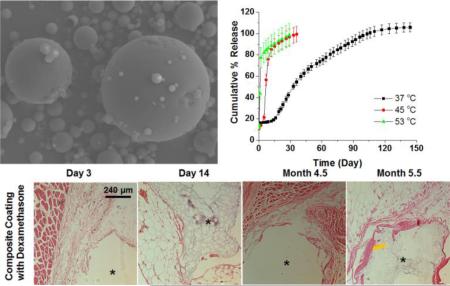
Abstract
The foreign body reaction is the major cause of the dysfunction and relatively short lifetime associated with implanted glucose biosensors. An effective strategy to maintain sensor functionality is to apply biocompatible coatings that elute drug to counter the negative tissue reactions. This has been achieved using dexamethasone releasing poly (lactic-co-glycolic acid) (PLGA) microspheres embedded in a poly vinyl alcohol (PVA) hydrogel coating. Accordingly, the biosensor lifetime relies on the duration and dose of drug release from the coating. To achieve long-term drug release mixed populations of microspheres have been used. In the current study, microspheres were prepared by blending low (25 KDa) and high (113 KDa) molecular weight PLGA at different mass ratios to overcome problems associated with mixing multiple populations of microspheres. “Real-time” in vitro studies demonstrated dexamethasone release for approximately 5 months. An accelerated method with discriminatory ability was developed to shorten drug release to less than 2 weeks. An in vivo pharmacodynamics study demonstrated efficacy against the foreign body reaction for 4.5 months. Such composite coatings composed of PLGA microspheres prepared using polymer blends could potentially be used to ensure long-term performance of glucose sensors.
Keywords: polymer blends, PLGA, microspheres, foreign body reaction, glucose biosensors, long-term drug release, accelerated release testing
Graphical Abstract
1. Introduction
Continuous glucose monitoring (CGM) devices can provide tremendous assistance in the management of diabetes through the availability of real-time blood glucose levels (Vaddiraju et al., 2010). The current CGM technology is based on semi-implantable glucose sensors with limited life-time (maximum 7 days). Those sensors suffer from instability as a result of the tissue trauma caused by implantation and a series of negative tissue responses triggered by the persistent presence of the implant (Morais et al., 2010). The series of immunological events that include acute inflammation (e.g. infiltration of inflammatory cells), chronic inflammation (e.g. activation of fibroblasts), and fibrous encapsulation (e.g. collagen capsule formation surrounding the implants) are called the foreign body reaction (FBR) (Anderson et al., 2008). Various approaches have been attempted to overcome the foreign body reaction to extend the life-time of glucose sensors. These include application of biocompatible coatings (Kastellorizios et al., 2015a; Wang et al., 2013), release of anti-inflammatory drugs (Bhardwaj et al., 2010; Galeska et al., 2005; Patil et al., 2004; Zolnik and Burgess, 2008) as well as delivery of angiogenic agents (Laschke et al., 2014; Patil et al., 2007).
Local delivery of dexamethasone, a potent corticosteroid, has been shown to effectively control the FBR in normal rats, diabetic/fatty rats as well as mini pigs (Kastellorizios et al., 2015a; Wang et al., 2013). However, delayed tissue reaction can develop after exhaustion of the drug (Bhardwaj et al., 2007). A dexamethasone loaded PLGA microsphere/PVA hydrogel coating has been developed to control the FBR for 3 months (Bhardwaj et al., 2010). This was achieved using a mixture of two populations of microspheres, one prepared with low Mw PLGA and the other with high Mw PLGA. Dexamethasone release from PLGA microspheres typically exhibits a tri-phasic profile with a burst release phase followed by a lag phase and a secondary release phase. A long lag phase is usually associated with high Mw PLGA based microspheres because the time required for sufficient bulk erosion to allow drug release. For example, the lag phase for dexamethasone from the microspheres was extended from 10 days to 41 days when the Mw of PLGA was changed from 25 KDa to 70 KDa (Wang et al., 2014). The limited drug release during the long lag phase of high Mw PLGA microspheres was compensated by drug release from the low Mw PLGA microspheres during that period and thus a 3-month effective formulation was achieved. Further extending drug release beyond 3 months using this strategy would be difficult since this would probably require at least three batches of microspheres and the drug release profiles of these batches would need to match one another to achieve continuous release with no lag phase. In addition, this would provide challenges in terms of processing and product quality control. An alternative strategy is therefore needed to produce a single formulation with a short lag phase and long duration of drug release.
Drug release from PLGA microspheres is controlled by polymer degradation, which is a result of the polymer backbone hydrolyzing into oligomers and monomers. Bulk erosion of the PLGA polymer matrix usually occurs from water penetration followed by autocatalysis of the ester bonds. The long lag phase associated with the degradation of high Mw PLGAs is a result of the relatively slow water penetration into these polymers due to their increased hydrophobicity compared to low Mw PLGAs. This can be shortened by enhancing the hydrophilicity of the polymer using approaches such as decreasing polymer Mw and introducing hydrophilic groups. Blending low Mw PLGA with high Mw PLGA is an intriguing prospect since the low Mw PLGA may facilitate degradation of the high Mw PLGA via increased water absorption and generation of acidic oligomers that will result in autocatalysis of the polymer matrix. A design of experiment (DoE) approach was used to generate a design space to optimize drug release profiles of PLGA microspheres prepared using polymer blends.(Gu and Burgess) Sustained dexamethasone release for approximately 3 months in vitro was achieved by blending 25 KDa PLGA with 70 KDa PLGA (Wang et al., 2014).
Drug release profiles are important product performance indicators. Since real-time release testing of the target product would last for months, it is essential to develop accelerated release testing methods for quality control purposes. Accelerated release testing for microsphere products can be achieved by changing various release conditions (such as pH, temperature, organic solvent, and surfactant). For example adjusting the media to acidic pH conditions has been shown to expedite drug release as PLGA degradation is catalyzed under acidic conditions (Zolnik and Burgess, 2007). However the drug release mechanism was shown to change from bulk erosion to surface erosion when lower pH condition were used. Elevating temperature can facilitate drug diffusion from the microspheres as well as expedite polymer matrix degradation. It has been shown that accelerated drug release from PLGA microspheres can be achieved under elevated temperature conditions (Rawat et al., 2012; Shen and Burgess, 2012; Zolnik et al., 2006).
In the current study, the in vitro and in vivo performance of microspheres prepared by blending low (25 KDa) and high (113 KDa) Mw PLGA was investigated. The microspheres were prepared with different polymer mass ratios and characterized for physicochemical properties such as particle size, glass transition temperatures and morphology. “Real-time” and accelerated release testing was performed. The accelerated release method was able to shorten the duration of drug release while maintaining discriminatory ability. The “Real-time” release profiles provided guidance for formulation screening towards in vivo testing. The selected formulation was evaluated in vivo and histological results demonstrated that the foreign body reaction was inhibited for at least 4.5 months.
2. Material and Methods
2.1. Materials
Dexamethasone was purchased from Cayman Chemical (Ann Arbor, MI), poly (vinyl alcohol) (PVA, Mw 30–70 KD), sodium chloride (NaCl, ACS grade), sodium azide (NaN3), sodium phosphate dibasic dihydrate (Na2HPO4·2H2O), sodium phosphate monobasic (NaH2PO4) and dimethyl sulfoxide (DMSO) were purchased from Sigma–Aldrich (St. Louis, MO). PVA (99% hydrolyzed, Mw 133 KD) was purchased from Polysciences, Inc. (Warrington, PA). PLGA Resomer® RG503H 5050 (RG503H, inherent viscosity 0.32–0.44 dl/g) was a gift from Boehringer-Ingelheim. PLGA 9010 DLG7E (DLG7E, inherent viscosity 0.6-0.8 dL/g) was purchased from Lakeshore Biomaterials (Birmingham, AL). RG503H has carboxylic acid end groups and DLG7E is end-capped with a lauryl ester group. Methylene chloride (DCM), acetonitrile (ACN, HPLC grade), and tetrahydrofuran (THF, HPLC grade) were purchased from Fisher Scientific (Pittsburgh, PA). NanopureTM quality water (Barnstead, Dubuque, IA) was used for all studies.
2.2. Methods
2.2.1. Preparation of microspheres and composite coated implants
2.2.1.1. Preparation of PLGA microspheres
Dexamethasone loaded microsphere formulations were prepared using an oil-in-water (o/w) emulsion solvent extraction/evaporation technique according to the compositions listed in Table 1. The PLGA polymer was dissolved in 2 ml of methylene chloride and dexamethasone was dispersed in this solution. Following 20-minute sonication in a bath sonicator, the dispersion was further dispersed using a T 25 digital ULTRA-TURRAX homogenizer (IKA Works, Inc., Wilmington, NC) at 10,000 rpm for 1 min. In order to form an emulsion, the organic phase was homogenized into a 10-ml PVA solution (1% (w/v), average Mw 30–70 KDa) at 10,000 rpm for 2.5 min. The emulsion was then transferred into a 125 ml aqueous PVA solution (0.1% (w/v), Mw 30-70 KDa) and stirred at 600 rpm under vacuum. After 2.5 hours, hardened microspheres were transferred to 50 mL centrifuge tubes and collected through centrifugation under 1500 rpm for 2 minutes. The microspheres were then washed three times with deionized water (10 mL each time), recollected using the same centrifugation procedure and dried via freeze drying. The prepared microspheres were stored at 4°C until further use. Blank microspheres were prepared following the same procedure except that dexamethasone was not added.
Table 1.
Composition and physical characteristics of microsphere formulations using polymer blends
| Formulation | Dexamethasone (mg) | PLGA (DLG7E, mg) | PLGA (RG503H, mg) | Drug Loading (w/w) | Particle Size (by number, μm) | Tg (°C) |
|---|---|---|---|---|---|---|
| F-1 | 50 | 500 | - | 8.34 ± 0.27 | 5.38 ± 5.27 | 51.3 |
| F-2 | 50 | 375 | 125 | 8.09 ± 0.07 | 4.78 ± 4.62 | 51.3 |
| F-3 | 50 | 333 | 167 | 8.11 ± 0.11 | 5.08 ± 4.88 | 50.3 |
| F-4 | 50 | 250 | 250 | 7.86 ± 0.04 | 4.43 ± 4.42 | 48.1 |
2.2.1.2. Preparation of PLGA microsphere/PVA hydrogel composite coated implants
Cylindrical implants were prepared using a two-piece grooved mold based method after three freeze-thaw cycles (Kastellorizios et al., 2015b). Briefly, 150 mg of microspheres (F-3 in Table 1) was suspended using 1 ml of 5% w/w PVA solution (133 KDa) and filled in a 1-mL syringe. The PVA solution was pre-filtrated using 0.22-μm sterile filters to ensure sterility. The suspension was sonicated in a bath sonicator for 10 seconds followed by one freeze–thaw cycle (2 hour at - 20 °C and 1 hour at ambient temperature). The partially thickened suspension was fed into a 2-piece mold with 1.5-mm grooves. The dummy sensors (silicon chips with dimension of 5 × 0.5 × 0.5 mm) were sandwiched between the two mold pieces and were then subjected to additional two freeze thaw cycles. The coated dummy sensors were air dried and cut into 7-mm length implants. The grooves in the mold are 1.5-mm diameter. To ensure size consistency of each implant, dummy sensors are placed in the center of the groove between the two pieces of the mold. Sterile tools (e.g. vials, tubes, twizzles and containers, etc.) were used in the coating process. All the procedures were conducted in a laminar flow hood under sterile conditions. Blank formulations were also prepared using blank PLGA microspheres without dexamethasone.
2.2.2. In vitro characterization of microspheres and composite coatings
2.2.2.1. Drug loading
Approximately 5 mg of dexamethasone-loaded PLGA microspheres was dissolved in 10 ml THF for drug loading determination. A previously reported HPLC method was used for analysis of dexamethasone concentration for the loading calculation (Gu and Burgess). Briefly, the solution was filtered (Millex® HV, PVDF 0.45 μm syringe filter) and subjected to HPLC analysis with 5 μl of injection volume. A Perkin Elmer series 200 HPLC system (Shelton, CT) was equipped with a UV absorbance detector (240 nm wave length for dexamethasone analysis). Acetonitrile/water/phosphoric acid (35/70/0.5, v/v/v) was used as the mobile phase. A Zobax C18 (4.6 mm × 15 cm, Agilent, Santa Clara, CA) analytical column was used with a flow rate of 1 ml/min. The chromatographs were analyzed by PeakSimple™ Chromatography System (SRI instruments, Torrance, CA).
Drug loading was determined as: % drug loading = (weight of drug loaded/weight of microspheres) × 100%.
2.2.2.2. Microsphere characterization
The particle size and size distribution was determined using an AccuSizer 780A autodiluter particle sizing system (Nicomp, Santa Barbara, CA). Approximately 5 mg of microspheres were dispersed in 1 ml of 0.1% (w/v) PVA solution (30-70 KDa) and 100 μl of the dispersion was injected into the system for particle size analysis. All measurements were conducted in triplicate. A TA Q1000 differential scanning calorimeter (DSC) (TA, New Castle, DE) was used to determine the glass transition temperature (Tg) of the prepared microspheres. Modulated DSC was performed with the cycle below: the samples were heated at a rate of 3 °C/min from 4°C to 80 °C at a modulating oscillatory frequency of 1°C/min. The thermograms were analyzed using Universal Analysis software (TA Instruments) to determine the glass transition temperature. The morphology of the microspheres were determined using a scanning electron microscopy (a JEOL JSM-6335F unit). Samples were mounted on carbon taped aluminum stubs and sputter coated with gold for 1.5 min at 6 mA before imaging.
2.2.2.3. In vitro release testing
In vitro release testing was performed on the PLGA microsphere/PVA hydrogel (99% hydrolyzed, Mw 133 KD) composites since the purpose was to test the performance of the coatings rather than the microspheres alone. The composites were prepared using a freeze-thaw method described previously (Wang et al., 2014). Briefly, approximately 75 mg of PLGA microspheres were dispersed into the PVA hydrogel (5% w/v) solution, then this suspension was filled into a pre-made mold (15×38×2 mm) and subjected to three freeze-thaw cycles consisting of 2 h freezing at −20°C followed by 1 h thawing at room temperature to form a physically crosslinked hydrogel resulting from PVA crystallization. The composites were then air dried. Approximately 8 mg composite samples were immersed in 5 ml of 10 mM PBS (pH 7.4) with 0.1% NaN3 and incubated at both elevated temperature (45 and 53 °C) and body temperature (37 °C) under constant agitation. At pre-determined time points, all the release media was removed and replenished with the same volume of fresh media. Sink conditions were maintained throughout. The samples were filtrated through 0.45 μm syringe filters and the concentration of dexamethasone in each sample was determined using the HPLC method as described above. The release profiles were plotted as cumulative % release vs. time. Cumulative percent release at a given time point was calculated as: cumulative percent release = (total amount released at sampling time/total amount loaded) × 100. The values are reported as mean ± standard deviation (n=3).
2.2.3. In vivo pharmacodynamics study of composite coated dummy sensors
PLGA/PVA composite coated dummy sensors prepared in section 2.2.1.2 were implanted into the interscapular subcutaneous tissue of male Sprague-Dawley rats (weighing ~ 200 g, n=3) using 16-gauge thin wall needles. During the implantation procedure, the rats were under anesthesia using 2% isoflurane in oxygen. Before implantation, the back of each animal was shaved and wiped with betadine solution. All of the procedures were conducted under aseptic conditions. One composite was implanted per rat on days 0, 35, 151 and 162 and the rats were sacrificed at day 165. Three rats were used for one group and each rat had a total of 4 composite implants at the end of the study. Accordingly, the duration of implantation for each of these implants is 165, 130, 14, and 3 days, respectively. The implants with surrounding subcutaneous tissue were harvested and fixed in 10% buffered formalin solution (Sigma-Aldrich Co. LLC.). All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) within the University of Connecticut prior to beginning the experiments. Paraffin blocks were prepared for tissue sections and a blinded histological evaluation was performed following hematoxylin and eosin (H&E) staining. Tissue samples were observed and digitally stored using an Olympus microscope (model BX51, Olympus America, Melville, NY).
3. Results and Discussion
3.1. In vitro characterization of microspheres
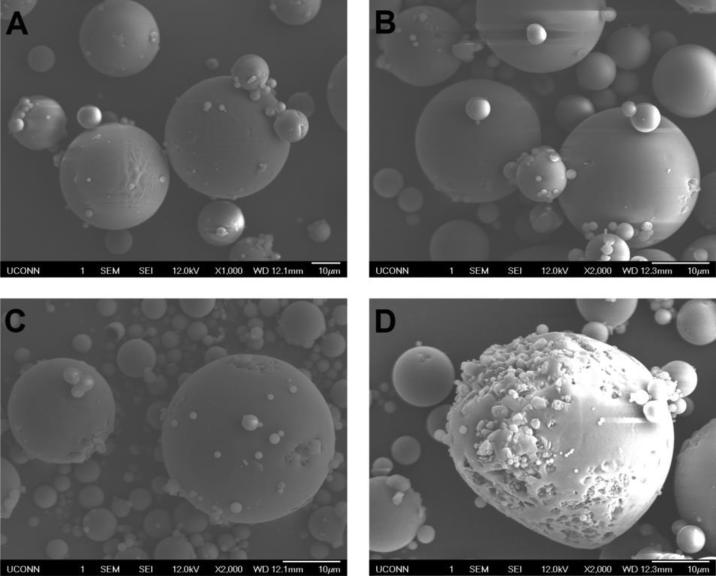
The microspheres were characterized for drug loading, particle size and thermal properties (Table 1). No significant differences were observed in the average particle size of all formulations investigated. For F-1, F-2 and F-3, the drug loading was approximately 8% (w/w) with a slight increase in loading with increase in the ratio of the more hydrophobic DLG7E polymer in the formulation. The drug loading of the F-4 formulation was significantly lower than that of the other formulations as determined by the paired student T-test (p<0.05 as significant). This increase hydrophobicity results in faster polymer solidification and therefore drug encapsulation may be enhanced. The glass transition temperatures for microspheres prepared with polymer blends are between the glass transition temperatures of DLG7E (52.97 °C) and RG503H (42.1 °C). Although the rank order for the Tg(s) is consistent with the empirical Tg(s) calculated from Fox Equation for polymer blends (Hiemenz, 2007), polymer segregation is still possible considering that the Tg(s) of the two polymers are very close. SEM examination will provide more detailed information regarding microsphere morphology. SEM micrographs (Figure 1) showed spherical microspheres with smooth morphology for most of the particles except for those of F-4. Large pores and crevasses were observed for the F-4 microspheres which can be explained by possible polymer phase separation in this formulation considering that equal amounts of the low and high Mw polymers were added in F-4. The peculiar structure of the F-4 formulation is an indication of phase separation. In addition, much higher burst release was observed for the F-4 formulation when compared to the other formulations.
Figure 1.
Scanning electron micrographs of microsphere formulations prepared using blends of PLGA polymers at various mass ratios: DLG7E/RG503H (w/w)=1/0, 3/1, 2/1 and 1/1 for F-1 (A), F-2 (B), F-3 (C) and F-4 (D), respectively.
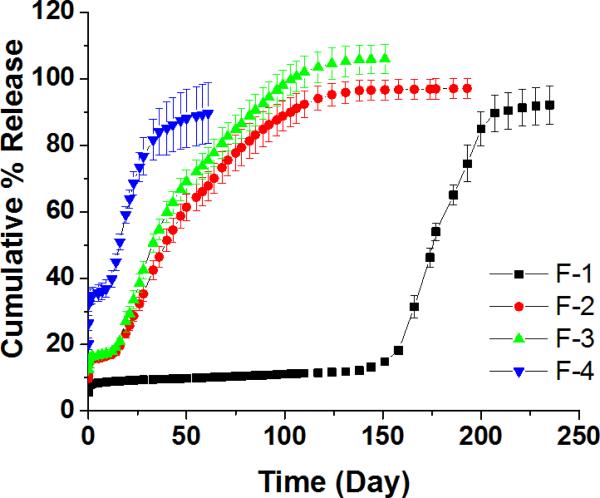
3.2. “Real-time” in vitro drug release from composites
The release profiles obtained for the microsphere formulations under “real-time” conditions (37 °C) are shown in Figure 2. Dexamethasone release from PLGA microspheres typically has an initial burst phase, followed by a lag phase and then a secondary zero-order release phase. These three distinct phases were identified for all the formulations. The F-4 formulation exhibited the highest burst release (approximately 35%) and the shortest lag phase (approximately 10 days).
Figure 2.
In vitro release profiles of microsphere/PVA hydrogel composite formulations at 37 °C. The cumulative % release was plotted as mean ± SD (n=3). Different ratios of polymers were used to prepare the microspheres: DLG7E/RG503H (w/w) = 1/0, 3/1, 2/1 and 1/1 for F-1, F-2, F-3 and F-4, respectively.
The pores and crevasses observed in the surface of F-4 formulation (Figure 1) are considered to be responsible for the high burst release associated with this formulation since easier drug diffusion from the surface is expected. The release of dexamethasone from the F-4 formulation reached a plateau within 1.5 months. Microspheres prepared using 100% RG503H have previously been shown to have a burst release of approximately 40% and a lag phase of approximately 7 days and the release was complete within 35 days. (Shen and Burgess, 2012) The release profile of the F-4 formulation is closer to that of the microspheres prepared with 100% RG503H compared to the other two polymer blend microspheres (F-2 and F-3). This may be a result of polymer phase separation in this microsphere formulation, as discussed above, as well as the relatively higher hydrophilicity of this formulation increasing water uptake and consequent polymer degradation. The F-1 formulation (prepared with 100% of the high Mw DLG7E polymer) has the lowest burst release (approximately 8%), the longest lag phase (approximately 4 months) and the entire release profile lasts for approximately 7 months. The DLG7E polymer has high hydrophobicity due to its high Mw and high lactic:glycolic acid ratio (90:10). Accordingly, polymer degradation is significantly slower compared to microspheres prepared with the polymer blends as the rate of water penetration and subsequent polymer hydrolysis are significantly reduced. The long lag phase of the F-1 formulation prohibits its use for the purpose of continuous drug release. The drug release profiles of the F-2 and F-3 formulations are in between those of the F-1 and F-4 formulations. These formulations have burst release phases of approximately 15%, lag phases of less than 2 weeks and their entire release profiles last for approximately 4.5 months. Water penetration and hence polymer degradation is more rapid compared to the F-1 formulation, since these two formulations were prepared by blending the more hydrophilic RG503H polymer with the DLG7E polymer. The relatively high hydrophilicity of the RG503H is attributed to three factors: 1) a relatively small Mw of ~25 KDa; 2) a lactic/glycolic acid ratio of 50/50; and 3) the fact that it is capped with hydrophilic carboxylic groups (Makadia and Siegel, 2011). In the blended polymers, the low Mw RG503H facilitates water penetration and the subsequent degradation of the RG503H polymer generates lactic/glycolic acid oligomers and monomers. The resultant decrease in pH catalyzes the degradation of the high Mw polymer (DLG7E). Therefore, the long lag phase associated with the high Mw polymer is eliminated and the microspheres prepared with polymer blends exhibit continuous release profiles following a small burst and a short lag phase. Although the release profiles of the F-2 and F-3 formulations were similar, the F-3 formulation was selected for further studies.
3.3. Accelerated in vitro drug release from composites
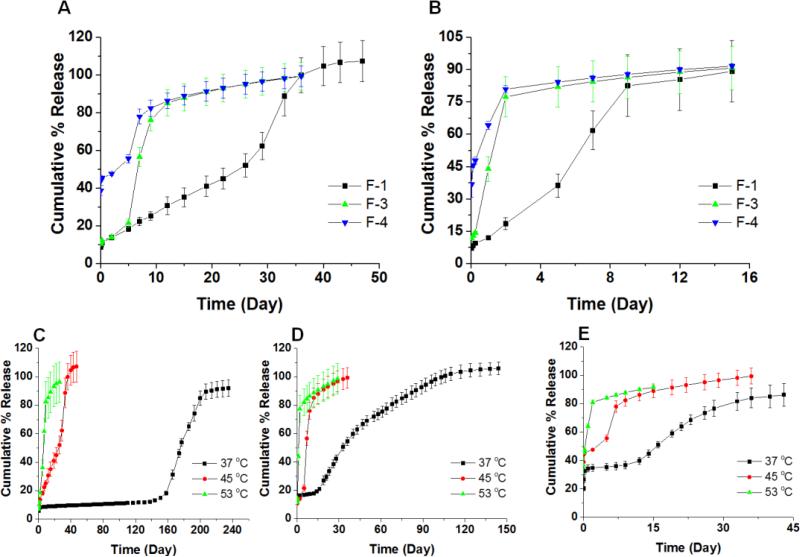
Drug release profiles are important product performance indicators. A real-time release profile is typically a good indication for the in vivo performance of the formulation. Considering that real-time release tests for microsphere products can last for months, it is essential to develop accelerated release testing methods for quality control purposes. Maintaining the discriminatory ability of the method while shortening the testing duration is essential. Accelerated release tests can be conducted by adjusting parameters such as temperature, solvent, ionic strength, pH, and agitation rate as well as addition of enzymes and surfactants.(Burgess et al., 2004) It has been shown that elevating temperature is an effective method of accelerating drug release from PLGA microsphere/PVA hydrogel composites (Shen and Burgess, 2012; Zolnik et al., 2006).
The F-1, F-3 and F-4 formulations were tested under elevated temperature conditions. Figure 3-A and B shows the release profiles for the three formulations at 45 °C and 53 °C, respectively. In order to investigate the effect of the glass transition temperature on the drug release profiles, two temperatures (one higher and another lower than the Tg of the formulations) were chosen for accelerated release testing method development. The release profiles for the individual formulations at real time (37 °C) as well as accelerated (45 and 53 °C) conditions are plotted in Figure 3-C, D and E for direct comparison. At 45 °C, although there were no lag phases, three distinct drug release phases were observed for all formulations. The burst release for F-1 and 3 is close to 10%, which is similar to that obtained at 37 °C. A slightly higher burst release was observed for the F-4 formulation with approximately 45% released after 24 hours compared to 35% at 37 °C. The middle phases for the F-3 and F-4 formulations at 45 °C lasted for approximately 5 days and this can be compared to their lag phases at 37 °C which lasted for approximately 3 weeks. The middle phase for the F-1 formulation lasted approximately 1 month, which can be compared to the 4-month lag phase observed at 37 °C. It is speculated that the drug diffusion plays a significant role in the middle phase at 45 °C, while the third phase is both diffusion and erosion controlled. At 53 °C, drug release from all formulations was further accelerated and the typical three phase release profile was lost. Instead, formulations F-3 and F-4 showed two distinct release phases and the release was complete in less than 1 week. Formulation F-1 also showed two release phases with a slight inflection point around day 5 and release from this formulation was complete in less than 2 weeks. The glass transition temperatures of the F-1, F-3 and F-4 formulations are below 53 °C (51.31 °C, 50.25°C, and 48.06°C, respectively). In addition to acceleration of polymer degradation, polymer chain mobility increases greatly at temperatures above the Tg which facilitates drug diffusion from the polymer matrix. Both of these factors contribute to change in the drug release mechanism and hence the changes observed in the release profiles of all three formulations. Nevertheless, the accelerated tests at both 45 and 53 °C were able to distinguish the three formulations. Drug release testing performed at 53 °C may be more suitable for quality assurance purposes due to the significant reduction in the duration of release testing.
Figure 3.
In vitro release profiles of microsphere formulations at elevated temperatures of 45 °C (A) and 53 °C (B). Different ratios of polymers were used to prepare the microspheres: DLG7E/RG503H (w/w) = 1/0, 2/1, and 1/1 for F-1, F-3 and F-4, respectively. In vitro release profiles of microsphere formulations at both “real-time” and elevated temperatures (45 °C and 53 °C) of the F-1 (C), F-3 (D) and F-4 (E) formulations.
3.4. In vivo pharmacodynamics
From the in vitro release tests the F-3 formulation was selected as the most promising formulation to achieve long-term continuous dexamethasone release in vivo. The F-1 formulation is unsuitable due to the extremely long lag phase (5 months). The F-4 formulation is unsuitable due to the short duration of drug release (1.5 months). The F-3 formulation is superior to the F-2 formulation since it has a slightly higher daily dose. The F-3 formulation was able to control the foreign body reaction for 4.5 months while the blank formulations triggered both acute and chronic inflammation. (Figure 4) The control group exhibited different stages of the foreign body reaction: 1) inflammatory cell recruitment (mostly neutrophils as indicated by the red arrows) at day 3; 2) fibroblast activation and alignment at day 14 (indicated by green arrows); and 3) fibrous encapsulation (indicated by yellow arrows) at 4.5 and 5.5 months. The composites prepared using the F-3 formulation were able to prevent both acute inflammation (no inflammatory cell accumulation at day 3) and chronic inflammation (no activated fibroblast or formation of fibrous encapsulation) up to 4.5 months. At 5.5 months post implantation, fibrous encapsulation was observed for the F-3 formulation composites indicating that dexamethasone was depleted or was released at an insufficient dose at this point. The in vivo performance of the F-3 formulation is therefore consistent with its in vitro release profile. This continuously releasing (4.5-month) formulation has the longest duration of action of any dexamethasone microsphere formulation reported thus far. An even longer duration of action (approximately 7 months) could be achieved using a mixture of the F-1 and F-3 formulations.
Figure 4.
In vivo pharmacodynamics of implanted composite coated dummy sensors in rats. Stars indicate where the implants were located, the red arrows indicate inflammatory cell infiltration during the acute inflammatory phase, the green arrow indicates activated fibroblasts present during the transitional phase from acute to chronic inflammation, and the yellow arrows indicate a fibrous capsule formed around the implants.
4. Conclusions
The current study established the concept of blending different PLGA polymers to achieve continuous and long-term drug release from PLGA microspheres by integrating the benefits of both small and high Mw polymers. The low Mw PLGA increases water absorption into the polymer matrix and generates acidic oligomers/monomers which facilitates the autocatalysis of the high Mw polymer resulting in a smoother release profile. A polymer blend based dexamethasone microsphere/hydrogel formulation with continuous drug release and in vivo efficacy of 4.5 months was achieved. This formulation has the longest duration of action among any reported dexamethasone microsphere formulations thus far. In addition, it may be possible to further extend the duration of action in vivo to approximately 7 months by mixing two of the microsphere formulations reported here. The composition of the polymer blends is critical to the release profile. One formulation blending the low and high Mw polymer at a 50:50 ratio exhibited high burst and short duration of drug release due to polymer phase separation during the microsphere formation process. In addition, accelerated drug release methods were developed under elevated temperature conditions to significantly shorten the testing period from months to days while retaining the ability to discriminate among the various formulations. The successful development of a long-term effective composite coating paves the way for the realization of long-term, totally implantable continuous glucose monitoring systems.
Acknowledgements
The authors thank US Army Medical Research (W81XWH0710688, W81XWH0910711), NIH (1R21HL09045801, R43EB011886, 9R01EB014586) and NSF/SBIR (1046902, 1230148) for funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations:
poly (lactic-co-glycolic acid) (PLGA), continuous glucose monitoring (CGM), foreign body reaction (FBR), polyvinyl alcohol (PVA), dimethyl sulfoxide (DMSO), methylene chloride (DCM), acetonitrile (ACN), tetrahydrofuran (THF), molecular weight (MW), differential scanning calorimeter (DSC), glass transition temperature (Tg), scanning electron microscopy (SEM).
References
- Anderson JM, Rodriguez A, Chang DT. FOREIGN BODY REACTION TO BIOMATERIALS. Seminars in immunology. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. Controlling Acute Inflammation with Fast Releasing Dexamethasone-PLGA Microsphere/PVA Hydrogel Composites for Implantable Devices. Journal of Diabetes Science and Technology. 2007;1:8–17. doi: 10.1177/193229680700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation. International Journal of Pharmaceutics. 2010;384:78–86. doi: 10.1016/j.ijpharm.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Burgess DJ, Crommelin DJ, Hussain AS, Chen ML. Assuring quality and performance of sustained and controlled release parenterals: EUFEPS workshop report. AAPS J. 2004;6:100–111. doi: 10.1208/ps060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeska I, Kim T-K, Patil S, Bhardwaj U, Chatttopadhyay D, Papadimitrakopoulos F, Burgess D. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J. 2005;7:E231–E240. doi: 10.1208/aapsj070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Burgess DJ. Prediction of dexamethasone release from PLGA microspheres prepared with polymer blends using a design of experiment approach. International Journal of Pharmaceutics. doi: 10.1016/j.ijpharm.2015.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemenz PTL. Polymer Chemistry. CRC Press; Boca Raton, Florida: 2007. [Google Scholar]
- Kastellorizios M, Papadimitrakopoulos F, Burgess DJ. Prevention of foreign body reaction in a pre-clinical large animal model. J Control Release. 2015a;202:101–107. doi: 10.1016/j.jconrel.2015.01.038. [DOI] [PubMed] [Google Scholar]
- Kastellorizios M, Papadimitrakopoulos F, Burgess DJ. Prevention of foreign body reaction in a pre-clinical large animal model. Journal of Controlled Release. 2015b;202:101–107. doi: 10.1016/j.jconrel.2015.01.038. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Augustin V, Kleer S, Tschernig T, Menger MD. Locally applied macrophage-activating lipopeptide-2 (MALP-2) promotes early vascularization of implanted porous polyethylene (Medpor(R)). Acta Biomater. 2014;10:4661–4669. doi: 10.1016/j.actbio.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais J, Papadimitrakopoulos F, Burgess D. Biomaterials/Tissue Interactions: Possible Solutions to Overcome Foreign Body Response. AAPS J. 2010;12:188–196. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil SD, Papadimitrakopoulos F, Burgess DJ. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol Ther. 2004;6:887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. Journal of Controlled Release. 2007;117:68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Rawat A, Bhardwaj U, Burgess DJ. Comparison of in vitro–in vivo release of Risperdal® Consta® microspheres. International Journal of Pharmaceutics. 2012;434:115–121. doi: 10.1016/j.ijpharm.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Shen J, Burgess DJ. Accelerated in vitro release testing of implantable PLGA microsphere/PVA hydrogel composite coatings. International Journal of Pharmaceutics. 2012;422:341–348. doi: 10.1016/j.ijpharm.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F. Technologies for Continuous Glucose Monitoring: Current Problems and Future Promises. Journal of Diabetes Science and Technology. 2010;4:1540–1562. doi: 10.1177/193229681000400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gu B, Burgess D. Microspheres Prepared with PLGA Blends for Delivery of Dexamethasone for Implantable Medical Devices. Pharm Res. 2014;31:373–381. doi: 10.1007/s11095-013-1166-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Papadimitrakopoulos F, Burgess DJ. Polymeric “smart” coatings to prevent foreign body response to implantable biosensors. Journal of Controlled Release. 2013;169:341–347. doi: 10.1016/j.jconrel.2012.12.028. [DOI] [PubMed] [Google Scholar]
- Zolnik BS, Burgess DJ. Effect of acidic pH on PLGA microsphere degradation and release. Journal of Controlled Release. 2007;122:338–344. doi: 10.1016/j.jconrel.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Zolnik BS, Burgess DJ. Evaluation of in vivo–in vitro release of dexamethasone from PLGA microspheres. Journal of Controlled Release. 2008;127:137–145. doi: 10.1016/j.jconrel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Zolnik BS, Leary PE, Burgess DJ. Elevated temperature accelerated release testing of PLGA microspheres. Journal of Controlled Release. 2006;112:293–300. doi: 10.1016/j.jconrel.2006.02.015. [DOI] [PubMed] [Google Scholar]