Abstract
Pantothenate kinase-associated neurodegeneration (PKAN) is a progressive movement disorder that is due to mutations in PANK2. Pathologically, it is a member of a class of diseases known as neurodegeneration with brain iron accumulation (NBIA) and features increased tissue iron and ubiquitinated protein aceous aggregates in the globuspallidus. We have previously determined that these aggregates represent condensed residue derived from degenerated pallidal neurons. However, the protein content, other than ubiquitin, of these aggregates remains unknown. In the present study, we performed biochemical and immunohistochemical studies to characterize these aggregates and found them to be enriched in apolipoprotein E that is poorly soluble in detergent solutions. However, did not determine a significant association between APOE genotype and the clinical phenotype of disease in our database of 81 cases. Rather, we frequently identified similar ubiquitin- and apolipoprotein E-enriched lesions in these neurons in non-PKAN patients in the penumbrae of remote infarcts that involve the globuspallidus, and occasionally in other brain sites that contain large γ-aminobutyric acid (GABA)ergic neurons. Our findings, taken together, suggest that tissue or cellular hypoxic/ischemic injury within the globuspallidus may underlie the pathogenesis of PKAN.
Keywords: Pantothenate kinase-associated neurodegeneration, apoliprotein E, globuspallidus
1. Introduction
Pantothenate kinase-associated neurodegeneration (PKAN) is the most common subtype of a spectrum of diseases known collectively as neurodegeneration with brain iron accumulation (NBIA) (Gregory and Hayflick, 2013). Common clinical symptoms in PKAN include parkinsonism with generalized dystonias that typically begin in childhood with a progressively disabling and ultimately fatal course (Gregory and Hayflick, 2002). Cognitive function in PKAN is relatively intact (Freeman et al., 2007). Diagnosis is enabled by magnetic resonance imaging (MRI), which reveals a characteristic “eye of the tiger” appearance with a T2 hyperintense signal core surrounded by a hypointense region in the globuspallidus, which is compatible with the clinical phenotype of a predominantly movement disorder (Hayflick et al., 2003).
Histologically, all NBIA disorders feature iron accumulation in the basal ganglia with neuroaxonal spheroids (Kruer, 2013). Additional neuropathologic features may be specific to NBIA subtypes and include Lewy bodies and neurofibrillary tangles (Kruer, 2013). As the genetic bases of NBIA disorders have been elucidated, the specificity of these features has become more apparent. PKAN is caused by recessively inherited mutations in PANK2, encoding pantothenate kinase 2, a mitochondrial enzyme that functions as the rate-limiting step in coenzyme A (CoA) biosynthesis (Dansie et al., 2014; Hayflick, 2014). PANK2 serves as a putative sensor of matrix CoA levels for fatty acid β-oxidation (Leonardi et al., 2007). In PKAN, the loss of PANK2 function would mimic the basal state signaling sufficient CoA independent of matrix CoA status. The functional basis of the association of this defect with clinical and pathologic disease, in particular the localization to the basal ganglia, remains unknown (Hayflick, 2014).
We recently reported detailed histologic features in the brain autopsies of six patients with genetically confirmed PKAN (Kruer et al., 2011). Specifically, we observed iron deposition and neuraxonal spheroids in the globuspallidus, as described above, but no specific or significant features of other neurodegenerative diseases, such as amyloid plaques, neurofibrillary tangles, or Lewy bodies. Antibodies to ubiquitin strongly labeled protein aceous aggregates found in structures that we identified as degenerating globuspallidus neurons. However, in keeping with the absence in PKAN of other histopathologic lesions found in other neurodegenerative diseases, convincingly positive staining for tau, α-synuclein, β-amyloid, or TAR DNA-binding protein 43 (TDP-43) failed to co-localize with ubiquitin expression in degenerating neurons.
We report here that ubiquitin expression in inclusions of degenerating pallidal neurons in PKAN brain tissue co-localizes with apolipoprotein E (apoE). The detergent-insoluble fraction of tissue extracts from PKAN patients was found to be enriched in apoE; insolubility in detergent solutions is a biochemical feature common to abnormal misfolded protein species found in other neurodegenerative diseases (Woltjer et al., 2009). No APOE allele-specific association with PKAN phenotype severity was evident. Morphologically identical inclusions were identified in degenerating neurons in the vicinity of remote infarcts in the globuspallidus in non-PKAN patients, and these were also found to contain ubiquitin and apoE. These findings indicate that the pathologic phenotype of PKAN recapitulates that of chronic neuronal hypoxia and/or ischemia involving the globuspallidus.
2. Materials and methods
2.1. Human subjects
Subjects were enrolled pre- or post-mortem after consent was obtained from surviving family members. The brain autopsies of most subjects were performed at Oregon Health & Science University (OHSU) in accordance with the requirements of the local Institutional Review Board, with informed consent for brain autopsy obtained from the legal next of kin. Other tissue samples were obtained from the National Institute of Child Health and Human Development Brain and Tissue Bank for Developmental Disorders, administered at the University of Maryland. Patient histories were obtained via direct interview, review of medical records, and/or correspondence with surviving family members.
2.2. APOE genotyping
Patient APOE genotypes were determined by polymerase chain reaction (PCR) amplification of genomic DNA and sequencing. Primers were designed to amplify exon 4 of APOE, and sequences were determined by the OCTRI Sequencing Core at OHSU using a 3130XL Genetic Analyzer (Applied Biosystems, Foster City, CA).
Patients were classified as classic or atypical PKAN using published criteria (Hayflick, 2013). The frequencies of APOE E2, E3, and E4 alleles in patients with classic or atypical PKAN were compared to each other as well as to published frequencies in the general population and analyzed by chi-square tests. General population frequencies were obtained from a meta-analysis compiled by AlzGene (Bertram et al., 2007).
2.3. Postmortem tissue processing
Fixed tissue was prepared by immersion of brain tissue from PKAN and non-PKAN patients in 10% neutral buffered formalin for at least ten days, followed by dissection into sections containing the basal ganglia and other brain regions.
2.4. Histologic and immunohistochemical evaluation
Standard methods were used to prepare 6-µm paraffin-embedded sections, that were stained with hematoxalin and eosin (H&E) with luxol fast blue (LFB) myelin stain to evaluate the presence of characteristic PKAN-associated findings.
Immunohistochemical stains were applied to paraffin sections after deparaffinization and antigen retrieval (5 min treatment at room temperature with 95% formic acid, followed by 30 min incubation in citrate buffer [pH 6.0] at 90°C) Tissue sections were blocked with 5% nonfat dry milk in phosphate-buffered saline and labeled with antibodies to ubiquitin (Dako, Glostrup, Denmark) and apoE (Academy Biomedical, Houston, TX) (Holthofer et al., 1982). All antibodies were applied at 1:5,000 dilution. Results were visualized after application of appropriate secondary antibodies using diaminobenzidine (brown) or Vector Red (Vector Laboratories) as chromagens.
2.5. Immunofluorescent imaging
The 6-µm tissue sections were deparaffinized and subjected to antigen retrieval as described above. Tissue sections were blocked with 10% bovine serum albumin (BSA) and simultaneously incubated with antibodies against ubiquitin (1:5,000, Invitrogen/Life Technologies, Carlsbad, CA) and apoE (1:10,000, Biomedical, Houston, TX) diluted in 1% BSA. Antibody labeling was visualized using Alexa-conjugated fluorescent secondary antibodies (1:500, Life Technologies). Sections were mounted using Prolong Gold antifade agent with 4',6-diamidino-2-phenylindole (DAPI, Life Technologies). Tissue was imaged using a Zeiss LSM 780 confocal microscope (Carl Zeiss, Oberkochen, Germany) at the OHSU Advanced Light Microscopy core facility.
2.6. Biochemical characterization of apoE
For biochemical studies performed on fresh frozen tissue, archived frozen globuspallidus from PKAN and non-PKAN control subjects was thawed, and proteins were liberated by probe sonication in 10 mL denaturing tissue lysis buffer containing 62.5 mM Tris (pH 6.8), 2% sodium dodecyl sulfate (SDS), and 10% glycerol per gram tissue. Extracts were separated by centrifugation for 10 min at 14,000 × g, and protein assays of supernatants were performed in triplicate using standard methods (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific Inc., Rockford, IL). Tissue protein was normalized to 2 mg/mL, Dithiothreitol (DTT) was added to a final concentration of 50 mM, and bromophenol blue was added to 0.01%. This method afforded near complete tissue protein extraction in extracts of known protein concentration in commonly used denaturing gel electrophoresis sample buffer (Laemmli, 1970). Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, transfer to polyvinylidinedifluoride (PVDF) membranes, and immunoblotting for apoE were performed on 40 µg total extracted protein using standard methods (Laemmli, 1970).
To prepare detergent-insoluble extracts of tissue, ten volume sice-cold 100 mM Tris, (pH 7.4)/10% sucrose “buffer A” containing protease and phosphatase inhibitors was added per gram tissue samples as previously described (Yang et al., 2007) The tissue was sonicated with a probe sonicator for 30 s and then separated by ultracentrifugation at 450,000 × g for 20 min at 4°C. Supernatants were removed, and three subsequent serial extractions of the insoluble pellets were performed with the same volume of buffer A with 1% Triton X-100 followed by ultracentrifugation at each step. The remaining pellets were resuspended in 10 mM Tris, (pH 8.0) to remove residual detergent, and the detergent-insoluble proteins were liberated from the final pellet by sonication in 70% formic acid. Aliquots of extracted protein were dried by vacuum centrifugation and resolubilized by sonication in 5 M guanidine hydrochloride and 100 mM Tris (pH 8.0) in a volume equal to the original extract volume. Enzyme-linked immunosorbent assays (ELISAs) to quantify apoE and ubiquitin were performed using 200 ng total detergent-insoluble protein per assay as previously described (Woltjer et al., 2009)
ApoE was immunoprecipitated from the detergent-insoluble protein fraction after resolubilization in guanidine as described above. The 40-µL resolubilized samples were first diluted with 10 volumes Tris-buffered saline (pH 7.4) containing 0.1% Tween 20 detergent (TBST), followed by the addition of 10 µL anti-apoE antibody and 40 µL agarose-bound protein G slurry (Santa Cruz Biotechnology, Santa Cruz, CA). After incubation with gentle agitation for 1 h at 4°C, agarose beads and associated immunoprecipitated material were collected by centrifugation for 5 min at 500× g at 4 degrees C, the supernatants were discarded, and agarose-bound immunoprecipitates were washed by resuspensionin 1 m Lice-cold TBST. After 4 washes, the beads were eluted by the addition of 20 mM ethanolamine (pH 12.5) and centrifugation as described above, and the eluates (supernatants) were collected. These were neutralized with the addition of 256 volumes of 100 mM Tris (pH 8.0). To confirm the specificity of immunoprecipitation, additional triplicate immunoprecipitations of Tris/guanidine buffer without brain extracts were prepared in parallel and washed and eluted exactly as described above for brain extracts. ELISAs for ubiquitin were performed from 200 µL neutralized immunoprecipitates as previously describe (Woltjer et al., 2009).
2.7. Statistical methods
Statistical analyses were carried out using Prism software (GraphPad Software, Inc., La Jolla, CA) unless otherwise noted.
3. Results
3.1. Patient characterization
All PKAN subjects had typical clinical, imaging, and histologic features as described previously, including ubiquitin-positive degenerating neurons in the globuspallidus (Kruer et al., 2011). The clinical and molecular features of PKAN subjects whose tissues were used in biochemical and histochemical studies are summarized in Table 1.
Table. 1.
Clinical and molecular genetic features of PKAN cases used in biochemical and immunohistochemical studies.
| Case | Sex | Age of onset (y) |
Symptoms | Age at death (y) |
Mutation | Molecular features |
|---|---|---|---|---|---|---|
| 120 | Female | 1 | Dystonia, pigmentary retinopathy | 10 | c.943_945delCTT | In-frame deletion |
| 158 | Female | 3 | Dystonia, parkinsonism | 10 | c.1231G>A | Missense |
| 23 | Male | 6 | Dystonia, parkinsonism, pigmentary retinopathy | 20 | 1231G>A (hom) | Missense |
| 367 | Female | Unknown | Dystonia | 8 | c.440_441insCT c.943_945delCTT |
Premature stop codon; In-frame deletion |
| 146 | Female | 32 | Dystonia, parkinsonism | 48 | c.1231G>A c.370A>G |
Premature stop codon; In-frame deletion |
Tissue from patients without evidence of neurodegenerative disease or with clinically diagnosed and neuropathologically confirmed Alzheimer’s disease was used for protein biochemical studies. For immunohistochemical studies of remote infarcts, we used tissue from elderly patients with grossly ascertained and microscopically confirmed brain lesions without additional neurodegenerative disease. Patients with neurologic disease were clinically evaluated in the OHSU Layton Aging and Alzheimer’s Disease Center as previously described (Silbert et al., 2008). Brain tissue from all non-PKAN cases was obtained from the Oregon Brain Bank and selected based on diagnoses of ischemic injury, infarction, and neurodegenerative disease as established by standard histopathologic assessment performed by the Neuropathology Core of the Layton Aging and Alzheimer’s Disease Center.
3.2. Immunohistochemical characterization
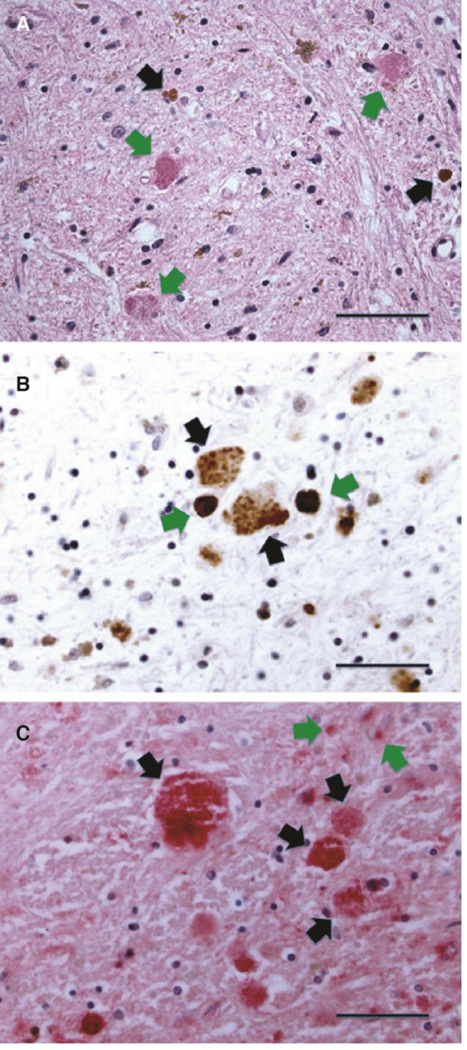
The globuspallidus, specifically the interna, was most heavily affected in PKAN brain, as previously described (Kruer et al., 2011). H&E-stained sections showed rarefied brain parenchyma with iron deposits, gliosis, and variably compact eosinophilic spheroidal structures that roughly approximated the size and spacing of large pallidal neurons (Fig. 1A) (Kruer et al., 2011). We immunohistochemically confirmed the presence of ubiquitin in these structures (Fig. 1B) in all cases. The presence of apoE was also established by immunohistochemical labeling and was found in degenerating neurons in a distribution that was similar to that of ubiquitin (Fig. 1C). In addition, apoE expression was increased in reactive astrocytes, the major apoE-expressing cells of the brain (Boyles et al., 1985; Pitas et al., 1987; Grehan et al., 2001). No abnormal apoE expression was identified in globuspallidus tissue from age-matched control subjects who died without neurologic disease.
Figure 1. Histologic and immunohistochemical features of globuspallidus affected by PKAN.
(A) High-magnification view of hematoxylin- and eosin (H&E)-stained section of the degenerating neurons harboring eosinophilic protein aceous aggregates (green arrows). Reactive gliosis and rust-colored iron deposits (black arrows) are present in the background neuropil (scale: 50 µm).
(B) Ubiquitin-immunohistochemical staining of the globuspallidus in PKAN, highlighting granular ubiquitin-positive inclusions in degenerating neurons (black arrows) as well as more condensed iron deposits (green arrows) (scale: 33 µm). Anti-ubiquitin immunostaining was developed with brown chromagen; hematoxalin counterstain stains background glial nuclei blue.
(C) ApoE-immunohistochemical staining of degenerating neurons in the globuspallidus in PKAN. Staining was developed with Vector Red chromagen to distinguish apoE-positive deposits from brown iron deposits in the background, with hematoxalin as counterstain. Granular protein aceous material is enriched in apoE (black arrows). Staining in the atrophic neuropil in the background reflects expression by reactive astrocytes (green arrows) (scale: 33 µm).
ApoE- and ubiquitin-positive structures were widespread thoughout the globuspallidus in PKAN but displayed a variety of morphologies. Granular clusters were found only in the globuspallid us interna and likely represent remnants of more disintegrated neurons in the area of maximal tissue damage that corresponds to the “eye of the tiger” sign seen in MRI scans. Compact or aggregated structures were more widespread throughout the globuspallidus along a morphologic spectrum that suggests progressive aggregation and ubiquitination of apoE in intermediate to advanced stages of neuronal degeneration.
3.3. Immunofluorescent labeling of neuronal aggregated protein in PKAN
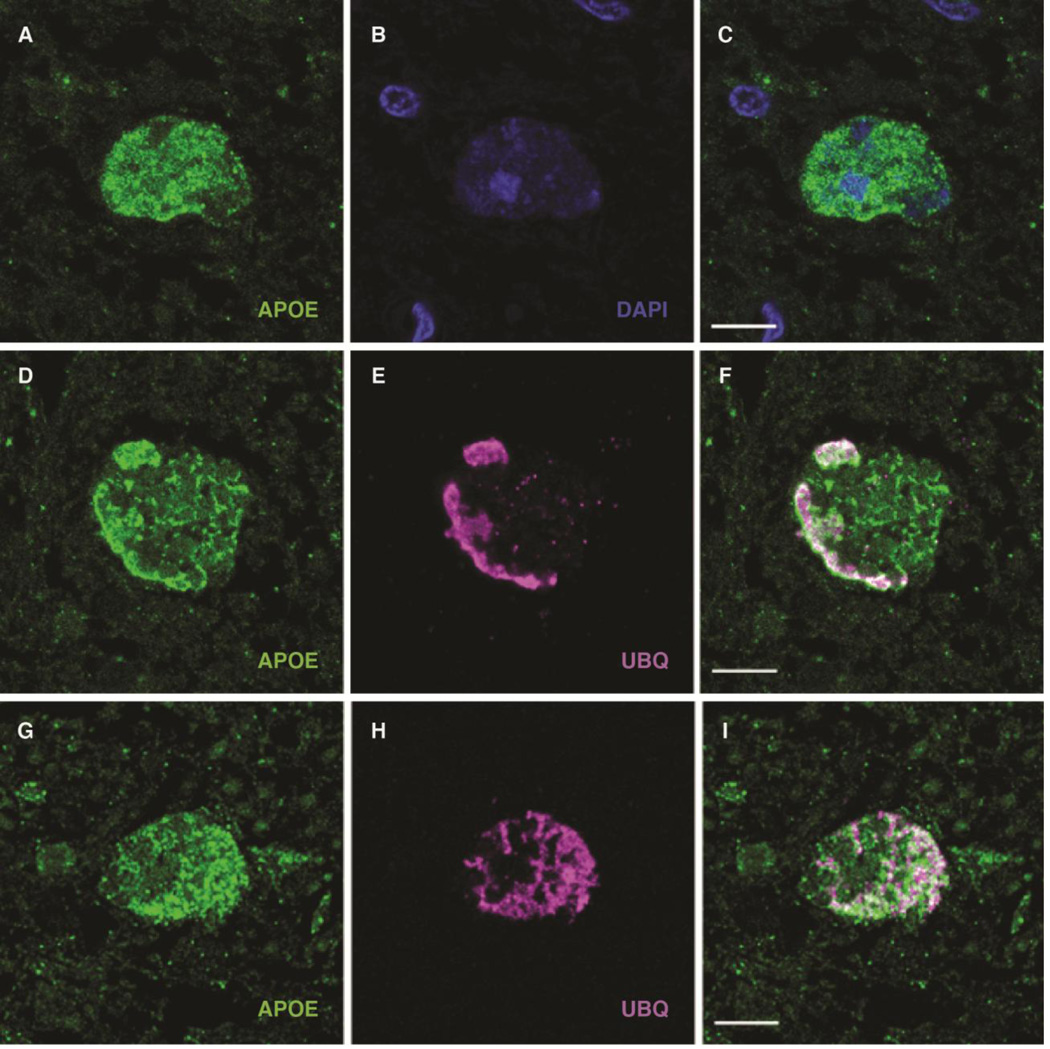
Next, dual-label confocal immunofluorescence was performed on paraffin sections of PKAN globuspallidus. The results confirmed the presence of apoE in ubiquitin-positive structures (Fig. 2). In addition, occasional less compact inclusions were identified, typically at the periphery of the most heavily affected areas of the globuspallidus. These inclusions were compatible with less developed lesions and were variably positive for ubiquitin but strongly positive for apoE. Occasionally, DAPI-positive nuclear material was associated with these aggregates, confirming their localization to cell bodies rather than neuronal processes, as we previously ascertained (Kruer et al., 2011). The findings are compatible with the interpretation that apoE aberrantly accumulates in PKAN neurons and that a large subset of aggregated apoE is ubiquitinated or associated with another ubiquitinated protein to constitute the hallmark cellular lesion of PKAN.
Figure 2. Demonstration by confocal immunofluorescence of apoE-positive protein aceous aggregates in the globuspallidus in PKAN.
(A–C) Confocal micrographs of anti-apoE (green) immunofluorescent staining of granular deposits (A), demonstrating widespread cytoplasmic distribution with scant residual DAPI-positive nuclear material (B; A and B merged in C); the size and structure of the apoE-positive material is compatible with cytoplasmic aggregation of apoE in degenerating neurons (scale: 10 µm).
(D–I) Association of apoE (green, E and G) and ubiquitin (magenta, E and H, and merged in F and I). D–F demonstrate a degenerating neuron with widespread apoE-containing aggregates, as well as more densely aggregated apoE on the left aspect of the periphery that feature more intense ubiquitination. G–I depict a neuron with more uniform colocalization of apoE and ubiquitin signals in larger aggregated material throughout the cell (scale: 10 µm).
3.4. Biochemical characterization of apoE protein in PKAN
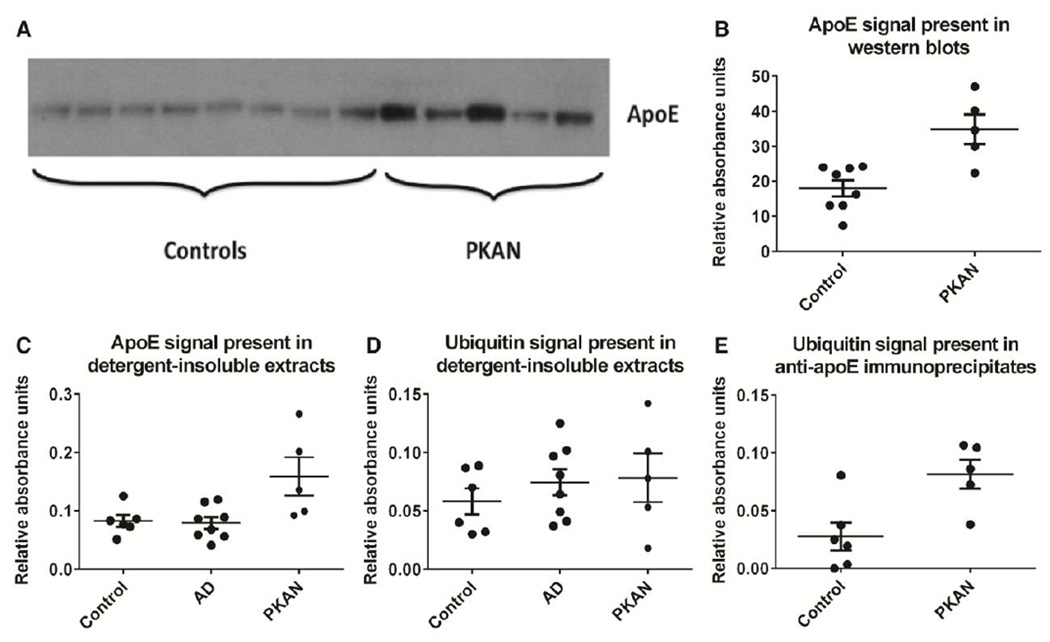
Aggregated proteins in morphologically identifiable lesions are common in many neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and various forms of frontotemporal lobar degeneration; these protein aceous deposits are attributed to cellular processes that lead to protein damage, inducing misfolding that typically results in reduced solubility of accumulated protein in detergent solutions (Woltjer et al., 2009). To determine whether this is true of apoE in the PKAN globuspallidus, we prepared total (sodium dodecyl sulfate, or SDS-extracted) and detergent (Triton X-100)-insoluble fractions of this brain region from PKAN and control subjects and performed western blotting and ELISAs on these extracts. ApoE was increased in PKAN subjects compared to controls in both SDS extracts of total globuspallidus protein (Fig. 3A and 3B) and detergent-insoluble extracts (Fig. 3C). Despite the presence of occasional apoE-positive beta-amyloid plaques in the deep gray matter in the brains of Alzheimer’s disease patients, detergent-insoluble apoE was not significantly increased in eight patients with Alzheimer’s disease who were included as controls for nonspecific tissue effects of other neurodegenerative disease. There was no increased ubiquitin ELISA signal in PKAN compared to control groups (Fig. 3D); this could be due to lower antibody sensitivity compared to that used for apoE ELISAs, a smaller tissue amount of abnormal ubiquitin compared to apoE, or a combination of these factors. To increase assay sensitivity and specificity, we immunoprecipitated apoE from detergent-insoluble fractions of PKAN and control globuspallid us prior and performed ELISAs to determine the ubiquitin content of immunoprecipitates (Fig. 3E). This method revealed increased ubiquitin signal in samples from PKAN patients compared to controls, which is consistent with ubiquitination of apoE or a co-immunoprecipitating apoE-associated protein species in PKAN compared to controls. It should be noted that the association of any co-immunoprecipitating proteins would be very tight, having been maintained throughout the detergent and formic acid extraction, and guanidine resolubilization, steps.
Figure 3. Biochemical features of apoE in globuspallidus afffected by PKAN.
(A) Total SDS-soluble protein was extracted from frozen globuspallidus of eight neurologically intact control and five PKAN patients. Controls ranged in age from 41 to 85 years but these and other experiments did not demonstrate age-related changes in apoE content of the globuspallidus (not shown). ApoE migrated as an approximately 34-kD band in reducing, denaturing SDS gels.
(B) Evaluation of band density using ImageJ software (National Institutes of Health) revealed the total apoE content of the globuspallidus to be significant increased in PKAN compared to neurologically intact controls (Mann-Whitney test, p=0.011). Data are presented as means ± SEM.
(C) 200 ng detergent-insoluble protein from six control, eight Alzheimer’s disease, and five PKAN patients was subjected to ELISA for quantitative determination of apoE. Control subjects without neurologic disease ranged in age from 41 to 91 years and did not differ significantly in age from AD subjects, who ranged from 56 to 92 years. No association of insoluble apoE was identified as a function of Alzheimer’s disease diagnosis (Mann-Whitney test, p=0.88) or age of any subject group (not shown). Detergent-insoluble apoE was significantly increased in PKAN subjects compared to controls (Mann-Whitney test, p=0.017). Data are presented as means ± SEM.
(D) 200 ng detergent-insoluble protein from six control, eight Alzheimer’s disease, and five PKAN patients as in C was subjected to ELISA for quantitative determination of ubiquitin. No association of insoluble ubiquitin was identified as a function of patient age or any diagnosis (Mann-Whitney test, p>0.05 for all comparisons). Data are presented as means ± SEM.
(E) Immunoprecipitation of apoE from detergent-insoluble extracts of globuspallidus from control and PKAN patients was carried out, followed by ELISA determination of the ubiquitin content of immunoprecipitates. Control and PKAN subjects were those of panels C and D. ApoE-associated ubiquitin was significantly increased in extracts from PKAN patients compared to controls (Mann-Whitney test, p=0.017). Data are presented as means ± SEM.
3.5. APOE genotype and PKAN phenotype
The ε4 allele of APOE is associated with an increased risk of various neurodegenerative diseases, most notably Alzheimer’s disease. To determine whether the presence of the ε4 allele was associated with PKAN, we determined APOE genotypes in the known available population of patients with classic or atypical PKAN. The classic PKAN group (n=81) had an allele distribution of 9 ε2 (5.6%), 140 ε3 (86.4%), and 13 ε4 (8%). The atypical PKAN group (n=41) had an allele distribution of 6 ε2 (7.3%), 70 ε3 (85.4%), and 6 ε (7.3%). Chi-square analysis revealed that none of the allele frequencies differed significantly: atypical versus classic PKAN allele frequencies (p=0.26), atypical PKAN versus general population frequencies (p=0.58), and classic PKAN versus general population frequencies (p=0.06). We also did not detect an association of age of onset or death with APOE genotype in these populations (data not shown); nor, in our limited patient set, was there an obvious association between the nature of the genetic lesion (in-frame deletion, missense, or premature stop codon) and the amount of detergent-insoluble apoE.
3.6. Recapitulation of ubiquitin- and apoE-positive aggregates in chronic hypoxic/ischemic injury affecting the globuspallidus
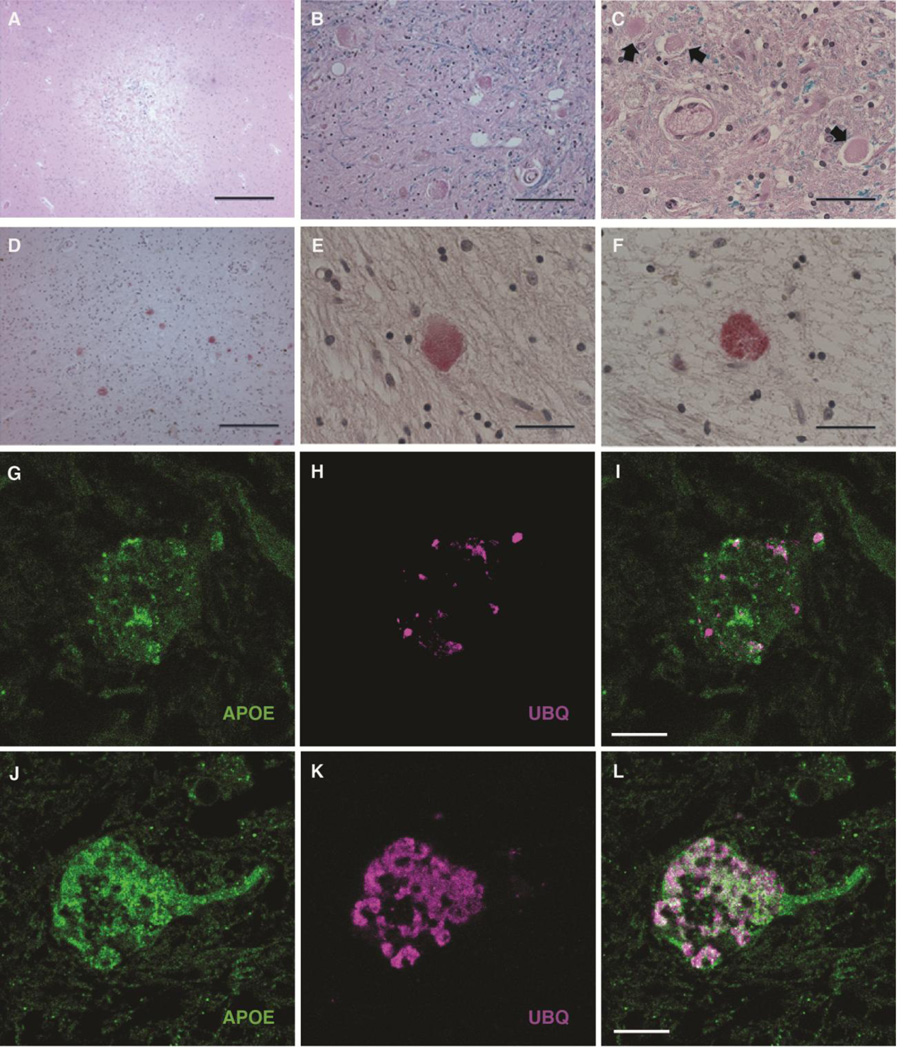
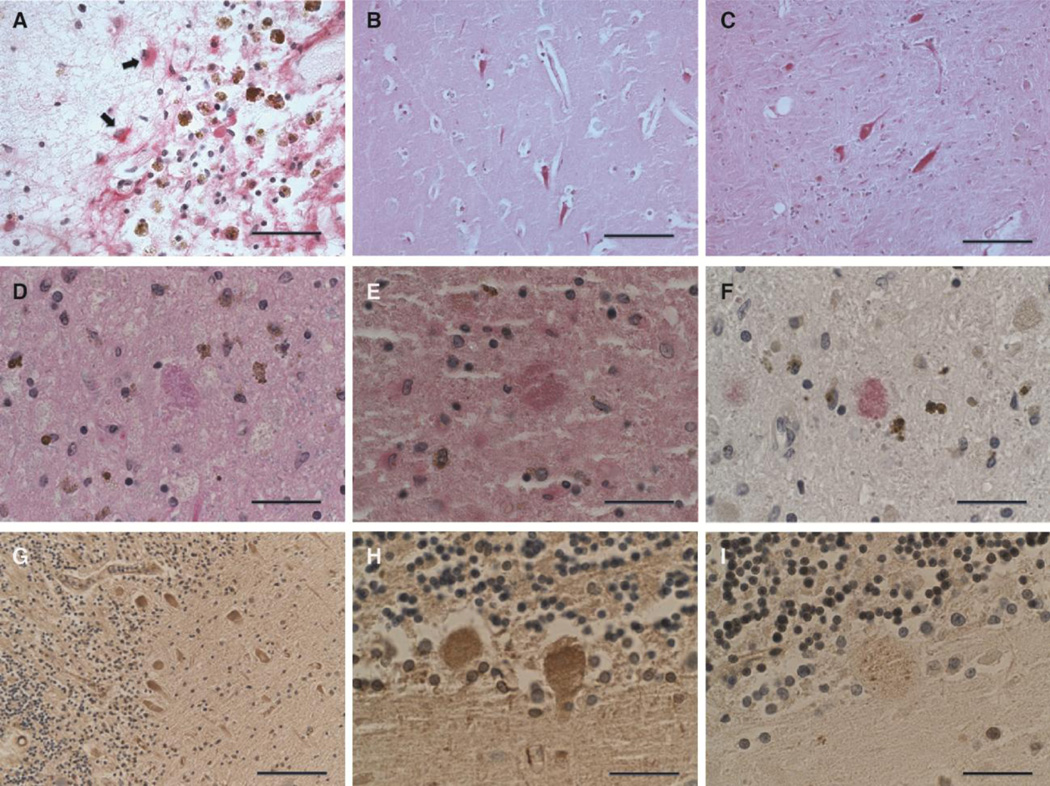
Neuronal changes that resemble those found in PKAN were serendipitously recognized in globipallidi from elderly patients with vascular ischemic injury. Typically, these were observed in the penumbrae of medium-sized subacute and older infarcts (Fig. 4A). The spectrum of neuronal changes was similar to that observed in PKAN and ranged from neurons with recognizable morphology and dense, eosinophilic cytoplasm to structures that were no longer recognizable as neurons but contained more compact aggregated protein aceous deposits of similar size and eosinophilic staining (Fig. 4B and 4C). Immunohistochemical and immunofluorescent labeling of brain tissue from these subjects revealed a spectrum of ubiquitin- and apoE-positive aggregates that was indistinguishable from lesions observed in PKAN (Fig. 4D–4L), with the exception of the widespread distribution in the globuspallidus in PKAN versus circumscribed localization to and around infarcts in non-PKAN subjects.
Figure 4. Association of PKAN-type protein aggregates with chronic hypoxic/ischemic injury involving the globuspallidus.
(A) H&E-stained section of a remote infarct at the interface of the put a men and globuspallidus in an 82-year-old male patient with cognitive impairment attributed to vascular brain injury. Morphologic features that recapitulated findings in PKAN were most commonly encountered at the periphery of lesions of approximately this size and age (scale: 500 µm).
(B) Higher magnification of H&E-stained section, with LFB myelin stain, of widespread granular eosinophilic aggregates in the penumbra of a remote ischemic lesion involving the globuspallidus, highly reminiscent of the cellular pathology of PKAN (scale: 50 µm).
(C) Higher magnification of H&E/LFB stained-section near a subacute ischemic lesion of the globuspallidus in a non-PKAN patient, depicting a spectrum of lesions, ranging from relatively homogeneous eosinophilia in a degenerating neuron (right), to more condensed protein aggregates (center), to eosinophilic granular protein aggregation (left) (scale: 33 µm).
(D) ApoE immunohistochemical stain of an area of granular degenerating neurons near a remote pallidal infarct in a non-PKAN patient, showing widespread apoE-positive protein aggregates similar to that observed in PKAN (Vector Red development; scale: 100 µm).
(E) High magnification of anti-apoE immunohistochemical stain of degenerating neuron in the globuspallidus near a remote infarct in a non-PKAN patient (Vector Red development; scale: 33 µm).
(F) High magnification of anti-ubiquitin immunohistochemical stain of degenerating neuron in the globuspallidus near a remote infarct in a non-PKAN patient, showing increased expression in a pattern very similar to that of apoE (Vector Red development; scale: 33 µm).
(G–L) Confocal micrographs of degenerating neurons in the globuspallidus of a 56-year old man with a remote infarct, demonstrating association of apoE (green, G and J) and ubiquitin (magenta, H and K, and merged in I and L). G–I depict a neuron with relatively modest apoE expression and focal ubiquitin-positive puncta. J–L demonstrate a degenerating neuron with more widespread apoE- and ubiquitin-containing aggregates, with widespread colocalization (scale: 10 µm).
3.7. Identification of PKAN-type protein aggregates in other brain regions
We performed a broad survey of brain tissues containing infarcts in various locations. Small to medium-sized infarcts with similar size and chronicity to those described for the globuspallidus were sought in subjects who had died without significant additional neurodegenerative disease. Neocortical and hippocampal subacute or older infarcts were not associated with identifiable ubiquitin- or apoE-positive granular aggregates in neurons, although apoE was widely expressed in reactive astrocytes (Fig. 5A) and increased with diffuse cytoplasmic distribution in hippocampal pyramidal and other neurons, including those of the globuspallidus, that displayed other morphologic features such as cytoplasmic contraction and hypereosinophilia associated with acute hypoxic/ischemic injury (Fig. 5B and 5C). However, granular ubiquitin- and apoE-positive protein aggregates similar to those of PKAN were observed in neurons in association with remote infarcts in the pars reticulata of the substantianigra (Fig. 5D–5F) and cerebellar Purkinje cells (Fig. 5G–5I). These neuronal populations, like those of the affected neurons of the globuspallidus, are characterized by a relatively large size that supports longer intracerebral projections, as well as GABAergic neurotransmission (Huang et al., 2007;Uusisaari and Knopfel, 2008; Zhou and Lee, 2011).
Figure 5. PKAN-type protein aggregates in other brain regions.
(A) Immunohistochemical staining for apoE demonstrated a high degree of expression in astrocytes (arrows) in reactive conditions, including this cortical infarct, in many regions of the brain; however, neuronal expression was found in limited circumstances in a survey of ischemic lesions (scale: 50 µm).
(B) ApoE immunohistochemistry performed on the CA1 sector of the hippocampus of this 47-year-old cognitively intact patient show increased apoE expression in neurons with contracted cytoplasm and loss of nuclear detail, morphologic features of acute terminal ischemia (Vector Red development; scale: 50 µm). Additional immunohistochemical staining did not reveal the presence of ubiquitin expression in these neurons (not shown).
(C) ApoE immunohistochemistry performed on condensed ischemic neurons in a section of globuspallidus of a 68-year-old patient with a nearby acute infarct (Vector Red development; scale: 50 µm). Acute lesions here also did not demonstrate ubiquitin expression (not shown).
(D–F) A remote infarct involving the pars reticulata of the substantianigra of an 88-year-old female contains neurons with granular eosinophilic aggregates similar to those of PKAN (D, H&E/LFB stain), that are shown by tissue immunohistochemistry to contain apoE (E) and ubiquitin (F) (Vector Red development in E and F; scale: 33 µm).
(G–I) Immunohistochemical studies of a remote cerebellar infarct in a 76-year-old female showing Purkinje neurons with widespread diffusely increased apoE (G and H) and focal, punctate ubiquitin (I) expression. Immunohistochemical stains were developed with brown chromagen and hematoxalin counterstain (scale: G, 100 µm; H and I, 33 µm).
4. Discussion
Common neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, other synucleinopathies, and various forms of frontotemporal lobar degeneration are characterized by the deposition of protein aceous aggregates of specific proteins in cell type- and location-specific distributions that correspond to symptoms attributable to dysfunction involving the brain regions in which these lesions are found (Soto, 2013; Ross and Poirier, 2004). Protein damage, misfolding, and abnormal association are hypothesized to be the basis of aggregate formation. Previously, we determined that decreased tissue protein solubility, another feature of misfolded aggregated protein, was widespread in Alzheimer’s disease and even affected proteins for which an abnormal protein distribution was not discernable (Woltjer et al., 2005). However, although many proteins display mild changes in solubility, the most striking changes causing a redistribution of a large percentage of protein into the insoluble tissue fraction were limited to just a few proteins, including beta-amyloid, tau, and apoE in Alzheimer’s disease (Woltjer et al., 2005); alpha-synuclein in diseases that feature Lewy bodies, neurites, and specific glial inclusions; and TDP-43 in neurons affected by the most common form of frontotemporal lobar degeneration. All of these protein aceous deposits, with the exception of the diffuse plaques containing beta-amyloid and apoE in Alzheimer’s disease, are associated with co-localization of ubiquitin in their respective pathologic lesions (Mori et al., 1987; Sampathy et al., 2003; Tofaris et al., 2003; Neumann et al., 2006). We previously reported the absence of significant deposition of any of these proteins, with the exception of apoE, in PKAN (Kruer et al., 2011).
The identification of lesions containing aggregated apoE with substantially increased insolubility in PKAN is reminiscent of its properties in Alzheimer’s disease, where it appears heavily co-deposited with beta-amyloid in both diffuse and neuritic plaques and in vessels affected by amyloid angiopathy (Nambra et al., 1991; Strittmatter et al., 1993; Kida et al., 1994). However, although dystrophic tau-positive neurites associated with neuritic plaques are ubiquitinated (Perry et al., 1987), to our knowledge this is the first report of neurodegenerative disease that features the co-deposition of ubiquitin and apoE.
ApoE is the major lipoprotein of the brain, where its endogenous function is to traffic lipids and lipophilic substances (Holtzman et al., 2012; Liu et al., 2013). Its most recognized association with disease is as the major genetic risk factor for development of sporadic Alzheimer’s disease, in which the ε2 and ε4 alleles convey substantially decreased and increased Alzheimer’s disease risk, respectively. Among PKAN patients, we found no evidence for an APOE allele-specific correlation with phenotype severity. Patients with PKAN harbor a multitude of mutations in PANK2, and it is likely that specific mutations determine disease phenotype much more strongly than alleles of APOE.
Besides PKAN, significant neuronal expression of apoE has been described for acutely ischemic hippocampal pyramidal and cortical neurons (Xu et al., 1999; Aoki et al., 2003). The degree of expression in these cells correlates with the apparent degree of ischemic injury, as manifested in the degree of cytoplasmic eosinophilia and contraction, with cornuammonis (CA) 1 neurons being most susceptible to injury and showing the greatest degree of apoE expression. Our survey of various brain regions also demonstrated that apoE expression is not found in the form of inclusions in the hippocampus, even in and around infarcts that are commonly encountered at that site in elderly subjects. It is possible that injured hippocampal pyramidal neurons have either a capacity for recovery or are cleared from the tissue through mechanisms that are not available to large GABAergic neurons of the globuspallidus.
Recapitulation of the morphologic and immunohistochemical features of PKAN neurons in the globuspallidus and other brain regions containing remote infarcts suggests that these features are markers of cell death in association with a marked degree of hypoxic/ischemic injury that appears specific to large GABAergic neurons. Neurodegeneration in PKAN may be attributable to cellular-based or tissue hypoxia; the function of PANK2 in mitochondrial metabolism would be compatible with a flaw in neuronal oxygen utilization and energy production as a plausible outcome of PKAN (Brunetti et al., 2012;Leoni et al., 2012). It is also conceivable that features of CoA activity in brain, apart those that involve oxidative metabolism, could promote morphologic changes that mimic those of tissue hypoxia as a “final pathway” of various toxic mechanisms that involving the globuspallidus, of which ischemic injury is by far the most commonly recognized.
The current results suggest that the specific localization of brain injury in PKAN to the globuspallidus reflects an intrinsic susceptibility of the large collection of GABAergic neurons at that site to injury, but they do not account for the lack of an apparent effect of either clinical or pathological manifestations of PKAN on larger GABAeric neurons at specific locations elsewhere or on the numerous small GABAergic neurons that are abundantly present throughout the brain. It is tempting to speculate that the combination of relatively poor vascular collateralization of the globuspallidus (Wolfram-Gabel and Maillot, 1994) with an intrinsic susceptibility of large GABAergic neurons accounts for the selective degeneration of these cells in PKAN.
Other toxic or metabolic disorders that selectively damage the globuspallidus may inform our understanding of PKAN pathogenesis. These include carbon monoxide poisoning, manganese toxicity, cyanide poisoning, unconjugated hyperbilirubinemia, pyruvate dehydrogenase deficiency, methylmalonicacidemia, and succinic semialdehyde deficiency. All of these conditions are associated with mitochondrial dysfunction and oxidative stress but preserved glycolysis (Rodrigues et al., 2002; Alonso et al., 2003; Pearl et al., 2004; Head et al., 2005; Prockop and Chichkov, 2007; Leavesley et al., 2008; Hamel, 2011; Patel et al., 2012; Martinez-Finley et al., 2013). Brain MRI scans of patients with these disorders show evidence of cytotoxic edema in the globuspallidus, which is also observed in PKAN. The high baseline firing rate of the large GABAergic neurons in globuspallidus distinguishes them from similar neurons in other regions. Such tonic activity creates high-energy demands, making these cells vulnerable to subacute energy losses that are tolerated by other neurons (Vitek et al., 1999; Johnston and Hoon, 2000).
Currently, no effective treatment exists for PKAN. Although our findings are based on histologic and biochemical analogies between PKAN and ischemic injury, they invite other studies of experimental models of PKAN to determine whether treatments that promote oxygenation or perfusion of the basal ganglia may be effective treatments that could be translated into human therapies. Alternately, several strategies have been proposed or are in development with the goal of enhancing tissue survival or recovery after stroke (Moskowitz et al., 2010); it is conceivable that these might also have efficacy as neuroprotectants in PKAN.
Acknowledgements
We acknowledge the patients and families whose thoughtful acts led to the procurement of this tissue for our study. We thank the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD, for providing some of the tissues described here. It should be noted that the role of that institution is to collect and distribute tissue; it cannot endorse studies performed or the interpretation of results. This work was made possible with support from the Oregon Clinical and Translational Research Institute (UL1 RR024140 NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. We thank Otto Szekely, MD for stimulating and insightful discussions.
Funding
This work was funded by the NBIA Disorders Association (to S.J.H.), with acquisition of brain tissue supported by NIH P30AG008017 (to R.L.W. of the Oregon Alzheimer’s Disease Center, directed by Jeffrey Kaye).
Abbreviations
- ApoE
Apolipoprotein E
- BSA
bovine serum albumin
- CA
cornuammonis
- CoA
coenzyme A
- DAPI
4’,6-diamidino-2-phenylindole
- DTT
dithiothreitol
- ELISA
enzyme-linked immunosorbent assay
- GABA
Gamma-aminobutyric acid
- H&E
hematoxalin and eosin
- LFB
luxol fast blue
- NBIA
Neurodegeneration with brain iron accumulation
- OHSU
Oregon Health & Science University
- PANK2
Pantothenate kinase, isoform 2
- PKAN
Pantothenate kinase-associated neurodegeneration
- PVDF
polyvinylidinedifluoride
- SDS
sodium dodecyl sulfate
- TBST
Tris-buffered saline (pH 7.4) containing 0.1% Tween 20
- TDP-43
TAR DNA-binding protein 43
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso JR, Cardellach F, Lopez S, Casademont J, Miro O. Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain. Pharmacol Toxicol. 2003;93:142–146. doi: 10.1034/j.1600-0773.2003.930306.x. [DOI] [PubMed] [Google Scholar]
- Aoki K, Uchihara T, Sanjo N, Nakamura A, Ikeda K, Tsuchiya K, et al. Increased expression of neuronal apolipoprotein E in human brain with cerebral infarction. Stroke. 2003;34:875–880. doi: 10.1161/01.STR.0000064320.73388.C6. [DOI] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76:1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti D, Dusi S, Morbin M, Uggetti A, Moda F, D'Amato I, et al. Pantothenate kinase-associated neurodegeneration: altered mitochondria membrane potential and defective respiration in Pank2 knock-out mouse model. Hum Mol Genet. 2012;21:5294–5305. doi: 10.1093/hmg/dds380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansie LE, Reeves S, Miller K, Zano SP, Frank M, Pate C, et al. Physiological roles of the pantothenate kinases. Biochem Soc Trans. 2014;42:1033–1036. doi: 10.1042/BST20140096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi H, Koyano S, Miyatake S, Matsumoto N, Kameda T, Tomita A, et al. Siblings with the adult-onset slowly progressive type of pantothenate kinase-associated neurodegeneration and a novel mutation, Ile346Ser, in PANK2: clinical features and (99m)Tc-ECD brain perfusion SPECT findings. J Neurol Sci. 2010;290:172–176. doi: 10.1016/j.jns.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Freeman K, Gregory A, Turner A, Blasco P, Hogarth P, Hayflick S. Intellectual and adaptive behaviour functioning in pantothenate kinase-associated neurodegeneration. J Intellect Disabil Res. 2007;51:417–426. doi: 10.1111/j.1365-2788.2006.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A, Hayflick S. Neurodegeneration with Brain Iron Accumulation Disorders Overview. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, et al., editors. Gene Reviews. Seattle: University of Washington; 2013. (Updated 2014). [PubMed] [Google Scholar]
- Gregory A, Hayflick SJ. Pantothenate Kinase-Associated Neurodegeneration. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, et al., editors. Gene Reviews. Seattle: University of Washington; 2002. (Updated 2013). [PubMed] [Google Scholar]
- Grehan S, Tse E, Taylor JM. Two distal downstream enhancers direct expression of the human apolipoprotein E gene to astrocytes in the brain. J Neurosci. 2001;21:812–822. doi: 10.1523/JNEUROSCI.21-03-00812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel J. A review of acute cyanide poisoning with a treatment update. Crit Care Nurse. 2011;31:72–81. doi: 10.4037/ccn2011799. [DOI] [PubMed] [Google Scholar]
- Hayflick SJ. Pantothenate kinase-associated neurodegeneration (formerly Hallervorden-Spatz syndrome) J Neurol Sci. 2003;207:106–107. doi: 10.1016/s0022-510x(02)00433-1. [DOI] [PubMed] [Google Scholar]
- Hayflick SJ. Defective pantothenate metabolism and neurodegeneration. Biochem Soc Trans. 2014;42:1063–1068. doi: 10.1042/BST20140098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick SJ, Westaway SK, Levinson B, Zhou B, Johnson MA, Ching KH, et al. Genetic, clinical, and radiographic delineation of Hallervorden-Spatz syndrome. N Engl J Med. 2003;348:33–40. doi: 10.1056/NEJMoa020817. [DOI] [PubMed] [Google Scholar]
- Head RA, Brown RM, Zolkipli Z, Shahdadpuri R, King MD, Clayton PT, et al. Clinical and genetic spectrum of pyruvate dehydrogenase deficiency: dihydrolipoamide acetyltransferase (E2) deficiency. Ann Neurol. 2005;58:234–241. doi: 10.1002/ana.20550. [DOI] [PubMed] [Google Scholar]
- Holthofer H, Virtanen I, Kariniemi AL, Hormia M, Linder E, Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest. 1982;47:60–66. [PubMed] [Google Scholar]
- Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci. 2007;8:673–686. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Hoon AH., Jr Possible mechanisms in infants for selective basal ganglia damage from asphyxia, kernicterus, or mitochondrial encephalopathies. J Child Neurol. 2000;15:588–591. doi: 10.1177/088307380001500904. [DOI] [PubMed] [Google Scholar]
- Kida E, Golabek AA, Wisniewski T, Wisniewski KE. Regional differences in apolipoprotein E immunoreactivity in diffuse plaques in Alzheimer's disease brain. Neurosci Lett. 1994;167:73–76. doi: 10.1016/0304-3940(94)91030-8. [DOI] [PubMed] [Google Scholar]
- Kruer MC. The neuropathology of neurodegeneration with brain iron accumulation. Int Rev Neurobiol. 2013;110:165–194. doi: 10.1016/B978-0-12-410502-7.00009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruer MC, Hiken M, Gregory A, Malandrini A, Clark D, Hogarth P, et al. Novel histopathologic findings in molecularly-confirmed pantothenate kinase-associated neurodegeneration. Brain. 2011;134:947–958. doi: 10.1093/brain/awr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leavesley HB, Li L, Prabhakaran K, Borowitz JL, Isom GE. Interaction of cyanide and nitric oxide with cytochrome c oxidase: implications for acute cyanide toxicity. Toxicol Sci. 2008;101:101–111. doi: 10.1093/toxsci/kfm254. [DOI] [PubMed] [Google Scholar]
- Leonardi R, Rock CO, Jackowski S, Zhang Y-M. Activation of human mitochondrial pantothenate kinase 2 by palmitoylcarnitine. Proc Natl Acad Sci U S A. 2007;104:1494–1499. doi: 10.1073/pnas.0607621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni V, Strittmatter L, Zorzi G, Zibordi F, Dusi S, Garavaglia B, et al. Metabolic consequences of mitochondrial coenzyme A deficiency in patients with PANK2 mutations. Mol Genet Metab. 2012;105:463–471. doi: 10.1016/j.ymgme.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Finley EJ, Gavin CE, Aschner M, Gunter TE. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic Biol Med. 2013;62:65–75. doi: 10.1016/j.freeradbiomed.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Kondo J, Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987;235:1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991;541:163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Patel KP, O'Brien TW, Subramony SH, Shuster J, Stacpoole PW. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol Genet Metab. 2012;106:385–394. doi: 10.1016/j.ymgme.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl PL, Dorsey AM, Barrios ES, Gibson KM. Succinic Semialdehyde Dehydrogenase Deficiency. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, et al., editors. Gene Reviews. Seattle: University of Washington; 2004. (Updated 2013). [Google Scholar]
- Perry G, Friedman R, Shaw G, Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci U S A. 1987;84:3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917:148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- Prockop LD, Chichkova RI. Carbon monoxide intoxication: an updated review. J Neurol Sci. 2007;262:122–130. doi: 10.1016/j.jns.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Rodrigues CM, Sola S, Brites D. Bilirubin induces apoptosis via the mitochondrial pathway in developing rat brain neurons. Hepatology. 2002;35:1186–1195. doi: 10.1053/jhep.2002.32967. [DOI] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;(10 Suppl):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Sampathu DM, Giasson BI, Pawlyk AC, Trojanowski JQ, Lee VM. Ubiquitination of alpha-synuclein is not required for formation of pathological inclusions in alpha-synucleinopathies. Am J Pathol. 2003;163:91–100. doi: 10.1016/s0002-9440(10)63633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71:108–113. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofaris GK, Razzaq A, Ghetti B, Lilley KS, Spillantini MG. Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem. 2003;278:44405–44411. doi: 10.1074/jbc.M308041200. [DOI] [PubMed] [Google Scholar]
- Uusisaari M, Knopfel T. GABAergic synaptic communication in the GABAergic and non-GABAergic cells in the deep cerebellar nuclei. Neuroscience. 2008;156:537–549. doi: 10.1016/j.neuroscience.2008.07.060. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46:22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Wolfram-Gabel R, Maillot C. Vascular networks of the nucleus lentiformis. Surg Radiol Anat. 1994;16:373–377. doi: 10.1007/BF01627656. [DOI] [PubMed] [Google Scholar]
- Woltjer RL, Cimino PJ, Boutte AM, Schantz AM, Montine KS, Larson EB, et al. Proteomic determination of widespread detergent-insolubility including A beta but not tau early in the pathogenesis of Alzheimer's disease. FASEB J. 2005;19:1923–1925. doi: 10.1096/fj.05-4263fje. [DOI] [PubMed] [Google Scholar]
- Woltjer RL, Sonnen JA, Sokal I, Rung LG, Yang W, Kjerulf JD, et al. Quantitation and mapping of cerebral detergent-insoluble proteins in the elderly. Brain Pathol. 2009;19:365–374. doi: 10.1111/j.1750-3639.2008.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PT, Gilbert JR, Qiu HL, Ervin J, Rothrock-Christian TR, Hulette C, et al. Specific regional transcription of apolipoprotein E in human brain neurons. Am J Pathol. 1999;154:601–611. doi: 10.1016/S0002-9440(10)65305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Woltjer RL, Sokal I, Pan C, Wang Y, Brodey M, et al. Quantitative proteomics identifies surfactant-resistant alpha-synuclein in cerebral cortex of Parkinsonism-dementia complex of Guam but not Alzheimer's disease or progressive supranuclear palsy. Am J Pathol. 2007;171:993–1002. doi: 10.2353/ajpath.2007.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Lee CR. Intrinsic and integrative properties of substantia nigra pars reticulata neurons. Neuroscience. 2011;198:69–94. doi: 10.1016/j.neuroscience.2011.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]