Abstract
Atrial fibrillation increases the risk of stroke, which is a leading cause of death and disability worldwide. The use of oral anticoagulation in patients with atrial fibrillation at moderate or high risk of stroke, estimated by established criteria, improves outcomes. However, to ensure that the benefits exceed the risks of bleeding, appropriate patient selection is essential. Vitamin K antagonism has been the mainstay of treatment; however, newer drugs with novel mechanisms are also available. These novel oral anticoagulants (direct thrombin inhibitors and factor Xa inhibitors) obviate many of warfarin’s shortcomings, and they have demonstrated safety and efficacy in large randomized trials of patients with non-valvular atrial fibrillation. However, the management of patients taking warfarin or novel agents remains a clinical challenge. There are several important considerations when selecting anticoagulant therapy for patients with atrial fibrillation. This review will discuss the rationale for anticoagulation in patients with atrial fibrillation; risk stratification for treatment; available agents; the appropriate implementation of these agents; and additional, specific clinical considerations for treatment.
Introduction
Summary points
Stroke is a major cause of morbidity and mortality in patients with atrial fibrillation
Oral systemic anticoagulation provides significant clinical benefit by reducing stroke or systemic embolism in patients with atrial fibrillation at moderate or high risk
Although warfarin has been the agent of choice in the past, several newly available oral anticoagulants (direct thrombin and factor Xa inhibitors) have shown superior safety and efficacy in clinical trials
Although novel oral anticoagulants are a major advance over warfarin, attention to dosing, potential interactions, and adherence are important
Management of bleeding in patients receiving oral systemic anticoagulation is an ongoing challenge to providers
Atrial fibrillation is the most common disturbance of cardiac rhythm in adults, and its prevalence is increasing.1 Patients with this condition have a significantly increased risk of stroke, and thromboembolic events are a major source of morbidity and mortality.2 3 4 5 Strokes caused by atrial fibrillation affect a larger part of the brain and are therefore more likely to be fatal or leave patients bedridden than non-cardioembolic strokes.6 7 8 The use of long term oral anticoagulation reduces the risk of stroke or systemic embolism in patients with atrial fibrillation.9 10 However, the use of these drugs can be challenging because they significantly increase the risk of bleeding, which can be fatal.11 The appropriate selection of patients for treatment represents an important clinical dilemma. In this review, we will discuss the background and rationale for long term anticoagulation in patients with atrial fibrillation; appropriate risk stratification for such patients; and the selection and management of oral anticoagulants, including emerging treatments.
Sources and selection criteria
We based this review on a comprehensive literature review and prioritized well conducted studies of high impact and clinical relevance to the topic. Data sources included PubMed, as well as reference lists from included articles. Searches were limited to English language results. Our search terms included atrial fibrillation, prevalence, incidence, stroke, bleeding, and names of individual anticoagulants (such as warfarin, dabigatran, and rivaroxaban). We included MeSH terms, where applicable. In addition, we searched the clinicaltrials.gov database for ongoing trials of novel agents. Most clinical data on novel oral anticoagulants were derived from large randomized clinical trials and retrospective analyses of such trials. The data were supplemented with expert interpretation of the results and summary of the cumulative data.
Epidemiology
The prevalence of atrial fibrillation in the United States has been projected to increase 2.5-fold during the first half of the 21st century.1 This trend was confirmed in a recent review of worldwide rates of atrial fibrillation in the past 20 years.12 Cohort studies in North America and Europe show the high burden of disease, which translates into a lifetime risk of about one in four.13 14 15 Recently the incidence of atrial fibrillation has been shown to vary by race.16 Nevertheless, the association between atrial fibrillation and adverse events, including all cause mortality and stroke, has been well described. The most recent data suggest that atrial fibrillation related mortality is about 1.6 per 100 000, a twofold increase over the past 20 years.3 11 12 17
Background and rationale for anticoagulation in atrial fibrillation
The association between atrial fibrillation and stroke was first described in analyses from the Framingham Heart Study cohorts.4 5 17 18 The earliest study detailed a fivefold increased risk of stroke in patients with non-rheumatic atrial fibrillation, and a 17-fold increase in patients whose atrial fibrillation was rheumatic in origin.4 Subsequent studies have tried to elucidate the causal pathway between atrial fibrillation and stroke. Many patients with atrial fibrillation also have traditional risk factors for atherosclerotic cerebrovascular disease, and some ischemic events have been attributed to carotid stenoses.19 However, the most often cited link is the high prevalence of left atrial appendage thrombus in patients with atrial fibrillation. This is attributed mainly to relative stasis of flow in the fibrillating atrium,20 although in vitro studies have also shown a relative hypercoagulable state in patients with atrial fibrillation.21 22 Nevertheless, cardiac thromboembolism causes most atrial fibrillation related strokes, so patients are also at increased risk of non-central nervous system systemic embolism.
The association between atrial fibrillation and stroke led to the pursuit of treatments that could reduce this risk. Several randomized trials established the efficacy of antithrombotic drugs for preventing stroke in patients with atrial fibrillation, with both antiplatelet agents and oral anticoagulants showing benefit. Concurrently, risk factors were identified that could stratify patients with atrial fibrillation on the basis of stroke risk, as a way to pinpoint those patients most likely to benefit from anticoagulation despite the increased risks of bleeding.
Risk stratification for stroke, bleeding, and net clinical benefit
The risk of stroke conveyed by atrial fibrillation is not uniform; event rates vary across populations and subgroups. The first observation, in the Framingham cohort, was the pronounced increase in risk for patients with rheumatic heart disease and atrial fibrillation.4 This association, predominantly related to rheumatic mitral stenosis, has led to the discrete characterization of “valvular atrial fibrillation.” Further risk stratification in these patients is not helpful—their risk is very high irrespective of additional risk factors. In contrast, for patients with non-valvular atrial fibrillation, additional risk stratification can yield better resolution of estimated event rates and identify patients at higher versus lower risk.
Stroke risk scores
The most widely used algorithm for estimation of stroke risk in patients with non-valvular atrial fibrillation has been the CHADS2 score.23 It assigns 1 point to each of congestive heart failure, hypertension, age over 75 years, and diabetes mellitus; and 2 points for a history of stroke or transient ischemic attack. The score was developed from risk factors identified in clinical trials and was subsequently measured and validated in 1733 patients in the national registry of atrial fibrillation, a combined dataset of Medicare patients participating in a quality improvement initiative. The score robustly identifies patients at significantly increased risk of stroke and has been validated repeatedly in other cohorts.24
However, in an effort to improve risk stratification in seemingly low risk patients, the CHADS2 score was expanded to incorporate additional risk factors.25 The CHA2DS2-VASc score further stratified age (1 point for age 65-74 years, 2 points for age ≥75 years) and added 1 point each for female sex and the presence of vascular disease (coronary, peripheral, or aortic plaque on imaging). The CHA2DS2-VASc score was tested and validated in 1084 Euro Heart Survey patients with non-valvular atrial fibrillation who were not taking oral anticoagulation. It showed improved resolution of patients at the lowest scores—no patients with a CHA2DS2-VASc score of 0 had events during follow-up and the adjusted rate of stroke was 0.7% for patients with a score of 1 (fig 1).
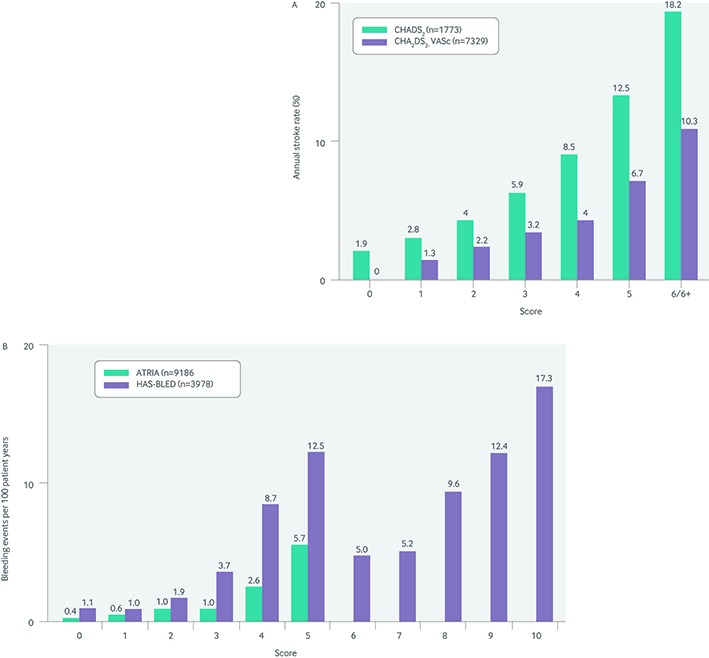
Fig 1 Event rates, according to scores on the various risk stratification algorithms, for (A) stroke and (B) bleeding23 25 26 27
However, the CHADS2 and CHA2DS2-VASc scores have limitations. Although they have been validated across many different cohorts of thousands of patients, the discriminatory power of the scores is limited (c statistics range 0.5-0.6). This may be partly because of the relatively narrow cohorts from which they were derived.24 28 In addition, although the CHA2DS2-VASc score improved discrimination at the lower end of the risk spectrum, stratification remains challenging—confidence intervals for the rate of stroke in patients with a CHA2DS2-VASc score of 1 range from 0% to 3.4%.25 Thus, guidelines committees have modified the CHA2DS2-VASc score further, downplaying the risk conveyed by a CHA2DS2-VASc score of only 1 when the single point comes from female sex.29 This is because studies have yielded conflicting estimates on the risk conveyed by female sex. In summary, risk factors can vary in the severity of risk they convey; and the same risk factor may not be equivalent across populations.24 28 30
Bleeding scores
Because the risk of stroke needs to be balanced against the risk of bleeding, several scoring algorithms have been developed to identify patients at highest risk of bleeding in the setting of oral anticoagulation. The most commonly cited algorithms are the ATRIA, HAS-BLED, and HEMORR2HAGES scores.26 27 31 Each combines several potential markers for future bleeding and has performed relatively well in large derivation and validation cohorts.
However, in clinical practice not all data points from these scores are readily available or calculable, and no robust prospective studies have shown any benefit for withholding anticoagulation on the basis of a high bleeding score. Furthermore, large observational datasets have failed to identify a group of patients whose risk of intracranial hemorrhage from anticoagulation outweighs the risk of stroke from withholding anticoagulation.32
Thus, it is difficult to identify patients with atrial fibrillation whose bleeding risk represents a true contraindication to antithrombotic therapy, and the decision is largely up to the subjective judgment of the individual provider (see box). Guidelines cite bleeding scores as potential tools to reduce the subjectivity involved in the decision but emphasize that these scores should not be the sole basis for a patient being excluded from treatment.33 Although stroke and bleeding risk factors overlap (for example, advanced age), current evidence suggests net clinical benefit for all but those with the highest risk of bleeding.32
Scores for risk stratification in atrial fibrillation
Stroke risk scores
-
CHADS223
Congestive heart failure (1 point)
Hypertension (1 point)
Age ≥75 years (1 point)
Diabetes (1 point)
Stroke or transient ischemic attack (2 points)
-
CHA2DS2-VASc25
Congestive heart failure (1 point)
Hypertension (1 point)
Age ≥75 (2 points)
Diabetes (1 point)
Stroke or transient ischemic attack (2 points)
Vascular disease (1 point)
Age 65-74 (1 point)
Sex category (1 point for female)
Bleeding risk scores
-
ATRIA27
• Anemia (3 points)
• Severe renal disease (3 points)
• Age ≥75 (2 points)
• Previous bleeding (1 point)
• Hypertension (1 point)
-
HAS-BLED26
• Hypertension (1 point)
• Abnormal liver or renal function (1 point each)
• Stroke (1 point)
• Bleeding (1 point)
• Labile international normalized ratio (1 point)
• Elderly: >65 years (1 point)
• Drugs or alcohol (1 point each)
Available agents
Warfarin
Vitamin K antagonists have been the mainstay of oral anticoagulation for nearly half a century, particularly for patients with atrial fibrillation. Several randomized clinical trials showed that warfarin was significantly better than placebo and antiplatelet agents (aspirin) for the prevention of stroke in patients with atrial fibrillation. The most important of these were the SPAF-I, SPAF-II, SPINAF, and AFASAK trials.34 35 36 37 In a meta-analysis of these and other randomized trials, warfarin reduced stroke in untreated patients at intermediate risk from 4.3% to 1.1% (1.4% for aspirin), and in high risk patients from 12% to 4% (10% for aspirin).38 An updated meta-analysis of 29 trials across comparators confirmed these findings; dose adjusted warfarin was associated with a 64% (95% confidence interval 49% to 74%) relative risk reduction for stroke compared with placebo and a 39% (22% to 52%) relative risk reduction compared with antiplatelet drugs.10
Warfarin was also compared with newer antiplatelet regimens, including the combination of aspirin and clopidogrel in the ACTIVE W trial.39 Warfarin was significantly better than dual antiplatelet therapy for the prevention of stroke, without a significant increased risk of bleeding. In fact, in patients who had previously taken warfarin, the risk of bleeding was numerically higher in the dual antiplatelet group. Therefore, despite the development of highly potent oral antiplatelet agents, warfarin remained the standard for stroke prevention in patients with atrial fibrillation through the turn of the century.
Subsequent analyses of the bleeding risk associated with warfarin have shown minimal additional risk in patients treated in the community, outside the setting of well conducted clinical trials. An observational study of 11 526 outpatients with non-valvular atrial fibrillation found a significant benefit of warfarin for the prevention of stroke or systemic embolism (51% reduction compared with no treatment or aspirin, 95% confidence interval 39% to 60%).9 Furthermore, although warfarin use was associated with a small but significant increased risk of intracranial hemorrhage (0.46 v 0.23 events/100 person years; adjusted hazard 1.97, 1.24 to 3.13), it was associated with lower all cause mortality (0.69, 0.61 to 0.77).
Dabigatran
In 2010, the direct thrombin inhibitor dabigatran etexilate became the first alternative to vitamin K antagonism approved for the prevention of stroke or systemic embolism in patients with non-valvular atrial fibrillation. The RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial, a prospective open label randomized trial compared dose adjusted warfarin to dabigatran at 150 mg twice daily or 110 mg twice daily in 18 113 patients with non-valvular atrial fibrillation.40 The primary endpoint of stroke or systemic embolism was similar between warfarin and dabigatran 110 mg (relative risk 0.91 for dabigatran 110 mg; P<0.001 for non-inferiority) and lower in patients receiving dabigatran 150 mg (0.66; P<0.001). Rates of major bleeding were lowest in the 110 mg dabigatran group (2.71%) and equivalent between warfarin (3.36%) and dabigatran 150 mg (3.11%; P=0.31 for warfarin v dabigatran 150 mg), whereas intracranial hemorrhage was highest in the warfarin group (0.31 for dabigatran 110 mg, 0.4 for dabigatran 150 mg; P<0.001 for both comparisons with warfarin). Risk of gastrointestinal bleeding was significantly higher in patients receiving both doses of dabigatran compared with warfarin (1.50 for dabigatran 150 mg, 1.36 for dabigatran 110 mg; P<0.001 and P=0.007, respectively). Largely on the basis of this trial, dabigatran was approved in the US at doses of 150 mg and 75 mg (for patients with creatinine clearance 15-30 mL/min/1.73 m2) and in the European Union at doses of 110 and 150 mg (dose based on clinical judgment).41 42
Several additional analyses have provided further data on the use of dabigatran. Firstly, the efficacy of the 150 mg dose and the safety of the 110 mg dose were both consistent with the overall trial across the spectrum of warfarin management quality (as assessed by time in therapeutic range).43 An in-depth review of intracerebral haemorrhage events in the RE-LY trial found a similar spectrum of severity of bleeding between warfarin and dabigatran.44 Patients in this trial who needed an invasive procedure had equivalent rates of periprocedural bleeding events (after carefully scripted discontinuation and restart of their anticoagulant),45 although this has not been a consistent finding across procedures and anticoagulation indications.46
In an observational extension of the RE-LY trial, the RELY-ABLE (Long-term Multicenter Extension of Dabigatran Treatment in Patients with Atrial Fibrillation) trial demonstrated efficacy of dabigatran up to 28 months beyond the original RE-LY follow-up period, with a consistent lower risk of bleeding in patients taking 110 mg (v 150 mg).47
Of note, the primary results of the RE-LY trial suggested a potential signal for increased risk of myocardial ischemic events. A subsequent meta-analysis of dabigatran trials across disease states confirmed such an association,48 although a mechanism has yet to be confirmed. To date, observational “real world” cohort studies of dabigatran users have demonstrated safety and effectiveness consistent with the RE-LY trial, without a signal for increased risk of myocardial events or higher than expected bleeding.49 50 51 Further investigation of this potential signal for myocardial events is warranted.
Rivaroxaban
In 2011, rivaroxaban became the first oral factor Xa inhibitor approved for the prevention of stroke in patients with non-valvular atrial fibrillation. The ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism) trial is a double blind double dummy trial, which randomized 14 246 patients with non-valvular atrial fibrillation to dose adjusted warfarin (international normalized ratio (INR) 2-3) or rivaroxaban, 20 mg daily (15 mg for creatinine clearance 30-49 mL/min/1.73 m2).52 This trial enrolled a population with a moderate to high risk of stroke (mean CHADS2 score 3.5; 55% had previous stroke or transient ischemic attack). In the intention to treat analysis, rivaroxaban was non-inferior to warfarin for the endpoint of stroke or systemic embolism (1.7% for rivaroxaban v 2.2% for warfarin; P<0.001 for non-inferiority).53 Overall major and clinically relevant non-major bleeding was similar between rivaroxaban and warfarin (14.9 v 14.5 events/100 patient years; P=0.44), although rates of intracerebral hemorrhage were significantly lower with rivaroxaban (hazard ratio 0.67; P=0.02). In contrast, gastrointestinal bleeding was significantly more common in the rivaroxaban group (3.15% v 2.16%; P<0.001). The treatment effects were consistent in patients with renal impairment treated with the 15 mg dose.54
When interpreting the ROCKET AF data, it should be noted that the time in therapeutic range (a well known marker of anticoagulation quality55 56 57) in those randomized to warfarin was lower than in other randomized trials of anticoagulation therapy (mean 55% of the time). However, the treatment effects of rivaroxaban were consistent over the spectrum of low and high time in therapeutic range.58 Secondly, the number of strokes in the rivaroxaban group increased after mandatory discontinuation of rivaroxaban at the end of the trial. This increase in stroke events was attributed to the delayed therapeutic effect of transitioning to open label warfarin (median 13 days to therapeutic INR when transitioning from rivaroxaban v 3 days in the warfarin arm).59 60 On the basis of these and other data, careful attention to the transition from a short acting anticoagulant to warfarin is warranted, regardless of the agent(s) used.61
An additional randomized trial of rivaroxaban was performed separately in Japan, the J-ROCKET AF trial.62 This was done for two reasons—firstly, pharmacokinetic data suggested that Japanese patients required lower dosing to achieve target drug concentrations; and, secondly, anticoagulation goals are generally lower in Japanese practice. The investigators randomized 1280 patients to rivaroxaban 15 mg daily or dose adjusted warfarin (INR goals 2-3 for patients <70 years, 1.6-2.6 for age >70 years). Rivaroxaban was non-inferior for the efficacy endpoint of stroke or systemic embolism (hazard ratio 0.49 for rivaroxaban, 0.24 to 1.00), and the safety endpoint of major bleeding or non-major clinically relevant bleeding (1.11 for rivaroxaban; P<0.001 for non-inferiority). Finally, rivaroxaban is the only once daily dosed novel anticoagulant available for stroke prevention in patients with atrial fibrillation.63
Apixaban
In 2012, apixaban became the second factor Xa inhibitor to be approved for patients with non-valvular atrial fibrillation. It was shown to be safe and effective in two large phase III trials of patients with non-valvular atrial fibrillation: the AVERROES (Apixaban Versus Acetylsalicylic Acid (ASA) to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment) trial and the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial.
In the AVERROES trial, 5599 patients with non-valvular atrial fibrillation, who were deemed by their physician not to be candidates for warfarin (40% had previously taken warfarin), were blindly randomized to daily aspirin or twice daily apixaban (5 mg).64 Reasons for not using warfarin included: difficulty with performing INR testing (43%); inability to maintain appropriate INR therapeutic range (42%); only moderate risk for stroke (CHADS2 score 1, 21%); and refusal to take warfarin (37%; sole reason in 15%). The trial was stopped prematurely, and at one year follow-up the primary outcome of stroke or systemic embolism significantly favored apixaban (1.6% v 3.7%; P<0.001). Rates of bleeding were low, but statistically equivalent between the two groups (hazard ratio for apixaban 1.13, 0.74 to 1.75; P=0.57), including gastrointestinal bleeding (0.86, 0.40 to 1.86; P=0.71).
In the ARISTOTLE trial, 18 201 patients with non-valvular atrial fibrillation were blindly randomized to dose adjusted warfarin (INR 2-3) or apixaban 5 mg twice daily.65 Patients with at least two of three select risk factors (age ≥80 years, weight ≤60 kg, and serum creatinine ≥1.5 mg/dL; 1 mg/dL=88.4 µmol/L) received 2.5 mg twice daily. After a median follow-up of 1.8 years, apixaban was superior for the reduction of stroke or systemic embolism (hazard ratio for apixaban 0.79; P=0.01 for superiority). Major bleeding occurred in 2.13% of patients taking apixaban each year versus 3.09% in those taking warfarin (P<0.001); gastrointestinal bleeding was equivalent between the groups (0.89, 0.70 to 1.15; P=0.37). Furthermore, the ARISTOTLE trial was the only study of an approved novel anticoagulant to show significantly lower rates of all cause mortality in the novel anticoagulant group (3.52% v 3.94%; P=0.047); however, a non-significant trend has been consistent in trials of other novel agents. Additional analyses of ARISTOTLE have shown consistently positive results across different types of atrial fibrillation (paroxysmal v persistent),66 degrees of warfarin anticoagulation control (time in therapeutic range),67 and stroke and bleeding risk score groups.68
Emerging treatments
In addition to the approved agents above, several anticoagulants are at various stages of development. The most advanced is edoxaban, another oral factor Xa inhibitor. The ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction) trial is a randomized double blind double dummy trial that compared two different once daily doses (30 mg and 60 mg) to dose adjusted warfarin.69 A total of 21 105 patients with moderate to high risk non-valvular atrial fibrillation were randomized, and the trial mandated a dose reduction (by half) for patients with any of the following: creatinine clearance 30-50 mL/min; weight ≤60 kg; or concomitant use of verapamil, quinidine, or dronedarone. Rates of stroke or systemic embolism were similar to those of warfarin for edoxaban 30 mg (hazard ratio 1.07; P=0.005 for non-inferiority) and 60 mg (0.79; P<0.001 for non-inferiority), with an increased risk of ischemic stroke in the low dose edoxaban group compared with warfarin (1.77; P<0.001). Compared with warfarin, rates of major bleeding were lower for both doses (30 mg edoxaban 0.47, 60 mg edoxaban 0.80; P<0.001 for both), and mortality was lower for low dose edoxaban (0.87; P=0.006), with a similar trend for high dose edoxaban (0.92; P=0.08).
Other novel oral anticoagulants are at earlier stages of development, including the factor Xa inhibitors betrixaban and darexaban,70 71 and the direct thrombin inhibitor AZD0837.72 Interventional treatments for stroke prevention are also emerging, including left atrial appendage closure devices.73 74 75 76 77
Comparative pharmacology and choosing an agent
Warfarin has relatively non-specific anticoagulant properties, inhibiting the production of factors II, VII, IX, and X. Although this mechanism leads to its notorious long time to onset and offset, warfarin has robust therapeutic effectiveness across many disease states.78 79 Furthermore, this pharmacodynamic profile is relatively forgiving of missed doses or poor adherence—the anticoagulant effect persists for days after the last warfarin dose. In addition, clearance of warfarin is not affected by renal function, so it can be used in patients across the spectrum of kidney disease.
For these reasons, warfarin remains the preferred anticoagulant for several specific patient groups. Firstly, patients with concomitant valve disease, including valve replacement or valvular atrial fibrillation (current or previous mitral stenosis), should be treated only with warfarin. Current data do not support the use of any of the novel anticoagulants in these patients. The only randomized trial of dabigatran in mechanical valves to date was halted prematurely owing to an increased risk of thrombosis and bleeding in patients assigned to dabigatran (v warfarin).46
Secondly, patients with the most severe renal dysfunction (estimate glomerular filtration rate <30 mL/min/1.73 m2) were not included in randomized trials of novel anticoagulants, so these agents should be avoided in such patients. Lastly, providers may choose to use warfarin in patients for whom the ability to readily and objectively monitor the extent of anticoagulation is paramount (for example, for adherence or safety reasons). However, in the remaining patients with atrial fibrillation, the choice of anticoagulant for stroke prevention can be tailored to individual needs and may include new drugs. Regardless of the ultimate choice, the selection of anticoagulant should always be patient centered—no single approach is optimal for all patients, and the subtleties of each patient’s characteristics and preferences should be considered.
The novel anticoagulants (dabigatran, rivaroxaban, apixaban, and edoxaban) have been shown collectively to be safer (for avoiding intracerebral hemorrhage) and more effective than warfarin,80 leading some guidelines to recommend them preferentially.33 However, these drugs have much shorter time to onset and offset compared with warfarin, and each exhibits some level of renal clearance (table). In addition, none of these agents can be easily monitored using readily available commercial assays for anticoagulation. However, there are additional drug specific considerations when selecting among them. Although some characteristics may favor one agent over another, no prospective randomized head to head comparisons have been made, so any conclusions about comparative safety and effectiveness are putative and remain inadequately tested. Indirect comparisons of these agents have been performed, but caution is needed when interpreting the results.86 87 Characteristics of individual patients or drugs that may favor one agent over another are therefore subject to provider judgment.
Pharmacologic properties of approved anticoagulants available for prevention of thromboembolism in atrial fibrillation*
| Property | Drug | |||
|---|---|---|---|---|
| Warfarin | Dabigatran | Rivaroxaban | Apixaban | |
| Mechanism | Vitamin K antagonist | Direct thrombin inhibitor | Factor Xa inhibitor | Factor Xa inhibitor |
| Dosing† | Variable (dose adjusted on the basis of international normalised ratio) | 150 mg; 110 mg bid (Europe only); 75 mg bid for creatinine clearance‡ 15-30 (US only), not recommended if <15 | 20 mg daily; 15 mg daily for creatinine clearance‡ 15-50, not recommended if <15 | 5 mg bid; 2.5 mg bid for patients with >2 of the following: creatinine ≥133 µmol/L, age ≥80 years, or weight ≤60 kg; creatinine clearance‡ <15: no data available |
| Oral bioavailability | 100% | 3-7% | 60% | 58% |
| Time to effect (h) | 72-96 | 1-2 | 2-4 | 3-4 |
| Half life (h) | ~40 | 12-17 | 5-9 | 8-15 |
| Notable drug interactions | Numerous | Strong P-glycoprotein inducers | ||
| Strong P-glycoprotein inhibitors with concomitant kidney dysfunction | Strong P-glycoprotein inhibitors; strong cytochrome P450 inducers and inhibitors | |||
*As detailed in their regulatory approval packages (may differ from clinical trials protocols).41 63 81-85
†Patients with creatinine clearance <30 mL/min/1.73 m2 were not included in any of the clinical trials of novel oral anticoagulants.
‡Creatinine clearance measured in mL/min/1.73 m2.
Abbreviation: bid=twice daily.
Competing risks
All anticoagulants increase the risk of bleeding. However, apixaban is the only agent to demonstrate safety and efficacy in a randomized trial of patients thought to be suboptimal candidates for warfarin (the AVERROES trial).64 In these patients, apixaban showed greater efficacy and equivalent safety compared with aspirin. Furthermore, the ARISTOTLE trial showed similar results with a reduced dosing regimen of apixaban in patients at particular risk of bleeding (based on age, weight, and serum creatinine, as noted above).65 Thus, in patients considered at higher risk of bleeding and those who are not candidates for vitamin K antagonism, apixaban may have several advantages, particularly with respect to bleeding.
By contrast, for patients at particular risk of ischemic stroke, dabigatran may be the most suitable novel anticoagulant. Although apixaban and rivaroxaban prevented more strokes than warfarin through a reduction in the number of hemorrhagic events, dabigatran was the only one of the three drugs that also significantly reduced non-hemorrhagic stroke events (as seen in the RE-LY trial).40 However, patients at high risk of non-hemorrhagic stroke may also be at high risk of acute coronary syndromes, and these risks must be balanced. Because of the observed association between dabigatran and increased risk of myocardial infarction, some investigators advocate that dabigatran should be avoided in patients with, or at risk of, coronary artery disease.48 It is important to note though, that the association between dabigatran and acute coronary events stems from retrospective analyses of randomized trials, and subsequent observational data have been conflicting.49 50 51
Other populations
Patients with chronic kidney disease represent a challenging population for the prescription and management of anticoagulation. Chronic kidney disease increases the risks of both stroke and bleeding,27 88 and patients with advanced disease are often under-represented in clinical trials. Each of the novel anticoagulant trials excluded patients with the most severe renal dysfunction, although both the ROCKET AF and ARISTOTLE trials tested reduced dosing of the drugs that they were testing.
In the ROCKET AF trial, the dose of rivaroxaban was reduced to 15 mg daily in patients with creatinine clearance of 30-49 mL/min/1.73 m2 (and received regulatory approval down to 15 mL/min/1.73 m2).53 63 In the ARISTOTLE trial, the dose of apixaban was reduced to 2.5 mg twice daily for patients with two or more of the following: age ≥80 years, body weight ≤60 kg, or serum creatinine ≥133 µmol/L.65 Although the use of serum creatinine as a measure of renal function has limitations, it does provide supporting data for the use of apixaban in such patients.
For dabigatran, pharmacokinetic studies were used to identify the preferred, renally adjusted dose in the US (75 mg), as is common practice for the Food and Drug Administration.89 Thus, although some dose adjustment for renal impairment is available for each of the novel anticoagulants, the evidence base for such recommendations varies, and this should be taken into account for patients with marginal kidney function.
Although as a group novel agents have fewer drug-drug and food-drug interactions than warfarin, there are specific, major interactions to be aware of. Antifungal agents almost all have major interactions with novel anticoagulants, as do HIV protease inhibitors and rifampin (US) and rifampicin (UK) (although fewer data are available). In addition, both rivaroxaban and apixaban interact with drugs that are strong inhibitors or inducers of cytochrome P450 3A4. More specific interactions include those between dabigatran and verapamil, quinidine, amiodarone, and dronedarone. However, data on interactions with rivaroxaban or apixaban are more limited,81 and many drugs have yet to be rigorously tested for interactions with these newer anticoagulants. The requirement for long term concomitant treatment with an interacting agent may guide the choice of anticoagulant in certain patients (table 1).
Additional considerations
Overall, the novel oral anticoagulants are well tolerated with few common side effects; however, dyspepsia is a problem in about 10% of patients receiving dabigatran and is relieved by a change of drug.40 Thus, it may be best to avoid dabigatran in patients with known dyspepsia, reflux, or gastrointestinal motility disease. Apixaban is the only approved novel anticoagulant that is not associated with an increased risk of gastrointestinal bleeding. Adherence can be a challenge for patients managing anticoagulation. In those patients who find it difficult to manage multiple daily dosages or monitoring, rivaroxaban is the only once daily alternative to warfarin currently available. Lastly, in regions lacking a single payer healthcare system, cost may influence the decision of which, if any, novel anticoagulant to use. To date, cost effectiveness data have favored novel anticoagulants (mostly because they do not need to be monitored),90 and as more become available they may become more affordable.
Patients stable on warfarin
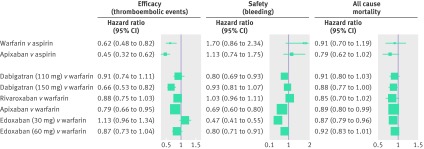
The management of patients who are stable on warfarin is a challenge for clinicians. The decision to switch a stable patient (with adequate time in therapeutic range) to a new drug should be individually tailored; however, there are several considerations. Most importantly, clinical data to date show that novel oral anticoagulants are more efficacious and safe than dose adjusted warfarin (fig 2). Furthermore, stability on warfarin is highly variable—many patients may tolerate the drug but their time in therapeutic range is insufficient to confer a clinical benefit.43 55 58 67
Fig 2 Evidence from major randomized comparisons of anticoagulants for stroke prevention in patients with atrial fibrillation.10 40 53 64 65 69 The efficacy endpoint includes stroke or systemic embolism (except warfarin v aspirin, stroke only). Safety includes major bleeding, as defined by the trial (except warfarin v aspirin, extracranial bleeding only). Estimates for warfarin versus aspirin are approximate conversions from risk reduction to relative risk (hazard). ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism trial) efficacy includes intention to treat analysis; safety and mortality include the on treatment population. ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48 trial) efficacy includes intention to treat analysis. CI=confidence interval
Providers should consider exactly how well controlled the INR is in these patients because novel drugs may be more beneficial in patients with poor control on warfarin.55
Nevertheless, switching has additional implications for the individual patient, who may be accustomed to warfarin and reluctant to fix “what isn’t broke.” For some, an agent that does not require repeated blood draws or dietary restrictions will be appealing. However, all patients should be advised about the availability of these new drugs, their risks, and their benefits and should be offered these alternatives where clinically appropriate. Patients’ engagement and subsequent adherence are paramount to the effective implementation of these newer treatments.
Adherence, planned interruptions, and transitions between drugs
Adherence to oral anticoagulation, particularly vitamin K antagonists, is a major problem. Vitamin K antagonists have myriad drug-drug and drug-food interactions that can dramatically modify the pharmacologic effect, so regular blood sampling and monitoring are required. However, novel oral anticoagulants do not require monitoring and interact with fewer foods and drugs. Yet because commercial assays specific to their activity are not widely available, adherence cannot be easily and objectively measured. Generic coagulation assays (such as prothrombin time and partial thromboplastin time) can be evaluated in patients taking these drugs, but they are not drug or dose specific.63 91
Management of planned and unplanned interruptions in chronic anticoagulation can be difficult. Data suggest a low risk of thrombotic events in patients who stop treatment for a short time (≤5 days),92 and trials have failed to show a benefit of bridging anticoagulation therapy with shorter acting drugs (a larger, more conclusive trial is ongoing).93 However, US guidelines call for a tailored approach because subgroups of patients seem to be at serious risk of short term thromboembolic events in the absence of anticoagulation.78 They recommend bridging in patients at high risk of thromboembolic events, such as those who previously experienced an event while not receiving anticoagulation. The guidelines also state that bridging may be considered in those at moderate risk, such as patients with an increased CHADS2 score but no high risk features.94 Nonetheless, there may be risks to starting and stopping multiple anticoagulants with varying degrees of overlap.
The bridging paradigm is less applicable to novel anticoagulants. Unlike warfarin, which takes a long time to reach maximum effect and has a long half life, newer agents exert systemic anticoagulation within hours—a similar pharmacodynamic profile to agents used for bridging (such as low molecular weight heparins). Thus, there is probably little benefit in substituting a low molecular weight heparin for one of the novel agents. Furthermore, early use of a novel anticoagulant may put patients at risk in the postoperative period—whereas warfarin can be started shortly after surgery with little effect on hemostasis for several days, newer anticoagulants could precipitate bleeding acutely within hours. This is why labels contain warnings against restarting these drug soon after certain types of invasive procedures (for example, neurosurgery and spinal procedures).41 84 95
Retrospective analyses of currently available randomized trials show favorable outcomes in patients taking novel anticoagulants who undergo invasive procedures when managed carefully. In 4591 patients in the RE-LY trial who underwent an invasive procedure, rates of major bleeding from a week before the procedure to 30 days after were similar across treatment groups (3.8% for dabigatran 110 mg, 5.1% for dabigatran 150 mg, and 4.6% for warfarin; P>0.05 for each two way comparison).45 Of note, in this open label trial patients receiving dabigatran stopped the agent a median of 49 (35-85) hours before the procedure, compared with 114 (87-144) hours for patients treated with warfarin (P<0.001). Similar analyses from the ROCKET AF and ARISTOTLE trials are forthcoming, and preliminary results have consistently yielded favorable findings.61 96
Management of bleeding events
The management of bleeding in the setting of systemic anticoagulation is an ongoing clinical challenge. In patients receiving warfarin, providers have long experience with reversing the agent using blood products (acutely and temporarily) or replacing vitamin K (gradually and permanently). However, the clinical evidence to support these approaches is not robust. Although they can improve laboratory markers of systemic anticoagulation (INR), their impact on durable clinical outcomes has not been firmly established.97 Nevertheless, the availability of, and experience with, such reversal strategies serve as a comfort to many clinicians, particularly interventional and surgical specialists.
By contrast, none of the novel oral anticoagulants (direct thrombin inhibitors or factor Xa inhibitors) has an effective reversal strategy that is supported by prospective data. Several approaches have been tested, including recombinant hemostatic factor concentrates and developmental small molecules.91 95 98 However, studies are limited by their observational nature, small sample size, and lack of long term durable outcomes. None of these compounds is commercially available for this indication. Although more robust trials are planned, the limited data to date do not show increased morbidity or mortality in patients receiving novel oral anticoagulants who experience bleeding events compared with those taking warfarin.
The most recent large analysis of bleeding in patients receiving a novel anticoagulant included a combined population of more than 1000 patients with bleeding events from five phase III clinical trials of dabigatran versus warfarin.99 It found that patients receiving dabigatran required more red blood cell transfusions, although they had lower rates of plasma transfusion and shorter stays in intensive care. Mortality at 30 days was lower in patients receiving dabigatran who experienced bleeding (9.1% v 13.0%; adjusted odds ratio 0.66; P=0.051). These data are limited because they are confined to the well controlled randomized trial population. Although rates of bleeding in several large observational analyses of dabigatran have not been higher than for warfarin,50 conclusions regarding the outcomes in these patients remain tentative. Furthermore, similar data are not yet available for the factor Xa inhibitors.
Therefore, although guidelines include the reversal of vitamin K antagonists in patients who are bleeding,78 recommendations for patients taking novel oral anticoagulants are mostly limited to supportive care (including volume resuscitation, hemodynamic support, and primary intervention).33 The administration of plasma is unlikely to be useful in patients without a primary coagulopathy. Factor concentrates have attracted much attention but remain untested, and they carry a serious risk of thrombotic complications (stroke) that counterbalances their antihemorrhagic properties.98
Summary
Thromboembolism is the major source of morbidity and mortality in patients with atrial fibrillation. Oral anticoagulation is beneficial in patients at risk for stroke. However, safe and effective implementation of oral anticoagulation requires appropriate risk stratification. Additional research is needed on stratifying the risk of bleeding in these patients. Novel oral anticoagulants represent an important breakthrough in medical treatment and are generally more effective than warfarin, but the agent and dose must be chosen carefully. Although these agents may reduce the most serious intracranial bleeding, management of hemorrhage in patients taking anticoagulants remains a challenge and further development of reversal strategies is a priority.
Guidelines
Anticoagulation is universally recommended for patients with valvular atrial fibrillation or those with mechanical heart valves (who do not have a contraindication).27 100 The most recent US guidelines for patients with non-valvular atrial fibrillation recommend stroke prophylaxis with oral anticoagulation for patients with a CHADS2 score of 2 or more and state that prophylaxis is reasonable for patients with a CHADS2 score of 1. In female patients with coronary artery disease or age 65-74 (CHA2DS2-VASc components), the guidelines suggest that anticoagulation is considered and weighed against its risks.100 However, an update to these guidelines is expected imminently. The most recent European guidelines for patients with non-valvular atrial fibrillation recommend no treatment for patients with a CHA2DS2-VASc score of 0; oral anticoagulation with warfarin or a novel anticoagulant (preferred) is recommended for those with a CHA2DS2-VASc score of 2 or more. In patients with a CHA2DS2-VASc score of 1, oral anticoagulation therapy should be considered and weighed against the risks (except for women under 65 years of age, in whom it is reasonable to withhold treatment). Lastly, in patients who decline oral anticoagulation, dual antiplatelet therapy with aspirin and clopidogrel is a reasonable alternative.33
Research questions
What is the benefit of oral anticoagulation for stroke prevention in patients with atrial fibrillation at low to moderate risk of stroke?
What role should formal assessment of bleeding risk play in the treatment decision?
What is the comparative effectiveness of the novel oral anticoagulants in patients with atrial fibrillation being treated in the community?
What is the optimal management of bleeding in patients receiving oral anticoagulation?
Contributors: BAS performed the literature searches and compiled source documents. BAS and JPP jointly interpreted the data and drafted and revised the manuscript. Both authors approve the final version of the manuscript and are guarantors. The authors certify that the manuscript represents their own work and are solely responsible for its content.
Competing interests: We have read and understood the BMJ Group policy on declaration of interests and declare the following interests: JPP discloses institutional research grant support from Janssen Scientific, GE Healthcare, ARCA pharmaceuticals, and ResMed; and consulting relationships with Johnson & Johnson, Pfizer/BMS, Medtronic, and Spectranetics. BAS was funded by NIH T-32 training grant #5 T32 HL 7101-38.
Provenance and peer review: Commissioned; externally peer reviewed.
Cite this as: BMJ 2014;348:g2116
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA 2001;285:2370-5. [DOI] [PubMed] [Google Scholar]
- 2.Conen D, Chae CU, Glynn RJ, Tedrow UB, Everett BM, Buring JE, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA 2011;305:2080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946-52. [DOI] [PubMed] [Google Scholar]
- 4.Wolf PA, Dawber TR, Thomas HE Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology 1978;28:973-7. [DOI] [PubMed] [Google Scholar]
- 5.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med 1987;147:1561-4. [PubMed] [Google Scholar]
- 6.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27:1760-4. [DOI] [PubMed] [Google Scholar]
- 7.Tu HT, Campbell BC, Christensen S, Collins M, De Silva DA, Butcher KS, et al. Pathophysiological determinants of worse stroke outcome in atrial fibrillation. Cerebrovasc Dis 2010;30:389-95. [DOI] [PubMed] [Google Scholar]
- 8.McGrath ER, Kapral MK, Fang J, Eikelboom JW, O’Conghaile A, Canavan M, et al. Association of atrial fibrillation with mortality and disability after ischemic stroke. Neurology 2013;81:825-32. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003;290:2685-92. [DOI] [PubMed] [Google Scholar]
- 10.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67. [DOI] [PubMed] [Google Scholar]
- 11.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, et al. Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke 2012;43:1795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 2014;129:837-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd-Jones DM. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042-46. [DOI] [PubMed] [Google Scholar]
- 14.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes 2012;5:85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 2001;86:516-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation 2013;128:2470-7. [DOI] [PubMed] [Google Scholar]
- 17.Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation. 30-year follow-up in the Framingham study. JAMA 1985;254:3449-53. [PubMed] [Google Scholar]
- 18.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8. [DOI] [PubMed] [Google Scholar]
- 19.Anderson DC, Kappelle LJ, Eliasziw M, Babikian VL, Pearce LA, Barnett HJ. Occurrence of hemispheric and retinal ischemia in atrial fibrillation compared with carotid stenosis. Stroke 2002;33:1963-7. [DOI] [PubMed] [Google Scholar]
- 20.Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. The stroke prevention in atrial fibrillation investigators committee on echocardiography. Ann Intern Med 1998;128:639-47. [DOI] [PubMed] [Google Scholar]
- 21.Uno M, Tsuji H, Sawada S, Toyoda T, Nakagawa M. Fibrinopeptide A (FPA) levels in atrial fibrillation and the effects of heparin administration. Jpn Circ J 1988;52:9-12. [DOI] [PubMed] [Google Scholar]
- 22.Mitusch R, Siemens HJ, Garbe M, Wagner T, Sheikhzadeh A, Diederich KW. Detection of a hypercoagulable state in nonvalvular atrial fibrillation and the effect of anticoagulant therapy. Thromb Haemost 1996;75:219-23. [PubMed] [Google Scholar]
- 23.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001;285:2864-70. [DOI] [PubMed] [Google Scholar]
- 24.Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010;137:263-72. [DOI] [PubMed] [Google Scholar]
- 26.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093-100. [DOI] [PubMed] [Google Scholar]
- 27.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011;58:395-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish atrial fibrillation cohort study. Eur Heart J 2012;33:1500-10. [DOI] [PubMed] [Google Scholar]
- 29.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429. [DOI] [PubMed] [Google Scholar]
- 30.Keogh C, Wallace E, Dillon C, Dimitrov BD, Fahey T. Validation of the CHADS2 clinical prediction rule to predict ischaemic stroke. A systematic review and meta-analysis. Thromb Haemost 2011;106:528-38. [DOI] [PubMed] [Google Scholar]
- 31.Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, et al. Clinical classification schemes for predicting hemorrhage: results from the national registry of atrial fibrillation (NRAF). Am Heart J 2006;151:713-9. [DOI] [PubMed] [Google Scholar]
- 32.Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation 2012;125:2298-307. [DOI] [PubMed] [Google Scholar]
- 33.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47. [DOI] [PubMed] [Google Scholar]
- 34.Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet 1994;343:687-91. [PubMed] [Google Scholar]
- 35.Design of a multicenter randomized trial for the Stroke Prevention in Atrial Fibrillation Study. The Stroke Prevention in Atrial Fibrillation Investigators. Stroke 1990;21:538-45. [DOI] [PubMed] [Google Scholar]
- 36.Ezekowitz MD, Bridgers SL, James KE, Carliner NH, Colling CL, Gornick CC, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med 1992;327:1406-12. [DOI] [PubMed] [Google Scholar]
- 37.Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet 1989;1:175-9. [DOI] [PubMed] [Google Scholar]
- 38.Ezekowitz MD, Levine JA. Preventing stroke in patients with atrial fibrillation. JAMA 1999;281:1830-5. [DOI] [PubMed] [Google Scholar]
- 39.Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367:1903-12. [DOI] [PubMed] [Google Scholar]
- 40.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [DOI] [PubMed] [Google Scholar]
- 41.Boehringer-Ingelheim. Dabigatran prescribing information. Secondary dabigatran prescribing information 2010. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf.
- 42.Boehringer-Ingelheim. Dabigatran prescribing information for stroke prevention in atrial fibrillation in the United Kingdom. 2013. www.pradaxa.co.uk/downloads/pi-spaf.pdf.
- 43.Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010;376:975-83. [DOI] [PubMed] [Google Scholar]
- 44.Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, Reilly PA, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke 2012;43:1511-7. [DOI] [PubMed] [Google Scholar]
- 45.Healey JS, Eikelboom J, Douketis J, Wallentin L, Oldgren J, Yang S, et al. Peri-procedural bleeding and thromboembolic events with dabigatran compared to warfarin: results from the RE-LY randomized trial. Circulation 2012;126:343-8. [DOI] [PubMed] [Google Scholar]
- 46.Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013;369:1206-14. [DOI] [PubMed] [Google Scholar]
- 47.Connolly SJ, Wallentin L, Ezekowitz MD, Eikelboom J, Oldgren J, Reilly PA, et al. The long-term multicenter observational study of dabigatran treatment in patients with atrial fibrillation (RELY-ABLE) study. Circulation 2013;128:237-43. [DOI] [PubMed] [Google Scholar]
- 48.Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012;172:397-402. [DOI] [PubMed] [Google Scholar]
- 49.Larsen TB, Rasmussen LH, Skjoth F, Due KM, Callreus T, Rosenzweig M, et al. Efficacy and safety of dabigatran etexilate and warfarin in “real-world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol 2013;61:2264-73. [DOI] [PubMed] [Google Scholar]
- 50.Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med 2013;368:1272-4. [DOI] [PubMed] [Google Scholar]
- 51.Sorensen R, Gislason G, Torp-Pedersen C, Olesen JB, Fosbol EL, Hvidtfeldt MW, et al. Dabigatran use in Danish atrial fibrillation patients in 2011: a nationwide study. BMJ Open 2013;3:2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.ROCKET AF Study Investigators. Rivaroxaban-Once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J 2010;159:340-47. [DOI] [PubMed] [Google Scholar]
- 53.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. [DOI] [PubMed] [Google Scholar]
- 54.Fox KA, Piccini JP, Wojdyla D, Becker RC, Halperin JL, Nessel CC, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 2011;32:2387-94. [DOI] [PubMed] [Google Scholar]
- 55.Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008;118:2029-37. [DOI] [PubMed] [Google Scholar]
- 56.Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 2009;124:37-41. [DOI] [PubMed] [Google Scholar]
- 57.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993;69:236-9. [PubMed] [Google Scholar]
- 58.Food and Drug Administration. FDA draft briefing document for the cardiovascular and renal drugs advisory committee (CRDAC): rivaroxaban for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation. 2011. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/drugs/CardiovascularandRenalDrugsAdvisoryCommittee/ucm270796.pdf.
- 59.Patel MR, Hellkamp AS, Lokhnygina Y, Piccini JP, Zhang Z, Mohanty S, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013;61:651-8. [DOI] [PubMed] [Google Scholar]
- 60.Mahaffey KW, Hellkamp AS, Patel MR, Hannan KL, Schwabe K, Nessel CC, et al. End of study transition from study drug to open-label vitamin K antagonist therapy: the ROCKET AF experience. Circ Cardiovasc Qual Outcomes 2013;6:470-8. [DOI] [PubMed] [Google Scholar]
- 61.Lopes RD, Garcia DA, Wojdyla D, Dorian P, Alexander JH, Wallentin L, et al. Use of apixaban and warfarin in patients undergoing invasive procedures: insights from ARISTOTLE. Eur Heart J 2013;34(suppl 1) 10.1093/eurheartj/eht307.P535. [Google Scholar]
- 62.Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation—the J-ROCKET AF study. Circ J 2012;76:2104-11. [DOI] [PubMed] [Google Scholar]
- 63.Pharmaceuticals J. Rivaroxaban prescribing information. Secondary rivaroxaban prescribing information 2011. www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100.
- 64.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806-17. [DOI] [PubMed] [Google Scholar]
- 65.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. [DOI] [PubMed] [Google Scholar]
- 66.Al-Khatib SM, Thomas L, Wallentin L, Lopes RD, Gersh B, Garcia D, et al. Outcomes of apixaban vs warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J 2013;34:2464-71. [DOI] [PubMed] [Google Scholar]
- 67.Wallentin L, Lopes RD, Hanna M, Thomas L, Hellkamp A, Nepal S, et al. Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation 2013;127:2166-76. [DOI] [PubMed] [Google Scholar]
- 68.Lopes RD, Al-Khatib SM, Wallentin L, Yang H, Ansell J, Bahit MC, et al. Efficacy and safety of apixaban compared with warfarin according to patient risk of stroke and of bleeding in atrial fibrillation: a secondary analysis of a randomised controlled trial. Lancet 2012;380:1749-58. [DOI] [PubMed] [Google Scholar]
- 69.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104. [DOI] [PubMed] [Google Scholar]
- 70.Hashimoto T, Suzuki K, Kihara Y, Iwatsubo T, Miyashita A, Heeringa M, et al. Absorption, metabolism and excretion of darexaban (YM150), a new direct factor Xa inhibitor in humans. Xenobiotica 2013;43:534-47. [DOI] [PubMed] [Google Scholar]
- 71.Connolly SJ, Eikelboom J, Dorian P, Hohnloser SH, Gretler DD, Sinha U, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa). Eur Heart J 2013;34:1498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johansson S, Cullberg M, Eriksson UG, et al. Single-dose pharmacokinetics, pharmacodynamics and safety of AZD0837, a novel oral direct thrombin inhibitor, in young healthy male subjects. Int J Clin Pharmacol Ther 2011;49:258-67. [DOI] [PubMed] [Google Scholar]
- 73.Bartus K, Han FT, Bednarek J, Elg M, Duner K, Jensen E, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62:108-18. [DOI] [PubMed] [Google Scholar]
- 74.Bartus K, Bednarek J, Myc J, Myc J, Kapelak B, Sadowski J, et al. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm 2011;8:188-93. [DOI] [PubMed] [Google Scholar]
- 75.Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber K, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) trial. Circulation 2013;127:720-9. [DOI] [PubMed] [Google Scholar]
- 76.Reddy VY, Holmes D, Doshi SK, , Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the continued access registry. Circulation 2011;123:417-24. [DOI] [PubMed] [Google Scholar]
- 77.Landmesser U, Holmes DR Jr. Left atrial appendage closure: a percutaneous transcatheter approach for stroke prevention in atrial fibrillation. Eur Heart J 2012;33:698-704. [DOI] [PubMed] [Google Scholar]
- 78.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis (9th ed). American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141(suppl 2):7S-47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed). Chest 2008;133(suppl 6):546S-92S. [DOI] [PubMed] [Google Scholar]
- 80.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2013; published 4 Dec. [DOI] [PubMed]
- 81.Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, et al. European Heart Rhythm Association practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625-5. [DOI] [PubMed] [Google Scholar]
- 82.Squibb B-M. Apixaban prescribing information. Secondary apixaban prescribing information 2012. http://packageinserts.bms.com/pi/pi_eliquis.pdf.
- 83.Perzborn E, Roehrig S, Straub A, Kubitza D, Misselwitz F. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat Rev Drug Discov 2011;10:61-75. [DOI] [PubMed] [Google Scholar]
- 84.Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet 2009;48:1-22. [DOI] [PubMed] [Google Scholar]
- 85.Pinto DJ, Orwat MJ, Koch S, Rossi KA, Alexander RS, Smallwood A, et al. Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H -pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem 2007;50:5339-56. [DOI] [PubMed] [Google Scholar]
- 86.Lip GY, Larsen TB, Skjoth F, Rasmussen LH. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2012;60:738-46. [DOI] [PubMed] [Google Scholar]
- 87.Dogliotti A, Paolasso E, Giugliano RP. Current and new oral antithrombotics in non-valvular atrial fibrillation: a network meta-analysis of 79 808 patients. Heart 2013; published online 5 Sep. [DOI] [PubMed]
- 88.Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation 2013;127:224-32. [DOI] [PubMed] [Google Scholar]
- 89.Beasley BN, Unger EF, Temple R. Anticoagulant options—why the FDA approved a higher but not a lower dose of dabigatran. N Engl J Med 2011;364:1788-90. [DOI] [PubMed] [Google Scholar]
- 90.Limone BL, Baker WL, Kluger J, Coleman CI. Novel anticoagulants for stroke prevention in atrial fibrillation: a systematic review of cost-effectiveness models. PLoS One 2013;8:e62183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573-9. [DOI] [PubMed] [Google Scholar]
- 92.Garcia DA, Regan S, Henault LE, Upadhyay A, Baker J, Othman M, et al. Risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med 2008;168:63-9. [DOI] [PubMed] [Google Scholar]
- 93.Wysokinski WE, McBane RD, Daniels PR, Litin SC, Hodge DO, Dowling NF, et al. Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation. Mayo Clin Proc 2008;83:639-45. [DOI] [PubMed] [Google Scholar]
- 94.Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis (9th ed). American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141(suppl 2):e326S-50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marlu R, Hodaj E, Paris A, Albaladejo P, Crackowski JL, Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost 2012;108:217-24. [DOI] [PubMed] [Google Scholar]
- 96.Sherwood MW, Douketis JD, Patel MR, Piccini JP, Hellkamp AS, Lokhnygina Y, et al. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from ROCKET AF. Circulation 2014; published online 19 Feb. [DOI] [PMC free article] [PubMed]
- 97.Bauer KA. Reversal of antithrombotic agents. Am J Hematol 2012;87(suppl 1):S119-26. [DOI] [PubMed] [Google Scholar]
- 98.Lu G, DeGuzman FR, Hollenbach SJ, Karbarz MJ, Abe K, Lee G, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013;19:446-51. [DOI] [PubMed] [Google Scholar]
- 99.Majeed A, Hwang HG, Connolly SJ, Eikelboom JW, Ezekowitz MD, Wallentin L, et al. Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation 2013;128:2325-32. [DOI] [PubMed] [Google Scholar]
- 100.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 2011;123:e269-367. [DOI] [PubMed] [Google Scholar]