Abstract
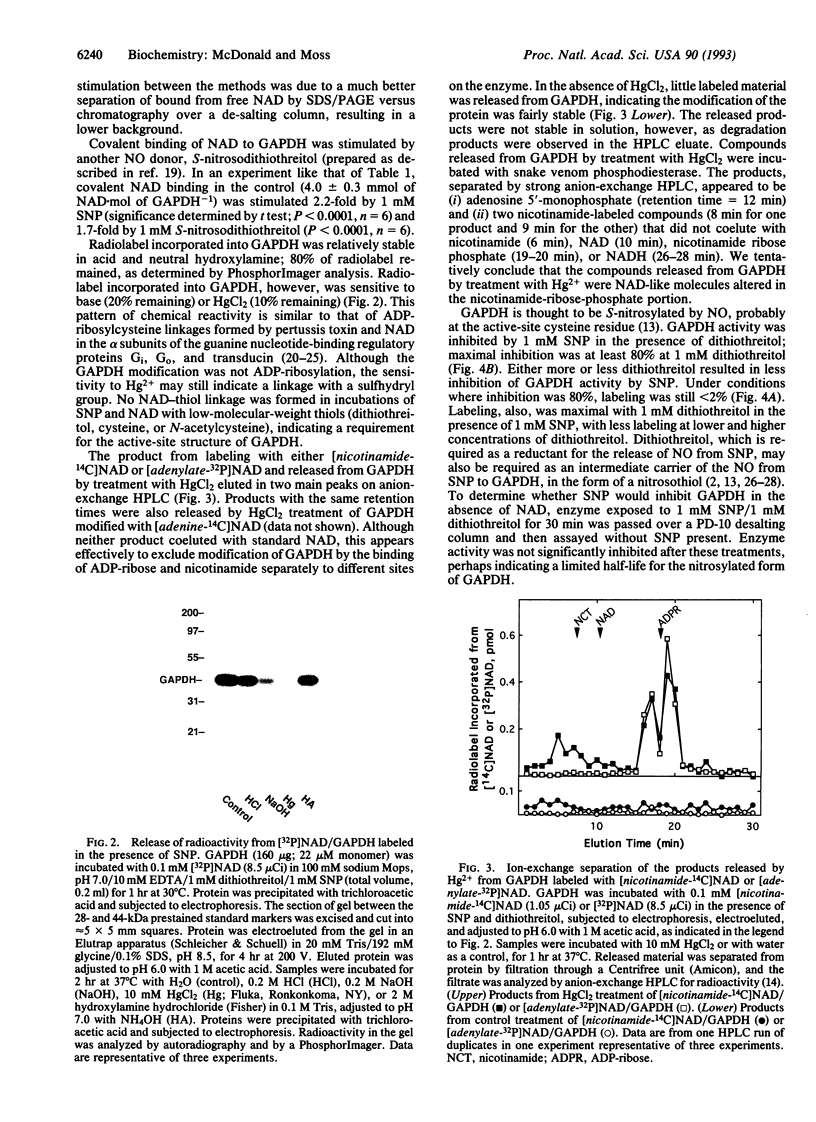
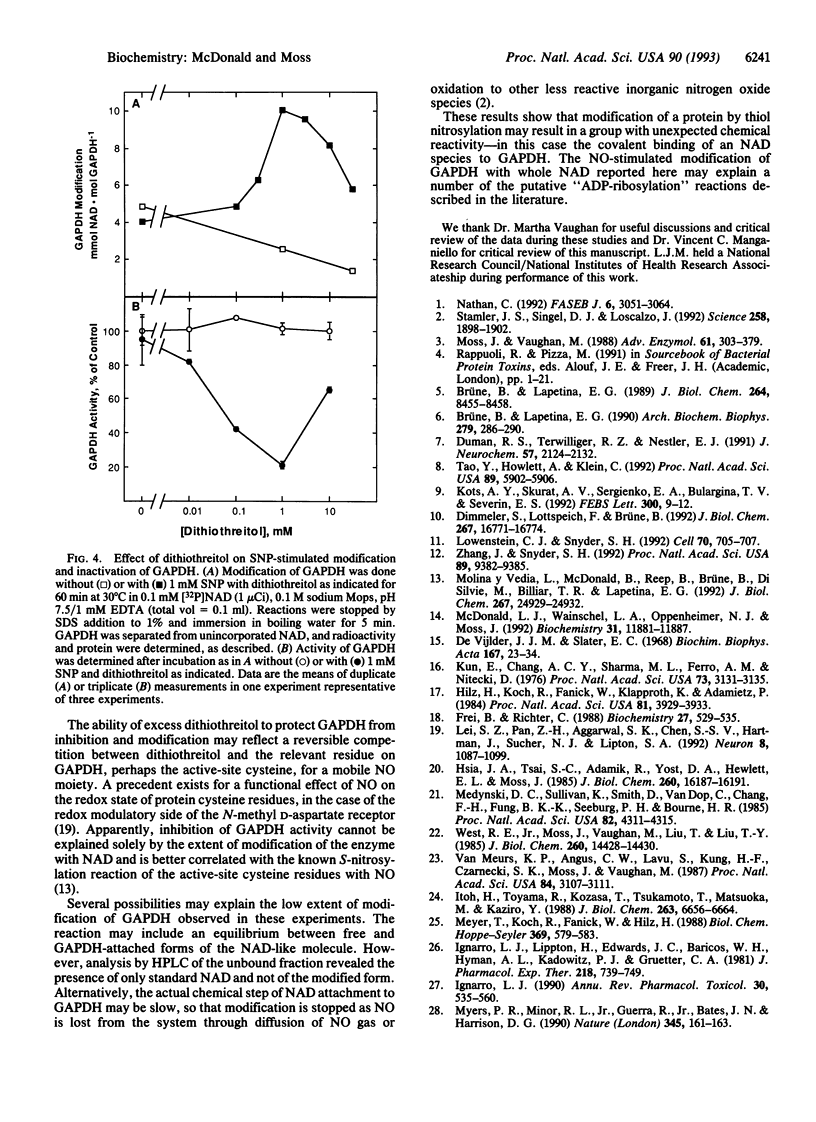
Nitric oxide-stimulated modification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by [adenylate-32P]NAD has been interpreted in recent reports as ADP-ribosylation. Incubations of GAPDH with the NO-releasing agent sodium nitroprusside (SNP) and NAD resulted, however, in essentially equal incorporation of radiolabel from the adenine, phosphate, and nicotinamide moieties to the extent of approximately 0.02 mol of NAD.mol of GAPDH-1. Modification of GAPDH by free adenosine 5'-diphosphoribose (ADP-ribose) was only 10% of that by NAD. Exposure of GAPDH modified by NAD in the presence of SNP to HgCl2, which acts at thiol linkages, released two products. Both contained nicotinamide and adenylate but did not cochromatograph with NAD. GAPDH activity was inhibited by SNP in a dose-dependent manner in the presence of NAD. When inhibition was 80%, with 1 mM SNP and 1 mM dithiothreitol, covalent modification with NAD was < 2%. This result is consistent with the conclusion that inhibition of GAPDH activity by SNP in the presence of NAD is due primarily to active-site nitrosylation, as reported by other workers, and is not due to the minor modification with NAD. These results demonstrate that NO-stimulated modification of GAPDH with NAD is not ADP-ribosylation as previously reported but rather is covalent binding of NAD through a NO-dependent thiol intermediate, possibly providing an example of an unexpected, altered reactivity of a nitrosylated protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brüne B., Lapetina E. G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem. 1989 May 25;264(15):8455–8458. [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Properties of a novel nitric oxide-stimulated ADP-ribosyltransferase. Arch Biochem Biophys. 1990 Jun;279(2):286–290. doi: 10.1016/0003-9861(90)90493-i. [DOI] [PubMed] [Google Scholar]
- Dimmeler S., Lottspeich F., Brüne B. Nitric oxide causes ADP-ribosylation and inhibition of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1992 Aug 25;267(24):16771–16774. [PubMed] [Google Scholar]
- Duman R. S., Terwilliger R. Z., Nestler E. J. Endogenous ADP-ribosylation in brain: initial characterization of substrate proteins. J Neurochem. 1991 Dec;57(6):2124–2132. doi: 10.1111/j.1471-4159.1991.tb06431.x. [DOI] [PubMed] [Google Scholar]
- Frei B., Richter C. Mono(ADP-ribosylation) in rat liver mitochondria. Biochemistry. 1988 Jan 26;27(2):529–535. doi: 10.1021/bi00402a004. [DOI] [PubMed] [Google Scholar]
- Hilz H., Koch R., Fanick W., Klapproth K., Adamietz P. Nonenzymic ADP-ribosylation of specific mitochondrial polypeptides. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3929–3933. doi: 10.1073/pnas.81.13.3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia J. A., Tsai S. C., Adamik R., Yost D. A., Hewlett E. L., Moss J. Amino acid-specific ADP-ribosylation. Sensitivity to hydroxylamine of [cysteine(ADP-ribose)]protein and [arginine(ADP-ribose)]protein linkages. J Biol Chem. 1985 Dec 25;260(30):16187–16191. [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Itoh H., Toyama R., Kozasa T., Tsukamoto T., Matsuoka M., Kaziro Y. Presence of three distinct molecular species of Gi protein alpha subunit. Structure of rat cDNAs and human genomic DNAs. J Biol Chem. 1988 May 15;263(14):6656–6664. [PubMed] [Google Scholar]
- Kots AYa, Skurat A. V., Sergienko E. A., Bulargina T. V., Severin E. S. Nitroprusside stimulates the cysteine-specific mono(ADP-ribosylation) of glyceraldehyde-3-phosphate dehydrogenase from human erythrocytes. FEBS Lett. 1992 Mar 23;300(1):9–12. doi: 10.1016/0014-5793(92)80153-8. [DOI] [PubMed] [Google Scholar]
- Kun E., Chang A. C., Sharma M. L., Ferro A. M., Nitecki D. Covalent modification of proteins by metabolites of NAD+. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3131–3135. doi: 10.1073/pnas.73.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S. Z., Pan Z. H., Aggarwal S. K., Chen H. S., Hartman J., Sucher N. J., Lipton S. A. Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron. 1992 Jun;8(6):1087–1099. doi: 10.1016/0896-6273(92)90130-6. [DOI] [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- McDonald L. J., Wainschel L. A., Oppenheimer N. J., Moss J. Amino acid-specific ADP-ribosylation: structural characterization and chemical differentiation of ADP-ribose-cysteine adducts formed nonenzymatically and in a pertussis toxin-catalyzed reaction. Biochemistry. 1992 Dec 1;31(47):11881–11887. doi: 10.1021/bi00162a029. [DOI] [PubMed] [Google Scholar]
- Medynski D. C., Sullivan K., Smith D., Van Dop C., Chang F. H., Fung B. K., Seeburg P. H., Bourne H. R. Amino acid sequence of the alpha subunit of transducin deduced from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4311–4315. doi: 10.1073/pnas.82.13.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Koch R., Fanick W., Hilz H. ADP-ribosyl proteins formed by pertussis toxin are specifically cleaved by mercury ions. Biol Chem Hoppe Seyler. 1988 Jul;369(7):579–583. doi: 10.1515/bchm3.1988.369.2.579. [DOI] [PubMed] [Google Scholar]
- Molina y Vedia L., McDonald B., Reep B., Brüne B., Di Silvio M., Billiar T. R., Lapetina E. G. Nitric oxide-induced S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. J Biol Chem. 1992 Dec 15;267(35):24929–24932. [PubMed] [Google Scholar]
- Moss J., Vaughan M. ADP-ribosylation of guanyl nucleotide-binding regulatory proteins by bacterial toxins. Adv Enzymol Relat Areas Mol Biol. 1988;61:303–379. doi: 10.1002/9780470123072.ch6. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Tao Y., Howlett A., Klein C. Nitric oxide stimulates the ADP-ribosylation of a 41-kDa cytosolic protein in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5902–5906. doi: 10.1073/pnas.89.13.5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meurs K. P., Angus C. W., Lavu S., Kung H. F., Czarnecki S. K., Moss J., Vaughan M. Deduced amino acid sequence of bovine retinal Go alpha: similarities to other guanine nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1987 May;84(10):3107–3111. doi: 10.1073/pnas.84.10.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R. E., Jr, Moss J., Vaughan M., Liu T., Liu T. Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J Biol Chem. 1985 Nov 25;260(27):14428–14430. [PubMed] [Google Scholar]
- Zhang J., Snyder S. H. Nitric oxide stimulates auto-ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9382–9385. doi: 10.1073/pnas.89.20.9382. [DOI] [PMC free article] [PubMed] [Google Scholar]