Summary
Objective
Understanding the neural mechanisms that support human consciousness is an important frontier in neuroscience and medicine. We previously developed a rodent model of temporal lobe seizures that recapitulates the human electroencephalography (EEG) signature of ictal and postictal neocortical slow waves associated with behavioral impairments in level of consciousness. The mechanism of slow-wave production in epilepsy may involve suppression of the subcortical arousal systems including the brainstem and intralaminar thalamic nuclei. We hypothesized that intralaminar thalamic stimulation may lead to electrophysiologic and functional rescue from postictal slow waves and behavioral arrest.
Methods
We electrically stimulated the central lateral thalamic nucleus (a member of the intralaminar nuclei) under anesthesia and after electrically induced hippocampal seizures in anesthetized and in awake-behaving animal model preparations.
Results
We demonstrated a proof-of-principle restoration of electrophysiologic and behavioral measures of consciousness by stimulating the intralaminar thalamic nuclei after seizures. We measured decreased cortical slow waves and increased desynchronization and multiunit activity in the cortex with thalamic stimulation following seizures. Functionally, thalamic stimulation produced resumption of exploratory behaviors in the postictal state.
Significance
Targeting of nodes in the neural circuitry of consciousness has important medical implications. Impaired consciousness with epilepsy has dangerous consequences including decreased school/work performance, social stigmatization, and impaired airway protection. These data suggest a novel therapeutic approach for restoring consciousness after seizures. If paired with responsive neurostimulation, this may allow rapid implementation to improve level of consciousness in patients with epilepsy.
Keywords: Deep brain stimulation, Epilepsy, Postictal, Consciousness, Thalamus, Cortical slow waves
The neural network involved in maintaining level of consciousness is important in epilepsy and other disorders of consciousness. The “network inhibition hypothesis” proposes a mechanism by which subcortical arousal circuits are depressed in ictal and postictal unconsciousness.1 Supported by human studies2,3 and animal model data that recapitulate the cortical electroencephalography (EEG) signature of ictal and postictal neocortical slow waves,4,5 this hypothesis rests on functional de-afferentation of the cortex.6,7 Inhibition of the brainstem reticular-activating system and one of its major targets, the intralaminar thalamic nuclei, may produce a cortical state resembling deep sleep, coma, or encephalopathy during and after seizures.1 This suggests that therapeutic neurostimulation of subcortical arousal structures could be a promising avenue for rapidly restoring consciousness following seizures.
The importance of the thalamus as a major network hub in ictal and postictal states of impaired consciousness has long been postulated.8 The rostral intralaminar thalamic group, composed of the central medial, paracentral, and central lateral nuclei, receives the densest input among thalamic nuclei from brainstem arousal structures.9 Early stimulation studies of the brainstem reticular formation and intralaminar thalamic nuclei showed decreased cortical synchronization under anesthesia.10,11 Central thalamic deep brain stimulation in a patient in a minimally conscious state after traumatic brain injury improved functional measures of arousal.12
Although neurostimulation has become commonplace for movement disorders, its use for epilepsy is emerging as a promising and safe technology for interrupting or abating seizure initiation or propagation. Chronic and responsive stimulation to cortical and deep brain structures has proven effective in some patients.13–18 No prior studies have considered neurostimulation for restoring impaired consciousness in the context of the epilepsies. This approach may be important for patients with seizure foci in eloquent or inaccessible brain areas, seizures refractory to conventional surgical therapies, generalized epilepsies refractory to medications, or surgically prohibitive comorbidities.
Herein we present a proof-of-principle preclinical study of electrophysiologic and behavioral rescue of measures of arousal in an animal model of secondarily generalized limbic seizures using intralaminar thalamic deep brain stimulation. We electrically stimulated the central lateral nucleus under anesthesia and after seizures in acute and chronic awake-behaving model preparations to provide evidence of reduced cortical slow-wave activity and increased behavioral arousal.
Methods
Animals
All procedures were done with approval by the Yale University Institutional Animal Care and Use Committee. Healthy adult female Sprague-Dawley rats weighing 180–300 g (Charles River Laboratories, Frederick, MD, U.S.A.) were used.
Surgery and electrode implantation
The animal model of partial seizures was prepared as described previously4 with the following modifications. Animals were deeply anesthetized with intramuscular injection 90 mg/kg ketamine (Henry Schein Animal Health, Ashburn, VA, U.S.A.) and 15 mg/kg xylazine (AnaSed; Lloyd Laboratories, Quezon City, Philippines). Craniotomies were placed stereotaxically over sites of planned electrode placement, three stainless steel anchoring screws (0–80×3/32; PlasticsOne, Roanoke, VA, U.S.A.) and a ground screw (E363-20; PlasticsOne) (Fig. S1A).
For “acute preparations,” a tungsten monopolar microelectrode (UEWMGGSEDNNM; FHC, Bowdoin, ME, U.S.A.) with impedance of 3–4 MΩ was implanted at an approach angle 20 degrees from vertical in right lateral orbitofrontal cortex (Ctx); anterior-posterior (AP) +4.2 mm and medial-lateral (ML) −2.2 mm from bregma, superior-inferior (SI) −2.4 mm from pial surface.19 For “chronic preparations,” a stainless-steel twisted-pair bipolar electrode (E363-2-2TW; PlasticsOne) cut to 20 mm and with electrode tips separated by 1 mm in the coronal plane was implanted vertically in right lateral orbitofrontal cortex (AP +4.2; ML +2.2; SI −2.4 mm). For both acute and chronic preparations, two twisted-pair bipolar electrodes with electrode tips in the sagittal plane were implanted in bilateral central lateral thalami (CL) (AP −2.8; ML ±1.5; SI −4.8 mm). A twisted-pair bipolar electrode with electrode tips separated by 1 mm, insulation shaved to 0.5 mm, and electrode tips in the coronal plane, was implanted in the right dorsal hippocampus (HC) (AP −3.8; ML −2.5; SI −2.6 mm). In three animals, a twisted-pair bipolar electrode with tips in the sagittal plane was implanted into the left rostral caudate-putamen (CP) (AP −0.3, ML +3.4, SI −4.4 mm). Caudate-putamen electrodes were unilaterally placed because of limitations of space with contralateral anchoring screws.
In chronic experiments, electrodes were firmly cemented to nearby anchoring screws with dental cement (Lang Dental Manufacturing, Wheeling, IL, U.S.A.). Electrode pins were then carefully bent and placed into two, 6-pin pedestals (MS363; PlasticsOne). Skin was reapproximated with interrupted silk sutures (Surgical Specialties Corp., Vancouver, BC, Canada) around each pedestal. Chronic recordings began at least 1 week postoperatively.
Electrophysiology and video recording procedures
Recordings were performed under the following three conditions: (1) Acute experiments under deep anesthesia were performed with ketamine/xylazine 90/15 mg/kg with the presence of continuous cortical slow wave activity; (2) acute experiments under light anesthesia were done after animals had recovered to a state when slow waves decreased to <3 per 10 s of recordings as described previously4; and (3) experiments in chronically implanted behaving animals were performed in the awake state, under anesthesia, or during natural sleep. A graphical representation of acute and chronic experiments is depicted in Figure S2.
Central lateral thalami were stimulated with square biphasic pulse (0.5 msec/phase) at 100 Hz for 20 s.12 Transistor-transistor logic (TTL) pulses for stimulation were programmed with a pulse stimulator (AMPI Master-8, Jerusalem, Israel); current was generated with a stimulus isolator unit (A365; WPI, Sarasota, FL, U.S.A.) connected to the bilateral thalamic CL electrodes.
Seizures in acute experiments were induced with a two-second biphasic 60 Hz hippocampal stimulus (1 msec per phase)generated with an isolated pulse stimulator (A-M Systems Model 2100, Carlsborg, WA, U.S.A.), with current titrated for individual animals from 100 µA to 1.5 mA. In chronic experiments, seizures were elicited with a higher current (>2 mA, titrated individually per animal) needed to effectively evoke a postictal period in awake animals associated with postictal hippocampal depression, impaired behavior, and cortical slow waves. Stimulations done under anesthesia in chronically implanted animals were performed after intramuscular injection of solution containing approximately 15 mg/kg ketamine and 3 mg/kg xylazine. This dose was selected to attain sufficient anesthetic depth to produce a state of slow-wave activity and behavioral arrest, yet not as deep as that used during surgical procedures. A set of thalamic CL stimulations and sham stimulations were also performed during periods of naturally occurring sleep; alternating sham and thalamic CL stimulations were done in succession to grossly control for ambient noise and light conditions.
Cortical, hippocampal, and intralaminar thalamic CL signals were recorded on separate channels of microelectrode amplifiers (A-M Systems Model 1800). In chronically implanted animals, two, 6-pin connector wires (Plastic-sOne) were connected to two commutators (PlasticsOne), and to the head stages of the amplifiers using customized 6-pin connectors (363SL-6; PlasticsOne). Hippocampal and thalamic CL signals were filtered at 1–500 Hz on the recording amplifiers with 1,000× gain. Cortical activity was amplified 100× by the recording amplifier. The cortical local field potential (LFP) was then filtered 1–100 Hz with 15× gain (Model 3364; Krohn-Hite, Brockton, MA, U.S.A.). For acute recordings where higher impedance electrodes were used for the cortex, we split the signal and filtered the same recording for LFP (as above) as well as for multiunit activity (MUA) at 400–10,000 Hz with 20× gain. All signals were digitized with analog-to-digital converter (Power 1401; CED, Cambridge, United Kingdom) using Spike2 (Version 5.2; CED); cortical LFP, thalamic CL LFP, and hippocampal LFP were acquired at sampling frequency of 1,000 Hz; cortical MUA was acquired at 20,000 Hz. For chronic recordings, electrophysiology-synchronized video was captured with a digital camera (Hercules, La Gacilly, France) and recorded with Spike2 Video Recorder (CED).
After completion of electrophysiologic recordings, the animal was sacrificed with a 0.5 mL intraperitoneal injection of pentobarbital sodium and phenytoin sodium solution (Euthasol; Virbac, Fort Worth, TX, U.S.A.) for histologic localization of electrodes.
Histology
Implanted animals were transcardially perfused with 0.1% heparinized phosphate-buffered saline (PBS) (APP Pharmaceuticals, Lake Zurich, IL, U.S.A.) followed by 4% paraformaldehyde (JT Baker, Center Valley, PA, U.S.A.) in PBS. Brains were then removed and post-fixed for 3 days in 4% paraformaldehyde (PFA) in PBS at 4°C. Brains were washed thrice in PBS, placed in 2% agarose gel (American Bioanalytical, Natick, MA, U.S.A.) and cut at 100 µm on a Vibratome (Leica Microsystems, Wetzlar, Germany). Slices were mounted on polarized slides (ThermoScientific, Waltham, MA, U.S.A.) and stained with cresyl violet to confirm electrode location using a manufacturer-recommended protocol for reagents (FD NeuroTechnologies, Columbia, MD, U.S.A.). Slides were then briefly dried and coverslipped with Permount (Fisher Chemicals, Pittsburgh, PA, U.S.A.). Images were taken at 40× magnification on a compound light microscope (Carl Zeiss, Oberkochen, Germany), imaged with a digital camera (Motic, Hong Kong), and digitally stitched together (Microsoft Image Composite Editor, Redmond, WA, U.S.A.). Electrode locations for the central lateral thalamus were confirmed using the following landmarks: location ventral to the hippocampus in the dorsal thalamus, and lateral to the stria medullaris/habenula complex (Fig. S1B,C).
Data analysis and statistics
Data were analyzed in Spike2 (CED) with supplementary custom scripts in MATLAB (MathWorks, Natick, MA, U.S.A.). Power was measured from the cortical LFP (FFT size 1.024s, Hanning window). Prestimulus period was designated as 10 s immediately prior to stimulation; intrastimulus was designated as 10 s, beginning 1 s after initiation of stimulation to allow for amplifier recovery; poststimulus was designated as 10 s immediately after stimulation ended. Power was normalized by average prestimulus power. Delta-band (0–4 Hz) time-course analysis was performed using 10 s epochs with a custom script written in MATLAB. Average root-mean-square amplitude of cortical MUA (sliding analysis window of 200 msec) over the same 10 s pre-, intra- and poststimulus epochs described earlier were used as an estimate of action potential firing. Root-meansquare of MUA has been shown to correspond well to spiking rate as measured by template matching.20
Seizures elicited in chronically implanted animals were behaviorally staged by the Racine scale.21 In addition, behavioral level of arousal was measured by rating exploratory activity using a scale previously developed and used in this animal model.5 A score of 0–3 was assigned for each 10 s epoch before, during, and after thalamic CL stimulation, according to the following: 0, no exploratory movements; 1, forepaw exploration of cage floor; 2, forepaw used to explore or climb sides of cage; and 3, locomotion requiring both forelimbs and hind limbs. For behavioral analysis, two 10-s epochs (20-s total) prior to stimulation, during the stimulation, and after stimulation were analyzed, and average scores were obtained for each state. These epochs included the 10-s windows for which cortical LFP power was analyzed. Ratings were performed by two observers (one unblinded and one blinded to stimulations) and averaged. Interobserver reliability was measured and reported as a kappa statistic.
In all experiments, if more than one trial was replicated within an animal, intra-animal data were averaged prior to performing group statistics. Data were reported as group mean ± standard error of mean. Statistical significance between groups/states was assessed using two-tailed Student’s t-test with α = 0.05 and uncorrected p-values are reported; however, significance threshold was corrected for multiple comparisons with the Bonferroni method where appropriate. In comparing exploratory behavior between groups, Fisher’s exact test was performed on behavioral ratings with scores of 0 versus scores ≥1.
Results
Intralaminar thalamic CL stimulation activates the cortex during deep anesthesia
While the animals were deeply anesthetized we electrically stimulated bilateral intralaminar thalamic CL electrodes to determine effective stimulus parameters to produce physiologic cortical arousal. Initial experiments used a range of stimulus amplitudes from 100 µA to 1 mA (data not shown) with unilateral and bilateral stimulation paradigms; 400 µA stimulus (approximately 200 µA per side with bilateral stimulation) was found to be optimal so was used in subsequent recordings. This was compared to a 0.1 µA control CL stimulus (sham stimulation) in the same animals. A subset of animals was also stimulated in the rostral caudate-putamen, a region not hypothesized to be part of the circuit directly involved in modulating arousal, to serve as a negative control of target-nonspecific deep brain stimulation.
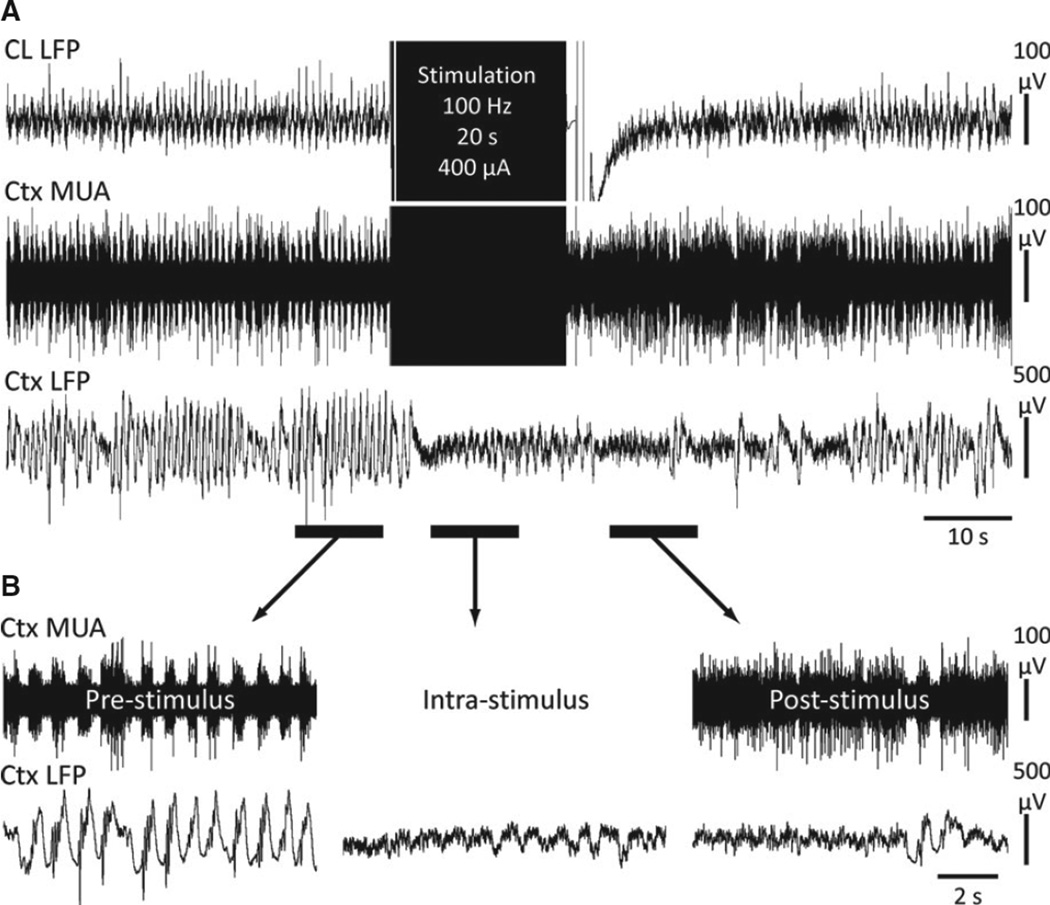
The large-amplitude 1–3 Hz slow waves that are prominent and continuous in the cortical LFP during deep anesthesia were eliminated during and after thalamic CL stimulation (Fig. 1). Persistent cortical desynchronization following the stimulus was associated with a sustained increase in neuronal activity measured in cortical MUA (Fig. 1B, right trace). In the stimulation current range used in our experiments, no thalamic afterdischarges or seizure-like activity were measured in the thalamic CL or hippocampal electrodes after stimulation. Cortical desynchronization of LFP was not seen in control stimuli with sham stimulation in thalamic CL or with 200 µA unilateral stimulation in caudate-putamen (data not shown). Again during caudate-putamen stimulation, no afterdischarges or seizure-like activity was measured in the hippocampus or thalamic CL in the current ranges tested.
Figure 1.
Central lateral thalamic stimulation during deep anesthesia decreases cortical slowing and increases cortical neuronal firing. (A) Example stimulation of CL under deep ketamine/xylazine anesthesia. (B) Magnified insets of the marked pre-, intra-, and poststimulus epochs exemplify desynchronized cortical LFP intrastimulus which is persistent post-stimulation and is then associated with sustained tonic cortical MUA. Ctx, frontal cortex; CL, central lateral thalamus; MUA, multiunit activity; LFP, local field potential.
Epilepsia © ILAE
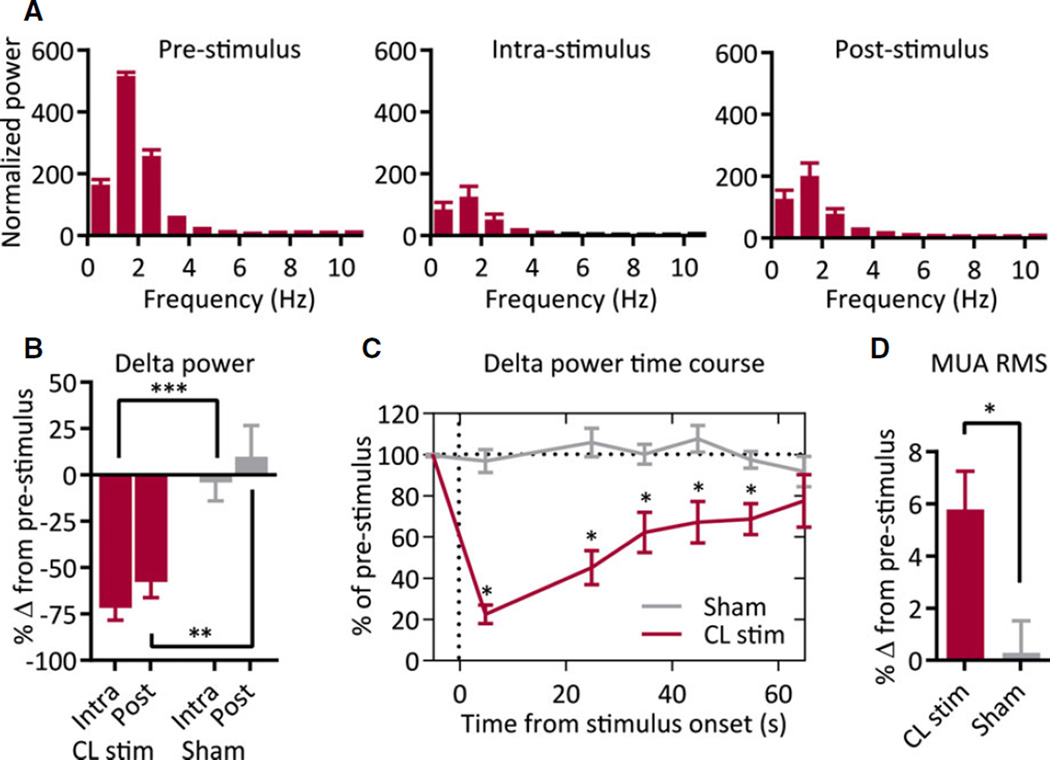
To quantify loss of low-frequency cortical activity, LFP power (normalized to the average prestimulus power within animal) was measured in 10-s epochs immediately prior to, during, and after stimulation. We observed decreases in low-frequency power during and after stimulation, especially notable at delta- and theta-band frequencies (Fig. 2A; n = 12 animals). This pattern was not present with CL sham stimulation (n = 12) or caudate-putamen stimulation (n = 3). Average delta-band power (0–4 Hz) was significantly decreased during (−72.1 ± 6.7%) and after (−58.1 ± 8.6%) stimulation compared to prestimulus (Fig. 2B; n = 12; intrastimulus, p < 0.0001; poststimulus p < 0.0001) and compared to sham stimulation (Fig. 2B; n = 12; intrastimulus, p < 0.0001; poststimulus, p = 0.008). No significant change in delta-band power was measured with thalamic CL sham stimulation compared to prestimulus (Fig. 2B; CL sham; n = 12; intrastimulus, p = 0.67; poststimulus, p = 0.59) or caudate-putamen stimulation compared to prestimulus (n = 3; intrastimulus, p = 0.73; poststimulus, p = 0.44).
Figure 2.
Group data for thalamic CL stimulation under anesthesia. (A) Thalamic CL stimulation under anesthesia decreases low-frequency frontal cortical power during intrastimulus and poststimulus epochs; n = 12 animals. (B) Delta-band (0–4 Hz) power significantly decreases during intrastimulus and poststimulus epochs as compared to sham stimulation. (C) Time course of decreases in delta-band power is sustained for >50 s after 20 s stimulation. Stimulus-onset time = 0 s. (D) Decrease in low-frequency power is associated with a significant increase in MUA; n = 12 animals. All results are mean ± standard error of the mean (SEM). MUA, multiunit activity; RMS, root-mean-square amplitude; *p < 0.05, **p < 0.01, ***p < 0.001. Comparisons are two-tailed Student’s t-test.
Epilepsia © ILAE
The loss of cortical slow waves persisted after stimulation, and slow waves gradually resumed over the 60 s after stimulation ended. Delta-band power after thalamic CL stimulation remained significantly lower than prestimulus delta-band power and sham-stimulation delta-band power until >50 s poststimulus (Fig. 2C; n = 12/group, p < 0.001 for epochs 0–30 s, p < 0.01 for epochs 30–60 s).
In acute experiments under anesthesia, we also investigated the effects of thalamic CL stimulation on cortical neuronal firing to confirm that the observed physiologic changes represented cortical arousal. Prior to stimulation, the slow waves present during anesthesia were accompanied by alternating UP and DOWN states of neuronal firing as described previously.22 After stimulation, tonic cortical MUA was seen (Fig. 1B), similar to activity described previously for a lightly anesthetized or awake state.4 To quantify increased cortical activity associated with LFP desynchronization, we calculated the root-mean-square of cortical MUA as a proxy for cortical neuronal firing.4,20 We measured a significantly increased amount of cortical unit activity after CL stimulation compared to the prestimulus epoch by 5.8 ± 1.5% (n = 12; p = 0.002) and compared to sham stimulation (Fig. 2D; n = 12; p = 0.01). Cortical firing after sham thalamic CL stimulation displayed no significant difference from that before stimulation (0.3 ± 1.3%; n = 12; p = 0.81).
Thalamic CL stimulation is effective in eliminating postictal cortical slowing
Animals that were initially anesthetized to surgical depth were allowed to recover to a level of light anesthesia indicated by predominance of cortical fast activity on LFP as described previously.4,5, Seizures were then induced with brief hippocampal stimulation to produce secondarily generalized seizures. Ten seconds after seizure termination, bilateral CL thalami were again stimulated with 400 µA (approximately 200 µA per side) or with 0.1 µA sham stimulation, and a subset of animals was also stimulated with 200 µA unilateral caudate-putamen stimulation to control for nonspecific stimulus effects.
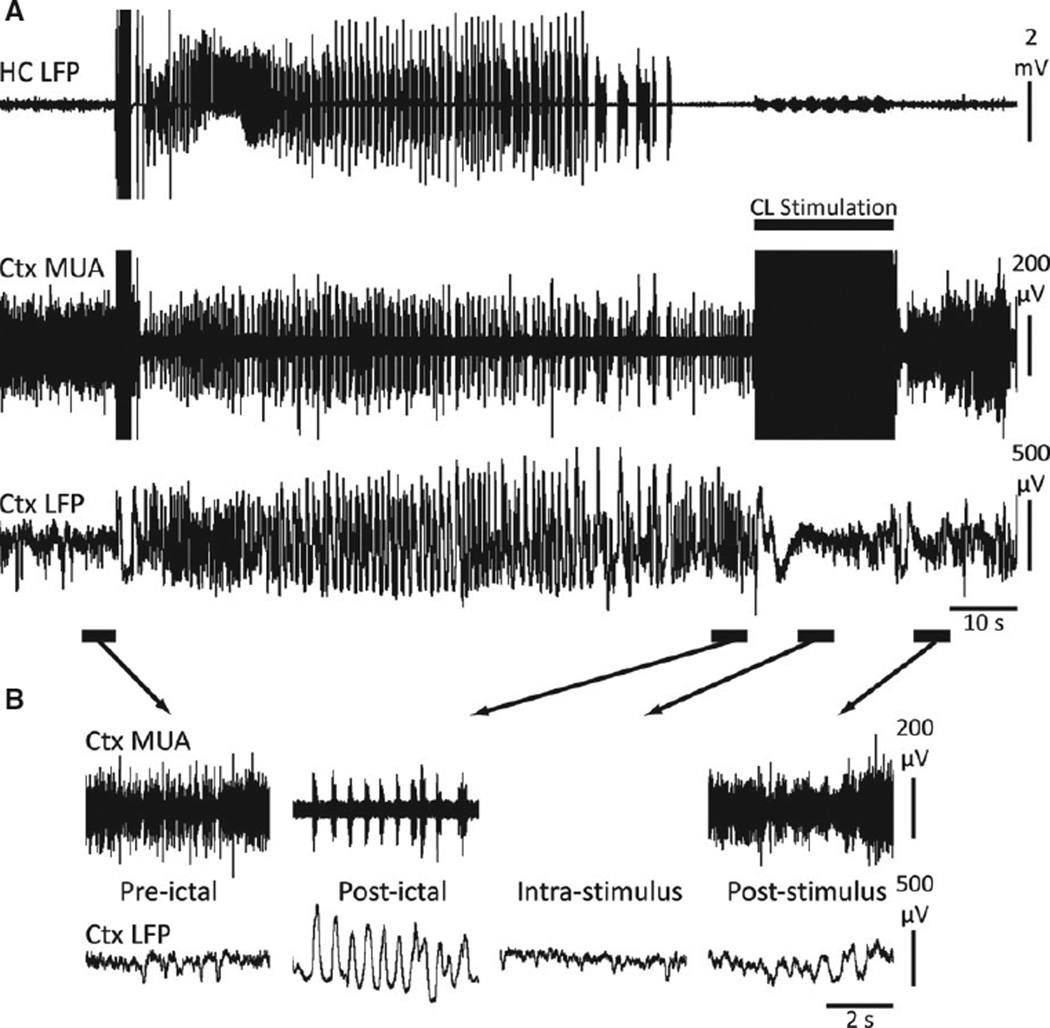
After seizure termination, we observed postictal cortical slow waves (Fig. 3B), previously known to occur following acute seizures. 4,5 Intralaminar thalamic CL stimulation during the postictal state abolished cortical slow waves during and after stimulation (Fig. 3A,B). CL stimulations were associated with persistently increased cortical activity following stimulation as indicated by sustained MUA increases (Fig. 3B).
Figure 3.
Central lateral thalamic stimulation in the postictal period decreases cortical slowing and increases cortical MUA. (A) Example of central lateral thalamic stimulation in the postictal period. Similar to thalamic stimulation under anesthesia, postictal slow waves are abolished with CL stimulation. (B) Magnified insets of the indicated preictal, postictal, intrastimulus, and poststimulus epochs. “Postictal” slowing (which resembles the cortical slow waves of anesthesia) are eliminated in the “intrastimulus” and “poststimulus” epochs. After stimulation, cortical MUA shows persistent activity similar to the “preictal” epoch. HC, hippocampus; CL, central lateral thalamus; Ctx, frontal cortex; MUA, multiunit activity; LFP, local-field potential.
Epilepsia © ILAE
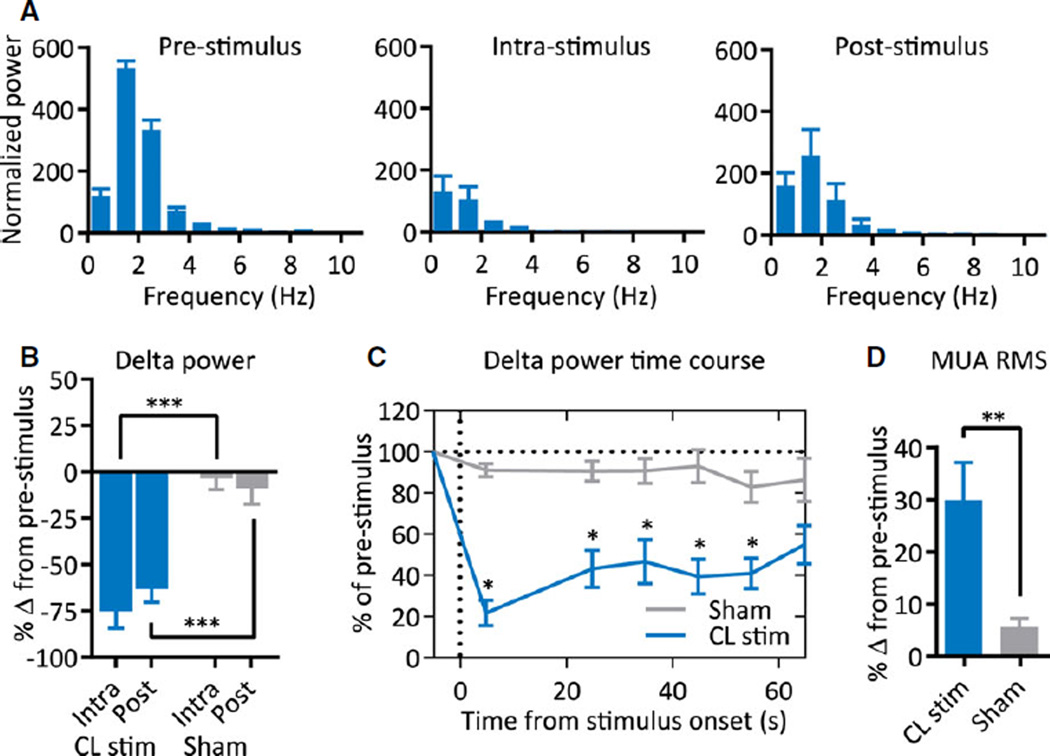
Group analysis of cortical LFP power showed decreases of low-frequency power during and after stimulation in the postictal period (Fig. 4A; n = 8). These changes appeared similar to those seen with stimulation under deep anesthesia (Fig. 2). Delta-band power significantly decreased during (−75.1 ± 9.2%) and after (−63.1 ± 7.1%) thalamic CL stimulation in the postictal period (n = 8; intrastimulus and poststimulus, p < 0.0001). These decreases were significantly greater than the decreases seen with sham stimulation (Fig. 4B; n = 8; intra-stimulus, p = 0.0001; post-stimulus, p = 0.0006). Control stimulation of caudate-putamen in the postictal period produced no decreases in delta-band power compared to prestimulus (n = 3; intrastimulus, p = 0.68, poststimulus, p = 0.37). Delta-band power after thalamic CL stimulation in the postictal period remained significantly lower than prestimulus delta-band power and sham-stimulation delta-band power until >50 s poststimulus (Fig. 4C; n = 8, p < 0.001 for epochs 0–50 s, p < 0.01 for 50–60 s epoch).
Figure 4.
Group data for thalamic CL stimulation in the postictal period. (A) Postictal thalamic CL stimulation decreases low-frequency power during intrastimulus and poststimulus epochs; n = 8 animals. (B) Delta-band (0–4 Hz) power significantly decreases during intrastimulus and poststimulus epochs as compared to sham stimulation. (C) Time course of decreases in delta-band power is sustained for >50 s after 20 s stimulation. Stimulus-onset time = 0 s. (D) Decrease in low-frequency power is associated with a significant increase in MUA; n = 8 animals. All results are mean ± SEM. MUA, multiunit activity; RMS, root-mean-square amplitude; *p < 0.05, **p < 0.01, ***p < 0.001. Comparisons are two-tailed Student’s t-test.
Epilepsia © ILAE
We again measured root-mean-square of cortical MUA as a proxy for cortical firing, which showed a dramatic increase following thalamic CL stimulation compared to sham stimulation (Fig. 4D; see also MUA in Fig. 3B, post-stimulus inset). The increase in cortical neuronal firing was greater with stimulation in the postictal period compared to prestimulus (n = 8; 29.9 ± 7.1%; p = 0.004) and sham stimulation (Fig. 4D; n = 8; p = 0.005). Of interest, cortical MUA was also increased from prestimulus with sham stimulation (n = 8; 5.5 ± 1.7%; p = 0.01), although notably less than with CL stimulation. This slight MUA increase was likely due to the beginning stages of natural recovery from the postictal state.
Thalamic CL stimulation improves postictal behavioral arousal
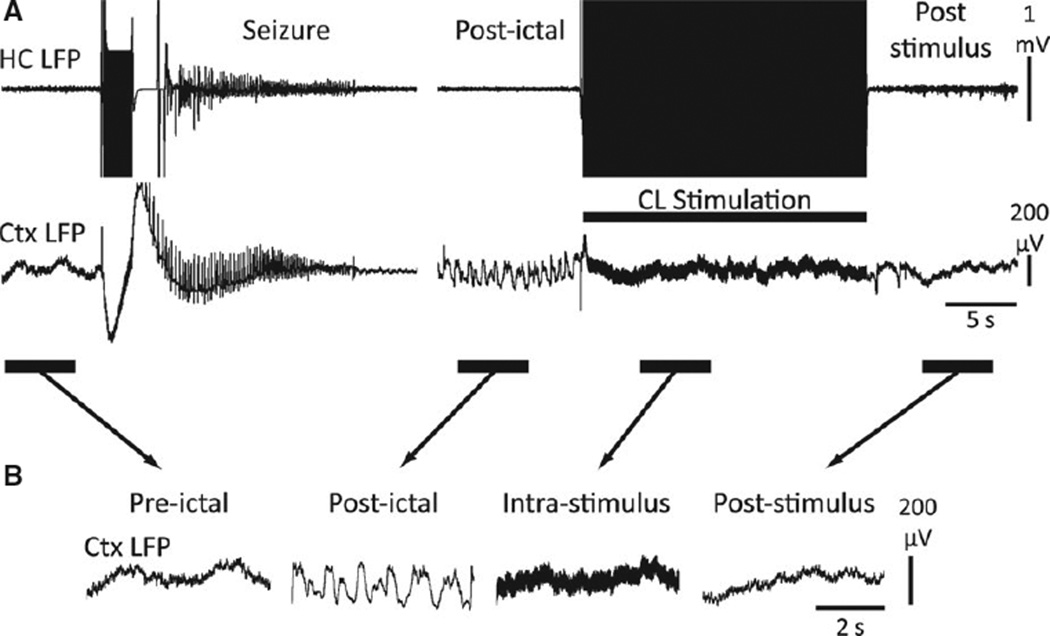
We next aimed to answer if electrophysiologic markers of improved consciousness (cortical desynchronization and increased multiunit cortical activity) during postictal stimulation translated to behavioral signs of arousal. To produce a consistent state of postictal slow wave activity and behavioral arrest in the awake-behaving animal, we used hippocampal current sufficient to produce secondarily generalized seizures (Racine class 5). In the postictal period, the animals showed behavioral arrest associated electrophysiologically with increased low-frequency large-amplitude cortical slow waves that occurred within minutes after the seizure (Fig. 5A). These slow waves were similar to those that appeared in the lateral orbital frontal cortex as the animal was naturally sleeping or under ketamine/xylazine anesthesia.
Figure 5.
Thalamic CL stimulation in the postictal period abolishes cortical slow-wave activity. (A) Example of central lateral thalamic stimulation in the postictal period after a secondarily generalized seizure in an awake-behaving animal. Break in recording display is 255 s to show postictal slow-wave activity just prior to CL stimulation. (B) Magnified insets of the indicated preictal, postictal, intrastimulus, and poststimulus epochs. Cortical slow waves are seen in the “postictal” epoch, which is dramatically reduced during “intrastimulus” and “poststimulus” epochs. The poststimulus epoch resembles the baseline preictal epoch. HC, hippocampus; CL, central lateral thalamus; Ctx, frontal cortex; LFP, local field potential.
Epilepsia © ILAE
In the postictal period, we then stimulated bilateral CL thalami with a stimulus (current titrated individually per animal; range 400–1,000 µA) or 0.1 µA sham stimulus. We observed resumption of normal-appearing exploratory behaviors that began to increase during thalamic CL stimulation, often accompanied by increased respirations, and then exploratory behavior increased more frequently after stimulation. Thalamic CL stimulation abolished postictal slow waves in the cortical LFP during and after the stimulus (Fig. 5B).
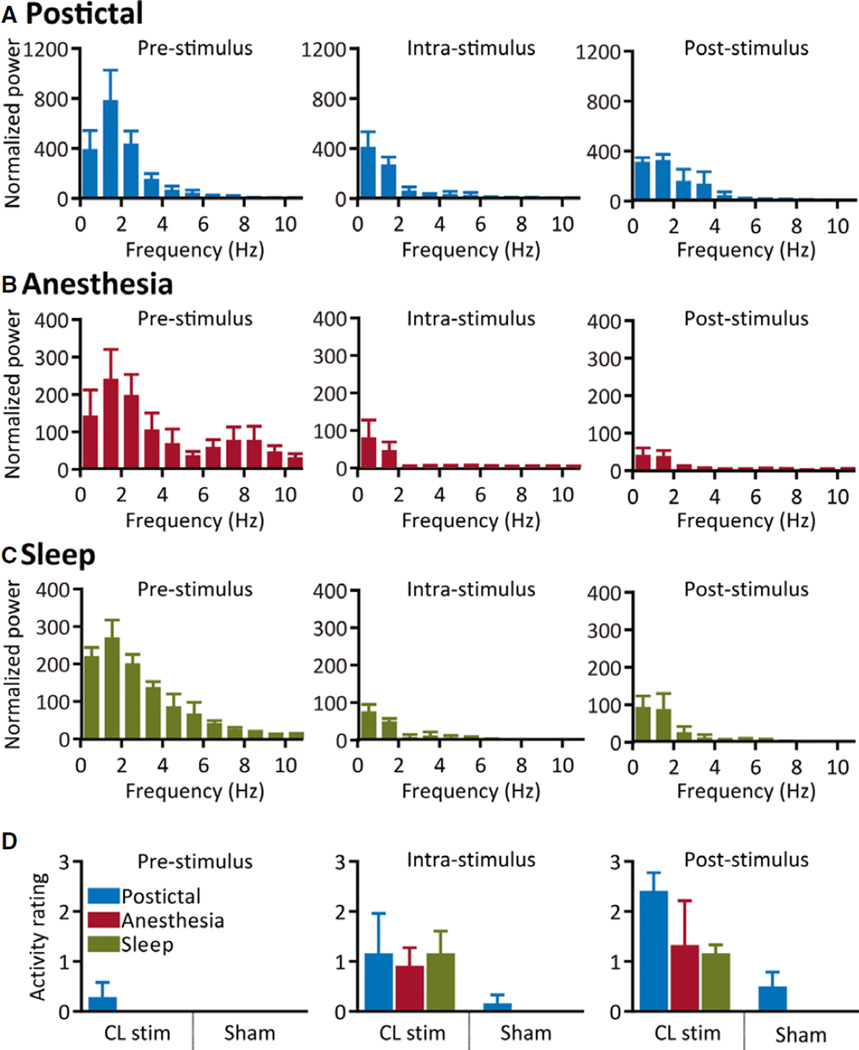
The decreases in low-frequency power following intralaminar thalamic CL stimulation in awake chronically implanted animals (Fig. 6A) resembled the effect of stimulation in the acute preparation. In the postictal period, we found significantly decreased cortical delta-band power during CL stimulation and a trend to significance after stimulation compared to prestimulus (n = 3; intrastimulus, p = 0.001; poststimulus, p = 0.06). Postictal exploratory behavior after thalamic CL stimulation significantly improved compared to sham stimulations (n = 3; intrastimulus, p = 0.15; poststimulus, p < 0.001, Fisher’s exact test; Fig. 6D). Ratings were performed by two observers with high interobserver reliability (κ = 0.86).
Figure 6.
Group data for thalamic CL stimulation in awake-behaving and chronically implanted animals. (A) Postictal central lateral thalamic stimulation in the awake-behaving animal decreases low-frequency power during and after CL stimulation. (B, C) Similar patterns of low-frequency power decreases are measured with thalamic CL stimulation during anesthesia (B) or natural sleep (C) in chronically implanted animals. (D) Significant increases in exploratory behaviors are observed following stimulation (“poststimulus”) from the postictal state and sleep; a small (nonsignificant) increase is also measured after stimulations during anesthesia. No changes are seen with sham stimulation. All results are plotted as mean ± SEM, n = 3 animals per condition.
Epilepsia © ILAE
We noted comparable decreases in cortical low-frequency signals with stimulation from light anesthesia (Fig. 6B) and from naturally occurring sleep (Fig. 6C) in chronically implanted animals. Delta activity decreased in thalamic CL stimulations during anesthesia (n = 3; intrastimulus p = 0.0001; poststimulus p = 0.02) and sleep (n = 3; intrastimulus p = 0.03; poststimulus p = 0.007) compared to prestimulus. Exploratory activity ratings above 0 also tended to increase after CL stimulation from light anesthesia or sleep, but not with sham stimulations (anesthesia: n = 3; intrastimulus, p = 0.18; poststimulus, p = 0.06, Fisher’s exact test) (sleep: n = 3; intrastimulus, p = 0.02; poststimulus, p = 0.002; Fig. 6D). Ratings were performed by two observers with high interobserver reliability (anesthesia: κ = 0.94; sleep: κ = 1.0).
Discussion
In summary, these results demonstrated that high-frequency intralaminar thalamic stimulation increased cortical physiologic and behavioral arousal in the postictal state. Acute electrophysiologic recordings provided local field potential and multiunit activity data from the cortex to show an awake-like state during and after intralaminar thalamic stimulations during anesthesia as well as in the postictal period. Thalamic stimulation in chronically implanted animals demonstrated that the stimulation-induced cortical de-synchronization on local field potential recordings translated to behavioral increases in activity from the postictal state. To our knowledge, these results are the first preclinical data of neurostimulation to improve postictal consciousness.
The stimulation parameters used in these experiments are comparable to those used in the single-patient human study of central thalamic deep brain stimulation (DBS) after traumatic brain injury.12 The mechanism of high frequency DBS is undoubtedly more complex than activating or inactivating a nucleus or pathway, and is highly location specific. Our results support the hypothesis that low-amplitude high-frequency stimulation has greater effect on depolarizing axons leaving the central lateral thalamic nucleus to activate the cortex.23 The nonspecific nature of electrical stimulation acting on local elements leads to more complete activation of the nucleus (compared to optogenetic approaches), however, is less selective. We did not discern any clear relationship between electrode location within the central lateral nuclei and efficacy of stimulation, although this merits further investigation. Prior work on the mechanism of DBS has indicated that stimulation of a variety of targets results in cortical desynchronization as a consequence of the network effects invoked by neurostimulation.23 We concluded that there was target specificity in the circuit proposed by the network inhibition hypothesis1 based on our observations that intralaminar thalamic but not rostral caudate-putamen stimulation is associated with loss of cortical slow waves during anesthesia and the postictal period.
The relation of the nonspecific intralaminar thalamocortical circuitry to arousal including sleep, anesthesia, awareness, and attention has been well described with anatomic, neuroimaging, and electrophysiologic evidence, which has indicated its role in organizing cortical activation.9,22,24–26 Prior animal studies of rostral intralaminar thalamic stimulation targeting the same nuclei and with similar stimulation frequencies showed increased measures in working memory and visual object recognition.27,28 Electrophysiologic evidence from nonhuman primates showed that central lateral thalamic firing and stimulation were related to attention and task performance29; central thalamic stimulation in a human patient with traumatic brain injury demonstrated improved functional outcomes including arousal.12 Neuroimaging evidence from patients with epilepsy supports the hypothesis that changes in thalamic nuclei underlie ictal and postictal cortical dysfunction after partial and secondarily generalized seizures associated with impaired levels of consciousness.30,31 We add that cortical activation following postictal thalamic stimulation leads to improved exploratory behavior accompanied by decreased cortical slow-wave power during and after stimulation. Our results, in the context of many studies relating intralaminar thalamic stimulation to arousal, suggest a plausible new avenue for addressing postictal changes in consciousness and cognition.
Future studies may address optimal stimulation parameters and paradigms, including time of stimulation after seizure onset. In preliminary studies (not shown), we noted more limited effects of thalamic stimulation on ictal cortical activity, possibly because of the magnitude of direct inhibition of subcortical arousal structures, including the intralaminar thalamus during seizures. Determining optimal stimulus parameters to potentially improve arousal during seizures requires further study. As an alternative, neurostimulation of brainstem reticular nuclei such as the pedunculopontine tegmental nucleus may provide another target to improve arousal.32 Another set of questions regards the effects of intralaminar thalamic neurostimulation on interictal discharges and ictal seizure frequency and severity in models of spontaneous epilepsy. A randomized controlled trial of intermittent high-frequency stimulation in the anterior thalamus reduced seizure frequency in patients with epilepsy (SANTE trial)33; in contrast, we observe improvement in level of arousal with intralaminar thalamic stimulation, but its effect on seizure frequency may also be worth investigating. Finally, more detailed behavioral testing involving task performance by awake-behaving animals would be helpful as an extension of the straightforward behavioral observations reported here.
The postictal state often persists for a far greater duration than its preceding seizure. Impairments in consciousness after seizures have significant consequences including decreased productivity, school/work performance, and social stigmatization, and may even put the patient’s life at risk. However, few studies and treatments directly address postictal morbidity.34 Studies indicate the postictal period is an impaired state of consciousness with electrophysiologic cortical depression and slowing,2 decreased cortical blood flow and metabolism,35 analgesia,36 and neurocognitive disturbances.37 Impaired arousal in combination with respiratory depression during and after seizures requires further study, as it may underlie postictal asphyxia and sudden unexplained death in epilepsy (SUDEP) occurrence.38,39 Improving the level of consciousness in the postictal state may have a major benefit on quality of life, cognition, and complications. For patients with medically or surgically refractory epilepsies, neurostimulation of the intralaminar thalamus to improve or prevent impairments in consciousness could have immense therapeutic potential. Recent advances in functional neurosurgery and responsive neurostimulation set an exciting stage for rapid translation of preclinical findings to therapies for patients with epilepsy.
Supplementary Material
Acknowledgments
We thank Robert Kim and Will Chen for scripts used in time-course data analysis; Florian Serout and Adeolu Morawo for assistance with awake recordings; Dr. Dennis Spencer, Dr. Jason Gerrard, and John Andrews for helpful comments on the manuscript. This work was supported by Howard Hughes Medical Institutes – Citizens United for Research against Epilepsy (HHMI-CURE) Medical Student Fellowship(AG), NIH R01 NS066974 (HB), and R21 NS083783 (HB), and by the Betsy and Jonathan Blattmachr Family.
Biography

Abhijeet Gummadavelli is an MD student at Yale School of Medicine pursuing a career in neurosurgery.
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Additional Contributors
AG, JEM, QZ, NDS, and HB designed experiments. AG performed experiments, analyzed data, and composed figures. AG and NS performed histology. AG and HB revised figures and wrote and revised the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Summary of electrode placements.
Figure S2. Schematic of experimental design for acute and chronic experiments.
References
- 1.Blumenfeld H. Impaired consciousness in epilepsy. Lancet Neurol. 2012;11:814–826. doi: 10.1016/S1474-4422(12)70188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenfeld H, Rivera M, McNally KA, et al. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004;63:1015–1021. doi: 10.1212/01.wnl.0000141086.91077.cd. [DOI] [PubMed] [Google Scholar]
- 3.Englot DJ, Yang L, Hamid H, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–3777. doi: 10.1093/brain/awq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Englot DJ, Mishra AM, Mansuripur PK, et al. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28:9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Englot DJ, Modi B, Mishra AM, et al. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 2009;29:13006–13018. doi: 10.1523/JNEUROSCI.3846-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giacino JT, Fins JJ, Laureys S, et al. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol. 2014;10:99–114. doi: 10.1038/nrneurol.2013.279. [DOI] [PubMed] [Google Scholar]
- 8.Penfield W. Epilepsy and surgical therapy. Arch Neurol Psychiatry. 1936;36:449–484. [Google Scholar]
- 9.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 10.Morison RS, Dempsey EW, Morison BR. Cortical responses from electrical stimulation of the brain stem. Am J Physiol. 1941;131:0732–0743. [Google Scholar]
- 11.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 12.Schiff ND, Giacino JT, Kalmar K, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 13.Fisher RS, Velasco AL. Electrical brain stimulation for epilepsy. Nat Rev Neurol. 2014;10:261–270. doi: 10.1038/nrneurol.2014.59. [DOI] [PubMed] [Google Scholar]
- 14.Heck CN, King-Stephens D, Massey AD, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55:432–441. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kossoff EH, Ritzl EK, Politsky JM, et al. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia. 2004;45:1560–1567. doi: 10.1111/j.0013-9580.2004.26104.x. [DOI] [PubMed] [Google Scholar]
- 16.Labar D, Dakov P, Kobylarz E, et al. Effects of responsive electrical brain stimulation on intracranial electroencephalogram spikes. Neuromodulation. 2013;16:355–361. doi: 10.1111/ner.12039. discussion 362. [DOI] [PubMed] [Google Scholar]
- 17.Morrell MJ Group RNSSiES. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 18.Rolston JD, Englot DJ, Wang DD, et al. Comparison of seizure control outcomes and the safety of vagus nerve, thalamic deep brain, and responsive neurostimulation: evidence from randomized controlled trials. Neurosurg Focus. 2012;32:E14. doi: 10.3171/2012.1.FOCUS11335. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 20.Schridde U, Khubchandani M, Motelow JE, et al. Negative BOLD with large increases in neuronal activity. Cereb Cortex. 2008;18:1814–1827. doi: 10.1093/cercor/bhm208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 22.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 23.Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002;17(Suppl. 3):S69–S72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- 24.Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- 25.Kinomura S, Larsson J, Gulyas B, et al. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- 26.Steriade M, Contreras D, Amzica F, et al. Synchronization of fast (30- 40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J Neurosci. 1996;16:2788–2808. doi: 10.1523/JNEUROSCI.16-08-02788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mair RG, Hembrook JR. Memory enhancement with event-related stimulation of the rostral intralaminar thalamic nuclei. J Neurosci. 2008;28:14293–14300. doi: 10.1523/JNEUROSCI.3301-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirvalkar P, Seth M, Schiff ND, et al. Cognitive enhancement with central thalamic electrical stimulation. Proc Natl Acad Sci U S A. 2006;103:17007–17012. doi: 10.1073/pnas.0604811103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah SA, Baker JL, Ryou JW, et al. Modulation of arousal regulation with central thalamic deep brain stimulation. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3314–3317. doi: 10.1109/IEMBS.2009.5333751. [DOI] [PubMed] [Google Scholar]
- 30.Blumenfeld H, Varghese GI, Purcaro MJ, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varghese GI, Purcaro MJ, Motelow JE, et al. Clinical use of ictal SPECT in secondarily generalized tonic-clonic seizures. Brain. 2009;132:2102–2113. doi: 10.1093/brain/awp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alessandro S, Ceravolo R, Brusa L, et al. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J Neurol Sci. 2010;289:44–48. doi: 10.1016/j.jns.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 34.Fisher RS, Schachter SC. The postictal state: a neglected entity in the management of epilepsy. Epilepsy Behav. 2000;1:52–59. doi: 10.1006/ebeh.2000.0023. [DOI] [PubMed] [Google Scholar]
- 35.Rowe CC, Berkovic SF, Austin MC, et al. Patterns of postictal cerebral blood flow in temporal lobe epilepsy: qualitative and quantitative analysis. Neurology. 1991;41:1096–1103. doi: 10.1212/wnl.41.7.1096. [DOI] [PubMed] [Google Scholar]
- 36.Coimbra NC, Castro-Souza C, Segato EN, et al. Post-ictal analgesia: involvement of opioid, serotoninergic and cholinergic mechanisms. Brain Res. 2001;888:314–320. doi: 10.1016/s0006-8993(00)03103-6. [DOI] [PubMed] [Google Scholar]
- 37.Helmstaedter C, Elger CE, Lendt M. Postictal courses of cognitive deficits in focal epilepsies. Epilepsia. 1994;35:1073–1078. doi: 10.1111/j.1528-1157.1994.tb02557.x. [DOI] [PubMed] [Google Scholar]
- 38.Devinsky O. Sudden, unexpected death in epilepsy. N Engl J Med. 2011;365:1801–1811. doi: 10.1056/NEJMra1010481. [DOI] [PubMed] [Google Scholar]
- 39.Massey CA, Sowers LP, Dlouhy BJ, et al. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol. 2014;10:271–282. doi: 10.1038/nrneurol.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.