Abstract
BACKGROUND
Plasma exchange (PE) is the first-line treatment for primary acquired thrombotic thrombocytopenic purpura (aTTP) with severe deficiency of ADAMTS13 activity. Some patients are poor-responders to PE, raising concern over multiple pathogenetic pathways.
METHODS
Based on 52 aTTP patients in our national cohort study, we monitored plasma levels of ADAMTS13, clinical and laboratory findings, and outcomes. In a representative poor-responder to PE, we examined an ADAMTS13-inhibitor complex in plasma milieu, by means of a large-pore isoelectric focusing (IEF) analysis.
RESULTS
Of 52 aTTP patients, 20 were well-responders and 32 were poor-responders. In the latter group, plasma ADAMTS13 activity levels never increased to more than 10% of normal during 14 days after PE initiation. Mean (±SD) plasma ADAMTS13 inhibitor titers (BU/ml) were 5.7 (±4.5) before PE, but decreased to 1.4 (±0.8) on 4th PE day, and then remarkably increased to 14.8 (±10.0) on 10th PE day, termed ‘inhibitor boosting’, and then slowly decreased to undetectable level over one month. On admission, none of the routinely available clinical and laboratory markers differentiated these two groups. However, elevated pre-PE levels of ADAMTS13 inhibitor were correlated with poor-response. We visualized an ADAMTS13-inhibitor (IgG) complex in a patient plasma by an IEF analysis, and found proteolytic fragment of ADAMTS13 antigen by a 2-dimentional IEF/SDS-PAGE analysis.
CONCLUSION
Findings from this cohort of aTTP patients demonstrated that inhibitor boosting often occurs in aTTP patients in Japan. Poor-responders could be predicted by elevated pre-PE ADAMTS13 inhibitor levels on admission, but not by routinely collected clinical or laboratory data.
Keywords: primary aTTP, ADAMTS13, inhibitor boosting, IEF, ADAMTS13 inhibitor complex
Introduction
Thrombotic thrombocytopenic purpura (TTP), a life-threatening generalized disorder originally characterized by a pentad of thrombocytopenia, microangiopathic hemolytic anemia, renal dysfunction, neurological signs and fever,1 is now primarily defined by severe deficiency of von Willebrand factor (VWF)-cleaving protease, termed ADAMTS13 (a disintegrin-like and metalloprotease with thrombospondin type 1 motifs 13) accompanied by thrombocytopenia.2–4 The ADAMTS13 specifically cleaves unusually large VWF multimer (UL-VWFM) with hyperaggregability of platelets,5 and down-regulates VWF function. Deficiency of ADAMTS13 activity (ADAMTS13:AC) is either caused by gene mutations in or acquired autoantibodies to this enzyme,6 occasionally without a known cause, termed primary acquired TTP (aTTP).7
Plasma exchange (PE) has long been the first-line treatment for aTTP, often with an adjunct of corticosteroid/corticosteroid pulse therapy,8 PE removes ADAMTS13 inhibitor (ADAMTS13:INH), UL-VWFM, and inflammatory cytokines that mediate UL-VWFM release from vascular endothelial cells, and replenishes ADAMTS13 and regular-sized VWFM required for normal hemostasis. Patients with aTTP frequently take several days to weeks of PE before platelet counts recover. However, one population of aTTP patients often shows an initial increase in the platelet count that is paradoxically followed by secondary thrombocytopenia. These patients have been categorized as having ‘PE-refractory aTTP,’ but the pathogenesis of secondary thrombocytopenia is unclear. Böhm et al.9 reported an increase in ADAMTS13:INH titers after PE, but no systematic studies on this potentially important issue have been conducted. In addition, 30% to 50% of aTTP patients will subsequently relapse, although these relapses occur at a time-point that is much longer than the secondary thrombocytopenia described herein.6,10 Due to concerns over both short-term thrombocytopenic events and long-term aTTP relapse, the anti-CD20 antibody rituximab, which removes inhibitory IgG-producing B lymphocytes from circulation,11,12has been used recently to treat aTTP patients in conjunction with PE and corticosteroid, in an effort to reduce IgG of ADAMTS13:INH with the goal of improving short-term responses as well as decreasing the rate of relapse.
We have recently shown that ADAMTS13 antigen (ADAMTS13:AG) in the plasma milieu consisted of 3 groups of bands with different isoelectric points (pI); Band I (pI 4.9 to 5.6) represents ADAMTS13 not bound to VWF; Band II (pI 5.8 to 6.7) remains unaddressed, and Band III (pI 7.0 or 7.5) corresponds to ADAMTS13 bound to larger VWFM.13 Further, during 1998–2012 we identified 52 patients with new onset of aTTP, whose plasma samples were followed for serial ADAMTS13 level measurements more than 3 times within 2 weeks after initiation of PE. These patients were classified into two groups based on the pre-PE levels of plasma ADAMTS13:AC measured on the 14th day after PE initiation; 20 well ADAMTS13:AC-responders (ADAMTS13:AC ≥10%) and 32 persons with poor ADAMTS13:AC-responders (ADAMTS13:AC <10%). We found that poor ADAMTS13:AC-responders were uniformly associated with a tremendous increase of ADAMTS13:INH titers (IgG1 subclass) on 10th PE day, termed ‘inhibitor boosting’. Further, using an IEF gel we visualized for the first time a complex of ADAMTS13:AG and ADAMTS13:INH (IgG), that underwent proteolytic cleavage followed by clearance from circulation. These studies may help to understand how treatment shortly after PE with corticosteroid and rituximab infusion works in aTTP patients who otherwise may develop secondary thrombocytopenia due to ‘inhibitor boosting’.
Patients, Materials, and Methods
Fifty-two aTTP patients enrolled in this national cohort study
Since 1998, our laboratory at Nara Medical University has served as a nationwide referral center for thrombotic microangiopathy (TMA) that analyzes ADAMTS13 as requested by clients across Japan. Using a chromogenic assay for ADAMTS13:AC,14 between January 2004 and March 2012, we were able to identify 184 new patients (89 males and 95 females) with aTTP, based on the following criteria; severe deficiency of plasma ADAMTS13:AC (<10% of normal) with positive ADAMTS13:INH (>1.0 Bethesda U/ml), but without the underlying diseases. Of 184 aTTP patients, 52 were able to have follow up determination of plasma ADAMTS13:AC levels more than 3 times at 3–5 days interval within 14 days after PE initiation, and were enrolled in this study (see the supplemental Table 1 and 2).
Patient outcome definitions
The study definitions were the same as those reported by Kremer-Hovinga et al15 and Froissart et al.16 Remission was defined as a complete resolution of laboratory and clinical data after cessation of PE. Durable remission was defined as laboratory and clinical resolution of aTTP evaluated at least 30 days following the first day of platelet count recovery (>150×109 /L) (this period included the time on maintenance PE). Relapse was considered a new episode of TTP if a patient had achieved a durable remission and subsequently experienced a laboratory relapse. Death from TTP was defined as any death that occurred within 30 days after a diagnosis of TTP.
Assays for plasma levels of ADAMTS13:AC, ADAMTS13:INH and ADAMTS13:AG
Plasma levels of ADAMTS13:AC were measured by chromogenic ADAMTS13-act-ELISA14 with a detection limit of 0.5% of normal, and the ADMTS13:INH titers were determined by means of Bethesda method17 using heat-inactivated patient plasmas, as previously described. One Bethesda unit (BU) was defined as the amount necessary to reduce ADAMTS13:AC to 50% of control levels, and the value of ≥1.0 BU/mL was assumed to be positive. Further, plasma levels of ADAMTS13:AG were measured using sandwich ELISA.18
Titers of anti-ADAMTS13 IgG, IgM, and IgA autoantibodies and IgG1–4 subclasses
Assays were performed as previously described19, with the following modifications. First, microtiter plates (Nunc Immuno Maxisorp, Nunc A/S, Roskilde, Denmark) were coated with 100 µL of human recombinant (r) ADAMTS13 (2µg/mL) dissolved in a coating buffer [carbonate-bicarbonate buffer, pH 9.6, Sigma C-3041 (Sigma-Aldrich, St. Louis, MO, USA)] and left overnight at 4°C. On the next day, 250 µL of blocking buffer (Pierce protein-free T20, Thermo Fisher Scientific Inc. Rockford, IL, USA) was added to the plates, and incubated for 2 hours at room temperature. Then, 100 µL of each diluted test sample was added to the each well and left for 3 hours at room temperature. After incubation, 100µL of diluted horseradish peroxidase (HRP)-conjugated goat anti-human IgG, IgG1–4, IgM, or IgA were added, and the plates were incubated for 2 hours at room temperature. Finally, 100 µL of 3,3',5,5'-tetramethylbenzidine (TMB)-H2O2 solution (TMB substrate kit, Thermo Fisher Scientific Inc. Rockford, IL, USA) was added, and the absorbance was measured at 450 nm with a reference filter of 620 nm on a Thermo microplate reader (Thermo Fisher Scientific Inc). Between each step, the plates were washed with phosphate-buffered saline (PBS, pH 7.2) 4 times. Titers were calculated as a ratio of the sample optical density (OD) at each dilution to the normal human plasma OD at each dilution. Ratios were determined for each individual dilution and compared to the corresponding cutoff values, and the results were expressed as the titer. The titer of a sample corresponded to the last dilution at which the ratio was above the cutoff level.19
IEF using an agarose-acrylamide composite gel
In some experiments, ADAMTS13 in patient plasma was analyzed by IEF using a large-pore agarose-acrylamide composite gel as recently described in detail.13 After IEF, the isolated proteins were electrophoretically transferred to nitrocellulose membranes, and then the blotted proteins were immunoreacted with anti-ADAMTS13 monoclonal antibody (WH2-11-1, epitope residing on the 4th thrombospondin type-1 domain of ADAMTS13),20 and visualized using a chemiluminescent detection kit (Perkin-Elmer Life Science, Boston, MA). Using this method, ADAMTS13:AG from normal plasma consistently shows the following 3 groups of bands; a major Band I representing unbound ADAMTS13 with a pI of 4.9–5.6, as in the case of purified plasma-derived ADAMTS13, as well as Band II at pI 5.8–6.7 and Band III at pI 7.0/7.5. The composition of Band II is not well defined yet, but Band III was shown to be a complex of ADAMTS13:AG and high-molecular-weight VWFM.13
Therapeutic regimen
The basic therapeutic regimen for our cohort patients with aTTP consisted of PE, corticosteroids, and immuno-suppressants such as rituximab (see the supplemental Table 1 and 2). All patients were initially treated with PE with fresh frozen plasma (FFP) at a dose of 40–60 ml/kg body weight/once per day, and mostly for the first 3 to 5 consecutive days. However, the subsequent regimen of PE therapy was variable based primarily on laboratory findings of patients (platelet count and serum LDH), and presence of neurological findings.
PE therapy was usually accompanied with corticosteroid therapy. In our cohort, 30 patients received high-dose methylprednisolone (mPSL, 0.5–1 g/day) pulse therapy, of whom 23 patients received it commonly for 3 consecutive days during 1–3 days after PE and 7 patients received it beyond this period. Another, 13 patients who did not receive corticosteroid pulse therapy received per os administration of predonisolone (0.5–1 mg/kg/day). One patient did not have corticosteroid therapy. For the remaining 8 patients, we were unable to retrieve information on corticosteroid therapy from the physicians-in-charge.
For immune-suppressants, rituximab infusion was most frequently used (18/52 patients with aTTP). The rituximab dose was 375 mg/m2 weekly for 4 weeks, although one patient (patient no. 34) died soon after the first rituximab infusion, and the one patient (patient no. 26) received 5 infusions of rituximab. Patient no. 28 (female) was an exception, who received a second course of rituximab therapy during 75–96 days (a total of 8 rituximab infusions), as the patient had persistence of high titers of ADAMTS13:INH (peak of 42.6 BU/mL). An additional 10 patients received vincristine (1–2 mg/body per day) one to four doses administered one week apart, and 4 patients received one or two cycles of cyclophoasphamide (CPA) pulse therapy (500 mg/body/day). Therapies administered to three additional patients included intravenous immunoglobulin (one patient), one dose of cyclosporine (one patient), and aspirin (one patient).
Statistical analysis
Laboratory data are expressed as the mean±SD. Comparisons between well ADAMTS13:AC-responders and poor ADAMTS13:AC-responders were analyzed using the Mann-Whitney U-test or Chi-square test. All analyses were carried out using StatView (SAS Institute Inc., Cary, NC, USA). A p-value <0.05 was considered significant.
Results
Two patient groups of aTTP: well ADAMTS13:AC-responders and poor ADAMTS13:AC-responders to PE
Follow-up clinical and laboratory findings were available for all 52 aTTP patients (between 7 and 2,607 days after the first admission). These follow-up data are summarized in the supplemental Table 1 and 2. The 52 aTTP patients were classified into two groups based on comparison of plasma levels of ADAMTS13:AC measured fourteen days after initiation of PE : 20 aTTP patients are characterized as well ADAMTS13:AC-responders (ADAMTS13:AC ≥10%) and 32 aTTP patients are characterized as poor ADAMTS13:AC-responders (ADAMTS13:AC <10%). Characteristics of these 2 aTTP patient groups are shown in Table 1. Most of the 2 patient groups received corticosteroids. Eighteen aTTP patients received rituximab, which was administered to poor ADAMTS13:AC-responders more frequently than well ADAMTS13:AC-responders (p<0.05).
Table 1.
Comparison of laboratory markers; well ADAMTS13:AC-responders and poor ADAMTS13:AC-responders to PE therapy
| ADAMTS13:AC (%) on 14th day after PE | ||||
|---|---|---|---|---|
| ≥10% (well-responders) |
<10% (poor-responders) |
p Value | ||
| Number | 20 | 32 | ||
| Age on admission | 53 (1–81) | 48 (13–84) | 0.44 | |
| Sex | ||||
| female | 13 (65%) | 19 (59%) | 0.91 | |
| male | 7 (35%) | 13 (41%) | ||
| PE (times) | total | 7.5 (3–15) | 14.3 (4–31) | <0.01 |
| during 14 days | 6.8 (3–11) | 9.9 (4–14) | <0.01 | |
| after 14 days | 0.7 (0–6) | 4.5 (0–20) | <0.01 | |
| Rituximab therapy | 3 | 15 | <0.05 | |
| Steroid/steroid pulse therapy | 18 | 30 | 0.62 | |
In well ADAMTS13:AC-responders (Fig. 1 left), plasma levels of ADAMTS13:AC (mean ± SD) increased to 15.0 ± 10.9% on the third day of PE, with a slight dip on the tenth day of PE, and then did not decrease below 10% within 14 days after PE initiation. As for plasma ADAMTS13:INH titer in well ADAMTS13:AC-responders, mean ± SD ADAMTS13:INH levels were 2.5 ± 1.5 BU/ml before PE, decreasing to 0.8 ± 0.4 BU/ml on 4th PE day, and then increasing to almost pre-PE levels (2.7 ± 4.4 BU/ml) on 10th PE day, followed by a consistent decrease to undetectable ADAMTS13:INH levels (<0.5 BU/ml) on the 14th PE day. Platelet recovery began one to two days later and mean platelet increased to 180×109 /L on 6th PE day, followed by a slight dip, and the mean platelet counts increased again to greater than 150×109 /L on the 14th PE day.
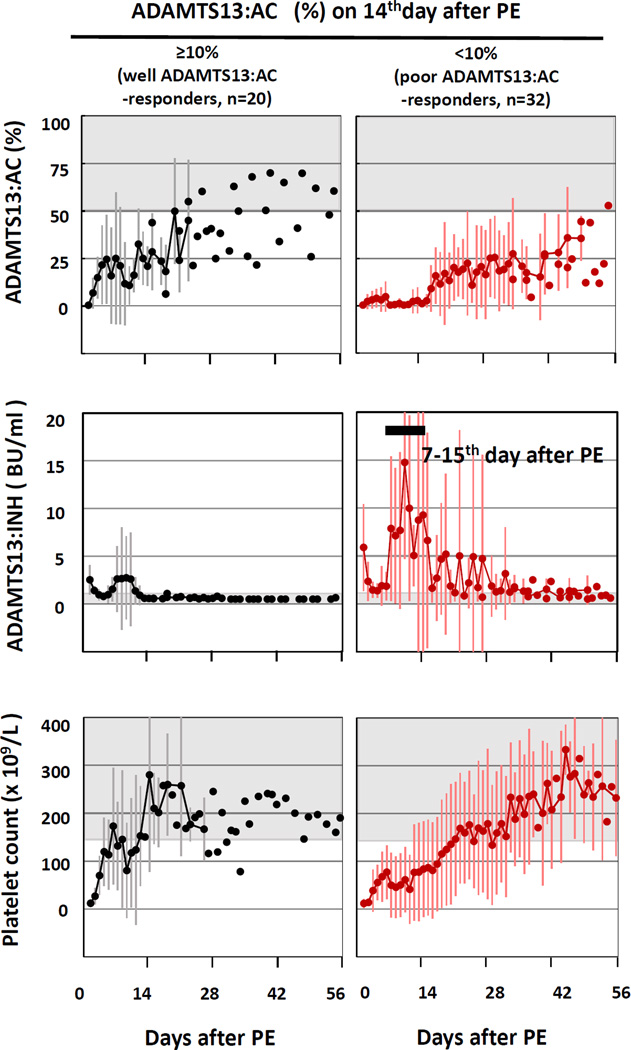
Fig. 1. Changes of plasma levels of ADAMTS13 activity (:AC), ADAMTS13 inhibitor (:INH) and platelet counts in 20 well ADAMTS13:AC-responder (left) and 32 poor ADAMTS13:AC-responder to PE (right).
In the figures, the shaded areas show normal ranges, and the vertical bars indicate values of mean ± SD.
In well ADAMTS13:AC-responders (left), plasma levels of ADAMTS13:AC increased to 15.0 ± 10.9% on 3rd PE day and then did not decrease below 10% within 14 days after PE initiation. Plasma ADAMTS13:INH titers decreased on 4th PE day, and then restored to almost pre-PE levels on 10th PE day, followed by a consistent decrease to the undetectable level on 14th PE day. Platelet counts increased to normal level on 6th PE day, followed by a slight dip, and then re-increased to normal on 14th PE day.
In poor ADAMTS13:AC-responders (right), plasma levels of ADAMTS13:AC never increased to more than 10% during 14 days after PE initiation. Further, plasma ADAMTS13:INH titer decreased from 5.9 ± 4.5 BU/ml before PE initiation to 1.4 ± 0.7 BU/ml on 4th PE day, and then remarkably increased to 14.8 ± 0.7 BU/ml on 10th PE day, termed ‘inhibitor boosting’, followed by a slow decrease to undetectable level over one-month after PE initiation. A recovery of platelet counts was very slow, and the count reached to more than 150×109 /L on 22nd PE day.
In poor ADAMTS13:AC-responders (Fig. 1 right), plasma levels of ADAMTS13:AC never increased to more than 10% of normal as measured at the fourteenth day after PE initiation. Further, mean ± SD plasma ADAMTS13:INH titers were 5.9 ± 4.5 BU/ml before PE initiation, but decreased to 1.4 ± 0.7 BU/ml on the 4th PE day, and then increased to 14.8 ± 0.7 BU/ml on 10th PE day. This is termed ‘inhibitor boosting’. Mean ADAMTS13:INH titers subsequently decreased to undetectable levels (<0.5 BU/ml) over a four week period following PE initiation. Among these aTTP patients, recovery of platelet counts was slow, and the platelet count was not greater than 150×109 /L in any of these patients by the 22nd PE day.
Sequential analysis of ADAMTS13:INH immunoglobulin class and IgG subclasses
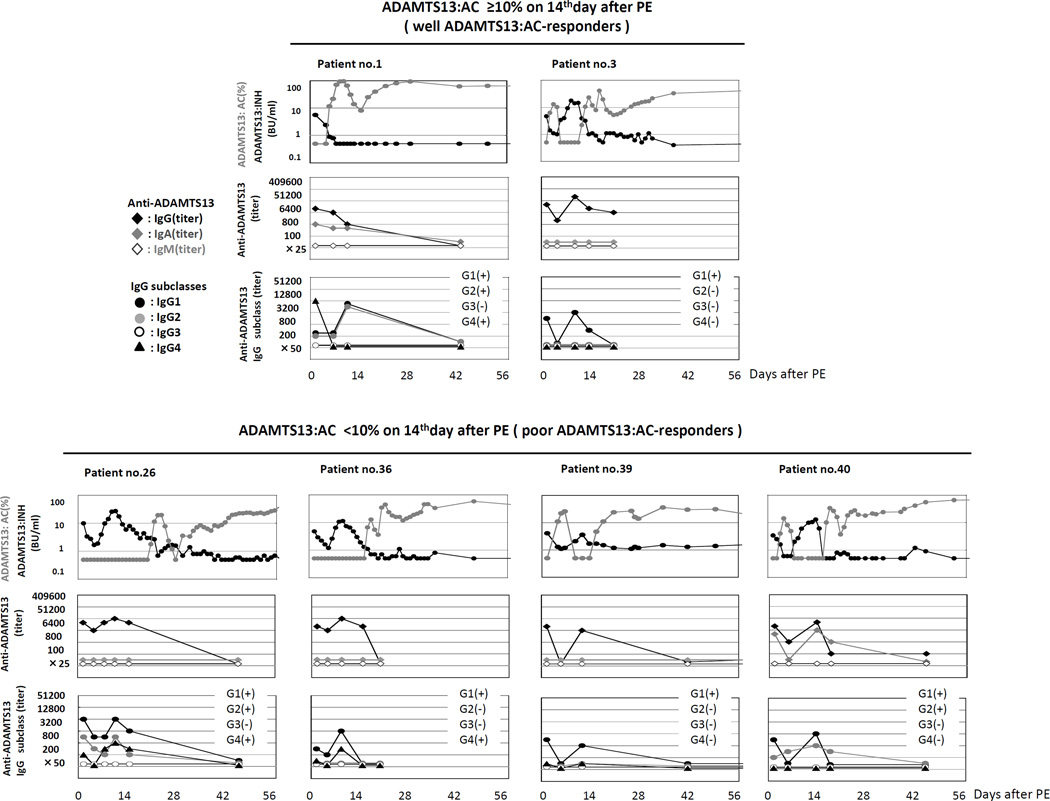
A previous report19 indicated clinical significance of the IgG4 subclass of ADAMTS13:INH (IgG) rather than others, and therefore we here sequentially measured the titers of immunoglobulins and IgG subclasses in 6 aTTP patients, including 2 well ADAMTS13:AC-responders (patient no. 1 and 3) and 4 poor ADAMTS13:AC -responders (patient no. 26, 36, 39, and 40) (Fig. 2).
Fig. 2. Sequential analysis of ADAMTS13:INH immunoglobulin class and IgG subclasses.
We sequentially analyzed the titers of immunoglobulins and IgG subclasses in 6 aTTP patients, including 2 well ADAMTS13:AC-responders (patient no. 1 and 3) and 4 poor ADAMTS13:AC-responders (patient no. 26, 36, 39, and 40). Both the patients in well ADAMTS13:AC-responders had IgG antibodies (IgG1 in both patients, IgG2 and IgG4 classes in one patient) and one had IgA antibodies. All 4 patients in poor ADAMTS13:AC-responders had IgG antibodies (IgG1 in 4 patients, IgG2 in 2 patients, and IgG4 in 2 patients) and one had IgA antibodies. In both the patient groups, the ADAMTS13:INH titers were consistently correlated with the IgG1 titers, and inconsistently with the titers of other IgG subclasses.
Both the patients in well ADAMTS13:AC-responders had IgG antibodies (IgG1 in both patients, IgG2 and IgG4 classes in one patient) and one had IgA antibodies. Over time, IgG and IgA antibody titers correlated with ADAMTS13:INH titers. Further, all 4 patients in poor ADAMTS13:AC-responders had IgG antibodies (IgG1 in 4 patients, IgG2 in 2 patients, and IgG4 in 2 patients) and one also had IgA antibodies, but none had IgM antibodies. Over time, IgG and IgA antibody titers correlated with ADAMTS13:INH titers.
Thus, in both the patient groups the ADAMTS13:INH titers were consistently correlated with the IgG1 titers, and inconsistently with the titers of other IgG subclasses.
Visualization of ADAMTS13:AG and its complex with ADAMTS13:INH (IgG) in plasma milieu during treatment
Plasma ADAMTS13:INH titers in aTTP patients correlated with titers of the IgG1 subclass. Further, despite severe deficiency of ADAMTS13:AC (<0.5% of normal) in all of our aTTP patients, these patients generally had low but variable levels of plasma ADAMTS13:AG (<0.1% to 84.8% of normal) (the supplemental Table 1 and 2).
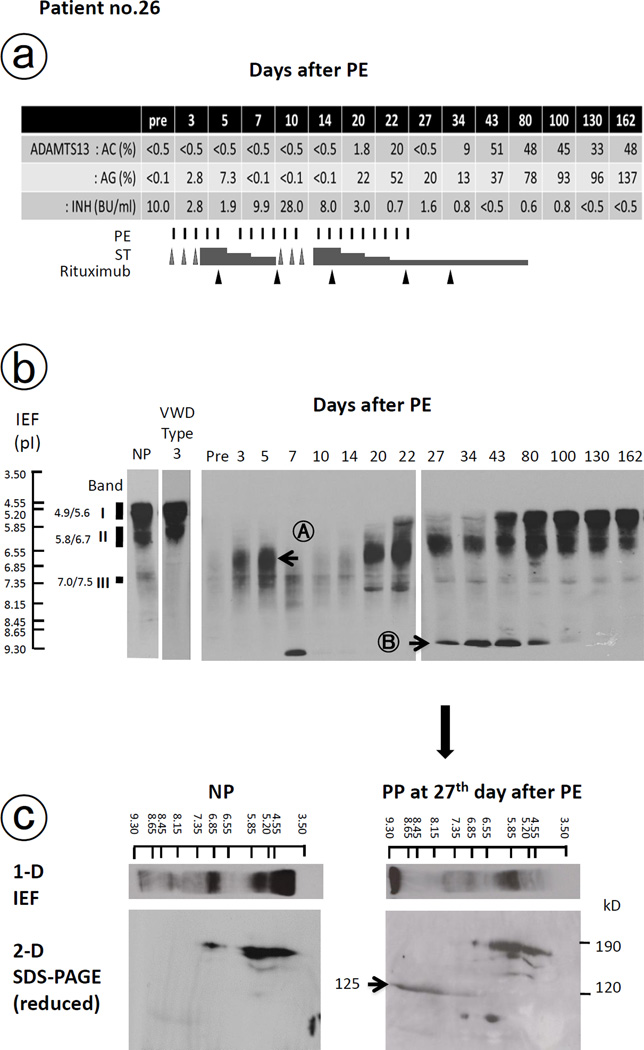
Most of the aTTP patients on admission had IEF patterns that differed from those of normal individuals (the supplemental Fig. 1). Notably, among the 52 aTTP patients, 3 poor ADAMTS13:AC-responders (patients no. 22, 26, and 47) had undetectably low plasma level of ADAMTS13:AG (<0.1% of normal). We report laboratory information herein for patient no. 26 (Fig. 3).
Fig. 3. Changes of ADAMTS13 parameters and isoelectric focusing (IEF) analysis of plasma ADAMTS13 antigen in patients with inhibitor boosting.
Changes of ADAMTS13 activity (:AC), antigen (:AG) and inhibitor (:INH) in addition to the therapy of patient with inhibitor boosting (patient no. 26) were shown in Fig. 3a. Before PE, this patient had no visible band of ADAMTS13:AG on IEF gel, and 3–5 days after initiation of PE, a new ADAMTS13 band at pI 6.0–7.3 (Band A) appeared, but on day 10 it almost totally disappeared again in accord with ‘inhibitor boosting’ as shown in Fig. 3b. After extensive treatment with PE, corticosteroid, and rituximab, ADAMTS13:AG patterns almost normalized on day 100 after PE initiation. Band B with pI >9.3 in Fig. 3b was identified as the degraded fragment of ADAMTS13:AG, showing by the presence of one ADAMTS13-related protein band (125 kD) by 2-dimensional analysis using sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis under reducing conditions in Fig. 3c.
VWD: von Willebrand disease, NP: normal plasma, PP: patient plasma,
Before PE, patient no. 26 had no visible band of ADAMTS13:AG on IEF gel, and 3–5 days after initiation of PE, a new ADAMTS13:AG band was detected at pI 6.0–7.3 (Band A). However, on day 10, the ADAMTS13:AG band was not detected in accord with ‘inhibitor boosting’ (Fig. 3b). During this period, plasma levels of ADAMTS13:AC were consistently below 0.5% of normal, suggesting that Band A was a complex of ADAMTS13:AG and ADAMTS13:INH (IgG). Band A was no longer detected after passing the patient’s plasma through a Protein G column to adsorb IgG (data not shown). After extensive treatment with PE, corticosteroid, and rituximab, ADAMTS13:AG patterns almost normalized on day 100 after PE initiation, along with improvements of other aTTP-related clinical and laboratory findings. In fact, aTTP remission was achieved at this point. Furthermore, Band B with pI >9.3 was identified as the degraded fragment of ADAMTS13:AG as shown by the presence of one ADAMTS13-related protein band (125kD) by 2-dimensional analysis, using sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis under reducing conditions (Fig. 3c).
Although remission was initially achieved in patient no. 26, the patient subsequently experienced a relapse 2 years later, that was featured by thrombocytopenia and an ADAMTS:13 INH titer of 4.6 BU/mL. This relapse was successfully treated with PE and corticosteroids.
Discussion
Using a large cohort of a TTP registry21, we conducted a retrospective analysis of 52 patients in Japan with aTTP. These patients were classified into two groups: 20 well ADAMTS13:AC-responders and 32 poor ADAMTS13:AC-responders, based on plasma levels of ADAMTS13:AC measured on the 14th day after PE initiation. aTTP patients who had ADAMTS13:AC levels less than 10% of normal on the 14th day after PE initiation were operationally considered to be ‘PE-refractory TTP’. We also characterized the nature of ADAMTS13:INH antibodies, and identified a complex of ADAMTS13:AG and ADAMTS13:INH (IgG), using a large-pore IEF gel analysis.13 In interpreting our findings, several factors should be considered.
First, measured pre-PE levels of ADAMTS13:INH appeared to have prognostic implications for measured ADAMTS13:AC levels following PE. As shown in Table 2, on admission, none of the clinical and laboratory markers except for the pre-PE levels of ADAMTS13:INH could predict well ADAMTS13:AC-responders versus poor ADAMTS13:AC-responders.
Table 2.
Comparison of laboratory markers between; well ADAMTS13:AC-responders and poor ADAMTS13:AC-responders to PE therapy
| on admission | at 14th day after PE | |||||
|---|---|---|---|---|---|---|
| ≥10% (well-responders) |
<10% (poor-responders) |
p Value | ≥10% (well-responders) |
<10% (poor-responders) |
p Value | |
| ADAMTS13 | ||||||
| : AC (%) | <0.5 | <0.5 | - | 31.7 (12.0–68.0) | 1.9 (<0.5–12.4) | <0.01 |
| : AG (%) | 12.3 (0.2–84.8) | 7.9 (<0.1–37.9) | 0.39 | 90.3 (36.0->100) | 42.1(0.1->100) | <0.01 |
| : INH (BU/ml) | 2.5 (1.0–5.6) | 5.7 (1.1–20.0) | <0.01 | 0.54(<0.5–0.9) | 6.9(0.7–51.0) | <0.01 |
| Neurologic | 0.23 | <0.01 | ||||
| symptoms (+) (−) |
15 (15/20) 5 (5/20) |
29 (29/32) 3 (3/32) |
0 (0/20) 20 (20/20) |
14 (14/32) 13(13/32) |
||
| Platelets (×109/L) | 12.3 (3–36) | 11.6 (3–54) | 0.78 | 225.1 (70–772) | 84.6 (3–330) | <0.01 |
| LDH (IU/ml) | 1696 (494–7768) | 1262 (478–3126) | 0.30 | 290 (165–891) | 536 (161–1941) | <0.05 |
| Hb(g/dl) | 7.3 (4.2–14.3) | 7.3 (4.9–10.1) | 0.99 | 9.1 (7.0–11.9) | 8.3 (5.1–12.6) | 0.11 |
| BUN (mg/dl) | 23.1 (9.5–58.7) | 26.5 (8.8–122.0) | 0.47 | 16.4 (3.7–32.0) | 26.1 (9.3–83.0) | <0.05 |
| Cre(mg/dl) | 0.88 (0.31–1.73) | 1.12 (0.39–7.70) | 0.33 | 0.65 (0.20–0.97) | 0.95 (0.43–4.30) | 0.10 |
mean (minimum–maximum)
Second, Ferrari et al.19 reported that IgG4 was the most prevalent IgG subclass in their sample of patients with aTTP (90%, 52/58), followed by IgG1 (52%), IgG2 (50%), and IgG3 (33%). They further showed that patients with high IgG4 and undetectable IgG1 levels were more prone to relapse than those with low IgG4 levels and detectable IgG1; they proposed that IgG4 could be a potentially useful indicator of patients at risk of relapse. In our serial study of 2 well ADAMTS13:AC-responders (without inhibitor boosting) and 4 poor ADAMTS13:AC-responders (with inhibitor boosting), ADAMTS13:INH titers were most strongly correlated with the IgG class, especially the IgG1 subclass. The difference in IgG subclass findings between the study by Ferrari et al.19,22 from Europe and our study from Japan is unclear, but predisposing genetic factors might be involved.
Third, we identified a complex of ADAMTS13:AG and ADAMTS13:INH (IgG) in patient plasma of poor ADAMTS13:AC-responders, using a large-pore IEF gel analysis. This finding has not been reported previously, because most of aTTP patients have low but a significant plasma level of ADAMTS13:AG (as shown in the supplemental Table 1 and 2). We reported 3 patients who were poor ADAMTS13:AC-responders (patients no. 22, 26, and 47) who had low levels of plasma ADAMTS13:AG (<0.1% of normal) before PE. Using the patient post-PE plasma level for patient no. 26, we reported that Band A was a complex of ADAMTS13:AG and ADAMTS13:INH (IgG), and Band B was the fragment of ADAMTS13:AG, as evidenced by a 2-dimentional IEF/SDS-PAGE analysis (Fig. 3). These results indicate that the ADAMTS13:AG-ADAMTS13:INH complex undergoes proteolytic modification, by which ADAMTS13:AG disappears from circulation.
We conclude that ADAMTS13:INH boosting is a frequent occurrence noted among aTTP patients, primarly occurring among aTTP patients who were poor ADAMTS13:AC-responders. Our results also identified that high ADAMTS13:INH titers (approximately >5 BU/ml) measured prior to PE might be a good indicator to predict poor ADAMTS13:AC-responders, for whom earlier administration of intensive immunosuppressive therapy such as rituximab appeared to facilitate reduction of the time and volume of PE. Further, durable remission of aTTP in patients who had measurable levels of ADAMTS13:AC may be more consistently identified by a new laboratory marker consisting of normal ADAMTS13:AG IEF patterns in the plasma milieu, but this does not guarantee freedom from relapse in the long term.
Supplementary Material
ADAMTS13:AG exists in plasma milieu as 3 major bands shown by IEF analysis; Band I (free or unbound to VWF), Band II (not featured), and Band III (bound to high-molecular-weight VWF) as shown in normal plasma (NP). However, our aTTP patients in both patients with well ADAMTS13:AC-responders (upper panel) and poor ADAMTS13:AC-responders (lower panel) had IEF patterns totally different from those of normal individuals. Among 52 our aTTP patients, 3 poor ADAMTS13:AC-responders (patient no. 22, 26, and 47) had undetectably low plasma level of ADAMTS13:AG (<0.1% of normal). Thus, we chose patient no. 26 as a representative to analyze an interaction between ADAMTS13:AG and ADAMTS13:INH (IgG) in plasma milieu using IEF as shown in Fig. 3.
Acknowledgements
The authors would like to express our gratitude to Dr. Kenji Soejima, who kindly provided anti-ADAMTS13 mAb (WH2-11-1) to us. This study was supported in part by research grants from the Ministry of Health, Labor, and Welfare of Japan, from the Ministry of Education, Culture, Sports, Science and Technology of Japan, from the Takeda Science Foundation, and from the National Heart Lung and Blood Institute (1R01 HL-096717) and the Doris Levkoff Meddin Center Medication Safety.
ABBREVIATIONS
- aTTP
primary acquired thrombotic thrombocytopenic purpura
- ADAMTS13
a disintegrin-like and metalloprotease with thrombospondin type-1 motifs 13
- VWF
von Willebrand factor
- IEF
isoelectric focusing
Footnotes
Disclosure of conflict of Interests
AI, CLB, MM, and YF: None. FS and BP are employees of Baxter Bioscience.
Addendum
A. Isonishi: performed research, analyzed data and wrote the manuscript. C.L. Bennett: interpreted data and wrote manuscript. F. Scheiflinger and B. Plaimauer: material support and reviewed manuscript. M. Matsumoto: collected and analyzed data, and wrote the manuscript. Y. Fujimura: designed study concept and interpreted data, and wrote the manuscript.
References
- 1.Amorosi EL, Ultmann JE. Thrombotic thrombocytopenic purpura:report of 16 cases and review of the literature. Medicine. 1966;45:139–159. [Google Scholar]
- 2.Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U, Solenthaler M, Lammle B. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339:1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 3.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–1594. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Ginsburg D, Tsai HM. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 5.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, López JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 6.Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112:11–18. doi: 10.1182/blood-2008-02-078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blombery P, Scully M. Management of thrombotic thrombocytopenic purpura: current perspectives. J Blood Med. 2014;5:15–23. doi: 10.2147/JBM.S46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppo P, Bussel A, Charrier S, Adrie C, Galicier L, Boulanger E, Veyradier A, Leblanc T, Alberti C, Azoulay E, Le Gall JR, Schlemmer B. High-dose plasma infusion versus plasma exchange as early treatment of thrombotic thrombocytopenic purpura/hemolytic-uremic syndrome. Medicine (Baltimore) 2003;82:27–38. doi: 10.1097/00005792-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Böhm M, Betz C, Miesbach W, Krause M, von Auer C, Geiger H, Scharrer I. The course of ADAMTS-13 activity and inhibitor titre in the treatment of thrombotic thrombocytopenic purpura with plasma exchange and vincristine. Br J Haematol. 2005;129:644–652. doi: 10.1111/j.1365-2141.2005.05512.x. [DOI] [PubMed] [Google Scholar]
- 10.Coppo P, Wolf M, Veyradier A, Bussel A, Malot S, Millot GA, Daubin C, Bordessoule D, Pène F, Mira JP, Heshmati F, Maury E, Guidet B, Boulanger E, Galicier L, Parquet N, Vernant JP, Rondeau E, Azoulay E, Schlemmer B, l'Adulte RdEdMTd. Prognostic value of inhibitory anti-ADAMTS13 antibodies in adult-acquired thrombotic thrombocytopenic purpura. Br J Haematol. 2006;132:66–74. doi: 10.1111/j.1365-2141.2005.05837.x. [DOI] [PubMed] [Google Scholar]
- 11.Scully M, McDonald V, Cavenagh J, Hunt BJ, Longair I, Cohen H, Machin SJ. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118:1746–1753. doi: 10.1182/blood-2011-03-341131. [DOI] [PubMed] [Google Scholar]
- 12.Westwood JP, Webster H, McGuckin S, McDonald V, Machin SJ, Scully M. Rituximab for thrombotic thrombocytopenic purpura: benefit of early administration during acute episodes and use of prophylaxis to prevent relapse. J Thromb Haemost. 2013;11:481–490. doi: 10.1111/jth.12114. [DOI] [PubMed] [Google Scholar]
- 13.Hori Y, Hayakawa M, Isonishi A, Soejima K, Matsumoto M, Fujimura Y. ADAMTS13 unbound to larger von Willebrand factor multimers in cryosupernatant: implications for selection of plasma preparations for thrombotic thrombocytopenic purpura treatment. Transfusion. 2013;53:3192–3202. doi: 10.1111/trf.12182. [DOI] [PubMed] [Google Scholar]
- 14.Kato S, Matsumoto M, Matsuyama T, Isonishi A, Hiura H, Fujimura Y. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion. 2006;46:1444–1452. doi: 10.1111/j.1537-2995.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 15.Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115:1500–1511. doi: 10.1182/blood-2009-09-243790. quiz 662. [DOI] [PubMed] [Google Scholar]
- 16.Froissart A, Buffet M, Veyradier A, Poullin P, Provôt F, Malot S, Schwarzinger M, Galicier L, Vanhille P, Vernant JP, Bordessoule D, Guidet B, Azoulay E, Mariotte E, Rondeau E, Mira JP, Wynckel A, Clabault K, Choukroun G, Presne C, Pourrat J, Hamidou M, Coppo P, Center FTMR. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit Care Med. 2012;40:104–111. doi: 10.1097/CCM.0b013e31822e9d66. [DOI] [PubMed] [Google Scholar]
- 17.Kasper CK, Pool JG. Letter: Measurement of mild factor VIII inhibitors in Bethesda units. Thromb Diath Haemorrh. 1975;34:875–876. [PubMed] [Google Scholar]
- 18.Yagi H, Ito S, Kato S, Hiura H, Matsumoto M, Fujimura Y. Plasma levels of ADAMTS13 antigen determined with an enzyme immunoassay using a neutralizing monoclonal antibody parallel ADAMTS13 activity Levels. Int J Hematol. 2007;85:403–407. doi: 10.1532/IJH97.06210. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari S, Mudde GC, Rieger M, Veyradier A, Kremer Hovinga JA, Scheiflinger F. IgG subclass distribution of anti-ADAMTS13 antibodies in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2009;7:1703–1710. doi: 10.1111/j.1538-7836.2009.03568.x. [DOI] [PubMed] [Google Scholar]
- 20.Soejima K, Nakamura H, Hirashima M, Morikawa W, Nozaki C, Nakagaki T. Analysis on the molecular species and concentration of circulating ADAMTS13 in Blood. J Biochem. 2006;139:147–154. doi: 10.1093/jb/mvj013. [DOI] [PubMed] [Google Scholar]
- 21.Fujimura Y, Matsumoto M. Registry of 919 patients with thrombotic microangiopathies across Japan: database of Nara Medical University during 1998–2008. Intern Med. 2010;49:7–15. doi: 10.2169/internalmedicine.49.2706. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari S, Palavra K, Gruber B, Kremer Hovinga JA, Knöbl P, Caron C, Cromwell C, Aledort L, Plaimauer B, Turecek PL, Rottensteiner H, Scheiflinger F. Persistence of circulating ADAMTS13-specific immune complexes in patients with acquired thrombotic thrombocytopenic purpura. Haematologica. 2014;99:779–787. doi: 10.3324/haematol.2013.094151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ADAMTS13:AG exists in plasma milieu as 3 major bands shown by IEF analysis; Band I (free or unbound to VWF), Band II (not featured), and Band III (bound to high-molecular-weight VWF) as shown in normal plasma (NP). However, our aTTP patients in both patients with well ADAMTS13:AC-responders (upper panel) and poor ADAMTS13:AC-responders (lower panel) had IEF patterns totally different from those of normal individuals. Among 52 our aTTP patients, 3 poor ADAMTS13:AC-responders (patient no. 22, 26, and 47) had undetectably low plasma level of ADAMTS13:AG (<0.1% of normal). Thus, we chose patient no. 26 as a representative to analyze an interaction between ADAMTS13:AG and ADAMTS13:INH (IgG) in plasma milieu using IEF as shown in Fig. 3.