Abstract
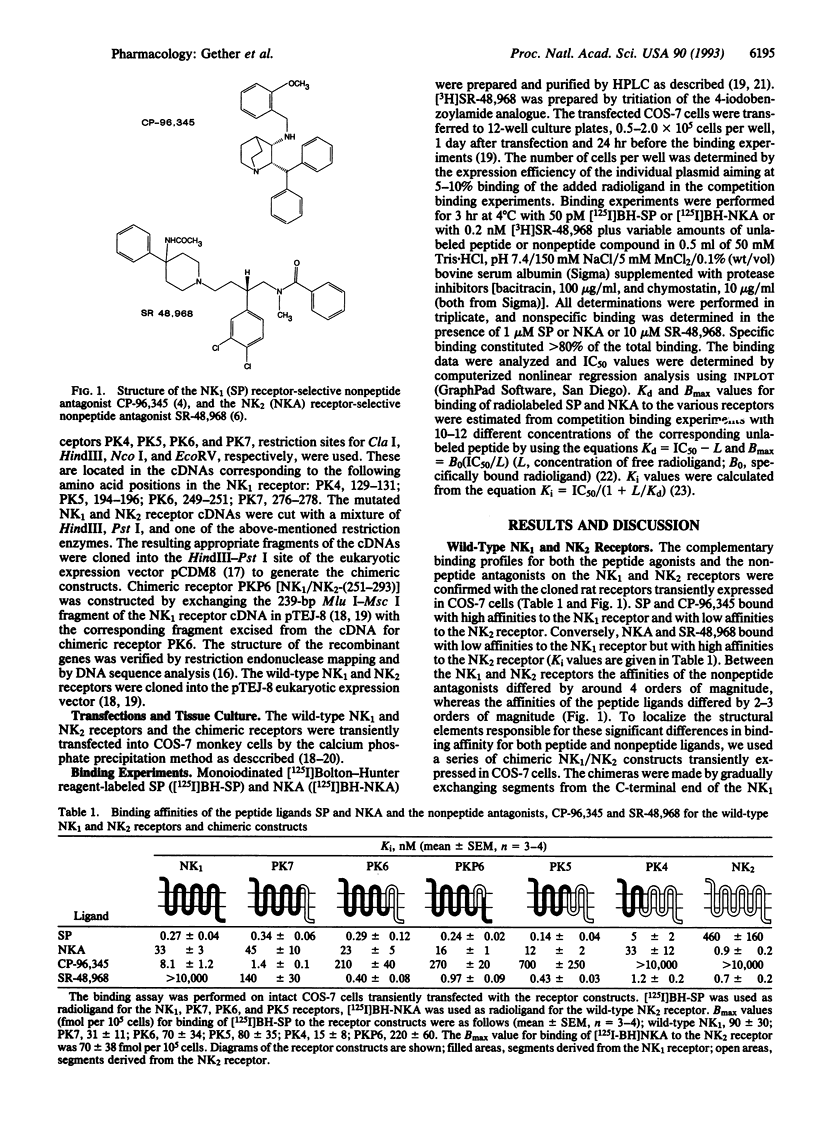
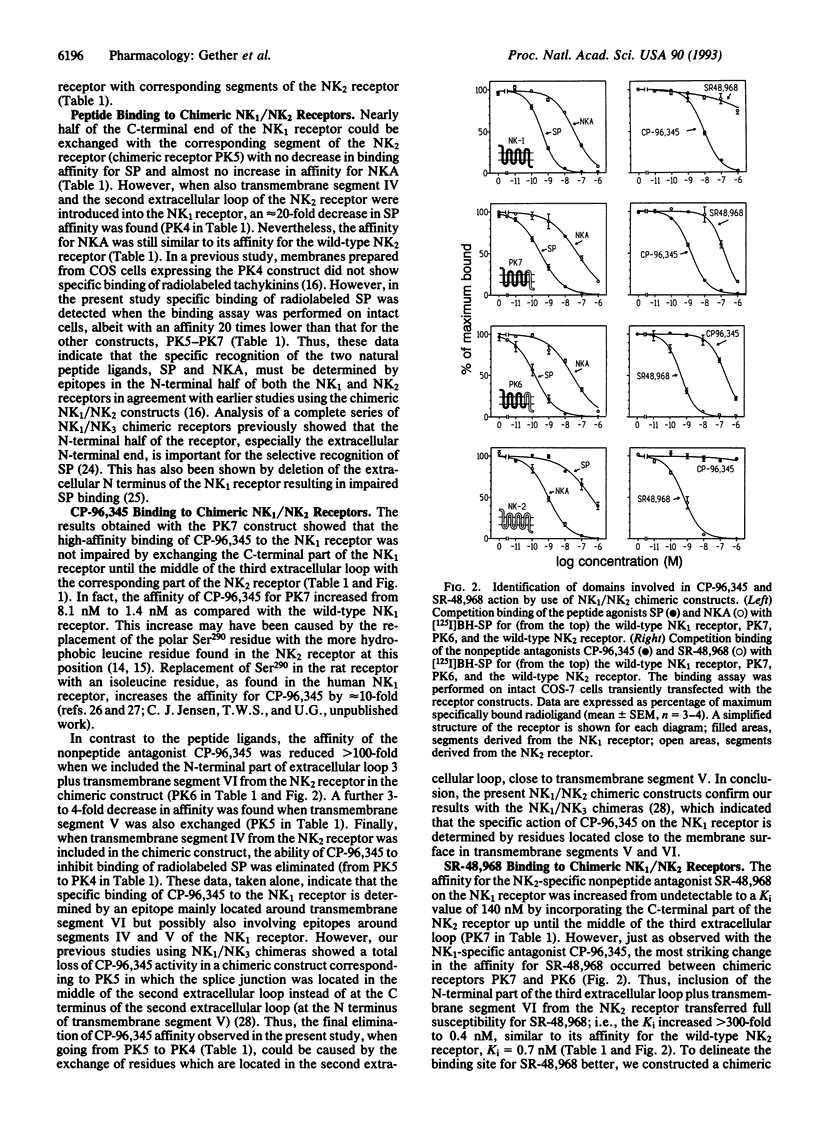
The molecular mechanism of action for two chemically distinct and highly selective, nonpeptide antagonists, CP-96,345 and SR-48,968, was studied by development of a series of chimeric constructs between their respective target receptors, the NK1 (substance P) and NK2 (neurokinin A) receptors. The binding affinities of the natural peptide ligands, substance P and neurokinin A, were not affected by exchanging almost the entire C-terminal half of the NK1 receptor with the corresponding segment of the NK2 receptor. In contrast, it was found that transfer from the NK2 to the NK1 receptor of a segment corresponding to transmembrane segment VI, the amino-terminal half of transmembrane segment VII, and the connecting extracellular loop 3 completely switched the susceptibility for the nonpeptide antagonists. This chimeric exchange, corresponding to 17 nonconserved residues, conveyed full susceptibility for the NK2-specific compound SR-48,968 to the previously unresponsive NK1 receptor--i.e., the Ki value for inhibition of binding of 125I-labeled substance P decreased from > 10,000 to 0.97 nM. At the same time the affinity for the NK1-selective compound CP-96,345 decreased > 30-fold. The actual binding site for SR-48,968 was localized to this region of the NK2 receptor by use of [3H]SR-48,968, which did not bind to the NK1 receptor but bound with similar high affinities to the wild-type NK2 receptor and to the chimeric NK1 receptor with the NK2 receptor segment incorporated around transmembrane segments VI and VII, Kd = 1.5 nM and 1.0 nM, respectively. Our data indicate that two chemically very different nonpeptide antagonists, CP-96,345 and SR-48,968, act through epitopes located around transmembrane segment VI on their respective target receptors and that at least the nonconserved residues in these epitopes are not important for the binding of the natural peptide ligands, substance P and neurokinin A.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinborn M., Lee Y. M., McBride E. W., Quinn S. M., Kopin A. S. A single amino acid of the cholecystokinin-B/gastrin receptor determines specificity for non-peptide antagonists. Nature. 1993 Mar 25;362(6418):348–350. doi: 10.1038/362348a0. [DOI] [PubMed] [Google Scholar]
- Birch P. J., Harrison S. M., Hayes A. G., Rogers H., Tyers M. B. The non-peptide NK1 receptor antagonist, (+/-)-CP-96,345, produces antinociceptive and anti-oedema effects in the rat. Br J Pharmacol. 1992 Mar;105(3):508–510. doi: 10.1111/j.1476-5381.1992.tb09008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascieri M. A., Chicchi G. G., Liang T. Demonstration of two distinct tachykinin receptors in rat brain cortex. J Biol Chem. 1985 Feb 10;260(3):1501–1507. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- DeBlasi A., O'Reilly K., Motulsky H. J. Calculating receptor number from binding experiments using same compound as radioligand and competitor. Trends Pharmacol Sci. 1989 Jun;10(6):227–229. doi: 10.1016/0165-6147(89)90266-6. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Sigal I. S., Candelore M. R., Register R. B., Scattergood W., Rands E., Strader C. D. Structural features required for ligand binding to the beta-adrenergic receptor. EMBO J. 1987 Nov;6(11):3269–3275. doi: 10.1002/j.1460-2075.1987.tb02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Emonds-Alt X., Vilain P., Goulaouic P., Proietto V., Van Broeck D., Advenier C., Naline E., Neliat G., Le Fur G., Brelière J. C. A potent and selective non-peptide antagonist of the neurokinin A (NK2) receptor. Life Sci. 1992;50(15):PL101–PL106. doi: 10.1016/0024-3205(92)90352-p. [DOI] [PubMed] [Google Scholar]
- Fong T. M., Cascieri M. A., Yu H., Bansal A., Swain C., Strader C. D. Amino-aromatic interaction between histidine 197 of the neurokinin-1 receptor and CP 96345. Nature. 1993 Mar 25;362(6418):350–353. doi: 10.1038/362350a0. [DOI] [PubMed] [Google Scholar]
- Fong T. M., Yu H., Huang R. R., Strader C. D. The extracellular domain of the neurokinin-1 receptor is required for high-affinity binding of peptides. Biochemistry. 1992 Dec 1;31(47):11806–11811. doi: 10.1021/bi00162a019. [DOI] [PubMed] [Google Scholar]
- Fong T. M., Yu H., Strader C. D. Molecular basis for the species selectivity of the neurokinin-1 receptor antagonists CP-96,345 and RP67580. J Biol Chem. 1992 Dec 25;267(36):25668–25671. [PubMed] [Google Scholar]
- Frielle T., Daniel K. W., Caron M. G., Lefkowitz R. J. Structural basis of beta-adrenergic receptor subtype specificity studied with chimeric beta 1/beta 2-adrenergic receptors. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9494–9498. doi: 10.1073/pnas.85.24.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garret C., Carruette A., Fardin V., Moussaoui S., Peyronel J. F., Blanchard J. C., Laduron P. M. Pharmacological properties of a potent and selective nonpeptide substance P antagonist. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10208–10212. doi: 10.1073/pnas.88.22.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gether U., Johansen T. E., Schwartz T. W. Chimeric NK1 (substance P)/NK3 (neurokinin B) receptors. Identification of domains determining the binding specificity of tachykinin agonists. J Biol Chem. 1993 Apr 15;268(11):7893–7898. [PubMed] [Google Scholar]
- Gether U., Johansen T. E., Snider R. M., Lowe J. A., 3rd, Nakanishi S., Schwartz T. W. Different binding epitopes on the NK1 receptor for substance P and non-peptide antagonist. Nature. 1993 Mar 25;362(6418):345–348. doi: 10.1038/362345a0. [DOI] [PubMed] [Google Scholar]
- Gether U., Marray T., Schwartz T. W., Johansen T. E. Stable expression of high affinity NK1 (substance P) and NK2 (neurokinin A) receptors but low affinity NK3 (neurokinin B) receptors in transfected CHO cells. FEBS Lett. 1992 Jan 27;296(3):241–244. doi: 10.1016/0014-5793(92)80295-r. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Johansen T. E., Schøller M. S., Tolstoy S., Schwartz T. W. Biosynthesis of peptide precursors and protease inhibitors using new constitutive and inducible eukaryotic expression vectors. FEBS Lett. 1990 Jul 16;267(2):289–294. doi: 10.1016/0014-5793(90)80947-h. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Kobilka T. S., Daniel K., Regan J. W., Caron M. G., Lefkowitz R. J. Chimeric alpha 2-,beta 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity. Science. 1988 Jun 3;240(4857):1310–1316. doi: 10.1126/science.2836950. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Donnerer J., Tsuchiya M., Nagahisa A. The non-peptide tachykinin antagonist, CP-96,345, is a potent inhibitor of neurogenic inflammation. Br J Pharmacol. 1992 Mar;105(3):527–530. doi: 10.1111/j.1476-5381.1992.tb09013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J. A., 3rd, Drozda S. E., Snider R. M., Longo K. P., Zorn S. H., Morrone J., Jackson E. R., McLean S., Bryce D. K., Bordner J. The discovery of (2S,3S)-cis-2-(diphenylmethyl)-N-[(2-methoxyphenyl)methyl]-1- azabicyclo[2.2.2]-octan-3-amine as a novel, nonpeptide substance P antagonisst. J Med Chem. 1992 Jul 10;35(14):2591–2600. doi: 10.1021/jm00092a009. [DOI] [PubMed] [Google Scholar]
- McGillis J. P., Mitsuhashi M., Payan D. G. Immunomodulation by tachykinin neuropeptides. Ann N Y Acad Sci. 1990;594:85–94. doi: 10.1111/j.1749-6632.1990.tb40470.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Mammalian tachykinin receptors. Annu Rev Neurosci. 1991;14:123–136. doi: 10.1146/annurev.ne.14.030191.001011. [DOI] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983 Jun;35(2):85–141. [PubMed] [Google Scholar]
- Sachais B. S., Snider R. M., Lowe J. A., 3rd, Krause J. E. Molecular basis for the species selectivity of the substance P antagonist CP-96,345. J Biol Chem. 1993 Feb 5;268(4):2319–2323. [PubMed] [Google Scholar]
- Sasai Y., Nakanishi S. Molecular characterization of rat substance K receptor and its mRNAs. Biochem Biophys Res Commun. 1989 Dec 15;165(2):695–702. doi: 10.1016/s0006-291x(89)80022-1. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Snider R. M., Constantine J. W., Lowe J. A., 3rd, Longo K. P., Lebel W. S., Woody H. A., Drozda S. E., Desai M. C., Vinick F. J., Spencer R. W. A potent nonpeptide antagonist of the substance P (NK1) receptor. Science. 1991 Jan 25;251(4992):435–437. doi: 10.1126/science.1703323. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Candelore M. R., Rands E., Hill W. S., Dixon R. A. Conserved aspartic acid residues 79 and 113 of the beta-adrenergic receptor have different roles in receptor function. J Biol Chem. 1988 Jul 25;263(21):10267–10271. [PubMed] [Google Scholar]
- Watling K. J. Nonpeptide antagonists herald new era in tachykinin research. Trends Pharmacol Sci. 1992 Jul;13(7):266–269. doi: 10.1016/0165-6147(92)90082-h. [DOI] [PubMed] [Google Scholar]
- Wess J., Gdula D., Brann M. R. Structural basis of the subtype selectivity of muscarinic antagonists: a study with chimeric m2/m5 muscarinic receptors. Mol Pharmacol. 1992 Feb;41(2):369–374. [PubMed] [Google Scholar]
- Yokota Y., Akazawa C., Ohkubo H., Nakanishi S. Delineation of structural domains involved in the subtype specificity of tachykinin receptors through chimeric formation of substance P/substance K receptors. EMBO J. 1992 Oct;11(10):3585–3591. doi: 10.1002/j.1460-2075.1992.tb05442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y., Sasai Y., Tanaka K., Fujiwara T., Tsuchida K., Shigemoto R., Kakizuka A., Ohkubo H., Nakanishi S. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem. 1989 Oct 25;264(30):17649–17652. [PubMed] [Google Scholar]


