Abstract
Upregulation of utrophin A is an attractive therapeutic strategy for treating Duchenne muscular dystrophy (DMD). Over the years, several studies revealed that utrophin A is regulated by multiple transcriptional and post-transcriptional mechanisms, and that pharmacological modulation of these pathways stimulates utrophin A expression in dystrophic muscle. In particular, we recently showed that activation of p38 signaling causes an increase in the levels of utrophin A mRNAs and protein by decreasing the functional availability of the destabilizing RNA-binding protein called K-homology splicing regulatory protein, thereby resulting in increases in the stability of existing mRNAs. Here, we treated 6-week-old mdx mice for 4 weeks with the clinically used anticoagulant drug heparin known to activate p38 mitogen-activated protein kinase, and determined the impact of this pharmacological intervention on the dystrophic phenotype. Our results show that heparin treatment of mdx mice caused a significant ∼1.5- to 3-fold increase in utrophin A expression in diaphragm, extensor digitorum longus and tibialis anterior (TA) muscles. In agreement with these findings, heparin-treated diaphragm and TA muscle fibers showed an accumulation of utrophin A and β-dystroglycan along their sarcolemma and displayed improved morphology and structural integrity. Moreover, combinatorial drug treatment using both heparin and 5-amino-4-imidazolecarboxamide riboside (AICAR), the latter targeting 5′ adenosine monophosphate-activated protein kinase and the transcriptional activation of utrophin A, caused an additive effect on utrophin A expression in dystrophic muscle. These findings establish that heparin is a relevant therapeutic agent for treating DMD, and illustrate that combinatorial treatment of heparin with AICAR may serve as an effective strategy to further increase utrophin A expression in dystrophic muscle via activation of distinct signaling pathways.
Introduction
Duchenne muscular dystrophy (DMD) is the most common form of muscular dystrophy as it afflicts ∼1 in 3500 male live births (1). Due to progressive muscle wasting, patients become wheelchair bound by early teens and death ensues in their second or third decade of life most often as a result of respiratory or heart failure (2–6). DMD is an X-linked recessive disorder caused by mutations/deletions in the dystrophin gene that prevent the production of functional dystrophin protein at the sarcolemma of individual muscle fibers (7–9). In these fibers, dystrophin plays a key role in maintaining their structural integrity by linking cytoplasmic actin filaments to the extracellular matrix via a multifunctional signaling complex called dystrophin-associated protein complex (DAPC) (10–13). A lack of dystrophin protein as seen in DMD muscle fibers, induces cycles of muscle degeneration and regeneration with an eventual failure of the regenerative capacity thereby leading to a loss of muscle mass and function. Several therapeutic interventions are currently being developed for treating DMD including suppression of premature termination codons (14,15), exon skipping (16–20) and gene-based therapies (21–23).
An alternative strategy for treating DMD is focused on increasing the endogenous levels of the autosomal homolog of dystrophin, named utrophin (24,25), in order to compensate functionally for the lack of dystrophin. Unlike dystrophin, which is expressed along the entire length of the sarcolemma in adult muscle fibers, expression of utrophin A (the muscle isoform) is mainly restricted to neuromuscular and myotendinous junctions (26,27). In addition to differences in their localization, dystrophin and utrophin A also differ in their ability to bind microtubules and in their ability to localize neuronal nitric oxide synthase (nNOS) at the sarcolemma (28). However, similar to dystrophin, utrophin A associates with members of DAPC (29,30) and with cytoskeletal F-actin filaments (31,32) and can thus serve to provide functional integrity to individual muscle fibers. As an eventual effective therapeutic strategy, expression of utrophin A needs to extend from synaptic and myotendinous sites well into extrasynaptic regions, all along the sarcolemma of dystrophic fibers. Interestingly, utrophin A expression is naturally upregulated in dystrophic muscles of mdx mice and DMD patients. However, this upregulation is not sufficient to compensate for the loss of dystrophin in these dystrophic muscles (33–35). In this context, several studies have shown that transgenic overexpression of utrophin A in skeletal muscle of mdx mice alleviates the dystrophic phenotype (36–40). As a therapeutic strategy, utrophin A upregulation offers the distinct advantage that it would be beneficial for all Duchenne and Becker patients since this approach is independent of the exact nature of the patient's mutation.
Given the therapeutic potential of increasing expression of utrophin A in dystrophic muscle, many laboratories around the world are devoting considerable efforts to identify drugs/small molecules that can stimulate utrophin A expression. One approach consists in screening libraries of chemical compounds in attempts to identify such active drugs/small molecules. To date, one such compound, i.e. SMT C1100, was shown to upregulate utrophin A in various experimental models and has since moved into clinical trials (41–44). Alternatively, the characterization of molecular events and signaling pathways represents an attractive route to identify pharmacologically active molecules that can act upon these pathways. In this context, several transcriptional regulatory mechanisms have been characterized including those that involve MyoD/myogenin and an E-box (45), GA-binding protein (GABP) and an N-Box (46,47), calcineurin/nuclear factor of activated T-cells (NFAT) signaling (48,49), peroxisome proliferator-activated receptor-β/δ (PPAR-β/δ) (50–52), peroxisome proliferator-activated receptor ϒ coactivator 1α (PGC-1α) (53–55), sirtuin 1 (Sirt1) (56) and Ets-2 repressor factor (ERF) and E2F1 (57,58). Collectively, these studies have led to the idea that promotion of a slower, more oxidative phenotype, which concomitantly upregulates utrophin A, is a viable therapeutic strategy for DMD (48,53,56,59–61). Over the last few years, several studies have used specific drugs to activate these various pathways in order to explore their therapeutic potential in mdx mice. In particular, treatment of mdx mice with either GW501516, 5-amino-4-imidazolecarboxamide riboside (AICAR), metformin and resveratrol, which respectively target PPAR-β/δ, 5′ adenosine monophosphate-activated protein kinase (AMPK), and Sirt1 and hence act primarily at the transcriptional level, results in an upregulation of utrophin A in muscle and functional benefits (53,60–67).
In addition to transcriptional regulatory mechanisms, earlier work from our laboratory has also shown the important contribution of post-transcriptional events in controlling utrophin A expression. In fact, the 3′UTR and 5′UTR of utrophin A have both been shown to play key roles in the post-transcriptional regulation of utrophin A by regulating the stability and translation of utrophin A mRNAs, respectively (68–73). Of particular pharmacological and therapeutic relevance, we demonstrated that the 3′UTR of utrophin A transcripts contains the cis-acting AU-rich elements (ARE) (68,69), known to be important for post-transcriptionally regulating mRNA stability (74–76). In this context, we also showed recently that activation of p38 MAP kinase signaling in muscle reduces the binding of the destabilizing RNA-binding protein called K-homology splicing regulatory protein (KSRP), to the ARE which in turn, causes an increase in the stability of pre-synthesized utrophin A transcripts (77). In this latter study, we also noted that a short, 10-day regimen of heparin injections, which is currently used in clinical settings as an anticoagulant and also known to activate p38, induced a marked increase in utrophin A expression in the diaphragm muscle of mdx mice (77). These results, therefore, represent proof-of-principle that drugs that modulate post-transcriptional pathways can promote utrophin A expression in muscle. What remains unclear however, is whether a longer heparin treatment such as 4-week treatment periods used in recent pre-clinical studies (53,63,77,78), can sufficiently increase utrophin A levels to provide morphological and functional benefits to muscle cells.
The aim of the current study was 2-fold. First, we examined the impact of treating mdx mice for 4 weeks with heparin on the expression of utrophin A in a variety of muscles and determined its beneficial effects on the morphology and integrity of muscle fibers. Our second objective was to investigate the effect of combining two different drugs, namely heparin and AICAR, on utrophin A expression to determine whether they could instigate an additive effect on utrophin A levels in mdx mouse muscle. In this second series of experiments, we hypothesized that activating distinct yet, complementary pathways (AICAR acting transcriptionally via AMPK and PGC-1α, and heparin acting post-transcriptionally via KSRP and the ARE) would overcome the issue of saturating common downstream targets with multiple drugs/small molecules, thereby promoting additive effects on utrophin A expression in muscle.
Results
Heparin stimulates utrophin A expression in cultured muscle cells
Heparin, a naturally occurring polysaccharide, is one of the oldest drugs used as an anticoagulant for treatment of thrombosis (79). Heparin has been shown to activate p38 mitogen-activated protein kinase (MAPK) activity in skeletal muscle of wild-type mice (78). Our laboratory was first to demonstrate that short-term pharmacological activation of p38 by heparin, stimulates utrophin A expression in C2C12 muscle cells and mdx mouse muscle through a post-transcriptional mechanism involving inhibition of KSRP and enhanced stability of existing utrophin A mRNAs (77). In the present study, we first decided to extend these findings by examining whether a 4-week treatment with heparin upregulates utrophin A expression in several mdx mouse muscles and provides morphological and functional benefits to dystrophic muscles.
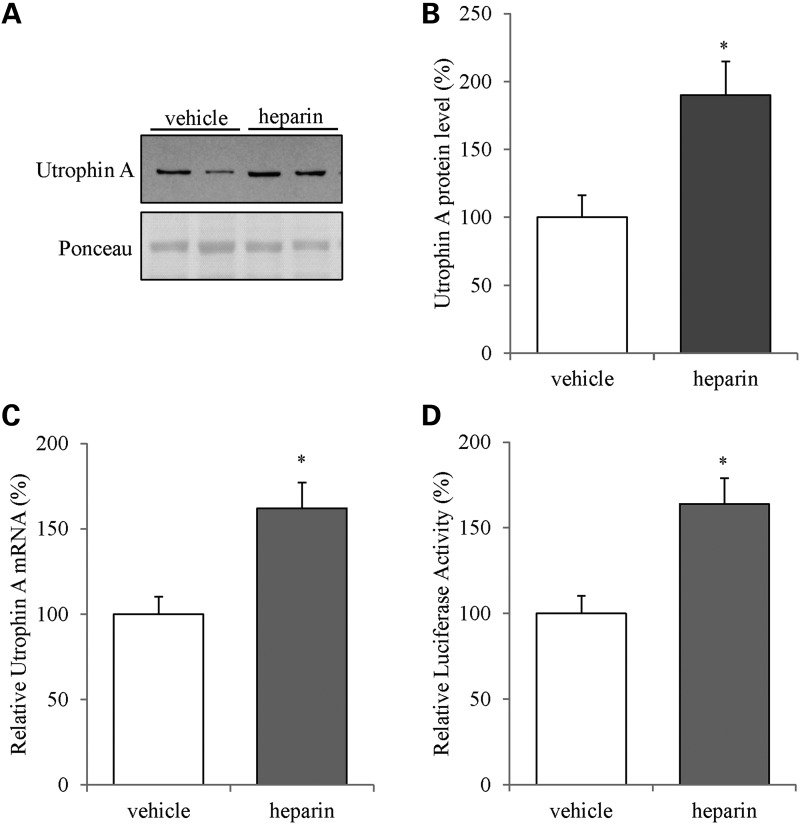
Initially, we treated C2C12 myoblasts for 24 h with 2.5 IU/mL of heparin and subsequently harvested the cells and analyzed utrophin A expression using western blot and RT-qPCR. Heparin treatment induced a significant ∼2-fold (P < 0.05) upregulation of utrophin A in C2C12 muscle cells compared with vehicle-treated cells (Fig. 1A and B). Moreover, treatment of C2C12 myoblasts with heparin also caused a significant increase (P < 0.05) in utrophin A mRNA levels (Fig. 1C). Finally, heparin treatment of cells transfected with a luciferase reporter construct containing the full-length 3′UTR of utrophin A, resulted in a ∼1.5-fold increase (P < 0.05) in luciferase activity compared with vehicle-treated control cells (Fig. 1D). In these latter experiments, we verified that heparin had no direct effect on the luciferase reporter activity per se by measuring the activity of the luciferase construct without the utrophin A 3′UTR in cells treated with or without heparin (luciferase activity (%): luciferase + vehicle = 100, luciferase + heparin = 107, ±2.49 (mean and SD). Together, these data demonstrate that heparin treatment enhances utrophin A expression in C2C12 muscle cells via a post-transcriptional mechanism thereby corroborating our earlier findings (77).
Figure 1.
Heparin increases utrophin A levels in C2C12 muscle cells. (A) Representative western blot of utrophin A and ponceau staining using protein extracts from C2C12 myoblasts treated with vehicle control (water) or heparin (2.5 IU/mL) for 24 h. (B) Quantification of utrophin A protein levels as shown in (A). (C) Utrophin A mRNA levels as measured by RT-qPCR using total RNA from C2C12 myoblasts treated with either vehicle (water) or heparin (2.5 IU/mL) for 24 h. The values are normalized to 18S mRNA levels and graphed as a percentage to control. (D) The activity of the luciferase reporter construct containing the full-length 3′UTR of utrophin A mRNA transfected into C2C12 myoblasts treated with either vehicle (water) or heparin (2.5 IU/mL) for 24 h. N = 3 (with three replicates each). Error bars represent SEM. *P < 0.05 versus vehicle control.
Heparin stimulates utrophin A expression in several mdx mouse muscles
Based on these results, we progressed to treat 6-week-old dystrophin-null, mdx mice with daily subcutaneous injections of heparin at 500 IU/kg for 4 weeks, which extends the duration of the short-term, 10-day treatment protocol used in our recent study (77). Here, we chose 4 weeks as a treatment duration since several pre-clinical studies from other labs using mdx mice (80,81), and ours (53,60), have previously also used such a time period to test the efficacy of various compounds thus allowing for appropriate comparison of results.
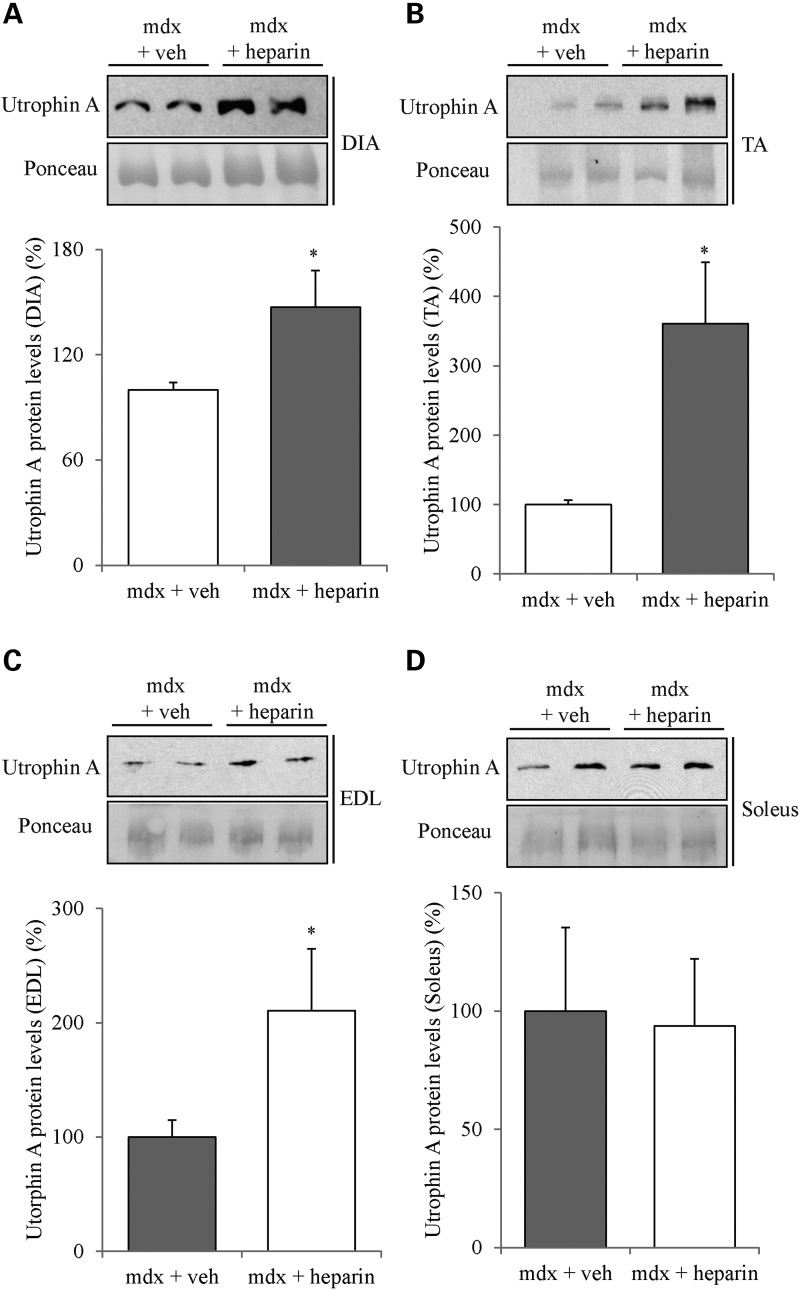
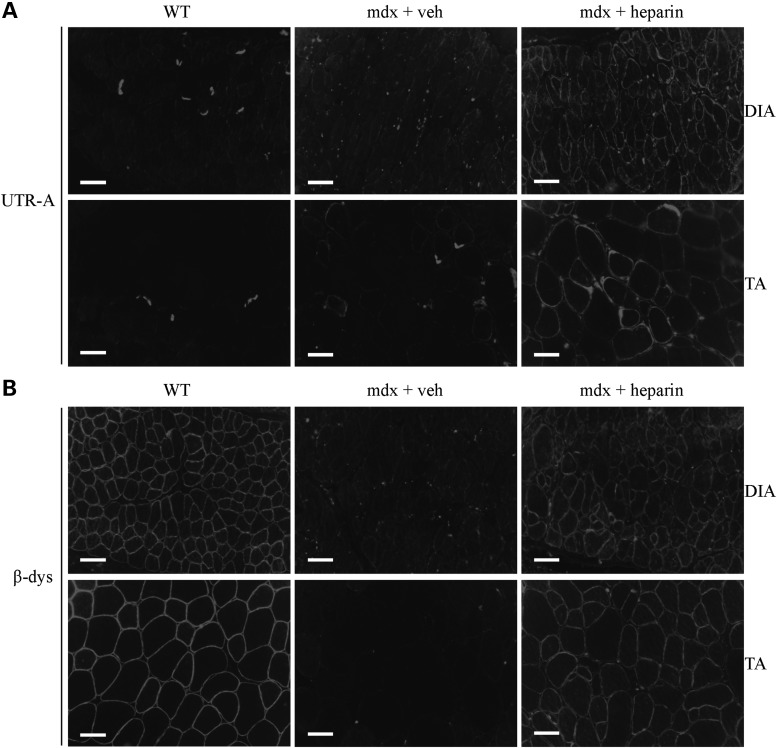
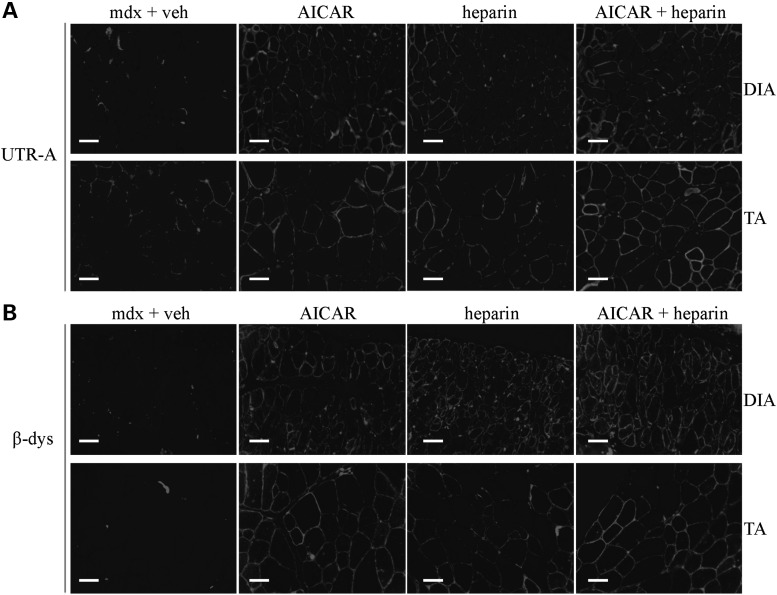
As illustrated in Figure 2, 4 weeks of heparin treatment significantly increased (P < 0.05) utrophin A protein levels in diaphragm and tibialis anterior (TA) muscles of mdx mice. Such an increase in utrophin A protein expression was also observed in the extensor digitorum longus (EDL) muscle. Remarkably, the increased expression of utrophin A in the fast TA and EDL muscles was greater than ∼3.5- and 2-fold, respectively. Nonetheless, there was no significant increase (P > 0.05) in utrophin A expression in the soleus most likely because utrophin A levels are already elevated in this slow oxidative muscle compared with fast glycolytic muscles (82). Immunofluorescence experiments further established that heparin treatment indeed caused an increase in utrophin A levels in mdx mouse muscles, while also showing that this increase occurred at the sarcolemma of both diaphragm and TA muscle fibers (Fig. 3A). Additionally, we assessed the localization of β-dystroglycan, a member of the DAPC, in order to examine whether heparin treatment caused reassembly of the DAPC along the sarcolemma. Similar to what we observed with utrophin A by immunofluorescence, heparin treatment enhanced β-dystroglycan expression along the sarcolemma of fibers from the diaphragm and TA muscles of mdx mice (Fig. 3B). Our data collectively show that a 4-week treatment with heparin stimulates utrophin A expression in several mdx mouse muscles and induces its accumulation at the sarcolemma of dystrophic fibers together with that of an important DAPC component.
Figure 2.
Heparin treatment induces upregulation of utrophin A protein levels in diaphragm, TA and EDL muscles from mdx mice. 6-week-old mdx mice were treated with heparin (500 IU/kg) or with vehicle (saline) for 4 weeks. Representative western blots and quantification of utrophin A protein levels standardized to ponceau using protein extracts from (A) diaphragm (DIA), (B) TA, (C) EDL and (D) soleus muscles. N = 4. Error bars represent SEM, *P < 0.05 versus mdx vehicle control. Note the significant ∼1.5- to ∼3-fold increase of utrophin A protein levels in diaphragm, TA and EDL muscles.
Figure 3.
Heparin increases sarcolemmal localization of utrophin A and promotes reassembly of the DAPC in mdx muscle fibers. (A) Representative examples of cross-sections obtained from diaphragm and TA muscles of wild-type (WT) mice and mdx mice treated with heparin or vehicle (saline), were immunostained with utrophin A (UTR-A) antibody. (B) Similar cross-sections from diaphragm and TA muscles were also immunostained with a β-dystroglycan (β-dys) antibody showing reassembly of the DAPC at the sarcolemma. Scale bar = 50 μm.
Heparin treatment improves morphological properties of mdx muscle
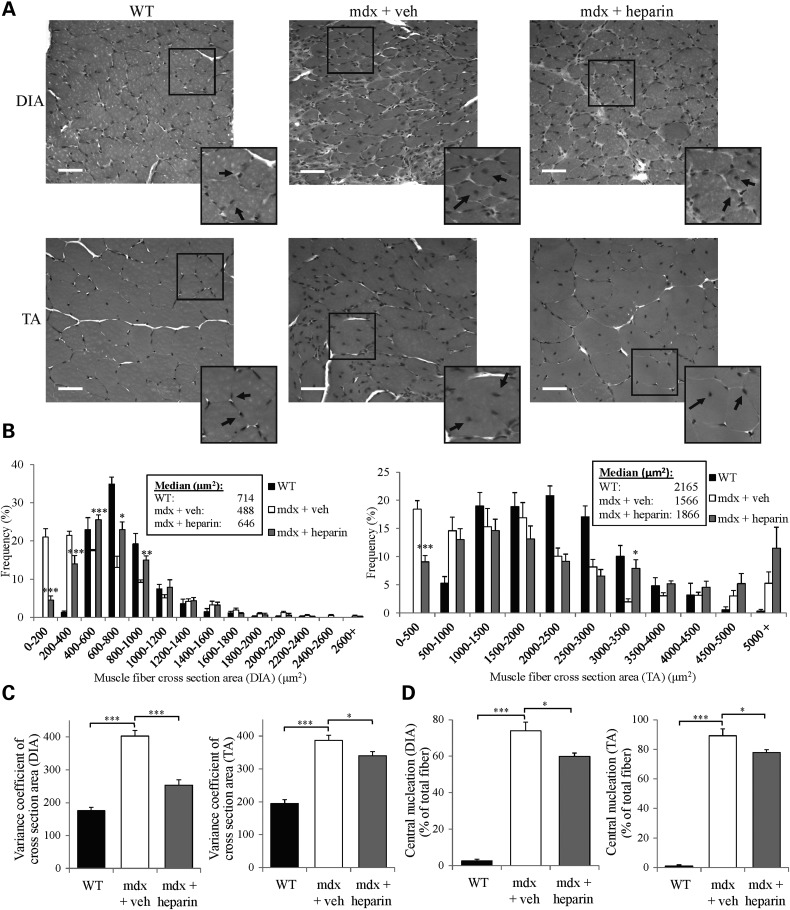
Given the findings shown above indicating assembly of utrophin A-containing DAPC at the sarcolemma, which implies an increase in the stability and integrity of dystrophic myofibers, we performed a series of hematoxylin and eosin (H and E) staining on cryostat sections of diaphragm and TA muscles from heparin- and vehicle-treated mdx mice. First, we measured the extent of central nucleation in these muscles. Central nucleation serves as a key indicator of muscle damage and regeneration in dystrophic muscle fibers (83). Our results revealed that heparin treatment caused a significant (P < 0.05) decrease in the percentage of central nucleation in both diaphragm and TA muscles of treated mdx mice as compared with control mdx mice (Fig. 4A and D). Since mdx muscle fibers go through cycles of muscle degeneration and regeneration, a decrease in central nucleation is an indication of reduced degeneration (83).
Figure 4.
Heparin treatment improves morphological features of mdx muscle fibers. (A) Representative examples of cross-sections of DIA and TA muscles from WT and mdx mice treated with heparin or with vehicle (saline) that were stained using hematoxylin and eosin. Black arrows indicate the position of nuclei (centrally nucleated or at the periphery of fibers). (B) Graphical summary of CSA (*P < 0.05, **P < 0.01, ***P < 0.001, significantly different from mdx vehicle control). Median CSAs of each muscle are displayed above the frequency histograms. (C) Variance coefficient measurements of the TA and diaphragm muscle fibers. (D) Percentage of central nucleation in TA and diaphragm muscle fibers. N = 4. Error bars represent SEM, *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from respective control (WT or mdx vehicle). Scale bar = 50 μm.
To determine whether heparin treatment provided additional morphological benefits to mdx muscle fibers, we also assessed the fiber size distribution by measuring the cross-sectional area (CSA) of individual fibers from diaphragm and TA muscles obtained from heparin- versus vehicle-treated mdx mice. Histopathological studies have shown that abnormal fiber size distribution is a hallmark of dystrophic muscles (83,84) since, for example, mdx mice display an abnormal proportion of small- and large-muscle fibers (83). Our analyses demonstrate that the CSA profiles of vehicle-treated mdx mice display large differences when compared with healthy wild-type mice (Fig. 4A and B). Four weeks of heparin treatment of mdx mice caused a rightward shift and normalization in the CSA frequency distribution of both diaphragm and TA muscles towards wild-type values with some fibers showing signs of hypertrophy. The reason why some of the mdx muscle fibers treated with heparin became hypertrophic is unclear. However, if toll-like receptors (TLR) are activated by heparin as suggested previously (78), it may be that activation of these receptors by heparin induces IL-6 production (85) which could promote muscle fiber hypertrophy (86,87).
To complement these data, we also calculated the variance coefficient (VC) based on individual CSA of muscle fibers. As illustrated in Figure 4C, our results show that diaphragm and TA fibers from vehicle-treated mdx mice display significantly (P < 0.001) higher VC as compared with wild-type mice. Moreover, heparin treatment caused a reduction of the VC in both diaphragm (P < 0.001) and TA (P < 0.05) muscles from treated mdx mice. Together, these data indicate that 4 weeks of heparin causes important improvements in the morphology of dystrophic muscle fibers.
In addition to this morphological analysis, we also performed a fiber typing study to determine whether heparin treatment leads to a change in the expression of the slow, oxidative versus fast, glycolytic myofiber program. In comparison to fast-twitch muscles, slow, oxidative fibers express considerably more utrophin A and show reduced dystrophic damage (82). Moreover, our recent work and that of others (53,59,60,88), had led to the idea that promotion of the slow oxidative phenotype is particularly beneficial to mdx mouse muscle since such a fiber type transition is accompanied by increases in utrophin A expression.
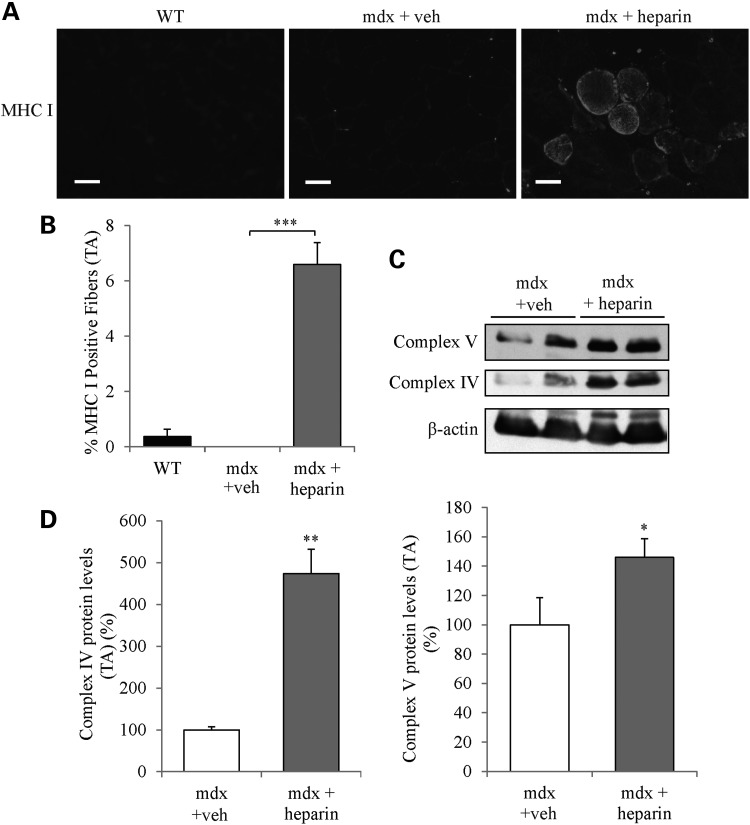
Immunofluorescence experiments using myosin heavy chain (MHC) antibodies showed that 4 weeks of heparin treatment of mdx mice caused a significant increase (P < 0.001) in the number of MHC type I fibers in TA muscles compared with vehicle-treated mdx mice (Fig. 5A and B). Moreover, western blotting revealed that expression of the oxidative phosphorylation (OXPHOS) markers, complex proteins IV and V were significantly (P < 0.05) elevated in TA muscles from heparin-treated mdx mice (Fig. 5C and D). These findings suggest that 4 weeks of heparin treatment induced an increase in the expression of the slow oxidative myogenic program in mdx mouse muscle.
Figure 5.
Heparin treatment promotes expression of the slow, oxidative myogenic program in mdx mouse muscle. (A) Immunofluorescence of TA muscle cross-sections was performed to detect MHC I in WT mice and mdx mice treated with heparin or vehicle. (B) Graphical summary of the percentage of MHC I-positive in TA muscles. (C and D) Representative western blots and quantitative analyses of the expression of OXPHOS complex IV subunit I, and OXPHOS complex V. Note the significant increase of MHC I-positive fibers (indicating a slow phenotype) and upregulation of the oxidative markers in TA muscles from heparin-treated mdx mice. N = 4. Scale bar = 50 μm. Error bars represent SEM. *P < 0.05, **P < 0.01, ***P < 0.001, (significantly different from mdx vehicle control).
Heparin enhances the sarcolemmal integrity of mdx muscle fibers
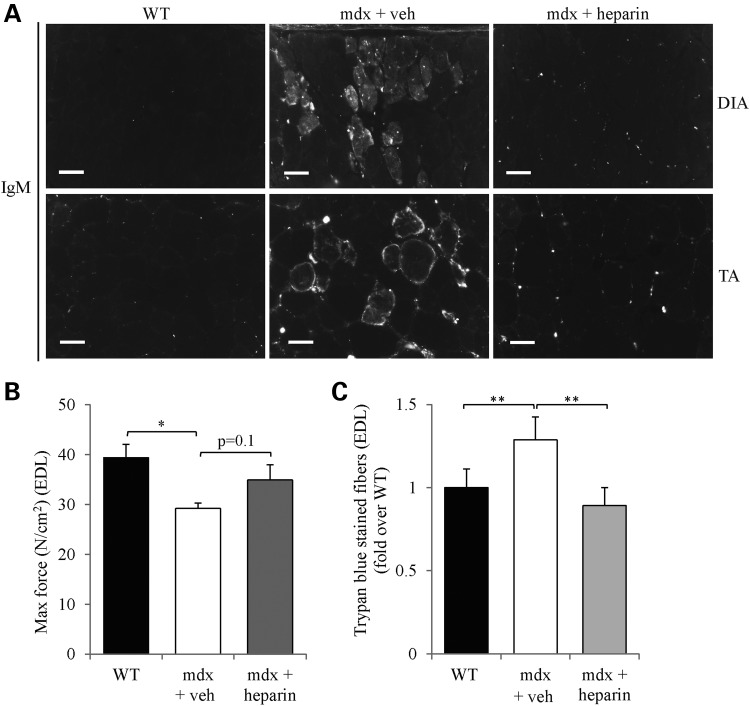
To functionally examine the beneficial impact of heparin treatment on the structural integrity of mdx myofibers, we conducted IgM immunostaining on diaphragm and TA muscle cryosections. IgM is typically an extracellular protein that can penetrate within the muscle fiber when the sarcolemmal integrity is compromised as seen in mdx myofibers (60,89). Detailed examination of the staining showed that IgM penetration within fibers was markedly reduced in both diaphragm and TA muscles from heparin- versus vehicle-treated mdx mice (Fig. 6A).
Figure 6.
Heparin improves functional parameters of mdx mouse muscle. (A) Representative examples of cross-sections of DIA and TA muscles from WT mice and mdx mice treated with heparin or vehicle that were immunostained with goat-anti-mouse IgM Alexa 594. (B) Shows the maximal force output of EDL muscles from WT mice and mdx mice treated with heparin or vehicle, obtained during the repetitive eccentric contraction protocol and generated at 200 Hz. (C) Quantitative analysis of trypan blue staining of muscle fibers (percent of total fibers). N = 4. Error bars represent SEM, *P < 0.05, **P < 0.01 versus respective control (WT or mdx vehicle). Scale bar = 50 μm.
In a final series of experiments, we further assessed sarcolemmal integrity by employing an ex vivo protocol of repetitive eccentric contractions with EDL muscles as used in an earlier study (90) and in ours (53,59,60). When determining maximal force, we observed that EDL muscles from heparin-treated mdx mice showed a trend toward normalization since force output was similar to wild-type mice (P > 0.05) and higher than vehicle-treated mdx mice (P = 0.1; Fig. 6B). During repetitive eccentric contractions, reduction of force over time is known to correlate with damage of mdx EDL myofibers (59,60,90). In our analysis, eccentric force measurements over time showed no difference (P > 0.05) between EDL muscles from heparin- and vehicle-treated mdx mice (data not shown). Such a discrepancy between force production and resistance to eccentric contractions has been observed in previous pre-clinical studies with the mdx mouse (91), including ours using AICAR treatment (53). During this eccentric contraction protocol, EDL muscles are bathed in 0.1% trypan blue staining solution which can penetrate into the cytoplasm of myofibers in response to contraction-induced muscle damage. Examination of the percentage of trypan blue-stained EDL muscle fibers revealed that heparin caused a ∼1.4-fold decrease (P < 0.01) in mdx mice versus vehicle-treated animals (Fig. 6C). Collectively, these results show that 4 weeks of heparin induced an increase in utrophin A expression in several mdx mouse muscles together with multiple morphological and functional benefits.
Combinatorial treatment with AICAR and heparin causes an additive effect on utrophin A expression
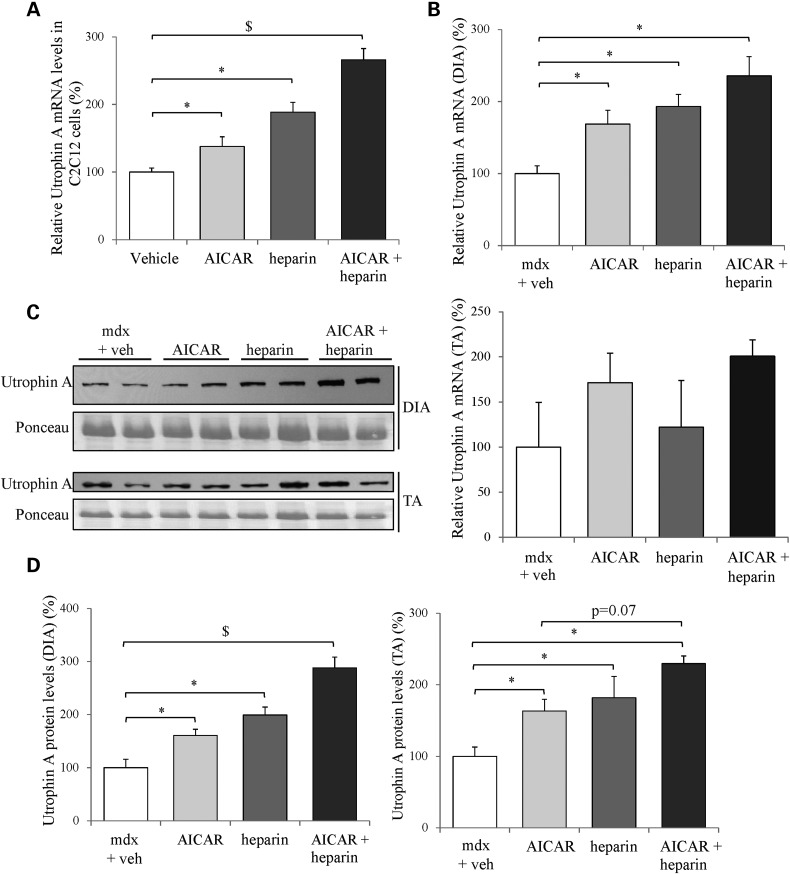
AICAR, a synthetic agonist, has been shown to stimulate AMPK activity in skeletal muscle (92) and recent work from our laboratory (53,59) and that of others (81), demonstrated that a 4-week AICAR treatment of mdx mice resulted in utrophin A upregulation in muscles as well as in an attenuation of the dystrophic phenotype. Thus, given the effective upregulation of utrophin A in response to individual AICAR and heparin treatments, we set out to determine whether a combinatorial treatment of AICAR plus heparin (A + H) causes greater increases (additive or synergistic) in utrophin A expression. Such a combinatorial A + H treatment is expected, based on recent findings (53,63,77,78,93,94), to activate distinct signaling pathways acting transcriptionally (AICAR) and post-transcriptionally (heparin). To this end, we first treated C2C12 myoblasts with AICAR, heparin and a combinatorial A + H treatment for 24 h, and examined utrophin A mRNA expression by RT-qPCR. As shown in Figure 7A, the combinatorial A + H treatment triggered an additive effect on utrophin A expression since it caused a ∼2.5-fold increase (P < 0.05) in its mRNA level (compared with vehicle), which was also significantly higher (P < 0.05) than the effect seen with individual AICAR or heparin treatments.
Figure 7.
Combinatorial treatment of mdx mice with AICAR and heparin increases utrophin A mRNA and protein expression. (A) C2C12 cells were treated with AICAR (1 mM), heparin (2.5 IU/mL), AICAR + heparin, or vehicle for 24 h. Utrophin A mRNA levels in treated C2C12 cells were measured by RT-qPCR and standardized to 18S mRNA levels. (B) Utrophin A mRNA levels in DIA and TA from mdx mice treated with AICAR (500 mg/kg), heparin (500 IU/kg), AICAR + heparin or vehicle for 4 weeks were measured by RT-qPCR. Values were standardized to 18S mRNA levels. (C) Representative western blots of utrophin A and ponceau stain of protein extracts from diaphragm and TA muscles from vehicle or treated mdx mice. (D) Quantification of utrophin A protein levels standardized to ponceau as shown in (C). N = 4 (3 replicates for each for RT-qPCR cell culture experiments). Error bars represent SEM. *P < 0.05 versus C2C12 cells + vehicle or mdx + vehicle, and $ = P < 0.05 different compared with all treatments and vehicle.
Given these positive findings obtained with cultured cells, we proceeded to test whether the combinatorial A + H treatment further stimulates utrophin A expression in mdx mouse muscles as compared with individual treatments. To achieve this, we treated 6-week-old mdx mice with subcutaneous injections of saline (control), AICAR (500 mg/kg body weight), heparin (500 IU/kg) and A + H for a duration of 4 weeks. The treatment and dosage protocols selected for these experiments were based on prior studies from our laboratory (53,77) and others (63,78,95–99). We first measured utrophin A mRNA levels in diaphragm and TA muscles from treated mdx mice. Our results showed that utrophin A mRNA levels were significantly (P < 0.05) stimulated in response to all three treatments (AICAR, heparin and A + H) in the diaphragm as compared with vehicle-treated mdx mice (Fig. 7B). Although the combinatorial treatment did not show a statistically significant greater increase in utrophin A mRNA expression, there was nonetheless a clear trend for an additive effect in comparison to individual AICAR and heparin treatments. In TA muscles, no significant difference in utrophin A mRNA levels of treated mdx mice was detected but there was a clear trend towards an increase in utrophin A transcripts with the combinatorial treatment (Fig. 7B).
We also measured utrophin A protein levels in both diaphragm and TA muscles of mdx mice treated with AICAR, heparin and A + H. In this case, and as seen with cultured myogenic cells, the combinatorial A + H treatment clearly resulted in a ∼2.9-fold additive effect on utrophin A protein expression (P < 0.05) in the diaphragm of mdx mice compared with vehicle-treated mdx mice. This increase was also significantly greater (P < 0.05) than the increase seen with AICAR and heparin alone (Fig. 7C and D). Similarly, all treatments induced an upregulation of utrophin A in the TA muscle with the combinatorial A + H treatment showing again an additive effect with a ∼2.3-fold increase (Fig. 7C and D).
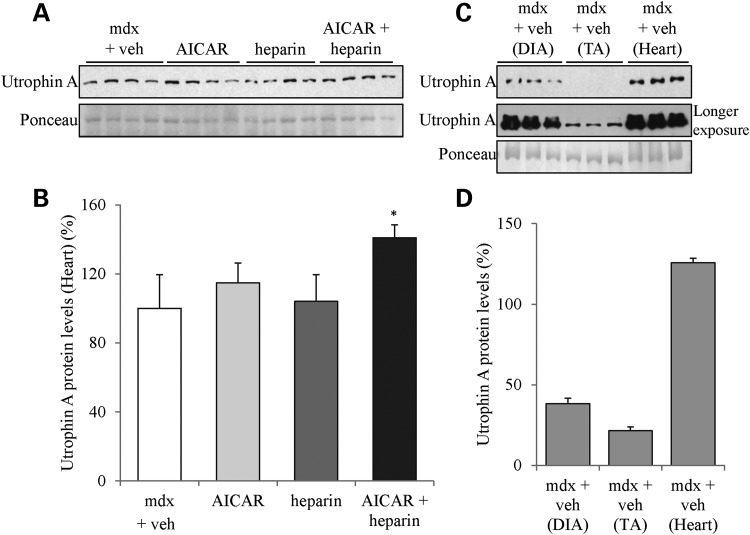
Since AICAR is associated with heart hypertrophy and that p38 MAPK signaling has also been reported to be connected to cardiac failure and hypertrophy (95,100–103), we also examined whether there was an increase in utrophin A expression in the heart following the various drug treatments. Our results show that individual treatments of mdx mice with heparin or AICAR did not increase utrophin A levels in the heart (Fig. 8). By contrast, the combinatorial therapy caused a more modest but significant increase in utrophin A expression (P < 0.05; Fig. 8A and B). In these experiments, we also noted that utrophin A in the mdx heart is relatively high compared with TA and diaphragm muscles (Fig. 8C and D) which, similar to the results obtained with the soleus (Fig. 2), suggests that muscles with endogenously high utrophin A levels may not respond robustly to individual heparin or AICAR treatments.
Figure 8.
Combinatorial treatment of mdx mice with AICAR and heparin increases utrophin A expression in the heart. (A) Representative western blot of utrophin A and ponceau stain of protein extracts from heart muscle from treated mdx mice with AICAR (500 mg/kg), heparin (500 IU/kg), AICAR + heparin or vehicle for 4 weeks. (B) Quantification of utrophin A protein levels standardized to ponceau as shown in (A). (C) Representative western blot of utrophin A and ponceau stain of protein extracts from diaphragm, TA and heart muscle from vehicle-treated mdx mice. (D) Quantification of utrophin A protein levels standardized to ponceau as shown in (C). N = 4. Error bars represent SEM. *P < 0.05 versus mdx + vehicle.
Next, we performed immunofluorescence experiments on both diaphragm and TA muscles to establish if the combinatorial A + H treatment stimulated utrophin A expression along the sarcolemma of treated mdx mice. As shown in Figure 9, utrophin A expression was enhanced in both muscles in response to all three treatments (AICAR, heparin, A + H) but, consistent with the western blotting data, the increase appeared even greater in the diaphragm and TA muscle from the A + H group of mdx mice. To determine whether the combinatorial A + H treatment leads to the reassembly of the DAPC complex, we also examined the localization of β-dystroglycan at the sarcolemma of mdx muscle fibers. As shown in Figure 9B, immunofluorescence experiments revealed that all treatments, including the combinatorial one with AICAR and heparin, enhanced β-dystroglycan expression along the sarcolemmal membrane in both diaphragm and TA muscles of treated mdx mice. Overall, these results indicate that in both cultured myogenic cells and mdx mouse muscles, the combinatorial A + H treatment is capable of inducing an additive effect on utrophin A expression as compared with individual treatments. The further increase in utrophin A expression seen following the combinatorial treatment, together with its localization at the sarcolemma and that of β-dystroglycan, suggest additional morphological and functional benefits over AICAR and heparin treatment alone.
Figure 9.
Combinatorial treatment of mdx mice with AICAR and heparin increases sarcolemmal localization of utrophin A in mdx muscle fibers. Representative cross-sections of DIA and TA muscles from mdx mice treated with AICAR, heparin, AICAR + heparin or vehicle that were immunostained with utrophin A (UTR-A) (A) and β-dystroglycan (β-dys) (B) antibodies. Scale bar = 50 μm.
AICAR and heparin treatments alters expression of key signaling molecules in mdx mouse muscles
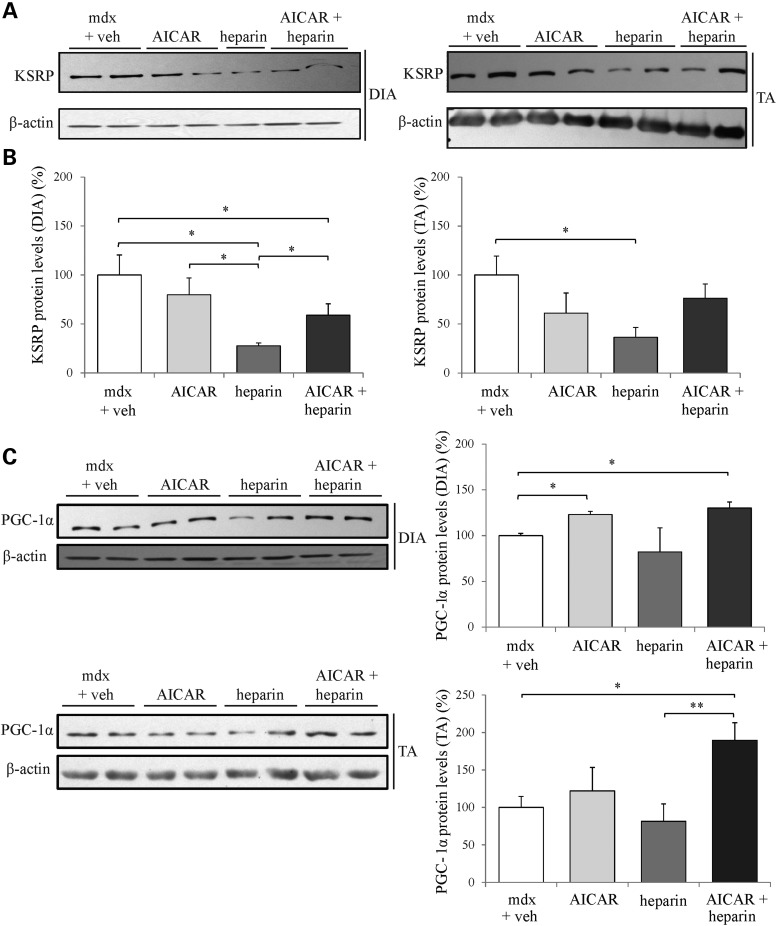
As mentioned above, we showed in our recent work that AICAR promotes utrophin A expression via a transcriptional mechanism involving PGC-1α (53,59); (see also 81,104) whereas heparin increases the stability of utrophin A mRNAs by decreasing the functional availability of KSRP (77). Here, we therefore verified that the combinatorial A + H treatment would similarly alter expression of these key molecules in treated mdx mice. First, we measured the expression levels of KSRP in muscles of mdx mice treated with AICAR, heparin and A + H. Our results show that heparin alone caused a ∼2- and 2.5-fold decrease (P < 0.05) in KSRP protein levels in the diaphragm and TA muscles of mdx mice, respectively, as compared with vehicle-treated mdx mice (Fig. 10A and B). Also, there was a trend towards a decrease in KSRP expression in both diaphragm and TA muscles following combinatorial A + H but this difference reached significance (P < 0.05) only in the diaphragm.
Figure 10.
Combinatorial treatment modulates expression of key signaling molecules in mdx mouse muscles. (A) Representative western blots of KSRP protein levels in protein extracts from DIA and TA muscles of mdx mice treated with AICAR, heparin, AICAR + heparin or vehicle. (B) Quantification of KSRP protein levels standardized to β-actin as shown in (A). (C) Representative western blots and quantification of PGC-1α protein levels standardized to β-actin from protein extracts of mdx diaphragm muscles. N = 4. Error bars represent SEM, *P < 0.05 versus mdx vehicle control.
AICAR treatment is known to drive a significant increase in PGC-1α protein expression in mdx mouse muscle (53,59,94,104,105). Here, we observed that similarly, the combinatorial A + H treatment led to an increase (P < 0.05) in PGC-1α levels in both mdx diaphragm and TA muscles that is comparable, at least in the diaphragm, to that observed following the individual AICAR treatment (Fig. 10C). Taken together, these findings suggest that the combinatorial A + H treatment regulates simultaneous expression of key and distinct signaling pathways that appear to ultimately converge onto the same slower, more oxidative phenotypic outcome in mdx mouse muscle which, in parallel, stimulate utrophin A expression.
Discussion
In the present study, we first determined whether a 4-week treatment regimen with heparin induces increases in utrophin A expression in several mdx mouse muscles and provides morphological and functional benefits. Our findings show that clinically used heparin upregulates utrophin A levels in a variety of muscles of mdx mice including the diaphragm. This 4-week treatment also improved several pathological features of mdx mouse muscles including reassembly of the DAPC at the sarcolemma as well as improved morphological parameters and sarcolemmal integrity. As part of this work, we also explored the therapeutic potential of using a combinatorial treatment with both heparin and AICAR. Our results clearly show that combination of heparin with AICAR instigates an additive effect on utrophin A transcript and protein levels in mdx mouse muscle that is greater than increases seen when these drugs are used in isolation. Taken together, these results suggest that combinatorial treatment of heparin and AICAR, which individually target distinct signaling pathways, may serve as an effective therapeutic strategy to treat DMD.
Heparin promotes the post-transcriptional upregulation of utrophin A in muscles and improves the dystrophic pathology
Initial evidence of possible post-transcriptional regulation of utrophin A came from earlier studies in which it was noted that altered expression of utrophin A protein in muscle was not mirrored by changes in levels of its mRNA (45,106,107). Additional work revealed the importance of the 5′ and 3′UTRs of utrophin A mRNAs in regulating its translation and stability, respectively (68–72). In this context, our laboratory showed that an ARE contained within the utrophin A 3′UTR plays a key role in controlling the longevity of utrophin A mRNAs in muscle (68,69). Studies from our laboratory and others have shown that p38 MAPK regulates the levels of utrophin A mRNA (77) and other pre-synthesized transcripts (108), by decreasing the functional availability of KSRP; an RNA-binding protein that interacts with AREs and known to induce decay of target transcripts. More specifically, we reported in our initial study that p38 activation with heparin decreases the ability of KSRP to interact with the ARE present in the utrophin 3′UTR thereby causing an increase in utrophin A mRNA and protein levels (77).
Here, we show that heparin is a potent utrophin A stimulator since treatment with heparin causes a clear increase in utrophin A expression in both C2C12 muscle cells and in several mdx mouse muscles. Such increase in utrophin A expression in dystrophic muscles was accompanied by important morphological and functional improvements. A central question raised by these findings concerns the identity of the signaling molecules upstream of p38 activation on which heparin acts. Although the complete nature of this pathway remains unclear, converging lines of evidence indicate that TLR may play a role in activating p38 MAPK (78,109). TLRs are transmembrane proteins highly expressed in immune cells, however, recent studies have also found TLRs to be present in other cell types including TLR2 and 4 that are expressed in skeletal muscle cells (109–111). In mdx mice, TLR1, 2, 3, 4, 7, 8 and 9 are expressed in a variety of skeletal muscles at different levels. In fact, slow-twitch muscles, such as the soleus have more TLRs (112). Of high relevance, heparin has been shown to activate p38 via TLR2 and TLR4 in C2C12 myotubes as well as skeletal muscle of C57BL/6J wild-type mice (78,109). More specifically, heparin treatment results in increases in extracellular levels of non-esterified fatty acid which in turn, directly activates the TLR receptors (78). This suggests that heparin may stimulate TLR which then activates p38 MAPK causing a decrease in the functional availability of KSRP and in its binding to the ARE located within the utrophin 3′UTR, culminating in increases in utrophin A expression. Interestingly, levels of TLR4 mRNA have been shown to be increased in mdx mouse muscles compared with wild type (113), thus providing greater target availability for heparin in dystrophic muscle. However, recent work has also shown that ablation of TLR or of the TLR adaptor protein called myeloid differentiation primary response gene 88 (myd88) in mdx mice, offers benefits to their muscle (112,113). Therefore, it seems clear that additional work is necessary to better understand the role and therapeutic potential of TLR's in dystrophic muscle.
Heparin promotes expression of the slow oxidative phenotype in dystrophic skeletal muscles
Our laboratory was first to demonstrate that slower, more oxidative fibers have overall higher content of utrophin A in extrasynaptic regions as compared with fast glycolytic fibers (82). Such increased expression of utrophin A correlates well with the fact that slow myofibers in both mdx mice and DMD patients show reduced damage in comparison to faster muscle fibers (82). Accordingly, we speculated several years ago (48,53,56,59–61) that strategies to promote expression of the slow oxidative phenotype, which includes increased utrophin A expression, would be highly beneficial to dystrophic muscle. Using a variety of transgenic mouse models and pharmacological interventions, our laboratory (53,59–61) and others (50–52,56,62,63,65,88,114) have shown that indeed such an approach is therapeutically relevant as increased expression of the slow oxidative phenotype provides important morphological and functional benefits to dystrophic muscle. In this context, beneficial adaptations have been seen previously in mitochondria, the contractile apparatus and in the structural integrity of fast glycolytic muscle fibers following an increase in utrophin A and β-dystroglycan expression throughout the sarcolemma (53,115).
In the present study, we also show that treatment of mdx mice with heparin promotes a shift towards a slower, more oxidative phenotype. This suggests that the protective effects of heparin treatment on the progression of the disease may relate to its ability to stimulate the slow oxidative myogenic program. How heparin signals to achieve this transition in fiber type and metabolic profile is unclear at the present time but it would seem important to examine whether TLR's play a role in such a signaling cascade. In agreement with our data, a recent study demonstrated that heparin induces mitochondrial membrane potential and increases expression of the slow oxidative marker cytochrome C oxidase subunit II in HUVECS cells (116). Thus, further studies are also warranted to determine if heparin, TLR's and/or the downstream regulators p38 MAPK and KSRP, are key modulators of the slow oxidative phenotype.
Combinatorial treatment of mdx mice with heparin and AICAR induces an additive effect on utrophin A expression by activating distinct pathways
In the present study, we also decided to treat mdx mice with a combination of the putative post-transcriptional activator heparin (77,78) with AICAR, a mediator of utrophin A expression via transcriptional pathways (53). In this work, we chose not to extensively analyze the individual AICAR treatment in mdx mice because this has already been studied in recent past by our laboratory (53,59) and that of others (63,81,96,99,117). In these combinatorial experiments, we postulated that by activating distinct pathways, utrophin A levels would be further increased in comparison to treatments using these two drugs in isolation. Our results show that individual treatments of AICAR and heparin increase utrophin A but at a lower extent to that seen with the combination of both drugs. In fact, our combinatorial treatment with AICAR + heparin stimulated a clear and important additive effect on utrophin A expression in C2C12 muscle cells and mdx mouse muscle. More specifically, utrophin A protein levels were increased by a maximum of nearly 3-fold in response to the AICAR plus heparin treatment which is particularly encouraging since previous studies reported that a 2-fold increase in utrophin A in muscle is sufficient to ameliorate the dystrophic phenotype in mdx mice (118). The fact that the diaphragm responded well to the combinatorial treatment is important given that maintaining functions of respiratory muscles in DMD patients is central to efficacious therapeutic interventions.
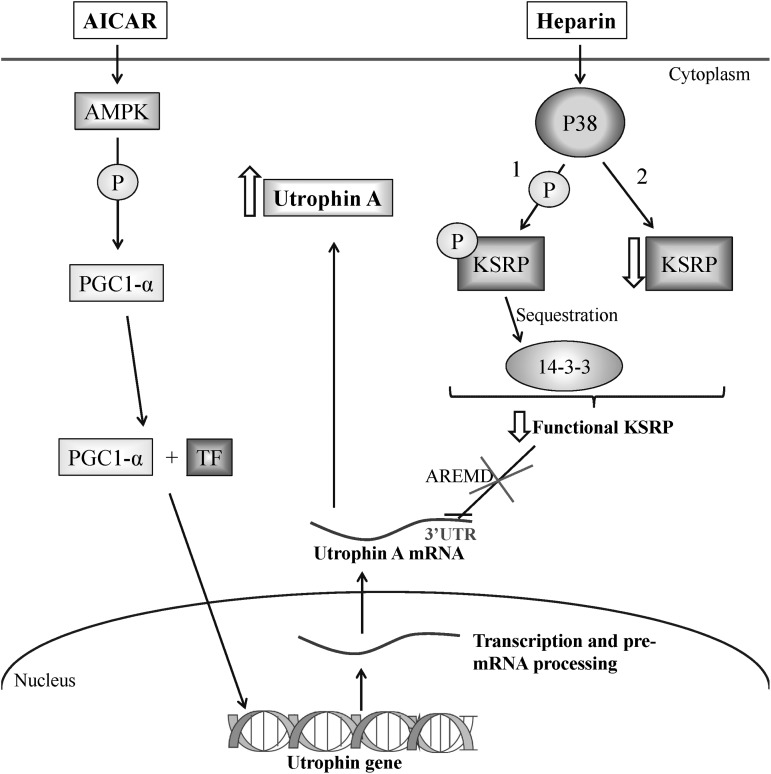
Since utrophin A expression can be modulated by both transcriptional and post-transcriptional events, we also examined expression levels of utrophin A regulators following AICAR and heparin treatment. As predicted based on recent work (53,98,105), the individual treatment with AICAR and the combinatorial treatment of AICAR plus heparin resulted in significant increases in PGC-1α levels in dystrophic muscle; a change not observed following treatment with heparin alone. In contrast, individual treatment with heparin and the combinatorial treatment both promoted a decrease of KSRP expression in treated mdx mouse muscle. However, the decrease of KSRP expression was greater following the individual heparin treatment compared with the combinatorial treatment, and varied between TA and diaphragm muscles. The fact that utrophin A levels are nonetheless additively increased following the combinatorial treatment indicates that at least one other mechanism controls the functional availability of KSRP in muscle, in particular its sequestration by 14-3-3 as we have shown recently (77). Taken together, these results further highlight the distinct mechanisms of action of AICAR and heparin on utrophin A upregulation and indicate that the combination of these two drugs activates both pathways. On the basis of these findings, we therefore propose a model (Fig. 11) by which AICAR and heparin stimulate utrophin A expression via distinct signaling cascades and molecular events. In this model, AICAR promotes utrophin A expression through activation of AMPK and PGC-1α signaling, whereas heparin acts mainly by activating p38 MAPK which subsequently phosphorylates KSRP and promotes its sequestration by 14-3-3 proteins while also decreasing its expression level. The combined effect of activating these pathways results in enhanced transcription of the utrophin gene with a parallel increase in the stability of synthesized utrophin A transcripts as illustrated in Figure 11.
Figure 11.
Model depicting the potential signaling and impact on utrophin A expression of the combinatorial treatment. AMPK activation by AICAR activates PGC-1α expression in muscle. Upon coactivation, PGC-1α and TFs (e.g. PPARβ/δ and GABP) stimulate utrophin A transcription. P38 activation by heparin mediates a decrease in the functional availability of the RNA-binding protein KSRP, which is involved in ARE-mediated decay of utrophin A transcripts. This decrease in the functional availability of KSRP is achieved by: (1) an increase in its phosphorylation status which promotes its sequestration by the regulatory protein 14-3-3; and (2) a decrease in its expression. Simultaneous activation of these pathways by AICAR and heparin leads to an additive effect on utrophin A expression in dystrophic muscle.
One of the highlights of our study is the identification and exploitation of clinically relevant drugs for treating DMD. Such a repurposing strategy exploits the off-target effects of clinically approved drugs, thereby providing opportunities to accelerate the development of new drugs for DMD while reducing health risks (119). Previous studies from our laboratory exploited the off-target effects of several drugs in upregulating utrophin A expression in muscle such as the PPAR-β/δ agonist GW501516, AMPK/Sirt1 activator Resveratrol, AMPK/PGC1α activators Metformin and AICAR, as well as heparin (53,60,61,67,77). In our current study, it appears that repurposing of AICAR and heparin may be an effective combinatorial therapeutic strategy to treat DMD. Heparin is an FDA approved drug that has been used to treat and prevent thrombosis for years (79). It is also a naturally occurring compound in the human body indicating that it is relatively safe with less severe side effects (79). On the other hand, AICAR is also being tested extensively in clinical trials for the treatment of other diseases such as type II diabetes (64,99,120). Despite the beneficial effects of the combinatorial treatment of AICAR with heparin on utrophin A expression, it is important to be mindful of the limitations of this strategy in long-term therapies. AICAR and heparin are associated with a few side effects. In particular, heparin can cause excessive bleeding, induce thrombocytopenia, skin necrosis and osteoporosis in treated patients. Conversely, and due to its anticoagulant properties, heparin could also be further beneficial by increasing the effective supply of metabolites and oxygen to working muscle. AICAR, on the other hand, is associated with liver and heart hypertrophy (95). In this case, metformin might represent an interesting alternative for activating AMPK (67,121). Nonetheless, one should remain well aware of these possible side effects and drug interactions, and ultimately balance the advantages versus disadvantages of using such drug therapies.
Materials and Methods
Cell culture, plasmid, transfection and luciferase assay
Mouse C2C12 cells (American Type Culture Collection, Manassas, VA, USA) were plated on 6-well culture dishes and maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (Wisent, St-Bruno, QC, Canada), 1% l-glutamine and 1% penicillin/streptomycin. The cells were incubated at 37°C with 5% CO2 in a humidified chamber.
The full-length 3′UTR from the utrophin A mRNA (2.1 kb), which was used in these experiments, was described in a previous study (77). Briefly, the 3′UTR was isolated by RT-PCR and subcloned in the PGL4 vector backbone, downstream of the Firefly luciferase gene. Transient transfections were performed using the transfection reagent Lipofectamine (Invitrogen) by following the manufacturer's instructions. Approximately 50 000 cells per well were seeded in a 6-well culture dish. The next day, C2C12 cells were transiently transfected at 50–60% confluence with a mixture of DNA (1 μg)/lipofectamine for 4 h and incubated at 37°C in a humidified chamber supplied with 5% CO2. Following the transfection, cells were treated for 24 h with heparin as described below. Cells were harvested 24 h later for further analyses.
C2C12 cells were homogenized in reporter lysis buffer (Dual Luciferase Assay System, Promega, Madison, WI, USA), followed by a freeze thaw treatment. The activity of Firefly luciferase was determined using the Dual Luciferase Assay kit and detected using a luminometer (Lumat LB 9507—Berthold Technologies, Große Enz, Germany). Cells were co-transfected with a control Renilla luciferase reporter vector (phRGtk-luc) to monitor transfection efficiency.
Heparin and AICAR treatment
Approximately, 75 000 cells per well were seeded in a 6-well culture dish. The next day, C2C12 cells (60–70% confluence) were treated with heparin (2.5 IU/mL) (LEO Pharma, Thornhill, Canada), or/and AICAR (1 mM) (TRC, Toronto, Canada), or sterile water (control) for 24 h as used in earlier studies (77,94). In vivo experiments were performed using mdx mice and C57BL10 WT mice (The Jackson Laboratory, Bar Harbor, USA), that were maintained in the Animal Care and Veterinary Service of University of Ottawa under a constant 12 h light–dark cycle with full access to water and food. The experimental protocols were approved by the University of Ottawa Animal Care Committee and were in accordance with the Canadian Council of Animal Care Guidelines. Six week-old mdx mice were treated daily with vehicle (sterile saline), AICAR (500 mg/kg), heparin (500 IU/kg) or AICAR + heparin by subcutaneous injections for 4 weeks (53,63,77,78). Both AICAR and heparin were dissolved in a volume of 50 mL of sterile saline prior to daily treatments. Muscles were dissected from euthanized treated mdx mice, flash frozen in liquid nitrogen, or in melting isopentane cooled with liquid nitrogen, for further analyses.
Ex vivo eccentric contractions
After 4 weeks, the heparin- and saline-treated mice were euthanized. The EDL muscle was dissected and attached at one end to a Dual mode lever system (model 300C, Aurora Scientific, Aurora, Canada) to measure force and to lengthen muscle, and the other end to a fixed rod. Throughout the experiment, the muscle was submerged in a saline solution containing (in mM): 118.5 NaCl, 4.7 KCl, 2.4 CaCl2, 3.1 MgCl2, 25 NaHCO3, 2 NaH2PO4, 5.5 D-glucose, 95% O2–5% CO2 (to maintain a pH of 7.4) and 0.1% trypan blue (Sigma-Aldrich, Oakville, Canada), with a flow rate of 15 mL/min at 25°C. Adjustments of the muscle length were performed in order to get maximal force output. Five maximal tetanic contractions (400 ms train duration, 10 V, 0.3 ms square pulse, 200 Hz) were elicited to determine muscle contractile kinetics. These contractions were executed every 100 s, followed by 12 eccentric contractions ever 120 s (700 ms train duration, 10 V, 0.3 ms square pulse, 200 Hz) Eccentric contractions were elicited by subjecting muscles to a 10% lengthening at a velocity of 0.5 Le/s throughout the last 200 ms. Electrical stimulation were generated across two platinum wires (positioned above and below muscles 4 mm apart) using a Grass stimulator (model S88X, Grass Technologies, West Warwick, USA). A Keithley data acquisition board (model KPCI-3104, Cleveland, USA) was used to detect the force at a sample rate of 5 KHz. Tetanic force was defined as the increase in force upon stimulation and was normalized to the CSA. The muscle CSA was calculated by dividing muscle volume by the muscle experimental weight; muscle volume was calculated from the muscle weight converted to volume using a density of 1.06 g/cm3. The trypan blue-stained EDL muscles were frozen in melting isopentane cooled with liquid nitrogen and cut into 10 μm cross-sections. The sections were observed with a fluorescent Zeiss Axioshop-2 microscope. Stained fibers were quantified compared with total fiber using image J (NIH version 1.0).
Western blotting
Frozen muscles were ground to powder with a BioPulverizer on dry ice. Muscle samples were suspended in 300 uL of urea extraction buffer (7 M urea, 2 M thiourea, 4 M CHAPS, 100 mM DTT, 125 mM Tris–HCl pH 6.8) supplemented with complete Mini Protease Inhibitor Cocktail and phosphatase inhibitor PhosSTOP (Roche, Laval, QC, Canada). The samples were vortexed for 30 min at room temperature and then centrifuged at 20 000 g for 15 min. Supernatants were collected and stored at −80°C. Protein concentrations were determined using CB-X Protein Assay Kit (G-Biosciences, St. Louis, MO, USA) and bovine serum albumin was used as a standard. Ten micrograms of extracted proteins were loaded on a sodium dodecyl sulfate polyacrylamide gel (6–8% polyacrylamide) and migrated at 80–100 V for 2–3 h. The protein transfer was performed on a nitrocellulose membrane (Bio-Rad, Mississauga, ON, Canada). After transfer, membranes were stained with Ponceau S (Sigma-Aldrich) to confirm equal loading between lanes. Membranes were subsequently washed four times with 1 × PBS-T (1× PBS, 0.2% Tween) and blocked for 1 h with a 5% skim milk in PBS-T solution. Blots were then incubated in blocking solution for 1 h or overnight with antibodies directed against utrophin A (1:500; Novocastra, Leica biosystems, Concord, ON, Canada), KSRP (1:1000; Bethyl Laboratories, Montgomery, TX, USA), PGC-1α (1:2000; Abcam, Toronto, ON, Canada) or OXPHO cocktail (1:1000; Abcam, Toronto, ON, Canada), with gentle rocking. The blots were incubated with appropriate Horse Radish Peroxidase-conjugated secondary antibody for 1 h at room temperature in blocking solution and washed 4 times with 1xPBS-T. When appropriate, the blots were also incubated with antibodies against β-actin (1:10 000; Santa Cruz, Dallas, Texas, USA) as a loading control. Ponceau staining was systematically used to verify equal loading for the utrophin A western blots. This was necessary to obtain better separation of large molecular mass proteins. The gels were run longer causing the lower mass proteins such as β-actin, GAPDH and tubulin to run out of the gels. We thus prefer staining the same membrane used for western blotting for utrophin A in order to assess loading. Protein quantification of utrophin A was normalized to protein levels as determined from the same membrane stained with ponceau. The Chemiluminescent detection of proteins was performed using ECL reagent (Perkin Elmer, Waltham, MA, USA). The films were quantified using ImageJ (NIH version 1.0) and/or Image Lab.
Immunofluorescence, and hematoxylin and eosin staining
Ten micrometer muscle cross-sections were processed for immunofluorescence using the M.O.M's Immunodetection kit (Vector Laboratories, Burlington, ON, Canada). Sections were incubated with primary antibodies against utrophin A (NCL-DRP2) (1:200; Novocastra, Leica biosystems), β-dystroglycan (1:400; NCL-B-DG, Novocastra, Leica biosystems) or myosin heavy chain type 1 (MHC I) (undiluted; BA-F8) (Hybridoma Bank, Iowa city, IA, USA), for 30 min at room temperature. A Texas Red conjugated Streptavidin antibody (1:500; Vector laboratories) was used for detection. A FITC-conjugated IgM anti-mouse secondary antibody was used (1:400; Sigma-Aldrich) for immunoglobulin (IgM) staining. The slides were mounted with Vectashield mounting medium (Vector Laboratories) and visualized using a Zeiss Axioskop-2- fluorescence microscope. MHC positive fibers, compared with total fiber, were quantified using image J (NIH version 1.0).
TA and diaphragm muscle cross-sections were also stained with hematoxylin and eosin dyes, dehydrated using a series of ethanol solutions (70%, 90%, 100%), and subsequently washed with toluene. The slides were then mounted using Permount and visualized using a Zeiss Axioshop-2 microscope. The percentage of central nucleation was determined by visually counting the total number of muscle fibers and the number of muscle fibers with central nucleation from 4–6 cross-sectional views using the Northern Eclipse Software (NES, Expix Imaging, Mississauga, Ontario, Canada). CSA of each fiber was measured using NES. The VC was calculated based on the CSA of muscle fibers using the formula ‘variance coefficient Z = 1000 × standard deviation of muscle fiber CSA/mean muscle fiber CSA’.
RNA extraction and RT-qPCR
Total RNA was extracted from muscle tissue and C2C12 cells using TRIzol reagent (Invitrogen) as recommended by the manufacturer. TRIzol extracted RNA was treated for 1 h with DNAse I (Invitrogen) to eliminate possible DNA contamination. Reverse transcription (RT) was carried out using an RT reaction mixture containing 5 mM MgCl2, 1× PCR buffer, 1 mM dNTP, 1 U/mL RNase inhibitor, 5 U/mL Moloney murine leukemia virus reverse transcriptase and 2.5 mM random hexamers (Applied Biosystems, CA, USA). A real-time quantitative PCR (qPCR) was performed on an MX3005p real-time PCR system (Stratagene, La Jolla, CA, USA) using a QuantiTect SYBR Green PCR kit (QIAGEN, Valencia, CA, USA). For these experiments, amplification of the 18S ribosomal subunit, GAPDH, utrophin A was performed in duplicates with the following primer sequences: utrophin A—forward 5′-ATCTTGTCGGGCTTTCCAC-3′ and reverse 5′-ATCCAAAGGCTTTCCCAGAT-3′, 18S Ribosomal—forward 5′-CGCCGCTAGAGGTGAAATC-3′ and reverse 5′-CCAGTCGGCATCGTTTATGG-3′, GAPDH—forward 5′-GGGTGTGAACCACGAGA AAT-3′ and reverse 5′-CCTTCCACAATGCCAAAGTT-3′.
Statistical analysis
The data were analyzed using paired and unpaired Student's t-test and analysis of variance with Fisher's post hoc tests as appropriate. Error bars represent standard error of the mean. Statistical analysis was done with StatView version 5.0 (SAS Institute Inc., Cary, NC, USA) on raw data prior to conversion to fold difference (compared with control). Significance was accepted at *P ≤ 0.05.
Funding
This work was supported by grants from Jesse's Journey: the Foundation for Cell and Gene Therapy, the Muscular Dystrophy Association (MDA USA) and the Canadian Institutes of Health Research. A.A. and T.E.C.P. benefited from Ontario Graduate Scholarship during the course of this work, and V.L. was the recipient of a Development Grant from the MDA.
Acknowledgements
The authors are grateful to John Lunde and Wei Lin for expert technical assistance.
Conflict of Interest statement. None declared.
References
- 1.Emery A.E.H. (1991) Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul. Disord., 1, 19–29. [DOI] [PubMed] [Google Scholar]
- 2.Anderson M.S., Kunkel L.M. (1992) The molecular and biochemical basis of Duchenne muscular dystrophy. Trends Biochem. Sci., 17, 289–292. [DOI] [PubMed] [Google Scholar]
- 3.Baxter P. (2006) Treatment of the heart in Duchenne muscular dystrophy. Dev. Med. Child Neurol., 48, 163. [DOI] [PubMed] [Google Scholar]
- 4.Gayraud J., Ramonatxo M., Rivier F., Humberclaude V., Petrof B., Matecki S. (2010) Ventilatory parameters and maximal respiratory pressure changes with age in Duchenne muscular dystrophy patients. Pediatr. Pulmonol., 45, 552–559. [DOI] [PubMed] [Google Scholar]
- 5.Ryan T.D., Jefferies J.L., Sawnani H., Wong B.L., Gardner A., Del Corral M., Lorts A., Morales D.L.S. (2014) Implantation of the HeartMate II and HeartWare left ventricular assist devices in patients with Duchenne muscular dystrophy: lessons learned from the first applications. ASAIO J., 60, 246–248. [DOI] [PubMed] [Google Scholar]
- 6.Bushby K., Finkel R., Birnkrant D.J., Case L.E., Clemens P.R., Cripe L., Kaul A., Kinnett K., McDonald C., Pandya S. et al. (2010) Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol., 9, 77–93. [DOI] [PubMed] [Google Scholar]
- 7.Worton R.G., Thompson M.W. (1988) Genetics of Duchenne muscular dystrophy. Annu. Rev. Genet., 22, 601–629. [DOI] [PubMed] [Google Scholar]
- 8.Straathof C.S.M., Van Heusden D., Elly P., Ippel F., Jan G., Voermans N.C., De Visser M., Brusse E. (2015) Diagnosis of Becker muscular dystrophy : results of re-analysis of DNA samples. Muscle Nerve., [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9.Nigro V., Piluso G. (2015) Spectrum of muscular dystrophies associated with sarcolemmal-protein genetic defects. Biochim. Biophys., 1852, 585–593. [DOI] [PubMed] [Google Scholar]
- 10.Yang B., Jung D., Rafael J.A., Chamberlain J.S., Campbell K.P. (1995) Identification of alpha-syntrophin binding to syntrophin triplet, dystrophin, and utrophin. J. Biol. Chem., 270, 4975–4978. [DOI] [PubMed] [Google Scholar]
- 11.Ehmsen J., Poon E., Davies K. (2002) The dystrophin-associated protein complex. J. Cell Sci., 115, 2801–2803. [DOI] [PubMed] [Google Scholar]
- 12.Johnson E.K., Li B., Yoon J.H., Flanigan K.M., Martin P.T., Ervasti J., Montanaro F. (2013) Identification of new dystroglycan complexes in skeletal muscle. PLoS One, 8, 15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strakova J., Dean J.D., Sharpe K.M., Meyers T.A., Odom G.L., Townsend D. (2014) Dystrobrevin increases dystrophin's binding to the dystrophin–glycoprotein complex and provides protection during cardiac stress. J. Mol. Cell. Cardiol., 76, 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barton-Davis E.R., Cordier L., Shoturma D.I., Leland S.E., Sweeney H.L. (1999) Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J. Clin. Invest., 104, 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik V., Rodino-Klapac L.R., Viollet L., Mendell J.R. (2010) Aminoglycoside-induced mutation suppression (stop codon readthrough) as a therapeutic strategy for Duchenne muscular dystrophy. Ther. Adv. Neurol. Disord., 3, 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyenvalle A., Vulin A., Fougerousse F., Leturcq F., Kaplan J.-C., Garcia L., Danos O. (2004) Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science, 306, 1796–1799. [DOI] [PubMed] [Google Scholar]
- 17.Lu Q.L., Rabinowitz A., Chen Y.C., Yokota T., Yin H., Alter J., Jadoon A., Bou-Gharios G., Partridge T. (2005) Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc. Natl. Acad. Sci. U. S. A., 102, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokota T., Lu Q.L., Partridge T., Kobayashi M., Nakamura A., Takeda S., Hoffman E. (2009) Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann. Neurol., 65, 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokota T., Nakamura A., Nagata T., Saito T., Kobayashi M., Aoki Y., Echigoya Y., Partridge T., Hoffman E.P., Takeda S. (2012) Extensive and prolonged restoration of dystrophin expression with vivo-morpholino-mediated multiple exon skipping in dystrophic dogs. Nucleic Acid Ther., 22, 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyenvalle A., Griffith G., Babbs A., Andaloussi S.E., Ezzat K., Avril A., Dugovic B., Chaussenot R., Ferry A., Voit T. et al. (2015) Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Nat. Med., 21, 270–275. [DOI] [PubMed] [Google Scholar]
- 21.Gregorevic P., Blankinship M.J., Allen J.M., Crawford R.W., Miller D.G., Russell D.W., Chamberlain J.S. (2004) Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med., 10, 828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregorevic P., Blankinship M.J., Allen J.M., Chamberlain J.S. (2008) Systemic microdystrophin gene delivery improves skeletal muscle structure and function in old dystrophic mdx mice. Mol. Ther., 16, 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodino-Klapac L.R., Janssen P.M.L., Shontz K.M., Canan B., Montgomery C.L., Griffin D., Heller K., Schmelzer L., Handy C., Clark K.R. et al. (2013) Micro-dystrophin and follistatin co-delivery restores muscle function in aged DMD model. Hum. Mol. Genet., 22, 4929–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love D.R., Forrest S.M., Smith T.J., England S., Flint T., Davies K.E. (1989) Molecular analysis of Duchenne and Becker muscular dystrophy. Br. Med. Bull., 45, 659–680. [DOI] [PubMed] [Google Scholar]
- 25.Blake D.J., Weir A., Newey S.E., Davies K.E. (2002) Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev., 82, 291–329. [DOI] [PubMed] [Google Scholar]
- 26.Khurana T.S., Watkins S.C., Chafey P., Chelly J., Tomé F.M., Fardeau M., Kaplan J.C., Kunkel L.M. (1991) Immunolocalization and developmental expression of dystrophin related protein in skeletal muscle. Neuromuscul. Disord., 1, 185–194. [DOI] [PubMed] [Google Scholar]
- 27.Ohlendieck K., Ervasti J.M., Matsumura K., Kahl S.D., Leveille C.J., Campbell K.P. (1991) Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron, 7, 499–508. [DOI] [PubMed] [Google Scholar]
- 28.Belanto J.J., Mader T.L., Eckhoff M.D., Strandjord D.M., Banks G.B., Gardner M.K., Lowe D.A., Ervasti J.M. (2014) Microtubule binding distinguishes dystrophin from utrophin. Proc. Natl. Acad. Sci. U. S. A., 111, 5723–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters M.F., Sadoulet-Puccio H.M., Grady R.M., Kramarcy N.R., Kunkel L.M., Sanes J.R., Sealock R., Froehner S.C. (1998) Differential membrane localization and intermolecular associations of α-dystrobrevin isoforms in skeletal muscle. J. Cell Biol., 142, 1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa-Sakurai M., Yoshida M., Imamura M., Davies K.E., Ozawa E. (2004) ZZ domain is essentially required for the physiological binding of dystrophin and utrophin to beta-dystroglycan. Hum. Mol. Genet., 13, 693–702. [DOI] [PubMed] [Google Scholar]
- 31.Winder S.J., Hemmings L., Maciver S.K., Bolton S.J., Tinsley J.M., Davies K.E., Critchley D.R., Kendrick-Jones J. (1995) Utrophin actin binding domain: analysis of actin binding and cellular targeting. J. Cell Sci., 108, 63–71. [DOI] [PubMed] [Google Scholar]
- 32.Perkins K.J., Davies K.E. (2002) The role of utrophin in the potential therapy of Duchenne muscular dystrophy. Neuromuscul. Disord., 12, 17–20. [DOI] [PubMed] [Google Scholar]
- 33.Mizuno Y., Nonaka I., Hirai S., Ozawa E. (1993) Reciprocal expression of dystrophin and utrophin in muscles of Duchenne muscular dystrophy patients, female DMD-carriers and control subjects. J. Neurol. Sci., 119, 43–52. [DOI] [PubMed] [Google Scholar]
- 34.De la Porte S., Morin S., Koenig J. (1999) Characteristics of skeletal muscle in mdx mutant mice. Int. Rev. Cytol., 191, 99–148. [DOI] [PubMed] [Google Scholar]
- 35.Kleopa K.A., Drousiotou A., Mavrikiou E., Ormiston A., Kyriakides T. (2006) Naturally occurring utrophin correlates with disease severity in Duchenne muscular dystrophy. Hum. Mol. Genet., 15, 1623–1628. [DOI] [PubMed] [Google Scholar]
- 36.Tinsley J.M., Potter A.C., Phelps S.R., Fisher R., Trickett J.I., Davies K.E. (1996) Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature, 384, 349–353. [DOI] [PubMed] [Google Scholar]
- 37.Rafael J.A., Tinsley J.M., Potter A.C., Deconinck A.E., Davies K.E. (1998) Skeletal muscle-specific expression of a utrophin transgene rescues utrophin-dystrophin deficient mice. Nat. Genet., 19, 79–82. [DOI] [PubMed] [Google Scholar]
- 38.Perkins K.J., Burton E.A., Davies K.E. (2001) The role of basal and myogenic factors in the transcriptional activation of utrophin promoter A: implications for therapeutic up-regulation in Duchenne muscular dystrophy. Nucleic Acids Res., 29, 4843–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Squire S., Raymackers J.M., Vandebrouck C., Potter A., Tinsley J., Fisher R., Gillis J.M., Davies K.E. (2002) Prevention of pathology in mdx mice by expression of utrophin: analysis using an inducible transgenic expression system. Hum. Mol. Genet., 11, 3333–3344. [DOI] [PubMed] [Google Scholar]
- 40.Krag T.O.B., Bogdanovich S., Jensen C.J., Fischer M.D., Hansen-Schwartz J., Javazon E.H., Flake A.W., Edvinsson L., Khurana T.S. (2004) Heregulin ameliorates the dystrophic phenotype in mdx mice. Proc. Natl. Acad. Sci. U. S. A., 101, 13856–13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tinsley J., Robinson N., Davies K.E. (2015) Safety, tolerability, and pharmacokinetics of SMT C1100, a 2-arylbenzoxazole utrophin modulator, following single- and multiple-dose administration to healthy male adult volunteers. J. Clin. Pharmacol., 10.1002/jcph.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khurana T.S., Davies K.E. (2003) Pharmacological strategies for muscular dystrophy. Nat. Rev. Drug Discov., 2, 379–390. [DOI] [PubMed] [Google Scholar]
- 43.Moorwood C., Lozynska O., Suri N., Napper A.D., Diamond S.L., Khurana T.S. (2011) Drug discovery for duchenne muscular dystrophy via utrophin promoter activation screening. PLoS One, 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moorwood C., Soni N., Patel G., Wilton S.D., Khurana T.S. (2012) A cell-based high-throughput screening assay for posttranscriptional utrophin upregulation. J. Biomol. Screen., 18, 400–406. [DOI] [PubMed] [Google Scholar]
- 45.Gramolini A.O., Jasmin B.J. (1999) Expression of the utrophin gene during myogenic differentiation. Nucleic Acids Res., 27, 3603–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dennis C.L., Tinsley J.M., Deconinck A.E., Davies K.E. (1996) Molecular and functional analysis of the utrophin promoter. Nucleic Acids Res., 24, 1646–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gramolini A.O., Dennis C.L., Tinsley J.M., Robertson G.S., Cartaud J., Davies K.E., Jasmin B.J. (1997) Local transcriptional control of utrophin expression at the neuromuscular synapse. J. Biol. Chem., 272, 8117–8120. [DOI] [PubMed] [Google Scholar]
- 48.Chakkalakal J.V., Stocksley M.A., Harrison M.-A., Angus L.M., Deschenes-Furry J., St-Pierre S., Megeney L.A., Chin E.R., Michel R.N., Jasmin B.J. (2003) Expression of utrophin A mRNA correlates with the oxidative capacity of skeletal muscle fiber types and is regulated by calcineurin/NFAT signaling. Proc. Natl. Acad. Sci. U. S. A., 100, 7791–7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Angus L.M., Chakkalakal J.V., Méjat A., Eibl J.K., Bélanger G., Megeney L.A., Chin E.R., Schaeffer L., Michel R.N., Jasmin B.J. (2005) Calcineurin-NFAT signaling, together with GABP and peroxisome PGC-1alpha, drives utrophin gene expression at the neuromuscular junction. Am. J. Physiol. Cell Physiol., 289, C908–C917. [DOI] [PubMed] [Google Scholar]
- 50.Luquet S., Lopez-Soriano J., Holst D., Fredenrich A., Melki J., Rassoulzadegan M., Grimaldi P.A. (2003) Peroxisome proliferator-activated receptor controls muscle development and oxidative capability. FASEB J., 17, 2299–2301. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y.X., Zhang C.L., Yu R.T., Cho H.K., Nelson M.C., Bayuga-Ocampo C.R., Ham J., Kang H., Evans R.M. (2004) Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol., 2, 1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ehrenborg E.W.A., Krook A. (2009) Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor. Pharmacol. Rev., 61, 373–393. [DOI] [PubMed] [Google Scholar]
- 53.Ljubicic V., Miura P., Burt M., Boudreault L., Khogali S., Lunde J.A., Renaud J.M., Jasmin B.J. (2011) Chronic AMPK activation evokes the slow, oxidative myogenic program and triggers beneficial adaptations in mdx mouse skeletal muscle. Hum. Mol. Genet., 20, 3478–3493. [DOI] [PubMed] [Google Scholar]
- 54.Lin J., Wu H., Tarr P.T., Zhang C.Y., Wu Z., Boss O., Michael L.F., Puigserver P., Isotani E., Olson E.N. et al. (2002) Transcriptional co-activator PGC-1alpha drives the formation of slow-twitch muscle fibres. Nature, 418, 797–801. [DOI] [PubMed] [Google Scholar]
- 55.Lira V.A., Benton C.R., Zhen Y., Bonen A. (2010) PGC-1α regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab., 299, E145–E161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chalkiadaki A., Igarashi M., Nasamu A.S., Knezevic J., Guarente L. (2014) Muscle-specific SIRT1 gain-of-function increases slow-twitch fibers and ameliorates pathophysiology in a mouse model of Duchenne muscular dystrophy. PLoS Genet., 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perkins K.J., Basu U., Budak M.T., Ketterer C., Baby S.M., Lozynska O., Lunde J.A., Jasmin B.J., Rubinstein N.A., Khurana T.S. (2007) Ets-2 Repressor factor silences extrasynaptic utrophin by N-Box-mediated repression in skeletal muscle. Mol. Biol. Cell, 18, 2991–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blanchet E., Annicotte J., Lagarrigue S., Aguilar V., Clapé C., Chavey C., Fritz V., Casas F., Apparailly F., Auwerx J. et al. (2011) E2F transcription factor-1 regulates oxidative metabolism. Nat. Cell Biol., 13, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Rewashdy H., Ljubicic V., Lin W., Renaud J.-M., Jasmin B.J. (2014) Utrophin A is essential in mediating the functional adaptations of mdx mouse muscle following chronic AMPK activation. Hum. Mol. Genet., 24, 1243–1255. [DOI] [PubMed] [Google Scholar]
- 60.Miura P., Chakkalakal J.V., Boudreault L., Bélanger G., Hébert R.L., Renaud J.M., Jasmin B.J. (2009) Pharmacological activation of PPARβ/δ stimulates utrophin A expression in skeletal muscle fibers and restores sarcolemmal integrity in mature mdx mice. Hum. Mol. Genet., 18, 4640–4649. [DOI] [PubMed] [Google Scholar]
- 61.Ljubicic V., Burt M., Lunde J.A., Jasmin B.J. (2014) Resveratrol induces expression of the slow, oxidative phenotype in mdx mouse muscle together with enhanced activity of the SIRT1-PGC-1α axis. Am. J. Physiol. Cell Physiol., 307, C66–C82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winder W.W., Holmes B.F., Rubink D.S., Jensen E.B., Chen M., Holloszy J.O. (2000) Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol., 88, 2219–2226. [DOI] [PubMed] [Google Scholar]
- 63.Jørgensen S.B., Treebak J.T., Viollet B., Schjerling P., Vaulont S., Wojtaszewski J.F.P., Richter E.A. (2007) Role of AMPKalpha2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am. J. Physiol. Endocrinol. Metab., 292, E331–E339. [DOI] [PubMed] [Google Scholar]
- 64.Boon H., Bosselaar M., Praet S.F.E., Blaak E.E., Saris W.H.M., Wagenmakers A.J.M., McGee S.L., Tack C.J., Smits P., Hargreaves M. et al. (2008) Intravenous AICAR administration reduces hepatic glucose output and inhibits whole body lipolysis in type 2 diabetic patients. Diabetologia, 51, 1893–1900. [DOI] [PubMed] [Google Scholar]
- 65.Riserus U., Sprecher D., Johnson T., Olson E., Hirschberg S., Liu A., Fang Z., Hegde P., Richards D., Sarov-Blat L. et al. (2008) Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes, 57, 332–339. [DOI] [PubMed] [Google Scholar]
- 66.Olson E.J., Pearce G.L., Jones N.P., Sprecher D.L. (2012) Lipid effects of peroxisome proliferator-activated receptor-delta agonist GW501516 in subjects with low high-density lipoprotein cholesterol: Characteristics of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol., 32, 2289–2294. [DOI] [PubMed] [Google Scholar]
- 67.Ljubicic V., Jasmin B.J. (2015) Metformin increases peroxisome proliferator-activated receptor γ Co-activator-1α and utrophin A expression in dystrophic skeletal muscle. Muscle Nerve, 52, 139–142. [DOI] [PubMed] [Google Scholar]
- 68.Chakkalakal J.V., Miura P., Bélanger G., Michel R.N., Jasmin B.J. (2008) Modulation of utrophin A mRNA stability in fast versus slow muscles via an AU-rich element and calcineurin signaling. Nucleic Acids Res., 36, 826–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gramolini A.O., Bélangera G., Jasmin B.J. (2001) Distinct regions in the 3′ untranslated region are responsible for targeting and stabilizing utrophin transcripts in skeletal muscle cells. J. Cell Biol., 154, 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miura P., Andrews M., Holcik M., Jasmin B.J. (2008) IRES-mediated translation of utrophin A is enhanced by glucocorticoid treatment in skeletal muscle cells. PLoS One, 3, e2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miura P., Coriati A., Bélanger G., de Repentigny Y., Lee J., Kothary R., Holcik M., Jasmin B.J. (2010) The utrophin A 5′-UTR drives cap-independent translation exclusively in skeletal muscles of transgenic mice and interacts with eEF1A2. Hum. Mol. Genet., 19, 1211–1220. [DOI] [PubMed] [Google Scholar]
- 72.Moorwood C., Khurana T.S. (2013) Duchenne muscular dystrophy drug discovery—the application of utrophin promoter activation screening. Expert Opin. Drug Discov., 8, 569–581. [DOI] [PubMed] [Google Scholar]
- 73.Miura P., Thompson J., Chakkalakal J.V., Holcik M., Jasmin B.J. (2005) The utrophin A 5′-untranslated region confers internal ribosome entry site-mediated translational control during regeneration of skeletal muscle fibers. J. Biol. Chem., 280, 32997–33005. [DOI] [PubMed] [Google Scholar]
- 74.Bakheet T., Williams B.R.G., Khabar K.S.A. (2003) ARED 2.0: an update of AU-rich element mRNA database. Nucleic Acids Res., 31, 421–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Apponi L.H., Corbett A.H., Pavlath G.K. (2011) RNA-binding proteins and gene regulation in myogenesis. Trends Pharmacol. Sci., 32, 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basu U., Lozynska O., Moorwood C., Patel G., Wilton S.D., Khurana T.S. (2011) Translational regulation of utrophin by miRNAs. PLoS One, 6, e29376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amirouche A., Tadesse H., Lunde J.A., Bélanger G., Côté J., Jasmin B.J. (2013) Activation of p38 signaling increases utrophin A expression in skeletal muscle via the RNA-binding protein KSRP and inhibition of AU-rich element-mediated mRNA decay: implications for novel DMD therapeutics. Hum. Mol. Genet., 22, 3093–3111. [DOI] [PubMed] [Google Scholar]
- 78.Zbinden-Foncea H., Raymackers J.M., Deldicque L., Renard P., Francaux M. (2012) TLR2 and TLR4 activate p38 MAPK and JNK during endurance exercise in skeletal muscle. Med. Sci. Sports Exerc., 44, 1463–1472. [DOI] [PubMed] [Google Scholar]
- 79.Gray E., Mulloy B., Barrowcliffe T.W. (2008) Heparin and low-molecular-weight heparin. Thromb. Haemost., 99, 807–818. [DOI] [PubMed] [Google Scholar]
- 80.Tinsley J.M., Fairclough R.J., Storer R., Wilkes F.J., Potter A.C., Squire S.E., Powell D.S., Cozzoli A., Capogrosso R.F., Lambert A. et al. (2011) Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS One, 6, e19189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jahnke V.E., Van Der Meulen J.H., Johnston H.K., Ghimbovschi S., Partridge T., Hoffman E.P., Nagaraju K. (2012) Metabolic remodeling agents show beneficial effects in the dystrophin-deficient mdx mouse model. Skelet. Muscle, 2, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gramolini A.O., Bélanger G., Thompson J.M., Chakkalakal J.V., Jasmin B.J. (2001) Increased expression of utrophin in a slow versus a fast muscle involves posttranscriptional events. Am. J. Physiol. Cell Physiol., 281, C1300–C1309. [DOI] [PubMed] [Google Scholar]
- 83.Briguet A., Courdier-Fruh I., Foster M., Meier T., Magyar J.P. (2004) Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul. Disord., 14, 675–682. [DOI] [PubMed] [Google Scholar]
- 84.Torres L.F., Duchen L.W. (1987) The mutant mdx: inherited myopathy in the mouse. Morphological studies of nerves, muscles and end-plates. Brain, 110, 269–299. [DOI] [PubMed] [Google Scholar]
- 85.Machida K., Cheng K.T.H., Sung V.M.H., Levine A.M., Foung S., Lai M.M.C. (2006) Hepatitis C virus induces Toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J. Virol., 80, 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Serrano A.L., Baeza-Raja B., Perdiguero E., Jardi M., Munoz-Canoves P. (2008) Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab., 7, 33–44. [DOI] [PubMed] [Google Scholar]
- 87.Pillon N.J., Bilan P.J., Fink L.N., Klip A. (2013) Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab, 304, E453–E465. [DOI] [PubMed] [Google Scholar]
- 88.Gordon B.S., Delgado D.C., Kostek M.C. (2013) Resveratrol decreases inflammation and increases utrophin gene expression in the mdx mouse model of Duchenne muscular dystrophy. Clin. Nutr., 32, 104–111. [DOI] [PubMed] [Google Scholar]
- 89.Straub V., Rafael J.A., Chamberlain J.S., Campbell K.P. (1997) Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol., 139, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petrof B.J., Shrager J.B., Stedman H.H., Kelly A.M., Sweeney H.L. (1993) Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. U. S. A., 90, 3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bogdanovich S., Krag T.O.B., Barton E.R., Morris L.D., Whittemore L.A., Ahima R.S., Khurana T.S. (2002) Functional improvement of dystrophic muscle by myostatin blockade. Nature, 420, 418–421. [DOI] [PubMed] [Google Scholar]
- 92.Merrill G.F., Kurth E.J., Hardie D.G., Winder W.W. (1997) AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol, 273, E1107–E1112. [DOI] [PubMed] [Google Scholar]
- 93.Holmes B.F., Kurth-Kraczek E.J., Winder W.W. (1999) Chronic activation of 5 ′ -AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J. Appl. Physiol., 87, 1990–1995. [DOI] [PubMed] [Google Scholar]
- 94.Irrcher I., Ljubicic V., Kirwan A.F., Hood D.A. (2008) AMP-activated protein kinase-regulated activation of the PGC-1α promoter in skeletal muscle cells. PLoS One, 3, e3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buhl E.S., Jessen N., Pold R., Ledet T., Flyvbjerg A., Pedersen S.B., Pedersen O., Schmitz O., Lund S. (2002) Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes, 51, 2199–2206. [DOI] [PubMed] [Google Scholar]
- 96.Bamford J.A., Lopaschuk G.D., MacLean I.M., Reinhart M.L., Dixon W.T., Putman C.T. (2003) Effects of chronic AICAR administration on the metabolic and contractile phenotypes of rat slow- and fast-twitch skeletal muscles. Can. J. Physiol. Pharmacol., 81, 1072–1082. [DOI] [PubMed] [Google Scholar]
- 97.Drake J.C., Alway S.E., Hollander J.M., Williamson D.L. (2010) AICAR treatment for 14 days normalizes obesity-induced dysregulation of TORC1 signaling and translational capacity in fasted skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol., 299, R1546–R1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fillmore N., Jacobs D.L., Mills D.B., Winder W.W., Hancock C.R. (2010) Chronic AMP-activated protein kinase activation and a high-fat diet have an additive effect on mitochondria in rat skeletal muscle. J. Appl. Physiol., 109, 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leick L., Fentz J., Bienso R.S., Knudsen J.G., Jeppesen J., Kiens B., Wojtaszewski J.F.P., Pilegaard H. (2010) PGC-1 is required for AICAR-induced expression of GLUT4 and mitochondrial proteins in mouse skeletal muscle. AJP Endocrinol. Metab., 299, E456–E465. [DOI] [PubMed] [Google Scholar]
- 100.Liao P., Georgakopoulos D., Kovacs A., Zheng M., Lerner D., Pu H., Saffitz J., Chien K., Xiao R.P., Kass D.A. et al. (2001) The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. PNAS, 98, 12283–12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang Q., Molkentin J.D. (2003) Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: Dichotomy between cultured myocytes and animal models. J. Mol. Cell. Cardiol., 35, 1385–1394. [DOI] [PubMed] [Google Scholar]
- 102.Clerk A., Sugden P.H. (2006) Inflame my heart (by p38-MAPK). Circ. Res., 99, 455–458. [DOI] [PubMed] [Google Scholar]
- 103.Streicher J.M., Ren S., Herschman H., Wang Y. (2010) MAPK-activated protein kinase-2 in cardiac hypertrophy and cyclooxygenase-2 regulation in heart. Circ. Res., 106, 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Irrcher I., Adhihetty P.J., Sheehan T., Joseph A.M., Hood D.A. (2003) PPARgamma coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am. J. Physiol. Cell Physiol., 284, C1669–C1677. [DOI] [PubMed] [Google Scholar]
- 105.Suwa M., Nakano H., Kumagai S. (2003) Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J. Appl. Physiol., 95, 960–968. [DOI] [PubMed] [Google Scholar]
- 106.Pasquini F., Guerin C., Blake D., Davies K., Karpati G., Holland P. (1995) The effect of glucocorticoids on the accumulation of utrophin by cultured normal and dystrophic human skeletal muscle satellite cells. Neuromuscul. Disord., 5, 105–114. [DOI] [PubMed] [Google Scholar]
- 107.Weir A.P., Burton E.A., Harrod G., Davies K.E. (2002) A- and B-utrophin have different expression patterns and are differentially up-regulated in mdx muscle. J. Biol. Chem., 277, 45285–45290. [DOI] [PubMed] [Google Scholar]
- 108.Briata P., Forcales S.V., Ponassi M., Corte G., Chen C.Y., Karin M., Puri P.L., Gherzi R. (2005) p38-Dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol. Cell, 20, 891–903. [DOI] [PubMed] [Google Scholar]
- 109.Senn J.J. (2006) Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J. Biol. Chem., 281, 26865–26875. [DOI] [PubMed] [Google Scholar]
- 110.Reyna S.M., Ghosh S., Tantiwong P., Meka R.C.S., Eagan P., Jenkinson C.P., Cersosimo E., Defronzo R.A., Coletta D.K., Sriwijitkamol A. et al. (2008) Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes, 57, 2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rawat R., Cohen T.V., Ampong B., Francia D., Henriques-Pons A., Hoffman E.P., Nagaraju K. (2010) Inflammasome up-regulation and activation in dysferlin-deficient skeletal muscle. Am. J. Pathol., 176, 2891–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Henriques-Pons A., Yu Q., Rayavarapu S., Cohen T.V., Ampong B., Cha H.J., Jahnke V., Van der meulen J., Wang D., Jiang W. et al. (2014) Role of toll-like receptors in the pathogenesis of dystrophin-deficient skeletal and heart muscle. Hum. Mol. Genet., 23, 2604–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giordano C., Mojumdar K., Liang F., Lemaire C., Li T., Richardson J., Divangahi M., Qureshi S., Petrof B.J. (2014) Toll-like receptor 4 ablation in mdx mice reveals innate immunity as a therapeutic target in Duchenne muscular dystrophy. Hum. Mol. Genet., 24, 2147–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reyes N.L., Banks G.B., Tsang M., Margineantu D., Gu H., Djukovic D., Chan J., Torres M., Liggitt H.D., Hirenallur-S D.K. et al. (2015) Fnip1 regulates skeletal muscle fiber type specification, fatigue resistance, and susceptibility to muscular dystrophy. Proc. Natl. Acad. Sci., 112, 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marusich M.F., Robinson B.H., Taanman J.W., Kim S.J., Schillace R., Smith J.L., Capaldi R.A. (1997) Expression of mtDNA and nDNA encoded respiratory chain proteins in chemically and genetically-derived Rho0 human fibroblasts: a comparison of subunit proteins in normal fibroblasts treated with ethidium bromide and fibroblasts from a patient with mtDNA depletion syndrome. Biochim. Biophys. Acta - Mol. Basis Dis., 1362, 145–159. [DOI] [PubMed] [Google Scholar]
- 116.Wang L., Liu Z., Dong Z., Pan J., Ma X. (2014) Azurocidin-induced inhibition of oxygen metabolism in mitochondria is antagonized by heparin. Exp. Ther. Med., 8, 1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Corton J.M., Gillespie J.G., Hawley S.A., Hardie D.G. (1995) 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem., 229, 558–565. [DOI] [PubMed] [Google Scholar]
- 118.Tinsley J., Deconinck N., Fisher R., Kahn D., Phelps S., Gillis J.M., Davies K. (1998) Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat. Med., 4, 1441–1444. [DOI] [PubMed] [Google Scholar]
- 119.Ashburn T.T., Thor K.B. (2004) Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov., 3, 673–683. [DOI] [PubMed] [Google Scholar]
- 120.Babraj J.A., Mustard K., Sutherland C., Towler M.C., Chen S., Smith K., Green K., Leese G., Hardie D.G., Rennie M.J. et al. (2009) Blunting of AICAR-induced human skeletal muscle glucose uptake in type 2 diabetes is dependent on age rather than diabetic status. Am. J. Physiol. Endocrinol. Metab., 296, E1042–E1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suwa M., Egashira T., Nakano H., Sasaki H., Kumagai S. (2006) Metformin increases the PGC-1alpha protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J. Appl. Physiol., 101, 1685–1692. [DOI] [PubMed] [Google Scholar]