Abstract
Background & Aims
Mounting evidence indicates that maternal exercise confers protection to adult offspring against various diseases. Here we hypothesized that maternal exercise during gestation would reduce high fat diet (HFD) induced hepatic steatosis in adult rat offspring.
Methods
Following conception, pregnant dams were divided into either voluntary wheel running exercise (GE) or wheel-locked sedentary (GS) groups throughout gestation (days 4-21). Post-weaning, offspring received either normal chow diet (ND; 10% fat, 70% carbohydrate, 20% protein) or high-fat diet (HFD; 45% fat, 35% carbohydrate, and 20% protein) until sacrifice at 4-or 8-months of age.
Results
GE did not affect offspring birth weight or litter size. HFD feeding in offspring increased weight gain, % body fat, and glucose tolerance test area under the curve (GTT-AUC). Male offspring from GE dams had reduced % body fat across all ages (p < 0.05). In addition, 8-mo male offspring from GE dams were protected against HFD-induced hepatic steatosis, which was associated with increased markers of hepatic mitochondrial biogenesis (PGC-1α and TFAM), autophagic potential (ATG12:ATG5 conjugation) and hepatic triacylglycerol secretion (MTTP).
Conclusions
The current study provides the first evidence that gestational exercise can reduce susceptibility to high fat diet induced hepatic steatosis in adult male offspring.
Keywords: NAFLD, Fetal Origins, Rat
INTRODUCTION
The American College of Sports Medicine (ACSM), the American College of Obstetricians and Gynecologists (ACOG), and the Center for Disease Control (CDC) recommend that healthy pregnant women engage in ≥ 30 minutes of moderate physical activity on most, if not all, days of the week (reviewed in [1]). However, a despairingly small proportion (~15%) of pregnant women actually achieve these recommendations [2] and a relatively low-proportion of obstetricians (52%) actually convey these recommendations to their pregnant patients [3]. Along these lines, it is necessary to address the current paucity in the literature regarding the long-term effects of maternal exercise on adult offspring health.
Adverse fetal stressors, such as maternal undernutrition or gestational diabetes, are well appreciated to negatively affect adult health outcomes of the offspring [4]. It is believed that the developing fetus responds to the in utero environment with epigenetic modifications to increase survival in the postpartum period that, when carried into adulthood, can increase disease susceptibility [5]. In contrast, evidence supporting the notion that healthy maternal behaviors may confer protection to the offspring against adult disease is comparatively understudied. Emerging evidence supports that maternal exercise may protect adult offspring against age and high-fat diet induced insulin resistance and adiposity [6-9]. Interestingly, despite the central importance of the liver to systemic metabolism, little it is currently known regarding the effects maternal exercise may have on the liver.
Nonalcoholic fatty liver disease (NAFLD) comprises a spectrum of liver phenotypes from simple steatosis to nonalcoholic steatohepatitis and cirrhosis [10]. It is present in the majority of obese individuals [11, 12] and is identified as an independent predictor of liver-related, cardiovascular, and all-cause mortality [13, 14]. Given the alarming modern obesity epidemic, the concept that maternal behaviors could affect NAFLD in adult offspring could have significant implications. Indeed, mice born to obese dams have elevated hepatic triacylglycerol (TAG) accumulation and reduced mitochondrial content and function compared to those born to dams of normal weight [15]. Whether maternal exercise can protect adult offspring from NAFLD, however, has not previously been addressed. Thus, in the current report we tested the hypothesis that gestational exercise during pregnancy would confer protection to adult offspring against high-fat diet induced NAFLD. Using a rat model of gestational exercise, we demonstrate the novel finding that high-fat fed adult male offspring from sedentary mothers are more susceptible to developing hepatic steatosis than are offspring from exercised mothers.
METHODS
Animal Protocol
Virgin female Sprague-Dawley rats were singled housed in a temperature controlled environment with a 12:12hr light-cycle and given ad libitum access to standard chow (Harlan Rodent Diet 2018; Harlan Laboratories, Indianapolis, Indiana, USA) and water. Prior to pregnancy, rats were acclimatized to running wheels with free access to running wheels for four days, at which point wheels were locked. Male rats were housed with dams for breeding purposes and removed thereafter. Pregnancy was confirmed via copulatory plug formation. Dams were randomly divided into either voluntary wheel running gestational exercise (GE; n=7) or wheel-locked sedentary (GS; n=7) groups. Daily running distance was recorded using a magnetic sensor system (Rat Activity Wheel; Lafayette Instrument, Lafayette, Indiana, USA or Schwinn 17 Function Bike Computer; Pacific Cycle, Madison, Wisconsin, USA). Wheels were locked following parturition in order to restrict exercise to the gestational period. Post-weaning (21d post-partum), four male and four female offspring were randomly selected from each litter and randomly divided to age to either 4- or 8-months. Offspring were single-housed to prevent potential confounding effects of increased spontaneous cage activity of group housing [16] and provided ad libitum access to either a normal diet (ND) of standard chow or a high-fat diet (HFD) containing 45% fat, 35% carbohydrate, and 20% protein (D12451; Open Source Diets, New Brunswick, New Jersey, USA). Subsets of offspring (n=8-10 per age and sex) were euthanized at 4- and 8-months of age following a 6-hour fast with an overdose of sodium pentobarbital and livers were rapidly excised and a portion snap frozen in liquid nitrogen or formalin-fixed for later processing and a segment processed for liver explant culture. Animal protocols were approved by the Purdue University Animal Care and Use Committee.
Body Composition
Body composition (fat-mass, fat-free-mass, and fluids) of offspring was assessed via an EchoMRI-900 machine (EchoMRI, Houston, TX, USA). The MRI test was administered to all offspring 3 weeks prior to the 4 month and 8 month sacrifices. Conscious rats were placed in a specialized holder that allowed for restricted movement on part of the animal. The holder was then placed in the MRI for up to 3 minutes. Rats were returned to their home cages immediately after the scan.
Offspring activity
At 2, 4, 6 and 8-months of age, rats were placed in cages with voluntary access to running wheels for three days. Mean daily running distance was monitored daily. To allow for acclimatization to the wheel, mean running distance over days two and three at each age is reported.
Glucose Tolerance Test (GTT)
A GTT was performed on the conscious offspring at 3 weeks prior to sacrifice. Baseline samples of whole blood were taken from a lateral tail vein and animals were given a single IP bolus of glucose (2 g/kg body weight). Blood glucose was measured at baseline, 10, 20, 30, 60, and 120 minutes post injection with a handheld glucometer (ACCU-CHEK Advantage, Roche, Indianapolis, IN). Glucose clearance during the GTT was evaluated by calculating the total area under the curve (AUC) using the trapezoidal method[17].
Hepatic TAG, Histology, and enzyme activities
Intrahepatic triacylglycerol (TAG) content, citrate synthase activity, and 3-hydroxyacyl-CoA dehydrogenase (β-HAD) activity were measured as described previously [18]. Formalin-fixed livers were embedded in paraffin, serially sliced, and stained with hematoxylin and eosin as previously referenced [18].
Palmitate Oxidation
At euthanasia, 500 mg of liver was excised and immediately placed on ice in 25 mL of DMEM containing 1% BSA (Millipore, Billerica, MA). Liver explant cultures were prepared within 10 minutes of sample collection by slicing liver tissue to a thickness of 0.5 mm in a Thomas Stadie-Riggs Tissue Slicer (Thomas Scientific, Swedesboro, NJ) and then subdividing to smaller pieces (20 to 30 mg) using razor blades. Explants were added to 20 mL flasks containing 2.0 mL DMEM and 1.0 mM [1-14C]palmitate (0.45 μCi; American Radiolabelled Chemicals, St Louis, MO) in complex with fatty acid-free BSA (Millipore, Billerica, MA) in a 4:1 molar ratio. Flasks were gassed for 2 sec with carbogen (95% O2 and 5% CO2), sealed with a stopper fitted with a Kontes hanging center well (Kimbal Chase, Vineland, NJ) and placed in a 37 C in an orbital shaking water bath. After 2 h, incubations were terminated by the addition of 0.2 mL of 40% HClO4 into the incubation medium and 14CO2 was collected by addition of 0.2 mL of phenethylamine to the hanging center well trap [19]. Accumulation of 14CO2 was determined by liquid scintillation counting. Tissue was removed, blotted and weighed and oxidization data expressed as nmol of [1-14C]palmitate converted to 14[CO2] · g tissue−1 · h−1.
Western blots
Western blot analysis was completed in whole liver homogenate. Primary antibodies used are as follows: acetyl coenzyme A carboxylase (ACC; #3662, Cell Signaling, Danvers, MA, USA), S79 phosphorylation specific ACC (p-ACC; #3661, Cell Signaling); fatty acid synthase (FAS; #3189, Cell Signaling, Danvers, MA), CD36 (ab133625, Abcam, Cambridge, MA), microsomal triglyceride transfer protein (MTTP; #135994, Santa Cruz Biotechnology, Dallas, TX, USA), apolipoprotein B100 (ApoB100, #11795, Santa Cruz), peroxisome proliferator activator receptor-γ (PPARγ; #7273, Santacruz), AuTophagy related Gene 12 (ATG12, #4180, Cell Signaling), adenosine monophosphate activated protein kinase-α (AMPK; #2603, Cell Signaling) and T172 phosphorylated AMPK (p-AMPK; #2531, Cell Signaling). Blots (n=7-10/group) were analyzed via densometric analysis (Image Lab 3.0). Amido-black staining was used to control for differences in protein loading and transfer as previously described [18].
RT-PCR
Quantitative real time PCR was completed with the ABI 7500 Fast Sequence Detection System (Applied Biosystems, Carlsbad, CA) using Fast Sybr Green Master Mix (Applied Biosystems, Carlsbad, CA). Primers pairs were obtained from Sigma (St. Louis, MO, USA) for β-Actin (Forward: 5′-CAG AGC AAG AGA GGC ATC CTC-3′, Reverse: 5′-GTC CAG ACG CAG GAT GGC ATG-3′), PGC-1α (Forward: 5′- TTG ACT GGC GTC ATT CAG GA-3′; Reverse: 5′-GGC AGC ACA CTC TAT GTC ACT-3′), and TFAM (Forward: 5′-GAA TGT GGG GCG TGC TAA GA-3′; Reverse: 5′-CAG ATA AGG CTG ACA GGC GA-3′). PCR product dissociation melt curves were used to assess primer pair-target specificity. β-actin transcript abundance was not different between groups and was used as the reference gene to calculate the expression levels of genes of interest using the 2−ΔΔCT method. Data are normalized to expression levels of GS-CD.
Statistical Analysis
Statistical analyses were completed in R (v.2.15.1) with P < 0.05 used to determine statistical significance of all comparisons. Within sex comparisons across age were assessed via three-way ANOVA (condition X diet X age). Across sex comparisons were not made unless otherwise noted. Variables that were assessed at a single age were analyzed with a two-way ANOVA (condition X diet). Significant interactions were followed post-hoc with a Fisher’s LSD test with pooled standard deviation to determine individual group differences. Data are presented as means ± SE. n=7-10 observations are represented per group.
RESULTS
Dam characteristics
Portions of dam and offspring characteristics of this cohort of rats used in the current report have been reported previously [20, 21] and for convenience pertinent data are summarized in Table 1. Dams voluntarily ran an average of 2.7 km/day during gestational days (GD) 4-14, and thereafter progressively reduced running distance to a nadir of ~0.5 km/day by GD21 (Table 1). Neither gestational weight gain (Table 1) nor food intake (not shown) was different between GE and GS dams.
Table 1.
Maternal and offspring characteristics. GD, gestational day. Data are means ± SE.
| GS | GE | |
|---|---|---|
| Mean daily running distance (km/day) | ||
| First trimester (GD 1-7) | - | 2.6 ± 1.2 |
| Second trimester (GD 8-14) | - | 2.8 ± 1.6 |
| Third trimester (GD 15-21) | - | 0.8 ± 0.6 |
| Total gestational weight gain (g) | 150 ± 10.5 | 140 ± 12.5 |
| Litter size (N) | 13.7 ± 0.8 | 13.8 ± 0.4 |
| Litter weight (g) | 92.9 ± 4.6 | 93.2 ± 3.5 |
Offspring characteristics and glucose tolerance
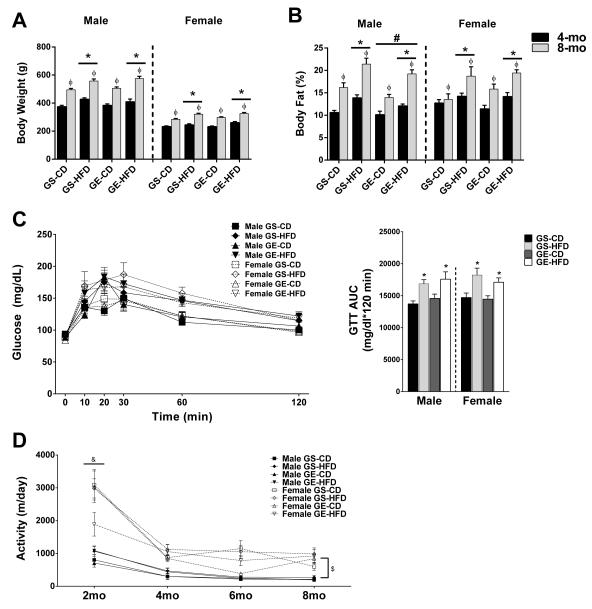
GE did not affect offspring birth weight, litter size, or male:female ratio (Table 1). HFD feeding significantly increased body weight (p<0.05, Figure 1A) and percent body fat (Figure 1B) in male and female offspring at both 4- and 8-months of age compared with CD fed counterparts. However, male offspring from GE dams had lower percent body fat than those from GS dams (Figure 1B; main effect for condition, p < 0.05). Based on reports of maternal exercise improving adult offspring glucose tolerance in an age and sex dependent manner, we assessed intraperitoneal (IP) glucose tolerance in offspring at 4- and 8-months of age. While GTT AUC was not different among groups in 4-mo rats (data not shown), HFD feeding resulted in significantly elevated glucose AUC in both male and female rats at 8-months of age (main effect for diet, p < 0.05) with no effect of GE (Figure 1C). To address the possibility that differences in spontaneous physical activity could account for reduced body fat percentage in GE male rats, we assessed voluntary wheel running activity for 3 day periods at 2-month intervals. While activity declined with age from 2 to 4 months in all groups and female rats voluntarily ran greater distances than male rats (Figure 1D), there was no effect of gestational exercise on this measure of spontaneous physical activity.
Figure 1.
Effects of age, GE, and HFD on offspring (A) bodyweight, (B) body fat percentage, (C) glucose tolerance in 8-mo offspring, and (D) voluntary physical activity. *, main effect of diet. ϕ, main effect of age. #, main effect of gestational condition. &, 2-mo activity greater than other ages. $, female activity greater than male activity.
Liver phenotype
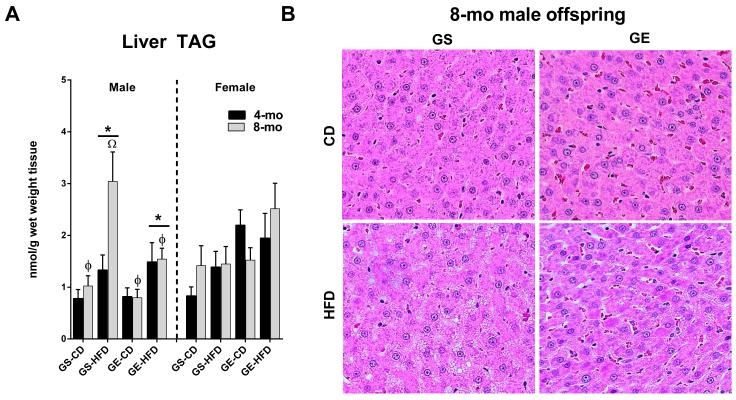
Male offspring fed the HFD had significantly increased hepatic TAG accumulation compared to CD fed male rats (Figure 2A, main effect for diet, p < 0.05). Remarkably, male offspring from GE dams were completely protected against the doubling in hepatic TAGs witnessed in the offspring from the GS dams that occurred from 4- to 8-mo of age (Figure 2A). This was paralleled by more advanced histological hepatic steatosis in GS-HFD rats relative to all other groups in 8-mo male cohort of offspring (Figure 2B). No differences were observed in hepatic TAG content in female offspring.
Figure 2.
Effects of age, GE, and HFD on offspring (A) liver TAG accumulation (B) histological hepatic steatosis in 8-mo male offspring. *, significant main effect of HFD. ϕ, main effect of age. Ω, 8-mo GS-HFD offspring greater than other groups by post-hoc pairwise comparison following significant 3-way interaction (age X diet X condition).
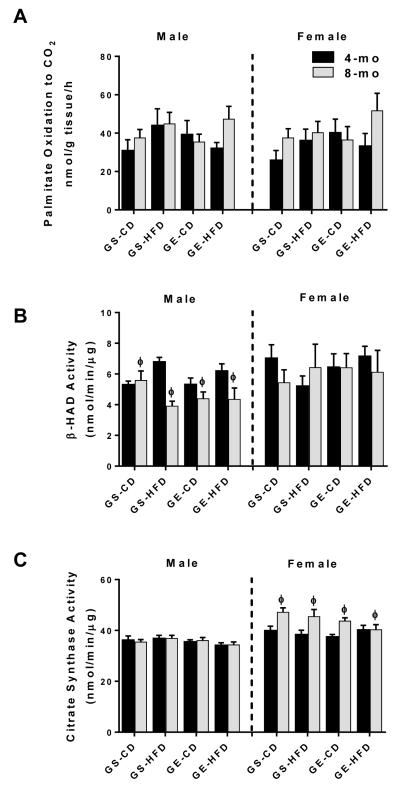
Reductions in hepatic mitochondrial content and function are common pathological features underlying NAFLD development and progression. Surprisingly, neither gestational exercise nor HFD feeding in male or female offspring significantly affected hepatic 1-[14C] palmitate oxidation to CO2. However, it was observed that β-HAD activity was reduced with age in male rats only (main effect for age, p < 0.05; Figure 3B), while citrate synthase activity was elevated with age in female rats (main effect for age, p < 0.05; Figure 3C). Collectively, these data suggest that the increase in hepatic steatosis in 8-mo male rats from GS mothers is not explained by these measures of β-oxidation or mitochondrial content.
Figure 3.
Effects of age, GE, and HFD on offspring (A) 1-[14C] palmitate oxidation, (B) β-HAD activity, and (C) citrate synthase activity in whole liver homogenate. ϕ, main effect of age.
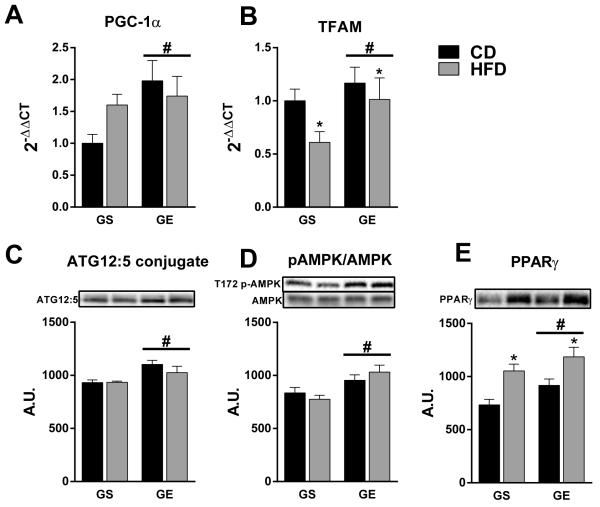
We then focused attention on evaluating the liver phenotype in 8-mo male offspring in order to gain mechanistic insight into the potential protective effects of gestational exercise on prevention of hepatic steatosis in older male offspring. PGC-1α is a putative marker of mitochondrial biogenesis that has previously been demonstrated to have a hepatoprotective role against NAFLD [22-24]. Accordingly, GE elevated hepatic expression of PGC-1α in 8-mo male offspring compared to counterparts from GS dams (main effect for condition, p < 0.05; Figure 4A). Similarly, TFAM mRNA was elevated by GE (main effect for condition, p < 0.05) and was reduced to a lesser extent by HFD (main effect for diet, p < 0.05) in GE (−13.2%) vs. GS offspring (−39.1%) (Figure 4B).
Figure 4.
Effects of GE and HFD on markers of mitochondrial biogenesis and autophagy in 8-mo male offspring. (A) PGC-1α mRNA, (B) TFAM mRNA, (C) ATG12:5 conjugation, (D) T172 p-AMPK/AMPK, (E) PPARγ protein. Representative western blots are presented above each respective graph. *, main effect of diet. #, main effect of gestational condition.
The observation that GE offspring had elevated markers of hepatic mitochondrial biogenesis without differences in mitochondrial content (Figure 3C) led us to hypothesize that autophagy may be elevated in order to maintain mitochondrial mass through enhanced turnover. Indeed, ATG12 conjugation to ATG5 was elevated in offspring from GE vs. GS dams (main effect for condition, p < 0.05; Figure 4C), indicating an increased potential for LC3 activation and autophagosomal formation. Further, activated (T172 phosphorylated) AMPK, which is stimulatory for autophagy, was elevated in GE offspring (Figure 4D; main effect for gestational condition, p < 0.05). Finally, we also observed an increase in PPARγ protein expression in offspring from GE dams (Figure 4E; main effects, p < 0.05). These data collectively suggest that offspring from GE mothers have elevated basal autophagic potential and enhanced mitochondrial biogenesis, which may serve to increase mitochondrial turnover and prevent the accumulation of dysfunctional mitochondria.
Next we sought to identify common proteins that are known to influence hepatic triglyceride balance. Eight-month old male offspring from GE dams had elevated hepatic MTTP expression (main effect for condition, p < 0.05) compared with GS offspring (Figure 5A), which is suggestive of increased hepatic triglyceride export. This was not related to ApoB100 levels, which was not influenced by gestational exercise and was reduced in HFD fed rats (main effect for diet, p < 0.05; Figure 5B). In addition, while there was no difference in hepatic CD36 among groups (Figure 5C), HFD feeding did result in expected increases S73 phosphorylated ACC (inactivation) and reduction in total ACC protein content and FAS protein content (Figure 5D-F).
Figure 5.
Effects of GE and HFD on markers of processes mediating hepatic triglyceride balance in 8-mo male offspring. (A) MTTP, (B) CD36, (D) S73 pACC/ACC, (E) ACC, and (F) FAS. Representative western blots are presented above each respective graph. *, significant main effect of HFD. #, significant main effect of GE.
DISCUSSION
Emerging pre-clinical evidence supports the concept that maternal exercise may improve the metabolic health of adult offspring [6-9]. The effect of maternal exercise on the adult offspring liver, however, has received little attention. Here, we report for the first time that gestational exercise in rats can protect adult offspring fed an obesogenic diet against the development of NAFLD. NAFLD is the most common chronic liver disease in the United States and is independently associated with increased mortality [14]. The underlying causes of NAFLD are an active area of investigation and the current findings suggest that a portion of obesity-related NAFLD risk may have maternal/fetal origins. Our data identify a potential role for gestational exercise-induced upregulation of hepatic mitochondrial biogenesis, autophagy, and TAG export that plays a protective role against HFD-induced hepatic steatosis in adult male offspring. These data indicate that the benefits of maternal exercise may extend well beyond the gestational/post-partum periods and into adulthood.
Hepatic mitochondrial dysfunction contributes to the development and progression of NAFLD [25]. We therefore sought to determine whether preserved mitochondrial function could account for the protection against hepatic steatosis seen in the HFD fed 8-mo male GE offspring. However, we did not observe any significant differences in our measures of hepatic β-oxidation or mitochondrial content (Figure 3). It is possible that other components of mitochondrial function, such as electron transport chain function and/or reactive oxygen species generation in isolated hepatic mitochondria, may be the primary site of dysfunction in this model and should be addressed in future studies. As we were unable to rule out a potential contribution of mitochondrial dysfunction, we shifted attention to focus on factors that could affect susceptibility to mitochondrial damage.
In the current study we demonstrate that gestational exercise increased hepatic PGC-1α mRNA in male offspring at 8-mo of age (Figure 4A) but not at 4-mo (data not shown) in association with protection against hepatic steatosis in response to chronic HFD feeding. This age dependent increase in PGC-1α mRNA is supported by a recent report by Laker et al. that demonstrated in mice that maternal exercise reduced PGC-1α promotor methylation in skeletal muscle of offspring and correspondingly increased PGC-1α transcript abundance in old (12-mo) but not young (4-mo) mice [7]. In contrast to the current report, however, these authors did not detect differences in hepatic PGC-1α promotor methylation or mRNA [7]. Differences in species and/or maternal exercise paradigm may explain this discrepancy (discussed in greater detail below). PGC-1α expression is known to be reduced in experimental NAFLD [22], and liver specific PGC-1α overexpression normalizes hepatic mitochondrial respiration and fat oxidation and prevents hepatic steatosis following an acute obesogenic challenge [26, 27]. One notable effect of PGC-1α activity is to increase mitochondrial biogenesis through augmented expression of the nuclear encoded mitochondrial transcription factor TFAM; the expression of which was concurrently increased with gestational exercise in the current study (Figure 4B). This finding suggests that gestational exercise may enhance the ability of offspring to generate new hepatic mitochondria.
Necessarily coupled to mitochondrial biogenesis is mitophagy (i.e. mitochondrial autophagy), which serves to prevent aberrant accumulation of mitochondria. Mice lacking the key mitophagy gene Bnip3 (BCL2/adenovirus E1B 19 kDa protein-interacting protein 3) have dramatic increases in hepatic mitochondrial mass, though most are fragmented, lack a membrane potential, and produce excess reactive oxygen species [28]. Given that mitochondrial content was not increased alongside increased mitochondrial biogenesis in GE offspring (Figure 3C and data not shown), we hypothesized that increased autophagy would be simultaneously increased to prevent the accumulation of excess, damaged mitochondria. Typically, assessment of autophagy requires the treatment of animals or cells with an inhibitor of autolysosomal degradation (e.g. chloroquine) in order to allow for the measurable accumulation of autophagic mediators, which we were unable to do post-hoc. Instead, we used the stable conjugation of ATG12 to ATG5 (ATG12:5) as a biomarker for autophagic potential. The ATG12:5 conjugate resides on isolation membranes (pre-autophagic structures) [29] and catalyzes the conjugation of LC3 to phosphadityl ethanolamine [30], which is the necessary committed step to mature autophagosome formation [31]. Consistent with our hypothesis, we demonstrate that offspring from GE dams have elevated ATG12:5 conjugation (Figure 4C), which we interpret to be indicative of increased autophagic potential. In further support of an increased autophagic potential in male offspring from GE dams is the finding that the activation status of hepatic AMPK, a canonical activator of autophagy [32], was elevated. Additionally, we observed elevated hepatic PPARγ expression, and while PPARγ has been shown to be pro-steatotic [15, 33], there are links between PPARγ (or PPARγ agonists) and autophagy stimulation [34-36]. When combined with amplified mitochondrial biogenesis, we propose a model in which gestational exercise may program male offspring to increased autophagic potential to prevent oxidant production and damage in response to a high-fat diet, presumably leading to preserved function of mitochondria and other organelles, and ultimately the mitigation of hepatic steatosis. Indeed, defective autophagy is linked to progression of NAFLD [37], while autophagy promotion can resolve hepatic steatosis [38]. This model needs more thorough examination in future investigations.
In addition to augmented mitochondrial biogenesis and autophagy in 8-mo male offspring from GE dams, we observed an upregulation in hepatic TAG export protein, MTTP, with both offspring HFD feeding and maternal gestational exercise. Pharmacological inhibitors of MTTP, which were developed in order to improve plasma lipid levels, increase hepatic steatosis owing to impaired TAG export (reviewed in [39]). In addition, patients with an MTTP polymorphism to reduce MTTP activity are more susceptible to hepatic steatosis [40, 41]. We also have recently demonstrated that prevention of western diet induced hepatic steatosis with dipeptidyl peptidase-4 inhibition was associated with increased hepatic MTTP expression and TAG secretion [42]. Thus, improved hepatic triglyceride export through enhanced MTTP expression may be another potential mechanism through which maternal exercise during gestation confers protection against HFD induced hepatic steatosis in male offspring.
A commonly held view regarding the underlying mechanism through which the maternal environment may influence the long term health of offspring is that various fetal exposures cause alterations in the DNA methylation status of various gene promotors (reviewed in [43]). Intriguingly, the expression of the key genes (PGC-1α, MTTP, PPARγ) described in the current study as being modulated by GE are sensitive to the methylation status of their respective promotors [7, 44, 45]. This observation further supports the current findings as lasting effects resulting from GE and should be examined in future investigations.
Contrary to a number of recent reports [6-9], gestational exercise did not affect offspring glucose tolerance in the current report, a finding likely related to differences in experimental design, age of testing, method of glucose delivery (oral vs. IP), paternal activity status, and/or animal strain/model. Our group demonstrated that 7 to 17 month old male and female ICR mice and 10 to 17 month old female CD/IGS Sprague–Dawley rats born to dams that voluntarily exercised [7-10 day prior to gestation, during gestation, and during lactation (approx. 45 days of wheel access)] had improved glucose homeostasis [6, 9]. Male rats were not tested as part of our previous studies. Goodyear and colleagues [8] have recently shown that gestational exercise only improved glucose tolerance in younger (8- and 12-wks) but not older (24-, 36-, 52-wks) male C57BL/6 mice offspring. In addition, offspring born to dams that were allowed wheel access before and during gestation had improved glucose tolerance at each age and were protected against maternal high fat diet-induced glucose intolerance in the male offspring [8]. In the present study, maternal exercise in Sprague-Dawley rats was restricted solely to the gestational period (days 4 to 21; 17 days of wheel access), which allows for the observed outcomes to be ascribed to engagement in exercise during gestation rather than increased maternal fitness prior to pregnancy. Thus, the present lack of differences in offspring glucose tolerance were likely because offspring did not reach the age (>10 months) at which we previously observed improvements and/or because dams only exercised during gestation and not prior to or during early pregnancy and nursing. These studies collectively provide insight into the potential role of intensity, timing, duration, and initiation of maternal exercise on different metabolic outcomes.
Healthy female rats commonly run >10 km/day [46], and previous reports by our group [6, 9] indicate that providing access to running wheels 7 to 10 days prior to pregnancy resulted in the exercise trained dams averaging ~4.5 km/day during pregnancy. This is in contrast to the current report, where wheel access beginning at day 4 of gestation resulted in much lower total volume of activity and lower average daily running distance (~2.5km/day). Despite this relatively low volume of gestational exercise and no differences observed in glucose tolerance assessed by IPGTT, 8-month old male offspring from exercising dams were protected against HFD-induced hepatic steatosis. Importantly, no adverse maternal or fetal outcomes were observed when previously sedentary rats began moderate physical activity concurrent with early pregnancy. Collectively, these observations suggest that engagement in moderate physical activity during healthy pregnancy, regardless of prior training status, may confer long-term benefits to hepatic health in the offspring without short-term complications.
In the current investigation we sought to detect potential mechanisms through which gestational exercise may influence the susceptibility of offspring to hepatic steatosis and identified hepatic mitochondrial quality control mechanisms and lipid export as promising candidates. In addition, GE modestly reduced % body fat in male offspring (main effect, Figure 1), which may contribute to the protection against HFD induced hepatic steatosis. However, it is unlikely that the modest reduction in % body fat in 8-mo GE-HFD vs GS-HFD (19.2% vs. 21.4%) accounts for the complete protection against HFD-induced hepatic steatosis. Other processes, including ketogenenesis which can be protective against NAFLD development [47], were not assessed in the current investigation and should be considered in subsequent studies. In addition, there is a paucity in the literature regarding the maternal exercise “signal” (e.g. altered uterine blood flow/oxygenation, catecholamines, nutrient composition, etc.) that reach the developing fetus to cause beneficial epigenetic and perhaps other lasting effects. Such efforts will be critical in order to design maternal exercise paradigms aimed at maximizing the symbiotic benefit to both mother and offspring.
In summary, here we provide the first evidence that moderate amounts of maternal exercise during gestation protect adult male offspring against high fat diet induced hepatic steatosis. This protection was associated with increased markers of hepatic mitochondrial biogenesis, autophagy, and hepatic TAG secretion/export. Our results encourage further study into the effects and underlying mechanisms responsible for maternal-exercise conferred protection against adult metabolic disease.
ACKNOWLEDGEMENTS
The authors would like to thank Grace Meers, Kayla Kanosky, Laura Taylor, and Tasnim Haq at the University of Missouri for their assistance with data collection. This work was supported with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO. Dr. R. Scott Rector is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
FUNDING
This work was supported by a University of Missouri Molecular Life Sciences pre-doctoral fellowship (RDS),VHA-CDA2 1 IK2 BX001299 (RSR), and the NIH R01 DK090460 (KJP).
Footnotes
DISCLOSURE STATEMENT
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Involved in the study concept and design (RDS, ANB, KJP, SD, SCN, RSR); acquisition of data (RDS, ANB, JAF, SD, SCN, RSR); analysis and interpretation of data (RDS, ANB, JAF, KJP, SD, SCN, RSR); drafting of the manuscript (RDS, RSR); critical revision of the manuscript for important intellectual content (RDS, ANB, JAF, KJP, SD, SCN, RSR); statistical analysis (RDS, RSR); obtained funding (RDS, RSR).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Mudd LM, Owe KM, Mottola MF, Pivarnik JM. Health benefits of physical activity during pregnancy: An international perspective. Med Sci Sports Exerc. 2013;45:268–277. doi: 10.1249/MSS.0b013e31826cebcb. [DOI] [PubMed] [Google Scholar]
- [2].Borodulin KM, Evenson KR, Wen F, Herring AH, Benson AM. Physical activity patterns during pregnancy. Med Sci Sports Exerc. 2008;40:1901–1908. doi: 10.1249/MSS.0b013e31817f1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Entin PL, Munhall KM. Recommendations regarding exercise during pregnancy made by private/small group practice obstetricians in the USA. J Sport Sci Med. 2006;5:449–458. [PMC free article] [PubMed] [Google Scholar]
- [4].Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gluckman PD, Hanson MA. Developmental origins of disease paradigm: A mechanistic and evolutionary perspective. Pediatr Res. 2004;56:311–317. doi: 10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- [6].Carter LG, Qi NR, De Cabo R, Pearson KJ. Maternal exercise improves insulin sensitivity in mature rat offspring. Med Sci Sports Exerc. 2013;45:832–840. doi: 10.1249/MSS.0b013e31827de953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Laker RC, Lillard TS, Okutsu M, Zhang M, Hoehn KL, Connelly JJ, et al. Exercise prevents maternal high-fat diet-induced hypermethylation of the pgc-1alpha gene and age-dependent metabolic dysfunction in the offspring. Diabetes. 2014;63:1605–1611. doi: 10.2337/db13-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stanford KI, Lee MY, Getchell KM, So K, Hirshman MF, Goodyear LJ. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes. 2015;64:427–433. doi: 10.2337/db13-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carter LG, Lewis KN, Wilkerson DC, Tobia CM, Ngo Tenlep SY, Shridas P, et al. Perinatal exercise improves glucose homeostasis in adult offspring. Am J Physiol Endocrinol Metab. 2012;303:E1061–1068. doi: 10.1152/ajpendo.00213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rector RS, Thyfault JP. Does physical inactivity cause nonalcoholic fatty liver disease? J Appl Physiol. 2011;111:1828–1835. doi: 10.1152/japplphysiol.00384.2011. [DOI] [PubMed] [Google Scholar]
- [11].Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the united states: Impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- [12].Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the united states from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. e521. doi: 10.1016/j.cgh.2011.03.020. quiz e560. [DOI] [PubMed] [Google Scholar]
- [13].Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- [14].Byrne CD, Targher G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease: Implications for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34:1155–1161. doi: 10.1161/ATVBAHA.114.303034. [DOI] [PubMed] [Google Scholar]
- [15].Alfaradhi MZ, Fernandez-Twinn DS, Martin-Gronert MS, Musial B, Fowden A, Ozanne SE. Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am J Physiol Regul Integr Comp Physiol. 2014;307:R26–34. doi: 10.1152/ajpregu.00049.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Spangenberg EM, Augustsson H, Dahlborn K, Essen-Gustavsson B, Cvek K. Housing-related activity in rats: Effects on body weight, urinary corticosterone levels, muscle properties and performance. Lab Anim. 2005;39:45–57. doi: 10.1258/0023677052886457. [DOI] [PubMed] [Google Scholar]
- [17].Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care. 1994;17:152–154. doi: 10.2337/diacare.17.2.152. [DOI] [PubMed] [Google Scholar]
- [18].Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, et al. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in otsuka long-evans tokushima fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G619–626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- [19].Donkin SS, Armentano LE. Regulation of gluconeogenesis by insulin and glucagon in the neonatal bovine. Am J Physiol. 1994;266:R1229–1237. doi: 10.1152/ajpregu.1994.266.4.R1229. [DOI] [PubMed] [Google Scholar]
- [20].Camarillo IG, Clah L, Zheng W, Zhou X, Larrick B, Blaize N, et al. Maternal exercise during pregnancy reduces risk of mammary tumorigenesis in rat offspring. Eur J Cancer Prev. 2014;23:502–505. doi: 10.1097/CEJ.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nicole Blaize A, Breslin E, Donkin SS, Cabot R, Pearson KJ, Newcomer SC. Maternal exercise does not significantly alter adult rat offspring vascular function. Med Sci Sports Exerc. 2015 doi: 10.1249/MSS.0000000000000665. [DOI] [PubMed] [Google Scholar]
- [22].Aharoni-Simon M, Hann-Obercyger M, Pen S, Madar Z, Tirosh O. Fatty liver is associated with impaired activity of ppargamma-coactivator 1alpha (pgc1alpha) and mitochondrial biogenesis in mice. Lab Invest. 2011;91:1018–1028. doi: 10.1038/labinvest.2011.55. [DOI] [PubMed] [Google Scholar]
- [23].Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, et al. Pgc-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2012;303:G979–G992. doi: 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Morris EM, Jackman MR, Meers GM, Johnson GC, Lopez JL, MacLean PS, et al. Reduced hepatic mitochondrial respiration following acute high-fat diet is prevented by pgc-1α overexpression. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2013;305:G868–G880. doi: 10.1152/ajpgi.00179.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52:727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morris EM, Jackman MR, Meers GM, Johnson GC, Lopez JL, MacLean PS, et al. Reduced hepatic mitochondrial respiration following acute high-fat diet is prevented by pgc-1alpha overexpression. Am J Physiol Gastrointest Liver Physiol. 2013;305:G868–880. doi: 10.1152/ajpgi.00179.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, et al. Pgc-1alpha overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol. 2012;303:G979–992. doi: 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Glick D, Zhang W, Beaton M, Marsboom G, Gruber M, Simon MC, et al. Bnip3 regulates mitochondrial function and lipid metabolism in the liver. Mol Cell Biol. 2012;32:2570–2584. doi: 10.1128/MCB.00167-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, et al. Dissection of autophagosome formation using apg5-deficient mouse embryonic stem cells. Journal of Cell Biology. 2001;152:657–667. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, et al. The atg12-atg5 conjugate has a novel e3-like activity for protein lipidation in autophagy. Journal of Biological Chemistry. 2007;282:37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- [31].Kabeya Y, Mizushima N, Uero T, Yamamoto A, Kirisako T, Noda T, et al. Lc3, a mammalian homologue of yeast apg8p, is localized in autophagosome membranes after processing. Embo Journal. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mack HI, Zheng B, Asara JM, Thomas SM. Ampk-dependent phosphorylation of ulk1 regulates atg9 localization. Autophagy. 2012;8:1197–1214. doi: 10.4161/auto.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Morán-Salvador E, López-Parra M, García-Alonso V, Titos E, Martínez-Clemente M, González-Périz A, et al. Role for pparγ in obesity-induced hepatic steatosis as determined by hepatocyte-and macrophage-specific conditional knockouts. The FASEB Journal. 2011;25:2538–2550. doi: 10.1096/fj.10-173716. [DOI] [PubMed] [Google Scholar]
- [34].Shin T, Kuboki S, Huber N, Eismann T, Galloway E, Schuster R, et al. Activation of peroxisome proliferator-activated receptor-gamma during hepatic ischemia is age-dependent. J Surg Res. 2008;147:200–205. doi: 10.1016/j.jss.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xu F, Li J, Ni W, Shen YW, Zhang XP. Peroxisome proliferator-activated receptor-gamma agonist 15d-prostaglandin j2 mediates neuronal autophagy after cerebral ischemia-reperfusion injury. PLoS One. 2013;8:e55080. doi: 10.1371/journal.pone.0055080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rovito D, Giordano C, Vizza D, Plastina P, Barone I, Casaburi I, et al. Omega-3 pufa ethanolamides dhea and epea induce autophagy through ppargamma activation in mcf-7 breast cancer cells. J Cell Physiol. 2013;228:1314–1322. doi: 10.1002/jcp.24288. [DOI] [PubMed] [Google Scholar]
- [37].Gonzalez-Rodriguez A, Mayoral R, Agra N, Valdecantos MP, Pardo V, Miquilena-Colina ME, et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of nafld. Cell Death & Disease. 2014;5:e1179. doi: 10.1038/cddis.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lin CW, Zhang H, Li M, Xiong X, Chen X, Chen X, et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol. 2013;58:993–999. doi: 10.1016/j.jhep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hussain MM, Bakillah A. New approaches to target microsomal triglyceride transfer protein. Curr Opin Lipidol. 2008;19:572–578. doi: 10.1097/MOL.0b013e328312707c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jun DW, Han JH, Jang EC, Kim SH, Kim SH, Jo YJ, et al. Polymorphisms of microsomal triglyceride transfer protein gene and phosphatidylethanolamine n-methyltransferase gene in alcoholic and nonalcoholic fatty liver disease in koreans. Eur J Gastroenterol Hepatol. 2009;21:667–672. doi: 10.1097/MEG.0b013e3283196adc. [DOI] [PubMed] [Google Scholar]
- [41].Hashemi M, Hoseini H, Yaghmaei P, Moazeni-Roodi A, Bahari A, Hashemzehi N, et al. Association of polymorphisms in glutamate-cysteine ligase catalytic subunit and microsomal triglyceride transfer protein genes with nonalcoholic fatty liver disease. DNA Cell Biol. 2011;30:569–575. doi: 10.1089/dna.2010.1162. [DOI] [PubMed] [Google Scholar]
- [42].Aroor AR, Habibi J, Ford DA, Nistala R, Lastra G, Manrique C, et al. Dipeptidyl peptidase-4 inhibition ameliorates western diet-induced hepatic steatosis and insulin resistance through hepatic lipid remodeling and modulation of hepatic mitochondrial function. Diabetes. 2015;64:1988–2001. doi: 10.2337/db14-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chmurzynska A. Fetal programming: Link between early nutrition, DNA methylation, and complex diseases. Nutrition Reviews. 2010;68:87–98. doi: 10.1111/j.1753-4887.2009.00265.x. [DOI] [PubMed] [Google Scholar]
- [44].Fujiki K, Kano F, Shiota K, Murata M. Expression of the peroxisome proliferator activated receptor gamma gene is repressed by DNA methylation in visceral adipose tissue of mouse models of diabetes. Bmc Biology. 2009;7:38. doi: 10.1186/1741-7007-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chang X, Yan H, Fei J, Jiang M, Zhu H, Lu D, et al. Berberine reduces methylation of the mttp promoter and alleviates fatty liver induced by a high-fat diet in rats. J Lipid Res. 2010;51:2504–2515. doi: 10.1194/jlr.M001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Manabe Y, Gollisch KS, Holton L, Kim YB, Brandauer J, Fujii NL, et al. Exercise training-induced adaptations associated with increases in skeletal muscle glycogen content. FEBS J. 2013;280:916–926. doi: 10.1111/febs.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cotter DG, Ercal B, Huang X, Leid JM, d’Avignon DA, Graham MJ, et al. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J Clin Invest. 2014;124:5175–5190. doi: 10.1172/JCI76388. [DOI] [PMC free article] [PubMed] [Google Scholar]