Abstract
Objective:
To study the safety of off-label IV thrombolysis in patients with very severe stroke (NIH Stroke Scale [NIHSS] scores >25) compared with severe stroke (NIHSS scores 15–25), where treatment is within European regulations.
Methods:
Data were analyzed from 57,247 patients with acute ischemic stroke receiving IV tissue plasminogen activator in 793 hospitals participating in the Safe Implementation of Thrombolysis in Stroke (SITS) International Stroke Thrombolysis Registry (2002–2013). Eight hundred sixty-eight patients (1.5%) had NIHSS scores >25 and 19,995 (34.9%) had NIHSS scores 15–25. Outcome measures were parenchymal hemorrhage, symptomatic intracerebral hemorrhage, mortality, and functional outcome.
Results:
Parenchymal hemorrhage occurred in 10.7% vs 11.0% (p = 0.79), symptomatic intracerebral hemorrhage per SITS-MOST (SITS–Monitoring Study) in 1.4% vs 2.5% (p = 0.052), death at 3 months in 50.4% vs 26.9% (p < 0.001), and functional independence at 3 months in 14.0% vs 29.0% (p < 0.001) of patients with NIHSS scores >25 and NIHSS scores 15–25, respectively. Multivariate adjustment did not change findings from univariate comparisons. Posterior circulation stroke was more common in patients with NIHSS scores >25 (36.2% vs 7.4%, p < 0.001), who were also more often obtunded or comatose on presentation (58.4% vs 7.1%, p < 0.001). Of patients with NIHSS scores >25, 26.2% were treated >3 hours from symptom onset vs 14.5% with NIHSS scores of 15–25.
Conclusions:
Our data show no excess risk of cerebral hemorrhage in patients with NIHSS score >25 compared to score 15–25, suggesting that the European contraindication to IV tissue plasminogen activator treatment at NIHSS levels >25 may be unwarranted. Increased mortality and lower rates of functional independence in patients with NIHSS score >25 are explained by higher stroke severity, impaired consciousness on presentation due to posterior circulation ischemia, and longer treatment delays.
Evidence has accumulated on treatment with IV thrombolysis for acute ischemic stroke in initially understudied patient groups: those older than 80 years, with a history of stroke and diabetes, and treated with anticoagulants.1–3 This is not yet the case for very severe stroke defined as NIH Stroke Scale (NIHSS) scores above 25 points. Here, the European Medicines Agency license for alteplase contraindicates treatment, stating: “patients with very severe stroke are at higher risk for intracerebral hemorrhage (ICH) and death and should not be treated.”4 Formulated in 2003, this was not based on high-grade evidence, as several trials at the time had excluded such patients.5,6 Later results from the Virtual International Stroke Trials Archive (VISTA) collaboration and the Third International Stroke Trial (IST-3) trial showed improved outcomes in patients who received thrombolysis with NIHSS scores ≥25, although lacking statistical significance; neither study reported on ICH or symptomatic ICH (SICH) in these patients.1,7
Increasing stroke severity is associated with higher risk of SICH.8,9 During the development of the SITS Risk Score, a predictive instrument for thrombolysis-related SICH,10 we found indications that the risk may plateau at high NIHSS levels. We hypothesized that there may not be a significant increase in ICH risk following thrombolysis in very severe stroke defined as NIHSS scores >25 (off-label in Europe), compared to severe stroke for which treatment is within EU regulations, i.e., NIHSS scores 15 to 25. The current study aims to investigate this hypothesis and compare clinical characteristics, mortality, causes of death, and functional outcomes between these patient groups.
METHODS
Patients.
All patients recorded in the SITS–International Stroke Thrombolysis Register (SITS-ISTR) between December 2002 and April 2013 treated with IV alteplase (Actilyse, Boehringer-Ingelheim, Germany) were considered (n = 58,293), and only patients with available baseline NIHSS score were included in this study (n = 57,247; 98.2%). These were enrolled at 793 centers in 44 countries, 95% in European hospitals.
The SITS-ISTR is an ongoing, prospective, academic-driven, multinational register for centers using thrombolysis for the treatment of acute ischemic stroke. The methodology of the SITS-ISTR, including procedures for data collection and management, patient identification, and verification of source data, has been described previously.11,12 Consecutive case reporting is a basic commitment for participating in the SITS-ISTR. We collected baseline and demographic characteristics, stroke severity per the standard 11-item, 42-point NIHSS, time logistics, medication history, and imaging data on admission and follow-up.
We defined very severe stroke as baseline NIHSS scores higher than 25 points and severe stroke as NIHSS scores 15 to 25. In total, 868 patients (1.5%) had very severe stroke, while 19,995 (34.9%) had severe stroke. In October 2010, the SITS-ISTR implemented the option to register information on the arterial territory affected by the stroke. Territory data were available in 23.6% of all cases with NIHSS scores 15–25 and >25 (total n = 4,922/20,863).
Standard protocol approvals, registrations, and patient consents.
Ethics approval and patient consent for participation in the SITS-ISTR were obtained in countries that required this; other countries approved the register for anonymized audit. The SITS International Coordination Office monitored the SITS-ISTR data online and checked individual patient data monthly to identify errors or inconsistencies.
Outcome measurements.
The following definitions of cerebral hemorrhage were used:
Any parenchymal hemorrhage (PH), local or remote, regardless of size or type (PH1, PHr1, PH2, PHr2) on any posttreatment imaging scans during hospitalization.
PH type 2 (PH2) or remote (extraischemic) PH type 2 (PHr2) on any posttreatment imaging scans during hospitalization. “Type 2” indicates hematoma exceeding 30% of the infarct (for PH2), with substantial space-occupying effect (both PH2 and PHr2).
SICH per SITS-MOST: PH2 or PHr2 on imaging 22 to 36 hours after treatment, or earlier if scanned because of clinical deterioration, combined with a neurologic deterioration of ≥4 NIHSS points or leading to death within 24 hours.
SICH per European Cooperative Acute Stroke Study II (ECASS II): any ICH on any posttreatment imaging after the start of thrombolysis and increase of ≥4 NIHSS points or leading to death, within 7 days.
SICH per National Institute of Neurological Diseases and Stroke (NINDS): any ICH on any posttreatment imaging and any deterioration in NIHSS or death within 7 days.
All SICH events were adjudicated centrally by the SITS International Coordination Office based on submitted clinical and imaging reports; images were not available for review.
Rates of mortality were assessed at 7 days and 3 months. Functional outcome per the modified Rankin Scale (mRS) was evaluated at 3 months. For patients deceased within 7 days and 3 months, we compared frequencies of various causes of death, as well as the median time from treatment to death. All assessments of imaging studies and neurologic and functional status were done according to clinical routine at centers participating in the SITS-ISTR.
Statistical analysis.
We performed descriptive univariate statistics for baseline clinical, demographic, and imaging data, as well as outcomes, causes of death, and time to death, comparing patients with very severe (NIHSS >25) and severe (NIHSS 15–25) stroke. For continuous and ordinal variables, median and interquartile range values were calculated. For categorical variables, we calculated percentage proportions by dividing the number of events by the total number of patients, excluding missing or unknown cases, as done in previous SITS publications.1,9 For calculation of significance of difference between medians and proportions, we used the Mann–Whitney U test and the Pearson χ2 method, respectively. For calculation of the 95% confidence intervals of proportions for PH and SICH, the Wilson score method with continuity correction was used.13 The association between NIHSS and all hemorrhagic outcome definitions was investigated graphically. We plotted the predicted probabilities of the respective hemorrhagic outcomes against individual values of the NIHSS. The fitted values were obtained from a univariate logistic model in which NIHSS was included by means of a natural cubic spline. Computation was implemented using the splines package in R, and the number of knots was selected based on the Akaike Information Criterion. To investigate the relationships between severe and very severe stroke and PH, SICH, functional outcomes, and mortality, multivariate logistic regression analyses were performed adjusting for factors known from previous publications to be associated with these endpoints. Specifically, analyses regarding all PH and SICH definitions were adjusted for age, blood glucose, systolic blood pressure, onset-to-treatment time, body weight, diabetes mellitus, hypertension, use of aspirin, hyperdense cerebral artery sign, and visible infarct signs on baseline CT.10,14 Analyses of mortality within 7 days and 3 months and functional outcomes at 3 months were adjusted for all factors listed above with the addition of atrial fibrillation, congestive heart disease, and previous stroke.3,8 Analyses were performed using STATISTICA 12 (StatSoft, Tulsa, OK) and R 3.1.1.
RESULTS
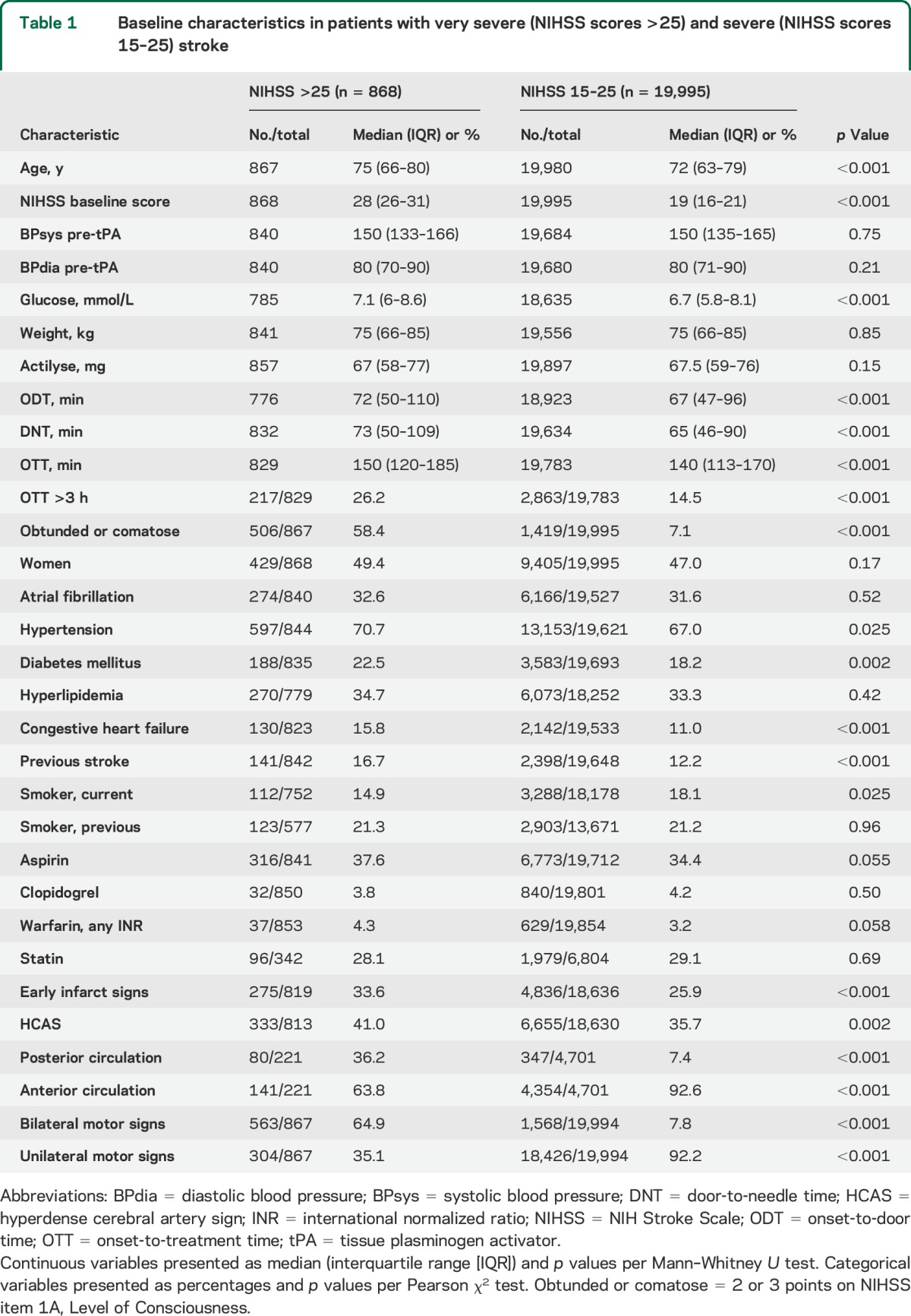
Baseline characteristics of the studied groups are shown in table 1. The very severe stroke group had a higher median age, 75 vs 72 years, and higher NIHSS scores, 28 vs 19 points. The proportion of patients presenting in an obtunded or comatose state, receiving 2 or 3 points on the 1A Level of Consciousness subscale of the NIHSS was 58.4% in the NIHSS >25 group compared to 7.1% in the NIHSS 15–25 group. Bilateral motor signs were present in 64.9% in the very severe and 7.8% in the severe stroke group. Stroke in the posterior circulation occurred in 36.2% of patients with NIHSS >25, compared to 7.4% in NIHSS 15–25. Patients with NIHSS scores >25 were treated later, with an onset-to-treatment time of 150 vs 140 minutes, and a longer door-to-needle time of 73 vs 65 minutes. CT was used as the initial imaging modality in 97.2% of cases. Early infarct signs on baseline imaging scans were observed in 33.6% of patients in the NIHSS >25 group and 25.9% in the NIHSS 15–25 group, while the hyperdense cerebral artery sign was seen in 41.0% vs 35.7%. The groups were similar regarding sex, blood pressure, body weight, and total alteplase dose, diagnoses of atrial fibrillation and hypertension, as well as baseline treatment with antiplatelet, anticoagulant, and cholesterol-lowering drugs. A total of 29 of 868 patients (3.3%) with NIHSS score >25 and 308 of 19,995 patients (1.5%) with NIHSS score 15–25 were recorded as treated with endovascular thrombectomy after IV thrombolysis.
Table 1.
Baseline characteristics in patients with very severe (NIHSS scores >25) and severe (NIHSS scores 15–25) stroke
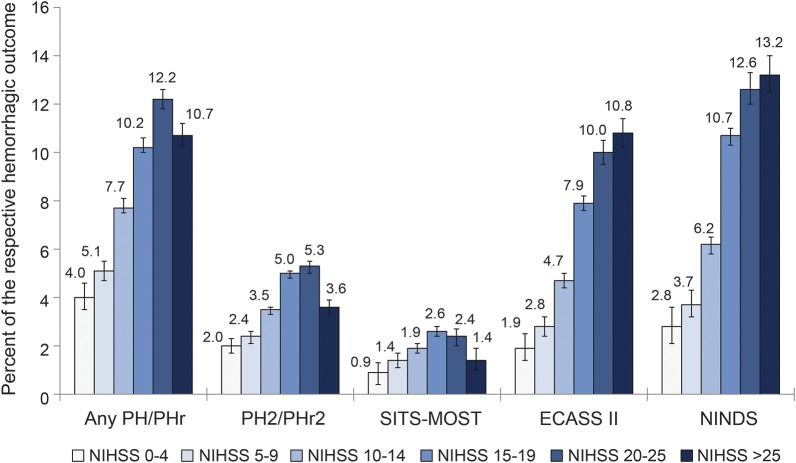
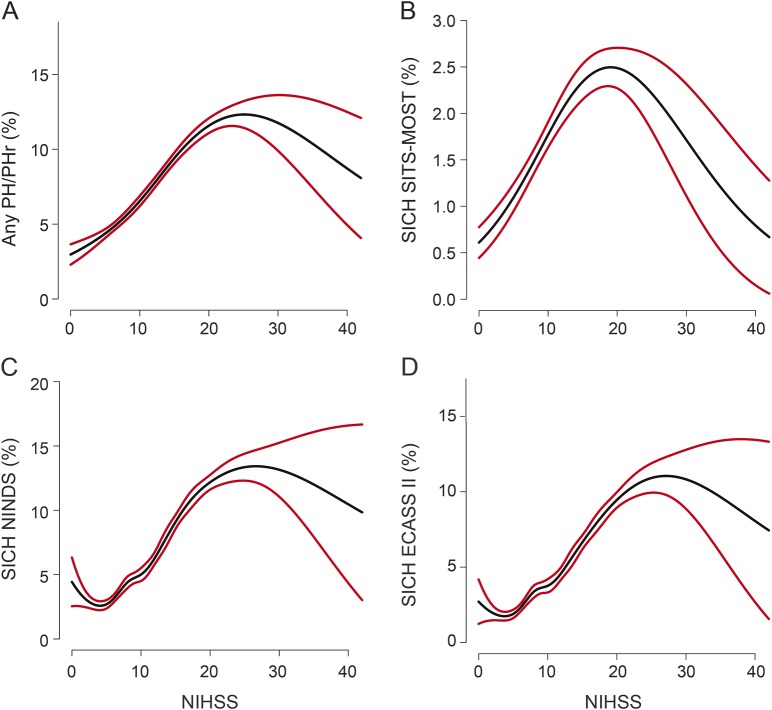
PH of any type or size occurred in 10.7% of patients with NIHSS score >25 and 11.0% with NIHSS score 15–25. Large PH with significant mass effect (PH2 or PHr2) was seen in 3.7% and 5.1% of patients with NIHSS >25 and NIHSS 15–25, respectively. Differences in the occurrence of SICH were small (table 2): SITS-MOST 1.4% vs 2.4%, ECASS II 10.8% vs 8.7%, and NINDS 13.2% vs 11.4%. Figure 1 illustrates the distribution of PH and SICH across the entire stratified range of the NIHSS. Graphical trends of the association between unadjusted baseline NIHSS score treated as a continuous variable and PH and SICH rates are shown in figure 2.
Table 2.
Outcomes by stroke severity group, unadjusted and adjusted analysis
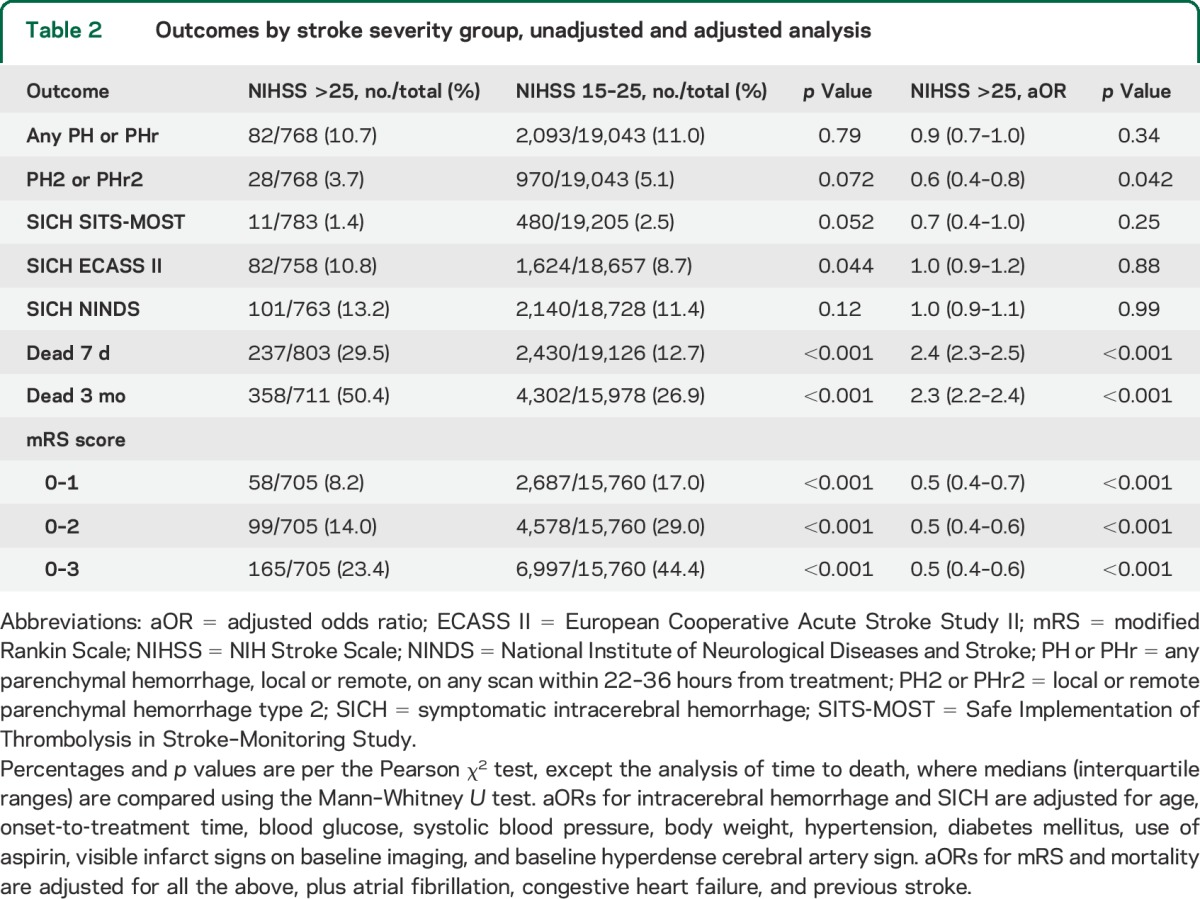
Figure 1. Frequency of PH and symptomatic intracerebral hemorrhage by stroke severity.
PH/PHr: any parenchymal hemorrhage, local or remote on any posttreatment scan during hospitalization. PH2/PHr2: Local or remote parenchymal hemorrhage type 2 on any posttreatment scan during hospitalization. “Type 2” indicates hematoma exceeding 30% of the infarct (for PH2), with substantial space-occupying effect (both PH2 and PHr2). ECASS II = European Cooperative Acute Stroke Study II; NINDS = National Institute of Neurological Diseases and Stroke; SITS-MOST = Safe Implementation of Thrombolysis in Stroke–Monitoring Study.
Figure 2. Relationship between baseline NIHSS score and risk of ICH/SICH following stroke thrombolysis.
(A) Any PH/Phr (%), (B) SICH SITS-MOST (%), (C) SICH ECASS II (%), and (D) SICH NINDS (%). Black line indicates risk of the respective hemorrhagic outcome; red lines indicate 95% confidence intervals. The graph was produced using univariate logistic regression, with NIHSS included by means of a natural cubic spline. ECASS II = European Cooperative Acute Stroke Study II; ICH = intracerebral hemorrhage; NIHSS = NIH Stroke Scale; NINDS = National Institute of Neurological Diseases and Stroke; PH = parenchymal hemorrhage; SICH = symptomatic ICH; SITS-MOST = Safe Implementation of Thrombolysis in Stroke–Monitoring Study.
Multivariate analysis adjusting for differences in known risk factors for PH and SICH between patients with NIHSS scores >25 and NIHSS scores 15–25 confirmed the findings from univariate comparisons (table 2). Following adjustment, there were no statistically significant differences in risk for any PH or SICH between the 2 severity strata, while the adjusted odds ratio (aOR) for PH2/PHr2 was actually lower in the NIHSS >25 group compared to NIHSS 15–25. In an additional multivariate analysis including only patients with NIHSS above 25, the well-established independent association of stroke severity with PH and SICH reported in numerous previous publications was no longer observed.
Because of limited numbers of patients with known stroke territory, we investigated the association of territory and PH/SICH using a simplified multivariate logistic regression model including stratified NIHSS (15–25 and >25), stroke territory, age, baseline blood glucose, systolic blood pressure, and aspirin. This included 152 patients with NIHSS >25 and 3,602 with NIHSS 15–25. Here, posterior circulation stroke was negatively associated with PH/SICH: with aOR 0.4 (0.1–0.6, p < 0.001) for SICH NINDS, aOR 0.5 (0.3–0.8, p = 0.008) for SICH ECASS II, aOR 0.6 (0.1–1.0, p = 0.22) for SICH SITS-MOST, aOR 0.7 (0.5–0.9, p = 0.064) for any PH/PHr, and aOR 0.64 (0.3–0.9, p = 0.14) for PH2/PHr2. In this analysis, NIHSS >25 remained without an independent association with all PH/SICH definitions, similar to results from the full multivariate model (table 2).
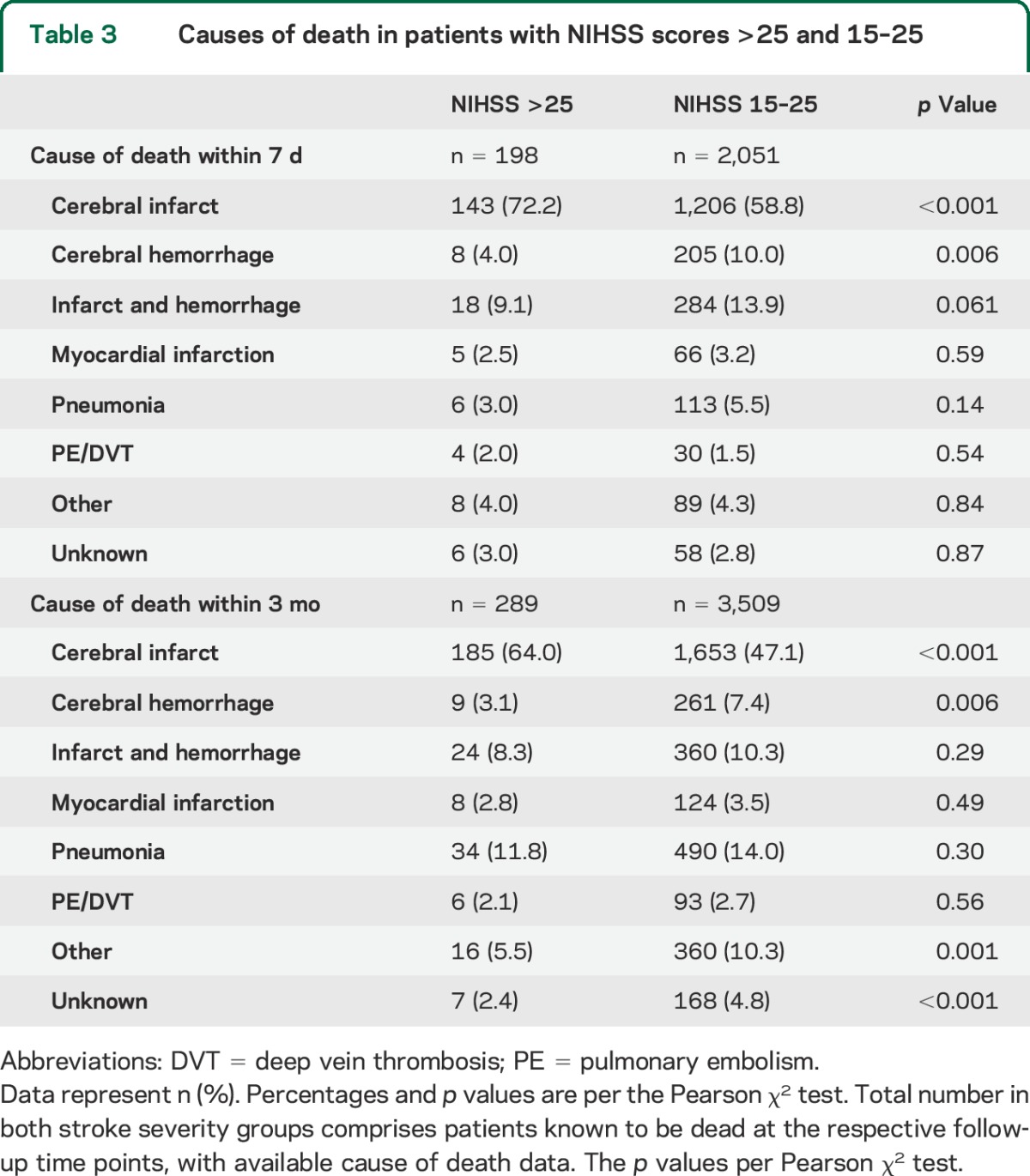
Among patients who died within 7 days and 3 months, ICH was reported to be the cause of death less frequently in the NIHSS >25 group compared to the NIHSS 15–25 group (table 3). There was no significant difference in the combined cause of death “cerebral infarct and hemorrhage without specification” between the groups.
Table 3.
Causes of death in patients with NIHSS scores >25 and 15–25
Mortality at 7 days (29.5% vs 12.7%) and 3 months (50.4% vs 26.9%) was higher in the NIHSS >25 group, while proportions of patients with excellent (mRS 0–1), functionally independent (mRS 0–2), and favorable (mRS 0–3) outcome at 3 months were lower compared to the NIHSS 15–25 group (table 2). Figure e-1 on the Neurology® Web site at Neurology.org shows functional outcomes and mortality across all stroke severity levels. In deceased patients with a reported time of death, survival time was shorter in the very severe stroke group: 3.8 days vs 6.1 days in NIHSS 15–25. Following multivariate adjustment, stroke severity above NIHSS 25 remained independently associated with higher short- and long-term mortality, and worse functional outcomes compared to NIHSS 15–25 (table 2).
DISCUSSION
We report a large observational study of outcomes following IV tissue plasminogen activator (tPA) treatment for very severe and severe stroke, and present results on treatment safety in patients with NIHSS score >25, in whom IV thrombolysis is currently off-label in Europe. Our main finding is that ICH risk is not increased following IV tPA treatment in patients with very severe stroke (NIHSS scores >25) compared to severe stroke (NIHSS scores 15–25). At high stroke severity, there is no further increase in ICH or SICH rates beyond NIHSS levels of 20–25, and the well-established relationship between increasing stroke severity and cerebral hemorrhagic complications is no longer seen beyond the NIHSS level of 25 points in multivariate analysis. Although mortality is higher in the NIHSS >25 group, it is not explained by an excess of hemorrhagic complications. The increased mortality in patients with NIHSS >25 is largely explained by the higher stroke severity itself, reflected by many patients with a decreased consciousness level at presentation. Patients with very severe stroke are also older and have more comorbidities such as congestive heart failure, previous stroke, and diabetes (table 1).
The lack of increase in cerebral hemorrhagic complications in the NIHSS >25 group is reflected by our finding that ICH, alone or together with the infarct, is not more often the cause of death within 7 days and 3 months than in patients with lower severity stroke.
Previous studies have demonstrated that time from stroke symptom onset to IV tPA treatment decreases with increasing stroke severity.15,16 Our findings show that this relationship is inverted at the upper extreme of stroke severity. More patients with NIHSS scores >25 were treated beyond 3 hours from symptom onset, compared to NIHSS scores 15–25 (26% vs 14%). This could be explained by more patients with very severe stroke presenting obtunded or comatose, increasing the complexity of initial diagnosis and management. In the NIHSS >25 group, the reduced level of consciousness and the high proportion of bilateral motor signs is in keeping with more posterior circulation strokes in this group compared to the NIHSS 15–25 group.
The differences in affected vascular territory are important for the interpretation of our results on SICH. In adjusted analysis, posterior territory stroke is associated with a lower risk of SICH per NINDS and ECASS, with nonsignificant trends in the same direction for all other hemorrhage definitions. Lower risk of hemorrhagic transformation and SICH in posterior circulation infarcts has previously been reported in patient cohorts who did and did not receive thrombolysis.17–19 The frequent occurrence of posterior circulation infarcts in patients with NIHSS score >25 is an important explanation for the lack of increase in ICH and SICH despite the higher stroke severity, which is otherwise strongly associated with the risk of ICH and SICH.8–10 Table e-1 shows that hemorrhagic complications are consistently less common in patients with posterior circulation stroke in both severity groups. Poor functional outcomes and high mortality in the NIHSS >25 group also reflect the higher proportion of major posterior circulation stroke, i.e., basilar artery occlusion. Among patients with basilar artery occlusion and very severe neurologic deficits treated with IV tPA in the BASICS (Basilar Artery International Cooperation Study) trial, only 26% achieved mRS score of 0 to 3 and 50% were dead at 1 month of follow-up, similar to 23% with mRS score of 0 to 3 and 50% dead at 3 months in the NIHSS >25 group in our study.20
At 1.5%, the proportion of patients with NIHSS score >25 in our study is lower than in previous IV thrombolysis publications from the IST-3 trialists and the VISTA trial data repository (2.9% and 4.2% with NIHSS scores ≥25, respectively).1,7 This could reflect a reluctance of physicians in routine practice, especially in Europe, to offer off-label treatment with IV tPA to patients with NIHSS score >25 outside clinical trials.
It is important to address the difficulty in establishing clinical deterioration in patients receiving thrombolysis with baseline NIHSS score >25 who subsequently have an ICH discovered on scheduled follow-up imaging at 22 to 36 hours. This may affect the calculation of SICH rates in this group. To compensate for this, we report 2 purely radiologic ICH definitions: any PH and PH type 2. Figures 1 and 2 show consistency between patterns of radiologic definitions of ICH and clinicoradiologic definitions of SICH across stroke severity strata.
Our study has several limitations. In 18% and 19% of patients, 3-month follow-up data for mortality and functional outcome are lacking. We attempt to mitigate the missing data for death within 3 months by including 7-day mortality rates, where records are 95% complete. Furthermore, the stroke territory variable, i.e., anterior or posterior circulation, was implemented in SITS-ISTR in 2010. To check for potential reporting bias, we performed a sensitivity analysis comparing outcomes in patients with and without territory data. Reassuringly, rates of PH, SICH, death, and functional outcomes were well-matched between the 2 groups (table e-2), indicating that patients with available territory data are representative of the registry. Until 2010, the SITS-ISTR lacked input possibilities for endovascular stroke treatment, and it was not widely practiced in Europe. Endovascular thrombectomy following IV tPA was uncommon in our material, registered in 1.6% of all patients. We believe this fraction is unlikely to have influenced our results.
There are important parallels between regulations surrounding IV thrombolysis for very severe stroke and those previously applied to treatment of patients older than 80 years, until recently contraindicated for similar safety reasons. Outcomes are inevitably poorer among the elderly as in those with very severe stroke. This has been conflated with a presumption of poorer response to treatment. Evidence undermining and eventually disproving that fear regarding the elderly has gradually accumulated.1,21,22 Meantime, thousands of elderly patients were denied effective treatment. Our results indicate that the same situation likely exists for very severe stroke.
Our findings suggest that the European label contraindication to IV thrombolysis treatment in patients with NIHSS score >25 based on safety concerns may be unwarranted. We found no excess risk of cerebral hemorrhage in this group compared to patients receiving thrombolysis for severe stroke with NIHSS score 15–25. Our findings are reinforced by the recent individual patient data meta-analysis by the Stroke Thrombolysis Trialists' Collaboration, showing a clear positive treatment effect of IV tPA in severe stroke, despite the increase in SICH inherent to thrombolytic therapy.22 Of note, patients with NIHSS score >25 frequently present with a reduced level of consciousness and receive thrombolysis with a longer delay than others, which contributes to poor functional outcomes and high mortality. Recent major studies have shown that endovascular thrombectomy in addition to IV tPA strongly improves the prognosis in severe large artery occlusion stroke. In light of this new evidence, efforts should be made to increase and accelerate the delivery of IV thrombolysis and thrombectomy for the most severely afflicted stroke patients. This study provides Class III evidence that treatment with IV thrombolysis in patients with NIHSS scores >25 holds no excess risk compared to patients with NIHSS scores 15–25.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all SITS-ISTR investigators and their centers for their participation and all patients who participated in SITS-ISTR. The authors gratefully acknowledge Dr. Paolo Frumento and Professor Matteo Bottai at the Biostatistics Core Facility and Institute for Environmental Medicine, Karolinska Institutet, Stockholm, Sweden, for excellent biostatistical consultations. The current SITS registry is developed, maintained, and upgraded by ZiteLab, Copenhagen, Denmark, in close collaboration with SITS.
GLOSSARY
- aOR
adjusted odds ratio
- ECASS II
European Cooperative Acute Stroke Study II
- ICH
intracerebral hemorrhage
- IST-3
Third International Stroke Trial
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- NINDS
National Institute of Neurological Diseases and Stroke
- PH
parenchymal hemorrhage
- SICH
symptomatic intracerebral hemorrhage
- SITS-ISTR
Safe Implementation of Thrombolysis in Stroke–International Stroke Thrombolysis Register
- SITS-MOST
Safe Implementation of Thrombolysis in Stroke–Monitoring Study
- tPA
tissue plasminogen activator
- VISTA
Virtual International Stroke Trials Archive
Footnotes
Supplemental data at Neurology.org
Editorial, page 2088
AUTHOR CONTRIBUTIONS
N. Ahmed and N. Wahlgren coordinated the study. M.V. Mazya performed the statistical analysis. M.V. Mazya, N. Ahmed, and N. Wahlgren wrote the initial draft of the manuscript. N. Ahmed, N. Wahlgren, K.R. Lees, and D. Toni were members of the SITS Scientific Committee. D. Collas and V.-M. Rand were local coordinators of leading recruiting centers. All authors have read and commented on the first draft, with regard to interpretation of the data and editing of the manuscript, and have seen and approved the final version. M.V. Mazya, N. Ahmed, and N. Wahlgren have direct access to the original data and attest to the accuracy and completeness of this report.
STUDY FUNDING
The SITS-ISTR is funded by an unrestricted grant from Boehringer Ingelheim, Ferrer, and by a grant from European Union Public Health Executive Authority (PHEA). Financial support was also provided through the regional agreement on medical training and research (ALF) between Stockholm County Council and the Karolinska Institute. This study is a part of the Fighting Stroke Project (Uppdrag: Besegra Stroke) supported by the Swedish Heart and Lung Foundation, Karolinska Institutet, Friends of Karolinska Institutet, USA, and Johanniterorden (Swedish Order of St. John).
DISCLOSURE
M. Mazya holds the position of researcher in SITS International, which receives a grant from Boehringer Ingelheim for the SITS-ISTR. K. Lees' institution is receiving a grant from Genentech. He has received fees from Boehringer Ingelheim for his role as member of DSMB boards. D. Collas, V. Rand, and R. Mikulik report no disclosures relevant to the manuscript. D. Toni has served as a consultant for Boehringer Ingelheim and has been paid lecture fees for attending and speaking at workshops held by Boehringer Ingelheim. N. Wahlgren is the Chairman of SITS International, and received a grant from Boehringer Ingelheim for the SITS-ISTR. N Wahlgren has also received lecture fees from Boehringer Ingelheim. N. Ahmed holds the position of vice chairman and senior researcher in SITS International, which receives a grant from Boehringer Ingelheim for the SITS-ISTR. Go to Neurology.org for full disclosures.
REFERENCES
- 1.IST-3 Collaborative Group, Sandercock P, Wardlaw JM, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the Third International Stroke Trial [IST-3]): a randomised controlled trial. Lancet 2012;379:2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra NK, Davis SM, Kaste M, Lees KR. Comparison of outcomes following thrombolytic therapy among patients with prior stroke and diabetes in the Virtual International Stroke Trials Archive (VISTA). Diabetes Care 2010;33:2531–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazya MV, Lees KR, Markus R, et al. Safety of intravenous thrombolysis for ischemic stroke in patients treated with warfarin. Ann Neurol 2013;74:266–274. [DOI] [PubMed] [Google Scholar]
- 4.EMEA, Committee for Proprietary Medicinal Products. Summary information on a referral opinion following an arbitration pursuant to article 29 of directive 2001/83/EC, for Actilyse. 2002. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Actilyse_29/WC500010327.pdf. Accessed May 30, 2015.
- 5.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–1025. [PubMed] [Google Scholar]
- 6.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–1251. [DOI] [PubMed] [Google Scholar]
- 7.Mishra NK, Lyden P, Grotta JC, Lees KR. Thrombolysis is associated with consistent functional improvement across baseline stroke severity: a comparison of outcomes in patients from the Virtual International Stroke Trials Archive (VISTA). Stroke 2010;41:2612–2617. [DOI] [PubMed] [Google Scholar]
- 8.Wahlgren N, Ahmed N, Eriksson N, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST). Stroke 2008;39:3316–3322. [DOI] [PubMed] [Google Scholar]
- 9.Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke 2012;43:2904–2909. [DOI] [PubMed] [Google Scholar]
- 10.Mazya M, Egido JA, Ford GA, et al. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: Safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke 2012;43:1524–1531. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed N, Wahlgren N, Grond M, et al. Implementation and outcome of thrombolysis with alteplase 3–4.5 h after an acute stroke: an updated analysis from SITS-ISTR. Lancet Neurol 2010;9:866–874. [DOI] [PubMed] [Google Scholar]
- 12.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007;369:275–282. [DOI] [PubMed] [Google Scholar]
- 13.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci 2001;16:101–133. [Google Scholar]
- 14.Mazya MV, Bovi P, Castillo J, et al. External validation of the SEDAN score for prediction of intracerebral hemorrhage in stroke thrombolysis. Stroke 2013;44:1595–1600. [DOI] [PubMed] [Google Scholar]
- 15.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013;309:2480–2488. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed N, Kellert L, Lees KR, Mikulik R, Tatlisumak T, Toni D. Results of intravenous thrombolysis within 4.5 to 6 hours and updated results within 3 to 4.5 hours of onset of acute ischemic stroke recorded in the Safe Implementation of Treatment in Stroke International Stroke Thrombolysis Register (SITS-ISTR): an observational study. JAMA Neurol 2013;70:837–844. [DOI] [PubMed] [Google Scholar]
- 17.Pagola J, Ribo M, Alvarez-Sabin J, et al. Thrombolysis in anterior versus posterior circulation strokes: timing of recanalization, ischemic tolerance, and other differences. J Neuroimaging 2011;21:108–112. [DOI] [PubMed] [Google Scholar]
- 18.Sarikaya H, Arnold M, Engelter ST, et al. Outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke. Stroke 2011;42:2498–2502. [DOI] [PubMed] [Google Scholar]
- 19.Sung SF, Chen CH, Chen YW, Tseng MC, Shen HC, Lin HJ. Predicting symptomatic intracerebral hemorrhage after intravenous thrombolysis: stroke territory as a potential pitfall. J Neurol Sci 2013;335:96–100. [DOI] [PubMed] [Google Scholar]
- 20.Schonewille WJ, Wijman CA, Michel P, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol 2009;8:724–730. [DOI] [PubMed] [Google Scholar]
- 21.Ford GA, Ahmed N, Azevedo E, et al. Intravenous alteplase for stroke in those older than 80 years old. Stroke 2010;41:2568–2574. [DOI] [PubMed] [Google Scholar]
- 22.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.