Abstract
Long-term alcohol use leads to persistent cognitive deficits that may be associated with maladaptive changes in the neurocircuitry that mediates executive functions. Impairments caused by these changes can persist well into abstinence and have a negative impact on quality of life and job performance, and can increase the probability of relapse. Many of the changes that affect cognitive function appear to involve dysregulation of the mesocortical dopamine system. This includes changes in dopamine release and alterations in dopamine receptor expression and function in the medial prefrontal cortex (PFC). This review summarizes the cellular effects of acute and chronic ethanol exposure on dopamine release and dopamine receptor function in the PFC with the goal of providing greater understanding of the effects of alcohol-use disorders on the dopamine system and how this relates to deficits in the executive function of the PFC.
Keywords: dopamine, prefrontal cortex, alcohol, cognition
1. Introduction
Cognitive dysfunction commonly occurs as a result of prolonged alcohol exposure and can persist well into abstinence, causing significant impairments in executive processes such as top-down inhibitory control, decision-making, and behavioral flexibility. Each of these aspects of executive function relies on a balance of excitatory and inhibitory activity in the prefrontal cortex (PFC) that supports synchronous activity of cellular networks within the cortex and with subcortical structures that mediate these processes. This balance is critically dependent upon a precise level of dopamine and dopamine receptor activity in this brain region, which tunes the networks by facilitating synchronous activation and by limiting activity in space and time to provide specificity over the flow of information in these networks (Arnsten, Cai, Murphy, & Goldman-Rakic, 1994; Floresco, 2013; Goldman-Rakic, 1998). Chronic alcohol-induced alterations in dopamine signaling produce deficits in executive function that not only affect quality of life, but also increase the probability of relapse to alcohol drinking (Fein, Bachman, Fisher, & Davenport, 1990; Rando et al., 2011). Studies in human alcoholics have demonstrated that higher levels of dopamine (DA) receptor binding in PFC may be protective against developing alcohol-use disorder (AUD) (Volkow et al., 2006). Human imaging studies have also reported decreases in prefrontal volume of alcoholics; the severity of such changes was also correlated with higher probability of relapse (Rando et al., 2011). Furthermore, alcoholics and control subjects show similar fMRI activity and similar performance on simpler tasks that do not require high cognitive demand. However, increased fMRI activity in frontal regions of alcoholics is observed as cognitive load increases that does not support enhanced performance on the task, but instead results in larger disparities between control and alcohol-dependent subjects (Parsons & Nixon, 1998).
Similar observations have been made in rodent models of alcohol dependence where confounds such as genetic predisposition and environmental influences can be controlled (Trantham-Davidson et al., 2014). A detailed understanding of the cellular effects of alcohol that contribute to cognitive dysfunction is important for the development of novel therapeutic strategies aimed at the mesocortical dopamine system to improve cognitive function and treat AUDs.
2. Dopamine and DA receptors
Dopamine (DA) is a catecholamine neurotransmitter that is primarily (although not exclusively) synthesized in the substantia nigra and ventral tegmental area (VTA). The release of DA from the substantia nigra principally controls motor function by modulating the activity of brain structures that make up the direct and indirect pathways that subserve planned motor sequences. The primary reward system in the brain is the VTA dopamine system. The VTA sends DAergic afferents to brain regions such as the PFC and nucleus accumbens (NAcc) that process cues associated with obtaining natural rewards such as food and various other forms of goal-directed behaviors. Exposure to substances that have rewarding properties, including alcohol and other drugs of abuse, enhances DA release from the VTA to subcortical structures such as the nucleus accumbens and promotes reward-seeking behavior. However, with prolonged exposure to addictive substances, persistent elevations in DA result in compensatory changes that take place in both subcortical and cortical regions that appear to promote anhedonia during withdrawal and prolonged deficits in cognitive function.
The VTA is a highly heterogeneous region that includes neurons that release dopamine, glutamate, or GABA, as well as neurons that co-release DA with glutamate or GABA. The GABAergic neurons make up a small percentage of cells in this region (approximately 20%) and include both local interneurons and projection neurons that modulate other brain structures. The interneurons play a very important role in tonic inhibition of activity of VTA DAergic and glutamatergic projection neurons that mediate reward signaling, and it appears that alcohol-induced changes in GABAergic activity in the VTA can indirectly affect DA and glutamate release to other brain regions. Therefore, contrary to the long-held view of the VTA as principally consisting of dopaminergic nuclei, it is becoming increasingly apparent that it may be more appropriately viewed as a heterogeneous structure composed mainly of amino-acid releasing neurons but with an additional DA-releasing component (Gorelova, Mulholland, Chandler, & Seamans, 2012). Regardless of the complexity of the neurotransmitter systems of the VTA, it is clear that DA plays a critical role in the rewarding effect of drugs of abuse, and that changes in this system may underlie many aspects of addiction (for review see Lapish, Seamans, & Chandler, 2006).
3. Acute ethanol enhances dopamine release
Extracellular levels of DA in target regions of the VTA such as the PFC and NAcc are controlled by two distinct firing patterns (Bannon & Roth, 1983; Chiodo, 1988; Chiodo, Bannon, Grace, Roth, & Bunney, 1984; Hoffman & Beninger, 1988; White & Wang, 1984). The tonic or low-frequency firing mode results in low ambient DA levels in target regions (Phillips & Wightman, 2004). In contrast, the phasic mode is associated with a much higher firing frequency that results in a transient increase in DA. This is thought to signal salience that is time-locked to the appearance of rewarding stimuli and reward-related cues. These initial observations of phasic DA signaling associated with reward learning lead to the development of the incentive salience hypothesis for the function of VTA DA. In particular, the pioneering studies by Shultz and colleagues revealed that in the early stages of learning a new task, DA neurons fire in phasic burst patterns in response to the presentation of a reward (Schultz, 1994, 1999). Over time, as the stimulus-action-reward relationship is encoded, the bursting mode of firing in DA neurons shifts to become time-locked to presentation of the stimulus or visual cue. If a reward is not delivered when it is expected, DA neuron firing falls below tonic/basal levels, signaling a reward-prediction error. This suggests that these fluctuations in DA cell firing support learning by providing information about the cues associated with rewards as well as the time when the stimuli no longer predict reward delivery, and this promotes engagement of a different strategy to obtain rewards.
Acute exposure to alcohol results in both reduced anxiety and a pleasurable hedonic state due to its rewarding properties. Together, these are thought to provide the subjective experience of alcohol intoxication that initially signals alcohol as a rewarding substance. While the anxiolytic effect of alcohol is due in part to its effects on various neurotransmitter systems (e.g., GABA or glutamate), increased DA release also contributes to reduced anxiety and mediates the reinforcing properties of ethanol. There are likely multiple mechanisms by which acute ethanol can enhance DA release that involve direct effects of ethanol on intrinsic excitability of VTA DA neurons. Acute ethanol itself has direct effects on DA VTA neurons that result in higher firing frequency and increased excitability. Under baseline conditions in the absence of acute alcohol, DA neurons in the VTA fire in spontaneous, low-frequency pacemaker-like bursts that occur in the absence of outside synaptic inputs. The pacemaker frequency is set by an inward cation current, termed Ih, that is activated by hyperpolarization (Neuhoff, Neu, Liss, & Roeper, 2002). Acutely, ethanol enhances this current resulting in increased firing and likely enhancement of dopamine release in target regions such as the NAcc core (NAccC) and PFC (Brodie & Appel, 1998; Okamoto, Harnett, & Morikawa, 2006). Recent work has shown that when non-pacemaker DA neurons (i.e., they do not exhibit Ih) are examined, ethanol also enhances firing in this population (Mrejeru, Martí-Prats, Avegno, Harrison, & Sulzer, 2015). This suggests that while the overall effect of acute ethanol is to increase DA release to target regions, the mechanism of how this occurs may be circuit-specific (Lammel et al., 2008; Mrejeru et al., 2015; Neuhoff et al., 2002).
In addition to its effects on intrinsic excitability, acute ethanol can also modulate GABAergic transmission and disinhibit DA neurons in the VTA. Since elevated levels of DA are observed in areas like the NAcc and PFC following ethanol exposure, one possibility is that DA neurons in the VTA are disinhibited from tonic control over their firing by GABAergic neurons in this region. Ethanol has different effects on GABAergic transmission in the VTA depending on which cell population is assessed, with some GABAergic neurons showing enhanced firing and others reduced firing (Xiao & Ye, 2008). When firing of GABAergic neurons of the VTA is attenuated, which presumably enhances DA release, it appears this is a delayed effect with respect to the time of ethanol administration such that the DA neurons respond to ethanol before the GABAergic cells respond. This suggests that while the increase in DA cell firing is not initially dependent on disinhibition, the prolonged reinforcing effects of alcohol may depend on this delayed and more persistent disinhibitory effect that maintains reinforcement and supports continued ethanol consumption (Adermark, Söderpalm, & Burkhardt, 2014; Burkhardt & Adermark, 2014; van Zessen, Phillips, Budygin, & Stuber, 2012). However, electrophysiological slice experiments revealed that acute bath application of alcohol increases firing only in a subset of VTA neurons that lack D2 autoreceptors. Again, this interesting observation suggests that acute ethanol may have distinct, circuit-specific effects, since PFC projections from the VTA lack D2 autoreceptors (Lammel et al., 2008; Mrejeru et al., 2015).
Interestingly, voltammetry experiments, where DA levels were measured in mPFC, have shown both increases (Robinson, Howard, McConnell, Gonzales, & Wightman, 2009) and decreases in mPFC DA release in response to acute ethanol administration (Shnitko, Kennerly, Spear, & Robinson, 2014). The opposing effects appear to be due to different firing modes such that under baseline firing conditions in the VTA, ethanol appears to enhance DA release in mPFC, but when firing is electrically stimulated, ethanol has an attenuating effect on mPFC DA. This appears to be due to differences in DA clearance or availability when the different firing modes are being examined (S. R. Wang et al., 2011). Another explanation is that while acute ethanol may increase firing rate in some VTA neurons, the amount of DA that is released with each stimulated pulse is actually reduced by acute ethanol. However, in an area like the nucleus accumbens where DA is cleared by the dopamine transporter (DAT), the clearance rate is attenuated by acute ethanol, ultimately producing increased DA levels. In contrast, in the PFC, where DA is cleared by the norepinephrine transporter and the enzyme COMT, acute ethanol may influence DA levels differently due to distinct ways in which DA is cleared from the synapse.
Additionally, a “first hit” hypothesis has been proposed in which metabolites of alcohol have reinforcing properties that may be distinct from the effects of alcohol itself (Israel, Quintanilla, Karahanian, Rivera-Meza, & Herrera-Marschitz, 2015). Acetaldehyde is a metabolite of alcohol that is formed by the oxidization of ethanol by alcohol dehydrogenase and has been shown to have its own reinforcing properties. For example, alcohol-preferring P rats will self-administer acetaldehyde into the posterior VTA (Rodd et al., 2005a, 2005b) and stimulate the release of DA into the NAcc (Deehan, Engleman, Ding, McBride, & Rodd, 2013; Deehan, Hauser, Wilden, Truitt, & Rodd, 2013). In addition, reducing acetaldehyde levels by decreasing catalase activity was shown to prevent the DA signal in the NAcc (Karahanian et al., 2015), indicating that acetaldehyde alone is sufficient to produce this reward signal. Follow-up studies have further shown that ethanol-induced DA release in NAcc could be blocked by reducing acetaldehyde levels in the VTA (Karahanian et al., 2011, 2015). This suggests that acetaldehyde, at least in part, directly contributes to the reinforcing properties of ethanol. In addition, an indirect mechanism for acetaldehyde facilitation of release could be the ability of acetaldehyde and DA to directly react to form the product salsolinol (Myers, 1985). Of note, salsolinol has been shown to support self-administration when delivered directly into the posterior VTA (Rodd et al., 2008). Interestingly, follow-up studies using patch clamp electrophysiology revealed that salsolinol acts at μ-opioid receptors on GABAergic neurons in the VTA to hyperpolarize the membrane potential, resulting in disinhibition of DA-releasing neurons and presumably elevated DA release to NAcc (Palm & Nylander, 2014; Xie et al., 2012). The ability of salsolinol to promote DA release and drinking is controversial, however, possibly due to difficulties in measuring brain concentrations of the compound. Newer methods of measurement will likely yield future pharmacological studies that will clarify its role in promoting drinking and the mechanisms which mediate its effects (Hipólito, Sánchez-Catalán, Martí-Prats, Granero, & Polache, 2012).
4. Chronic alcohol decreases DA release
While the effect of acute ethanol is to initially increase spontaneous DA transmission and promote reward (Robinson et al., 2009; Schier, Dilly, & Gonzales, 2013), allostatic changes in the DA circuitry occur with prolonged exposure. Interestingly, while somewhat normal levels of functioning in daily life can be observed under the influence of alcohol in chronic users (Koob, 2003a, b), anhedonia, aversion, and blunted reward processing accompany alcohol withdrawal that may relate to a hypodopaminergic state in target areas like cortex and nucleus accumbens (Koob & Volkow, 2010; Trevisan et al., 1998). While little is known about the mechanisms that mediate the decrease in DA transmission, one possible explanation is that chronic ethanol exposure decreases Ih current density, resulting in reduced firing of VTA DA neurons that has been observed during withdrawal (Diana et al., 1993; Okamoto et al., 2006). Sensitization of DA D2 autoreceptors has been shown to reduce DA release in monkeys (Budygin et al., 2003) and mice (Karkhanis et al., 2015), and to reduce DA release and increase uptake in the NAcc core in mice following exposure to chronic ethanol (Karkhanis et al., 2015). Taken together, these findings are consistent with the idea that chronic ethanol-induced attenuation of DA transmission may mediate the aversive state of withdrawal and, as discussed below, may contribute to cognitive deficits that are associated with increased vulnerability to relapse during abstinence.
5. Cognition relies on appropriate stimulation of DA receptors in PFC
The medial prefrontal cortex (mPFC) is generally divided into ventral and dorsal aspects that serve specific roles in mediating distinct components of executive function. In the rodent mPFC, the dorsal portion is composed of the anterior cingulate (ACC) and prelimbic regions, and the ventral aspect contains the infralimbic and orbitofrontal regions. The anterior cingulate cortex is primarily responsible for behavioral inhibition and decision-making when emotion-related cues can be used to guide responding. The prelimbic cortex is just ventral to the ACC and it has been implicated in execution of goal-directed behaviors and likely subserves some aspects of working memory. Ventral to the PrL cortex, the infralimbic cortex is generally thought to serve an opposing role on the action sequences generated by the PrL region. In particular, its outputs to the NAcc shell and BLA are thought to inhibit drug seeking and fear responding, respectively (Peters, Dieppa-Perea, Melendez, & Quirk, 2010; Peters, LaLumiere, & Kalivas, 2008). Interestingly, more recent studies into the circuitry of habitual responding reveal that the IfL outputs to NAcc shell also mediate habitual responding, and dopaminergic modulation of this pathway can significantly impact habitual vs. goal-directed responding (Barker, Taylor, & Chandler, 2014). The orbital frontal cortex mediates behavioral flexibility and valuation of reinforcers to promote formation of strategies aimed at maximizing reward magnitude. Dopamine afferents project to each of these cortical regions with a larger percentage of projection neurons targeting the deeper layers in addition to relatively less dense innervation to superficial layers. In addition, DA receptors are expressed on both excitatory and inhibitory neurons and can significantly modulate synchronization of network activity as well as overall activation and synaptic responses in both cell types.
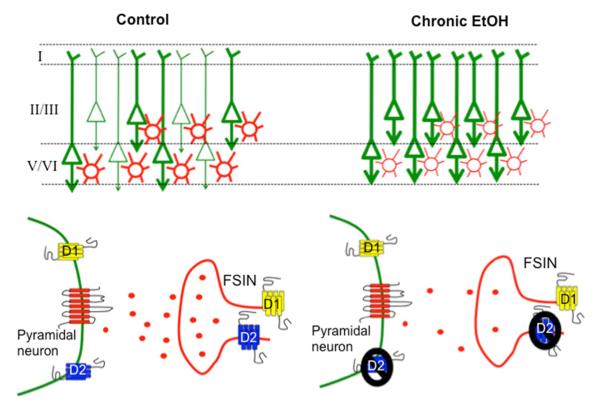
In the mPFC, stimulation of DA receptors influences neuronal firing in different ways that relate in part to the G-protein coupled signaling pathways associated with the different types of DA receptors. DA D1-like receptors, which include the D1 and D5 subtypes, are coupled to Gs and Gq proteins and enhance activation of PKA signaling. In both pyramidal neurons and GABAergic interneurons in the PFC, PKA activation results in increased firing and enhancement of glutamate and GABA-evoked currents (Chen, Bohanick, Nishihara, Seamans, & Yang, 2007; Gonzalez-Islas & Hablitz, 2001, 2003). Activation of DA D2-like receptors, which include the D2, D3, and D4 subtypes, is coupled to Gi/o proteins and inhibits PKA activity. In pyramidal neurons, inhibition of PKA results in decreased firing and attenuation of glutamatergic and GABAergic responses (Gonzalez-Islas & Hablitz, 2001; Wang, Zhong, & Yan, 2002). A potential cellular mechanism for the loss of behavioral flexibility observed in rats following chronic ethanol administration is the loss of D2/D4 receptor modulation of cellular targets such as glutamate receptors, GABA receptors, and ion channels that modulate excitability of pyramidal neurons (Fig. 1). While no changes in D2/D4 receptor expression were observed, it is possible that the G-protein coupled signaling pathways downstream of the D2/D4 receptors are affected by ethanol, and this disrupts the synchronization of pyramidal networks that mediates some aspects of executive function, such as behavioral flexibility (Trantham-Davidson et al., 2014).
Fig. 1.
Cellular mechanisms that mediate alterations in mPFC synchrony and cognitive disruption following chronic ethanol exposure. Under control conditions (left panel), synchronous activation of appropriate cortical networks mediates cognitive control over behavior. At the cellular level this is achieved via dopamine modulation of intrinsic and synaptic currents within pyramidal neurons (green) and FSINs (red) to promote temporal and spatial precision over networks to increase specificity of information flow by synchronizing specific groups of neurons (symbolized by bold lines). Following chronic alcohol exposure (right panel), network synchrony is disrupted due to the reduction in D2/D4 receptor modulation of excitability of pyramidal neurons and FSINs. The loss of D2/D4 receptor-mediated recruitment of FSINs (lighter red color) results in desynchronization of pyramidal networks and loss of specificity over information flow (all pyramidal cells in bold green).
In contrast to pyramidal neurons, the effects of D2-like receptors on interneurons in the PFC is not as clear cut, in large part due to the wide diversity of interneuron types. The interneuron subtype in the PFC that appears to be most responsive to D2/D4 receptor stimulation is the parvalbumin-positive, fast-spiking subtype (Gorelova, Seamans, & Yang, 2002). These cells undergo a developmental transition in responding to D2 receptor stimulation during adolescence that occurs in parallel with the maturation of cognitive function during the transition to adulthood. In rodents, D2 receptor modulation of the activity of fast-spiking interneurons (FSINs) is not observed until postnatal day 45 and this modulation increases until it plateaus in early adulthood (~postnatal day 60) (Tseng & O'Donnell, 2007a). Another interesting property of FSINs is that their activation is critical for the generation of gamma oscillations and the synchronous, recurrent excitatory activity between pyramidal neurons that is hypothesized to mediate working memory (Curley & Lewis, 2012; Lewis, Curley, Glausier, & Volk, 2012). FSINs have a distinct firing profile that includes short-duration action potentials and high firing frequency that is thought to be necessary for recruiting large populations of neurons simultaneously and with high temporal specificity. These neurons contain potassium channels of the Kv 3.1/3.2 family that confer these firing characteristics by limiting action potential duration and shortening the after-hyperpolarization to produce high firing rates (Harvey, Lau, Civillico, Rudy, & Contreras, 2012). Our laboratory recently showed that D4 receptor stimulation enhances firing in FSINs at least in part by enhancing the Kv3.2-mediated current. In addition, our data revealed that in slices from rats exposed to chronic ethanol, this D4-mediated enhancement of firing and Kv3.2 currents is absent but remains normal in control animals. This uncoupling of D4 receptors from their targets that modulate intrinsic excitability may explain the cognitive deficits that persist following chronic ethanol exposure, due to reductions in gamma oscillations that are critical for behavioral flexibility (Trantham-Davidson et al., 2014). These findings present another possibility for the cellular mechanisms that might mediate the loss of behavioral flexibility observed in rats that have received chronic alcohol exposure (Fig. 1), and restoring activity selectively in these neurons may restore at least some aspects of cognitive function.
Many aspects of cognitive function such as behavioral flexibility, planning of goal-directed strategies, and inhibitory control are dependent upon a balance of activation of D1 and D2 receptors in the PFC (Arnsten et al., 1994; Floresco & Magyar, 2006; Gao, Wang, & Goldman-Rakic, 2003). While earlier work established an inverted U-shaped relationship for DA levels such that optimal levels of DA transmission are needed to facilitate working memory, more recent work suggests that other cognitive processes such as behavioral flexibility and decision-making require different levels of tone on D1 and D2 receptors for optimal performance, and the amount needed depends on the specific aspect of cognition that is needed to perform the task (Floresco, 2013).
The prefrontal cortex receives dense DAergic projections to the deep layers of the medial regions, especially in the prelimbic and infralimbic regions that are involved in planning, execution of goal-directed behaviors, and habit formation (Arnsten & Li, 2005; Floresco & Phillips, 2001; Goldman-Rakic, 1996; Hitchcott, Quinn, & Taylor, 2007; Robbins, 2000; Roesch-Ely et al., 2005). Transient increases in DA stimulate D1 receptors to enhance both glutamatergic and GABAergic inhibition to increase synchronization and promote network excitability (Kroener, Chandler, Phillips, & Seamans, 2009; Lapish, Durstewitz, Chandler, & Seamans, 2008). This results in more efficient processing of highly salient/reward-related items in the environment, and this has been termed the D1-dominated state. In contrast, lower, ambient levels of DA target D2 receptors that decrease excitatory and inhibitory influences so that multiple items in the environment can be attended to at once. Because of this, networks under the influence of this D2-dominated state can flexibly respond to changes in environmental cues to execute updated strategies aimed at obtaining reinforcers (Durstewitz, Seamans, & Sejnowski, 2000).
6. Effects of chronic alcohol on PFC and striatum
Relatively little is known about the effects of chronic alcohol exposure on DA transmission in the PFC. There is an increasing appreciation of the critical role of cognitive function in addiction and relapse that has emphasized the need to gain a greater understanding of how alcohol affects DA signaling in PFC. Cognitive deficits in human alcoholics and enhanced risk for developing alcohol-use disorders may result, at least in part, from alterations in D2 receptor expression in dlPFC and striatum (Kraschewski et al., 2009; Volkow et al., 1996, 2002). Furthermore, low D2 receptor function has been shown to increase alcohol consumption in rodent models of alcohol dependence (Bice et al., 2006; Morganstern & Tejani-Butt, 2010). High levels of D2 receptor expression may protect against alcoholism and have been shown to reduce drinking in human subjects (Kraschewski et al., 2009; Volkow et al., 2006). To date, most of the studies of chronic ethanol-induced changes in DA receptor function have focused on striatal changes and very few have focused on changes in PFC. However, the more recent appreciation of the important role that cognitive dysfunction plays in addiction has suggested that changes in DA receptors in PFC may accompany these changes that occur in striatum. Consistent with this idea, rats that received chronic exposure to alcohol showed a reduction of D2 receptor function in PFC in both pyramidal neurons and fast-spiking interneurons that was accompanied by deficits in performance of PFC-dependent measures of cognitive flexibility (Trantham-Davidson et al., 2014). These observations are in general agreement with recent studies examining prefrontal function in chronic alcohol-exposed mice (Holmes et al., 2012; Kroener et al., 2012). Cognitive flexibility relies on proper tuning of excitatory networks of pyramidal neurons that is critically modulated by GABAergic inputs from fast-spiking interneurons (FSINs), and as discussed above, DA modulates the activity of both cell types through activation of D1 and D2-like receptors (Adell & Artigas, 2004; Trantham-Davidson, Kröner, & Seamans, 2008; Trantham-Davidson & Lavin, 2004). Interestingly, the reduction of D2 and D4 receptor function that we recently reported appeared immediately after cessation of chronic alcohol exposure and remained attenuated for up to 4 weeks after the last exposure to alcohol (Trantham-Davidson et al., 2014). Although speculative, it is reasonable to suggest that this loss of D2 receptor function could result in dysregulation of both persistent network activity and tuning of those networks. Interestingly, since there were no differences in D2 or D4 receptor expression as measured by receptor autoradiography, a likely explanation for the observed loss of D2/D4 function in the PFC is an uncoupling of these receptors from their signaling pathways. This uncoupling could result in alterations in phosphorylation of downstream targets that modulate activity. In the adult PFC, D2/D4 receptor stimulation increases firing in FSINs (Tseng & O'Donnell, 2007b), resulting in more precise regulation over pyramidal cell networks. Therefore, attenuated D2 receptor stimulation of FSINs would negatively affect prefrontal function and could contribute to cognitive deficits observed in abstinent alcoholics that appear to play a role in relapse.
7. Developmental changes in the DA system
The prefrontal DA system undergoes significant changes that primarily begin during adolescence that continue into early adulthood (Yetnikoff, Reichard, Schwartz, Parsely, & Zahm, 2014). This critical period of developmental change may render this system relatively vulnerable to environmental insults during childhood and adolescence. This may be especially important for the effects of alcohol exposure given the high prevalence of drinking during adolescence, especially binge-like consumption. In rats, the DA innervation of striatum occurs during embryogenesis but continues to develop in cortex throughout adolescence and into early adulthood (Kalsbeek, Voorn, Buijs, Pool, & Uylings, 1988). The expression of DA receptors in the striatum also peaks at P50 and then decreases until P90. In striatum and PFC, DA receptor expression appears to follow a similar developmental trajectory such that the system is relatively vulnerable well into adulthood (Tarazi & Baldessarini, 2000). Exposure to alcohol during these critical periods of development could significantly affect formation of dopaminergic synapses and development of cortical and striatal circuitry that critically regulate cognitive function and reward-related behavior in a way that permanently damages the system. Consistent with this, we recently showed that adolescent exposure to alcohol results in deficits in behavioral flexibility on several PFC-dependent tasks that might relate, at least in part, to changes in dopaminergic modulation of cortical activity.
8. Conclusion
Chronic alcohol-induced disruption of dopamine modulation of prefrontal activity plays a major role in the cognitive dysfunction that persists well into abstinence and may contribute to the high probability of relapse in dependent individuals. Despite this knowledge, relatively little is known about the mechanisms by which this occurs. While recent evidence suggests these deficits in cognitive control of behavior may be related to altered dopamine release and disruption of DA receptor functioning in the PFC, a better understanding is needed that can guide the development of highly selective pharmacological approaches to restore prefrontal function. Therapeutic restoration of cognitive control of behavior has the promise of reducing the rate of relapse and improving quality of life in individuals who suffer from alcohol-use disorders.
Highlights.
Acute exposure to alcohol results in increases in DA transmission in the striatum and PFC that contribute to the reinforcing properties of alcohol and controlled drinking patterns.
Chronic exposure to alcohol results in decreased DA transmission that leads to cognitive dysfunction and the anhedonic state of withdrawal that may contribute to relapse.
Pharmacological approaches aimed at resolving dysregulation of DA in the PFC could enhance treatment outcomes by improving cognition, thereby promoting control over goal-directed versus habitual alcohol-seeking behavior.
Acknowledgments
This research was supported by National Institutes of Health Grants AA010761, AA010983, AA022475, and NIAAA Conference Grant AA071581.
Abbreviations
- PFC
prefrontal cortex
- mPFC
medial prefrontal cortex
- PrL
prelimbic prefrontal cortex
- IfL
infralimbic prefrontal cortex
- NAcc
nucleus accumbens
- NAccSh
nucleus accumbens shell
- NAccC
nucleus accumbens core
- DA
dopamine
- VTA
ventral tegmental area
- D2
D2 dopamine receptor
- D1
D1 dopamine receptor
- Str
striatum
- FSIN
fast-spiking interneuron
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neuroscience and Biobehavioral Reviews. 2004;28:415–431. doi: 10.1016/j.neubiorev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Adermark L, Söderpalm B, Burkhardt JM. Brain region specific modulation of ethanol-induced depression of GABAergic neurons in the brain reward system by the nicotine receptor antagonist mecamylamine. Alcohol. 2014;48:455–461. doi: 10.1016/j.alcohol.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biological Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharmacological Reviews. 1983;35:53–68. [PubMed] [Google Scholar]
- Barker JM, Taylor JR, Chandler LJ. A unifying model of the role of the infralimbic cortex in extinction and habits. Learning & Memory. 2014;21:441–448. doi: 10.1101/lm.035501.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bice PJ, Foroud T, Carr LG, Zhang L, Liu L, Grahame NJ, et al. Identification of QTLs influencing alcohol preference in the High Alcohol Preferring (HAP) and Low Alcohol Preferring (LAP) mouse lines. Behavior Genetics. 2006;36:248–260. doi: 10.1007/s10519-005-9019-6. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcoholism: Clinical and Experimental Research. 1998;22:236–244. [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Daunais JB, Friedman DP, Grant KA, et al. Chronic ethanol exposure alters presynaptic dopamine function in the striatum of monkeys: a preliminary study. Synapse. 2003;50:266–268. doi: 10.1002/syn.10269. [DOI] [PubMed] [Google Scholar]
- Burkhardt JM, Adermark L. Locus of onset and subpopulation specificity of in vivo ethanol effect in the reciprocal ventral tegmental area-nucleus accumbens circuit. Neurochemistry International. 2014;76:122–130. doi: 10.1016/j.neuint.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Chen L, Bohanick JD, Nishihara M, Seamans JK, Yang CR. Dopamine D1/5 receptor-mediated long-term potentiation of intrinsic excitability in rat prefrontal cortical neurons: Ca2+-dependent intracellular signaling. Journal of Neurophysiology. 2007;97:2448–2464. doi: 10.1152/jn.00317.2006. [DOI] [PubMed] [Google Scholar]
- Chiodo LA. Dopamine-containing neurons in the mammalian central nervous system: electrophysiology and pharmacology. Neuroscience and Biobehavioral Reviews. 1988;12:49–91. doi: 10.1016/s0149-7634(88)80073-3. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bannon MJ, Grace AA, Roth RH, Bunney BS. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience. 1984;12:1–16. doi: 10.1016/0306-4522(84)90133-7. [DOI] [PubMed] [Google Scholar]
- Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. The Journal of Physiology. 2012;590(Pt 4):715–724. doi: 10.1113/jphysiol.2011.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA, Jr., Engleman EA, Ding ZM, McBride WJ, Rodd ZA. Microinjections of acetaldehyde or salsolinol into the posterior ventral tegmental area increase dopamine release in the nucleus accumbens shell. Alcoholism: Clinical and Experimental Research. 2013;37:722–729. doi: 10.1111/acer.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA, Jr., Hauser SR, Wilden JA, Truitt WA, Rodd ZA. Elucidating the biological basis for the reinforcing actions of alcohol in the mesolimbic dopamine system: the role of active metabolites of alcohol. Frontiers in Behavioral Neuroscience. 2013;7:104. doi: 10.3389/fnbeh.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proceedings of the National Academy of Sciences. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. Journal of Neurophysiology. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. The Western Journal of Medicine. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Frontiers in Neuroscience. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behavioral Neuroscience. 2001;115:934–939. [PubMed] [Google Scholar]
- Gao WJ, Wang Y, Goldman-Rakic PS. Dopamine modulation of perisomatic and peridendritic inhibition in prefrontal cortex. The Journal of Neuroscience. 2003;23:1622–1630. doi: 10.1523/JNEUROSCI.23-05-01622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Advances in Pharmacology. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. Journal of Neurophysiology. 2001;86:2911–2918. doi: 10.1152/jn.2001.86.6.2911. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine enhances EPSCs in layer II-III pyramidal neurons in rat prefrontal cortex. The Journal of Neuroscience. 2003;23:867–875. doi: 10.1523/JNEUROSCI.23-03-00867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Mulholland PJ, Chandler LJ, Seamans JK. The glutamatergic component of the mesocortical pathway emanating from different subregions of the ventral midbrain. Cerebral Cortex. 2012;22:327–336. doi: 10.1093/cercor/bhr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. Journal of Neurophysiology. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Harvey M, Lau D, Civillico E, Rudy B, Contreras D. Impaired long-range synchronization of gamma oscillations in the neocortex of a mouse lacking Kv3.2 potassium channels. Journal of Neurophysiology. 2012;108:827–833. doi: 10.1152/jn.00102.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipólito L, Sánchez-Catalán MJ, Martí-Prats L, Granero L, Polache A. Revisiting the controversial role of salsolinol in the neurobiological effects of ethanol: old and new vistas. Neuroscience and Biobehavioral Reviews. 2012;36:362–378. doi: 10.1016/j.neubiorev.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Hitchcott PK, Quinn JJ, Taylor JR. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cerebral Cortex. 2007;17:2820–2827. doi: 10.1093/cercor/bhm010. [DOI] [PubMed] [Google Scholar]
- Hoffman DC, Beninger RJ. Conditional tolerance to haloperidol-induced catalepsy: striatal dopamine receptor supersensitivity is a possible explanation. Psychopharmacology (Berl) 1988;95:142–145. doi: 10.1007/BF00212784. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, et al. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nature Neuroscience. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Y, Quintanilla ME, Karahanian E, Rivera-Meza M, Herrera-Marschitz M. The “first hit” toward alcohol reinforcement: role of ethanol metabolites. Alcoholism: Clinical and Experimental Research. 2015;39:776–786. doi: 10.1111/acer.12709. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. The Journal of Comparative Neurology. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Karahanian E, Quintanilla ME, Tampier L, Rivera-Meza M, Bustamante D, Gonzalez-Lira V, et al. Ethanol as a prodrug: brain metabolism of ethanol mediates its reinforcing effects. Alcoholism: Clinical and Experimental Research. 2011;35:606–612. doi: 10.1111/j.1530-0277.2011.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahanian E, Rivera-Meza M, Tampier L, Quintanilla ME, Herrera-Marschitz M, Israel Y. Long-term inhibition of ethanol intake by the administration of an aldehyde dehydrogenase-2 (ALDH2)-coding lentiviral vector into the ventral tegmental area of rats. Addiction Biology. 2015;20:336–344. doi: 10.1111/adb.12130. [DOI] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, Jones SR. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug and Alcohol Dependence. 2015;150:24–30. doi: 10.1016/j.drugalcdep.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. European Neuropsychopharmacology. 2003a;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcoholism: Clinical and Experimental Research. 2003b;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraschewski A, Reese J, Anghelescu I, Winterer G, Schmidt LG, Gallinat J, et al. Association of the dopamine D2 receptor gene with alcohol dependence: haplotypes and subgroups of alcoholics as key factors for understanding receptor function. Pharmacogenetics and Genomics. 2009;19:513–527. doi: 10.1097/fpc.0b013e32832d7fd3. [DOI] [PubMed] [Google Scholar]
- Kroener S, Chandler LJ, Phillips PE, Seamans JK. Dopamine modulates persistent synaptic activity and enhances the signal-to-noise ratio in the prefrontal cortex. PLoS One. 2009;4:e6507. doi: 10.1371/journal.pone.0006507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lapish CC, Durstewitz D, Chandler LJ, Seamans JK. Successful choice behavior is associated with distinct and coherent network states in anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11963–11968. doi: 10.1073/pnas.0804045105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish CC, Seamans JK, Chandler LJ. Glutamate-dopamine cotransmission and reward processing in addiction. Alcoholism: Clinical and Experimental Research. 2006;30:1451–1465. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in Neurosciences. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Tejani-Butt S. Differential patterns of alcohol consumption and dopamine-2 receptor binding in Wistar-Kyoto and Wistar rats. Neurochemical Research. 2010;35:1708–1715. doi: 10.1007/s11064-010-0233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrejeru A, Martí-Prats L, Avegno EM, Harrison NL, Sulzer D. A subset of ventral tegmental area dopamine neurons responds to acute ethanol. Neuroscience. 2015;290:649–658. doi: 10.1016/j.neuroscience.2014.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RD. Multiple metabolite theory, alcohol drinking and the alcogene. Progress in Clinical and Biological Research. 1985;183:201–220. [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. The Journal of Neuroscience. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. Journal of Neurophysiology. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm S, Nylander I. Alcohol-induced changes in opioid peptide levels in adolescent rats are dependent on housing conditions. Alcoholism: Clinical and Experimental Research. 2014;38:2978–2987. doi: 10.1111/acer.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA, Nixon SJ. Cognitive functioning in sober social drinkers: a review of the research since 1986. Journal of Studies on Alcohol. 1998;59:180–190. doi: 10.15288/jsa.1998.59.180. [DOI] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. The Journal of Neuroscience. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Wightman RM. Extrasynaptic dopamine and phasic neuronal activity. Nature Neuroscience. 2004;7:199. doi: 10.1038/nn0304-199a. author reply 199. [DOI] [PubMed] [Google Scholar]
- Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, et al. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. The American Journal of Psychiatry. 2011;168:183–192. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Experimental Brain Research. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcoholism: Clinical and Experimental Research. 2009;33:1187–1196. doi: 10.1111/j.1530-0277.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, et al. Chronic ethanol drinking by alcohol-preferring rats increases the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol. Alcoholism: Clinical and Experimental Research. 2005a;29:358–366. doi: 10.1097/01.alc.0000156127.30983.9d. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, et al. Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. The Journal of Pharmacology and Experimental Therapeutics. 2005b;315:648–657. doi: 10.1124/jpet.105.084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Oster SM, Ding ZM, Toalston JE, Deehan G, Bell RL, et al. The reinforcing properties of salsolinol in the ventral tegmental area: evidence for regional heterogeneity and the involvement of serotonin and dopamine. Alcoholism: Clinical and Experimental Research. 2008;32:230–239. doi: 10.1111/j.1530-0277.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Roesch-Ely D, Scheffel H, Weiland S, Schwaninger M, Hundemer HP, Kolter T, et al. Differential dopaminergic modulation of executive control in healthy subjects. Psychopharmacology (Berl) 2005;178:420–430. doi: 10.1007/s00213-004-2027-z. [DOI] [PubMed] [Google Scholar]
- Schier CJ, Dilly GA, Gonzales RA. Intravenous ethanol increases extracellular dopamine in the medial prefrontal cortex of the Long-Evans rat. Alcoholism: Clinical and Experimental Research. 2013;37:740–747. doi: 10.1111/acer.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavior-related activity of primate dopamine neurons. Revue Neurologique (Paris) 1994;150:634–639. [PubMed] [Google Scholar]
- Schultz W. The Reward Signal of Midbrain Dopamine Neurons. News in Physiological Sciences. 1999;14:249–255. doi: 10.1152/physiologyonline.1999.14.6.249. [DOI] [PubMed] [Google Scholar]
- Shnitko TA, Kennerly LC, Spear LP, Robinson DL. Ethanol reduces evoked dopamine release and slows clearance in the rat medial prefrontal cortex. Alcoholism: Clinical and Experimental Research. 2014;38:2969–2977. doi: 10.1111/acer.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. International Journal of Developmental Neuroscience. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, et al. Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. The Journal of Neuroscience. 2014;34:3706–3718. doi: 10.1523/JNEUROSCI.0623-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Kröner S, Seamans JK. Dopamine modulation of prefrontal cortex interneurons occurs independently of DARPP-32. Cerebral Cortex. 2008;18:951–958. doi: 10.1093/cercor/bhm133. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Lavin A. Acute cocaine administration depresses cortical activity. Neuropsychopharmacology. 2004;29:2046–2051. doi: 10.1038/sj.npp.1300482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan LA, Boutros N, Petrakis IL, Krystal JH. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health and Research World. 1998;22:61–66. [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007a;61:843–850. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cerebral Cortex. 2007b;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Archives of General Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcoholism: Clinical and Experimental Research. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Wang SR, Yao W, Huang HP, Zhang B, Zuo PL, Sun L, et al. Role of vesicle pools in action potential pattern-dependent dopamine overflow in rat striatum in vivo. Journal of Neurochemistry. 2011;119:342–353. doi: 10.1111/j.1471-4159.2011.07440.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Yan Z. Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. The Journal of Neuroscience. 2002;22:9185–9193. doi: 10.1523/JNEUROSCI.22-21-09185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Wang RY. A10 dopamine neurons: role of autoreceptors in determining firing rate and sensitivity to dopamine agonists. Life Sciences. 1984;34:1161–1170. doi: 10.1016/0024-3205(84)90088-2. [DOI] [PubMed] [Google Scholar]
- Xiao C, Ye JH. Ethanol dually modulates GABAergic synaptic transmission onto dopaminergic neurons in ventral tegmental area: role of mu-opioid receptors. Neuroscience. 2008;153:240–248. doi: 10.1016/j.neuroscience.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Hipólito L, Zuo W, Polache A, Granero L, Krnjevic K, et al. Salsolinol stimulates dopamine neurons in slices of posterior ventral tegmental area indirectly by activating μ-opioid receptors. The Journal of Pharmacology and Experimental Therapeutics. 2012;341:43–50. doi: 10.1124/jpet.111.186833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetnikoff L, Reichard RA, Schwartz ZM, Parsely KP, Zahm DS. Protracted maturation of forebrain afferent connections of the ventral tegmental area in the rat. The Journal of Comparative Neurology. 2014;522:1031–1047. doi: 10.1002/cne.23459. [DOI] [PMC free article] [PubMed] [Google Scholar]