Abstract
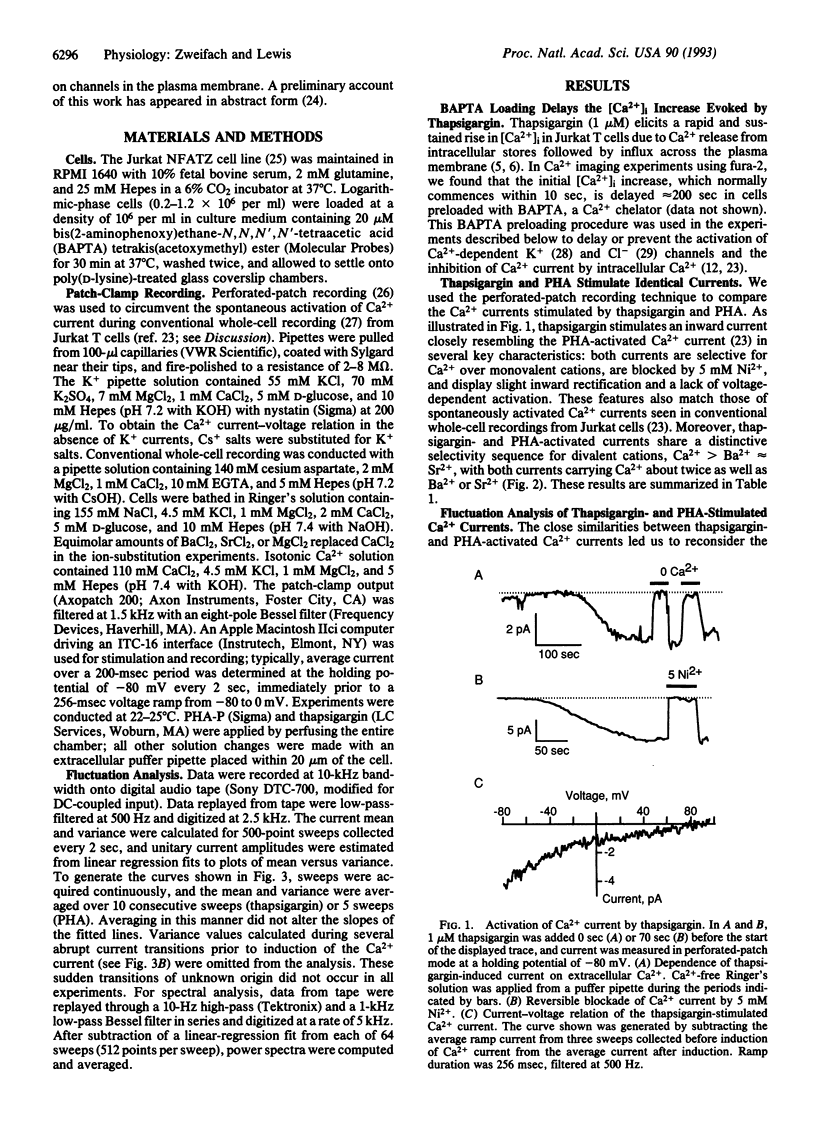
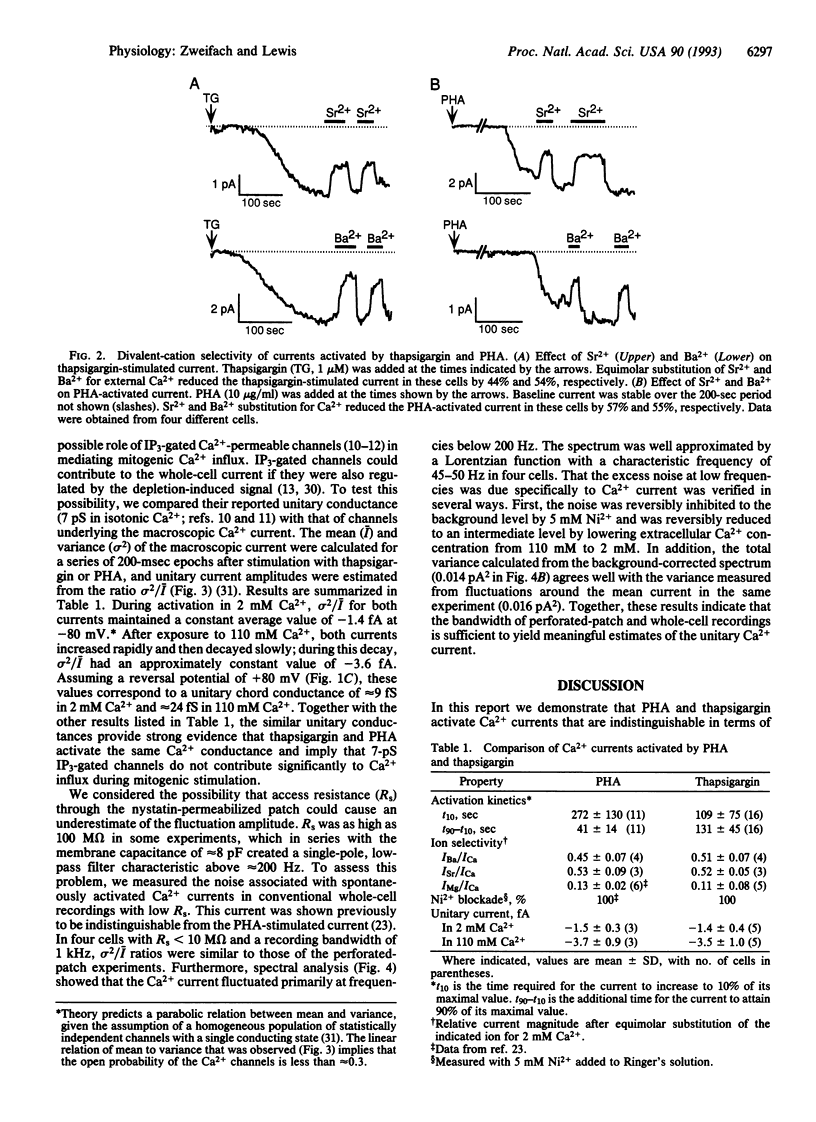
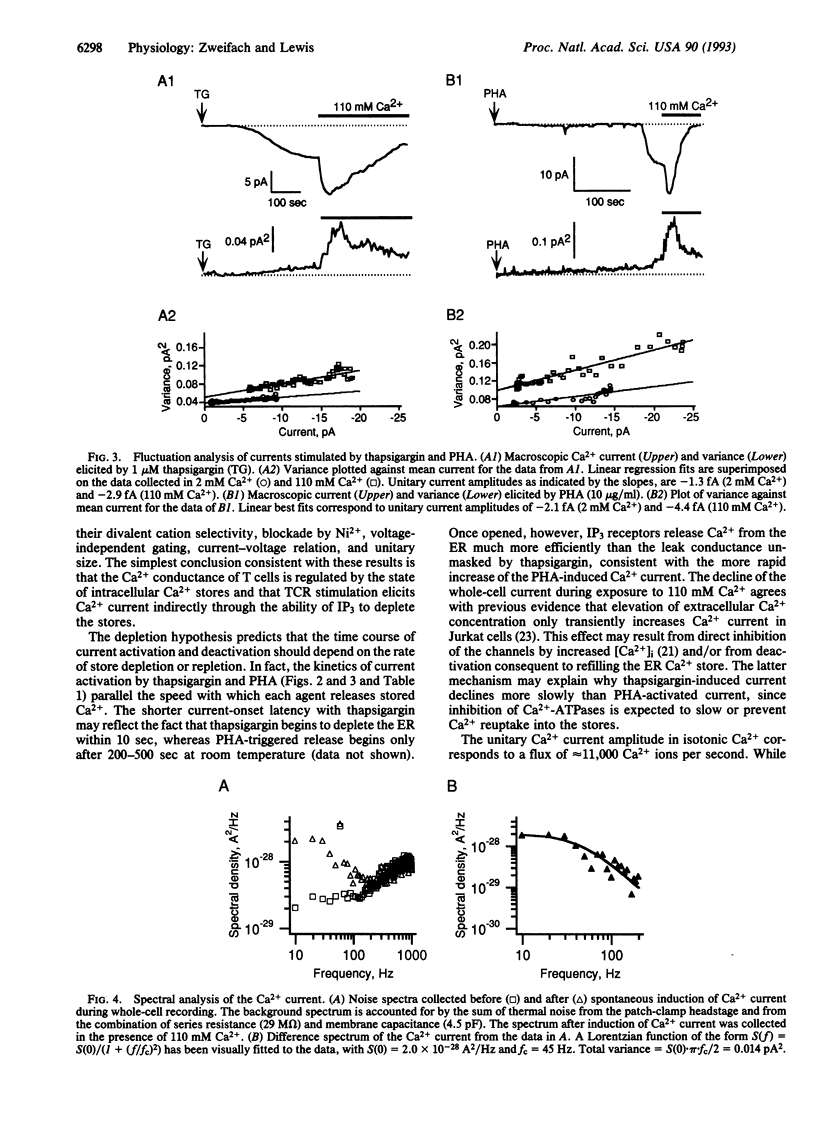
Stimulated influx of Ca2+ across the plasma membrane of T lymphocytes is an essential triggering signal for T-cell activation by antigen. Regulation of the T-cell Ca2+ conductance is not understood; conflicting evidence supports direct activation by inositol 1,4,5-trisphosphate (IP3) or by a signal generated by the depletion of intracellular Ca2+ stores. We have used the perforated-patch recording technique to compare the biophysical properties of Ca2+ currents activated by T-cell receptor stimulation and by thapsigargin, a Ca(2+)-ATPase inhibitor that depletes intracellular stores without generating IP3. Both currents are blocked by Ni2+, are inwardly rectifying, are highly Ca(2+)-selective, and exhibit voltage-independent gating with a unitary chord conductance of approximately 24 fS in isotonic Ca2+. Fluctuation analysis suggests that the underlying Ca2+ transporter is a channel rather than an iron carrier. Thus, in terms of ion permeation, gating, and unitary conductance, the Ca2+ current activated by thapsigargin is indistinguishable from the elicited by crosslinking of T-cell receptors. Moreover, the unitary Ca2+ conductance is > 100-fold smaller than that of previously described IP3-gated, Ca(2+)-permeable channels in T cells [Kuno, M. & Gardner, P. (1987) Nature (London) 326, 301-304]. These results demonstrate that mitogen-activated Ca2+ influx is controlled by the state of intracellular Ca2+ stores rather than by the direct action of IP3 on Ca2+ channels in the plasma membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Ehrlich B. E., Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988 Dec 8;336(6199):583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Fiering S., Northrop J. P., Nolan G. P., Mattila P. S., Crabtree G. R., Herzenberg L. A. Single cell assay of a transcription factor reveals a threshold in transcription activated by signals emanating from the T-cell antigen receptor. Genes Dev. 1990 Oct;4(10):1823–1834. doi: 10.1101/gad.4.10.1823. [DOI] [PubMed] [Google Scholar]
- Gardner P., Alcover A., Kuno M., Moingeon P., Weyand C. M., Goronzy J., Reinherz E. L. Triggering of T-lymphocytes via either T3-Ti or T11 surface structures opens a voltage-insensitive plasma membrane calcium-permeable channel: requirement for interleukin-2 gene function. J Biol Chem. 1989 Jan 15;264(2):1068–1076. [PubMed] [Google Scholar]
- Goldsmith M. A., Weiss A. Early signal transduction by the antigen receptor without commitment to T cell activation. Science. 1988 May 20;240(4855):1029–1031. doi: 10.1126/science.3259335. [DOI] [PubMed] [Google Scholar]
- Gouy H., Cefai D., Christensen S. B., Debré P., Bismuth G. Ca2+ influx in human T lymphocytes is induced independently of inositol phosphate production by mobilization of intracellular Ca2+ stores. A study with the Ca2+ endoplasmic reticulum-ATPase inhibitor thapsigargin. Eur J Immunol. 1990 Oct;20(10):2269–2275. doi: 10.1002/eji.1830201016. [DOI] [PubMed] [Google Scholar]
- Grissmer S., Lewis R. S., Cahalan M. D. Ca(2+)-activated K+ channels in human leukemic T cells. J Gen Physiol. 1992 Jan;99(1):63–84. doi: 10.1085/jgp.99.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Rabinovitch P. S., Martin P. J., Beatty P. G., Hansen J. A. Distinct patterns of transmembrane calcium flux and intracellular calcium mobilization after differentiation antigen cluster 2 (E rosette receptor) or 3 (T3) stimulation of human lymphocytes. J Clin Invest. 1986 Apr;77(4):1224–1232. doi: 10.1172/JCI112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. A., Steiner J. P., Klein M. G., Schneider M. F., Snyder S. H. IP3 receptor: localization to plasma membrane of T cells and cocapping with the T cell receptor. Science. 1992 Aug 7;257(5071):815–818. doi: 10.1126/science.1323146. [DOI] [PubMed] [Google Scholar]
- Khan A. A., Steiner J. P., Snyder S. H. Plasma membrane inositol 1,4,5-trisphosphate receptor of lymphocytes: selective enrichment in sialic acid and unique binding specificity. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2849–2853. doi: 10.1073/pnas.89.7.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Kuno M., Goronzy J., Weyand C. M., Gardner P. Single-channel and whole-cell recordings of mitogen-regulated inward currents in human cloned helper T lymphocytes. Nature. 1986 Sep 18;323(6085):269–273. doi: 10.1038/323269a0. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Cahalan M. D. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989 Nov;1(1):99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopis J., Chow S. B., Kass G. E., Gahm A., Orrenius S. Comparison between the effects of the microsomal Ca(2+)-translocase inhibitors thapsigargin and 2,5-di-(t-butyl)-1,4-benzohydroquinone on cellular calcium fluxes. Biochem J. 1991 Jul 15;277(Pt 2):553–556. doi: 10.1042/bj2770553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Mason M. J., Garcia-Rodriguez C., Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991 Nov 5;266(31):20856–20862. [PubMed] [Google Scholar]
- Mason M. J., Mahaut-Smith M. P., Grinstein S. The role of intracellular Ca2+ in the regulation of the plasma membrane Ca2+ permeability of unstimulated rat lymphocytes. J Biol Chem. 1991 Jun 15;266(17):10872–10879. [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Second messenger-activated calcium influx in rat peritoneal mast cells. J Physiol. 1989 Nov;418:105–130. doi: 10.1113/jphysiol.1989.sp017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Nishimoto I., Wagner J. A., Schulman H., Gardner P. Regulation of Cl- channels by multifunctional CaM kinase. Neuron. 1991 Apr;6(4):547–555. doi: 10.1016/0896-6273(91)90057-7. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Tordai A., Homolya L., Scharff O., Gárdos G. Calcium influx and intracellular calcium release in anti-CD3 antibody-stimulated and thapsigargin-treated human T lymphoblasts. J Membr Biol. 1991 Jul;123(1):9–21. doi: 10.1007/BF01993958. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B., Thastrup O., Hofmann B., Møller J., Ryder L. P., Jacobsen K. D., Langhoff E., Dickmeiss E., Christensen S. B. Effect of thapsigargin on cytoplasmic Ca2+ and proliferation of human lymphocytes in relation to AIDS. Biochim Biophys Acta. 1988 Dec 9;972(3):257–264. doi: 10.1016/0167-4889(88)90200-5. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Hess P., McCleskey E. W., Rosenberg R. L. Calcium channels: mechanisms of selectivity, permeation, and block. Annu Rev Biophys Biophys Chem. 1987;16:265–290. doi: 10.1146/annurev.bb.16.060187.001405. [DOI] [PubMed] [Google Scholar]
- Weiss A., Koretzky G., Schatzman R. C., Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-gamma 1. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5484–5488. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]


