Abstract
The term “Wernicke's area” is most often used as an anatomical label for the gyri forming the lower posterior left sylvian fissure. Although traditionally this region was held to support language comprehension, modern imaging and neuropsychological studies converge on the conclusion that this region plays a much larger role in speech production. This evidence is briefly reviewed, and a simple schematic model of posterior cortical language processing is described.
In an influential 1976 article called “Wernicke's region: Where is it?” Bogen and Bogen1 defined the Wernicke area (commonly known as Wernicke's area) unequivocally as “the area where a lesion will cause language comprehension deficit.” They reviewed the large literature on this topic, emphasizing how anatomical evidence up to that time had variously implicated the left posterior superior temporal gyrus (pSTG), supramarginal gyrus (SMG), middle temporal gyrus (MTG), angular gyrus (AG), and inferior temporal gyrus in language comprehension. Like many authors before them,2–6 Bogen and Bogen concluded that language comprehension is not highly localized, but involves large regions of the left temporal and inferior parietal lobe.
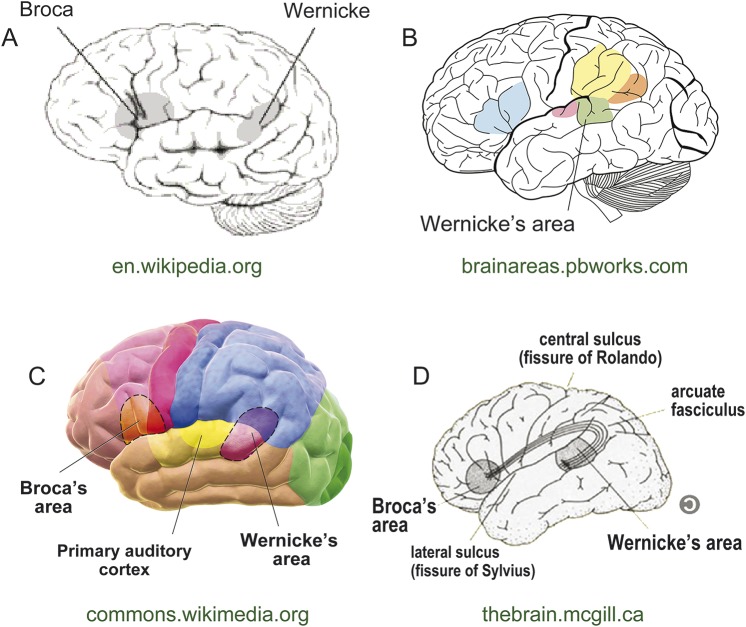
In contrast, the widely accepted definition of the Wernicke area has in recent decades become an anatomical rather than a functional one. Rather than “the area where language comprehension occurs,” the Wernicke area has come to be synonymous with the left pSTG and SMG, i.e., the cortices that surround the left posterior sylvian fissure (figure 1). The reasons for the persistence of this anatomical label are complex and need not be considered in detail here (see reference 7 for discussion). They include Wernicke's original claim that the left superior temporal gyrus (STG) is the site of speech comprehension8 and the particular emphasis placed on the pSTG by later authors.9–12 Today the label is reinforced in standard neuroscience textbooks and on numerous Internet sites, including Wikipedia (http://en.wikipedia.org/wiki/Wernicke's_area).
Figure 1. Current depictions of the Wernicke area.
A representative sample of Internet images depict the Wernicke area, found using a Google search for “Wernicke's area images.” All highlight the posterior superior temporal gyrus, with variable extension into the posterior supramarginal gyrus and occasionally the angular gyrus. Web locations are shown in green below each image. A–C are in the public domain; D is reproduced with permission from the Web page manager.
But does this de facto anatomically defined Wernicke area truly have the functional properties traditionally ascribed to it? As this brief review will make clear, there is now compelling evidence, and a general consensus among language researchers, that the region currently labeled the Wernicke area plays little or no role in language comprehension. This new understanding should motivate a revision of standard teaching at medical schools and neurology residency programs. Practical benefits will include a better understanding of the vascular and degenerative fluent aphasia syndromes, and improved understanding and application of clinical brain mapping data.
In the following discussion, “the Wernicke area” refers to the anatomical site now labeled the Wernicke area, specifically the pSTG (posterior portion of Brodmann area 22) and the SMG (Brodmann area 40).
THE WERNICKE AREA IS CRITICAL FOR SPEECH PRODUCTION
Although the end product of speech production is a series of muscle movements, the brain mechanisms involved in speech production should not be seen as limited to motor commands that move muscles. Before such commands can be sent, the speaker must momentarily activate knowledge about the sequence of consonant and vowel speech sounds (phonemes) that form the word to be spoken. This mental stage prior to articulation is known as phonologic retrieval.13 Its existence can be demonstrated by the fact that one knows that the word “snow” rhymes with “blow” but not with “plow” without needing to say these words aloud. In the jargon of language scientists, this knowledge reflects activation of a phonologic “representation” or mental image of the sounds comprising the words. Partial disruption of this phonologic retrieval process causes a speech production impairment called phonemic paraphasia, in which the phonemes of the spoken word are chosen incorrectly or are incorrectly ordered.14,15 Phonemic paraphasia is a cardinal feature of both Wernicke aphasia and conduction aphasia. Although these are fluent aphasias because there is no slowing of overall word output, the paraphasic component is nevertheless a deficit of speech production, not speech comprehension. Thus Wernicke aphasia, though often thought of as a syndrome affecting comprehension, also includes a prominent speech production impairment.
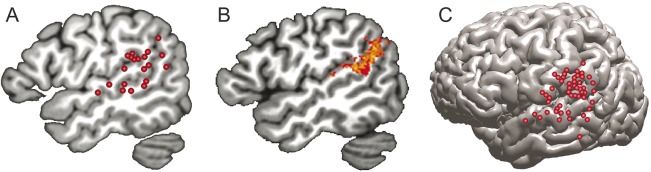
Functional neuroimaging methods, including functional MRI, PET, and magnetoencephalography, have provided compelling and consistent evidence that the Wernicke area is involved in phonologic retrieval in healthy participants.16,17 Figure 2A shows a summary of 14 functional imaging studies that isolated the phonologic retrieval stage by incorporating controls for semantic processing, speech articulation, and auditory perception (see appendix e-1 on the Neurology® Web site at Neurology.org for a description of these studies). The structures most commonly implicated are pSTG, posterior MTG, and SMG.
Figure 2. Involvement of the Wernicke area in speech production.
Example data indicate a prominent role for the Wernicke area in speech production. (A) A summary of 14 functional neuroimaging studies shows peak locations where activation was related to phonologic retrieval independent of semantic processing, speech articulation, or auditory perception (see appendix e-1 for a description of these studies). (B) The lesion location in 40 left hemisphere stroke patients where damage was associated with failure to retrieve phonologic information without other (semantic or articulation) deficits (adapted with permission from reference 7). (C) Sites where electrical stimulation of the cortex in 14 patients produced phonologic production errors during reading aloud (adapted with permission from reference 8).
Anatomical observations in patients with conduction aphasia have long implicated the left pSTG and inferior parietal lobe as the main regions where damage produces phonemic paraphasia without impairing comprehension.16,18–20 A recent localization study21 provided specific evidence that this region supports prearticulatory phonologic retrieval. Using a quantitative statistical method called voxel-based lesion-symptom mapping,22 the authors showed that damage to a focal region in the left pSTG and SMG causes impairment on a silent visual rhyme judgment task similar to the “snow/blow/plow” example given above (figure 2B). Patients with lesions in this region were unable to perform this task normally, indicating an inability to retrieve an internal mental image of the phonemes.21 Other patient-based evidence comes from direct cortical electrical stimulation studies in patients undergoing brain surgery, which show that stimulation in the Wernicke area elicits phonemic paraphasia typical of conduction aphasia, without impairing comprehension23,24 (figure 2C).
Finally, selective involvement of the Wernicke area in phonologic retrieval explains many of the behavioral and imaging manifestations of logopenic-variant primary progressive aphasia (lvPPA). Patients with this syndrome have difficulty retrieving words and make phonemic paraphasias in speech production tasks, but have little or no impairment of word comprehension.25,26 Pathologic changes are concentrated in the inferior parietal and posterior superior temporal region.25,27–29 Thus converging evidence from clinical studies in conduction aphasia, localization of phonologic retrieval in stroke patients, structural imaging in lvPPA, and functional imaging studies in healthy participants all indicate a central role for the pSTG and SMG in the phonologic retrieval stage of speech production.
THE WERNICKE AREA IS NOT CRITICAL FOR WORD COMPREHENSION
As compelling as the evidence in favor of a role for the Wernicke area in speech production is the evidence against a role in speech comprehension. By definition, patients with conduction aphasia and patients with lvPPA have relatively intact word comprehension; therefore, if the lesions associated with these syndromes are centered in the Wernicke area, it follows that lesions in the Wernicke area do not as a rule impair comprehension. As an example, damage to the region shown in figure 2B, which was associated with phonologic retrieval impairment, was not associated with word comprehension deficits.21 Conversely, several large voxel-based lesion-symptom mapping studies have demonstrated an association between comprehension impairment and damage to MTG, AG, anterior STG, and several areas in left prefrontal cortex, but no association with damage to pSTG or SMG.30–32 Finally, a large body of imaging evidence links the semantic variant of primary progressive aphasia, in which language comprehension is severely impaired, with pathologic changes focused in the anterior half of the temporal lobe, well outside the standard Wernicke area.27,33,34 The logical conclusion to be drawn from these and other lesion studies is that comprehension impairment can result from damage in many brain regions, but not from damage restricted to the anatomically defined Wernicke area.
Functional neuroimaging studies have explored many aspects of language comprehension. Speech comprehension is best viewed as involving at least 2 distinct processing stages. The first of these is a sensory process that analyzes the auditory input for phoneme content, independent of word meaning. A wide range of evidence suggests that this “phoneme perception” process involves high-level auditory areas in the STG and adjacent superior temporal sulcus in both hemispheres.35,36 The STG regions responsible for this process are anterior to those involved in speech production, and anterior to the classical Wernicke area.37 The bilateral localization of this perceptual stage makes it more resistant to unilateral lesions, which accounts for the relative rarity of pure speech perception deficits.38
The second distinct processing stage in speech comprehension is the retrieval of semantic information, or meaning, associated with the input. A meta-analysis of 120 neuroimaging studies on this topic identified a large network of brain regions involved in semantic processing, including AG, MTG, ventral temporal lobe, medial parietal cortex, medial prefrontal cortex, and inferior lateral prefrontal regions.39 These results closely mirror the previously mentioned lesion data. Together, the data converge on the conclusion that many cortical regions support speech comprehension, whereas the classical Wernicke area is one of the few brain regions that does not.
A SIMPLE MODEL OF POSTERIOR CORTICAL LANGUAGE NETWORKS
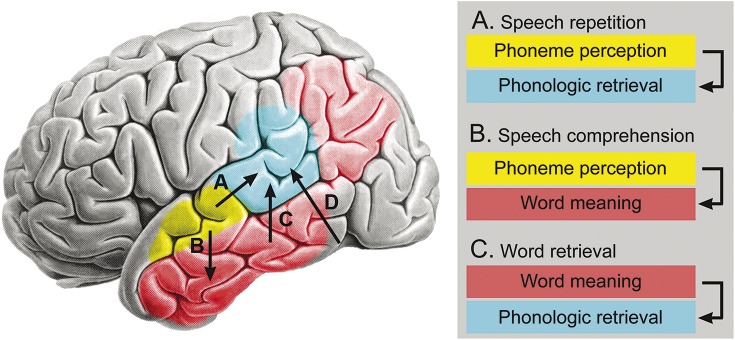
Figure 3 presents a visual summary of some key conclusions regarding the classical Wernicke area and other posterior language regions, derived from modern lesion localization and functional imaging studies of word comprehension and production. The take-home points are as follows:
The brain region known as the Wernicke area, shown in blue, supports a critical component of speech production, referred to as phonologic retrieval, in which the phonemes to be articulated, and their temporal order, are represented mentally. This process is required for all speech production tasks, including repetition, word retrieval (e.g., in spontaneous speech or naming), and reading aloud.
Speech repetition (pathway A) involves input to the phonologic retrieval system from the auditory phoneme perception system (shown in yellow). A similar mechanism supports reading aloud, except that the input to the phonologic retrieval system comes from a visual letter perception system in the ventral occipitotemporal region (pathway D).
Communicative speech production (as in spontaneous speech and naming) involves a stage prior to phonologic retrieval, in which a concept is retrieved that expresses what the speaker wants to say. Word retrieval is then accomplished by mapping these word meanings onto phonologic representations (pathway C). Thus, unlike with repetition and reading, the input to the phonologic retrieval system in these tasks comes from an internal semantic (word meaning) system. This semantic processing network is widely distributed across higher-order association cortices in the temporal, parietal, and frontal lobes (temporal and parietal components shown in red in figure 3).
Speech comprehension (pathway B) involves mapping sequences of phonemes (perceived in the auditory phoneme perception system) onto word meanings (represented in the semantic system).
Figure 3. Posterior language systems.
A functional model of major posterior language systems. Yellow indicates a bilateral speech phoneme perception system. Blue indicates the Wernicke area, which supports prearticulatory phonologic retrieval. Red indicates the temporal and parietal components of a distributed system for word meaning (semantic) representations. Speech repetition requires the pathway designated A in the figure, as well as more anterior parietal and frontal regions (not shown) that support articulatory preparation and execution. Spoken word comprehension involves the pathway marked B in the figure, which maps perceived phoneme sequences to word concepts. Communicative speech production, in which the speaker retrieves words to express concepts, requires the pathway marked C, which maps concept representations onto phonologic representations. Pathway D indicates a direct mapping from visual word forms to phonologic representations, required for reading aloud. Background brain image reproduced with permission from Springer.
Figure 4 summarizes the lesion correlates of 4 posterior aphasia syndromes arising from damage to these systems. Isolated damage to the phoneme perception system (region A in figure 4) produces pure word deafness. Because both hemispheres can support phoneme perception, this syndrome generally occurs only after bilateral lesions. Damage to the phonologic retrieval system (region B) results in phonemic paraphasia and impaired word retrieval (anomia). When this occurs in isolation, the result is conduction aphasia or lvPPA. Patients with lvPPA have more pronounced anomia than is typical with conduction aphasia, probably due to additional involvement of adjacent semantic regions. Isolated damage to the semantic network (region C or D) produces comprehension impairment with intact automatic speech production (i.e., repetition), a syndrome known as transcortical sensory aphasia. Damage to region C is characteristic of semantic-variant primary progressive aphasia.27,33,34 In addition to speech comprehension deficits, damage to this semantic processing network results in anomia and empty speech that lacks meaningful content. Finally, Wernicke aphasia, characterized by both paraphasic speech production and comprehension impairment, results from combined damage to the phonologic retrieval and semantic systems, typically within the zone indicated by the green line in figure 4.
Figure 4. Lesion correlates of posterior aphasia syndromes.
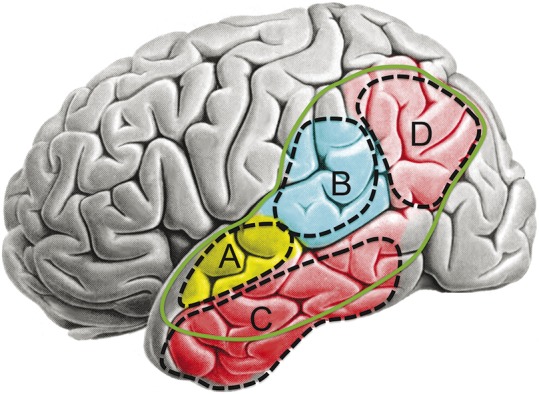
Bilateral damage restricted to region A produces pure word deafness. Strokes centered on region B produce conduction aphasia, and neurodegeneration in this region produces a similar syndrome called logopenic-variant primary progressive aphasia. Damage localized to the semantic system (regions C or D) produces transcortical sensory aphasia, and neurodegeneration focused on region C produces a similar syndrome known as semantic-variant primary progressive aphasia. The solid green line indicates the territory in which larger lesions produce Wernicke aphasia, a syndrome that combines damage to region B with partial damage to the surrounding semantic system. Background brain image reproduced with permission from Springer.
SHOULD THE WERNICKE AREA BE RELOCATED (AGAIN)?
If the posterior perisylvian region now labeled the Wernicke area does not support the main function traditionally ascribed to it (i.e., speech comprehension), one possible course of action is to apply the Wernicke area label instead to those regions that do support speech comprehension. The main problem with this approach is that speech comprehension is a highly distributed function, involving a bihemispheric phoneme perception system and a widely distributed semantic network. To refer to all of these regions as the Wernicke area seems to sacrifice any utility that the term might have, and furthermore these other brain networks were never the focus of Wernicke's claims. Given the pervasive application of the Wernicke area label to the posterior perisylvian region, which seems unlikely to change, and the fact that damage in this location produces one component of Wernicke aphasia (i.e., paraphasic production), a wiser course might be to retain the label while keeping in mind the true function of this brain region.
Supplementary Material
GLOSSARY
- AG
angular gyrus
- lvPPA
logopenic-variant primary progressive aphasia
- MTG
middle temporal gyrus
- pSTG
posterior superior temporal gyrus
- SMG
supramarginal gyrus
- STG
superior temporal gyrus
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Jeffrey R. Binder conceived, researched, and wrote the article.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
J.R. Binder receives honoraria for serving as an associate editor for the Journal of Cognitive Neuroscience and as a grant reviewer for the Dana Foundation, and is funded by NIH grants R01 NS035929, R01 DC003681, and R01 GM103894. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bogen JE, Bogen GM. Wernicke's region: where is it? Ann NY Acad Sci 1976;290:834–843. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein K. Language and Language Disturbances. New York: Grune & Stratton; 1948. [Google Scholar]
- 3.Henschen SE. Klinische und Anatomische Beitrage zur Pathologie des Gehirns. Stockholm: Nordiska Bokhandeln; 1920–1922. [Google Scholar]
- 4.Marie P. On aphasia in general and agraphia in particular according to the teaching of Professor Charcot: reprinted from Le Progres Medical, Series 2, 1888;7:81–84. In: Pierre Marie's Papers on Speech Disorders. New York: Hafner; 1971. [Google Scholar]
- 5.Nielsen JM. Agnosia, Apraxia, Aphasia: Their Value in Cerebral Localization. New York: Paul B. Hoeber; 1946. [Google Scholar]
- 6.Starr MA. The pathology of sensory aphasia, with an analysis of fifty cases in which Broca's centre was not diseased. Brain 1889;12:82–101. [Google Scholar]
- 7.Binder JR. Wernicke aphasia: a disorder of central language processing. In: D'Esposito ME, ed. Neurological Foundations of Cognitive Neuroscience. Cambridge, MA: MIT Press; 2002:175–238. [Google Scholar]
- 8.Wernicke C. Der aphasische Symptomenkomplex. Breslau: Cohn & Weigert; 1874. [Google Scholar]
- 9.Benson DF. Aphasia, Alexia and Agraphia. New York: Churchill Livingstone; 1979. [Google Scholar]
- 10.Geschwind N. Current concepts: aphasia. N Engl J Med 1971;284:654–656. [DOI] [PubMed] [Google Scholar]
- 11.Kleist K. Sensory Aphasia and Amusia. London: Pergamon Press; 1962. [Google Scholar]
- 12.Pick A. Aphasia. Berlin: Springer; 1931. [Google Scholar]
- 13.Levelt WJM. Speaking: from intention to articulation. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- 14.Kohn SE, ed. Conduction Aphasia. Hillsdale, NJ: Lawrence Erlbaum; 1992. [Google Scholar]
- 15.Wilshire CE, McCarthy RA. Experimental investigations of an impairment in phonological encoding. Cogn Neuropsychol 1996;13:1059–1098. [Google Scholar]
- 16.Buchsbaum BR, Baldo J, D'Esposito M, Dronkers N, Okada K, Hickok G. Conduction aphasia, sensory-motor integration, and phonological short-term memory: an aggregate analysis of lesion and fMRI data. Brain Lang 2011;119:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition 2004;92:101–144. [DOI] [PubMed] [Google Scholar]
- 18.Axer H, Keyserlingk AG, Berks G, Keyserlingk DF. Supra- and infrasylvian conduction aphasia. Brain Lang 2001;76:317–331. [DOI] [PubMed] [Google Scholar]
- 19.Damasio H, Damasio AR. The anatomical basis of conduction aphasia. Brain 1980;103:337–350. [DOI] [PubMed] [Google Scholar]
- 20.Fridriksson J, Kjartansson O, Morgan PS, et al. Impaired speech repetition and left parietal lobe damage. J Neurosci 2010;30:11057–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pillay SB, Stengel BC, Humphries C, Book DS, Binder JR. Cerebral localization of impaired phonological retrieval during rhyme judgment. Ann Neurol 2014;76:738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bates E, Wilson S, Saygin AP, et al. Voxel-based lesion-symptom mapping. Nat Neurosci 2003;6:448–450. [DOI] [PubMed] [Google Scholar]
- 23.Roux FE, Durand JB, Jucla M, Réhault E, Reddy M, Démonet JF. Segregation of lexical and sub-lexical reading processes in the left perisylvian cortex. PLoS One 2012;7:e50665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson JM, Gilmore R, Roper S, et al. Conduction aphasia and the arcuate fasciculus: a reexamination of the Wernicke-Geschwind model. Brain Lang 1999;70:1–12. [DOI] [PubMed] [Google Scholar]
- 25.Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology 2008;71:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology 2011;76:1804–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohrer JD, Ridgway GR, Crutch SJ, et al. Progressive logopenic/phonological aphasia: Erosion of the language network. Neuroimage 2010;49:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teichmann M, Kas A, Boutet C, et al. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain 2013;136:3474–3488. [DOI] [PubMed] [Google Scholar]
- 30.Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition 2004;92:179–229. [DOI] [PubMed] [Google Scholar]
- 31.Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition 2004;92:145–177. [DOI] [PubMed] [Google Scholar]
- 32.Thothathiri M, Kimberg DY, Schwartz MF. The neural basis of reversible sentence comprehension: evidence from voxel-based lesion symptom mapping in aphasia. J Cogn Neurosci 2012;24:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohrer JD, Warren JD, Modat M, et al. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology 2009;72:1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 2002;58:198–208. [DOI] [PubMed] [Google Scholar]
- 35.Binder JR, Frost JA, Hammeke TA, et al. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 2000;10:512–528. [DOI] [PubMed] [Google Scholar]
- 36.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci 2007;8:393–402. [DOI] [PubMed] [Google Scholar]
- 37.DeWitt I, Rauschecker JP. Phoneme and word recognition in the auditory ventral stream. Proc Natl Acad Sci USA 2012;109:E505–E514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poeppel D. Pure word deafness and the bilateral processing of the speech code. Cogn Sci 2001;25:679–693. [Google Scholar]
- 39.Binder JR, Desai R, Conant LL, Graves WW. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 2009;19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.