Abstract
Polycyclic aromatic hydrocarbons, like benzo[a]pyrene (BaP), are ubiquitous environmental pollutants and potent ovarian toxicants. The transcription factor NRF2 is an important regulator of the cellular response to electrophilic toxicants like BaP and to oxidative stress. NRF2 regulates transcription of genes involved in the detoxification of reactive metabolites of BaP and reactive oxygen species. We therefore hypothesized that Nrf2−/− mice have accelerated ovarian aging and increased sensitivity to the ovarian toxicity of BaP. A single injection of BaP dose-dependently depleted ovarian follicles in Nrf2+/+ and Nrf2−/− mice, but the effects of BaP were not enhanced in the absence of Nrf2. Similarly, Nrf2−/− mice did not have increased ovarian BaP DNA adduct formation compared to Nrf2+/+ mice. Ovarian follicle numbers did not differ between peripubertal Nrf2−/− and Nrf2+/+ mice, but by middle age, Nrf2−/− mice had significantly fewer primordial follicles than Nrf2+/+ mice, consistent with accelerated ovarian aging.
Keywords: NRF2, ovarian aging, benzo[a]pyrene, oxidative stress, polycyclic aromatic hydrocarbon, DNA adduct
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants, which are formed during the incomplete combustion of organic materials. Tobacco smoke is rich in PAHs [1]. Inhalation exposure to PAHs also occurs in non-smokers, due to exposure to second hand tobacco smoke and to air pollution. PAHs in ambient urban air are more than 10-fold higher than in rural air [2, 3]. Grilling or smoking of food also generates PAHs, and frequent consumption of grilled or smoked foods is another major source of PAH exposure [2, 3].
Women who smoke have decreased per menstrual cycle probability of pregnancy compared to women who do not smoke [4–6]. The onset of menopause occurs several years earlier in women who smoke [7, 8]. The adverse ovarian effects of smoking may be caused by PAHs present in tobacco smoke. The PAHs dimethylbenzanthracene (DMBA), 3-methylcholanthrene (3MC), and benzo[a]pyrene (BaP) all destroy follicles in mice after single high doses [9–12] and after repeated low doses [13]. DMBA also destroyed ovarian follicles in human ovarian explants, which were implanted subcutaneously in mice [14]. Prenatal and postnatal exposure to PAHs also causes ovarian tumors later in life [15, 16]. We recently showed that prenatal exposure to BaP causes ovarian tumors, which exhibit high levels of the epithelial marker cytokeratin by immunostaining [17]. The latter results suggest that PAH-induced ovarian tumors may arise from the ovarian surface epithelial (OSE) cells from which most malignant ovarian tumors in women are believed to arise [18, 19]. BaP has been shown to induce proliferation of mammary tumor cells in vitro [20–22], but effects of BaP on OSE cell proliferation in vivo have not been examined.
PAHs are oxidized by microsomal cytochrome P450 enzymes (chiefly P450s 1A1 and 1B1) to epoxides, which are converted to diols by microsomal epoxide hydrolase (Ephx1) [23, 24]. Diols can undergo further oxidation by P450 enzymes to mutagenic and ovotoxic diol epoxides [23, 25]. Alternatively, they can be metabolized by diol dehydrogenases, ultimately yielding catechols, which can undergo two sequential one-electron oxidations to o-semiquinone and then to o-quinone, generating hydrogen peroxide and superoxide anion radical [24]. The o-quinone can be reduced back to the semiquinone or catechol, resulting in redox cycles. All of the enzymes required to activate PAHs are present in the ovary [26–32]. Glutathione-S-transferase (GST)-catalyzed conjugation with glutathione (GSH) is an important Phase II detoxification mechanism for the diol epoxide, diol, and quinone metabolites of PAHs [33], and GSH also reduces and detoxifies reactive oxygen species (ROS) via direct reactions or as a cofactor for glutathione peroxidases.
The Cap ‘n Collar basic leucine zipper transcription factor Nuclear Factor 2 Erythroid Related Factor 2 (NRF2) regulates transcription of GSTs, the enzymes of GSH synthesis, superoxide dismutase, NADPH quinone oxidoreductase, and UDP-glucuronosyltransferase, which are important in detoxifying PAH metabolites and ROS, and of Ephx1, which is required to generate ovotoxic metabolites of PAHs [34–36]. Nrf2 null mice are more susceptible than wild type mice to chemical toxicity, including cancer induction by the PAH BaP [37], ovarian toxicity by vinylcyclohexene diepoxide [38], and liver toxicity by acetaminophen [39]. They are also more susceptible to autoimmune diseases [40, 41] than wild type mice. Nrf2−/− mice also exhibit increased oxidative stress compared to Nrf2+/+ mice [42]. We previously reported that young male Nrf2−/− mice had normal spermatogenesis, but developed age-related defects in spermatogenesis compared to wild type littermates, which was associated with increased testicular oxidative lipid damage [43]. We and others have shown that oxidative stress is associated with normal ovarian aging [44–46] and that deletion of the antioxidant gene Gclm causes accelerated ovarian aging [47].
Herein we report on the results of experiments testing the hypotheses that 1) Nrf2−/− mice are more sensitive to ovarian DNA damage, apoptosis, and follicle destruction by BaP than Nrf2+/+ mice due to their decreased ability to detoxify reactive metabolites of BaP; 2) BaP stimulates OSE cell proliferation and this occurs to a greater extent in Nrf2−/− ovaries than in Nrf2+/+ ovaries; 3) Nrf2 deletion accelerates the age-related decline in ovarian follicle numbers.
METHODS
Materials
All chemicals and reagents were purchased from Sigma Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA), unless otherwise noted.
Animals
Nrf2 null mice were generated by disrupting the Nrf2 gene by homologous recombination in embryonic stem cells, using a targeting vector that results in deletion of part of exon 4 and all of exon 5, replacing them with a LacZ reporter gene [48]. Mice for these experiments were generated in our breeding colony by mating Nrf2+/− males with Nrf2+/− females. Nrf2+/− breeder mice had been backcrossed 8 times onto a C57BL/6Crl genetic background. Genotyping of tail snip DNA by PCR was carried out as described [48]. The mice were housed in an American Association for the Accreditation of Laboratory Animal Care-accredited facility, with free access to deionized water and laboratory chow (Prolab RMH 2500), on a 14:10h light-dark cycle. Temperature was maintained at 21–24°C. Experimental females were group-housed with their female littermates after weaning (up to 5 mice per cage). The experimental protocols were carried out in accordance with the Guide for the Care and Use of Laboratory Animals [49] and were approved by the Institutional Animal Care and Use Committee at the University of California Irvine.
Experimental protocol - ovarian effects of BaP treatment in Nrf2+/+ and Nrf2−/− mice
To investigate the effects of lack Nrf2 on the ovarian sensitivity to BaP, 28-day old Nrf2−/− and Nrf2+/+ female mice were injected intraperitoneally with 0, 2, or 50 mg/kg BaP dissolved in sesame oil (N=5 to 9/group). These doses were chosen based on a prior study, which showed that a single dose of 50 mg/kg depleted primordial follicles by 56%, while a 5 mg/kg dose depleted primordial follicles by 18% [12]. Seven days later at 35 days of age, mice were euthanized using carbon dioxide inhalation. One ovary per animal was randomly chosen for fixation in Bouin’s fixative for 24 h, followed by 4 washes in 50% ethanol, and storage in 70% ethanol. The other ovary was fixed in 4% paraformaldehyde (PFA) in PBS for 1 hour, then cryoprotected in 15% sucrose in PBS for 4 hours prior to being embedded in Tissue-Tek OCT (Sakura Finetek, Torrance CA). The embedded ovaries were serially sectioned at 10 microns for immunohistochemistry and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). Another set of mice was treated identically with 0 or 50 mg/kg BaP, and both ovaries were snap frozen on dry ice for subsequent DNA extraction for 32P-postlabeling to detect BaP-related DNA adducts.
GSH assays
For GSH assays, ovaries from 6 Nrf2+/+ and 7 Nrf2−/− 18–22 day old mice were homogenized in 20 mM Tris, 1 mM EDTA, 250 mM sucrose, 2 mM L-serine, 20 mM boric acid (TES-SB). After removal of aliquots for protein assay, supernatants were acidified with one quarter volume 5% sulfosalicylic acid for GSH assays [50]. Total GSH was measured using a modification of an enzymatic recycling assay developed by Griffith [50–52]. Triplicates of samples or standards were combined with 33 μl metal free water and incubated for 10 min at 30°C. The samples were mixed with 140 μl of 0.3 mM NADPH, 20 μl of 6 mM DTNB (5,5′-dithiobis(2-nitrobenzoic acid), and 2 μl of 50 units/mL GSH reductase. The rate of thiobis(2-nitrobenzoic acid) (TNB) formation from DTNB is proportional to the total GSH concentration in each sample. TNB formation was monitored by measuring the absorbance at 412 nm for 5 min every 10 sec using a VersaMax microplate spectrophotometer (Molecular Devices, Sunnyvale, CA). The concentrations of total GSH in the samples were calculated from a standard curve generated from the slopes of the standards.
Immunoblotting for GCLC, GCLM, SOD2 in ovarian extracts
Ovaries from 34–36 day old Gclm−/− and Gclm+/+ littermates were homogenized in RIPA buffer (PBS, 0.5% sodium deoxycholate, 0.1% SDS, 1% NP40) with protease inhibitors (Pefablock, TLCK, pepstatin, aprotonin, leupeptin) using a Kontes handheld dounce homogenizer (Kimble-Chase, Vineland, NJ) on ice. Protein concentration was determined by Pierce BCA (bicinchoninic acid) Assay. Twenty to forty micrograms of each protein sample were separated by SDS-PAGE, transferred to polyvinylidine difluoride membranes, blocked in 5% nonfat milk in phosphate buffered saline with 0.1% Tween-20, incubated with primary antibodies diluted in 3% nonfat dry milk, 3% BSA, 1% ovalbumin, 1% normal goat serum, 0.1% sodium azide, and then visualized with ECL chemiluminescence (GE Healthcare Lifesciences, Piscataway, New Jersey). GCL subunit antibodies were raised against ovalbumin conjugates and were a kind gift of Dr. Terrance J. Kavanagh, University of Washington [53]. GCLC subunit antibodies were used at a dilution of 1:40,000 and GCLM at 1:20,000. Antibody directed against manganese containing superoxide dismutase (SOD2) was purchased from Stressgen (#SOD-110, Ann Arbor, MI) and used at a 1:5000 dilution. All immunoblots were re-probed with monoclonal anti-β-actin mouse antibody (Sigma, #A5441) at a 1:160K dilution for normalization of protein loading.
Quantitative real time RT-PCR
For quantitative real time RT PCR, total RNA was extracted from ovaries of 27 day old mice using the Qiagen RNEasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The quality and quantity of the total RNA were assessed by spectrometry. Purified total RNA (900 ng) was reverse-transcribed to cDNA with Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) using an oligo dT12–18 primer (Invitrogen) after digestion with DNAse I (Roche, Indianapolis, IN). Twenty nanograms of cDNA were subjected to PCR using gene-specific forward and reverse primers and the Roche SYBR Green RT-PCR reagent (Roche) in 20 μl reaction volumes in duplicate. Gene-specific primers used are listed in Supplemental Table S1. The PCR amplification of all transcripts was performed on the AB StepOne Plus PCR machine (Applied Biosystems, Foster City, CA) using the following program: 1) initial incubation at 95°C for 10 min to activate FastStart Taq DNA polymerase; 2) each cycle (total 40 cycles) at 95°C for 10 sec, followed by incubation at an average annealing temperature of forward and reverse primers for 30 sec according to the primers used, and final elongation at 72°C for 10 sec. The quality and identity of each PCR product was determined by melting curve analysis. Expression of each target gene was calculated by the delta-delta Ct method [54, 55]. All data were normalized to expression of glyceraldehyde-3-phosphate dehydrogenase (Gapdh).
Ovarian histomorphometry
In addition to ovaries from 35 day old Nrf2+/+ and Nrf2−/− mice treated with BaP or vehicle, ovaries were harvested on the day of estrus from 10 and 12 month old, untreated Nrf2+/+ and Nrf2−/− mice. After fixation with Bouin’s fluid, ovaries were embedded in paraffin and complete serial sections (6 μm thick) were cut. Sections were stained with hematoxylin and eosin. Histomorphometry was carried out by a skilled observer, blind to genotype and BaP dose. Follicles were classified as primordial (oocyte surrounded by single layer of fusiform granulosa cells), primary (oocyte surrounded by single layer of granulosa cells of predominantly cuboidal morphology), secondary (oocyte surrounded by more than one layer of granulosa cells), or antral (follicle with multiple fluid-filled vesicles or single antrum) according to established criteria [56–58]. Follicles were also classified as healthy or atretic according to established criteria [59, 60]. Healthy and atretic primordial, primary, and secondary follicles were counted in every 5th section. Healthy and atretic antral follicles were counted in every section, Only secondary and antral follicles in which the oocyte nucleolus was visible were counted to avoid overcounting. Multiovular follicles (follicles with more than one oocyte) of all stages were identified and counted.
32P postlabeling
DNA was extracted from ovaries and purified using a Qiagen DNeasy Tissue Kit (Qiagen, Valencia, CA). 32P-postlabeling for detection of BaP DNA adducts was performed according to previously described methods [61–63]. Five μg of the purified DNA was dried using a Speedvac evaporator. Digestion of DNA was accomplished by incubation in hydrolysis buffer, micrococcal nuclease, and spleen phosphodiesterase for 3.5 hours at 37°C. To the digested material was added ZnCl2, sodium acetate, and nuclease P1, which was used to separate adduct nucleotides from normal nucleotides. This incubation was performed for 1 hour at 37°C. Addition of Tris base was then performed, followed by preparation of a labeling mixture consisting of kinase buffer, polynucleotide kinase (United States Biomedical), [γ-32P]ATP (MP Biomedicals, Solon, OH), and bicine. Labeling mixture was added to each sample and incubated for 45 min at 37°C, followed by addition of apyrase for 30 min. at 37°C. Thin layer chromatography (TLC) was then performed using methanol-washed cellulose PEI TLC plates (Selecto Scientific, Georgia, USA) with various plate orientations and solvents: to the origin in 1 M NaH2PO4 overnight, in opposite direction in 3.6 M lithium formate/8.5 M urea, 90° to the previous direction in 0.8 M LiCl/0.5 M Tris-Cl/8.5 M urea, and in same direction in 1.7 M NaH2PO4. Phosphorimaging was then used to measure the intensity of radioactive labeling. To determine the ratio of DNA adducts to normal nucleotides, normal nucleotides were also imaged. This was done by incubating a portion of the digested DNA with labeling mixture for 45 min. at 37°C, followed by TLC using LiCl solvent. Phosphorimaging was used to visualize the total nucleotides and DNA adducts on each TLC plate. Phosphorimaging software was used to measure the density of the corresponding spots for each sample. The relative adduct labeling was then determined by dividing the density of the adducted nucleotides by that of the total nucleotides for the same plate.
Immunohistochemistry
Paraformaldehyde-fixed cryosections were immunostained with primary antibodies directed against PCNA (Santa Cruz Biotechnology, #SC-56 at a dilution of 1:1500) using the MOM Immunodetection Kit (Vector Laboratories, Burlingame, CA). Antigen retrieval was first performed by incubating slides in 10 mM citrate buffer for 10 min at 95°C, followed by blocking with MOM Mouse Ig Blocking Reagent, then Avidin/Biotin blocking, incubation with blocking serum, incubation with primary antibody, incubation with biotinylated anti-mouse Ig, blocking with 3% hydrogen peroxide in PBS, incubation with Vectastain ABC reagent, DAB conversion, and counterstaining. Negative controls included slides incubated with non-immune mouse IgG in place of primary antibody.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL)
Paraformaldehyde-fixed cryosections were used for in situ detection of DNA fragmentation by the TUNEL method using the In Situ Cell Death Detection Kit POD (Roche) as previously described [56, 64].
Quantification of TUNEL and immunostaining
All counting was conducted by an observer blind to genotype and treatment using light microscopy. Secondary and antral follicles were counted if the oocyte was present in a section for a total of 4 to 16 sections per animal. Follicles were considered TUNEL-positive if there were 3 or more TUNEL-positive granulosa cells per cross-section. The total number of TUNEL-positive and negative secondary and antral follicles was counted for each section. The average percentage of positive secondary and antral follicles was then calculated for each mouse and was used for statistical analyses.
For counting of PCNA or TUNEL positive OSE cells, a site lying above a growing follicle was chosen and considered an over-site. The total numbers of positive and negative OSE cells in the over-site were counted in one 400x microscope field. After an over-site was counted for a section, a site 180° away from the over-site was found that contained OSE between two growing follicles. This site was called the adjacent site. For the next section, the first site to be located would be an adjacent site, and following that would be an over-site 180° away. 8 to 20 sections were counted per experimental animal per experiment to determine the numbers of PCNA or TUNEL positive OSE cells. There were 3–4 experimental animals per group for TUNEL and PCNA-immunostaining. The average percentage of positive cells per animal was calculated, and these averages were used for statistical analyses.
Statistics
Continuous dependent variables with homogenous variances were analyzed by ANCOVA with independent variables genotype and covariate BaP dose or by t-test, as appropriate. When variances were not homogeneous non-parametric tests were used. Data expressed as fractions or percentages (fraction of atretic, TUNEL positive or PCNA positive follicles or OSE cells) were subjected to arcsine square root transformation prior to analysis [65].
RESULTS
Effect of Nrf2 deletion on ovarian GSH synthesis and antioxidant gene expression
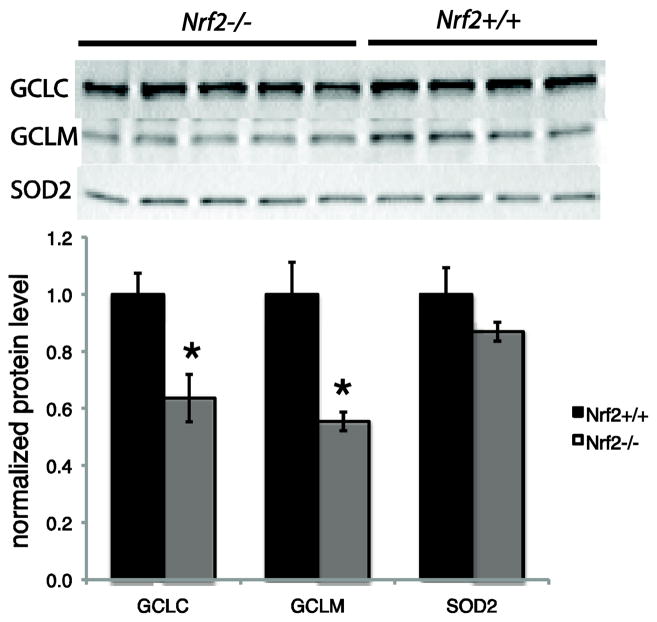
Total ovarian GSH concentrations in Nrf2−/− females were about 15% lower than in Nrf2+/+ females (2.7±0.15 nmol GSH/mg ovary compared to 3.2±0.13 nmol GSH/mg ovary, respectively; P<0.05 by t-test). A similar, although not statistically significant, trend was observed when GSH concentrations were expressed in terms of protein content (57 ± 4.7 nmol GSH/mg protein in Nrf2−/− versus 67 ± 6.9 nmol GSH/mg protein in Nrf2+/+, P=0.245 by t-test). Consistent with the lower GSH concentrations, Nrf2−/− ovaries also had lower protein levels of the catalytic and modifier subunits of GCL, the rate-limiting enzyme in GSH synthesis. Compared to Nrf2+/+ ovaries, Nrf2−/− ovaries had 36% lower protein levels of GCLC (P=0.017) and 45% lower protein levels of GCLM (P=0.024; Figure 1). In contrast, ovarian SOD2 (also called Mn-SOD) protein levels were not affected by lack of Nrf2 (Figure 1).
Figure 1. Protein levels of GCLC and GCLM, but not SOD2 are decreased in ovaries of Nrf2−/− mice compared to Nrf2+/+ mice.
Ovaries of Nrf2−/− and Nrf2+/+ mice were processed for immunoblotting as described in Materials and Methods. Images of blots are shown at top. The graph shows mean ± SEM densitometry of protein of interest normalized to β-actin loading control. N=4–5/group. *P<0.05 versus Nrf2+/+ by t-test.
Ovarian mRNA levels of genes involved in BaP metabolism were measured in Nrf2−/− and Nrf2+/+ mice (Table 1). Ovarian mRNA levels of Gstm1 (P=0.006 by t-test) and Ephx1 (P=0.07) were 67% and 91% lower, respectively, in Nrf2−/− than in Nrf2+/+ mice. When the Ephx1 outlier that fell outside of the 95% confidence interval for the Nrf2+/+ group was excluded, the effect of Nrf2 genotype was statistically significant (P=0.021). Ovarian mRNA levels of Gstm2 and Gsta4 were 36% and 54% lower, respectively, in the Nrf2−/− mice, but the differences were not statistically significant. The mRNA levels of Gclc, Gclm, and Gstp1 did not differ between Nrf2−/− and Nrf2+/+ ovaries.
Table 1.
Effects of Nrf2 deletion on ovarian expression of metabolic genes
| Nrf2+/+ | Nrf2−/− | |
|---|---|---|
| Gclc | 1.00±0.13 | 0.79±0.27 |
| Gclm | 1.00±0.18 | 0.90±0.25 |
| Gstm1a | 1.00±0.14 | 0.34±0.08 |
| Gstm2 | 1.00±0.16 | 0.64±0.29 |
| Gsta4 | 1.00±0.26 | 0.46±0.10 |
| Gstp1 | 1.00±0.22 | 1.04±0.14 |
| Ephx1b | 1.00±0.38 | 0.11±0.05 |
N= 4–5/group
P=0.006, t-test
P=0.07, t-test
Nrf2−/− mice are not more sensitive to the ovarian toxicity of BaP than Nrf2+/+ mice
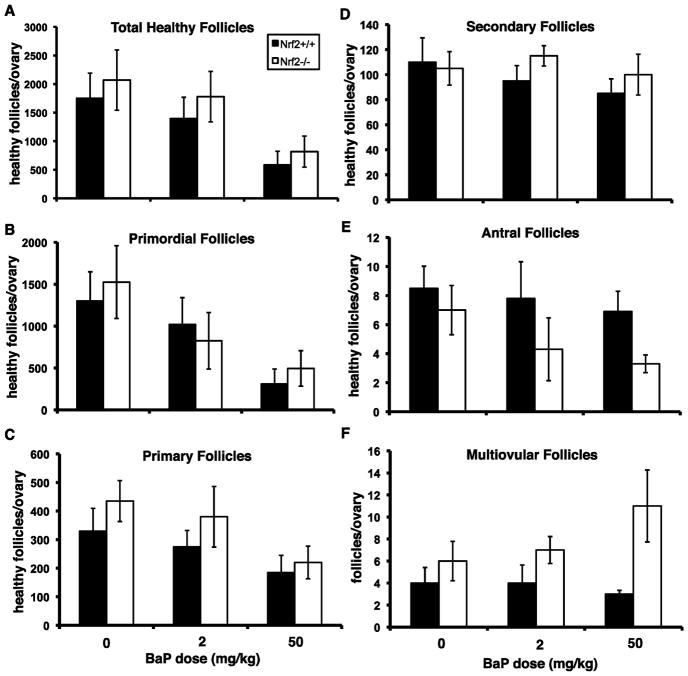
As expected, BaP treatment caused statistically significant dose-dependent declines in the total numbers of healthy follicles per ovary (Figure 2A) and the numbers of healthy primordial and primary follicles per ovary (Figure 2B,C). The numbers of healthy secondary (Figure 2D) and antral follicles (Figure 2E) also decreased with BaP dose, but the effects were not statistically significant. The effects of Nrf2 genotype on numbers of primordial, primary, secondary, or total healthy follicles were not statistically significant; however, Nrf2−/− mice had significantly greater numbers of multiovular follicles and fewer healthy antral follicles regardless of BaP dose (Figure 2E,F).
Figure 2. Effects of Nrf2 deletion and BaP treatment on numbers of healthy ovarian follicles.
Graphs show mean ± SEM follicle counts. Data were analyzed by ANCOVA with BaP dose as a continuous variable and Nrf2 genotype as a nominal variable. There was a BaP dose-dependent decrease in total healthy follicles (A) and healthy primordial follicles (B), p=0.001, effect of BaP dose. There was no effect of genotype. C) Healthy primary follicles also decreased with increasing BaP dose (p=0.020). There was no effect of genotype. D) Healthy secondary follicle numbers did not vary significantly with dose or genotype. E) Nrf2−/− mice had fewer healthy antral follicles than their wild type littermates (p=0.037). The effect of BaP treatment on antral follicle numbers was not statistically significant (p=0.23). F) Nrf2−/− ovaries had significantly greater numbers of multiovular follicles than wild type ovaries (p=0.045, effect of genotype). There was no effect of BaP dose on multiovular follicles.
No statistically significant Nrf2 genotype- or BaP treatment-related differences in the percentages of TUNEL-positive, apoptotic secondary or antral follicles were observed (data not shown).
Effects of Nrf2 genotype and BaP treatment on ovarian DNA adducts
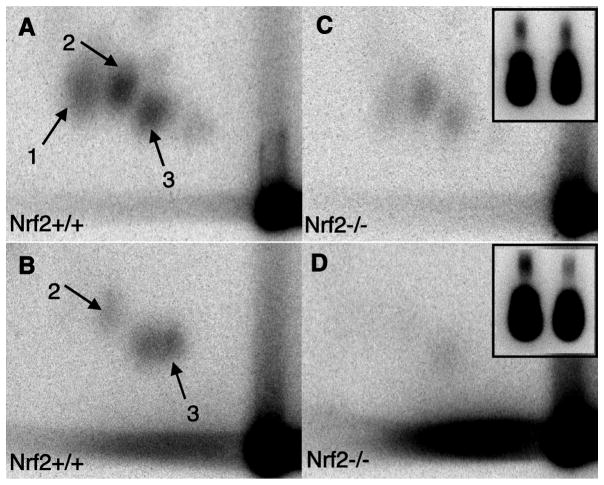
Nrf2+/+ and Nrf2−/− mice treated with 50 mg/kg BaP had two or three distinct ovarian DNA adducts as demonstrated by 32P-postlabeling (Figure 3), whereas no adducts were observed in DNA from ovaries of control, vehicle-treated mice (not shown). However, DNA adduct levels were not greater in ovaries from Nrf2−/− mice treated with BaP (Figure 3C,D) compared to Nrf2+/+, BaP-treated mice (Figure 3A,B). The relative levels of adducts in Nrf2−/− versus Nrf2+/+ ovaries were 0.8 for adduct 2 and 0.7 for adduct 3 (mean of two samples per genotype).
Figure 3. Representative images of 32P-postlabeling detection of DNA adducts in ovaries 7 days after i.p. injection with 50 mg/kg BP.
on postnatal day 28 of Nrf2+/+ (A,B) or Nrf2−/− (C,D) mice. Three adducts are clearly visible (1,2,3) in A and C, while only two adducts are visible in B and D. The insets show total nucleotides for each TLC plate, with the total nucleotides for the Nrf2+/+ ovary on the left and the Nrf2−/− ovary on the right. 32P-postlabeling and TLC of A and C were performed on the same day and B and D were performed on a different day.
Effects of Nrf2 genotype and BaP treatment on ovarian surface epithelial (OSE) cell proliferation and apoptosis
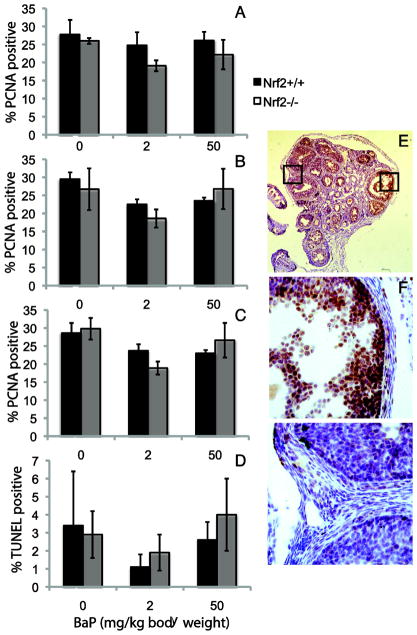
Immunostaining for PCNA was used to detect OSE cell proliferation. PCNA positive and negative OSE cells located over antral follicles or away from antral follicles were counted and the percentages of positive cells were calculated. The percentages of PCNA positive OSE cells were lower in ovaries of BaP-treated mice. The effect of BaP dose on the percentage of PCNA positive OSE cells overlying antral follicles was statistically significant (P=0.018; Figure 4A), but the effect of BaP dose was not statistically significant for OSE cells not overlying antral follicles (P=0.286; Figure 4B). The effect of BaP dose was also statistically significant for all OSE cells combined (P=0.047; Figure 4C). The effect of Nrf2 genotype was not statistically significant.
Figure 4. Effects of BaP treatment and Nrf2 deletion on ovarian surface epithelial cell proliferation and apoptosis.
A) Mean ± SEM percentage of PCNA positive OSE cells in site located over an antral follicle, as shown in F. Statistical analyses were performed by ANCOVA as for Figure 2. The effect of BaP dose was statistically significant, P=0.018; the effect of genotype was not. B) Mean ± SEM percentage of PCNA positive OSE cells in site not located over an antral follicle, as shown in G. There were no statistically significant effects of BaP or genotype. C) Mean ± SEM percentage of PCNA positive OSE cells overall. The effect of BaP dose was statistically significant, P=0.047; the effect of genotype was not. D) Mean ± SEM percentage of TUNEL positive OSE cells. E) View of an entire ovarian section after PCNA immunostaining. Original magnification, 40X. F and G show higher magnification (400X) views of the areas indicated by squares in E.
TUNEL was used to detect OSE cell apoptosis. TUNEL-positive and negative OSE cells located over antral follicles (data not shown), away from antral follicles (data not shown), or at all sites (Figure 4D) were counted and the percentages of positive cells were calculated. Neither the effects of BaP dose nor the effects of Nrf2 genotype were statistically significant.
Nrf2 deletion accelerates the age-related decline in ovarian follicles
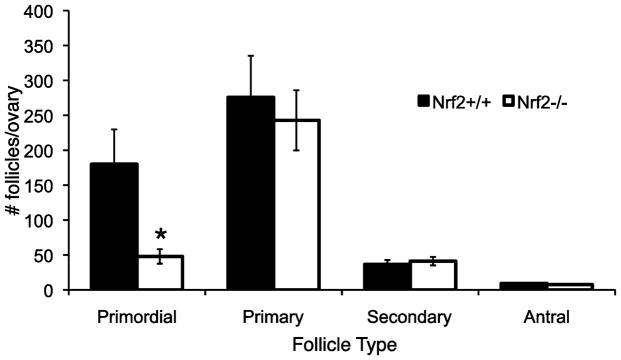
Follicle counts did not differ by Nrf2 genotype in 35 day old mice, as described above (Figure 2). However, we hypothesized that Nrf2−/− female mice, due to decreased ability to respond to oxidative stress, display a more rapid decline in follicle numbers with aging than Nrf2+/+ mice. We therefore counted follicles in 10 to 12 month old (middle-aged) Nrf2+/+ and Nrf2−/− mice. The results are shown in Figure 5. Middle-aged Nrf2−/− female mice had statistically significantly fewer healthy primordial follicles (P=0.011) than age-matched Nrf2+/+ females, indicating greater depletion of the ovarian reserve in Nrf2−/− mice.
Figure 5. Middle-aged Nrf2−/− mice have fewer ovarian follicles than Nrf2+/+ mice of the same age.
Healthy primordial, primary, secondary and antral follicles were counted as described in Methods in ovaries from 10–12 month old Nrf2−/− and Nrf2+/+ mice. The graph shows mean ± SEM follicles in each category per ovary. * P<0.02 versus Nrf2+/+ by Mann Whitney test. N=2 twelve month old and N=4 ten month old mice of each genotype.
DISCUSSION
NRF2 is a master regulator of the antioxidant response and serves as a key sensor detecting chemical-induced toxicity in various organs, including ovary [38, 39]. In the presence of oxidative stress, the affinity of KEAP-1 for cytosolic NRF2 decreases, which promotes relocation of cytosolic NRF2 to the nucleus, where it induces the expression of target antioxidant genes, including GSTs and GSH synthesis genes [34–36]. The lack of NRF2 disrupts antioxidant signaling, leading to a decreased ability to mount an antioxidant defense [34, 42] and decreased ability to metabolize xenobiotics, such as PAHs [37, 66, 67]. In the present study, Nrf2−/− female mice had similar numbers of ovarian follicles to Nrf2+/+ mice at 35 days of age, but Nrf2−/− mice had fewer remaining primordial follicles at 10–12 months of age compared to Nrf2+/+ littermates. This may indicate that sustained oxidative stress during aging accelerates depletion of primordial follicles in the middle-aged Nrf2−/− female mice. In contrast, our results show that Nrf2−/− mice do not have greater sensitivity to the ovarian toxicity induced by treatment with the PAH BaP.
GSTs are a major group of Phase II detoxification enzymes induced by NRF2 activation, and GST-catalyzed conjugation with GSH is an important Phase II biotransformation pathway for diol epoxide, diol, and quinone metabolites of PAHs [33]. GSH also detoxifies ROS produced as a result of BaP metabolism. We therefore hypothesized that Nrf2−/− mice, having decreased ovarian expression of several GSH-related genes would have increased ovarian sensitivity to BaP. We observed decreased mRNA levels of several GST genes, decreased protein expression of GCLC and GCLM, and decreased GSH concentrations in Nrf2−/− ovaries. However, in contrast to their reportedly greater sensitivity to the ovarian toxicant vinylcyclohexene diepoxide [38], Nrf2−/− mice did not have increased sensitivity to ovarian follicle destruction by BaP. In addition, ovarian BaP-related DNA adduct formation was not increased in the ovaries of Nrf2−/− mice compared to Nrf2+/+ littermates. This may reflect the greater importance of downregulation of Ephx1, which is required to generate the reactive diol epoxide metabolite of BaP [24], than downregulation of GSTs and GSH, which detoxify BaP metabolites [33]. Indeed, ovarian mRNA levels of Ephx1 in Nrf2−/− mice were less than 15% of those in Nrf2+/+ mice, and Ephx1 null mice are resistant to PAH-induced carcinogenesis [68]. The absence of BaP treatment-related increases in the percentages of apoptotic follicles or ovarian surface epithelial cells in mice of either Nrf2 genotype in the present study are consistent with apoptotic cells having already been cleared by 7 days after BaP treatment.
BaP or its metabolites have been reported to stimulate proliferation of cultured breast epithelial cells and breast cancer cells via estrogen receptor dependent mechanisms [21, 22, 69, 70] BaP treatment was shown to induce anchorage independent growth of normal human OSE cells that overexpressed CYP1A1 [71]. In contrast to these in vitro findings, we did not observe increased OSE proliferation after in vivo treatment with BaP measured by PCNA immunostaining 7 days after BaP injection. Rather, we observed decreased percentages of PCNA positive OSE cells after BaP treatment.
We and others have shown that ovarian aging is associated with decreased ovarian antioxidant defense capacity and increased oxidative damage [44–46, 72–74]. We have also shown that Nrf2−/− male mice have normal spermatogenesis as young adults, but subsequently demonstrate an age-related decline in spermatogenesis, which is associated with testicular oxidative damage [43]. NRF2 is activated by ROS, and the activation of NRF2-dependent antioxidant signaling is an adaptive response to oxidative stress [67, 75]. Thus NRF2 signaling may have important implications in countering aging in the ovary. In the present study, we observed downregulation of key antioxidants in Nrf2−/− ovaries. We found that Nrf2−/− mice had similar numbers of ovarian follicles at 35 days of age, but fewer follicles at 10–12 months of age compared to Nrf2+/+ littermates. This is consistent with an accelerated age-related decline in ovarian follicle numbers in the Nrf2−/− mice. The accelerated decline in the total number of healthy follicles in Nrf2−/− mice was driven by a more rapid decline in the numbers of quiescent primordial follicles, which constitute the ovarian reserve. We recently reported an accelerated age-related decline in ovarian follicle numbers in another mouse model genetically deficient in a key antioxidant gene, glutamate cysteine ligase modifier subunit (Gclm), a subunit of the rate limiting enzyme in GSH synthesis [47]. Gclm−/− mice have greatly decreased ovarian GSH concentrations and ratio of reduced to oxidized glutathione and increased ovarian oxidative protein and lipid damage, consistent with ovarian oxidative stress. Our current findings raise the possibility that NRF2 signaling is required for resistance to oxidative stress during aging in the ovary. Together our results from these two mouse models provide strong support for the hypothesis that increased ovarian oxidative stress can cause premature ovarian senescence.
We observed significantly more multiovular follicles in ovaries of Nrf2−/− mice than Nrf2+/+ mice at 35 days of age. Multiovular follicles are thought to arise due to incomplete oocyte cyst breakdown during primordial follicle formation [76]. Previous studies have shown that neonatal treatment with estradiol or with the soy isoflavone and phytoestrogen genistein increases the numbers of multiovular follicles [77]. Fetal exposure to gamma-radiation has been reported to increase multiovular follicle formation in rats [78]. Ionizing radiation is well known to cause oxidative stress [79, 80], suggesting that oxidative stress may disrupt oocyte cyst breakdown leading to multiovular follicle formation.
In conclusion, our results show that Nrf2−/− and Nrf2+/+ mice have similar numbers of ovarian follicles at 35 days of age. However, by 10–12 months of age, the remaining primordial follicle pool is significantly smaller in Nrf2−/− compared to Nrf2+/+ mice, consistent with accelerated ovarian aging in Nrf2−/− mice. We also showed the ovarian concentrations of GSH and protein levels of the subunits of the rate-limiting enzyme in GSH synthesis are decreased in Nrf2−/− mice. Messenger RNA levels of several GSTs and of Ephx1, which are involved in BaP metabolism, are also decreased in Nrf2−/− ovaries. Unlike their reportedly greater sensitivity to the ovarian toxicity of vinylcyclohexene diepoxide, Nrf2−/− mice do not have increased sensitivity to the ovarian toxicity of BaP. This may be due to lower ovarian expression of Ephx1 in Nrf2−/− mice, which is required to generate the toxic diol epoxide metabolite of BaP, but is involved in detoxification of vinylcyclohexene diepoxide.
Supplementary Material
Highlights.
The effects of deletion of Nrf2 on ovarian follicle dynamics were investigated.
Nrf2 deletion alone caused accelerated age-related depletion of ovarian follicles.
Nrf2 deletion did not modify follicle depletion by exposure to benzo[a]pyrene.
Acknowledgments
The authors thank Kira Pate for optimizing the 32P-postlabeling, Lee Sutherland and Jeffrey Kim for preparation of histological specimens.
Supported by NIH K08ES10963, NIH R21AG032087 and NIH R01ES020454 (to UL); NIH P30CA062203, the University of California Irvine (UC Irvine) Chao Family Comprehensive Cancer Center; University of California CRCC Research Grant (to UL); and the Center for Occupational and Environmental Health, UC Irvine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shopland DR, Burns DM, Benowitz NL, Amacher RH. Smoking and Tobacco Control Monographs. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 2001. Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine; pp. 1–236. [Google Scholar]
- 2.ATSDR. Toxicological Profile for Polycyclic Aromatic Hydrocarbons. Atlanta, GA: US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 1995. [PubMed] [Google Scholar]
- 3.Menzie CA, Potocki BB, Santodonato J. Ambient Concentrations and Exposure to Carcinogenic PAHs in the Environment. Environ Sci Technol. 1992;26:1278–84. [Google Scholar]
- 4.Baird DD, Wilcox AJ. Cigarette Smoking Associated with Delayed Conception. JAMA. 1985;253:2979–83. [PubMed] [Google Scholar]
- 5.Alderete E, Eskenazi B, Sholtz R. Effect of Cigarette Smoking and Coffee Drinking on Time to Conception. Epidemiology. 1995;6:403–8. doi: 10.1097/00001648-199507000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Jensen TK, Henriksen TB, Hjollund NH, Scheike T, Kolstad H, Giwercman A, et al. Adult and Prenatal Exposures to Tobacco Smoke as Risk Indicators of Fertility among 430 Danish Couples. Am J Epidemiol. 1998;148:992–7. doi: 10.1093/oxfordjournals.aje.a009576. [DOI] [PubMed] [Google Scholar]
- 7.Mattison DR, Plowchalk DR, Meadows MJ, Miller MM, Malek A, London S. The Effect of Smoking on Oogenesis, Fertilization, and Implantation. Semin Reprod Endocrinol. 1989;7:291–304. [Google Scholar]
- 8.Harlow BL, Signorello LB. Factors Associated with Early Menopause. Maturitas. 2000;35:3–9. doi: 10.1016/s0378-5122(00)00092-x. [DOI] [PubMed] [Google Scholar]
- 9.Mattison DR. Difference in Sensitivity of Rat and Mouse Primordial Oocytes to Destruction by Polycyclic Aromatic Hydrocarbons. Chem Biol Interact. 1979;28:133–7. doi: 10.1016/0009-2797(79)90120-0. [DOI] [PubMed] [Google Scholar]
- 10.Mattison DR, Nightingale MS. Oocyte Destruction by Polycyclic Aromatic Hydrocarbons is not Linked to the Inducibility of Ovarian Aryl Hydrocarbon (Benzo(a)pyrene) Hydroxylase Activity in (DBA/2N x C57BL/6N) F1 x DBA/2N Backcross Mice. Pediatr Pharmacol. 1982;2:11–21. [PubMed] [Google Scholar]
- 11.Mattison DR, Thorgeirsson SS. Ovarian Aryl Hydrocarbon Hydroxylase Activity and Primordial Oocyte Toxicity of Polycyclic Aromatic Hydrocarbons in Mice. Cancer Res. 1979;39:3471–5. [PubMed] [Google Scholar]
- 12.Mattison DR, White NB, Nightingale MR. The Effect of Benzo(a)pyrene on Fertility, Primordial Oocyte Number, and Ovarian Response to Pregnant Mare’s Serum Gonadotropin. Pediatr Pharmacol. 1980;1:143–51. [PubMed] [Google Scholar]
- 13.Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in Female Fischer Rats and B6 Mice Induced by Low-Dose Exposure to Three Polycyclic Aromatic Hydrocarbons: Comparison through Calculation of an Ovotoxic Index. Toxicol Appl Pharmacol. 2000;167:191–8. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- 14.Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu H-Y, et al. Aromatic Hydrocarbon Receptor-Driven Bax Gene Expression is Required for Premature Ovarian Failure Caused by Biohazardous Environmental Chemicals. Nat Genet. 2001;28:355–60. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- 15.Taguchi O, Michael SD, Nishizuka Y. Rapid Induction of Ovarian Granulosa Cell Tumors by 7,2-Dimethylbenz(a)anthracene in Neonatally Estrogenized Mice. Cancer Res. 1988;48:425–9. [PubMed] [Google Scholar]
- 16.Shorey LE, Castro DJ, Baird WM, Siddens LK, Löhr CV, Matzke MM, et al. Transplacental Carcinogenesis with Dibenz [def,p]chrysene (DBC): Timing of Maternal Exposures Determines Target Tissue Response in Offspring. Cancer Lett. 2012;317:49–55. doi: 10.1016/j.canlet.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim J, Lawson GW, Nakamura BN, Ortiz L, Hur JA, Kavanagh TJ, et al. Glutathione-Deficient Mice Have Increased Sensitivity to Transplacental Benzo[a]pyrene-Induced Premature Ovarian Failure and Ovarian Tumorigenesis. Cancer Res. 2013;73:908–17. doi: 10.1158/0008-5472.CAN-12-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen VW, Ruiz B, Killeen JL, Cote TR, Wu XC, Correa CN. Pathology and Classification of Ovarian Tumors. Cancer. 2003;97:2631–42. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- 19.Flesken-Nikitin A, Hwang C-I, Cheng C-Y, Michurina TV, Enikolopov G, Nikitin AY. Ovarian Surface Epithelium at the Junction Area Contains a Cancer-Prone Stem Cell Niche. Nature. 2013;495:241–5. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tannheimer SL, Ethier SP, Caldwell KK, Burchiel SW. Benzo[a]pyrene and TCDD-Induced Alterations in Tyrosine Phosphorylation and Insulin-Like Growth Factor Signaling Pathways in the MCF-7a Human Mammary Epithelial Cell Line. Carcinogenesis. 1998;19:1291–7. doi: 10.1093/carcin/19.7.1291. [DOI] [PubMed] [Google Scholar]
- 21.Tannheimer SL, Barton SL, Ethier SP, Burchiel SW. Carcinogenic Polycyclic Aromatic Hydrocarbons Increase Intracellular Ca2+ and Cell Proliferation in Primary Human Mammary Epithelial Cells. Carcinogenesis. 1997;18:1177–82. doi: 10.1093/carcin/18.6.1177. [DOI] [PubMed] [Google Scholar]
- 22.Plí kovÆ M, VondrÆcek J, Vojtc ek B, Kozubík A, Machala M. Deregulation of Cell Proliferation by Polycyclic Aromatic Hydrocarbons in Human Breast Carcinoma MCF-7 Cells Reflects Both Genotoxic and Nongenotoxic Events. Toxicol Sci. 2005;83:246–56. doi: 10.1093/toxsci/kfi040. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner HE, Vulimiri S, Hatten WB, Reed MJ, Nebert DW, Jefcoate CR, et al. Role of Cytochrome P4501 Family Members in the Metabolic Activation of Polycyclic Aromatic Hydrocarbons in Mouse Epidermis. Chem Res Toxicol. 2004;17:1667–74. doi: 10.1021/tx049919c. [DOI] [PubMed] [Google Scholar]
- 24.Xue W, Warshawsky D. Metabolic Activation of Polycyclic Aromatic Hydrocarbon and Heterocyclic Aromatic Hydrocarbons and DNA Damage: A Review. Toxicol Appl Pharmacol. 2005;206:73–93. doi: 10.1016/j.taap.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Takizawa K, Yagi H, Jerina DM, Mattison DR. Murine Strain Differences in Ovotoxicity following Intraovarian Injection with Benzo(a)pyrene, (+)-(7R,8S)-oxide, (−)-(7R,8S)-Dihydrodiol, or (+)-(7R,8S)-Diol-(9S,10R)-epoxide-2. Cancer Res. 1984;44:2571–6. [PubMed] [Google Scholar]
- 26.Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and Activity of Microsomal Epoxide Hydrolase in Follicles Isolated from Mouse Ovaries. Toxicol Sci. 2002;68:24–31. doi: 10.1093/toxsci/68.1.24. [DOI] [PubMed] [Google Scholar]
- 27.Coller JK, Fritz P, Zanger UM, Siegle I, Eichelbaum M, Kroemer HK, et al. Distribution of Microsomal Epoxide Hydrolase in Humans: An Immunohistochemical Study in Normal Tissues, and Benign and Malignant Tumours. Histochem J. 2001;33:329–36. doi: 10.1023/a:1012414806166. [DOI] [PubMed] [Google Scholar]
- 28.Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of Apoptosis in 4-Vinylcyclohexene Diepoxide-Induced Ovotoxicity in Rats. Toxicol Appl Pharmacol. 1996;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- 29.Shimada T, Sugie A, MS, Nakajima T, Azuma E, Hashimoto M, et al. Tissue-Specific Induction of Cytochromes P450 1A1 and 1B1 by Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls in Engineered C57BL/6J Mice of Arylhydrocarbon Receptor. Gene Toxicol Appl Pharmacol. 2003;187:1–10. doi: 10.1016/s0041-008x(02)00035-2. [DOI] [PubMed] [Google Scholar]
- 30.Otto S, Bhattacharyya KK, Jefcoate CR. Polycyclic Aromatic Hydrocarbon Metabolism in Rat Adrenal, Ovary, and Testis Microsomes is Catalyzed by the Same Novel Cytochrome P450 (P450RAP) Endocrinology. 1992;131:3067–76. doi: 10.1210/endo.131.6.1332854. [DOI] [PubMed] [Google Scholar]
- 31.Muskhelishvili L, Thompson PA, Kusewitt DF, Wang C, Kadlubar FF. In Situ Hybridization and Immunohistochemical Analysis of Cytochrome P450 1B1 Expression in Human Normal Tissues. J Histochem Cytochem. 2001;49:229–36. doi: 10.1177/002215540104900210. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharyya KK, Brake PB, Eltom SE, Otto SA, Jefcoate CR. Identification of a Rat Adrenal Cytochrome P450 Active in Polycyclic Aromatic Hydrocarbon Metabolism as Rat CYP1B1. J Biol Chem. 1995;270:11595–602. doi: 10.1074/jbc.270.19.11595. [DOI] [PubMed] [Google Scholar]
- 33.Seidel A, Friedberg T, Löllman B, Schwierzok A, Funk M, Frank H, et al. Detoxification of Optically Active Bay- and Fjord-Region Polycyclic Aromatic Hydrocarbon Dihydrodiol Epoxides by Human Glutathione Transferase P1-1 Expressed in Chinese Hamster V79 Cells. Carcinogenesis. 1998;19:1975–81. doi: 10.1093/carcin/19.11.1975. [DOI] [PubMed] [Google Scholar]
- 34.Chan JY, Kwong M. Impaired Expression of Glutathione Synthetic Enzyme Genes in Mice with Targeted Deletion of the Nrf2 Basic-Leucine Zipper Protein. Biochim Biophys Acta. 2000;1517:19–26. doi: 10.1016/s0167-4781(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 35.Ramos-Gomez M, Dolan PM, Itoh K, Yamamoto M, Kensler TW. Interactive Effects of nrf2 Genotype and Oltipraz on Benzo[a]pyrene DNA Adducts and Tumor Yield in Mice. Carcinogenesis. 2003;24:461–7. doi: 10.1093/carcin/24.3.461. [DOI] [PubMed] [Google Scholar]
- 36.Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 Signaling in Regulation of Antioxidants and Phase 2 Enzymes in Cardiac Fibroblasts: Protection against Reactive Oxygen and Nitrogen Species-Induced Cell Injury. FEBS Lett. 2005;579:3029–36. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 37.Ramos-Gomez M, Kwak M-K, Dolan PM, Itoh K, Yamamoto M, Talalay P, et al. Sensitivity to Carcinogenesis Is Increased and Chemoprotective Efficacy of Enzyme Inducers Is Lost in nrf2 Transcription Factor-Deficient Mice. Proc Natl Acad Sci U S A. 2001;98:3410–5. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu X, Roberts JR, Apopa PL, Kan YW, Ma Q. Accelerated Ovarian Failure Induced by 4-Vinyl Cyclohexene Diepoxide in Nrf2 Null Mice. Mol Cell Biol. 2006;26:940–54. doi: 10.1128/MCB.26.3.940-954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan K, Han X-D, Kan YW. An Important Function of Nrf2 in Combating Oxidative Stress: Detoxification of Acetaminophen. Proc Natl Acad Sci. 2001;98:4611–6. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J-M, Chan K, Kan YW, Johnson JA. Targeted Disruption of Nrf2 Causes Regenerative Immune-Mediated Hemolytic Anemia. Proc Natl Acad Sci U S A. 2004;101:9751–6. doi: 10.1073/pnas.0403620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Q, Battelli L, Hubbs AF. Multiorgan Autoimmune Inflammation, Enhanced Lymphoproliferation, and Impaired Homeostasis of Reactive Oxygen Species in Mice Lacking the Antioxidant-Activated Transcription Factor Nrf2. Am J Pathol. 2006;168:1960–74. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung L, Kwong M, Hou S, Lee CJ, Chan JY. Deficiency of the Nrf1 and Nrf2 Transcription Factors Results in Early Embryonic Lethality and Severe Oxidative Stress. J Biol Chem. 2003;278:48021–9. doi: 10.1074/jbc.M308439200. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura BN, Lawson G, Chan JY, Banuelos J, Cortés MM, Hoang YD, et al. Knockout of the Transcription Factor Nrf2 Disrupts Spermatogenesis in an Age-Dependent Manner. Free Radic Biol Med. 2010;49:1368–79. doi: 10.1016/j.freeradbiomed.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim J, Luderer U. Oxidative Damage Increases and Antioxidant Gene Expression Decreases with Aging in the Mouse Ovary. Biol Reprod. 2011;84:775–82. doi: 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarín JJ. Potential Effects of Age-Associated Oxidative Stress on Mammalian Oocytes/Embryos. Mol Hum Reprod. 1996;2:717–24. doi: 10.1093/molehr/2.10.717. [DOI] [PubMed] [Google Scholar]
- 46.Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, et al. Age-Associated Alteration of Gene Expression Patterns in Mouse Oocytes. Hum Mol Genet. 2004;13:2263–78. doi: 10.1093/hmg/ddh241. [DOI] [PubMed] [Google Scholar]
- 47.Lim J, Nakamura BN, Mohar I, Kavanagh TJ, Luderer U. Glutamate Cysteine Ligase Modifier Subunit (Gclm) Null Mice Have Increased Ovarian Oxidative Stress and Accelerated Age-related Ovarian Failure. Endocrinology. 2015 doi: 10.1210/en.2015-1206. Epub ahead of print, June 17, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan K, Lu R, Chang JC, Kan YW. NRF2, a Member of the NFE2 Family of Transcription Factors, Is not Essential for Murine Erythropoiesis, Growth, and Development. Proc Natl Acad Sci U S A. 1996;93:13493–948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.NRC. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Research Council, National Academy of Sciences; 1996. [Google Scholar]
- 50.Tsai-Turton M, Luderer U. Gonadotropin Regulation of Glutamate Cysteine Ligase Catalytic and Modifier Subunit Expression in the Rat Ovary is Subunit and Follicle Stage-Specific. Am J Physiol. 2005;289:E391–E402. doi: 10.1152/ajpendo.00531.2004. [DOI] [PubMed] [Google Scholar]
- 51.Griffith OW. Determination of Glutathione and Glutathione Disulfide using Glutathione Reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–12. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 52.Luderer U, Kavanagh TJ, White CC, Faustman EM. Gonadotropin Regulation of Glutathione Synthesis in the Rat Ovary. Reprod Toxicol. 2001;15:495–504. doi: 10.1016/s0890-6238(01)00157-5. [DOI] [PubMed] [Google Scholar]
- 53.Thompson SA, White CC, Krejsa CM, Diaz D, Woods JS, Eaton DL, et al. Induction of Glutamate-Cysteine Ligase (γ-Glutamylcysteine Synthetase) in the Brains of Adult Female Mice Subchronically Exposed to Methylmercury. Toxicol Lett. 1999;110:1–9. doi: 10.1016/s0378-4274(99)00133-2. [DOI] [PubMed] [Google Scholar]
- 54.Pfaffl MW. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-??CT Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 56.Lopez SG, Luderer U. Effects of Cyclophosphamide and Buthionine Sulfoximine on Ovarian Glutathione and Apoptosis. Free Radic Biol Med. 2004;36:1366–77. doi: 10.1016/j.freeradbiomed.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 57.Pedersen T, Peters H. Proposal for a Classification of Oocytes in the Mouse Ovary. J Reprod Fertil. 1968;17:555–7. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 58.Plowchalk DR, Smith BJ, Mattison DR. Assessment of Toxicity to the Ovary Using Follicle Quantitation and Morphometrics. In: Heindel JJ, Chapin RE, editors. Female Reproductive Toxicology. San Diego, CA: Academic Press; 1993. pp. 57–68. [Google Scholar]
- 59.Hirshfield AN. Size-Frequency Analysis of Atresia in Cycling Rats. Biol Reprod. 1988;38:1181–8. doi: 10.1095/biolreprod38.5.1181. [DOI] [PubMed] [Google Scholar]
- 60.Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL. Ablation of bcl-2 Gene Expression Decreases the Numbers of Oocytes and Primordial Follicles Established in the Post-Natal Female Mouse Gonad. Endocrinology. 1995;136:3665–8. doi: 10.1210/endo.136.8.7628407. [DOI] [PubMed] [Google Scholar]
- 61.Randerath K, Randerath E. 32P-Postlabeling Methods for DNA Adduct Detection: Overview and Critical Evaluation. Drug Metab Rev. 1994;26:67–85. doi: 10.3109/03602539409029785. [DOI] [PubMed] [Google Scholar]
- 62.Reddy MV, Randerath K. Nuclease P1-Mediated Enhancement of Sensitivity of 32P-Postlabeling Test for Structurally Diverse DNA Adducts. Carcinogenesis. 1986;7:1543–51. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- 63.Randerath K, Reddy MV, Gupta RC. 32P-Labeling Test for DNA Damage. Proc Natl Acad Sci U S A. 1981;78:6126–9. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luderer U, Diaz D, Faustman EM, Kavanagh TJ. Localization of Glutamate Cysteine Ligase Subunit mRNA within the Rat Ovary and Relationship to Follicular Atresia. Mol Reprod Dev. 2003;65:254–61. doi: 10.1002/mrd.10298. [DOI] [PubMed] [Google Scholar]
- 65.Pasternack BS, Shore RE. Analysis of Dichotomous Response Data from Toxicological Experiments Involving Stable Laboratory Mouse Populations. Biometrics. 1982;38:1057–67. [PubMed] [Google Scholar]
- 66.Aoki Y, Hashimoto AH, Amanuma K, Matsumoto M, Hiyoshi K, Takano H, et al. Enhanced Spontaneous and Benzo(a)pyrene-Induced Mutations in the Lung of Nrf2-Deficient gpt Delta Mice. Cancer Res. 2007;67:5643–8. doi: 10.1158/0008-5472.CAN-06-3355. [DOI] [PubMed] [Google Scholar]
- 67.Kensler TW, Wakabayashi N. Nrf2: Friend or Foe for Chemoprevention? Carcinogenesis. 2010;31:90–9. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyata M, Kudo G, Lee Y-H, Yang TJ, Gelboin HV, Fernandez-Salguero P, et al. Targeted Disruption of the Microsomal Epoxide Hydrolase Gene. J Biol Chem. 1999;274:23963–8. doi: 10.1074/jbc.274.34.23963. [DOI] [PubMed] [Google Scholar]
- 69.van Lipzig MMH, Vermeulen NPE, Gusinu R, Legler J, Frank H, Seidel A, et al. Formation of Estrogenic Metabolites of Benzo [a]pyrene and Chrysene by Cytochrome P450 Activity and Their Combined and Supra-maximal Estrogenic Activity. Environ Toxicol Pharmacol. 2005;19:41–55. doi: 10.1016/j.etap.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 70.Burdick AD, Davis JWI, Liu KJ, Hudson LG, Shi H, Monske ML, et al. Benzo(a)pyrene Quinones Increase Cell Proliferation, Generate Reactive Oxygen Species, and Transactivate the Epidermal Growth Factor Receptor in Breast Epithelial Cells. Cancer Res. 2003;63:7825–33. [PubMed] [Google Scholar]
- 71.Leung Y-K, Lau K-M, Mobley J, Jiang Z, Ho S-M. Overexpression of Cytochrome P450 1A1 and Its Novel Spliced Variant in Ovarian Cancer Cells: Alternative Subcellular Enzyme Compartmentation May Contribute to Carcinogenesis. Cancer Res. 2005;65:3726–34. doi: 10.1158/0008-5472.CAN-04-3771. [DOI] [PubMed] [Google Scholar]
- 72.Wiener-Megnazi Z, Vardi L, Lissak A, Shnizer S, Reznick AZ, Ishai D, et al. Oxidative Stress Indices in Follicular Fluid as Measured by the Thermochemi-luminescence Assay Correlate with Outcome Parameters in in Vitro Fertilization. Fertil Steril. 2004;82(Suppl 3):1171–6. doi: 10.1016/j.fertnstert.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 73.Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, et al. Age-Dependent Changes in the Expression of Superoxide Dismutases and Catalase Are Associated with Ultrastructural Modifications in Human Granulosa Cells. Mol Reprod Dev. 2006;12:655–60. doi: 10.1093/molehr/gal080. [DOI] [PubMed] [Google Scholar]
- 74.Das S, Chattopadhyay R, Ghosh S, Ghosh S, SKG, Chakravarty BN, et al. Reactive Oxygen Species Level in Follicular Fluid--Embryo Quality Marker in IVF? Hum Reprod. 2006;21:2403–7. doi: 10.1093/humrep/del156. [DOI] [PubMed] [Google Scholar]
- 75.Kobayashi M, Yamamoto M. Nrf2-Keap1 Regulation of Cellular Defense Mechanisms against Electrophiles and Reactive Oxygen Species. Adv Enzyme Regul. 2006;46:113–40. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 76.Pepling ME. Follicular Assembly: Mechanisms of Action. Reproduction. 2012;143:139–49. doi: 10.1530/REP-11-0299. [DOI] [PubMed] [Google Scholar]
- 77.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, Progesterone, and Genistein Inhibit Oocyte Nest Breakdown and Primordial Follicle Assembly in the Neonatal Mouse Ovary in Vitro and in Vivo. Endocrinology. 2007;148:3580–90. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- 78.Mazaud-Guittot S, Guigon CJ, Coudouel N, Magre S. Consequences of Fetal Irradiation on Follicle Histogenesis and Early Follicle Development in Rat Ovaries. Biol Reprod. 2006;75:749–59. doi: 10.1095/biolreprod.105.050633. [DOI] [PubMed] [Google Scholar]
- 79.Ward JF. The Complexity of DNA Damage: Relevance to Biological Consequences. Int J Radiat Biol. 1994;66:427–32. doi: 10.1080/09553009414551401. [DOI] [PubMed] [Google Scholar]
- 80.Riley PA. Free Radicals in Biology: Oxidative Stress and the Effects of Ionizing Radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.