Abstract
Objective
The long-term impact of burn trauma on skeletal muscle bioenergetics remains unknown. Here, we determined respiratory capacity and function of skeletal muscle mitochondria in healthy individuals and in burn victims for up to two years post-injury.
Methods
Hypermetabolism was determined by the difference in predicted and measured metabolic rate. Biopsies were collected from the m. vastus lateralis of 16 healthy men (26±4 years) and 69 children (8±5 years) with burns encompassing ≥30% of their total body surface area. 79 biopsies were collected from cohorts of burn victims at 2 weeks (n=18), 6 months (n=18), 12 months (n=25) and 24 months (n=18) post-burn. Mitochondrial respiration was determined in saponin-permeabilized myofiber bundles. Outcomes were modeled by analysis of variance, with differences in groups assessed by Tukey-adjusted contrasts.
Results
Burn patients were hypermetabolic for up to two years post injury. Coupled mitochondrial respiration was lower at 2 weeks (17 (8) pmol/s/mg, P<0.001), 6 months (41 (30) pmol/s/mg, P=0.03) and 12 months (35 (14) pmol/s/mg, P<0.001) post-burn compared to healthy controls (58 (13) pmol/s/mg). Coupled respiration was greater at 6, 12 and 24 months post-burn vs. 2 weeks post-burn (P<0.001). Mitochondrial ADP and oligomycin sensitivity (measures of coupling control) were lower at all time-points post-burn vs. control (P<0.05), but greater at 6, 12 and 24 months post burn vs. 2 weeks post burn (P<0.05).
Conclusions
Muscle mitochondrial respiratory capacity remains significantly lower in burn victims for one-year post injury. Mitochondrial coupling control is diminished for up to two years post-injury in burn victims, resulting in greater mitochondrial thermogenesis. These quantitative and qualitative derangements in skeletal muscle bioenergetics likely contribute to the long-term pathophysiological stress response to burn trauma.
Keywords: Burn trauma, hypermetabolism, skeletal muscle, mitochondria, thermogenesis
Introduction
Burns encompassing more than 30% of the total body surface area (TBSA) induce a pathophysiological stress response that persists for several years post-injury 1,2. Hypermetabolism, an increase in resting energy expenditure (REE), is a hallmark of this stress response 1-4. Moreover, hypermetabolism is thought to contribute to cachexia post injury 5, where providing burn survivors with adequate nutrition is not readily achievable 6,7, resulting in the erosion of lean tissue. In particular, skeletal muscle cachexia is associated with delayed wound healing and prolonged morbidity in burn survivors 8. Thus, managing hypermetabolism and preventing cachexia in burn survivors is of clinical importance.
Mechanistically, burn-induced hypermetabolism is not fully understood. Increases in ATP dependent processes such as myocardial contractility 9, protein synthesis 10,11 and substrate cycling 12-14 significantly contribute to increased metabolic rate in burn victims. However, elevated ATP turnover cannot fully explain post-burn hypermetabolism 15. This suggests that O2 consumption out-paces ATP production following burn injury. Almost all oxygen consumption and ATP production in vivo occurs within mitochondria, suggesting that these organelles play a central role in post-burn hypermetabolism. Indeed, we have recently shown in a cohort of adults with massive burns that skeletal muscle mitochondrial function is acutely altered, where the coupling of oxygen consumption to ATP production is diminished 16, suggesting that mitochondrial thermogenesis increases within skeletal muscle of burn survivors. Further, increased skeletal muscle thermogenesis was associated with hypermetabolism in burn survivors 16, indicating that skeletal muscle mitochondria contribute to hypermetabolism in burn victims.
Burn induced hypermetabolism is known to persist for up to two years post injury 2. However, whether altered skeletal muscle mitochondrial function persists beyond the acute hospitalization period post-burn, remains unknown. The purpose of the current study was to determine skeletal muscle mitochondrial functional in burn victims for up to two years post burn. We hypothesize that like hypermetabolism, derangements in skeletal muscle mitochondrial function persists for several months post injury in burn survivors.
Methods
Burn patients
Children with burns encompassing more that 30% of their total body surface area (TBSA) who were admitted to Shriners hospitals for Children – Galveston between 2012 and 2014 were included in the current study, which was approved by the Institutional Review Board at the University of Texas Medical Branch. Informed written consent was obtained from the parent or legal guardian of the burned children prior to participation in this study. All burn victims received standard burn care on admission to our hospital, which included fluid resuscitation, excision of all full-thickness burn wounds, and auto-grafting to close burn wounds. Throughout this acute hospitalization, patients were fed 1500 kcal/m2 body area plus 1500 kcal/m2 body area burned of a total enteral nutrition formula (82% carbohydrates, 3% fat, 15% protein) via a nasogastric feeding tube (Vivonex T.E.N., Nestlé Health Science, Minneapolis, MN).
Cohorts of burn patients were studied during the acute hospitalization period, approximately 2 weeks post injury, and then at 6, 12 and 24 months post-burn, when they returned to our institution for follow-up care. On each occasion, indirect calorimetry was performed to calculate the degree of hypermetabolism, and a biopsy from the m. vastus lateralis was collected under sedation and local anesthesia 17 to determine mitochondrial respiratory capacity and function.
Healthy participants
Sixteen young healthy males were recruited to obtain a skeletal muscle sample to act as a reference control value. These participants reported to the clinical research center at the University of Texas Medical Branch the evening prior to being studied. The next morning, following an overnight fast, a muscle biopsy of the m. vastus lateralis was collected under local anesthesia using a suction adapted Bergstrom needle. Healthy participants were young adults and not children. Due to ethical constraints, we were unable to obtain quadriceps skeletal muscle samples from healthy children. We chose to use healthy adults as controls and not since we wanted to study the impact of burn on locomotive skeletal muscle.
Indirect calorimetry
The resting metabolic rate (REE) of burn victims was calculated from the whole body oxygen consumption and carbon dioxide production rates using the Weir equations 18. REE calculated from respiratory gas exchange was compared to predicted REE, estimated using modified Harris-Benedict equations 19. This approach allows the degree of hypermetabolism to be computed.
Muscle biopsy preparation
Ten to twenty mg of muscle tissue was immediately sub-merged in an ice-cold preservation buffer containing 10 mM Ca-EGTA, 0.1 μM free Ca2+, 20 mM imidazole 20 mM taurine, 50 mM K-MES, 0.5 mM DTT, 6.56 mM MgCl2, 5.77 mM ATP and 15 mM creatine phosphate (pH 7.1). Muscle samples were transferred to the laboratory where they were manually separated into myofiber bundles using sharp forceps. To fully permeabolize the sarcolemmal membranes, myofiber bundles were agitating in a sucrose buffer containing 5 μM saponin for 30 min at 4°C. Thereafter, approximately 1-3 mg of muscle tissue was blotted on filter paper, weighed and transferred to an Oxygraph O2K respirometer chamber (Oroboros Instruments, Innsbruck, Austria) containing 2 ml of a respiration buffer (0.5 mM EGTA, 3 mM MgCl2, 60 mM lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 10 mM sucrose and 1 mg/ml bovine serum albumin) for high-resolution respirometry measurements.
High-resolution respirometry
A detailed description of the experimental protocol used in the current study has been reported elsewhere 16,20. Briefly, mitochondrial substrates and inhibitors were added sequentially to the respirometer chambers to determine various respiratory states and mitochondrial coupling control. First, after a baseline respiratory flux was recorded, state 2 (leak) respiration was determined after the titration of octanoyl-l-carnitine (1.5 mM), pyruvate (5 mM), malate (2 mM) and glutamate (10 mM). Then, a saturating concentration of ADP (5 mM) was titrated into the respirometer chamber in order to transition to state 3I respiration, where complex I supported respiration is linked to phosphorylation. Thereafter, 10 mM succinate was titrated into the respirometer chamber in order to stimulate maximal state 3I+II respiration with electron input through complex I and II of the electron transport chain. Finally, 5 μM oligomycin was titrated into the respirometer chamber in order to determine state 4O (leak) respiration. Oligomycin inhibits the FO unit of ATP synthase, blocking ATP synthase dependent inner mitochondrial proton transfer. Thus, state 4O respiration represents inner mitochondrial proton conductance not linked to ATP production.
The interrogation of mitochondrial respiratory flux by high-resolution respirometry allows flux or coupling control ratios to be calculated, which represent a robust index of intrinsic mitochondrial function. The respiratory control ratio (RCR) for ADP was calculated as state 3I/state 2, providing an index of mitochondrial ADP sensitivity and thus coupling control. The substrate control ratio (SCR) for succinate was calculated as state 3I+II/state 3I, providing and index of succinate sensitivity and thus mitochondrial electron-transfer system function. Finally, the coupling control ratio (CCR) for oligomycin was calculated as state 4O/state 3I+II, and from this the coupling control factor (CCF) for oligomycin was calculated as 1 – CCR. The CCR for oligomycin represents the proportion of coupled respiration sensitive to oligomycin, and thus linked to ADP phosphorylation. The CCF for oligomycin represents the proportion of coupled respiration insensitive to oligomycin and therefore linked to mitochondrial thermogenesis.
Statistical analysis
Patient demographics are reported as group means ± the standard deviation. Outcome measures are presented as group medians with the interquartile range unless otherwise stated. For heart rate and resting energy expenditure, a multiple linear regression modeled the relation between outcome and time point (2 week, 6 month, 12 month, and 14 months), while adjusting for the effects of potentially prognostic covariates age and burned percentage of total surface area. Mitochondrial respiration measures were modeled by analysis of variance with relation to healthy reference or burned patient’s time point (healthy, 2 week, 6 month, 12 month, and 24 months). Differences among time points were assessed by Tukey-adjusted contrasts. Normality of outcomes was assessed by normal quantile plots, and some outcomes were transformed to better approximations of normality prior to analysis, with results inverted for interpretation. Resting energy expenditure, including percent predicted REE, was log-transformed. Coupled state 3I+II and RCR were log-transformed. CCR was transformed by raising to the −3 power. Predicted and calculated REE for burn victims were compared using a paired t-test. The correlations presented in Figure 5 were assessed by Spearman rank correlation. Regression and analysis of variance were performed using R statistical software (R Foundation for Statistical Computing, Vienna, Austria). t-tests and Spearman correlation analysis were performed in GraphPad Prism version 6 (GraphPad Software, Inc., La Jolla, CA)
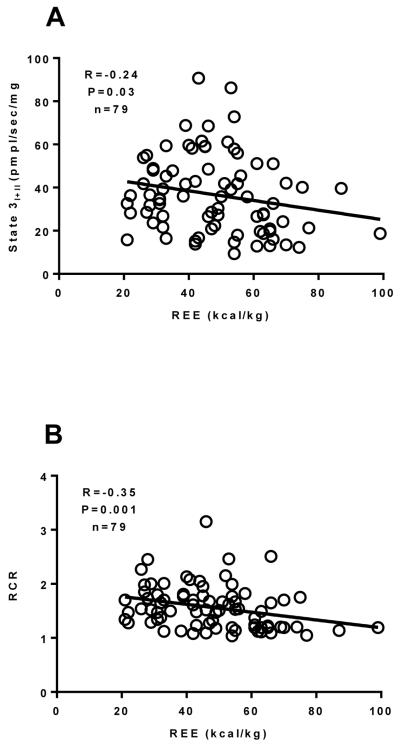
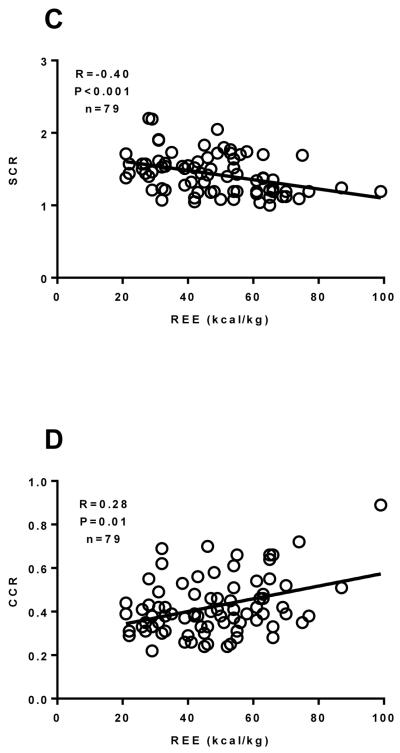
Figure 5.
The relationship between the REE and State 3I+II respiration (Panel A), the RCR (Panel B), the SCR (Panel C) and the CCR (Panel D) in burned children (n=79) ranging from one week to two years post burn was determined by performing Spearman rank correlation analysis. There was a significant correlation between State 3I+II respiration the RCR, the SCR and the CCR. The Spearman correlation coefficient and P value is reported on each trace. The straight line on each trace is the slope computed by linear regression. Values are presented as group medians with the interquartile range.
Results
Patient demographics
Burn patient demographics are reported in Table 1. In total, 69 children (8±5 years; mean±SD) were studied (77% males, 23% females). Patients had large burns, encompassing on average 44±12% of the TBSA, 26±20% of which were full-thickness burn lesions. Burn patients were hospitalized for 25±17 days on average, or 0.57 day per % TBSA burned. Muscle biopsies were collected from 16 young healthy men (26±4 years) to serve as healthy control participants. Demographics for these healthy participants and patient cohorts studied at 2 weeks, 6 months, 12, months, and 24 months post burn are presented in Table 2.
Table 1.
| Patient demographics (mean±SD) | |
|---|---|
| Age (years) | 8±5 |
| Sex (males/females) | 50/15 |
| Height (cm) | 123±30 |
| Weight (kg) | 32±21 |
| Burn size (% TBSA) | 44±12 |
| % Full-thickness | 26±20 |
| Length of stay (days) | 25±17 |
Table 2.
| Controls (n=16) |
2 weeks (n=18) |
6 months (n=25) |
12 months (n=18) |
24 months (n=18) |
|
|---|---|---|---|---|---|
| Age (years) | 26±4 | 5±2 | 7±5 | 11±5 | 9±5 |
| Height (cm) | 179±8 | 109±2 | 122±28 | 138±28 | 131±28 |
| Weight (kg) | 82±13 | 21±8 | 31±26 | 44±27 | 37±26 |
| Days post burn | - | 12±7 | 191±83 | 391±103 | 686±121 |
Burn induced hypermetabolism
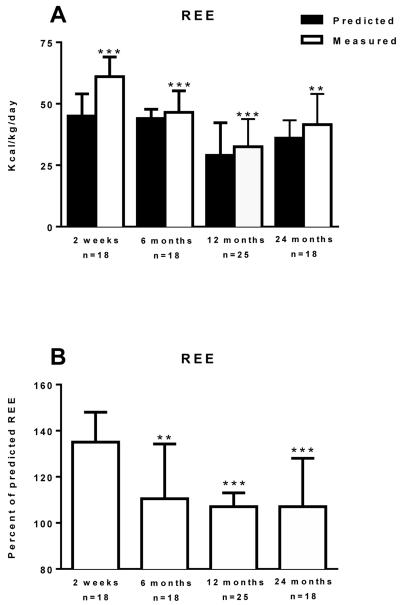
Hypermetabolism was determined in burn victims by estimating REE using established equations and determining REE indirectly by respiratory gas exchange. The discrepancy in these two values infers the degree of hypermetabolism. Hypermetabolism was most pronounced at 2 weeks post burn, where measured REE was 36% greater than estimated REE (46 (10) vs. 62 (15) kcal/kg/day; P<0.001). Burn victims remained hypermetabolic, albeit to a lesser extent, at 6 months (42 (13) vs. 48 (11) kcal/kg/day; P<0.01), 12 months (36 (15) vs. 39 (13) kcal/kg/day; P<0.01), and 24 months (36 (11) vs. 44 (23) kcal/kg/day; P<0.01) post burn (Figure 1A). The degree of hypermetabolism was significantly lower at 6 months (35% (33%) vs. 11% (29%, P=0.01), 12 months (35% (33%) vs. 8±2%, P<0.001), and 24 months (35% (33%) vs. 7% (27%), P=0.001), respectively, when compared to the 2 weeks post burn cohort (Figure 1B), suggesting that hypermetabolism was largely resolved over time post burn.
Figure 1.
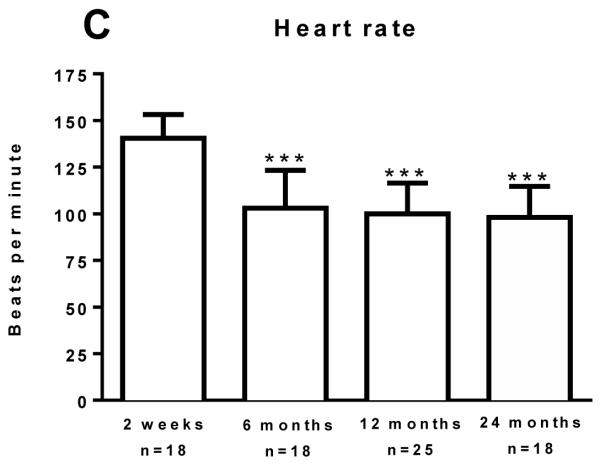
Resting energy expenditure (REE) predicted by modified Harris-Benedict equations (Predicted) and measured from respiratory gas exchange (Measured) is shown in Panel A. Measured and Predicted REE were compared in each cohort by a Paired t-test. **P<0.01 and ***P<0.001 vs. Predicted REE. The percent of predicted REE and heart rate for burn patients is shown in Panel 1B and Panel 1C, respectively. Data was modeled by analysis of variance to account for age, TBSA and repeated measures. **P<0.01 and ***P<0.001 vs. 2 weeks post burn group. Values are presented as group medians with the interquartile range.
Elevated heart rate (HR), another index of the hypermetabolic stress response to burn injury is shown in Figure 1C. HR was highest in the 2 weeks post burn cohort (141 (28) beats per minute, bpm). Compared to the two-week post burn time-point, HR was significantly lower at 6 months (103 (30) bpm, P<0.001), 12 months (98 (27) bpm, P=0.001), and 24 months (98 (41) bpm, P<0.001) post burn time-points, further highlighting a partial resolution of burn induced hypermetabolism.
Skeletal muscle mitochondrial respiratory capacity
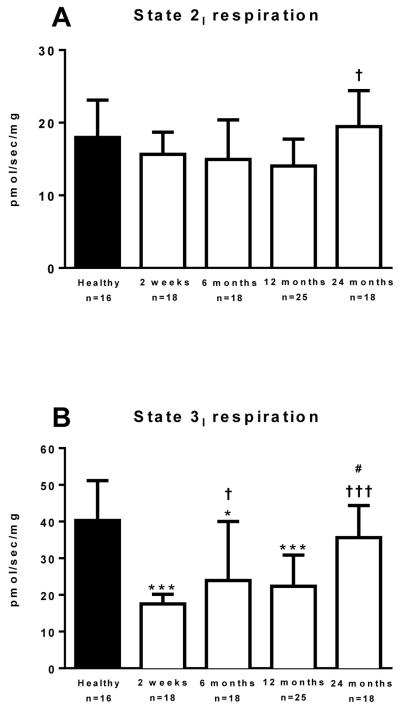
Mitochondrial respiration data is presented in Figure 2. Leak state 2 respiration in the presence of pyruvate, octanoyl-carnitine, malate and glutamate, but in the absence of ADP was similar between groups; although was significantly elevated in the 24 months post burn cohort vs. the 12 month post burn cohort (Figure 2A, P=.007).
Figure 2.
State 2 respiration in the leak state (Panel 2A), State 3I coupled respiration with electron input through complex I of the electron transport chain (Panel 2B), State 3I+II coupled respiration with electron input through complex I and II of the electron transport chain (Panel 2C), and state 4O leak respiration (Panel 2D) where ATP synthase is inhibited by oligomycin. Data was modeled by analysis of variance to account for age, TBSA and repeated measures. *P<0.05, **P<0.01 and ***P<0.001 vs. healthy control group. †P<0.05 and †††P<0.001 vs. 2 weeks post burn group. #P<0.05 vs. 12 months post burn group. Values are presented as group medians with the interquartile range.
Coupled state 3I respiration following the addition of ADP was significantly lower in the 2 weeks (17.5 (5.7) vs. 40.3 (18.6) pmol/s/mg; P<0.001), 6 months (24.1 (19.4) vs. 40.3 (18.6) pmols/s/mg; P=0.01), and 12 months (22.3 (14.5) vs. 40.3 (18.6) pmol/sec/mg; P<0.001), post burn in comparison to healthy individuals (Figure 2B). State 3I respiration at 24 months post burn was not significantly different to the healthy control group (35.6 (20.1) vs. 40.3 (18.6) pmols/s/mg; Figure 2B). Compared to the 2-week post burn cohort, state 3I respiration was greater in the 6 months (P=0.017) and 24 months (P<0.001) post burn cohorts. Further, state 3I respiration was significantly greater in the 24 months post burn cohort when compared to the 12-month post burn cohort (P=0.03).
Coupled state 3I+II respiration following the addition of succinate is shown in Figure 2C. State 3I+II respiration was significantly lower in the 2 weeks (17.0 (8.3) vs. 58.1 (12.7) pmols/s/mg; P<0.001), 6 months (41.8 (30.4) vs. 58.1 (12.7) pmol/s/mg; P=0.03), and 12 months (35.8 (13.6) vs. 58.1 (12.7) pmol/sec/mg; P=0.001), post burn in comparison to healthy individuals. State 3I+II respiration at 24 months post burn was not significantly different when compared to the healthy control group (49.5 (23.0) vs. 58.1 (12.7) pmol/s/mg; Figure 2C), suggesting that skeletal muscle respiratory capacity is largely restored at 24 months post-burn. State 3I+II respiration was significantly greater at 6 months (P<0.001), 12 months (P<0.001) and 24 months (P<0.001) post burn when compared to the 2 weeks post burn group.
Leak state 4O respiration following the addition of the ATP synthase inhibitor oligomycin is shown in Figure 2D. State 4O respiration was significantly lower in the 2 weeks (8.9 (4.3) vs. 19.7 (7.4) pmol/s/mg; P<0.001) and 12 months (13.7 (4.4) vs. 19.7 (7.4) pmol/s/mg; P=0.036) post burn groups, but not the 6 months (14.9 (2.6) vs. 19.7 (7.4) pmol/s/mg) and 24 months 18.4 (8.3) vs. 19.7 (7.4) pmol/s/mg) post burn groups, when compared to healthy individuals. State 4O respiration was significantly greater at 24 months post burn when compared to the 2 weeks (P<0.001) and 12 months (P=0.009) post burn groups.
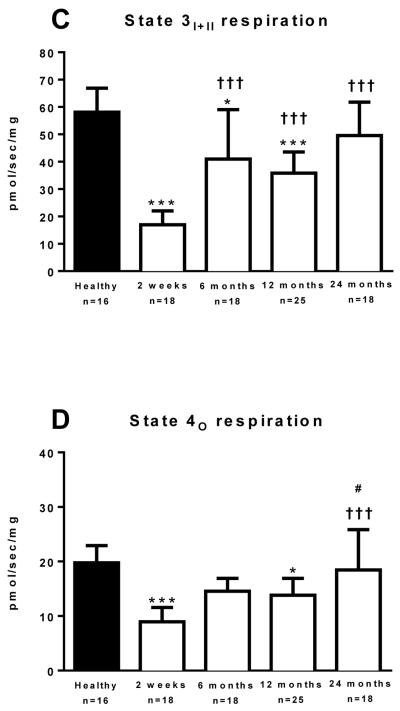
Skeletal muscle mitochondrial coupling control
Mitochondrial respiratory capacity, while informative in terms of the maximal oxidative capacity of the tissue sample, is influenced by both mitochondrial number and mitochondrial quality. However, since skeletal muscle mitochondria rarely function at maximal capacity in vivo, measures of mitochondrial function in vitro likely better reflect mitochondrial myopathies in vivo than measures of maximal respiration in vitro. Thus, we calculated the RCR for ADP, the SCR for succinate, and the CCR (and CCF) for oligomycin, as measures of mitochondrial quality. The RCR for ADP is shown in Figure 3A. The RCR was significantly lower in the 2 weeks (1.19 (0.06 vs. 2.11 (0.81); P<0.001), 6 months (1.68 (0.41) vs. 2.11 (0.81); P=0.005), 12 months (1.61 (0.47) vs. 2.11 (0.81); P<0.001), and 24 months (1.70 (0.52) vs. 2.11 (0.81); P<0.001), post burn in comparison to healthy individuals, suggesting that the ability of mitochondria to make ATP is diminished following severe burn injury. Further, the RCR for ADP was significantly greater in the 6 (P=0.001), 12 (P=0.005) and 24 (P<0.001) months post burn groups when compared to the 2 weeks post burn group, indicating that mitochondrial respiratory control is somewhat recovered at approximately 6 months post burn.
Figure 3.
The respiratory control ratio (RCR) for ADP (Panel 3A) and the substrate control ratio (SCR) for succinate (Panel 3B). Data was modeled by analysis of variance to account for age, TBSA and repeated measures. ***P<0.001 vs. healthy control group. †P<0.05, ††P<0.01 and †††P<0.001 vs. 2 weeks post burn group. Values are presented as group medians with the interquartile range.
The SCR for succinate is shown in Figure 3B. The SCR was significantly lower in the 2 weeks post (1.19 (0.08) vs. 1.42 (0.53); P<0.001), in comparison to healthy individuals, suggesting that mitochondrial substrate sensitivity is acutely reduced following severe burn injury, something which is largely restored at 6 months post burn. Indeed, the SCR for succinate was significantly greater at 6 (1.44 (0.30) vs. 1.19 (0.08); P=0.02), 12 (1.54 (0.44) vs. 1.19 (0.08); P=0.002) and 24 (1.48 (0.24) vs. 1.19 (0.08); P=0.02) months post burn groups when compared to the 2 weeks post burn group.
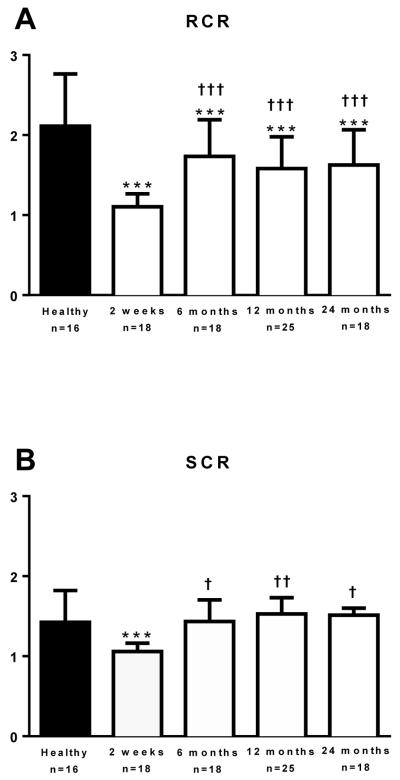
Mitochondrial sensitivity to the ATP synthase inhibitor oligomycin was determined as a further measure of coupling control. The CCR for oligomycin is a ratio that falls within the confines of 0 to 1. Further, the CCF, which is equal to 1 minus the CCR, is therefore also subject to this confine. Thus, the CCR represents the fraction of respiration sensitive to ATP synthase inhibition, i.e., respiration linked to phosphorylation; whereas the CCF represents the fraction of respiration insensitive to oligomycin, i.e., respiration linked to thermogenesis. These two fractions are presented as a percentage of maximal respiration in Figure 4. The FCR was significantly lower in the 2 weeks (0.53 (0.19) vs. 0.32 (0.10); P<0.001), 12 months (0.38 (0.13) vs. 0.32 (0.10); P=0.050), and 24 months (0.39 (0.08) vs. 0.32 (0.10); P=0.043), post burn in comparison to healthy individuals. The FCR was also numerically lower in the 6 months post burn cohort compared to the control group, but this was not a statistically significant finding (0.32 (0.10) vs. 0.35 (0.11); P=0.26). Further, compared to the 2 weeks post burn group, the FCR for oligomycin was significantly lower in the 6 month post burn group (P=0.02), suggesting a marginal recovery of coupling control at this time point post injury.
Figure 4.
The flux control ratio (FCR) and flux control factor (FCF) for oligomycin are shown in Figure 4. The FCR, shown as phosphorylation and the FCF, shown as thermogenesis, are graphed as a percentage of maximal state 3I+II respiration. *P<0.05 and ***P<0.001 vs. healthy control group. †P<0.05 vs. 2 weeks post burn group. Values are presented as group medians with the interquartile range.
Spearman correlation analysis was performed between REE data and mitochondrial respiratory function and coupling control parameters. There was no significant correlation between state 2I, state 3I, or state 4O respiration (data no shown). However, there was a significant negative correlation (R=−0.24, P=0.03) between state 3I+II respiration and REE (Figure 5A), suggesting a relationship between the degree of hypermetabolism and muscles ability to produce ATP. Further, the RCR (R=−0.35, P=0.001; Figure 5B), SCR (R=−0.40, P<0.001; Figure 5C), and the CCR (R=0.28, P=0.01; Figure 5D) were all significantly correlated with REE, suggesting that diminished skeletal muscle mitochondrial coupling control is associated with hypermetabolism in burn survivors.
Discussion
Hypermetabolism is a hallmark of the pathophysiological stress response to severe burn trauma 1-4. Currently, a complete biochemical understanding of burn-induced hypermetabolism is lacking. Here, we determined skeletal muscle mitochondrial respiratory capacity and function in cohorts of severely burned children for up to two years post injury, with the working hypothesis that skeletal muscle mitochondrial thermogenesis contributes to burn induced hypermetabolism. Our current findings demonstrate an association between hypermetabolism and skeletal muscle mitochondrial dysfunction in burn survivors, which persists for up to two years post-injury. Specifically, skeletal muscle mitochondria of burn survivors remain insensitive to ADP and the ATP synthase inhibitor oligomycin for two years post injury, where mitochondria exhibit a thermogenic phenotype post-burn. Since this alteration in skeletal muscle mitochondrial coupling control occurs in parallel to increased whole body REE, we suggest that altered skeletal mitochondrial function contributes to hypermetabolism in burn victims.
Hypermetabolism has previously been shown to persist for up to two years post injury in burn victims 2. Acutely, hypermetabolism complicates the process of providing patients with sufficient energy and macronutrients 6,7, which may worsen lean tissue catabolism and impede wound healing. Further, the long-term impact of hypermetabolism may have a negative impact of the rehabilitation of burn victims, especially in pediatric cohorts, where post-burn growth and body composition are known to be altered from that of age matched children 21. Thus, blunting thi hypermetabolic response will likely hasten recovery in burn survivors.
Mechanistically, post-burn hypermetabolism is only partially understood 15. Pioneering work of Wilmore and colleagues 4 demonstrated a clear relationship between burn size, adrenergic stress and the degree of hypermetabolism. Further, these investigators showed a step-wise reduction in hypermetabolism with increasing ambient temperature 19. Thus, destruction of the body’s skin barrier and the resultant adrenergic stress appear to mediate post-burn hypermetabolism, a significant portion of which can be attributed to thermogenesis, most likely to maintain core temperature in the absence of a patent skin barrier. In support of this supposition, our current data show that in the leak state, i.e., mitochondrial respiration linked to thermogenesis, respiration is similar in burn victims in comparison to healthy individuals, despite an overall reduction in mitochondrial respiratory capacity in the coupled state. Thus, it would appear that the maximal thermogenic capacity of skeletal muscle is maintained post burn, even when overall oxidative capacity is diminished. In further support of a qualitative shift in skeletal muscle respiratory function, the coupling effect of ADP on muscle mitochondrial respiration is markedly diminished acutely post burn, which is in line with our previous findings in burned adults 16. Further, while improved at 6 months, one and two years post burn relative to the acute setting, ADP exerts a diminished coupling response on skeletal muscle mitochondria for up to 2 years post burn. This strongly suggests a phenotypic shift in skeletal muscle mitochondria of burn victims, away from ATP production and towards thermogenesis.
While marked differences in coupled ATP producing respiration are seen in the current study, it is unlikely that this will limit ATP availability in vivo. For example, muscle mitochondria only work at near capacity during intense muscular contraction. At rest, in an immobilized patient, ATP availability will likely be unaffected by a ~50% decrease in maximal ATP producing capacity. Indeed, ATP concentration in muscle of burned rodents are slightly, albeit not significantly greater, than in unburned controls 22, further supporting that reduced ATP producing capacity does not impact ATP availability (at rest) in vivo. With that said, the long-term decrease in maximal ATP producing capacity and ADP sensitivity of skeletal muscle from burn survivors may impact functional capacity. By way of example, skeletal muscle mitochondrial respiratory capacity correlates with whole body VO2 max and insulin sensitivity in vivo 23-25. Clearly then, restoration of muscle respiratory capacity will likely have a favorable impact on muscle and whole body metabolic function in burn survivors. Indeed, exercise training has been repeatedly shown to increase whole body O2 consumption rates in burn victims 26, likely mediated in part by increased mitochondrial capacity in locomotive muscles.
Approximately 90% of whole body O2 consumption occurs within mitochondria, of which, the vast majority (~80%) is coupled to ATP production 27. Thus, considering that a significant portion of hypermetabolism in burn victims appears to be the result of O2 consumption rates out-pacing those of ATP production, this suggests a central role for mitochondrial coupling control in burn induced hypermetabolism. Skeletal muscle represents approximately 40% of body mass in healthy humans, and is responsible for ~20% of whole body O2 consumption in the rested state. Thus, altered mitochondrial coupling control in skeletal muscle would likely have a significant impact on whole body O2 consumption. Indeed, rare mitochondrial myopathies have been reported where severe hypermetabolism is associated with derangements in skeletal muscle mitochondrial coupling control, such as insensitivity to ADP and oligomycin 28,29. Similarly, we have shown a loss in mitochondrial coupling control acutely post injury in burned adults, where hypermetabolism was associated with altered mitochondrial coupling control, specifically, reduced ADP and oligomycin sensitivity 16. In the current study, we extend these observations, demonstrating that altered skeletal muscle mitochondrial coupling control persists for up to two years post injury in burned children, which is associated with hypermetabolism. Specifically, ADP has a reduced stimulatory effect on mitochondrial respiration for two years post-burn, while the ATP synthase inhibitor oligomycin has a diminished inhibitory effect on mitochondrial respiration, which also persists for two years post injury. Collectively, these findings demonstrate poorer coupling control in skeletal muscle mitochondria of burn victims, where mitochondrial respiration is insensitive to stimulation (ADP) and inhibition (oligomycin) of ATP synthase, indicative of mitochondria becoming more uncoupled or thermogenic.
While this cross-sectional cohort study does not directly prove that skeletal muscle mitochondrial thermogenesis contributes to hypermetabolism in burn victims, our current data demonstrate a clear association. In further support of this, we see that hypermetabolism decreases with time post burn, where there is a concurrent improvement in skeletal muscle mitochondrial coupling control. This concomitant decay in hypermetabolism and improvement in skeletal muscle mitochondrial coupling control over an extended period post injury (2 years), offers further robust support for our hypothesis that skeletal muscle mitochondrial thermogenesis does indeed contribute to hypermetabolism in burn victims. Indeed, correlation analysis revealed a significant negative association between maximal coupled (ATP producing) respiration and REE. In other words, lower muscle ATP producing capacity was associated with greater hypermetabolism. Further, reduced coupling control in response to both ADP and oligomycin were associated with greater hypermetabolism, again linking altered skeletal muscle mitochondria with increased energy expenditure in burn victims.
Mechanistically, a full explanation of the mediators of skeletal muscle mitochondrial uncoupling post-burn is lacking. The inner mitochondrial membrane is inherently leaky, allowing protons to re-enter the matrix independently of ATP synthase. However, this ‘leakiness’ is dependent upon the number and function of specific inner mitochondrial proteins, such as adenine nucleotide translocase and uncoupling proteins (UCPs). Specifically, UCPs function to dissipate the electro-chemical potential of the mitochondrial membranes as heat, and are thus potential mediators of skeletal muscle mitochondrial coupling control in burn victims. Indeed, others have reported an increase in skeletal muscle UCP2 expression in burn victims 30. The probing of mitochondrial UCP expression in skeletal muscle of burn victims will likely be an exciting avenue for future research.
A limitation of the current study was that we could only study young healthy adults (mean age 26 years) since is not possible to collect quadriceps biopsies from healthy children. While abdominal muscle can be collected from children undergoing elective surgeries such as hernia repairs, we feel it is imperative to compare the same muscle in control and burn patients, since locomotive and postural muscles differ in terms of mitochondrial function 31. While it is known that skeletal muscle mitochondrial function declines with advancing age in adults 32, this most likely attributable to reducing physical activity and fitness, since younger and older adults matched for aerobic fitness have similar skeletal muscle mitochondrial function 33. Thus, we think it is reasonable to suggest that skeletal muscle mitochondrial respiratory capacity and function will be comparable in physically active healthy children and adults. Indeed, we saw similar detriments in skeletal muscle mitochondrial function in both burned adults and children, leading us to the conclusion that our current findings are not an artefact of the differences in age between our healthy control participants and our burned children.
To summarize, despite significant strides in scientific understanding and clinical practice, hypermetabolism remains a significant clinical problem for burn survivors. A better mechanistic understanding of post-burn hypermetabolism precedes the development of novel interventions aimed at blunting this stress response. Here, we provide evidence in a large cohort of severely burned human patients that altered skeletal muscle mitochondrial function likely contributes to burn-induced hypermetabolism. Going forward, future murine and human studies need to underscore the physiological role for this phenomenon, while better characterizing the genomic and proteomic alterations in skeletal muscle tissue which contribute to this phenotypic alteration.
Acknowledgements
We sincerely thank the patients and healthy participants who took part in the current study. We also acknowledge the expert technical support of the clinical research staff at Shriners Hospitals for Children and The University of Texas Medical Branch. Funding was provided by the National Institutes of Health (P50 GM060388, R01 HD049471, R01 AR049877, P30 AG024832, T32-GM8256), The Institute for Translational Sciences at UTMB (supported in part by a Clinical and Translational Science Award [UL1TR000071] from the National Center for Advancing Translational Sciences, NIH), Shriners Hospitals for Children (84080, 84090, 71006, 85310), and the National Institute for Disability and Rehabilitation Research (H133A120091). CP was supported in part by an Interdisciplinary Rehabilitation Research Postdoctoral Training Grant (H133P110012) from the National Institute for Disability and Rehabilitation Research.
Supported by the National Institutes of Health, the National Institute of Disability and Rehabilitation Research, and Shriners of North America.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goran MI, Peters EJ, Herndon DN, Wolfe RR. Total energy expenditure in burned children using the doubly labeled water technique. Am J Physiol. 1990;259:576–85. doi: 10.1152/ajpendo.1990.259.4.E576. [DOI] [PubMed] [Google Scholar]
- 4.Wilmore D, Long J, Mason AJ, Skreen R, Pruitt BJ. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180:653–69. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–65. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson BW, Nguyen T, Pierre E, Herndon DN, Wolfe RR. Urea and protein metabolism in burned children: effect of dietary protein intake. Metabolism. 1997;46:573–8. doi: 10.1016/s0026-0495(97)90196-7. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe RR, Goodenough RD, Burke JF, Wolfe MH. Response of protein and urea kinetics in burn patients to different levels of protein intake. Ann Surg. 1983;197:163–71. doi: 10.1097/00000658-198302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter C, Hurren N, Herndon D, Børsheim E. Whole body and skeletal muscle protein turnover in recovery from burns. Int J Burns Trauma. 2013;3:9–17. [PMC free article] [PubMed] [Google Scholar]
- 9.Williams FN, Herndon DN, Suman OE, et al. Changes in cardiac physiology after severe burn injury. J Burn Care Res. 2011;32:269–74. doi: 10.1097/BCR.0b013e31820aafcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002;87:3378–84. doi: 10.1210/jcem.87.7.8699. [DOI] [PubMed] [Google Scholar]
- 11.Gore DC, Chinkes DL, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Quantification of protein metabolism in vivo for skin, wound, and muscle in severe burn patients. JPEN J Parenter Enteral Nutr. 2006;30:331–8. doi: 10.1177/0148607106030004331. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe RR, Herndon DN, Jahoor F, Miyoshi H, Wolfe M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317:403–8. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe RR, Herndon DN, Peters EJ, Jahoor F, Desai MH, Holland OB. Regulation of lipolysis in severely burned children. Ann Surg. 1987;206:214–21. doi: 10.1097/00000658-198708000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe RR, Jahoor F, Herndon DN, Miyoshi H. Isotopic evaluation of the metabolism of pyruvate and related substrates in normal adult volunteers and severely burned children: effect of dichloroacetate and glucose infusion. Surgery. 1991;110:54–67. [PubMed] [Google Scholar]
- 15.Yu YM, Tompkins RG, Ryan CM, Young VR. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr. 1999;23:160–8. doi: 10.1177/0148607199023003160. [DOI] [PubMed] [Google Scholar]
- 16.Porter C, Herndon D, Borscheim E, et al. Uncoupled skeletal muscle mitochondria contribute to hypermetabolism in severely burned adults. Am J Physiol Endocrinol Metab. 2014;307:462–7. doi: 10.1152/ajpendo.00206.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergström J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–16. [PubMed] [Google Scholar]
- 18.Weir J. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roza A, Shizgal H. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr. 1984;40:168–82. doi: 10.1093/ajcn/40.1.168. [DOI] [PubMed] [Google Scholar]
- 20.Porter C, Reidy P, Bhattarai N, Sidossis L, Rasmussen B. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc. 2014 doi: 10.1249/MSS.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Przkora R, Barrow RE, Jeschke MG, et al. Body composition changes with time in pediatric burn patients. J Trauma. 2006;60:968–71. doi: 10.1097/01.ta.0000214580.27501.19. [DOI] [PubMed] [Google Scholar]
- 22.Padfield K, Astrakas L, Zhang Q, et al. Burn injury causes mitochondrial dysfunction in skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:5368–73. doi: 10.1073/pnas.0501211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanza I, Short D, Short K, et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–42. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rooyackers O, Adey D, Ades P, Nair K. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93:15364–9. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritov VB, Menshikova EV, Azuma K, et al. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab. 2009;298:49–58. doi: 10.1152/ajpendo.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter C, Hardee J, Herndon D, Suman O. The role of exercise in the rehabilitation of patients with severe burns. Exerc Sport Sci Rev. 2015;43:34–40. doi: 10.1249/JES.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolfe D, Brown G. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–58. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 28.Luft R. The development of mitochondrial medicine. Proc Natl Acad Sci U S A. 1994;13:8731–8. doi: 10.1073/pnas.91.19.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luft R, Ikkos D, Palmieri G, Ernster L, Aafeluis B. A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: a correlated clinical, biochemical, and morphological study. J Clin Invest. 1962;41:1776–804. doi: 10.1172/JCI104637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzika A, Mintzopoulos D, Mindrinos M, Zhang J, Rahme L, Tompkins R. Microarray analysis suggests that burn injury results in mitochondrial dysfunction in human skeletal muscle. Int J Mol Med. 2009;24:387–92. doi: 10.3892/ijmm_00000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabøl R, Larsen S, Højberg P, et al. Regional anatomic differences in skeletal muscle mitochondrial respiration in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2010;95:857–63. doi: 10.1210/jc.2009-1844. [DOI] [PubMed] [Google Scholar]
- 32.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–23. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsen S, Hey-Mogensen M, Rabøl R, Stride N, Helge J, Dela F. The influence of age and aerobic fitness: effects on mitochondrial respiration in skeletal muscle. Acta Physiol. 2012;205:423–32. doi: 10.1111/j.1748-1716.2012.02408.x. [DOI] [PubMed] [Google Scholar]