Abstract
Objective
Sleep curtailment has been linked to obesity, but underlying mechanisms remain to be elucidated. We assessed whether sleep restriction alters 24-hour profiles of appetite-regulating hormones ghrelin, leptin and pancreatic polypeptide during a standardized diet, and whether these hormonal alterations predict food intake during ad libitum feeding.
Methods
Nineteen healthy, lean men were studied under normal sleep and sleep restriction in a randomized crossover design. Blood samples were collected for 24-hours during standardized meals. Subsequently, participants had an ad libitum feeding opportunity (buffet meals and snacks) and caloric intake was measured.
Results
Ghrelin levels were increased after sleep restriction as compared to normal sleep (p<0.01). Overall, sleep restriction did not alter leptin or pancreatic polypeptide profiles. Sleep restriction was associated with an increase in total calories from snacks by 328 ± 140 Kcal (p=0.03), primarily from carbohydrates (p=0.02). The increase in evening ghrelin during sleep restriction was correlated with higher consumption of calories from sweets (r=0.48, p=0.04).
Conclusions
Sleep restriction as compared to normal sleep significantly increases ghrelin levels. The increase in ghrelin is associated with more consumption of calories. Elevated ghrelin may be a mechanism by which sleep loss leads to increased food intake and the development of obesity.
Keywords: Ghrelin, sleep restriction, obesity, food intake
Introduction
There is strong epidemiologic evidence suggesting that reduced sleep duration is associated with an increased risk for obesity and weight gain (1–4). One possible mechanism by which short sleep may predispose to weight gain is through alterations in appetite-regulating hormones, leading to increased food intake. Indeed, short-term experimental studies have consistently shown that sleep restriction increases caloric intake when free access to food is allowed (5–11). However, findings regarding the direct effects of sleep restriction on appetite-regulating hormones have been inconsistent. Although some studies have shown increased levels of the appetite-stimulating hormone ghrelin (10, 12) and decreased levels of the satiety hormone leptin (13–15) during controlled caloric intake, the majority of studies have reported that sleep restriction is not associated with these hormonal changes, as measured by a single morning blood sample (16, 17) or under ad libitum feeding conditions (5, 7–9, 18, 19). These inconsistent findings may be due to methodological variations in feeding conditions during hormonal measurements (e.g. standardized vs. ad libitum caloric intake) or the timing and frequency of blood sampling. Likewise, it is possible that the effects of sleep restriction on appetite-regulating hormones may be undetectable when measurements are taken during uncontrolled caloric intake.
In the present study, we have first examined the effects of sleep restriction on frequently sampled 24-hour blood profiles of appetite-regulating hormones ghrelin, leptin and pancreatic polypeptide during standardized meals. Subsequently, we measured caloric intake during an ad libitum feeding opportunity. We hypothesized that sleep restriction as compared to normal sleep, would alter appetite-regulating hormones during the controlled diet period and that these hormonal alterations would predict increased caloric intake during the subsequent ad libitum eating opportunity.
Methods
Participants
Nineteen healthy young lean men (mean age: 23.5 ± 0.7 years) who had a BMI between 19.0 and 24.9 kg/m2 (mean BMI: 23.1 ± 0.4 kg/m2) were recruited from the local community through advertisements. All participants had self-reported habitual bedtimes between 7.5 to 8.5 hours. Exclusion criteria were: history of any acute or chronic medical condition, current or past shift work, travel across time zones during the past 4 weeks, depressed mood (as assessed by a score on the Center for Epidemiologic Studies of Depression scale above 16), use of any medications or supplements known to affect sleep or glucose metabolism, current smoking, excessive alcohol (>2 drinks per day) or caffeine (>300mg per day) consumption or abnormal findings on physical examination or routine laboratory testing. Sleep disorders were excluded by laboratory polysomnography. Normal glucose tolerance status was confirmed with a standard 75-g oral glucose tolerance test. All participants had normal findings on routine laboratory tests (complete blood counts, comprehensive metabolic panel, thyroid function tests, lipid panel and hemoglobin A1c), as well as a 12-lead electrocardiogram. The Institutional Review Board of the University of Chicago approved the protocol and written informed consent was obtained from each participant. We previously reported the effects of sleep restriction on insulin signaling in adipocytes, total body insulin sensitivity, and 24-hour profiles of free fatty acids and lipolytic hormones from the participants who are included in this report (20, 21).
Study protocol
Participants were studied under normal sleep and sleep restriction conditions in a randomized order spaced at least 4 weeks apart. Participants were admitted to the University of Chicago Clinical Research Center for the entire duration of the study. The normal sleep condition involved four consecutive nights of 8.5 hours in bed (2300 - 0730h) and the sleep restriction condition involved four consecutive nights of 4.5 hours in bed (0100 – 0530h). During each sleep condition, body weight was measured with a digital scale (Scale-Tronix, Model 5002, Wheaton, IL) in the morning following all experimental nights except on the day of 24-hour blood sampling. Percent body fat was assessed by bioimpedance (Quantum X, RJL Systems, Clinton Township, MI) at the beginning of each sleep condition.
Sleep was recorded each night by polysomnography, as previously described (20, 21). Under both sleep conditions, participants remained in the laboratory and engaged in only sedentary activities (e.g. reading, watching TV, computer work, board or card games, etc.). Activity levels were measured continuously using accelerometer-based monitors attached to the wrist (Actiwatch; Philips-Respironics, Bend, OR) and waist (Actical; Philips-Respironics, Bend, OR). Research staff was continuously present to monitor wakefulness during scheduled wake periods. During the week preceding each laboratory period, participants maintained standardized bedtimes at home in accordance with their usual habits. Naps were not allowed. Compliance with this schedule was verified by continuous wrist activity monitoring and sleep diaries.
Standardized diet and 24-hour blood sampling
Blood samples were collected at 15 to 30 min intervals for 24 hours (from 2130h starting on the evening prior to the 3rd night of each condition) as previously described (20) for measurements of ghrelin, leptin and pancreatic polypeptide. At 1000h on the day following the 4th night of each sleep condition, a frequently sampled intravenous glucose tolerance test was performed as previously described (21). Diet was strictly controlled and caloric intake was identical under both sleep conditions from admission until the ad libitum feeding period starting at 1500h on the day following the 4th night of each sleep condition. During the screening process, each study participant met with a registered dietitian to review the standardized diet and to determine if they had any food allergies or intolerances. Subjects’ individual energy requirements were determined using the Schofield equation (22). The standardized study diet consisted of a two-day cycle menu of three isocaloric meals per day (55–60% carbohydrate, 15–20% protein and 30–35% fat). Participants received identical carbohydrate-rich meals (68% carbohydrate, 12% protein and 20% fat) at 1900h prior to 24-hour blood sampling and at 0900h, 1400h and 1900h during blood sampling. Participants were required to consume each meal in its entirety within 20 minutes.
Ad libitum feeding
Following the 4th night of each sleep condition, participants were presented with an ad libitum lunch buffet (served at 1500h) and dinner buffet (served at 1930h). Participants were given a 1-hour period during both buffet meals without any distraction by the research team to eat as much as they wanted. Participants also had unlimited access to a snack bar between the two buffet meals. To allow for ad libitum intake, participants were presented with excessive portion sizes of all food items. A registered dietitian met with each participant to plan the buffet meals based on individual food preferences. For each buffet meal, participants were required to choose three entrees (e.g. hot or cold sandwich, pizza, pasta, hot meat, poultry or fish), two breads (e.g. bread, dinner roll), one raw vegetable (e.g. salad or raw vegetable plate), one cooked vegetable, one starch (e.g. rice or potato), two fruits, two dairy products (e.g. milk, cheese or yogurt), two desserts (one of which was chocolate), one non-caffeinated beverage and condiments. For the snack bar, participants were asked to choose a variety of ten items that they found appealing from the following food categories including sweets (e.g. cookies, candy, ice cream), salty snacks (e.g. chips, popcorn), starches (e.g. bread, bagels), dairy (e.g. yogurt, cheese), protein and meats (e.g. hummus, nut butter, beef jerky), fruits and vegetables (e.g. fresh, canned or dried fruit, fresh vegetables), and beverages (e.g. juice, soda). The same individually customized assortment of foods was given for all buffet meals and the snack bar in both sleep conditions.
Food was weighed before and after the buffet meals and snacking period to determine food consumption. The Food Processor SQL software (ESHA Research, Salem, OR) was used to calculate total caloric content and macronutrient composition of each food item. Food categories for snacks were defined as sweets, salty snacks, starches, fruits and vegetables, dairy, protein/meat, beverages and condiments/fats/oils. Participants were only permitted to consume food and beverages that were provided by the metabolic kitchen. Beverages containing caffeine were not allowed. All meals were prepared under the supervision of a registered dietitian from the metabolic kitchen at University of Chicago Clinical Research Center.
Hormonal Assays
Blood samples were immediately centrifuged at 4°C. Plasma and serum samples were frozen and stored at −80°C until assayed. Serum total ghrelin and leptin levels were measured by radioimmunoassay (EMD Millipore, St Charles, MI) with the limit of sensitivity of 93 pg/mL and 0.5 ng/mL respectively, and the intra-assay coefficient of variations of 5%. Plasma pancreatic polypeptide levels were measured by radioimmunoassay (Alpco, Salem, NH) with the limit of sensitivity of 3 pmol/L with an intra-assay coefficient of variation of less than 3%. For each 24-hour profile, all samples obtained from the same participant were measured in the same assay.
Data Analysis and Statistical Methods
All hormonal profiles were interpolated at 15 min intervals to facilitate chronobiological analyses. The meal-related peak values for ghrelin and pancreatic polypeptide were defined as the maximum concentration measured during the 60 min after each meal. The post-meal nadir values for ghrelin and pancreatic polypeptide were defined as the minimum concentration measured between 60 and 240 min after each meal. The meal responses for ghrelin and pancreatic polypeptide were calculated as the areas under the curve (AUC) during the 2.5 hours after each meal using the trapezoidal method. Nocturnal mean values for each hormone were calculated from the overnight fasting period between 2130h and 0900h. To quantify the 24-hour profiles of serum leptin, a best-fit curve was fitted for each individual profile using a robust locally weighted regression procedure with a 4-hour window (23). The leptin amplitude was defined as half of the difference between the acrophase (maximum of the best-fit curve) and the nadir (minimum of the best-fit curve).
The effects of sleep restriction on all variables were assessed using a mixed model ANOVA with restricted maximum likelihood estimates of variance components. Participants were treated as random effects and sleep condition was treated as a fixed effect. Relationships between changes in hormone levels and changes in food intake were examined using the Pearson coefficient. Data are expressed as mean ± standard error of the mean (SEM). All statistical analyses were performed using JMP statistical software for Macintosh (SAS Institute, version 9.0.3). A p-value less than 0.05 was considered significant.
Results
Body weight and percent body fat
During the standardized diet period, average body weight over the four days of each sleep condition was similar (75.0 ± 2.5 kg during normal sleep vs. 74.4 ± 2.4 kg during sleep restriction, p=0.09) and percent body fat measured at admission was not different between conditions (19.3 ± 1.1 % before normal sleep vs. 19.2 ± 1.0 % before sleep restriction, p=0.86). Morning body weight measured prior to ad libitum feeding did not differ between conditions (75.0 ± 2.5 kg in normal sleep vs. 74.6 ± 2.4 kg in sleep restriction, p=0.27).
Sleep characteristics
Over 4 nights, participants slept an average of 7.8 ± 0.1 hours during normal sleep vs. 4.3 ± 0.0 hours during sleep restriction (p<0.0001). Overall, the duration of REM sleep was reduced by 54% during sleep restriction relative to normal sleep (p<0.0001), whereas the duration of slow wave sleep did not differ between sleep conditions (p=0.13). The effects of sleep restriction on all sleep stages have been previously reported in more detail (20).
24-hour profiles of ghrelin, leptin and pancreatic polypeptide during controlled diet period
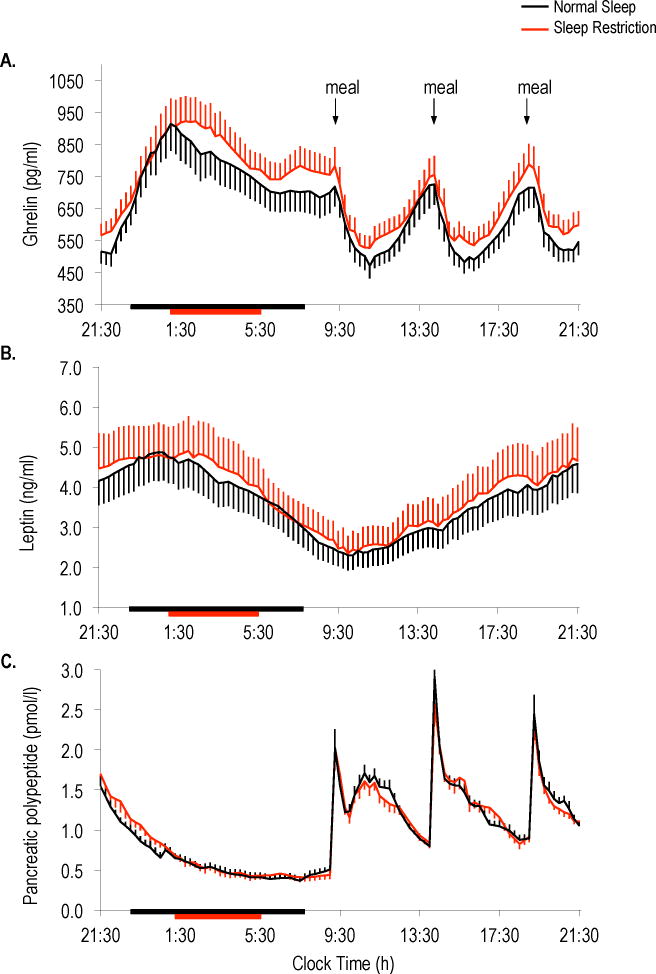
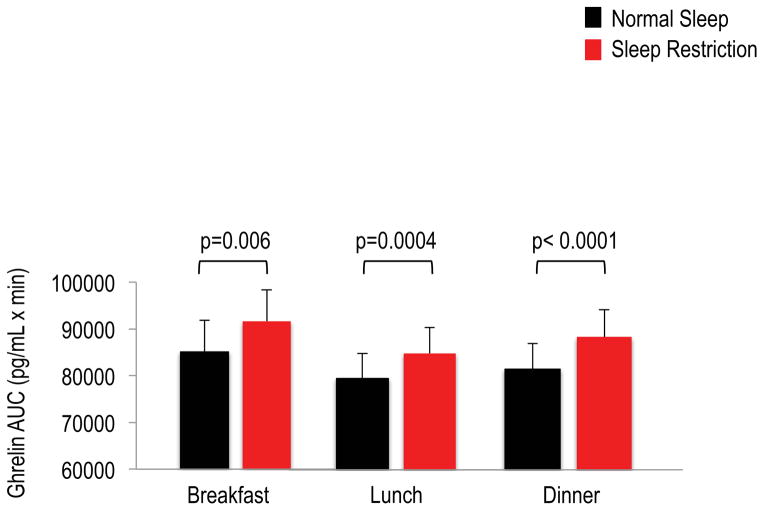
Twenty-four hour ghrelin profiles were altered during sleep restriction (Figure 1A) with higher mean 24-hour and nocturnal levels occurring during sleep restriction as compared to normal sleep (Table 1). Sleep restriction also resulted in elevated postprandial ghrelin after breakfast, lunch and dinner (Figure 2). The meal-related peaks in ghrelin were significantly higher for breakfast and dinner, and post-meal nadir ghrelin levels were increased for all meals during sleep restriction relative to normal sleep (Table 1).
Figure 1.
Twenty-four hour profiles of (A) ghrelin, (B) leptin and (C) pancreatic polypeptide under normal sleep (black lines) and sleep restriction (red lines). Horizontal black bars indicate time in bed under normal sleep (2300h to 0730h) and red bars indicate time in bed under sleep restriction (0100h to 0530h). Black arrows represent identical meals served at 0900h, 1400h and 1900h. Data are shown as mean ± SEM.
Table 1.
Effects of sleep restriction on 24-hour profiles of ghrelin, leptin and pancreatic polypeptide
| Hormonal Characteristic | Normal Sleep | Sleep Restriction | p value |
|---|---|---|---|
| Ghrelin | |||
| 24-hour mean (pg/mL) | 658 ±54 | 704 ±52 | 0.005 |
| Nocturnal mean (pg/mL) | 741 ±63 | 787 ±59 | 0.04 |
| Meal-related peaks (pg/mL) | |||
| Breakfast | 739 ± 64 | 804 ± 62 | 0.01 |
| Lunch | 757 ± 69 | 788 ± 59 | 0.30 |
| Dinner | 739 ± 62 | 810 ± 65 | 0.0006 |
| Post-meal nadirs (pg/mL) | |||
| Breakfast | 454 ± 38 | 491 ± 35 | 0.04 |
| Lunch | 452 ± 35 | 496 ± 39 | 0.01 |
| Dinner | 484 ± 38 | 535 ± 41 | < 0.0001 |
| Leptin | |||
| 24-hour mean (ng/mL) | 3.6 ± 0.6 | 3.8 ± 0.7 | 0.62 |
| Nocturnal mean (ng/mL) | 4.0 ± 0.6 | 4.2 ± 0.7 | 0.65 |
| Acrophase (ng/mL) | 5.0 ± 0.7 | 5.0 ± 0.8 | 0.92 |
| Nadir (ng/mL) | 2.4 ± 0.4 | 2.5 ± 0.5 | 0.68 |
| Amplitude (ng/mL) | 1.3 ± 0.2 | 1.3 ± 0.2 | 0.72 |
| Pancreatic polypeptide | |||
| 24-hour mean (pg/mL) | 47.5 ± 4.4 | 47.2 ± 4.3 | 0.84 |
| Nocturnal mean (pg/mL) | 29.1 ± 3.1 | 31.0 ± 3.1 | 0.17 |
| Meal-related peaks (pg/mL) | |||
| Breakfast | 100.9 ± 12.6 | 106.4 ± 13.5 | 0.55 |
| Lunch | 136.4 ± 13.5 | 126.5 ± 13.4 | 0.19 |
| Dinner | 117.7 ± 12.9 | 115.3 ± 12.8 | 0.51 |
| Post-meal nadirs (pg/mL) | |||
| Breakfast | 40.9 ± 4.4 | 46.5 ± 5.4 | 0.11 |
| Lunch | 42.5 ± 5.1 | 40.9 ± 3.7 | 0.58 |
| Dinner | 44.6 ± 4.5 | 46.9 ± 4.7 | 0.25 |
Data represent mean ± SEM.
Figure 2.
Postprandial ghrelin responses to breakfast, lunch and dinner meals under normal sleep (black bars) and sleep restriction (red bars). The AUCs were calculated during the 2.5 hours after each meal using the trapezoidal method. Identical meals were served at 0900h, 1400h and 1900h. Data are shown as mean ± SEM.
Leptin profiles displayed the expected circadian rhythm with peak levels occurring at night under both sleep conditions (Figure 1B). Mean 24-hour and nocturnal leptin levels were unaffected by sleep duration. The leptin acrophase, nadir and amplitude did not differ between sleep conditions (Table 1).
Sleep restriction did not alter mean 24-hour or nocturnal pancreatic polypeptide levels (Figure 1C) and neither the meal-related peaks nor the post-meal nadirs were different between sleep conditions (Table 1). In contrast, sleep restriction resulted in a small, but significant reduction in post-dinner pancreatic polypeptide levels (p=0.04); however, postprandial pancreatic polypeptide responses to breakfast (p=0.43) and lunch (p=0.71) did not differ between conditions.
Caloric intake during ad libitum feeding period
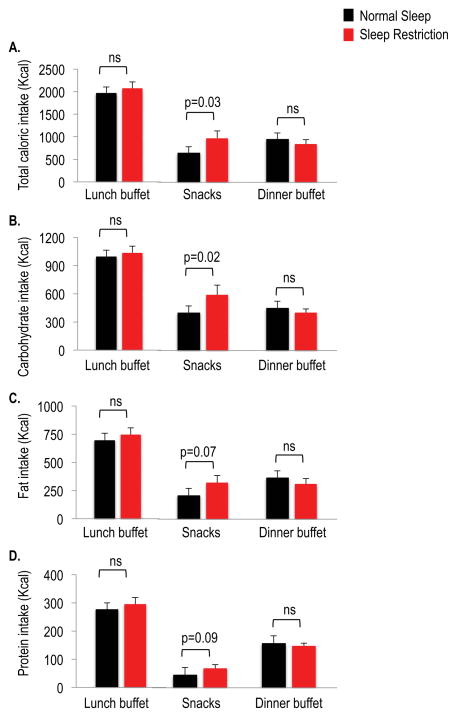
Sleep restriction was associated with an increase in total caloric intake by 340 ± 131 Kcal as compared to normal sleep (3888 ± 208 Kcal vs. 3548 ± 205 Kcal, p=0.02) during the ad libitum feeding period. Overall energy intake was higher for calories from carbohydrates when sleep was restricted (2026 ± 106 Kcal vs. 1833 ± 103 Kcal, p=0.005), but did not differ for calories from fat (1379 ± 100 Kcal vs. 1264 ± 98 Kcal, p=0.12) or protein (485 ± 28 Kcal vs. 451 ± 36 Kcal, p=0.20). Total caloric and macronutrient intake did not differ between sleep conditions for the lunch or dinner buffet meals (Figure 3). Total caloric intake from snacks was higher with more consumption of carbohydrates during sleep restriction relative to normal sleep, whereas fat and protein consumption from snacks were not significantly increased during sleep restriction.
Figure 3.
Caloric intake during the ad libitum feeding period under normal sleep (black bars) and sleep restriction (red bars). (A) Total caloric intake, (B) carbohydrate intake, (C) fat intake, (D) protein intake. The ad libitum feeding opportunity included buffet meals (lunch buffet served at 1500h and dinner buffet served at 1930h) and unlimited access to a snack bar between the two buffet meals. Data are shown as mean ± SEM.
On average, the snack items served under both sleep conditions (selected based on subjects’ individual preferences), consisted of 39% sweets, 25% salty snacks, 0% starches, 14% fruits and vegetables, 7% dairy, 2% protein/meat, 12% beverages and 1% condiments/fats/oils. When sleep was restricted, the consumption of sweet and salty snacks was higher by 283 ± 130 Kcal (p=0.04).
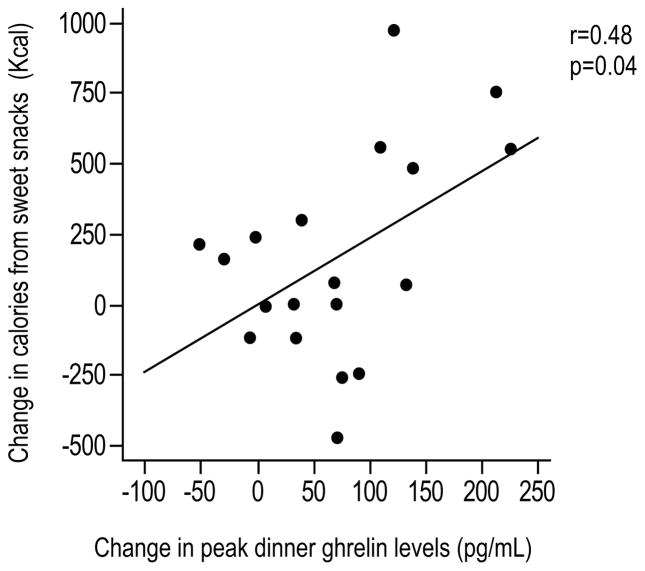
When we examined the relationships between the changes in ghrelin and the changes in caloric intake during sleep restriction, we found that the increase in evening ghrelin (i.e. dinner-related peak) was correlated with more consumption of calories from sweets (r=0.48, p=0.04; Figure 4). A trend for a correlation was also apparent between the change in evening ghrelin and carbohydrate calories from snacks (r=0.42, p=0.07), but not with the consumption of calories from salty snacks. We did not find any significant relationships between the changes in ghrelin profiles and the changes in total caloric intake, fat or protein intake from snacks.
Figure 4.
Correlation between the change in evening ghrelin levels (i.e. dinner-related peak) and the change in caloric intake from sweet snacks. Change values are expressed as the difference between sleep restriction and normal sleep.
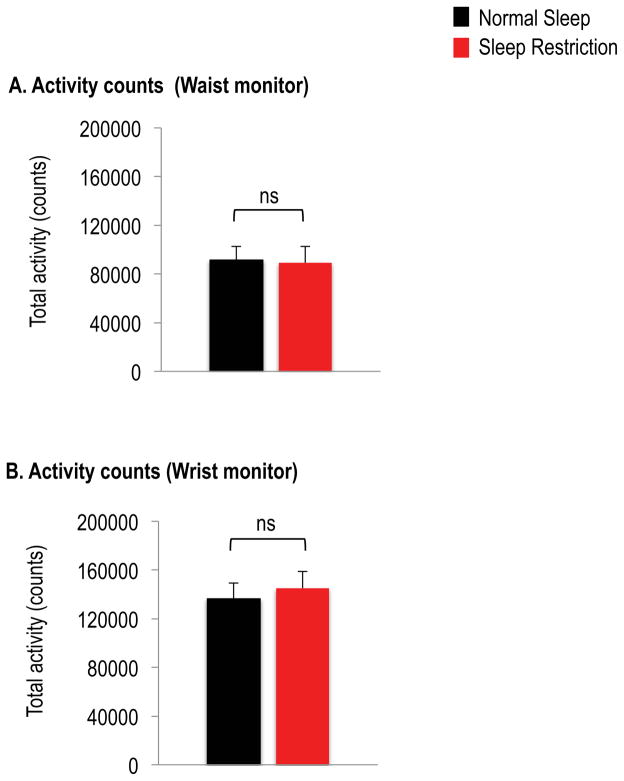
Activity levels
Activity counts, as assessed by accelerometer-based monitors attached to the waist (p= 0.86) or wrist (p=0.68), were similar between sleep conditions (Figure 5).
Figure 5.
Total activity counts from the (A) waist monitor and (B) wrist monitor under normal sleep (black bars) and sleep restriction (red bars). Data are shown as mean ± SEM. Data are from n=16 for the waist monitor and n=18 for the wrist monitor.
Discussion
We have demonstrated that experimental sleep restriction in healthy men alters 24-hour ghrelin profiles during a standardized diet resulting in higher ghrelin levels during sleep restriction as compared to normal sleep. We also found that the increase in ghrelin levels during sleep restriction was associated with more consumption of calories when ad libitum access to food was allowed. To our knowledge, this is the first demonstration that elevated ghrelin levels may predict food intake during sleep restriction. These findings suggest a potential mechanism by which sleep loss may lead to increased food intake and the development of weight gain.
We found that sleep restriction was associated with increases in both nocturnal and daytime ghrelin levels during the standardized diet. Postprandial ghrelin levels after breakfast, lunch and dinner remained elevated during sleep restriction as compared to normal sleep. In agreement with our findings, higher daytime ghrelin levels during experimental sleep restriction have previously been observed in healthy men under conditions of controlled caloric intake (12, 15). In contrast, prior studies with ad libitum feeding conditions reported ghrelin levels to be either decreased (8) or unchanged (5, 7, 9, 18) during sleep restriction. It is possible that the effects of sleep duration on ghrelin were masked in these studies due to uncontrolled caloric intake.
In our study, 24-hour leptin profiles were not significantly affected by sleep duration during a standardized diet. Previous findings on the effects of sleep restriction on the satiety hormone leptin are mixed. Under controlled feeding conditions, leptin levels have been shown to be decreased (13, 15), increased (16, 17) or unchanged (12). Under ad libitum feeding conditions, both increased (5, 8, 19) and unchanged (7, 9, 18) leptin levels have been reported. These inconsistent findings may be due to methodological variations in blood sampling timing and frequency as well as the nutritional status and energy balance of the study participants.
Pancreatic polypeptide is a gut hormone thought to play an important role in appetite regulation (24). To our knowledge, our study is the first to examine the impact of sleep restriction on pancreatic polypeptide levels. Overall, we did not observe a significant change in pancreatic polypeptide profiles. However, we did find a small but significant reduction in post-dinner pancreatic polypeptide levels during sleep restriction, which could conceivably stimulate food intake.
When free access to food was allowed, we found that total caloric intake was increased during sleep restriction. This finding is in agreement with multiple prior studies that report increased caloric intake during sleep restriction under ad libitum feeding conditions (5–12). In our participants, the increase in food intake was primarily driven by a higher caloric intake from snack and carbohydrate consumption, which has previously been found (8, 9).
Insufficient sleep has been demonstrated to increase energy expenditure as assessed by whole room calorimetry (8, 25). Previous studies have also shown that short-term sleep restriction results in weight gain when free access to food is allowed throughout the experiment (5, 7, 8, 10), suggesting that energy intake exceeds the metabolic cost of extended wakefulness. In our study, diet was controlled and body weight was similar between sleep conditions prior to the ad libitum feeding period, which took place at the end of the experiment. In addition, activity levels, as measured by accelerometer-based monitors, did not differ between conditions.
Our study design allowed both detailed assessments of appetite-regulating hormones with frequent sampling across 24-hours during a standardized diet, as well as measurements of actual food intake during an ad libitum feeding opportunity. Importantly, we have demonstrated that the increase in ghrelin levels during sleep restriction is associated with more consumption of calories when free access to food is allowed. This finding suggests that elevations in ghrelin may be a potential mechanism by which sleep restriction increases food intake. Ghrelin can activate homeostatic and reward-related pathways, leading to increased food intake (26, 27). However, the current study was not designed to distinguish between the homeostatic vs. non-homeostatic pathways, and thus future studies will be needed to address this question. In support of the hypothesis that hedonic eating may be involved in overconsumption of food during sleep loss (2), neuroimaging studies have shown alterations in reward-related brain regions in sleep-deprived individuals (28–31). Taken together, it is likely that multiple neural and hormonal systems are involved in the alterations in appetite and food intake in the context of sleep loss (32). Additionally, due to the acute nature of our study, it remains unknown whether our current findings can be applied to chronic or repeated sleep restriction over a longer period or time.
In conclusion, we have demonstrated that experimental sleep restriction in healthy young lean men results in elevated ghrelin levels, promoting increased food intake. These findings provide evidence for potential factors by which sleep loss may lead to increased weight gain and the development of obesity. Future mechanistic studies are needed to identify potential mediators in the link between increased food intake and insufficient sleep.
What is already known about this subject?
Sleep loss has been linked to increased risk for obesity.
Experimental sleep restriction increases food intake, but the mechanisms remain to be elucidated
What does this study add?
Sleep restriction increases 24-hour ghrelin levels during a standardized diet
Increased ghrelin during sleep restriction is associated with an increase in caloric intake
Higher ghrelin levels may partly explain why sleep loss leads to increased food intake
Acknowledgments
Funding: This work was supported by NIH grants R01-HL-075079, P01-AG11412, CTSA-UL1 TR000430, P50-HD057796, P60-DK20595, Department of Defense award W81XWH-07-2-0071 and Society in Science, The Branco Weiss Fellowship, administered by the ETH Zürich (to JLB).
The authors wish to thank the nursing and dietary staff of the University of Chicago Clinical Research Center for their expert assistance, and the volunteers for participating in this study.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaput JP, St-Onge MP. Increased food intake by insufficient sleep in humans: are we jumping the gun on the hormonal explanation? Frontiers in endocrinology. 2014;5:116. doi: 10.3389/fendo.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magee L, Hale L. Longitudinal associations between sleep duration and subsequent weight gain: a systematic review. Sleep medicine reviews. 2012;16(3):231–41. doi: 10.1016/j.smrv.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164(10):947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, Settler U, Peters A, Kiosz D, Muller MJ. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obesity facts. 2008;1(5):266–73. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91(6):1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 7.Calvin AD, Carter RE, Adachi T, Macedo PG, Albuquerque FN, van der Walt C, Bukartyk J, Davison DE, Levine JA, Somers VK. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest. 2013;144(1):79–86. doi: 10.1378/chest.12-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP., Jr Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(14):5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89(1):126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaeth AM, Dinges DF, Goel N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep. 2013;36(7):981–90. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94(2):410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St-Onge MP, O’Keeffe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35(11):1503–10. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guilleminault C, Powell NB, Martinez S, Kushida C, Raffray T, Palombini L, Philip P. Preliminary observations on the effects of sleep time in a sleep restriction paradigm. Sleep Med. 2003;4(3):177–84. doi: 10.1016/s1389-9457(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 14.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89(11):5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 15.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 16.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiology & behavior. 2010;99(5):651–6. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 17.van Leeuwen WM, Hublin C, Sallinen M, Harma M, Hirvonen A, Porkka-Heiskanen T. Prolonged sleep restriction affects glucose metabolism in healthy young men. International journal of endocrinology. 2010;2010:108641. doi: 10.1155/2010/108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, Born J, Schultes B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90(6):1476–82. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 19.Simpson NS, Banks S, Dinges DF. Sleep restriction is associated with increased morning plasma leptin concentrations, especially in women. Biological research for nursing. 2010;12(1):47–53. doi: 10.1177/1099800410366301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broussard JL, Chapotot F, Abraham V, Day A, Delebecque F, Whitmore HR, Tasali E. Sleep restriction increases free fatty acids in healthy men. Diabetologia. 2015;58(4):791–8. doi: 10.1007/s00125-015-3500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157(8):549–57. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Human nutrition Clinical nutrition. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 23.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. Journal of the American Statistical Association. 1979;74:829–36. [Google Scholar]
- 24.Batterham RL, Le Roux CW, Cohen MA, Park AJ, Ellis SM, Patterson M, Frost GS, Ghatei MA, Bloom SR. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab. 2003;88(8):3989–92. doi: 10.1210/jc.2003-030630. [DOI] [PubMed] [Google Scholar]
- 25.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. The Journal of physiology. 2011;589(Pt 1):235–44. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiology & behavior. 2006;89(1):71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. The Journal of clinical investigation. 2007;117(1):13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benedict C, Brooks SJ, O’Daly OG, Almen MS, Morell A, Aberg K, Gingnell M, Schultes B, Hallschmid M, Broman JE, et al. Acute sleep deprivation enhances the brain’s response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97(3):E443–7. doi: 10.1210/jc.2011-2759. [DOI] [PubMed] [Google Scholar]
- 29.St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95(4):818–24. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.St-Onge MP, Wolfe S, Sy M, Shechter A, Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int J Obes (Lond) 2014;38(3):411–6. doi: 10.1038/ijo.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nature communications. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berthoud HR. The neurobiology of food intake in an obesogenic environment. The Proceedings of the Nutrition Society. 2012;71(4):478–87. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]