Abstract
Epoxides from polyunsaturated fatty acids (PUFAs) are potent lipid mediators. In vivo stabilization of these epoxides by blockade of the soluble epoxide hydrolase (sEH) leads to anti-inflammatory, analgesic and normotensive effects. Therefore, sEH inhibitors (sEHi) are a promising new class of drugs. Herein, we characterized pharmacokinetic (PK) and pharmacodynamic properties of a commercially available potent sEHi 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU). Cell culture studies suggest its high absorption and metabolic stability. Following administration in drinking water to rats (0.2, 1, and 5 mg TPPU/L with 0.2% PEG400), TPPU’s blood concentration increased dose dependently within the treatment period to reach an almost steady state after 8 days. TPPU was found in all the tissues tested. The linoleic epoxide/diol ratios in the tissues were dose dependently increased, indicating significant sEH inhibition. Overall, administration of TPPU with the drinking water led to systemic distribution as well as high levels and thusmakes chronic sEH inhibition studies possible.
Keywords: Soluble epoxide hydrolase, eicosanoids, oxylipins, epoxy fatty acids, EETs, primary rat hepatocytes, Caco-2 intestinal absorption
Introduction
Oxidative metabolites (called oxylipins) of long chain polyunsaturated fatty acids (PUFA) are potent lipid mediators. While many research and commercial drugs have focused on the formation of oxylipins by cyclooxygenases (COX) and lipoxygenases (LOX) 1, in recent years, research on the biology of oxylipins produced by cytochrome P450s has increased. For example, 20-HETE, a product of arachidonic acid (ARA) by CYP4s, acts as a potent vasoconstrictor2. By contrast, conversion of PUFA by CYP2C, CYP2J and several other CYP families, predominantly leads to the formation of epoxy-fatty acids (EpFA)3. All double bonds of PUFAs can be epoxidized, and, at least for ARA all possible regioisomers are biologically formed3, 4.
The biology of the epoxides of ARA (epoxy eicosatrienoic acid, EpETrEs or EETs) is well characterized, and has been summarized in several recent reviews4–7 Maintaining EET levels has beneficial effects in animal models of hypertension, inflammation and pain. However, the molecular mechanisms of EpFAs are largely unknown. It seems that EpFA actions have different targets in different tissues, including nuclear receptors8 and large-conductance potassium (BK) channels,9 as well as inhibiting, by an unclear mechanism, NF-κB signaling10. In contrast to other potent eicosanoids, such as prostaglandins (PGs), no receptor for EpFAs has been found yet. Interestingly, not all EpFAs evoke the same physiological response. While both, EETs and epoxides derived from EPA, epoxy eicosatetraenoic acids (EEQs or EpETEs), are analgesic11 only EETs promote angiogenesis12 while DHA epoxides13 block it in the models reported to date. Furthermore it is unclear if EpFAs act as such, or are further processed e.g., by 12-LOX to 12-Hydroxy-17(18)-EpETE14 or conjugates such as amides,15 which could act as endocannabinoids.
Epoxy fatty acids are mostly degraded in vivo by the soluble epoxide hydrolase (sEH; EC 3.3.2.10) to the corresponding diols 16. Consequently, blockade of the sEH leads to maintain epoxy-FA level.4 In fact, most of the current knowledge about the biology of the EpFAs is based on sEH knockout/inhibition with/without epoxy-FAs or their stable analogs.
Starting from mechanistic transition-state analogs, more than 15 years of development of sEH inhibitors led to highly potent compounds with drug-like properties 16,4. The most promising class of compounds are 1,3-disubstituted ureas such as 1-trifluoromethoxyphenyl-3-(1-propionylpiperidine-4-yl)urea (TPPU)17, which possesses high potency,17–19 and adequate water solubility thus allowing easy formulation for animal studies.20, 21 sEHis are a promising class of new drugs, and their beneficial effects have been already described on animal models of hypertension20, 21 sepsis19 and cardiac fibrosis22. For drug development, studies of pharmacokinectics (PK, absorption, distribution, metabolism and excretion) and oral bioavailability are crucial. Therefore, we investigated here both intestinal absorption and metabolic stability of TPPU using cell culture models and a PK study in rats after administrating low-dose TPPU in drinking water. The study was focused on distribution and in vivo inhibition of sEH (pharmacodynamics) by TPPU.
Materials and methods
Chemicals
The sEHi TPPU, 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (Fig. 1D, Sigma # SML0750, Cayman # 11120) was synthesized in house as previously described17. HPLC grade acetonitrile (ACN), acetic acid (AcOH), methanol (MeOH) and polyethylene glycol 400 (PEG400) were from Fisher Scientific (Nidderau, Germany). Oxylipin standards and internal standards were obtained from Cayman Chemicals (local distributor: Biomol, Hamburg, Germany). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS) and all cell culture reagents were purchased from Biochrom (Berlin, Germany). β-glucuronidase (GUS) and sulfatase from Helix pomatia (HP-2) and all other chemicals were from Sigma Aldrich (Schnelldorf, Germany).
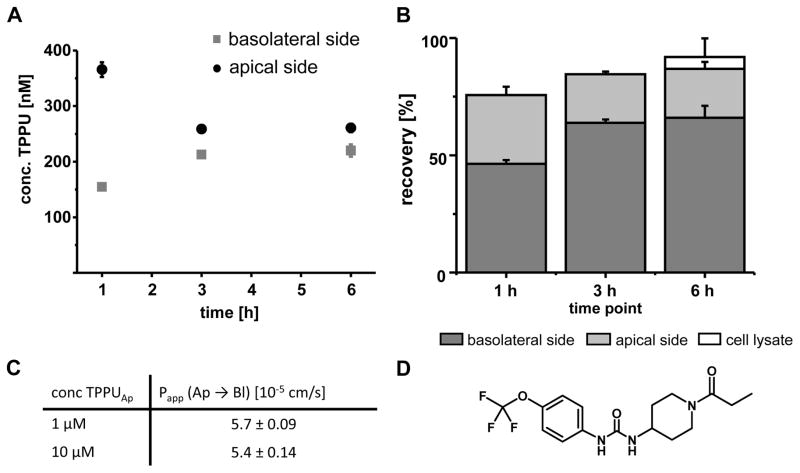
Fig. 1.
Intestinal absorption of TPPU in the Caco-2 transwell system. A: Concentrations of TPPU in the apical and basolateral compartment (initial apical concentration 1 μM). B: Mass balance: Recovery of TPPU in the basolateral and apical compartment as well as in the cells after 6 hours. C: Apparent permeability coefficient (Papp) for TPPU (1 μM and 10 μM) after one hour of incubation. All results are shown as mean ± SD (n=3). D: Structure of TPPU.
Incubation with primary rat hepatocytes (Metabolic stability)
Primary rat hepatocytes were prepared as previously described.23 Briefly, the animals was perfused with oxygen saturated 2 mM EDTA buffer (37°C, 40 mL/min) for 45 min through the portal vain.. The liver was cut into small pieces and the gently scratched-off cells werefiltered and washed. Hepatocytes were isolated by density gradient centrifugation at 800 × g for 5 min with a Percoll colloid (58%) gradient. 1×105 cells were platted in a 6-well dish (2 mL) and incubated for 4 hours. Thereafter, medium and non-adherent cells were removed and the cells were incubated with TPPU (1 μM and 10 μM) in DMEM medium (final DMSO concentration 0.1%). After 24 hours the medium was collected and one aliquot was directly frozen until analysis. The other was incubated with GUS (10.000 U/mL) and sulfatase (700 U/mL) in 1 M acetate buffer (pH 5.0) and incubated for 24 hours.
Caco-2 cell model (in vitro intestinal absorption)
A monolayer of Caco-2 cells (continuous cell of heterogeneous human epithelial colorectal adenocarcinoma cells) was grown on permeable membranes (“ThinCerts”, Greiner Bio One, Frickenhausen, Germany) with a pore size of 0.4 μm and a growth area of 1.13 cm2 within 23–27 days as described.24, 25 Cell monolayers that exceeded a resistance of 300 Ω cm−2 were incubated with either 1 μM or 10 μM of TPPU solution in DMSO on the apical side. Medium samples on the apical and basolateral side were collected and frozen immediately after 1, 3 and 6 hours. The apparent permeability coefficient (Papp) was calculated for t = 1 h as described.24–27 In order to assure that TPPU does not compromise the integrity of the monolayer additional experiments were performed in the presence of Lucifer yellow (LY; 100 μM in the apical compartment), a marker for paracellular diffusion and no LY could be detected in the basolateral chamber.
Preparation of drinking water (and chemical stability)
TPPU stock solutions were prepared by dissolving in PEG400 at a concentration of 0.1, 1, 2.5 and 5 mg/mL. At room temperature, up to 15 mg/mL yielded a clear and stable solution. Each drinking water solution was prepared by mixing 10 mL of the stock solution with tap water (pH 7.7, calcium 78 mg/L, sodium 28 mg/L magnesium 6.6 mg/L, chloride 45 mg/L, sulfate 95 mg/L) to a total solution volume of 5 L, yielding a final PEG400 concentration of 0.2%. Stability of TPPU was assessed using water solutions containing 0.2 mg of TPPU/L and 10 mg of TPPU/L in 750 mL polycarbonate animal water bottles (Bioscape, Castrop-Rauxel, Germany) that were kept at the animal facility. Samples were taken at 1, 3, 6 and 8 days without shaking the bottle, and then directly mixed with ACN containing internal standard (1:1, v:v) and frozen until analysis.
Animal experiments
6-week old male Sprague Dawley rats (n=6) were obtained from Charles River Laboratories International Inc. (Sulzfeld, Germany) and kept in groups in type IV polycarbonate cages (Bioscape). Before starting the TPPU treatment, the animals were allowed to acclimatize in our laboratory for one week. The animals had access to standard chow (#1324 Altromin, Lage, Germany) and were kept at 23 °C, in 55% humidity and a 12-hour light/dark cycle. Water was provided ad libitum containing 0.2% PEG400 with and without TPPU at a concentration of 0.2 mg/L, 1 mg/L and 5 mg/L during the treatment period (8 days). Before (0 h) and after 2 h, 4 h, 8 h, 1 d, 2 d, 4 d and 8 d, 10 μL blood were sampled from the tail vain. The blood was directly mixed with 50 μL deionzed water28 and frozen until analysis. On day 8, the animals were sacrificed by cardiac puncture after anesthesia with xylazine/ketamine (66/5 mg/kg/BW). Plasma and whole blood were sampled. Aliquots of whole blood (800 μL) were incubated with LPS (1 μg/mL) for 24 h and calcium ionophore A23187 (50 μM)29 for 5 minutes at 37 °C to stimulate oxylipin formation.
After blood collection the animal was directly perfused with 30 mL of ice-cold phosphate buffered saline (PBS). Brain, heart, spleen, kidney and muscle (biceps femoris) tissues were collected and snap frozen. In addition, the gut cleaned by perfusion from stool residuals and was transferred to ice cold PBS, and proximal and distal colon sections were also sampled.30 As an orthogonal model to monitor systemic effects on oxylipins by TPPU, a high dose (10 mg/kg BW) was administered at 0 h and 24 h by oral gavage (10 μL/g BW) to male CD-1 mice (10 weeks) and blood was collected after 48 hours by puncuture from the retrobulbar venous plexus. All animal experiments were approved by the animal welfare service of the state of Lower Saxony (Oldenburg, Germany; 33.9-42502-04-13/1134, 28.05.2013).
LC-MS Analysis of TPPU and oxylipin concentrations
Quantification of TPPU was carried out by online solid phase extraction (SPE)-LC-MS as described28. Briefly the analog 1-trifluoromethoxyphenyl-3-(1-acetylpiperidin-4-yl)urea was used as an internal standard (IS) at a final concentration of 300 nM. Liquid samples were mixed 1:1 (v/v) with IS in ACN and directly analyzed. Tissue samples were homogenized and extracted with 250 μL ethyl acetate (EtOAc)/250 μL water in a ball mill (25 Hz, 10 min, 22 °C, MM 400, Retsch, Haan, Germany) The dried EtOAc phase was reconstituted in 50 μL ACN/water 1/1 (v/v). 5 μL of the sample were injected and extracted on a HLB SPE column (1.0 × 50 mm, 25 μm particle) (Waters, Eschborn, Germany) and separation was carried out on a Kinetex column (150 × 2.1 mm, 2.6 μm particle, Phenomenex, Torrance, CA USA). TPPU and the IS were detected on a Micromass LC-Quattro (Waters) in selected reaction monitoring mode (SRM) following positive electrospray ionization (SI).
Quantitative targeted oxylipin metabolomics in plasma was carried out on a 1290 LC equipped with a Zorbax Eclipse Plus C18 reversed phase column (2.1 × 150 mm, particle size 1.8 μm, Agilent Waldbronn Germany) coupled to a 6500 QTRAP instrument (AB Sciex, Darmstadt, Germany) in scheduled selected reaction monitoring mode following negative electrospray ionization (ESI).31 Instrument control was performed with Analyst 1.6.2 and data analysis was carried out with Multiquant 2.1.1 (AB Sciex) as described. For oxylipin analysis 250 μL of plasma were extracted on 60 mg Oasis HLB cartridges (Waters) or 500 mg SepPak tC18 (Waters) as previously described.31
Effects of TPPU on tissue lipids were analyzed by online-SPE-LC-MS on a 6500 QTRAP instrument32 simultaneously determining the linoleic acid metabolites 9(10)-epoxy octadecenoic acid (EpOME), 12(13)-EpOME, 9,10-dihydroxy octadecenoic acid (DiHOME) and 12,13-DiHOME. Utilizing 2H4-9(10)-EpOME and 2H4-9,10-DiHOME as internal standards (final concentration of 20 nM), concentrations in tissues were determined in the EtOAc extracts (see above) and the epoxide/diol ratio was calculated as sum of EpOMEs/DiHOMEs.
Results
Cell culture models
In the Caco-2 cells permeability assay, TPPU rapidly passes the cell monolayer, suggesting it will have a good intestinal permeability (Fig. 1A–C). Only a minor portion of the compound was found in the cells and after 6 hours of incubation 66% of the added amount was detected on the basolateral side. Based on the flux after 1 h, a consistent apparent permeability coefficient (Papp) of 5.5 × 10−5 cm/s was calculated for incubations containing 1 μM and 10 μM of TPPU (Fig. 1C).
Metabolic stability of TPPU was investigated by incubating TPPU with rat hepatocytes (n=6). After 20 hours, 87 ± 3.5% of the initially added 1 μM TPPU, and 90 ± 1.6 % for 10 μM, remained intact. Treating the medium after incubation with glucuronidase/sulfatase did not change the concentrations of TPPU (recovery 93 ± 9.3% (1 μM) and 90 ± 5.9% (10 μM)), suggesting no relevant direct phase II metabolism.
In vivo pharmaceutical properties and tissues distribution
Before testing in animals, we first assessed the stability of the compound during the course of the experiment (8 days). The chemical stability of TPPU in water was assessed, under realistic conditions inside the animal facility, in plastic water bottles at a low concentration of 0.2 mg/L and a high concentration of 10 mg/L (both 0.2% PEG400 in water). TPPU concentrations remained stable over a period of 8 days, with a recovery of 103 ± 3.2% for 0.2 mg/L and 109 ± 4.9% for 10 mg/L, respectively (Fig. S1).
The PK of TPPU was then tested in animals. Administration of TPPU with the drinking water at a concentration of 0.2, 1 and 5 mg/L (0.2% PEG400) changed neither the water consumption nor the body weight gain nor the behavior of the animals (Table S1). Following administration of the drinking water, the TPPU blood concentration increased rapidly (Fig. 2A, Fig S2) in a dose-depend manner over 24 hours. After 8 days, a steady state was reached at blood concentrations of 31 ± 3.4 nM (11 ± 1.2 ng/mL; 0.02 mg/kg BW), 188 ± 22 nM (68 ± 7.9 ng/mL; 0.1 mg/kg BW) and 877 ± 47 nM (320 ± 17 ng/mL; 0.5 mg/kg BW). However, TPPU concentration in plasma was significantly lower, suggesting its absorption into the blood cells (Fig. 2B and C).
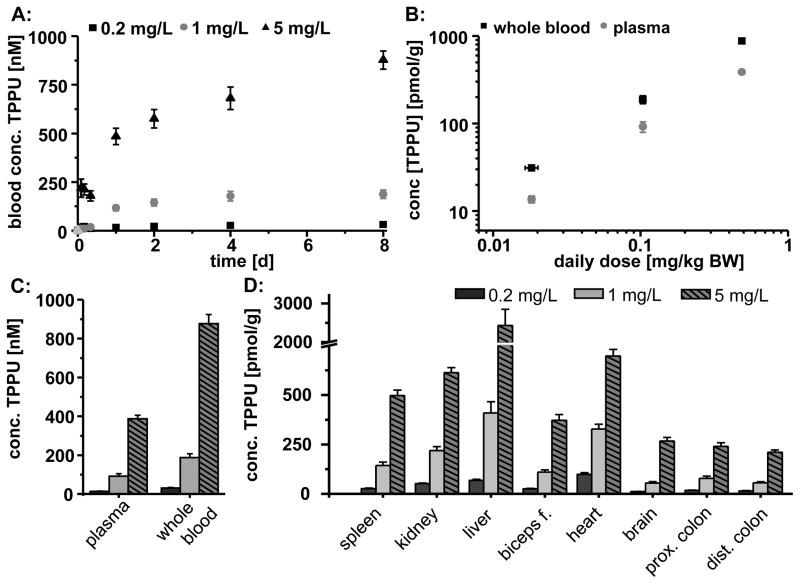
Fig. 2.
Absorption and distribution of TPPU following administration with the drinking water (0.2 mg/L, 1 mg/L and 5 mg/L) by male SD rats. A: Blood concentration over the 8 day treatment period. B: Linearity between daily dose and concentration in blood (day 8). C: Distribution of TPPU in blood and plasma (day 8). D: Distribution of TPPU in different tissues (day 8). All results are shown as mean ± SE (n=6).
TPPU was distributed in all tissues investigated (Fig. 2D). The compound reached similar levels in spleen, kidney and heart (30–700 pmol/g (11–250 ng/g), Fig. 2D). In the group with the highest TPPU concentration the liver tissue levels were about 3.5-fold higher in comparison to the heart tissue. TPPU levels of the other treatment groups were comparable in heart and liver tissue. In the intestinal tissue, concentrations were slightly lower, probably because of the higher amount of connective tissue in the investigated colon preparations. For plasma, whole blood and all tissues, the reached levels showed a remarkable linear relation of the TPPU concentration to the administered dose (Fig. 2B–D).
In vivo inhibition of sEH
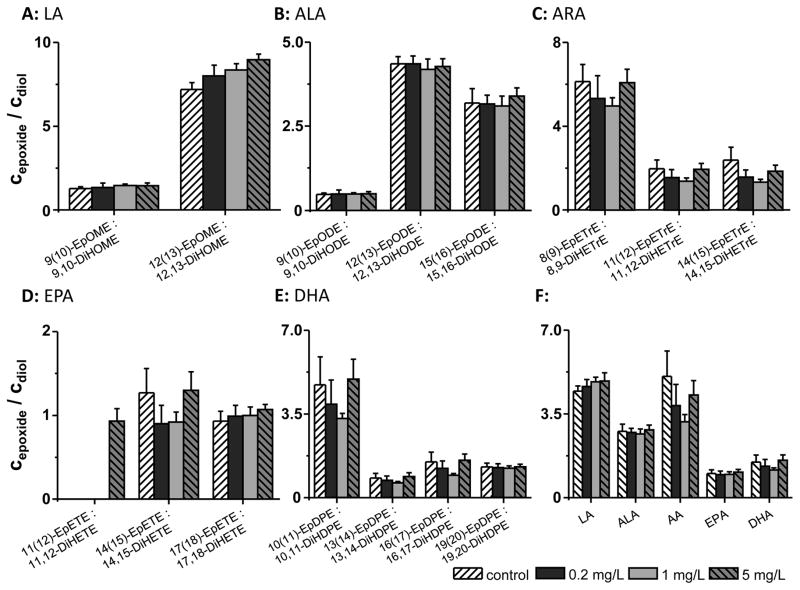
The target engagement of TPPU was assessed based on the plasma epoxide to diol ratios of PUFAs (Fig. 3) 6, 12, 13, 19–22, 33, 34. The concentrations of all oxylipins determined in rat plasma are shown in Table S2A and B. Analytes covered by the LC-MS method which did not exeed the LOQ are displayed in Table S3. Eight days administration of TPPU with the drinking water (0.02, 0.1 and 0.5 mg/kg BW/day) did not change the plasma epoxide/diol ratio of most n3- and n6-PUFA (Fig. 3). However, a dose dependent increase was observed for the EpOME/DiHOME ratio, which was most pronounced for 12(13)-EpOME/12,13-DiHOME (Fig. 3). Utilizing another sample preparation technique for plasma – because it has been shown that the solid phase extraction technique massively influences the determined epoxy-FA levels 31– led to consistent results (SI Table S2A and B, Fig. S3).
Fig. 3.
Modulation of plasma oxylipin levels by 8 day TPPU administration with the drinking water (0.2 mg/L, 1 mg/L and 5 mg/L) to SD rats. Shown are the epoxy-FA/dihydroxy-FA ratios of all biologically relevant PUFAs: A: linoleic acid (LA), B: α-linolenic acid (ALA), C: arachidonic acid (ARA), D: eicosapentaenoic acid (EPA) and E: docosahexaenoic acid (DHA). F: The ratio of the sum of all regioisomer epoxy-FAs to the sum of the corresponding dihydroxy-FAs of each precursor PUFA is shown. All results are shown as mean ± SE (n=6, one-way ANOVA followed by Tuckey test, for all p>0.05). Oxylipin extraction was performed on 500 mg SepPak tC18 cartridges.
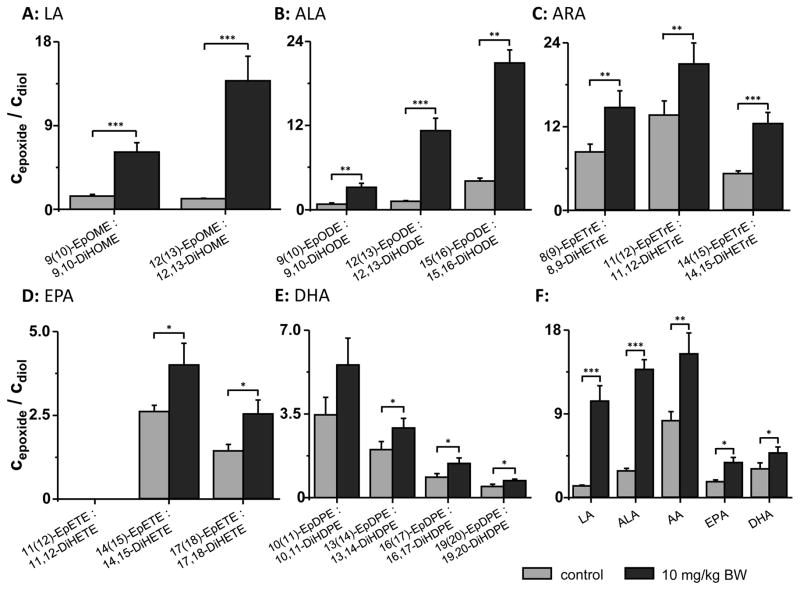
In order to compare the results to previous studies investigating changes in sEH activity by TPPU,19–21 effects of a 20-fold higher dose were tested in an acute exposure model. TPPU at 10 mg/kg BW administered twice via oral gavage to mice (at 0 h and 24 h) resulted in plasma TPPU concentrations of 1780 ± 400 nM after 48 h and led to significant shifts in the epoxide/diol ratios of all PUFAs (Fig. 4).
Fig. 4.
Modulation of plasma oxylipin levels by p.o. administration of TPPU 10 mg/kg BW to CD-1 mice. Shown are the epoxy-FA/dihydroxy-FA ratios of all biologically relevant PUFAs: A: linoleic acid (LA), B: α-linolenic acid (ALA), C: arachidonic acid (ARA), D: eicosapentaenoic acid (EPA) and E: docosahexaenoic acid (DHA). F: The ratio of the sum of all epoxy-FAs to the sum of dihydroxy-FAs of each precursor PUFA is shown. All results are shown as mean ± SE (n=4, Student t-test *** p < 0.001, ** p < 0.01, * p < 0.05). Oxylipin extraction was performed on 500 mg SepPak tC18 cartridges.
Following ex vivo activation of lipases and enzymes of the ARA cascade in blood cells by treatment with calcium ionophore (50 μM for 5 min), led to a reduction of most epoxide levels while diol levels were comparable to inactivated samples (Tab. S2B and C). This led overall to a reduction of epoxide/diol ratios (Table S2B and C and Fig. S4). However, following treatment with LPS (1 μg/ml for 24 h), most epoxide levels were slightly and diol concentrations were strongly elevated in comparison to the inactivated samples leading to reduced epoxide/diol ratios (Table S2B and D and Fig. S5). Moreover, observed effects of TPPU were much stronger for linoleic acid (LA) and ALA metabolite ratios, especially for 12(13)-EpOME/12,13-DiHOME and 12(13)-EpODE/12,13-DiHODE.
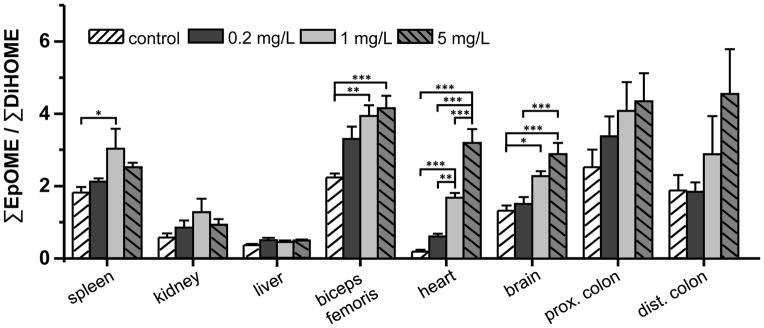
The modulation of sEH activity in tissues after oral treatment via the drinking water was investigated based on the EpOME/DiHOME ratio (Fig. 5). In heart, skeleton muscle, brain and proximal colon a dose dependent increase of the EpOME/DiHOME ratio was observed, being most pronounced in the heart (17-fold control vs. 0.5 mg/kg BW day). In this tissue, even the lowest dose tested (0.02 mg/kg BW/day) led to a pronounced elevation of the epoxide to diol ratio (Fig. 5).
Fig. 5.
Epoxy-linolenic acids to dihydroxy-linolenic acids ratio as marker for sEH activity in tissues following 8 day administration of TPPU to male SD rats. All results are shown as mean ± SE (n=6, one-way ANOVA followed by Tuckey test, *** p < 0.001, ** p < 0.01, * p < 0.05)
Discussion
In the present work, we present details on pharmacokinetics and pharmacodynamics of the potent sEHi, TPPU. With a molecular weight of 359 Da, four hydrogen bond accepting groups and two hydrogen bond donors as well as an n-octanol/water coefficient of 2.4 ± 0.5 17, it fulfills all criteria of Lipinski’s rule of five 35 of a drugable compound. Moreover, the potency to inhibit sEH is high (human IC50 0.9 nM; rat 5 nM and mice IC50 6 nM 36) and TPPU has been successfully used in several animal disease models such as LPS induced sepsis 19 and cardiac fibrosis22 in mice.
In the Caco-2 cell model, TPPU efficiently traverses through the intestinal barrier with an observed Papp of 5.5 × 10−5 cm/s. This value suggests that absorption should not to be a limiting factor for the oral bioavailability of TPPU27,26. This is consistent with the observed bioavailability of TPPU (31–41%) when administered by oral gavage (0.1–3 mg/kg) in mice, based on the area under the curve (AUC).19
Another major factor limiting oral bioavailability and in vivo potency of a drug is its metabolism (first pass effect) 37. TPPU shows good metabolic stability when incubated with primary rat hepatocytes. It is interesting that apparently no direct phase II conjugation by liver glucuronosyltransferases takes place, though the direct N-glucuronidation has been described for the structurally similar triclocarban38. Moreover, TPPU seems to be stable by phase I metabolism since 90% of TPPU was remained in the medium of primary rat hepatocytes after 20 h of incubation. This metabolic stability may provide one mechanistical explanation for the long half-life / mean residence time of more than 24 h for TPPU after a single dose by p.o. or i.v. administration.19
For long-term treatment, oral gavage as well as sub-cutaneous injections are not favorable. The resulting stress could have an influence on the animal model. Moreover, tissue injury and inflammation, particularly of the esophagus after oral gavage, can lead to artifacts and loss of animals. Administration with the drinking water circumvents these problems. Consistent with an earlier report39, TPPU could be well formulated in water using PEG400 as primary solvent. The resulting solution with 0.2% PEG400 in the drinking water was stable under realistic conditions in the animal facility. Over a period of 8 days, no concentration change of TPPU was observed. Moreover, TPPU in a concentration of 0.2–5 mg/L did not change the drinking behavior of the animals and no apparent signs of changed physiology or toxicity (altered body weight gain, health status) were observable after 8 days of treatment.
As commonly expected for a rat on a dry pellet diet, the animals drank about 10% of their body weight per day. Thus, TPPU concentrations in the drinking water can be easily translated to daily oral doses (10% of water concentration) over the whole dose range tested (0.02, 0.1 and 0.5 mg/kg BW/d). Because of the long half-life of TPPU19, its concentration increased during the treatment period. However, after 8 days an almost plateau of mean 31 ± 2.4, 188 ± 20, 877 ± 47 nM was reached in blood. The observed blood concentrations were similar to mice between a single dose (p.o.) of TPPU (Cmax = 270 nM and 1700 nM at doses of 0.1 mg/kg and 0.3 mg/kg, respectively)19 and 8–12 day drinking water treatment (Cmax = 200–1500 nM at doses of 0.02–0.2 mg/kg BW:).21 A single oral dose (0.3 mg/kg) led to similar Cmax (700 ± 600 nM) in cynomolgus monkeys,18 indicating that it might be possible to extrapolate these data to larger animals.
An excellent linearity between the administered dose and the blood levels was found, allowing to precisely adjusting the systemic TPPU levels by drinking water concentration. With a log P of 3.5,17 TPPU concentrates in the (more lipophilic) cellular fraction of whole blood resulting in a 2-fold lower plasma concentration. Interestingly, this distribution is comparable to the much less polar sEHi triclocarban40.
Consistent with its high flux through the Caco-2 monolayer and the short alpha half-life in a two component model after i.v. administration (indicating distribution)19, TPPU freely diffuses through membranes and a distribution in all investigated tissues was found. A significant portion also crosses the blood brain barrier. However, despite the high fat content of the brain, the levels were slightly lower than in other tissues, maybe due to an active efflux transport. Highest concentrations were found in liver, spleen, heart and kidney (dose 0.5 mg/kg BW: 0.37–2.4 nmol/g (0.14–0.86 μg/g)). Ulu et al. found after three weeks administration of TPPU in the drinking water to mice at the same daily doses (0.2 and 0.6 mg/kg BW) kidney concentrations of 7 ± 0.8 μg/g (20 ± 2 nmol/g) and 33 ± 0.8 μg/g (91 ± 2 nmol/g)39. The observed plasma levels were with 610 ± 130 nM and 1800 ± 445 nM also 100-fold higher than in our study. These concentrations are in the same range as observed in our experiments following two days treatment with 10 mg/kg BW administered by oral gavage (1800 ± 300 nM TPPU in plasma). Thus, three months chronic treatment with TPPU in the drinking water could lead to a stronger accumulation of TPPU in blood and tissues than indicated by the kinetics of the eight-day-treatment-period reported here.
Target engagement of TPPU treatment with the drinking water were investigated with respect to its intended mode of action, inhibition of the sEH. As commonly carried out,11–13, 18–21, 33, 34 the epoxy-FA to diol ratio was used as biomarker of in vivo sEH activity. Though plasma and blood concentrations of TPPU is higher than its IC50 value (6 nM for the rat sEH) 36, only moderate or no effects on the epoxide to diol ratios of LA, ALA, ARA, EPA and DHA were observed. Using an alternative sample preparation method 31 yielded the same result (SI). Only the 12(13)-EpOME/12,13-DiHOME and the overall EpOME/DiHOME ratio in plasma were increased in a dose-depend manner. By contrast, two-day treatment by oral gavage (10 mg/kg) in mice led to the expected dramatic increase in the epoxide/diol ratio of all PUFAs. Ex vivo activation of the freshly drawn blood boosted the detected concentration of oxylipins. However, even under these conditions mimicking an activation of the ARA cascade, no or only moderate effects of TPPU treatment on epoxide/diol ratio (except for LA and ALA oxylipins with LPS treatment) were observed.
8-day administration of up to 5 mg/L TPPU in the drinking water does not change the epoxide/diol ratios in plasma. This ratio, which is commonly used for target engagement of sEH inhibition,33, 34 is thus of little help for the investigation of possible effects of low oral doses of TPPU. Consistently, other studies giving TPPU doses below 1 mg/kg BW to monkeys (0.3 mg/kg BW)18 and mice,21 did not find an effect on the epoxide/diol ratio of most PUFA in plasma. As in our study, the EpOME/DiHOME ratio was strongest increased among all PUFA. Consequentially, using this ratio as biomarker for sEH activity, we found massive effects of all administered doses in tissues, except for liver. Particularly in the heart, expressing high amounts of EET forming CYP2J2,41 even a dose as low as 0.2 mg/kg BW led to an increase in the epoxide/diol ratio. In the kidney, a moderate increase in the EpOME/DiHOME ratio was observed, which is in line with the effects of TPPU (0.2–0.6 mg/kg BW) in an angiotensin II hypertension model20, 21.
Conclusion
The potent sEH inhibitor TPPU shows high metabolic stability, is intestinally efficiently absorbed and can be stably formulated in the drinking water. Moreover, the water concentrations correlate excellently with the resulting plasma and tissues levels of the compound. Besides having concentrations well above TPPU-IC50, the epoxide/diol ratios, the most commonly used marker for sEHi target engagement, were not changed in the plasma but increased for EpOMEs dose-dependently in some tissues. Though EpOMEs are not believed to significantly contribute to the biology of epoxy-ARA, such as anti-inflammatory, vasodilatory and analgesic effects, these results clearly suggest that the treatment with low doses of TPPU with the drinking water led to systemic sEH inhibition. If this is sufficient to cause a biological/physiological response will clearly depend on the physiological function/disease investigated. Some models might require a higher dose of TPPU to elicit a significant sEH inhibition in the targeted organ.
Supplementary Material
Acknowledgments
This work was supported by Fonds der Chemischen Industrie (IW and NHS), the European Union (Marie Curie Career Integration Grant CIG 293536 to NHS) and the German Research Foundation (Grant SCHE 1801 to NHS) and the Federal Ministry of Education and Research (BMBF grant 01FP09104C to FG). This work was in part supported by NIEHS grant ES002710 and NIEHS/Superfund Research Program grant ES004699 (to BDH).
References
- 1.Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50(6):1015–38. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoff U, Lukitsch I, Chaykovska L, et al. Inhibition of 20-HETE synthesis and action protects the kidney from ischemia/reperfusion injury. Kidney Int. 2011;79(1):57–65. doi: 10.1038/ki.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol Rep. 2010;62(3):536–47. doi: 10.1016/s1734-1140(10)70311-x. [DOI] [PubMed] [Google Scholar]
- 4.Morisseau C, Hammock BD. Impact of Soluble Epoxide Hydrolase and Epoxyeicosanoids on Human Health. Annu Rev Pharmacol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson SJ, Askari A, Bishop-Bailey D. Anti-inflammatory effects of epoxyeicosatrienoic acids. International journal of vascular medicine. 2012;2012:605101. doi: 10.1155/2012/605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner K, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibition, epoxygenated fatty acids and nociception. Prostaglandins & other lipid mediators. 2011;96(1–4):76–83. doi: 10.1016/j.prostaglandins.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming I. The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease. Pharmacological reviews. 2014;66(4):1106–40. doi: 10.1124/pr.113.007781. [DOI] [PubMed] [Google Scholar]
- 8.Fang X, Hu S, Watanabe T, et al. Activation of peroxisome proliferator-activated receptor alpha by substituted urea-derived soluble epoxide hydrolase inhibitors. J Pharmacol Exp Ther. 2005;314(1):260–70. doi: 10.1124/jpet.105.085605. [DOI] [PubMed] [Google Scholar]
- 9.Larsen BT, Miura H, Hatoum OA, et al. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. American journal of physiology Heart and circulatory physiology. 2006;290(2):H491–9. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Node K, Huo Y, Ruan X, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285(5431):1276–9. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morisseau C, Inceoglu B, Schmelzer K, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51(12):3481–90. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panigrahy D, Edin ML, Lee CR, et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. The Journal of clinical investigation. 2012;122(1):178–91. doi: 10.1172/JCI58128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang G, Panigrahy D, Mahakian LM, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(16):6530–5. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota T, Arita M, Isobe Y, et al. Eicosapentaenoic acid is converted via omega-3 epoxygenation to the anti-inflammatory metabolite 12-hydroxy-17,18-epoxyeicosatetraenoic acid. FASEB J. 2014;28(2):586–93. doi: 10.1096/fj.13-236224. [DOI] [PubMed] [Google Scholar]
- 15.Snider NT, Kornilov AM, Kent UM, Hollenberg PF. Anandamide metabolism by human liver and kidney microsomal cytochrome p450 enzymes to form hydroxyeicosatetraenoic and epoxyeicosatrienoic acid ethanolamides. J Pharmacol Exp Ther. 2007;321(2):590–7. doi: 10.1124/jpet.107.119321. [DOI] [PubMed] [Google Scholar]
- 16.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annual review of pharmacology and toxicology. 2005;45:311–33. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 17.Rose TE, Morisseau C, Liu JY, et al. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. Journal of medicinal chemistry. 2010;53(19):7067–75. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulu A, Appt S, Morisseau C, et al. Pharmacokinetics and in vivo potency of soluble epoxide hydrolase inhibitors in cynomolgus monkeys. British journal of pharmacology. 2012;165(5):1401–12. doi: 10.1111/j.1476-5381.2011.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JY, Lin YP, Qiu H, et al. Substituted phenyl groups improve the pharmacokinetic profile and anti-inflammatory effect of urea-based soluble epoxide hydrolase inhibitors in murine models. Eur J Pharm Sci. 2013;48(4–5):619–27. doi: 10.1016/j.ejps.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulu A, Harris TR, Morisseau C, et al. Anti-inflammatory effects of omega-3 polyunsaturated fatty acids and soluble epoxide hydrolase inhibitors in angiotensin-II-dependent hypertension. J Cardiovasc Pharmacol. 2013;62(3):285–97. doi: 10.1097/FJC.0b013e318298e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulu A, Stephen Lee KS, Miyabe C, et al. An omega-3 epoxide of docosahexaenoic acid lowers blood pressure in angiotensin-II-dependent hypertension. J Cardiovasc Pharmacol. 2014;64(1):87–99. doi: 10.1097/FJC.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirish P, Li N, Liu JY, et al. Unique mechanistic insights into the beneficial effects of soluble epoxide hydrolase inhibitors in the prevention of cardiac fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(14):5618–23. doi: 10.1073/pnas.1221972110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meredith MJ. Rat hepatocytes prepared without collagenase: prolonged retention of differentiated characteristics in culture. Cell biology and toxicology. 1988;4(4):405–25. doi: 10.1007/BF00117769. [DOI] [PubMed] [Google Scholar]
- 24.Willenberg I, Elsner L, Steinberg P, Schebb NH. Development of an online-SPE-LC-MS method for the investigation of the intestinal absorption of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PHIP) and its bacterial metabolite PHIP-M1 in a Caco-2 Transwell system. Food Chem. 2015;166:537–43. doi: 10.1016/j.foodchem.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 25.Willenberg I, Michael M, Wonik J, Bartel LC, Empl MT, Schebb NH. Investigation of the absorption of resveratrol oligomers in the Caco-2 cellular model of intestinal absorption. Food Chem. 2014 doi: 10.1016/j.foodchem.2014.06.103. [DOI] [PubMed] [Google Scholar]
- 26.Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochemical and Biophysical Research Communications. 1991;175(3):880–5. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- 27.Rubas W, Jezyk N, Grass G. Comparison of the Permeability Characteristics of a Human Colonic Epithelial (Caco-2) Cell Line to Colon of Rabbit, Monkey, and Dog Intestine and Human Drug Absorption. Pharmaceutical research. 1993;10(1):113–8. doi: 10.1023/a:1018937416447. [DOI] [PubMed] [Google Scholar]
- 28.Schebb NH, Inceoglu B, Rose T, Wagner K, Hammock BD. Development of an ultra fast online-solid phase extraction (SPE) liquid chromatography electrospray tandem mass spectrometry (LC-ESI-MS/MS) based approach for the determination of drugs in pharmacokinetic studies. Anal Methods. 2011;3(2):420–8. doi: 10.1039/C0AY00714E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomolka B, Siegert E, Blossey K, Schunck WH, Rothe M, Weylandt KH. Analysis of omega-3 and omega-6 fatty acid-derived lipid metabolite formation in human and mouse blood samples. Prostag Oth Lipid M. 2011;94(3–4):81–7. doi: 10.1016/j.prostaglandins.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Nicken P, Hamscher G, Breves G, Steinberg P. Uptake of the colon carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by different segments of the rat gastrointestinal tract: its implication in colorectal carcinogenesis. Toxicology letters. 2010;196(1):60–6. doi: 10.1016/j.toxlet.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Ostermann AI, Willenberg I, Schebb NH. Comparison of sample preparation methods for the quantitative analysis of eicosanoids and other oxylipins in plasma by means of LC-MS/MS. Anal Bioanal Chem. 2015 doi: 10.1007/s00216-014-8377-4. [DOI] [PubMed] [Google Scholar]
- 32.Schebb NH, Huby M, Morisseau C, Hwang SH, Hammock BD. Development of an online SPE-LC-MS-based assay using endogenous substrate for investigation of soluble epoxide hydrolase (sEH) inhibitors. Anal Bioanal Chem. 2011;400(5):1359–66. doi: 10.1007/s00216-011-4861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inceoglu B, Wagner K, Schebb NH, et al. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):5093–7. doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inceoglu B, Wagner KM, Yang J, et al. Acute augmentation of epoxygenated fatty acid levels rapidly reduces pain-related behavior in a rat model of type I diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):11390–5. doi: 10.1073/pnas.1208708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced drug delivery reviews. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 36.Sasso O, Wagner K, Morisseau C, Inceoglu B, Hammock BD, Piomelli D. Peripheral FAAH and soluble epoxide hydrolase inhibitors are synergistically antinociceptive. Pharmacol Res. 2015;97:7–15. doi: 10.1016/j.phrs.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibaldi M, Boyes RN, Feldman S. Influence of first-pass effect on availability of drugs on oral administration. J Pharm Sci. 1971;60(9):1338–40. doi: 10.1002/jps.2600600909. [DOI] [PubMed] [Google Scholar]
- 38.Schebb NH, Franze B, Maul R, Ranganathan A, Hammock BD. In vitro glucuronidation of the antibacterial triclocarban and its oxidative metabolites. Drug metabolism and disposition: the biological fate of chemicals. 2012;40(1):25–31. doi: 10.1124/dmd.111.042283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulu A, Harris TR, Morisseau C, et al. Anti-inflammatory Effects of Omega-3 Polyunsaturated Fatty Acids and Soluble Epoxide Hydrolase Inhibitors in Angiotensin-II Dependent Hypertension. J Cardiovasc Pharmacol. 2013 doi: 10.1097/FJC.0b013e318298e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schebb NH, Ahn KC, Dong H, Gee SJ, Hammock BD. Whole blood is the sample matrix of choice for monitoring systemic triclocarban levels. Chemosphere. 2012;87(7):825–7. doi: 10.1016/j.chemosphere.2011.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem. 1996;271(7):3460–8. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.