Abstract
The pleiotropic second messenger adenosine 3′,5′-cyclic monophosphate (cAMP) regulates a myriad of biological processes under both physiological and pathophysiological conditions. Exchange protein directly activated by cAMP 1 (EPAC1) mediates the intracellular functions of cAMP by acting as a guanine nucleotide exchange factor for the Ras-like Rap small GTPases. Recent studies suggest that EPAC1 plays important roles in immunomodulation, cancer cell migration/metastasis, and metabolism. These results, coupled with the successful development of EPAC-specific small molecule inhibitors, identify EPAC1 as a promising therapeutic target for cancer treatments.
Keywords: EPAC1, therapeutic target, cancer cAMP, PKA
Introduction
The prototypic second messenger adenosine 3′,5′-cyclic monophosphate (cAMP) controls a complex network of signaling pathways to ensure optimal cellular performance in response to environmental cues. In mammals, cAMP-mediated signaling cascades employ multiple large families of signaling molecules and enzymes that include G-protein coupled receptors, guanine nucleotide-binding proteins (G-proteins), adenylyl cyclases, and phosphodiesterases (PDEs), as well as scaffold proteins such as A-kinase anchoring proteins (AKAPs) [1]. The cellular functions of cAMP are mediated by two families of ubiquitously expressed cAMP effector proteins, namely protein kinase A (PKA), also known as cAMP-dependent protein kinase, and exchange protein directly activated by cAMP (EPAC), also known as cAMP-regulated guanine nucleotide exchange factor (cAMP-GEF), along with tissue-restricted cyclic nucleotide-gated (CNG) ion channels and hyperpolarization-activated cyclic nucleotide–modulated (HCN) channels [2].
While EPAC proteins are similar in sequence with PKA at their regulatory regions, i.e. the cAMP binding domains (CBDs), they possess distinct biochemical properties by acting as guanine nucleotide exchange factors for the Ras-like Rap small GTPases (RasGEF), Rap1 and Rap2 [3,4]. Unlike PKA whose regulatory and catalytic subunits are products of two separated genes, EPAC proteins are single chain multi-domain polypeptides that contain both the regulatory and catalytic elements. At the molecular level, the two mammalian EPAC isoforms, EPAC1 and EPAC2, share extensive sequence and domain homology. The N-terminal regulatory region of EPAC proteins contains a Dishevelled, Egl-10, Pleckstrin homology domain, and either one or two CBDs in EPAC1 or EPAC2, respectively. The C-terminal catalytic region comprises a RAS exchange motif, a RAS-association domain, and a CDC25 homology domain, also known as the RasGEF domain. Structural analyses reveal that EPAC proteins adopt an autoinhibitory conformation in which the regulatory half folds on top of the catalytic half, sterically blocking access to the catalytic site [5]. Binding of cAMP reorients the regulatory and catalytic lobes through a localized ‘hinge’ motion, frees the RasGEF domain from the autoinhibitory regulatory lobe, and allows the binding of downstream effectors, Rap1 or Rap2 [6–8].
The discovery of EPAC family cAMP sensors in 1998 significantly expanded the signaling pathways directly controlled by cAMP to include small GTPases. Rap1 GTPase, the main downstream EPAC effector, plays an important role in regulating cell adhesion and cell junction, as well as cell proliferation, depending upon the cell type and stimuli. Rap1 was initially identified as a gene (k-rev) capable of reversing the KRAS oncogene-mediated malignant transformation in fibroblasts. Sequence analysis further reveals that Rap1 and Ras share more than 50% sequence identity and high structural similarity [9]. One major difference between Rap1 and Ras signaling is the cellular loci where these GTPases function. While Ras is activated mostly at the plasma membrane, Rap1 activation occurs mainly inside the cell body and spreads toward the cell surface. Unlike its cousin Ras which is the most mutated human oncogene, the role of Rap1 in tumorigenesis has not been clearly defined and remains controversial. Rap1 was initially implicated in promoting tumorigenesis, as over-expression of Rap1 in Swiss 3T3 cells led to cellular transformation and tumor formation in nude mice [10]. Furthermore, constitutive activation of Rap1 by targeted degradation of E6TP1, a Rap1 negative regulator, by the human papillomavirus (HPV) E6 oncoprotein via E6AP ubiquitin ligase, correlates with the transformation of epithelial cells by HPV in vitro [11]. Perhaps, the most substantial evidence connecting Rap1 activation and malignancy is based on mouse genetic studies in which a Rap1 GTPase-activating protein (GAP), SPA-1, is deleted. SPA-1-deficient mice exhibit constitutive activation of Rap1 in the hematopoietic progenitors of bone marrow and develop a spectrum of myeloid disorders that resemble human chronic myelogenous leukemia (CML) [12]. Taken together, these studies suggest that Rap1 activation is associated with tumorigenesis. On the other hand, it is important to point out that Rap1 activation has also been shown to suppress malignancy and tumor invasiveness [13,14], therefore, the role of Rap1 in tumorigenesis appears to be context-dependent.
Alterations in the expression and/or activity of cAMP signaling components are common events in various cancers [15–19] and contribute to the prognosis of cancer treatment [20–23]. While the role of PKA in tumorigenesis has been confirmed through the revelation that mutations in PRKAR1A and PRKACA genes cause Carney complex [24–26] and Cushing's disease [27–29], respectively, the involvement of EPAC in cancer is emerging. While EPAC1 is ubiquitously expressed, EPAC2 has limited tissue expression profiles and is mainly found in neurons, pancreatic beta cells, and adrenal gland [3]. Due to their distinct tissue and cellular distributions, physiological functions of EPAC1 and EPAC2 are mostly non-redundant. To date, EPAC2 has not been implicated in cancer. This review focuses on recent findings of EPAC1's role in cancer and the potential of EPAC1 as a target for cancer therapeutics.
EPAC1 in Cancer Cell Proliferation and Apoptosis
cAMP signaling has been found to have either positive or negative effects on cancer cell growth and survival. In particular, it has been known that cAMP signaling is important for leukemia cell survival. However, the molecular mechanism of cAMP-induced cell killing in various leukemia cells is not clear. Tawari et al. [30] showed that B-cell chronic lymphocytic leukemia (B-CLL) cells undergo apoptosis following treatment with PDE4 inhibitors. Interestingly, whereas rolipram/forskolin and 8-Bromo-cAMP induce apoptosis in B-CLL cells, treatment with an EPAC-specific cAMP analog decreases basal apoptosis in B-CLL cells, suggesting an opposing relationship between PKA and EPAC in mediating cAMP's effect on B-CLL survival [30]. Similarly, cAMP synergizes strongly with glucocorticoids (GC) to induce apoptosis in normal or malignant lymphoid cells. Between the two major cAMP sensors, PKA and EPAC, PKA is shown to be responsible for the observed synergism with GC, whereas EPAC exerts a weak antagonistic effect against GC-induced apoptosis [31]. It has been further revealed that the PKA regulatory subunit isoform RIIβ is over-expressed in the human acute lymphoblastic leukemia (ALL) GC-sensitive clone cells, whereas other intracellular cAMP receptors, including EPAC, are expressed at similar levels in both GC-sensitive and GC-resistant clones. High RIIβ expression level is correlated with elevated PKA cellular activity and heightened cAMP sensitivity [32]. On the other hand, in the immature B lymphoma cell line WEHI-231, ligation of B cell antigen receptor (BCR) promotes growth arrest and apoptosis in an EPAC-dependent manner. In fact, activation of endogenous EPAC by an EPAC-selective cAMP analog, 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate (007), enhances BCR-induced growth arrest and apoptosis [33].
On the basis of limited published studies, the effects of EPAC1 on cell proliferation and survival in solid tumors are also cell type-dependent and context-dependent. It is reported that in A172 and U87MG human glioblastoma cells, PKA and EPAC1 pathways synergistically promote cAMP-induced cell death and cell cycle arrest [34], while in clear renal cell carcinoma (cRCC) A498 cells, growth-inhibitory response to vasoactive intestinal peptide (VIP) is shown to be mediated by EPAC/PI3K pathway [35]. In contrast, EPAC1 promotes cell proliferation and survival by up-regulating Ras/MAPK and PI3K/AKT/mTOR signaling in prostate cancer cells [36,37].
EPAC1 in Cancer Cell Migration and Metastasis
cAMP plays a complex and context-dependent role in regulating cell migration [38,39]. Traditionally the focus has been mainly on PKA-mediated migratory effects. Since the discovery of EPAC, several studies have shown that EPAC1 mediates cAMP's role in migration in a variety of cell types. For example, EPAC1 promotes adhesion and migration of white blood cells [40], cells of epithelial origin [41], and vascular smooth muscle cells [42]. When it comes to cancer, the past decade produced numerous studies elucidating a critical role for EPAC1 in the invasion and metastasis of several cancers, particularly those of epithelial origin [43–50] (Table 1).
Table 1.
Reported role of EPAC1 in regulating cell migration in various cancers
| Cancer type | Migration regulation | Reference |
|---|---|---|
| Cervical cancer | Promoting | [51] |
| Fibrosarcoma | Promoting | [52] |
| Melanoma | Promoting | [44,47–49,53,54] |
| Ovarian cancer | Promoting; suppressing | [43]; [45] |
| Pancreatic cancer | Promoting | [39,50,55] |
| Prostate cancer | Promoting; suppressinga | [56–58]; [46] |
aSubsequent study suggests that the apparent suppressive effect observed for EPAC1 may be attributed to PKA activation, as the EPAC-selective agonist used was shown to activate PKA [59].
In melanoma, there is an agreement that EPAC1 enhances invasion and metastasis [44,47–49,53,54]. In prostate cancer, while some results suggest that EPAC1 promotes metastasis and proliferation [56–58], one study contradicts these results by showing that activation of EPAC1 by 007, an EPAC-selective agonist, inhibits migration and proliferation of human prostate carcinoma cells [46]. Subsequently, it has been shown that the inhibitory effect observed for EPAC1 in the aforementioned contradictory study was actually the result of PKA activation. The inhibitory effects of 007 were not affected by EPAC1 and EPAC2 silencing using siRNA, but could be rescued by PKA inhibitors H89 and PKI [59]. This is not completely surprising because while 007 exerts about 100-fold selectivity towards EPAC1 over PKA, it still has the ability to activate PKA directly. In addition, as a cyclic nucleotide analog, 007 is known to inhibit PDEs, and consequently cause elevation of cAMP/cGMP and indirect activation of intracellular cyclic nucleotide sensors [60]. In ovarian cancer, EPAC1 seems to have pro-migratory effects in some cell lines (Ovcar3) [43], and anti-migratory effects in others (ES-2) [45]. However, the latter study also utilized 007 to activate EPAC1. So again, it is possible that the anti-migratory effects are in fact mediated by PKA rather than EPAC1. In fibrosarcoma cells, it has been shown that lysophosphatidic acid (LPA) receptor 4, LPA(4), signaling promotes invadopodia formation downstream of autotaxin (ATX), a secreted lysophospholipase involved in the production of LPA and whose level of expression within tumors correlates strongly with their aggressiveness and invasiveness. It was further demonstrated that ATX/LPA-induced invadopodia formation is mediated through the activation of EPAC by cAMP and subsequent Rac1 activation [52]. In pancreatic cancer, it has been recently shown that at some point during their malignant transformation, pancreatic ductal adenocarcinoma (PDA) cells up-regulate their expression of EPAC1 [61], a finding that spurred our group to investigate the functional implication of this over-expression. Our data, in agreement with the findings of Burdyga et al. [39], show that EPAC1 enhances PDA invasion and metastasis in vitro and in vivo [39,50,55]. The role of EPAC1 in cervical cancer has not been investigated extensively, but a recent report showed that the activation of this protein enhances migration of HeLa human cervical cancer cells [51].
A common theme that emerges from some of the aforementioned studies is that PKA and EPAC1 potentially work in opposition to each other in mediating cAMP's effect on cancer migration. Similarly to their contradictory actions in prostate and possibly ovarian cancer migration, these two signaling molecules also work antagonistically in controlling migration of cervical cancer cells [51] and PDA cell invasion, as PKA inhibits the ruffling and formation of focal adhesions in PDA cells, while EPAC potentiates these processes [39]. This notion is in agreement with previous studies showing that EPAC and PKA work antagonistically in certain cellular context [62–65].
Our theory that EPAC1 and PKA have pro- and anti-migratory roles on cancer migration, respectively, helps explain the complex role of cAMP in cancer invasion and metastasis. Hence, therapeutic strategies designed to reduce cancer metastasis must take a nuanced approach focusing on the downstream targets of cAMP and aim to inhibit EPAC1 and activate PKA for potential synergism. Approaches that aim at the receptor level or target PDEs to increase or decrease cAMP might be more likely to have unintended consequences and their overall impact on cancer migration will be difficult to predict.
The cellular and molecular mechanisms of EPAC1-mediated cell migration have been investigated extensively in melanoma. It appears that EPAC-induced cell migration is associated with the translocation of syndecan-2, a cell-surface heparan sulfate (HS) proteoglycan, to lipid rafts, as well as the production of HS, a major component of extracellular matrix. While syndecan-2 translocation is regulated by tubulin polymerization downstream of EPAC1/PI3K pathway, HS production is the result of an increased expression of N-deacetylase/N-sulfotransferase-1 (NDST-1) [53]. It was further revealed that the expression of EPAC1 is positively correlated with those of HS and NDST-1. Most importantly, in human melanoma tissue microarrays, levels of EPAC1 expression are up-regulated in metastatic melanoma, compared with primary melanoma, suggesting a role for EPAC1 in melanoma metastasis [48].
In addition to HS-related mechanism, EPAC1-mediated melanoma cell migration is also Ca2+-dependent. In various melanoma cell lines, but not in melanocytes, EPAC activation by 007 leads to a PLC/IP3 receptor-dependent increase in intracellular Ca2+, which promotes actin assembly and induces cell migration [47]. Subsequent analysis further suggests a cross-talk between EPAC1 and G-protein βγ subunits (Gβγ) in Ca2+ signaling and cell migration in melanoma: activation of Gβγ induces Ca2+ entry from the extracellular space and inhibits the EPAC-induced Ca2+ release from the endoplasmic reticulum, resulting in suppression of cell migration [54].
While a diverse array of signaling pathways may be involved, studies that have investigated the role of EPAC1 in cancer migration suggest that this cAMP sensor facilitates cancer migration ultimately through integrin-dependent pathways in various cancers [43,44,47,55,56]. Although several integrins have been implicated, integrin β1 plays a particularly important role in the process. Not only does EPAC1 enhance the trafficking of integrin β1, but it also promotes its activation [55]. These findings carry significant therapeutic implications, since this integrin mediates the malignant phenotype and facilitates the loss of epithelial integrity and oncogenic transformation in several epithelial cancers [66–68]. In fact, constitutive activation of integrin β1 is correlated with higher grade carcinomas [67]. There are currently no available small molecules that target integrin β1, but monoclonal antibodies and synthetic peptides against this integrin have shown significant clinical efficacy [69]. Hence, EPAC-specific inhibitors might provide a new approach to target integrin β1 in cancer treatment.
EPAC1 in T-cell Function: Implications for Anti-cancer Immunotherapy
Since the 1970s researchers have sought to exploit features of the body's spontaneous immune response to cancer to develop effective immunotherapies. Not only can immunotherapy eradicate existing tumor cells, but it also has the potential to provide the immune surveillance necessary to prevent cancer recurrence. A wide array of treatment modalities have undergone clinical trials, including the administration of cytokines that enhance the activity of tumor-specific immune cells, adoptive transfer of engineered autologous T-cells, and administration of monoclonal antibodies against cancer-specific antigens or whole-cell or synthetic peptide vaccines [70]. The recent major breakthroughs, particularly in the area of checkpoint anti-cancer immunotherapy, validate that it is an effective strategy to fight cancer via modulating the activity of host T-cells, in which EPAC1 has been implicated to play an important role [71,72]
One of the main hurdles facing the development of cancer immunotherapy is the fact that cross-talk between tumor infiltrating immune cells, tumor cells, and stromal cells leads to the reprogramming of the anti-tumor immune response and development of an immunosuppressive milieu that is suitable for tumor growth [73,74]. Indeed, the ability to escape immune surveillance is now considered as a hallmark of cancer [74]. Regulatory-T cells (Treg), a subset of T-cells with general suppressor function [75,76], are a major driver of the immunosuppressive tumor microenvironment and play an essential role in the development of cancer from an early stage [77,78]. Not only do these cells suppress the host's immune response to cancer cells, but they also reduce the efficacy of cell- and vaccine-based cancer immunotherapies [79]. For instance, several studies have shown that after the administration of tumor vaccines, the number of vaccine-specific Treg cells increases significantly; diminishing the effectiveness of the treatment [80–82]. Therefore, treatments that down-regulate Treg activity have great potential as cancer immunotherapies, especially when administered in combination with cancer vaccines. In fact, Ipilimumab, a monoclonal antibody recently approved by the FDA for cancer immunotherapy, is an anti-CTLA-4 antibody that eliminates Treg cells and has shown excellent efficacy in melanoma patients [83].
Recently, we showed that EPAC1 plays an essential role in modulating the activity of Treg cells using in vitro and in vivo EPAC1 KO models [71]. We examined Treg-mediated suppression utilizing genetic and pharmacologic approaches and our data showed that the suppressive capacity of Treg cells was reduced in the absence of EPAC1. Furthermore, the lack of EPAC1 in effector T-cells (Teff) rendered them resistance to suppression by Treg, and inhibition/suppression of EPAC1 in both cell populations had an additive effect on compromising Treg-mediated suppression. Our findings highlight a critical role for EPAC1 in mediating cAMP-regulated Treg suppression, in agreement with a recent report showing that significant inhibition of the PKA pathway has no impact on Treg suppression [75].
Our findings have significant potential clinical implications as they validate EPAC1 as a potential target for fine tuning Treg cell activity. Strategies that rely on depletion of Treg cells, even transiently, usually lead to serious systemic side effects and signs of severe autoimmune disease [84]. Not surprisingly, Ipilimumab, the anti-CTLA-4 antibody alluded to earlier, has potentially fetal autoimmune side effects and is only used in advanced non-resectable cases of melanoma [83]. On the other hand, neither genetic deletion nor long-term pharmacologic inhibition of EPAC1 had observable side effects in mice as we have shown previously [85]. Hence, pharmacologic modulators of this protein might have excellent therapeutic potential in modulating cancer immunity. It is important to note that our original study was not directly related to cancer, but the data suggest that EPAC signaling could influence inflammation and tumor microenvironment and should be further investigated in the context of cancer.
EPAC1 in Cancer Metabolism
Reprogramming of metabolic pathways, chief among them increasing glucose transport and metabolism, is a critical step in cancer progression. It has been more than half a century since the Warburg studies showed that cancer cells rely heavily on glycolysis and the conversion of pyruvate to lactate even in aerobic conditions [86,87]. The Warburg effect has since been observed in numerous neoplasms and it is now considered as one of the hallmarks of cancer [88]. Initially, this metabolic change was deemed a result of defective mitochondrial function that prevented completion of oxidative phosphorylation. However, over the past decade it has become apparent that changes in cancer metabolism are a direct result of oncogenic signaling pathways, which produce metabolites that play a role in the malignant transformation of tumor cells [89]. Up-regulation of aerobic glycolysis is thought to confer survivability to cancer cells in hypoxic conditions and provide anabolic metabolites necessary for cancer proliferation and NADPH to scavenge reactive oxygen species (ROS) [88,89]. A recent study revealed an unexpected role of the EPAC1/RAP1 signaling pathway in promoting oncogenesis via up-regulating aerobic glycolysis [68].
Onodera et al. [68] provided convincing evidence that increased glucose metabolism is not a passive consequence of multiple oncogenic signals involved in carcinogenesis, but it is actually a driver of the process and represents an oncogenic event in itself. Furthermore, the cellular transformation resulting from increased glucose uptake is mediated through the EPAC1/RAP1 pathway. Onodera et al. [68] used a 3D culture model, which had been validated in vivo, to show that inhibiting glucose metabolism pharmacologically, or decreasing glucose uptake by genetic knockdown, caused malignant breast epithelial cells (T4-2) to revert to their non-malignant phenotype. On the other hand, increasing glucose concentration in the culture medium activated oncogenic pathways and enhanced the malignant phenotype of T4-2 cells in a dose-dependent manner.
At the molecular level, glucose metabolism did not initiate oncogenesis through the canonical pathways AMPK, mTOR, and HIF-1α/2α, which are considered the main regulatory pathways of nutrient signaling [88]. Genetic or pharmacologic inhibition of these pathways did not significantly affect the phenotype of T4-2 cells. Instead, it was found that in response to glucose metabolism, a soluble form of adenylyl cyclase generates cAMP and activates EPAC1, which in turn activates RAP1 and triggers a signaling cascade that controls the malignant phenotype of T4-2 cells through integrin β1 [68]. Addition of increasing concentrations of glucose, after expressing a dominant-negative form of RAP1 in T4-2 cells, reestablished cell polarity and resulted in the loss of the malignant phenotype. On the other hand, addition of an EPAC-selective cAMP analog, 007, activated RAP1 and increased integrin β1 expression and caused loss of tissue polarity.
The results presented by Onodera et al. [68] signal a potential paradigm shift in how glucose metabolism in cancer is viewed, and show that aerobic glycolysis likely triggers an upstream oncogenic signaling pathway, rather than acting as a downstream event of other oncogenes during tumorigenesis. These findings still need to be further validated in in vivo models and expanded to different cancer cell types, but nonetheless, they point to yet another potential way of exploiting the EPAC pathway as a therapeutic target that directly influences the malignant transformation of cancer cells.
EPAC1 Inhibitors: the Next Big Thing in Anti-cancer Therapy?
The cAMP signaling pathway plays a role in mediating nearly all biological functions. The pervasiveness of this signaling network lends itself to therapeutic exploitation and the design of drugs that can target multiple cell functions in different systems within the body. In fact, drugs targeting proteins involved in the regulation of cAMP span a wide range of diseases, from simple headaches to schizophrenia and congestive heart failure [90]. However, the ubiquitous nature of cAMP signaling also means greater potential for detrimental systemic drug side effects, which might diminish the therapeutic index for a given treatment. Hence, targeting specific proteins downstream of cAMP might provide more successful treatment strategies than do those that alter cAMP levels in general. The studies summarized in this review identify EPAC1 as such a target for treatment of cancers.
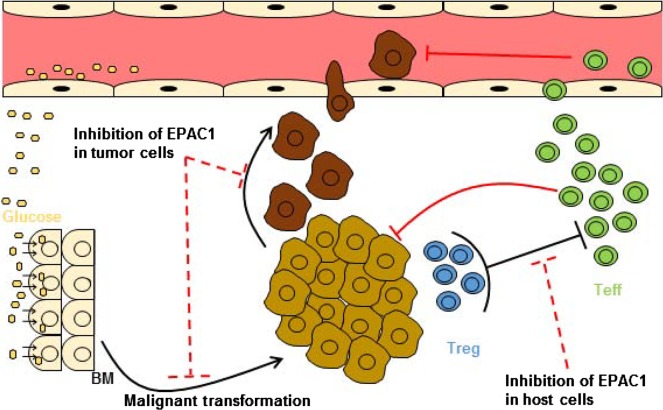
While further investigation is needed, especially in vivo, to better understand EPAC1's role in cancer migration and oncogenic transformation, we have enough evidence suggesting that small molecule inhibitors of EPAC1 might provide an efficient approach to combat cancer at multiple levels (Fig. 1). On the one hand, inhibition of EPAC1 in cancer cells has the potential to reduce metastasis and negate some of the oncogenic effects of deregulated glucose uptake, and on the other hand, its inhibition in the host's immune cells can reduce Treg-mediated suppression and augment anti-tumor immune responses. Such a multi-mechanistic approach can help negate the possible resistance commonly seen against therapies that work on one target/pathway and obviate the need for using multiple drugs simultaneously, which can lead to multiple side effects and contribute to the high costs of cancer treatment [91]. Nevertheless, EPAC1 inhibitors have the potential to act synergistically with other anti-cancer drugs, particularly those aiming at the integrin β1 signaling as explained previously.
Figure 1.
Proposed model for the therapeutic potential of EPAC1 inhibitors in cancer treatment Inhibition of EPAC1 in tumor cells has the potential to reduce the oncogenic effects of up-regulated glucose metabolism and to inhibit invasion/migration of cancer cells. On the other hand, inhibition of EPAC1 in the normal host cells has the potential to down-regulate the activity of Treg and consequently boosts the anti-tumor activity of Teff. BM: basement membrane.
In conclusion, EPAC1 presents a novel target for the development of cancer therapies and recently-developed small molecule inhibitors of this protein warrant investigation as potential new class of anti-neoplastic agents [50,92–95].
Funding
This work was supported by the grants from the National Institutes of Health (Nos. GM061770, GM106218 and AI111464).
References
- 1.Beavo JA, Brunton LL. Cyclic nucleotide research—still expanding after half a century. Nat Rev Mol Cell Biol 2002, 3: 710–718. [DOI] [PubMed] [Google Scholar]
- 2.Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu Rev Physiol 2006, 68: 375–401. [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE et al. . A family of cAMP-binding proteins that directly activate Rap1. Science 1998, 282: 2275–2279. [DOI] [PubMed] [Google Scholar]
- 4.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396: 474–477. [DOI] [PubMed] [Google Scholar]
- 5.Rehmann H, Das J, Knipscheer P, Wittinghofer A, Bos JL. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature 2006, 439: 625–628. [DOI] [PubMed] [Google Scholar]
- 6.Rehmann H, Arias-Palomo E, Hadders MA, Schwede F, Llorca O, Bos JL. Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nature 2008, 455: 124–127. [DOI] [PubMed] [Google Scholar]
- 7.Tsalkova T, Blumenthal DK, Mei FC, White MA, Cheng X. Mechanism of Epac activation: structural and functional analyses of Epac2 hinge mutants with constitutive and reduced activities. J Biol Chem 2009, 284: 23644–23651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Tsalkova T, White MA, Mei FC, Liu T, Wang D, Woods VL Jr et al. . Mechanism of intracellular cAMP sensor Epac2 activation: cAMP-induced conformational changes identified by amide hydrogen/deuterium exchange mass spectrometry (DXMS). J Biol Chem 2011, 286: 17889–17897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell 1989, 56: 77–84. [DOI] [PubMed] [Google Scholar]
- 10.Altschuler DL, Ribeiro-Neto F. Mitogenic and oncogenic properties of the small G protein Rap1b. Proc Natl Acad Sci USA 1998, 95: 7475–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh L, Gao Q, Kumar A, Gotoh T, Wazer DE, Band H, Feig LA et al. . The high-risk human papillomavirus type 16 E6 counters the GAP function of E6TP1 toward small Rap G proteins. J Virol 2003, 77: 1614–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishida D, Kometani K, Yang H, Kakugawa K, Masuda K, Iwai K, Suzuki M et al. . Myeloproliferative stem cell disorders by deregulated Rap1 activation in SPA-1-deficient mice. Cancer Cell 2003, 4: 55–65. [DOI] [PubMed] [Google Scholar]
- 13.Yajnik V, Paulding C, Sordella R, McClatchey AI, Saito M, Wahrer DC, Reynolds P et al. . DOCK4, a GTPase activator, is disrupted during tumorigenesis. Cell 2003, 112: 673–684. [DOI] [PubMed] [Google Scholar]
- 14.Mitra RS, Goto M, Lee JS, Maldonado D, Taylor JM, Pan Q, Carey TE et al. . Rap1GAP promotes invasion via induction of matrix metalloproteinase 9 secretion, which is associated with poor survival in low N-stage squamous cell carcinoma. Cancer Res 2008, 68: 3959–3969. [DOI] [PubMed] [Google Scholar]
- 15.Miller WR. Regulatory subunits of PKA and breast cancer. Ann N Y Acad Sci 2002, 968: 37–48. [DOI] [PubMed] [Google Scholar]
- 16.Chung S, Furihata M, Tamura K, Uemura M, Daigo Y, Nasu Y, Miki T et al. . Overexpressing PKIB in prostate cancer promotes its aggressiveness by linking between PKA and Akt pathways. Oncogene 2009, 28: 2849–2859. [DOI] [PubMed] [Google Scholar]
- 17.James MA, Lu Y, Liu Y, Vikis HG, You M. RGS17, an overexpressed gene in human lung and prostate cancer, induces tumor cell proliferation through the cyclic AMP-PKA-CREB pathway. Cancer Res 2009, 69: 2108–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merkle D, Hoffmann R. Roles of cAMP and cAMP-dependent protein kinase in the progression of prostate cancer: cross-talk with the androgen receptor. Cell Signal 2011, 23: 507–515. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez CI, Setaluri V. Cyclic AMP (cAMP) signaling in melanocytes and melanoma. Arch Biochem Biophys 2014, 563: 22–27. [DOI] [PubMed] [Google Scholar]
- 20.Michalides R, Griekspoor A, Balkenende A, Verwoerd D, Janssen L, Jalink K, Floore A et al. . Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell 2004, 5: 597–605. [DOI] [PubMed] [Google Scholar]
- 21.Troiani T, Vecchione L, Martinelli E, Capasso A, Costantino S, Ciuffreda LP, Morgillo F et al. . Intrinsic resistance to selumetinib, a selective inhibitor of MEK1/2, by cAMP-dependent protein kinase A activation in human lung and colorectal cancer cells. Br J Cancer 2012, 106: 1648–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terada N, Shiraishi T, Zeng Y, Mooney SM, Yeater DB, Mangold LA, Partin AW et al. . Cyr61 is regulated by cAMP-dependent protein kinase with serum levels correlating with prostate cancer aggressiveness. Prostate 2012, 72: 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johannessen CM, Johnson LA, Piccioni F, Townes A, Frederick DT, Donahue MK, Narayan R et al. . A melanocyte lineage program confers resistance to MAP kinase pathway inhibition. Nature 2013, 504: 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS et al. . Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet 2000, 26: 89–92. [DOI] [PubMed] [Google Scholar]
- 25.Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R et al. . Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 2009, 94: 2085–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey M, Vaughan CJ, He J, Hatcher CJ, Winter JM, Weremowicz S, Montgomery K et al. . Mutations in the protein kinase A R1alpha regulatory subunit cause familial cardiac myxomas and Carney complex. J Clin Invest 2000, 106: R31–R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y, He M, Gao Z, Peng Y, Li Y, Li L, Zhou W et al. . Activating hotspot L205R mutation in PRKACA and adrenal Cushing's syndrome. Science 2014, 344: 913–917. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y, Maekawa S, Ishii R, Sanada M, Morikawa T, Shiraishi Y, Yoshida K et al. . Recurrent somatic mutations underlie corticotropin-independent Cushing's syndrome. Science 2014, 344: 917–920. [DOI] [PubMed] [Google Scholar]
- 29.Beuschlein F, Fassnacht M, Assie G, Calebiro D, Stratakis CA, Osswald A, Ronchi CL et al. . Constitutive activation of PKA catalytic subunit in adrenal Cushing's syndrome. N Engl J Med 2014, 370: 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiwari S, Felekkis K, Moon EY, Flies A, Sherr DH, Lerner A. Among circulating hematopoietic cells, B-CLL uniquely expresses functional EPAC1, but EPAC1-mediated Rap1 activation does not account for PDE4 inhibitor-induced apoptosis. Blood 2004, 103: 2661–2667. [DOI] [PubMed] [Google Scholar]
- 31.Ji Z, Mei FC, Johnson BH, Thompson EB, Cheng X. Protein kinase A, not Epac, suppresses hedgehog activity and regulates glucocorticoid sensitivity in acute lymphoblastic leukemia cells. J Biol Chem 2007, 282: 37370–37377. [DOI] [PubMed] [Google Scholar]
- 32.Ji Z, Mei FC, Miller AL, Thompson EB, Cheng X. Protein kinase A (PKA) isoform RIIbeta mediates the synergistic killing effect of cAMP and glucocorticoid in acute lymphoblastic leukemia cells. J Biol Chem 2008, 283: 21920–21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grandoch M, López de Jesús M, Oude Weernink PA, Weber AA, Jakobs KH, Schmidt M. B cell receptor-induced growth arrest and apoptosis in WEHI-231 immature B lymphoma cells involve cyclic AMP and Epac proteins. Cell Signal 2009, 21: 609–621. [DOI] [PubMed] [Google Scholar]
- 34.Moon EY, Lee GH, Lee MS, Kim HM, Lee JW. Phosphodiesterase inhibitors control A172 human glioblastoma cell death through cAMP-mediated activation of protein kinase A and Epac1/Rap1 pathways. Life Sci 2012, 90: 373–380. [DOI] [PubMed] [Google Scholar]
- 35.Vacas E, Fernández-Martínez AB, Bajo AM, Sánchez-Chapado M, Schally AV, Prieto JC, Carmena MJ. Vasoactive intestinal peptide (VIP) inhibits human renal cell carcinoma proliferation. Biochim Biophys Acta 2012, 1823: 1676–1685. [DOI] [PubMed] [Google Scholar]
- 36.Flacke JP, Flacke H, Appukuttan A, Palisaar RJ, Noldus J, Robinson BD, Reusch HP et al. . Type 10 soluble adenylyl cyclase is overexpressed in prostate carcinoma and controls proliferation of prostate cancer cells. J Biol Chem 2013, 288: 3126–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misra UK, Pizzo SV. Evidence for a pro-proliferative feedback loop in prostate cancer: the role of Epac1 and COX-2-dependent pathways. PLoS One 2013, 8: e63150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howe AK. Cross-talk between calcium and protein kinase A in the regulation of cell migration. Curr Opin Cell Biol 2011, 23: 554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burdyga A, Conant A, Haynes L, Zhang J, Jalink K, Sutton R, Neoptolemos J et al. . cAMP inhibits migration, ruffling and paxillin accumulation in focal adhesions of pancreatic ductal adenocarcinoma cells: effects of PKA and EPAC. Biochim Biophys Acta 2013, 1833: 2664–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carmona G, Chavakis E, Koehl U, Zeiher AM, Dimmeler S. Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood 2008, 111: 2640–2646. [DOI] [PubMed] [Google Scholar]
- 41.Enserink JM, Price LS, Methi T, Mahic M, Sonnenberg A, Bos JL, Taskén K. The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the alpha3beta1 integrin but not the alpha6beta4 integrin. J Biol Chem 2004, 279: 44889–44896. [DOI] [PubMed] [Google Scholar]
- 42.Yokoyama U, Minamisawa S, Quan H, Akaike T, Jin M, Otsu K, Ulucan C et al. . Epac1 is upregulated during neointima formation and promotes vascular smooth muscle cell migration. Am J Physiol Heart Circ Physiol 2008, 295: H1547–H1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, Bos JL. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol 2003, 160: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao L, Feng Y, Bowers R, Becker-Hapak M, Gardner J, Council L, Linette G et al. . Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: two processes important to melanoma tumorigenesis and metastasis. Cancer Res 2006, 66: 7880–7888. [DOI] [PubMed] [Google Scholar]
- 45.Bastian P, Balcarek A, Altanis C, Strell C, Niggemann B, Zaenker KS, Entschladen F. The inhibitory effect of norepinephrine on the migration of ES-2 ovarian carcinoma cells involves a Rap1-dependent pathway. Cancer Lett 2009, 274: 218–224. [DOI] [PubMed] [Google Scholar]
- 46.Grandoch M, Rose A, ter Braak M, Jendrossek V, Rübben H, Fischer JW, Schmidt M et al. . Epac inhibits migration and proliferation of human prostate carcinoma cells. Br J Cancer 2009, 101: 2038–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baljinnyam E, De Lorenzo MS, Xie LH, Iwatsubo M, Chen S, Goydos JS, Nowycky MC et al. . Exchange protein directly activated by cyclic AMP increases melanoma cell migration by a Ca2+-dependent mechanism. Cancer Res 2010, 70: 5607–5617. [DOI] [PubMed] [Google Scholar]
- 48.Baljinnyam E, Umemura M, De Lorenzo MS, Iwatsubo M, Chen S, Goydos JS, Iwatsubo K. Epac1 promotes melanoma metastasis via modification of heparan sulfate. Pigment Cell Melanoma Res 2011, 24: 680–687. [DOI] [PubMed] [Google Scholar]
- 49.Baljinnyam E, Umemura M, Chuang C, De Lorenzo MS, Iwatsubo M, Chen S, Goydos JS et al. . Epac1 increases migration of endothelial cells and melanoma cells via FGF2-mediated paracrine signaling. Pigment Cell Melanoma Res 2014, 27: 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, Schwede F et al. . A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol 2013, 83: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JW, Lee J, Moon EY. HeLa human cervical cancer cell migration is inhibited by treatment with dibutyryl-cAMP. Anticancer Res 2014, 34: 3447–3455. [PubMed] [Google Scholar]
- 52.Harper K, Arsenault D, Boulay-Jean S, Lauzier A, Lucien F, Dubois CM. Autotaxin promotes cancer invasion via the lysophosphatidic acid receptor 4: participation of the cyclic AMP/EPAC/Rac1 signaling pathway in invadopodia formation. Cancer Res 2010, 70: 4634–4643. [DOI] [PubMed] [Google Scholar]
- 53.Baljinnyam E, Iwatsubo K, Kurotani R, Wang X, Ulucan C, Iwatsubo M, Lagunoff D et al. . Epac increases melanoma cell migration by a heparan sulfate-related mechanism. Am J Physiol Cell Physiol 2009, 297: C802–C813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baljinnyam E, Umemura M, De Lorenzo MS, Xie LH, Nowycky M, Iwatsubo M, Chen S et al. . Gβγ subunits inhibit Epac-induced melanoma cell migration. BMC Cancer 2011, 11: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Almahariq M, Chao C, Mei FC, Hellmich MR, Patrikeev I, Motamedi M, Cheng X. Pharmacological inhibition and genetic knockdown of exchange protein directly activated by cAMP 1 reduce pancreatic cancer metastasis in vivo. Mol Pharmacol 2015, 87: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bailey CL, Kelly P, Casey PJ. Activation of Rap1 promotes prostate cancer metastasis. Cancer Res 2009, 69: 4962–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Misra UK, Pizzo SV. Epac1-induced cellular proliferation in prostate cancer cells is mediated by B-Raf/ERK and mTOR signaling cascades. J Cell Biochem 2009, 108: 998–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Misra UK, Pizzo SV. Upregulation of mTORC2 activation by the selective agonist of EPAC, 8-CPT-2Me-cAMP, in prostate cancer cells: assembly of a multiprotein signaling complex. J Cell Biochem 2012, 113: 1488–1500. [DOI] [PubMed] [Google Scholar]
- 59.Menon J, Doebele RC, Gomes S, Bevilacqua E, Reindl KM, Rosner MR. A novel interplay between Rap1 and PKA regulates induction of angiogenesis in prostate cancer. PLoS One 2012, 7: e49893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F et al. . Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods 2008, 5: 277–278. [DOI] [PubMed] [Google Scholar]
- 61.Lorenz R, Aleksic T, Wagner M, Adler G, Weber CK. The cAMP/Epac1/Rap1 pathway in pancreatic carcinoma. Pancreas 2008, 37: 102–103. [DOI] [PubMed] [Google Scholar]
- 62.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 2002, 277: 11497–11504. [DOI] [PubMed] [Google Scholar]
- 63.Brennesvik EO, Ktori C, Ruzzin J, Jebens E, Shepherd PR, Jensen J. Adrenaline potentiates insulin-stimulated PKB activation via cAMP and Epac: implications for cross talk between insulin and adrenaline. Cell Signal 2005, 17: 1551–1559. [DOI] [PubMed] [Google Scholar]
- 64.Li J, O'Connor KL, Cheng X, Mei FC, Uchida T, Townsend CM, Evers BM. Cyclic adenosine 5′-monophosphate-stimulated neurotensin secretion is mediated through Rap1 downstream of both Epac and protein kinase A signaling pathways. Mol Endocrinol 2007, 21: 159–171. [DOI] [PubMed] [Google Scholar]
- 65.Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin 2008, 40: 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grzesiak JJ, Bouvet M. The alpha2beta1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines. Br J Cancer 2006, 94: 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee YC, Jin JK, Cheng CJ, Huang CF, Song JH, Huang M, Brown WS et al. . Targeting constitutively activated β1 integrins inhibits prostate cancer metastasis. Mol Cancer Res 2013, 11: 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Onodera Y, Nam JM, Bissell MJ. Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J Clin Invest 2014, 124: 367–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barkan D, Chambers AF. β1-integrin: a potential therapeutic target in the battle against cancer recurrence. Clin Cancer Res 2011, 17: 7219–7223. [DOI] [PubMed] [Google Scholar]
- 70.Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin 2012, 62: 309–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Almahariq M, Mei FC, Wang H, Cao AT, Yao S, Soong L, Sun J et al. . Exchange protein directly activated by cAMP modulates regulatory T-cell-mediated immunosuppression. Biochem J 2015, 465: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shirshev SV. Role of Epac proteins in mechanisms of cAMP-dependent immunoregulation. Biochemistry (Mosc) 2011, 76: 981–998. [DOI] [PubMed] [Google Scholar]
- 73.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010, 140: 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wörmann SM, Diakopoulos KN, Lesina M, Algül H. The immune network in pancreatic cancer development and progression. Oncogene 2014, 33: 2956–2967. [DOI] [PubMed] [Google Scholar]
- 75.Vang AG, Housley W, Dong H, Basole C, Ben-Sasson SZ, Kream BE, Epstein PM et al. . Regulatory T cells and cAMP suppress effector T cells independently of PKA-CREM/ICER: a potential role for Epac. Biochem J 2013, 456: 463–473. [DOI] [PubMed] [Google Scholar]
- 76.Ring S, Pushkarevskaya A, Schild H, Probst HC, Jendrossek V, Wirsdörfer F, Ledent C et al. . Regulatory T cell-derived adenosine induces dendritic cell migration through the Epac-Rap1 pathway. J Immunol 2015, 194: 3735–3744. [DOI] [PubMed] [Google Scholar]
- 77.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood 2006, 108: 804–811. [DOI] [PubMed] [Google Scholar]
- 78.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008, 8: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 2014, 27: 1–7. [DOI] [PubMed] [Google Scholar]
- 80.Ebert LM, MacRaild SE, Zanker D, Davis ID, Cebon J, Chen W. A cancer vaccine induces expansion of NY-ESO-1-specific regulatory T cells in patients with advanced melanoma. PLoS One 2012, 7: e48424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakraborty NG, Chattopadhyay S, Mehrotra S, Chhabra A, Mukherji B. Regulatory T-cell response and tumor vaccine-induced cytotoxic T lymphocytes in human melanoma. Hum Immunol 2004, 65: 794–802. [DOI] [PubMed] [Google Scholar]
- 82.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood 2006, 107: 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R et al. . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010, 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ellis JS, Wan X, Braley-Mullen H. Transient depletion of CD4+ CD25+ regulatory T cells results in multiple autoimmune diseases in wild-type and B-cell-deficient NOD mice. Immunology 2013, 139: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan J, Mei FC, Cheng H, Lao DH, Hu Y, Wei J, Patrikeev I et al. . Enhanced leptin sensitivity, reduced adiposity, and improved glucose homeostasis in mice lacking exchange protein directly activated by cyclic AMP isoform 1. Mol Cell Biol 2013, 33: 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Warburg O. On respiratory impairment in cancer cells. Science 1956, 124: 269–270. [PubMed] [Google Scholar]
- 87.Warburg O. On the origin of cancer cells. Science 1956, 123: 309–314. [DOI] [PubMed] [Google Scholar]
- 88.Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov 2012, 2: 881–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pierre S, Eschenhagen T, Geisslinger G, Scholich K. Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov 2009, 8: 321–335. [DOI] [PubMed] [Google Scholar]
- 91.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013, 13: 714–726. [DOI] [PubMed] [Google Scholar]
- 92.Tsalkova T, Mei FC, Li S, Chepurny OG, Leech CA, Liu T, Holz GG et al. . Isoform-specific antagonists of exchange proteins directly activated by cAMP. Proc Natl Acad Sci USA 2012, 109: 18613–18618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu Y, Chen H, Boulton S, Mei F, Ye N, Melacini G, Zhou J et al. . Biochemical and pharmacological characterizations of ESI-09 based EPAC inhibitors: defining the ESI-09 “therapeutic window”. Sci Rep 2015, 5: 9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen H, Ding C, Wild C, Liu H, Wang T, White MA, Cheng X et al. . Efficient synthesis of ESI-09, a novel non-cyclic nucleotide EPAC antagonist. Tetrahedron Lett 2013, 54: 1546–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Banerjee U, Cheng X. Exchange protein directly activated by cAMP encoded by the mammalian rapgef3 gene: structure, function and therapeutics. Gene 2015, 570: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]