abstracts. [Google Scholar]
- Hakan RL, Berg GI, Henriksen SJ. Electrophysiological evidence for reciprocal connectivity between the nucleus accumbens septi and ventral pallidal region. Brain Res. 1992;581:344–350. doi: 10.1016/0006-8993(92)90730-w. [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol. 1987;262(1):105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- Hamilton ME, Bozarth MA. Feeding elicited by dynorphin (1-13) microinjections into the ventral tegmental area in rats. Life Sci. 1988;43(11):941–6. doi: 10.1016/0024-3205(88)90271-8. [DOI] [PubMed] [Google Scholar]
- Hammer RP., Jr Cocaine alters opiate receptor binding in critical brain reward regions. Synapse. 1989;3(1):55–60. doi: 10.1002/syn.890030108. [DOI] [PubMed] [Google Scholar]
- Hars B, Maho C, Edeline JM, Hennevin E. Basal forebrain stimulation facilitates tone-evoked responses in the auditory cortex of awake rat. Neuroscience. 1993;56(1):61–74. doi: 10.1016/0306-4522(93)90562-t. [DOI] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu. Rev. Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Härtig W, Brauer K, Fritschy JM, Brückner G, Bigl V. Regional and cellular expression sites of the alpha 1 subunit of GABAA receptors in the rat basal forebrain: a cytochemical study with glutamic acid decarboxylase, choline acetyltransferase, calcium-binding proteins and nitric oxide synthase as second markers. Brain Res. 1995;692(1-2):215–26. doi: 10.1016/0006-8993(95)00631-y. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, 2nd, Grey C, Jones CM, McCane S, Cummings R, Mason D, Ma C, Cook JM, June HL. The GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci. 2002;22(9):3765–75. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL. Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharmacology (Berl) 2003;168(1-2):75–83. doi: 10.1007/s00213-002-1328-3. [DOI] [PubMed] [Google Scholar]
- Heidenreich BA, Napier TC. Effects of serotonergic 5-HT1A and 5-HT1B ligands on ventral pallidal neuronal activity. Neuroreport. 2000;11(13):2849–2853. doi: 10.1097/00001756-200009110-00005. [DOI] [PubMed] [Google Scholar]
- Heidenreich BA, Mitrovic I, Battaglia G, Napier TC. Limbic pallidal adaptations following long-term cessation of dopamine transmission. Alterations in basal firing without an upregulation of dopamine receptor function. Experimental Neurology. 2004;186:145–157. doi: 10.1016/j.expneurol.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Heimer L, Harlan RE, Alheid GF, Garcia MM, de Olmos J. Substantia innominata: a notion which impedes clinical-anatomical correlations in neuropsychiatric disorders. Neuroscience. 1997;76(4):957–1006. doi: 10.1016/s0306-4522(96)00405-8. [DOI] [PubMed] [Google Scholar]
- Heimer L, Wilson RD. The subcortical projections of the allocortex: similarities in the neural associations of the hippocampus, the piriform cortex, and the neocortex. In: Santini M, editor. In Golgi Centennial Symposium. Raven Press; New York: 1975. pp. 177–193. [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Heimer L. The olfactory cortex and the ventral striatum. In: Livingston KE, Hornykiewicz O, editors. Limbic Mechanisms. Plenum Press; New York and London: 1978. pp. 95–188. [Google Scholar]
- Heimer L, Zahm DS, Alheid GF. Basal ganglia. In: Paxinos G, editor. The Rat Nervous Syststem. 2nd ed. Academic Press; San Diego: 1995. pp. 579–628. [Google Scholar]
- Heimer L, Switzer RD, Van Hoesen GW. Ventral striatum and ventral pallidum Components of the motor system? TINS. 1982:83–87. [Google Scholar]
- Heimer L. The olfactory connections of the diencephalon in the rat An experimental light-and electron-microscopic study with special emphasis on the problem of terminal degeneration. Brain, Behav. Evol. 1972;6:484–523. doi: 10.1159/000123728. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Everitt BJ, Lee JL. Disrupting reconsolidation of conditioned withdrawal memories in the basolateral amygdala reduces suppression of heroin seeking in rats. J Neurosci. 2006;26(49):12694–9. doi: 10.1523/JNEUROSCI.3101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson Z. Expression of GABAA receptor subunit messenger RNA in non-cholinergic neurons of the rat basal forebrain. Neuroscience. 1995;65(4):1077–86. doi: 10.1016/0306-4522(94)00542-d. [DOI] [PubMed] [Google Scholar]
- Henry DJ, White FJ. Electrophysiological correlates of psychomotor stimulant-induced sensitization. Ann N Y Acad Sci. 1992;654:88–100. doi: 10.1111/j.1749-6632.1992.tb25958.x. [DOI] [PubMed] [Google Scholar]
- Henry P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27(3):654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Forebrain projections of the rostral nucleus raphe magnus shown by iontophoretic application of choleratoxin b in rats. Neurosci Lett. 1996 Oct 4;216(3):151–154. doi: 10.1016/0304-3940(96)13013-5. 1996. [DOI] [PubMed] [Google Scholar]
- Herrold AA, Voigt RM, Napier TC. Brain region-selective cellular redistribution of mGlu5 but not GABA(B) receptors following methamphetamine-induced associative learning. Synapse. 2011;65(12):1333–43. doi: 10.1002/syn.20968. [DOI] [PubMed] [Google Scholar]
- Herrold AA, Persons AL, Napier TC. Cellular distribution of AMPA receptor subunits and mGlu5 following acute and repeated administration of morphine or methamphetamine. J Neurochem. 2013;126(4):503–17. doi: 10.1111/jnc.12323. [DOI] [PubMed] [Google Scholar]
- Hill JM, Switzer RC., 3rd The regional distribution and cellular localization of iron in the rat brain. Neuroscience. 1984;11(3):595–603. doi: 10.1016/0306-4522(84)90046-0. [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO, Xia Y, Margolis EB, Fields HL. Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons. J Neurosci. 2013;33(15):6454–9. doi: 10.1523/JNEUROSCI.0178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: Electrophysiological properties and projections. JNeurosci. 2012;32(43):15076–15085. doi: 10.1523/JNEUROSCI.3128-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DC, West TE, Wise RA. Ventral pallidal microinjections of receptor-selective opioid agonists produce differential effects on circling and locomotor activity in rats. Brain Res. 1991;550(2):205–212. doi: 10.1016/0006-8993(91)91319-v. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci. 2003;17(8):1680–94. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav. 2002;76(1):117–29. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Abstinence from cocaine self-administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacology. 2005;30(8):1464–74. doi: 10.1038/sj.npp.1300748. [DOI] [PubMed] [Google Scholar]
- Holstege G. The mesopontine rostromedial tegmental nculeus and the emotional motor system: role in basic survival. J Comp Neurol. 2009;513:559–565. doi: 10.1002/cne.21990. [DOI] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Diverse sources of reward value signals in the basal ganglia nuclei transmitted to the lateral habenula in the monkey. Front Hum Neurosci. 2013;778 doi: 10.3389/fnhum.2013.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31(32):11457–71. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Kalivas PW. Involvement of dopamine and excitatory amino acid transmission in novelty-induced motor activity. J Pharmacol Exp Ther. 1994;269(3):976–88. [PubMed] [Google Scholar]
- Hooks MS, Kalivas PW. The role of mesoaccumbens--pallidal circuitry in novelty-induced behavioral activation. Neuroscience. 1995;64(3):587–97. doi: 10.1016/0306-4522(94)00409-x. [DOI] [PubMed] [Google Scholar]
- Houser CR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980;200(2):341–354. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- Hreib KK, Rosene DL, Moss MB. Basal forebrain efferents to the medial dorsal thalamic nucleus in the rhesus monkey. J Comp Neurol. 1988;277(3):365–90. doi: 10.1002/cne.902770304. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Manvich DF, Kuhar MJ. Cocaine and amphetamine-regulated transcript-containing neurons in the nucleus accumbens project to the ventral pallidum in the rat and may inhibit cocaine-induced locomotion. Neuroscience. 2009;165(1):179–187. doi: 10.1016/j.neuroscience.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EE, Záborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study. J Comp Neurol. 2005;483(3):351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Huston JP, Kiefer S, Buscher W, Muñoz C. Lateralized functional relationship between the preoptic area and lateral hypothalamic reinforcement. Brain Res. 1987;436(1):1–8. doi: 10.1016/0006-8993(87)91549-6. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci. 2003;23(28):9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Bonci A. Neurocircuitry of drug reward. Neuropharmacology. 2014;76B:329–341. doi: 10.1016/j.neuropharm.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui T, Shimura T, Yamamoto T. The role of the ventral pallidum GABAergic system in conditioned taste aversion: effects of microinjections of a GABA (A) receptor antagonist on taste palatability of a conditioned stimulus. Brain Research. 2007;1164:117–124. doi: 10.1016/j.brainres.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Inui T, Yamamoto T, Shimura T. GABAergic transmission in the rat ventral pallidum mediates a saccharin palatability shift in conditioned taste aversion. Eur J Neurosci. 2009;30(1):110–5. doi: 10.1111/j.1460-9568.2009.06800.x. [DOI] [PubMed] [Google Scholar]
- Inui T, Shimura T. Delta-opioid receptor blockade in the ventral pallidum increases perceived palatability and consumption of saccharin solution in rats. Behav Brain Res. 2014;269C:20–27. doi: 10.1016/j.bbr.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Isaacson RL, Danks AM, Oestreicher AB, Brakkee JH, Gispen WH. Spontaneous bodily rotations and direction of locomotion at different times after radio frequency lesions at sites in and near the substantia nigra. Physiol Behav. 1988;44(2):199–204. doi: 10.1016/0031-9384(88)90138-2. [DOI] [PubMed] [Google Scholar]
- Jenck F, Gratton A, Wise RA. Opposite effects of ventral tegmental and periaqueductal gray morphine injections on lateral hypothalamic stimulation-induced feeding. Brain Res. 1986;399(1):24–32. doi: 10.1016/0006-8993(86)90597-4. [DOI] [PubMed] [Google Scholar]
- Jenck F, Gratton A, Wise RA. Opioid receptor subtypes associated with ventral tegmental facilitation of lateral hypothalamic brain stimulation reward. Brain Res. 1987;423(1-2):34–8. doi: 10.1016/0006-8993(87)90821-3. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009a;513(6):566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbtain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009b;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Churchill L, Klitenick MA, Hooks MS, Kalivas PW. Involvement of the ventral tegmental area in locomotion elicited from the nucleus accumbens or ventral pallidum. J Pharmacol Exp Ther. 1996;277(2):1122–1131. [PubMed] [Google Scholar]
- Johnson PI, Napier TC. Morphine modulation of GABA-and glutamate-induced changes of ventral pallidal neuronal activity. Neuroscience. 1997b;77(1):187–197. doi: 10.1016/s0306-4522(96)00482-4. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Napier TC. Contribution of the nucleus accumbens to cocaine-induced responses of ventral pallidal neurons. Synapse. 1996;22(3):253–260. doi: 10.1002/(SICI)1098-2396(199603)22:3<253::AID-SYN8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Napier TC. GABA-and glutamate-evoked responses in the rat ventral pallidum are modulated by dopamine. Eur J Neurosci. 1997a;9(7):1397–1406. doi: 10.1111/j.1460-9568.1997.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Napier TC. Ventral pallidal injections of a mu antagonist block the development of behavioral sensitization to systemic morphine. Synapse. 2000;38(1):61–70. doi: 10.1002/1098-2396(200010)38:1<61::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Stellar JR, Paul AD. Regional reward differences within the ventral pallidum are revealed by microinjections of a mu opiate receptor agonist. Neuropharmacology. 1993;32(12):1305–14. doi: 10.1016/0028-3908(93)90025-x. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Stellar JR. N-methyl-D-aspartic acid-induced lesions of the nucleus accumbens and/or ventral pallidum fail to attenuate lateral hypothalamic self-stimulation reward. Brain Res. 1994;646(1):73–84. doi: 10.1016/0006-8993(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Stellar JR. Comparison of delta opiate receptor agonist induced reward and motor effects between the ventral pallidum and dorsal striatum. Neuropharmacology. 1994;33(10):1171–82. doi: 10.1016/s0028-3908(05)80007-3. [DOI] [PubMed] [Google Scholar]
- Jones BE, Cuello AC. Afferents to the basal forebrain cholinergic cell area from pontomesencephalic--catecholamine, serotonin, and acetylcholine--neurons. Neuroscience. 1989;31(1):37–61. doi: 10.1016/0306-4522(89)90029-8. [DOI] [PubMed] [Google Scholar]
- Jones DL, Mogenson GJ. Nucleus accumbens to globus pallidus GABA projection: electrophysiological and iontophoretic investigations. Brain Res. 1980;188(1):93–105. doi: 10.1016/0006-8993(80)90559-4. [DOI] [PubMed] [Google Scholar]
- June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, Eiler WJ, Grey C, Carroll MR, McCane S, Jones CM, Yin W, Mason D, Cummings R, Garcia M, Ma C, Sarma PV, Cook JM, Skolnick P. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28(12):2124–37. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- Jourdain A, Semba K, Fibiger HC. Basal forebrain and mesopontine tegmental projections to the reticular thalamic nucleus: an axonal collateralization and immunohistochemical study in the rat. Brain Res. 25. 1989;505(1):55–65. doi: 10.1016/0006-8993(89)90115-7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57(4):1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Jackson D, Romanidies A, Wyndham L, Duffy P. Involvement of pallidothalamic circuitry in working memory. Neuroscience. 2001;104(1):129–136. doi: 10.1016/s0306-4522(01)00054-9. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Klitenick MA, Hagler H, Austin MC. GABAergic and enkephalinergic regulation of locomotion in the ventral pallidum: involvement of the mesolimbic dopamine system. The Basal Forebrain: Anatomy to Function. In: Napier TC, Kalivas PW, Hanin I, editors. Advances in Experimental Medicine and Biology. Vol. 295. Plenum Press; New York, NY: 1991. pp. 315–26. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22(3):1126–36. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13(11):4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E, Jenner P, Marsden CD. Comparison of changes in locomotor activity with striatal homovanillic acid and 3,4-dihydroxyphenylacetic acid concentrations following the bilateral intranigral injection of dopamine agonist drugs in rats. J Pharm Pharmacol. 1987;39(3):196–202. doi: 10.1111/j.2042-7158.1987.tb06248.x. [DOI] [PubMed] [Google Scholar]
- Kemppainen H, Raivio N, Kiianmaa K. Role for ventral pallidal GABAergic mechanisms in the regulation of ethanol self-administration. Psychopharmacology (Berl) 2012;223(2):211–21. doi: 10.1007/s00213-012-2709-x. 2012. [DOI] [PubMed] [Google Scholar]
- Kemppainen H, Raivio N, Nurmi H, Kiianmaa K. GABA and glutamate overflow in the VTA and ventral pallidum of alcohol-preferring AA and alcohol-avoiding ANA rats after ethanol. Alcohol Alcohol. 2010;45(2):111–8. doi: 10.1093/alcalc/agp086. [DOI] [PubMed] [Google Scholar]
- Kemppainen H, Raivio N, Suo-Yrjo V, Kiianmaa K. Opioidergic modulation of ethanol self-administration in the ventral pallidum. Alcohol Clin Exp Res. 2012;36(2):286–93. doi: 10.1111/j.1530-0277.2011.01611.x. [DOI] [PubMed] [Google Scholar]
- Khaimova E, Kandov Y, Israel Y, Cataldo G, Hadjimarkou MM, Bodnar RJ. Opioid receptor subtype antagonists differentially alter GABA agonist-induced feeding elicited from either the nucleus accumbens shell or ventral tegmental area regions in rats. Brain Res. 2004;1026(2):284–94. doi: 10.1016/j.brainres.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Intracellular study of rat globus pallidus neurons: membrane properties and responses to neostriatal, subthalamic and nigral stimulation. Brain Res. 1991;564(2):296–305. doi: 10.1016/0006-8993(91)91466-e. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Ikeda H, Koshikawa N, Cools AR. GABA (A) agents injected into the ventral pallidum differently affect dopaminergic pivoting and cholinergic circling elicited from the shell of the nucleus accumbens. Neuroscience. 2001;104:117–127. doi: 10.1016/s0306-4522(01)00053-7. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, Deutch AY, Churchill L, Kalivas PW. Topography and functional role of dopaminergic projections from the ventral mesencephalic tegmentum to the ventral pallidum. Neuroscience. 1992;50(2):371–386. doi: 10.1016/0306-4522(92)90430-a. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, Kalivas PW. Behavioral and neurochemical studies of opioid effects in the pedunculopontine nucleus and mediodorsal thalamus. J Pharmacol Exp Ther. 1994;269(1):437–48. [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Ventral pallidal GABA-A receptors regulate prepulse inhibition of acoustic startle. Brain Res. 1995a;684(1):26–35. doi: 10.1016/0006-8993(95)00372-w. [DOI] [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Prepulse inhibition in the rat is regulated by ventral and caudodorsal striato-pallidal circuitry. Behav Neurosci. 1995b;109(5):912–28. doi: 10.1037//0735-7044.109.5.912. [DOI] [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Regulation of prepulse inhibition by ventral pallidal projections. Brain Res Bull. 1997;43(2):219–28. doi: 10.1016/s0361-9230(96)00440-6. [DOI] [PubMed] [Google Scholar]
- Kolomiets BP, Deniau JM, Mailly P, Menetry A, Glowinski J, Thierry AM. Segregation and convergence of information flow through the cortico subthalamic pathways. J Neurosci. 2001;21(15):5764–5772. doi: 10.1523/JNEUROSCI.21-15-05764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. 35(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23(1):7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowski AB, Geisler S, Krauss M, Veh RW. Differential projections from the subfields in the lateral preoptic area to the lateral habenular complex of the rat. J Comp Neurol. 2008;507:1465–1478. doi: 10.1002/cne.21610. [DOI] [PubMed] [Google Scholar]
- Kretschmer BD. NMDA receptor antagonist-induced dopamine release in the ventral pallidum does not correlate with motor activation. Brain Res. 2000;859(1):147–56. doi: 10.1016/s0006-8993(00)01989-2. [DOI] [PubMed] [Google Scholar]
- Kretschmer BD, Koch M. The ventral pallidum mediates disruption of prepulse inhibition of the acoustic startle response induced by dopamine agonists, but not by NMDA antagonists. Brain Res. 1998;798(1-2):204–10. doi: 10.1016/s0006-8993(98)00424-7. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21(14):RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H, Chang HT. Ventral pallido-striatal pathway in the rat brain: a light and electron microscopic study. J Comp Neurol. 1992;321:626–636. doi: 10.1002/cne.903210409. [DOI] [PubMed] [Google Scholar]
- Kupchik YM, Kalivas PW. The rostral subcommissural ventral pallidum is a mix of ventral pallidal neurons and neurons from adjacent areas: an electrophysiological study. Brain Struct Funct. 2012;218(6):1487–1500. doi: 10.1007/s00429-012-0471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Scofield MD, Rice KC, Cheng K, Roques BP, Kalivas PW. Cocaine dysregulates opioid gating of GABA neurotransmission in the ventral pallidum. JNeurosci. 2014;34(3):1057–1066. doi: 10.1523/JNEUROSCI.4336-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Schwartz D, Kalivas PW. The Indirect Pathway is not what you think: D1 medium spiny neurons of the nucleus accumbens project to the ventral pallidum. Society for Neuroscience Abstract. 2014 [Google Scholar]
- Kuroda M, Price JL. Synaptic organization of projections from basal forebrain structures to the mediodorsal thalamic nucleus of the rat. J Comp Neurol. 1991;303(4):513–533. doi: 10.1002/cne.903030402. [DOI] [PubMed] [Google Scholar]
- Lahti RA, Mickelson MM, Jodelis KS, McCall JM. Comparative neuroanatomical distribution of the kappa and mu opioid receptors in guinea pig brain sections. Eur J Pharmacol. 1989;166(3):563–6. doi: 10.1016/0014-2999(89)90377-4. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci. 2012;35(4):614–22. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat. Rev. Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Lamour Y, Dutar P, Rascol O, Jobert A. Basal forebrain neurons projecting to the rat frontoparietal cortex: electrophysiological and pharmacological properties. Brain Res. 1986;362(1):122–31. doi: 10.1016/0006-8993(86)91405-8. [DOI] [PubMed] [Google Scholar]
- Lardeux S, Paleressompoulle D, Pernaud R, Cador M, Baunez C. Different populations of subthalamic neurons encode cocaine vs. sucrose reward and predict future error. J Neurophysiol. 2013;110:1497–1510. doi: 10.1152/jn.00160.2013. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2010;171(3):830–9. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavín A, Grace AA. Modulation of dorsal thalamic cell activity by the ventral pallidum: its role in the regulation of thalamocortical activity by the basal ganglia. Synapse. 1994;18(2):104–127. doi: 10.1002/syn.890180205. [DOI] [PubMed] [Google Scholar]
- Lavezzi HN, Parsley KP, Zahm DS. Mesopontine rostromedial tegmental nucleus neurons projecting to the dorsal raphe and pedunculopontine tegmental nucleus: psychostimulant-elicited Fos expression and collateralization. Brain Struct Funct. 2012;217(3):719–34. doi: 10.1007/s00429-011-0368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavín A, Grace AA. Physiological properties of rat ventral pallidal neurons recorded intracellularly in vivo. J Neurophysiol. 1996;75(4):1432–1443. doi: 10.1152/jn.1996.75.4.1432. [DOI] [PubMed] [Google Scholar]
- Lavín A, Grace AA. Response of the ventral pallidal/mediodorsal thalamic system to antipsychotic drug administration: involvement of the prefrontal cortex. Neuropsychopharmacology. 1998;18(5):352–363. doi: 10.1016/S0893-133X(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Sharp T, Peters SP, Gray JA, Young AM. GABA transmission in the ventral pallidum is not involved in the control of latent inhibition in the rat. Neuroscience. 2003;122(1):267–75. doi: 10.1016/s0306-4522(03)00552-9. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26(22):5881–7. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung BK, Balleine BW. The ventral striato-pallidal pathway mediates the effect of predictive learning on choice between goal-directed actions. JNeurosci. 2013;33(34):13848–13860. doi: 10.1523/JNEUROSCI.1697-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Hallanger AE, Wainer BH. Cholinergic nucleus basalis neurons may influence the cortex via the thalamus. Neurosci Lett. 1987;74(1):7–13. doi: 10.1016/0304-3940(87)90042-5. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct. 2012;217(2):257–73. doi: 10.1007/s00429-011-0360-7. [DOI] [PubMed] [Google Scholar]
- Li L, Fulton JD, Yeomans JS. Effects of bilateral electrical stimulation of the ventral pallidum on acoustic startle. Brain Res. 1999;836:164–172. doi: 10.1016/s0006-8993(99)01651-0. [DOI] [PubMed] [Google Scholar]
- Li X, Li J, Peng XQ, Spiller K, Gardner EL, Xi ZX. Metabotropic glutamate receptor 7 modulates the rewarding effects of cocaine in rats: involvement of a ventral pallidal GABAergic mechanism. Neuropharmacology. 2009;34(7):1783–1796. doi: 10.1038/npp.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang H-L, Morales M. Heterogenous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct Funct. 2013;218(5):1159–1176. doi: 10.1007/s00429-012-0452-z. [DOI] [PubMed] [Google Scholar]
- Li X, Li J, Gardner EL, Xi ZX. Activation of mGluR7s inhibits cocaine-induced reinstatement of drug-seeking behavior by a nucleus accumbens glutamate-mGluR2/3 mechanism in rats. J Neurochem. 2010;114(5):1368–1380. doi: 10.1111/j.1471-4159.2010.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster). J Comp Neurol. 2004;468:555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Lin S-C, Nicolelis MAL. Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron. 2008;59:138–149. doi: 10.1016/j.neuron.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82(3):767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Lu XY, Churchill L, Kalivas PW. Expression of D1 receptor mRNA in projections from the forebrain to the ventral tegmental area. Synapse. 1997;25(2):205–14. doi: 10.1002/(SICI)1098-2396(199702)25:2<205::AID-SYN11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Lu L, Xue Y, Steketee JD, Rebec GV, Sun W. Regulation of cocaine-induced reinstatement by group II metabotropic glutamate receptors in the ventral tegmental area. Psychopharmacology. 2012;220(1):75–85. doi: 10.1007/s00213-011-2455-5. [DOI] [PubMed] [Google Scholar]
- MacAskill AF, Little JP, Cassel JM, Carter AG. Subcellular connectivity underlies pathway-specific signaling in the nucleus accumbens. Nat Neurosci. 2012;15(12):1624–6. doi: 10.1038/nn.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine-seeking in rats. J Neurosci. 2012;32(38):13309–13325. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2012;226(4):687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014 doi: 10.1038/nn.3664. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Kelley AE. Differential behavioral effects following microinjection of an NMDA antagonist into nucleus accumbens subregions. Psychopharmacology (Berl) 1994;116(1):65–72. doi: 10.1007/BF02244872. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Kelley AE. Excitotoxic lesions of the core and shell subregions of the nucleus accumbens differentially disrupt body weight regulation and motor activity in rat. Brain Res Bull. 1995;38(6):551–9. doi: 10.1016/0361-9230(95)02030-2. [DOI] [PubMed] [Google Scholar]
- Manns ID, Mainville L, Jones BE. Evidence for glutamate, in addition to acetylcholine and GABA, neurotransmitter synthesis in basal forebrain neurons projecting to the entorhinal cortex. Neuroscience. 2001;107(2):249–263. doi: 10.1016/s0306-4522(01)00302-5. [DOI] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, Watson SJ. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci. 1990;10(8):2587–600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI. Immunohistochemical localization of GABAB receptors in the rat central nervous system. J Comp Neurol. 1999;405:299–321. doi: 10.1002/(sici)1096-9861(19990315)405:3<299::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mariotti M, Gritti I, Mancia M. The synchronizing influence of Substantia Innominata on the thalamus of the cat. J Sleep Res. 2001;10(2):143–152. doi: 10.1046/j.1365-2869.2001.00246.x. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Levey AI, Huganir RL, Price DL. Cellular localizations of AMPA glutamate receptors within the basal forebrain magnocellular complex of rat and monkey. J Neurosci. 1993;13(5):2249–2263. doi: 10.1523/JNEUROSCI.13-05-02249.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Parvalbumin-immunoreactive neurons and GABAergic neurons of the basal forebrain project to the rat basolateral amygdala. Neuroscience. 2009;160(4):805–812. doi: 10.1016/j.neuroscience.2009.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski RJ, Napier TC. Effects of D1 and D2 antagonists on apomorphine-induced responses of ventral pallidal neurons. Neuroreport. 1991a;2:451–454. doi: 10.1097/00001756-199108000-00010. [DOI] [PubMed] [Google Scholar]
- Maslowski RJ, Napier TC. Dopamine D1 and D2 receptor agonists induce opposite changes in the firing rate of ventral pallidal neurons. European Journal of Pharmacology. 1991b;200:103–112. doi: 10.1016/0014-2999(91)90672-d. [DOI] [PubMed] [Google Scholar]
- Maslowski-Cobuzzi RJ, Napier TC. Activation of dopaminergic neurons modulates ventral pallidal response evoked by amygdala stimulation. Neuroscience. 1994;62:1103–1120. doi: 10.1016/0306-4522(94)90347-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447(7148):1111–5. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Deniau JM, Glowinski J, Thierry AM. Relationships between the prefrontal cortex and basal ganglia in the rat: physiology of the corticosubthalamic circuits. J Neurosci. 1998;18(22):9539–9546. doi: 10.1523/JNEUROSCI.18-22-09539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Deniau JM, Menetrey A, Glowinski J, Thierry AM. Position of the ventral pallidum in the rat prefrontal cortex-basal ganglia circuit. Neuroscience. 1997;80(2):523–534. doi: 10.1016/s0306-4522(97)00002-x. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Robbins TW, Everitt BJ. Effects of medial dorsal thalamic and ventral pallidal lesions on the acquisition of a conditioned place preference: further evidence for the involvement of the ventral striatopallidal system in reward-related processes. Neuroscience. 1993;52(3):605–620. doi: 10.1016/0306-4522(93)90410-h. [DOI] [PubMed] [Google Scholar]
- McDaid J, Dallimore JE, Mackie AR, Mickiewicz AL, Napier TC. Cross-sensitization to morphine in cocaine-sensitized rats: behavioral assessments correlate with enhanced responding of ventral pallidal neurons to morphine and glutamate, with diminished effects of GABA. J Pharmacol Exp Ther. 2005;313(3):1182–1193. doi: 10.1124/jpet.105.084038. [DOI] [PubMed] [Google Scholar]
- McDaid J, Dallimore JE, Mackie AR, Mickiewicz AL, Napier TC. Changes in accumbal and pallidal pCREB and deltaFosB in morphine-sensitized rats: correlations with receptor-evoked electrophysiological measures in the ventral pallidum. Neuropsychopharmacology. 2006;31:1212–1226. doi: 10.1038/sj.npp.1300854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, Tedford CE, Mackie AR, Dallimore JE, Mickiewicz AL, Shen F, Angle JM, Napier TC. Nullifying drug-induced sensitization: behavioral and electrophysiological evaluations of dopaminergic and serotonergic ligands in methamphetamine-sensitized rats. Drug Alcohol Depend. 2007;86(1):55–66. doi: 10.1016/j.drugalcdep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Zaric V. Subpopulations of somatostatin immunoreactive non-pyramidal neurons in the amygdala and adjacent external capsule project to the basal forebrain: evidence for the existence of GABAergic projection neurons in the cortical nuclei and basolateral nuclear complex. Front Neural Circuits. 2012;6:46. doi: 10.3389/fncir.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24(7):1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Roberts DC. Effect of medial prefrontal cortex injections of SCH 23390 on intravenous cocaine self-administration under both a fixed and progressive ratio schedule of reinforcement. Behav Brain Res. 1995;67(1):75–80. doi: 10.1016/0166-4328(94)00106-p. [DOI] [PubMed] [Google Scholar]
- McGregor A, Roberts DC. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res. 1993;624(1-2):245–52. doi: 10.1016/0006-8993(93)90084-z. [DOI] [PubMed] [Google Scholar]
- McKay JR, Rutheford MJ, Alterman AI, Cacciola JS, Kaplan MR. An examination of the cocaine relapse process. Drug and Alcohol Dependence. 1995;38(1):35–43. doi: 10.1016/0376-8716(95)01098-j. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168(1-2):57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd ZA, McBride WJ, Murphy JM. Involvement of the mesopallidal dopamine system in ethanol reinforcement. Alcohol. 2004;32(2):137–144. doi: 10.1016/j.alcohol.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd ZA, McBride WJ, Murphy JM. Dopamine receptor regulation of ethanol intake and extracellular dopamine levels in the ventral pallidum of alcohol preferring (P) rats. Drug Alcohol Depend. 2005;77(3):293–301. doi: 10.1016/j.drugalcdep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Michaud JC, Gueudet C, Soubrié P. Effects of neurotensin receptor antagonists on the firing rate of rat ventral pallidum neurons. Neuroreport. 2000;11(7):1437–41. doi: 10.1097/00001756-200005150-00017. [DOI] [PubMed] [Google Scholar]
- Mickiewicz AL, Dallimore JE, Napier TC. The ventral pallidum is critically involved in the development and expression of morphine-induced sensitization. Neuropharmacology. 2008;34(4):874–886. doi: 10.1038/npp.2008.111. [DOI] [PubMed] [Google Scholar]
- Mickiewicz AL, Dallimore JE, Napier TC. The ventral pallidum is critically involved in the development and expression of morphine-induced sensitization. Neuropsychopharmacology. 2009;34:874–886. doi: 10.1038/npp.2008.111. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on {beta}-adrenergic receptors. Learn Mem. 2008;15(2):88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM, Port RG, Sink KS, Bunce JG, Chrobak JJ, Salamone JD. Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neurosci. 2008;28(36):9037–9046. doi: 10.1523/JNEUROSCI.1525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Richardson RT, Baker FH, DeLong MR. The primate globus pallidus: neuronal activity related to direction of movement. Exp Brain Res. 1987;68(3):491–505. doi: 10.1007/BF00249793. [DOI] [PubMed] [Google Scholar]
- Mitrovic I, Napier TC. Electrophysiological demonstration of mu, delta and kappa opioid receptors in the ventral pallidum. J Pharmacol Exp Ther. 1995;272(3):1260–1270. [PubMed] [Google Scholar]
- Mitrovic I, Napier TC. Interactions between the mu opioid agonist DAMGO and substance P in regulation of the ventral pallidum. Synapse. 1996;23(3):142–151. doi: 10.1002/(SICI)1098-2396(199607)23:3<142::AID-SYN3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Mitrovic I, Napier TC. Substance P attenuates and DAMGO potentiates amygdala glutamatergic neurotransmission within the ventral pallidum. Brain Res. 1998;792(2):193–206. doi: 10.1016/s0006-8993(98)00130-9. [DOI] [PubMed] [Google Scholar]
- Mitrovic I, Napier TC. Mu and kappa opioid agonists modulate ventral tegmental area input to the ventral pallidum. Eur J Neurosci. 2002;15(2):257–68. doi: 10.1046/j.0953-816x.2001.01860.x. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(2-3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Mogenson G, Ciriello J, Garland J, Wu M. Ventral pallidum projections to mediodorsal nucleus of the thalamus: an anatomical and electrophysiological investigation in the rat. Brain Research. 1987;404:211–230. doi: 10.1016/0006-8993(87)91373-4. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Swanson LW, Wu M. Neural projections from the nucleus accumbens to globus pallidus, substantia innominata and lateral preoptic-lateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. J Neurosci. 1983;3:189–202. doi: 10.1523/JNEUROSCI.03-01-00189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Wu M, Tsai CT. Subpallidal-pedunculopontine projections but not subpallidal-mediodorsal thalamus projections contribute to spontaneous exploratory locomotor activity. Brain Res. 1989;485(2):396–398. doi: 10.1016/0006-8993(89)90584-2. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Wu M. Subpallidal projections to the mesencephalic locomotor region investigated with a combination of behavioral and electrophysiological recording techniques. Brain Res Bull. 1986;16(3):383–390. doi: 10.1016/0361-9230(86)90060-2. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. In: The Basal Forebrain: Anatomy to Function. In: Napier TC, Kalivas PW, Hanin I, editors. Advances in Experimental Medicine and Biology. Vol. 295. Plenum Press; New York: 1991. pp. 267–90. [DOI] [PubMed] [Google Scholar]
- Morales M, Root DH. Glutamate neurons within the midbrain dopamine regions. Neuroscience. 2014;282C:60–68. doi: 10.1016/j.neuroscience.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz AS, Goodman RR. Light microscopic autoradiographic localization of mu and delta opioid binding sites in the mouse central nervous system. J Neurosci. 1984;4(5):1331–42. doi: 10.1523/JNEUROSCI.04-05-01331.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Iversen SD. Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res. 1986;397(2):214–24. doi: 10.1016/0006-8993(86)90622-0. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Hodenpijl MG, Lopes da Silva FH. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation, and interaction of inputs. The Journal of Neuroscience. 1998;18:5095–5102. doi: 10.1523/JNEUROSCI.18-13-05095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muma NA, Lee JM, Gorman L, Heidenreich BA, Mitrovic I, Napier TC. 6-hydroxydopamine-induced lesions of dopaminergic neurons alter the function of postsynaptic cholinergic neurons without changing cytoskeletal proteins. Exp Neurol. 2001;168(1):135–143. doi: 10.1006/exnr.2000.7582. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Henry S, Hu J, Gallezot JD, Planeta-Wilson B, Neumaier JF, Neumeister A. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology (Berl) 2011;213(2-3):547–53. doi: 10.1007/s00213-010-1881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Llinas R. Electrophysiology of globus pallidus neurons in vitro. J Neurophys. 1994;72(3):1127–1139. doi: 10.1152/jn.1994.72.3.1127. [DOI] [PubMed] [Google Scholar]
- Nader K, van der Kooy D. Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J Neurosci. 1997;17(1):383–90. doi: 10.1523/JNEUROSCI.17-01-00383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, gabaergic and glutamatergic neurons in the ventral tegmental area, substantia nigra, and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Stanfield PR, Yamaguchi K, Nakajima S. Substance P excites cultured cholinergic neurons in the basal forebrain. In: The Basal Forebrain: Anatomy to Function. In: Napier TC, Kalivas PW, Hanin I, editors. Advances in Experimental Medicine and Biology. Vol. 295. Plenum Press; New York: 1991. pp. 157–67. [DOI] [PubMed] [Google Scholar]
- Napier TC. Dopamine receptors in the ventral pallidum regulate circling induced by opioids injected into the ventral pallidum. Neuropharmacology. 1992a;31(11):1127–1136. doi: 10.1016/0028-3908(92)90009-e. [DOI] [PubMed] [Google Scholar]
- Napier TC. Contribution of the amygdala and nucleus accumbens to ventral pallidal responses to dopamine agonists. Synapse. 1992b;10:110–119. doi: 10.1002/syn.890100205. [DOI] [PubMed] [Google Scholar]
- Napier TC. Functional pharmacology of basal forebrain dopamine. In: Levin ED, Butcher L, editors. Neurotransmitter Interactions and Cognitive Function. Birkhäuser Boston, Inc.; Cambridge, MA: 1992c. pp. 66–77. [Google Scholar]
- Napier TC, Chrobak JJ, Yew J. Systemic and microiontophoretic administration of morphine differentially effect ventral pallidum/substantia innominata neuronal activity. Synapse. 1992a;12(3):214–219. doi: 10.1002/syn.890120306. [DOI] [PubMed] [Google Scholar]
- Napier TC, Chrobak JJ. Evaluations of ventral pallidal dopamine receptor activation in behaving rats. Neuroreport. 1992;3(7):609–11. doi: 10.1097/00001756-199207000-00016. [DOI] [PubMed] [Google Scholar]
- Napier TC, Istre ED. Methamphetamine-induced sensitization includes a functional upregulation of ventral pallidal 5-HT(2A/2C) receptors. Synapse. 2008;62:14–21. doi: 10.1002/syn.20460. [DOI] [PubMed] [Google Scholar]
- Napier TC, Maslowski-Cobuzzi RJ. Electrophysiological verification of the presence of D1 and D2 dopamine receptors within the ventral pallidum. Synapse. 1994;17(3):160–166. doi: 10.1002/syn.890170304. [DOI] [PubMed] [Google Scholar]
- Napier TC, Mickiewicz AL. The role of the ventral pallidum in psychiatric disorders. Neuropsychopharmacology. 2010;35(1):337. doi: 10.1038/npp.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier TC, Mitrovic I. Opioid modulation of ventral pallidal inputs. Ann N Y Acad Sci. 1999;877:176–201. doi: 10.1111/j.1749-6632.1999.tb09268.x. [DOI] [PubMed] [Google Scholar]
- Napier TC, Mitrovic I, Churchill L, Klitenick MA, Lu XY, Kalivas PW. Substance P in the ventral pallidum: projection from the ventral striatum, and electrophysiological and behavioral consequences of pallidal substance P. Neuroscience. 1995;69(1):59–70. doi: 10.1016/0306-4522(95)00218-8. [DOI] [PubMed] [Google Scholar]
- Napier TC, Muench MB, Maslowski RJ, Battaglia G. Is dopamine a neurotransmitter in the ventral pallidum/substantia innominata? In: The Basal Forebrain: Anatomy to Function. In: Napier TC, Kalivas PW, Hanin I, editors. Advances in Experimental Medicine and Biology. Vol. 295. Plenum Press; New York: 1991a. pp. 183–196. [DOI] [PubMed] [Google Scholar]
- Napier TC, Rehman F. Motoric analysis of dopamine receptor subtype activation within the ventral pallidum and dorsal globus pallidus. Soc. for Neurosci. Abstr. 1992;18:994. [Google Scholar]
- Napier TC, Shippenberg TS, Chefer V. Dopamine transmission in the ventral pallidum of cocaine sensitized rats. Society for Neuroscience. 2001:441, 12. [Google Scholar]
- Napier TC, Simson PE, Givens BS. Dopamine electrophysiology of ventral pallidal/substantia innominata neurons: comparison with the dorsal globus pallidus. J Pharmacol Exp Ther. 1991b;258(1):249–262. [PubMed] [Google Scholar]
- Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3(4-5):385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5(12):1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Nguyen DP, Lin S-C. A frontal cortex event-related potential driven by the basal forebrain. Elife. 2014;3:e02148. doi: 10.7554/eLife.02148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaus S, Huston JP, Hasenöhrl RU. Reinforcing effects of neurokinin substance P in the ventral pallidum: mediation by the tachykinin NK1 receptor. Eur J Pharmacol. 1999;370(2):93–9. doi: 10.1016/s0014-2999(99)00105-3. [DOI] [PubMed] [Google Scholar]
- Noel MB, Wise RA. Ventral tegmental injections of morphine but not U-50,488H enhance feeding in food-deprived rats. Brain Res. 1993;632(1-2):68–73. doi: 10.1016/0006-8993(93)91139-j. [DOI] [PubMed] [Google Scholar]
- Noel MB, Wise RA. Ventral tegmental injections of a selective mu or delta opioid enhance feeding in food-deprived rats. Brain Res. 1995;673(2):304–12. doi: 10.1016/0006-8993(94)01442-k. [DOI] [PubMed] [Google Scholar]
- Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behavioral and Cognitive Neuroscience Reviews. 2006;5(4):163–190. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in Neuroendocrinology. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005;119(6):1588–604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav Brain Res. 2005;158(1):53–68. doi: 10.1016/j.bbr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS, Dellevigne AA, Correnti CM, Numan MJ. Temporary inactivation of ventral tegmental area neurons with either muscimol or baclofen reversibly disrupts maternal behavior in rats through different underlying mechanisms. Behav Neurosci. 2009;123(4):740–51. doi: 10.1037/a0016204. [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98(4):712–27. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519(18):3599–639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336(6085):1154–7. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Lavín A, Enquist LW, Grace AA, Card JP. Interconnected parallel circuits between rat nucleus accumbens and thalamus revealed by retrograde transynaptic transport of pseudorabies virus. J Neurosci. 1997;17(6):2143–2167. doi: 10.1523/JNEUROSCI.17-06-02143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel WH, Mugnaini E. Immunocytochemical studies of GABAergic neurons in rat basal ganglia and their relations to other neuronal systems. Neurosci Lett. 1984;47(3):233–238. doi: 10.1016/0304-3940(84)90519-6. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47(6):419–27. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Olive MF, Anton B, Micevych P, Evans CJ, Maidment NT. Presynaptic verses postsynaptic localization of mu and -delta opioid receptors in dorsal and ventral striatopallidal pathways. J Neurosci. 1997;17(19):7471–7479. doi: 10.1523/JNEUROSCI.17-19-07471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Munn EM, Franklin KB, Wise RA. Effects of pedunculopontine tegmental nucleus lesions on responding for intravenous heroin under different schedules of reinforcement. J Neurosci. 1998;18(13):5035–44. doi: 10.1523/JNEUROSCI.18-13-05035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24(37):8097–105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KJ, Everitt BJ. The distribution of neurons coexpressing immunoreactivity to AMPA-sensitive glutamate receptor subtypes (GluR1-4) and nerve growth factor receptor in the rat basal forebrain. Eur J Neurosci. 1995;7(5):1022–33. doi: 10.1111/j.1460-9568.1995.tb01090.x. [DOI] [PubMed] [Google Scholar]
- Panagis G, Spyraki C. Neuropharmacological evidence for the role of dopamine in ventral pallidum self-stimulation. Psychopharmacology (Berl) 1996;123(3):280–8. doi: 10.1007/BF02246582. [DOI] [PubMed] [Google Scholar]
- Panagis G, Kastellakis A, Spyraki C. Involvement of the ventral tegmental area opiate receptors in self-stimulation elicited from the ventral pallidum. Psychopharmacology (Berl) 1998;139(3):222–9. doi: 10.1007/s002130050708. [DOI] [PubMed] [Google Scholar]
- Panagis G, Kastellakis A. The effects of ventral tegmental administration of GABA(A), GABA(B), NMDA and AMPA receptor agonists on ventral pallidum self-stimulation. Behav Brain Res. 2002;131(1-2):115–123. doi: 10.1016/s0166-4328(01)00353-9. [DOI] [PubMed] [Google Scholar]
- Panagis G, Miliaressis E, Anagnostakis Y, Spyraki C. Ventral pallidum self-stimulation: a moveable electrode mapping study. Behav Brain Res. 1995;68(2):165–172. doi: 10.1016/0166-4328(94)00169-g. [DOI] [PubMed] [Google Scholar]
- Panagis G, Nomikos GG, Miliaressis E, Chergui K, Kastellakis A, Svensson TH, Spyraki C. Ventral pallidum self-stimulation induces stimulus dependent increase in c-fos expression in reward-related brain regions. Neuroscience. 1997;77(1):175–186. doi: 10.1016/s0306-4522(96)00471-x. [DOI] [PubMed] [Google Scholar]
- Pang K, Tepper JM, Záborszky L. Morphological and electrophysiological characteristics of noncholinergic basal forebrain neurons. J Comp Neurol. 1998;394(2):186–204. doi: 10.1002/(sici)1096-9861(19980504)394:2<186::aid-cne4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56(1):141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Slater P. Effects of GABA compounds injected into the subpallidal regions of rat brain on nucleus accumbens evoked hyperactivity. Behav Neurosci. 1988;102(4):596–600. doi: 10.1037//0735-7044.102.4.596. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th edition. Elsevier: Academic Press; New York NY.: 2007. [Google Scholar]
- Péczely L, Ollmanna T, Lászlóa K, Kovácsa A, Gálosia R, Szabóa A, Karádia Z, Lénárda L. Role of D1 dopamine receptors of the ventral pallidum in inhibitory avoidance learning. Behav Brain Res. 2014 doi: 10.1016/j.bbr.2014.04.054. in press. [DOI] [PubMed] [Google Scholar]
- Perry CJ, McNally GP. A role for the ventral pallidum in context-induced and primed reinstatement of alcohol-seeking. Eur J Neurosci. 2013;38(5):2762–2773. doi: 10.1111/ejn.12283. [DOI] [PubMed] [Google Scholar]
- Person AL, Gale SD, Farries MA, Perkel DJ. Organization of the songbird basal ganglia, including area X. J Comp Neurol. 2008;508(5):840–66. doi: 10.1002/cne.21699. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, et al. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316:904–906. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28(23):6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84(2):167–73. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82(2):443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Shobin ET, Lane DA, Mackie K. Cannabinoid-1 (CB1) receptors in the mouse ventral pallidum are targeted to axonal profiles expressing functionally opposed opioid peptides and contacting NAPE PLD terminals. Neuroscience. 2012;227:10–21. doi: 10.1016/j.neuroscience.2012.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilapil C, Welner S, Magnan J, Gauthier S, Quirion R. Autoradiographic distribution of multiple classes of opioid receptor binding sites in human forebrain. Brain Res Bull. 1987;19(5):611–5. doi: 10.1016/0361-9230(87)90080-3. [DOI] [PubMed] [Google Scholar]
- Pioli EY, Meissner W, Sohr R, Gross CE, Bezard E, Bioulac BH. Differential behavioral effects of partial bilateral lesions of ventral tegmental area or substantia nigra pars compacta in rats. Neuroscience. 2008;153(4):1213–24. doi: 10.1016/j.neuroscience.2008.01.084. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Napier TC, Corbus MJ. Brain stimulation as a cue for event-related potentials in rat cortex: amphetamine effects. Int J Neurosci. 1981;15(4):217–222. doi: 10.3109/00207458108985859. [DOI] [PubMed] [Google Scholar]
- Pirch JH. Basal forebrain and frontal cortex neuron responses during visual discrimination in the rat. Brain Res Bull. 1993;31(1-2):73–83. doi: 10.1016/0361-9230(93)90013-2. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Barnes PR. Steady potential responses from the rat cortex during conditioning. Experientia. 1972;28(2):164–165. doi: 10.1007/BF01935733. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Corbus MJ. Haloperidol antagonism of amphetamine-induced effects on event-related slow potentials from rat cortex. Int J Neurosci. 1983;18(1-2):137–42. doi: 10.3109/00207458308985887. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Corbus MJ, Ebenezer I. Conditioned cortical slow potential responses in urethane anesthetized rats. Int J Neurosci. 1985;25(3-4):207–18. doi: 10.3109/00207458508985372. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Corbus MJ, Rigdon GC, Lyness WH. Generation of cortical event-related slow potentials in the rat involves nucleus basalis cholinergic innervation. Electroencephalogr Clin Neurophysiol. 1986;63(5):464–75. doi: 10.1016/0013-4694(86)90128-8. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Corbus MJ, Napier TC. Auditory cue preceding intracranial stimulation induces event-related potential in rat frontal cortex: alterations by amphetamine. Brain Res Bull. 1981;7(4):399–404. doi: 10.1016/0361-9230(81)90037-x. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Napier TC, Corbus MJ. Brain stimulation as a cue for event-related potentials in rat cortex: amphetamine effects. Int J Neurosci. 1983;15(4):217–22. doi: 10.3109/00207458108985859. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Peterson SL. Event-related slow potentials and activity of singly neurons in rat frontal cortex. Int J Neurosci. 1981;15(3):141–6. doi: 10.3109/00207458108985906. [DOI] [PubMed] [Google Scholar]
- Pirch J, Rigdon G, Rucker H Turco K. Basal forebrain modulation of cortical cell activity during conditioning. In: The Basal Forebrain: Anatomy to Function. In: Napier TC, Kalivas PW, Hanin I, editors. Advances in Experimental Medicine and Biology. Vol. 295. Plenum Press; New York: 1991. pp. 219–231. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Turco K, Rucker HK. A role for acetylcholine in conditioning-related responses of rat frontal cortex neurons: microiontophoretic evidence. Brain Res. 1992;586(1):19–26. doi: 10.1016/0006-8993(92)91366-m. [DOI] [PubMed] [Google Scholar]
- Pirot S, Jay TM, Glowinski J, Thierry AM. Anatomical and electrophysiological evidence for an excitatory amino acid pathway from the thalamic mediodorsal nucleus to the prefrontal cortex in the rat. Eur J Neurosci. 1994;6(7):1225–34. doi: 10.1111/j.1460-9568.1994.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21(18):7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin A, Gurci A, Mestikawy SE, Semba K. Vesicular glutamate transporter 3 immunoreactivity is present in cholinergic basal forebrain neurons projecting to the basolateral amygdala in rat. J Comp Neurol. 2006;498:690–711. doi: 10.1002/cne.21081. [DOI] [PubMed] [Google Scholar]
- Powell EW, Leman RB. Connections of the nucleus accumbens. Brain Res. 1976;105(3):389–403. doi: 10.1016/0006-8993(76)90589-8. [DOI] [PubMed] [Google Scholar]
- Prensa L, Parent A. The nigrostriatal pathway in the rat: a single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J Neurosci. 2001;21(18):7247–7260. doi: 10.1523/JNEUROSCI.21-18-07247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossin AR, Love TM, Koeppe RA, Zubieta JK, Silk KR. Dysregulation of regional endogenous opioid function in borderline personality disorder. Am J Psychiatry. 2010;167(8):925–933. doi: 10.1176/appi.ajp.2010.09091348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvirenti L, Berrier R, Kreifeldt M, Koob GF. Modulation of locomotor activity by NMDA receptors in the nucleus accumbens core and shell regions of the rat. Brain Res. 1994;664(1-2):231–6. doi: 10.1016/0006-8993(94)91977-1. [DOI] [PubMed] [Google Scholar]
- Quinlan MG, Sharf R, Lee DY, Wise RA, Ranaldi R. Blockade of substantia nigra dopamine D1 receptors reduces intravenous cocaine reward in rats. Psychopharmacology (Berl) 2004;175(1):53–9. doi: 10.1007/s00213-003-1771-9. 2004. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Kovacs B, Shen F, Napier TC, Meredith GE. The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus-reward associations. Eur J Neurosci. 2006;24(7):2089–2097. doi: 10.1111/j.1460-9568.2006.05066.x. [DOI] [PubMed] [Google Scholar]
- Ray JP, Russchen FT, Fuller TA, Price JL. Sources of presumptive glutamatergic/aspartatergic afferents to the mediodorsal nucleus of the thalamus in the rat. J Comp Neurol. 1992;320(4):435–456. doi: 10.1002/cne.903200403. [DOI] [PubMed] [Google Scholar]
- Reiner A, Anderson KD. The patterns of neurotransmitter and neuropeptide co-occurrence among striatal projection neurons: conclusions based on recent findings. Brain Res Brain Res Rev. 1990;15(3):251–65. doi: 10.1016/0165-0173(90)90003-7. [DOI] [PubMed] [Google Scholar]
- Ricardo JA. Efferent connections of the subthalamic region in the rat. I. The subthalamic nucleus of Luys. Brain Res. 1980;202(2):257–71. doi: 10.1016/0006-8993(80)90140-7. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30(3):767–77. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Rigdon GC, Pirch JH. Nucleus basalis involvement in conditioned neuronal responses in the rat frontal cortex. J Neurosci. 1986;6(9):2535–42. doi: 10.1523/JNEUROSCI.06-09-02535.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigdon GC, Pirch JH. Microinjection of procaine or GABA into the nucleus basalis magnocellularis affects cue-elicited unit responses in the rat frontal cortex. Exp Neurol. 1984;85(2):283–96. doi: 10.1016/0014-4886(84)90141-9. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav. 1982;17(5):901–4. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12(5):781–7. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Jian M. D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience. 1995;64:1019–1034. doi: 10.1016/0306-4522(94)00426-6. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–7. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Koob GF. Two discrete nucleus accumbens projection areas differentially mediate cocaine self-administration in the rat. Behav Brain Res. 1993;55(2):159–166. doi: 10.1016/0166-4328(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Rodgers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151(2):579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BL, Stines SG, McGuire HB, King BM. Effects of amygdala lesions on body weight, conditioned taste aversion, and neophobia. Physiol Behav. 2001;72(5):735–42. doi: 10.1016/s0031-9384(01)00433-4. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Sanghera MK, Roper-Hall A. The latency of activation of neurons in the lateral hypothalamus and substantia innominata during feeding in the monkey. Brain Res. 1979;164:121–135. doi: 10.1016/0006-8993(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Root DH, Fabbricatore AT, Ma S, Barker DJ, West MO. Rapid phasic activity of ventral pallidal neurons during cocaine self-administration. Synapse. 2010;64:704–713. doi: 10.1002/syn.20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Barker DJ, Ma S, Coffey KR, Fabbricatore AT, West MO. Evidence for learned skill during cocaine self-administration in rats. Psychopharmacology. 2011;217:91–100. doi: 10.1007/s00213-011-2261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Fabbricatore AT, Pawlak AP, Barker DJ, Ma S, West MO. Slow phasic and tonic activity of ventral pallidal neurons during cocaine self-administration. Synapse. 2012;66(2):106–127. doi: 10.1002/syn.20990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Ma S, Barker DJ, Megehee L, Striano BM, Ralston CM, Fabbricatore AT, West MO. Differential roles of ventral pallidum subregions during cocaine self-administration behaviors. J Comp Neurol. 2013;521(3):558–588. doi: 10.1002/cne.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Zhang S, Wang H-L, Hoffman AF, Lupica CR, Morales M. Single rodent mesohabenular axons release glutamate and GABA. Nature Neurosci. 2014a;17(11):1543–1551. doi: 10.1038/nn.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Qi J, Morales M. Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. J Neurosci. 2014b;34(42):13906–13910. doi: 10.1523/JNEUROSCI.2029-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Herkenham M, Pert CB, Liang T, Cascieri MA. Visualization of rat brain receptors for the neuropeptide, substance P. Brain Res. 1984 Aug 20. 1984;309(1):47–54. doi: 10.1016/0006-8993(84)91009-6. [DOI] [PubMed] [Google Scholar]
- Rouaud T, Lardeux S, Panayotis N, Paleressompoulle D, Cador M, Baunez C. Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc Natl Acad Sci U S A. 2010;107(3):1196–200. doi: 10.1073/pnas.0908189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker HK, Corbus MJ, Pirch JH. Discriminative conditioning-related slow potential and single-unit responses in the frontal cortex of urethane-anesthetized rats. Brain Res. 1986;376(2):368–72. doi: 10.1016/0006-8993(86)90201-5. [DOI] [PubMed] [Google Scholar]
- Rudenga R, Green B, Nachtigal D, Small DM. Evidence for an integrated oral sensory module in the human anterior ventral insula. Chem Senses. 2010;35(8):693–703. doi: 10.1093/chemse/bjq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russchen FT, Amaral DG, Price JL. The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. J Comp Neurol. 1985;242(1):1–27. doi: 10.1002/cne.902420102. [DOI] [PubMed] [Google Scholar]
- Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13:627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol. 1987;259:483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry. BrainRes. 1982;252:367–372. doi: 10.1016/0006-8993(82)90404-8. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Sano H, Yokoi M. Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocredit-or melanin-concentrating hormone-containing neurons. J Neurosci. 2007;27(26):6948–6955. doi: 10.1523/JNEUROSCI.0514-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Vergé D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88(3):899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- Saunders A, Oldenburg IA, Berezovskii VK, Johnson CA, Kingery ND, Elliot HL, Xie T, Gerfen CR, Sabatini BL. Nature. 2015a doi: 10.1038/nature14179. doi:10.1038/nature14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Granger AJ, Sabatini BL. Corelease of acetylcholine and GABA from cholinergic forebrain neurons. eLife. 2015b doi: 10.7554/eLife.06412. 2015;10.7554/eLife.06412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Horger BA, Peltier R, Shelton K. Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats. Brain Res. 1991;543(2):227–35. doi: 10.1016/0006-8993(91)90032-q. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Famous KR, Pierce RC. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci. 2009;30(7):1358–69. doi: 10.1111/j.1460-9568.2009.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Seeley BJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–6671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Phillips AG. Selective memory impairments produced by transient lidocaine-induced lesions of the nucleus accumbens in rats. Behav Neurosci. 1994 Jun. 1994;108(3):456–68. doi: 10.1037//0735-7044.108.3.456. [DOI] [PubMed] [Google Scholar]
- See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience. 2003;117(2):477–83. doi: 10.1016/s0306-4522(02)00665-6. [DOI] [PubMed] [Google Scholar]
- Segall MA, Margules DL. Central mediation of naloxone-induced anorexia in the ventral tegmental area. Behav Neurosci. 1989;103(4):857–64. doi: 10.1037//0735-7044.103.4.857. [DOI] [PubMed] [Google Scholar]
- Seifert U, Härtig W, Grosche J, Brückner G, Riedel A, Brauer K. Axonal expression sites of tyrosine hydroxylase, calretinin-and calbindin immunoreactivity in striato-pallidal and septal nuclei of the rat brain: a double-immunolabelling study. Brain Res. 1998;795(1-2):227–46. doi: 10.1016/s0006-8993(98)00298-4. [DOI] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23(15):6295–303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, Reiner PB, McGeer EG, Fibiger HC. Brainstem afferents to the magnocellular basal forebrain studied by axonal transport, immunohistochemistry, and electrophysiology in the rat. J Comp Neurol. 1988;267(3):433–453. doi: 10.1002/cne.902670311. [DOI] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and pedunculopontine tegmental nuclei in the rat: a retro-and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:38–41. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290(2):213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shiflett MW, Balleine BW. At the limbic-motor interface: disconnection of basolateral amygdala from nucleus accumbens core and shell reveals dissociable components of incentive motivation. Eur J Neurosci. 2010;32(10):1735–43. doi: 10.1111/j.1460-9568.2010.07439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322(1):121–35. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci. 2006;23(6):1596–604. doi: 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- Shults CW, Quirion R, Chronwall B, Chase TN, O'Donohue TL. A comparison of the anatomical distribution of substance P and substance P receptors in the rat central nervous system. Peptides. 1984;5(6):1097–128. doi: 10.1016/0196-9781(84)90177-3. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Rapuano KM, Ingeholm JE, Avery J, Kallman S, Hall KD, Martin A. The ventral pallidum and orbitofrontal coretx support food pleasantness inferences. Brain Struct Function. 2014;219(2):473–483. doi: 10.1007/s00429-013-0511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disrupts prepulse inhibition of startle in rats via 5-HT2A receptors in the ventral pallidum. Brain Res. 1997;761(1):97–104. doi: 10.1016/s0006-8993(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Sizemore GM, Co C, Smith JE. Ventral pallidal extracellular fluid levels of dopamine, serotonin, gamma amino butyric acid, and glutamate during cocaine self-administration in rats. Psychopharmacology (Berl) 2000;150(4):391–398. doi: 10.1007/s002130000456. [DOI] [PubMed] [Google Scholar]
- Skoubis PD, Maidment NT. Blockade of ventral pallidal opioid receptors induces a conditioned place aversion and attenuates acquisition of cocaine place preference in the rat. Neuroscience. 2003;119(1):241–249. doi: 10.1016/s0306-4522(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Smeets WJAJ, Marin O, Gonzalez A. Evolution of the basal ganglia: new perspectives through a comparative approach. J Anat. 2000;196:501–517. doi: 10.1046/j.1469-7580.2000.19640501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JE, Koves TR, Co C. Brain neurotransmitter turnover rates during rat intravenous cocaine self-administration. Neuroscience. 2003;117(2):461–475. doi: 10.1016/s0306-4522(02)00819-9. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25(38):8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27(7):1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behavioral Brain Research. 2009;196(2):155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren WP, Piosik PA, Cools AR. Dopamine D1 receptors in the sub commissural part of the globus pallidus and their role in oro-facial dyskinesia in cats. Eur J Pharmacol. 1991;204(2):217–22. doi: 10.1016/0014-2999(91)90708-x. [DOI] [PubMed] [Google Scholar]
- Spooren WPJM, Cuypers E, Cools AR. Oro-facial dyskinesia and the sub-commissural part of the globus pallidus in the cat: role of acetylcholine and its interaction with GABA. Psychopharmacology (Berl) 1989;99:381–385. doi: 10.1007/BF00445562. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Lynd-Balta E, Mitchell S, Haber SN. Ventral pallidostriatal pathway in the monkey: evidence for modulation of basal ganglia circuits. J Comp Neurol. 1996;370(3):295–312. doi: 10.1002/(SICI)1096-9861(19960701)370:3<295::AID-CNE2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Staines WA, Atmadja S, Fibiger HC. Demonstration of a pallidostriatal pathway by retrograde transport of HRP-labeled lectin. Brain Res. 1981;206(2):446–450. doi: 10.1016/0006-8993(81)90545-x. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Lucki I, McGonigle P. Differential regulation of dopamine D2 and D3 receptors by chronic drug treatments. J Pharmacol Exp Ther. 2000;295:1232–1240. [PubMed] [Google Scholar]
- Stratford TR, Kelley AE, Simansky KJ. Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res. 1999;825:199–203. doi: 10.1016/s0006-8993(99)01239-1. [DOI] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Brown RM, Kalivas PW. Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. J Neurosci. 2013;33(34):13654–62. doi: 10.1523/JNEUROSCI.1570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci. 2013;7:213. doi: 10.3389/fnbeh.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J Comp Neurol. 1992;321(4):515–43. doi: 10.1002/cne.903210403. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19(24):11040–8. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Evidence that the nucleus accumbens shell, ventral pallidum, and lateral hypothalamus are components of a lateralized feeding circuit. Behav Brain Res. 2012;226(2):548–554. doi: 10.1016/j.bbr.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Lateral hypothalamic involvement in feeding elicited from the ventral pallidum. Eur J Neurosci. 2013;37(4):648–653. doi: 10.1111/ejn.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology. 2005;30(11):2073–81. doi: 10.1038/sj.npp.1300744. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;177(3):315–23. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23(32):10258–64. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;177(3):315–23. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology. 2005;30(11):2073–81. doi: 10.1038/sj.npp.1300744. [DOI] [PubMed] [Google Scholar]
- Sur C, Betz H, Schloss P. Immunocytochemical detection of the serotonin transporter in rat brain. Neuroscience. 1996;73(1):217–231. doi: 10.1016/0306-4522(96)00030-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. A note on the connections and development of the nucleus accumbens. Brain Res. 1975;92(2):324–330. doi: 10.1016/0006-8993(75)90278-4. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Köhler C. Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. J Neurosci. 1986;6(10):3010–3023. doi: 10.1523/JNEUROSCI.06-10-03010.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Mogenson GJ, Gerfen CR, Robinson P. Evidence for a projection from the lateral preoptic area and substantia innominata to the 'mesencephalic locomotor region' in the rat. Brain Res. 1984;295(1):161–178. doi: 10.1016/0006-8993(84)90827-8. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF. Lesions of the dorsomedial nucleus of the thalamus, medial prefrontal cortex and pedunculopontine nucleus: effects on locomotor activity mediated by nucleus accumbens-ventral pallidal circuitry. Brain Res. 1987;412(2):233–43. doi: 10.1016/0006-8993(87)91129-2. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. GABAergic projection from nucleus accumbens to ventral pallidum mediates dopamine-induced sensorimotor gating deficits of acoustic startle in rats. Brain Res. 1990;532(1-2):146–50. doi: 10.1016/0006-8993(90)91754-5. [DOI] [PubMed] [Google Scholar]
- Switzer RC, 3rd, Hill J, Heimer L. The globus pallidus and its rostroventral extension into the olfactory tubercle of the rat: a cyto-and chemoarchitectural study. Neuroscience. 1982;7(8):1891–1904. doi: 10.1016/0306-4522(82)90005-7. [DOI] [PubMed] [Google Scholar]
- Tachibana Y, Hikosaka O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron. 2012;76(4):826–837. doi: 10.1016/j.neuron.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Katsuura Y, Noorvash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience. 7. 2009;161(3):718–733. doi: 10.1016/j.neuroscience.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566(1-2):26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Tang XC, McFarland K, Cagle S, Kalivas PW. Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum. J Neurosci. 2005;25(18):4512–4520. doi: 10.1523/JNEUROSCI.0685-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Badurek S, DiLeone RJ, Nashmi R, Minichiello L, Picciotto MR. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J Comp Neurol. 2014 doi: 10.1002/cne.23603. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci. 2004;24(5):1058–1069. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005;22(10):2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Berridge KC, Aldridge JW. Dynamic computation of incentive salience: “wanting” what was never “liked”. J Neurosci. 2009;29(39):12220–12228. doi: 10.1523/JNEUROSCI.2499-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Peciña S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol. 2006;96(5):2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Tang XC, Kalivas PW. The glutamatergic projection from the prefrontal cortex to the nucleus accumbens core is required for cocaine-induced decreases in ventral pallidal GABA. Neurosci Lett. 2008;438(2):142–145. doi: 10.1016/j.neulet.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth A, Hajnik T, Záborszky L, Détári L. Effect of basal forebrain neuropeptide Y administration on sleep and spontaneous behavior in freely moving rats. Brain Res Bull. 2007;72(4-6):293–301. doi: 10.1016/j.brainresbull.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Prensa L, Cebrian C, Mengual E. Axonal branching patterns of nucleus accumbens neurons in rat. J Comp Neurol. 2010;518(22):4649–4673. doi: 10.1002/cne.22484. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Prensa L, Mengual E. Axonal branching patterns of ventral pallidal neurons in the rat. Brain Struct Funct. 2013;218:1133–1157. doi: 10.1007/s00429-012-0451-0. [DOI] [PubMed] [Google Scholar]
- Troiano R, Siegel A. Efferent connections of the basal forebrain in the cat: the nucleus accumbens. Exp Neurol. 1978a;61(1):185–197. doi: 10.1016/0014-4886(78)90190-5. [DOI] [PubMed] [Google Scholar]
- Troiano R, Siegel A. Efferent connections of the basal forebrain in the cat: the substantia innominata. Exp Neurol. 1978b;61(1):198–213. doi: 10.1016/0014-4886(78)90191-7. [DOI] [PubMed] [Google Scholar]
- Trojniar W, Staszewska M. Bilateral lesions of the pedunculopontine tegmental nucleus affect feeding induced by electrical stimulation of the ventral tegmental area. Acta Neurobiol Exp (Wars) 1995;55(3):201–6. doi: 10.55782/ane-1995-1076. [DOI] [PubMed] [Google Scholar]
- Turenius CI, Htut MM, Prodon DA, Ebersole PL, Ngo PT, Lara RN, Wilczynski JL, Stanley BG. GABA(A) receptors in the lateral hypothalamus as mediators of satiety and body weight regulation. Brain Res. 2009;1262:16–24. doi: 10.1016/j.brainres.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Turner MS, Lavin A, Grace AA, Napier TC. Regulation of limbic information outflow by the subthalamic nucleus: excitatory amino acid projections to the ventral pallidum. J Neurosci. 2001;21(8):2820–2832. doi: 10.1523/JNEUROSCI.21-08-02820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MS, Gray TS, Mickiewicz AL, Napier TC. Fos expression following activation of the ventral pallidum in normal rats and in a model of Parkinson's Disease: implications for limbic system and basal ganglia interactions. Brain Struct Funct. 2008;213(1-2):197–213. doi: 10.1007/s00429-008-0190-4. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: Dissection of D3 signal and anatomy. NeuroImage. 2010;54(1):264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Uchida T, Adachi K, Fujita S, Lee J, Gionhaku N, Cools AR, Koshikawa N. Role of GABA(A) receptors in the retrorubral field and ventral pallidum in rat jaw movements elicited by dopaminergic stimulation of the nucleus accumbens shell. Eur J Pharmacol. 2005;510(1-2):39–47. doi: 10.1016/j.ejphar.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Yang P, Robinson TE. Subthalamic nucleus lesions enhance the psychomotor-activating, incentive motivational, and neurobiological effects of cocaine. J Neurosci. 2005;25(37):8407–15. doi: 10.1523/JNEUROSCI.1910-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Research. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25(38):8665–70. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313(4):643–68. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Bockaert J, Dumuis A. Regional distribution and ontogeny of 5-HT4 binding sites in rat brain. Behav Brain Res. 1996;73(1-2):259–62. doi: 10.1016/0166-4328(96)00108-8. [DOI] [PubMed] [Google Scholar]
- Vives F, Mogenson GJ. Electrophysiological evidence that the mediodorsal nucleus of the thalamus is a relay between the ventral pallidum and the medial prefrontal cortex in the rat. Brain Res. 1985;344(2):329–337. doi: 10.1016/0006-8993(85)90811-x. [DOI] [PubMed] [Google Scholar]
- Walaas I, Fonnum F. The distribution and origin of glutamate decarboxylase and choline acetyltransferase in ventral pallidum and other basal forebrain regions. Brain Res. 1979;177(2):325–36. doi: 10.1016/0006-8993(79)90783-2. [DOI] [PubMed] [Google Scholar]
- Wallace LJ, Uretsky NJ. Effect of GABAergic and glutamatergic drugs injected into the ventral pallidum on locomotor activity. In: The Basal Forebrain: Anatomy to Function. In: Napier TC, Kalivas PW, Hanin I, editors. Advances in Experimental Medicine and Biology. Vol. 295. Plenum Press; New York: 1991. pp. 307–14. [DOI] [PubMed] [Google Scholar]
- Walter MG. The convergence and interaction of visual, auditory, and tactile responses in human nonspecific cortex. Ann NY Acad Sci. 1964;112:321–361. doi: 10.1111/j.1749-6632.1964.tb26760.x. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25(22):5389–96. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65(10):857–62. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waraczynski M, Demco C. Lidocaine inactivation of the ventral pallidum affects responding for brain stimulation reward more than it affects the stimulation's reward value. Behav Brain Res. 2006;173(2):288–98. doi: 10.1016/j.bbr.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Robbins TW, Everitt BJ. Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology (Berl) 1997;134(3):242–57. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Whitelaw RB, Robbins TW, Everitt BJ. Excitotoxic lesions of the mediodorsal thalamic nucleus attenuate intravenous cocaine self-administration. Psychopharmacology (Berl) 1998;140(2):225–32. doi: 10.1007/s002130050761. [DOI] [PubMed] [Google Scholar]
- Wenk GL. The nucleus basalis magnocellularis cholinergic system: one hundred years of progress. Neurobiol Learn Mem. 1997;67(2):85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57(5):774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- White FJ. Electrophysiological basis of the reinforcing effects of cocaine. Behav Pharmacol. 1990;1(4):303–315. doi: 10.1097/00008877-199000140-00004. [DOI] [PubMed] [Google Scholar]
- Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology (Berl) 1996;127(3):213–24. [PubMed] [Google Scholar]
- Wikler A. Recent progress in research on the neurophysiologic basis of morphine addiction. American Journal of Psychiatry. 1948;105(5):329–338. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23(7):2882–8. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. The amygdala is critical for opioid-mediated binge eating of fat. Neuroreport. 2004;15(12):1857–60. doi: 10.1097/00001756-200408260-00004. [DOI] [PubMed] [Google Scholar]
- Will MJ, Pritchett CE, Parker KE, Sawani AM, Ma H, Lai AY. Behavioral characterization of amygdala involvement in mediating intra accumbens opioid-driven feeding behavior. Behav Neurosci. 2009;123(4):781–93. doi: 10.1037/a0016060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DJ, Crossman AR, Slater P. The efferent projections of the nucleus accumbens in the rat. Brain Res. 1977;130(2):217–227. doi: 10.1016/0006-8993(77)90271-2. [DOI] [PubMed] [Google Scholar]
- Wilson FAW, Rolls ET. Neuronal responses related to reinforcement in the primate basal forebrain. Brain Res. 1990;509:213–231. doi: 10.1016/0006-8993(90)90546-n. [DOI] [PubMed] [Google Scholar]
- Wydra K, Golembiowska K, Zaniewska M, Kaminska K, Ferraro L, Fuxe K, Filip M. Accumbal and pallidal dopamine, glutamate and GABA overflow during cocaine self-administration and its extinction in rats. Addict Biol. 2013;18(2):307–324. doi: 10.1111/adb.12031. [DOI] [PubMed] [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1980;35(1-2):227–63. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic projections from the basal forebrain to the frontal cortex: a combined fluorescent tracer and immunohistochemical analysis in the rat. Neuroscience Letters. 1983;40:93–98. doi: 10.1016/0304-3940(83)90285-9. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Increased mesolimbic GABA concentration blocks heroin self-administration in the rat. J Pharmacol Exp Ther. 2000;294(2):613–9. [PubMed] [Google Scholar]
- Xie X, Lasseter HC, Ramirez DR, Ponds KL, Wells AM, Fuchs RA. Subregion-specific role of glutamate receptors in the nucleus accumbens on drug context-induced reinstatement of cocaine-seeking behavior in rats. Addict Biol. 2012;17(2):287–299. doi: 10.1111/j.1369-1600.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. JNeurosci. 2011;31(23):8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. Brain regions responsible for the expression of conditioned taste aversion in rats. Chem Senses. 2007;32:105–109. doi: 10.1093/chemse/bjj045. [DOI] [PubMed] [Google Scholar]
- Yang CR, Mogenson GJ. An electrophysiological study of the neural projections from the hippocampus to the ventral pallidum and the subpallidal areas by way of the nucleus accumbens. Neuroscience. 1985;15:1015–1024. doi: 10.1016/0306-4522(85)90250-7. [DOI] [PubMed] [Google Scholar]
- Yarovaya N, Schot R, Fodero L, McMahon M, Mahoney A, Williams R, Verbeek E, de Bondt A, Hampson M, van der Spek P, Stubbs A, Masters CL, Verheijen FW, Mancini GM, Venter DJ. Sialin, an anion transporter defective in sialic acid storage diseases, shows highly variable expression in adult mouse brain, and is developmentally regulated. Neurobio Dis. 2005;19(3):351–365. doi: 10.1016/j.nbd.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Yawata S, Yamaguchi T, Danjo T, Hikida T, Nakanishi S. Pathway-specific control of reward learning and its flexibility via selective dopamine receptors in the nucleus accumbens. Proc Natl Acad Sci U S A. 2012;109(31):12764–9. doi: 10.1073/pnas.1210797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Response of ventral pallidal neurons to amygdala stimulation and its modulation by dopamine projections to nucleus accumbens. J Neurophysiol. 1983;50(1):148–161. doi: 10.1152/jn.1983.50.1.148. [DOI] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J Neurosci. 2007;27(39):10546–55. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, 3rd, Alheid GF, Heimer L. The ventral pallidal projection to the mediodorsal thalamus: a study with fluorescent retrograde tracers and immunohistofluorescence. J Neurosci. 1984;4(6):1626–1638. doi: 10.1523/JNEUROSCI.04-06-01626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, 3rd, Alheid GF, Heimer L. The ventral pallidal projection to the mediodorsal thalamus: a study with fluorescent retrograde tracers and immunohistofluorescence. J Neurosci. 1984;4(6):1626–38. doi: 10.1523/JNEUROSCI.04-06-01626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J Neurosci. 2007;27(39):10546–55. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Fields HL. Basolateral amygdala lesions impair both cue-and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121(3):747–57. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
- Zaborszky L. Afferent connections of the forebrain cholinergic projection neurons, with special reference to monoaminergic and peptidergic fibers. In: Frotscher M, Misgeld U, editors. Central Cholinergic Synaptic Transmission. Birkhauser Verlag; Basel, Switzerland: 1989. pp. 12–32. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Buhl DL, Pobalashingham S, Bjaalie JG, Nadasdy Z. Three-dimensional chemoarchitecture of the basal forebrain: spatially specific association of cholinergic and calcium binding protein-containing neurons. Neuroscience. 2005;136:697–713. doi: 10.1016/j.neuroscience.2005.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Záborszky L, Carlsen J, Brashear HR, Heimer L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol. 1986;243(4):488–509. doi: 10.1002/cne.902430405. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE. Projections from the nucleus accumbens to cholinergic neurons of the ventral pallidun: a correlated light and electron microscopic double-immunolabeling study in rat. Brain Research. 1992;570:92–101. doi: 10.1016/0006-8993(92)90568-t. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE, Braun A. Afferents to basal forebrain cholinergic projection neurons: an update. In: The Basal Forebrain: Anatomy to Function. In: Napier TC, Kalivas PW, Hanin I, editors. Advances in Experimental Medicine and Biology. Vol. 295. Plenum Press; New York: 1991. pp. 43–100. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79(4):1051–1078. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Heimer L, Eckenstein F, Leranth C. GABAergic input to cholinergic forebrain neurons: an ultrastructural study using retrograde tracing of HRP and double immunolabeling. J Comp Neurol. 1986;250:282–295. doi: 10.1002/cne.902500303. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. NeuroImage. 2008;42:1127–1141. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Léránth C, Heimer L. Ultrastructural evidence of amygdalofugal axons terminating on cholinergic cells of the rostral forebrain. Neurosci Lett. 1984;52(3):219–225. doi: 10.1016/0304-3940(84)90165-4. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Ann N Y Acad Sci. 1999;877:339–367. doi: 10.1111/j.1749-6632.1999.tb09276.x. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, van den Pol A, Gyengesi E. The basal forebrain cholinergic projection system in mice. In: Watson C, et al., editors. The Mouse Nervous System. Elsevier Inc.; Amsterdam: 2012. pp. 684–718. [Google Scholar]
- Zaborszky L, Heimer L. IBAGS IInd Triennial. Univ. Victoria, B.C.; Canada: 1986. GABAergic neurons in the ventral pallidum. Abstracts 4l. [Google Scholar]
- Zaborszky L, Duque A, Gielow M, Gombkoto P, Nadasdy Z, Somogyi J. Organization of the basal forebrain cholinergic projection system: specific or diffuse? In: Paxinos G, editor. The Rat Nervous System. 4th edition Elsevier Inc.; Amsterdam: 2015a. pp. 491–507. [Google Scholar]
- Zaborszky L, Amunts K, Palomero-Gallagher N, Zilles K. Basal forebrain anatomical systems in MRI space. In: Zilles K, Amunts K, Toga A, editors. Brain Mapping: An Encyclopedic Reference. Elsevier; New York, NY: 2015b. pp. 395–409. in press. [Google Scholar]
- Zahm DS. The ventral striatopallidal parts of the basal ganglia in the rat-II. Compartmentation of ventral pallidal efferents. Neuroscience. 1989;30:33–50. doi: 10.1016/0306-4522(89)90351-5. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Grosu S, Williams EA, Qin S, Berod A. Neurons of origin of the neurotensinergic plexus enmeshing the ventral tegmental area in rat: retrograde labeling and in situ hybridization combined. Neuroscience. 2001;104(3):841–851. doi: 10.1016/s0306-4522(01)00118-x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Becker ML, Freiman AJ, Strauch S, DeGarmo B, Geisler S, Meredith GE, Marinelli M. Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the basal forebrain and recalibration of expression. Neuropsychopharmacology. 2009:1–19. doi: 10.1038/npp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50(4):751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Grosu S, Irving JC, Williams EA. Discrimination of striatopallidum and extended amygdala in the rat: a role for parvalbumin immunoreactive neurons? Brain Res. 2003;978(1-2):141–154. doi: 10.1016/s0006-8993(03)02801-4. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Ventral striatopallidal parts of the basal ganglia in the rat: I. Neurochemical compartmentation as reflected by the distributions of neurotensin and substance P immunoreactivity. J Comp Neurol. 1988;272:516–535. doi: 10.1002/cne.902720406. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. The ventral striatopallidothalamic projection. III. Striatal cells of the olfactory tubercle establish direct synaptic contact with ventral pallidal cells projecting to mediodorsal thalamus. Brain Res. 1987;404(1-2):327–331. doi: 10.1016/0006-8993(87)91388-6. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302(3):437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327(2):220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Cheng AY, Lee TJ, Ghobadi CQ, Shwartz ZM, Geisler S, Parsely KP, Gruber C, Veh RW. Inputs to the midbrain dopaminergic complex in the rat with emphasis on extended amygdala-recipient sectors. J Comp Neurol. 2011;519(16):3159–3188. doi: 10.1002/cne.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Annals NY Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Johnson SN. Asymmetrical distribution of neurotensin immunoreactivity following unilateral injection of 6-hydroxydopamine in rat ventral tegmental area (VTA). Brain Res. 1989;483(2):301–311. doi: 10.1016/0006-8993(89)90174-1. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Williams E, Wohltmann C. Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. J Comp Neurol. 1996;364(2):340–362. doi: 10.1002/(SICI)1096-9861(19960108)364:2<340::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Záborszky L, Alones VE, Heimer L. Evidence for the coexistence of glutamate decarboxylase and Met-enkephalin immunoreactivities in axon terminals of rat ventral pallidum. Brain Res. 1985;325(1-2):317–321. doi: 10.1016/0006-8993(85)90331-2. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Parsely KP, Schwartz ZM, Cheng AY. On lateral septum-like characteristics of outpute from the accumbal hedonic ‘hotspot’ of Pecina and Berridge with commentary on the transitional nature of basal forebrain ‘boundaries’. J Comp Neurol. 2012;521(1):50–68. doi: 10.1002/cne.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Schwartz ZM, Lavezzi HN, Yetnikoff L, Parsley KP. Comparison of the locomotor-activating effects of bicuculline infusions into the preoptic area and ventral pallidum. Brain Struct Func. 2013;219(2):511–526. doi: 10.1007/s00429-013-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Ebrahimi-Ghiri M, Rostami P, Rezayof A. Repeated pre-exposure to morphine into the ventral pallidum enhances morphine-induced place preference: involvement of dopaminergic and opioidergic mechanisms. Behav Brain Res. 2007;181(1):35–41. doi: 10.1016/j.bbr.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bailey KR, Toupin MM, Mair RG. Involvement of ventral pallidum in prefrontal cortex-dependent aspects of spatial working memory. Behav Neurosci. 2005;119(2):399–409. doi: 10.1037/0735-7044.119.2.399. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27(41):11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, et al. Regulation of human affective responses by anterior cingulate and limbic mu opioid neurotransmission. Arch Gen Psychiatry. 2003;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]