Case presentations
Case 1
A 48-year-old man presents with epistaxis, fatigue, and pancytopenia, and is diagnosed with acute myeloid leukemia (AML) with a t(9;11)(p22;q23) translocation in 16 of 20 metaphases. He has an excellent performance status and no comorbidities. Curative-intent chemotherapy with cytarabine and daunorubicin (“7 + 3”) is initiated. What is the most appropriate strategy to prevent fungal infections in this patient?
Case 2
The patient has achieved a morphologic complete remission with 2 cycles of induction chemotherapy. An HLA-matched unrelated donor has been identified, and he is planned to undergo myeloablative allogeneic hematopoietic cell transplantation (HCT). What strategy should be pursued to prevent fungal infections before and after engraftment?
Introduction
Invasive fungal infections (IFIs) occur in 5% to 40% of patients with hematologic malignancies and are most common in AML.1 Aspergillus and Candida species (spp) currently account for ∼95% of all cases, but the epidemiological characteristics of IFIs evolve under the selection pressure of antimicrobials and other factors.2,3 With increasing use of intensively immunosuppressive cancer therapies, IFIs have become more frequent and now constitute a leading cause of morbidity and mortality. An important reason for delays and reductions of antileukemia therapies, they can also reduce AML cure rates.1,4-6 High mortality from IFIs is attributed to diagnostic difficulties and protracted treatment initiation, limited activity of antifungal agents, drug side effects, and increasing use of high-dose corticosteroids.7 Primary prevention of fungal infections, repeatedly demonstrated to reduce IFIs as well as infection-attributable and all-cause mortality, therefore remains essential.8,9
The ideal prophylactic antifungal agent is safe and well tolerated with long-term use, effective against a wide spectrum of organisms, and manufactured as IV and oral formulations with good bioavailability.10 With multiple polyenes, echinocandins, and triazoles now available, several antifungal agents fulfill some of these requirements. In developing a rationale for antifungal prophylaxis, the potential risks need to be balanced against the benefits. Among the risks of antifungal prophylaxis are drug toxicities, selection for resistant pathogens, adverse drug-drug interactions, and costs. Among the key benefits of prevention of invasive fungal infections during neutropenia in AML induction therapy are the reduction of morbidity and mortality, and shortening of hospital stay. One must appreciate that there is no single agent that will prevent all mycoses; thus, careful monitoring throughout the risk period is essential with treatment of emergent breakthrough invasive fungal infections. Herein, we examine the evidence guiding the choices of antifungal prophylaxis in adults undergoing curative-intent AML therapy.
Methods
Literature search strategy and study selection criteria
A systematic literature search restricted to English language articles published since 1990 was conducted using MEDLINE (May 18, 2015; see supplemental Table, available on the Blood Web site). Two authors independently reviewed all abstracts for eligibility assessment, with a third mediating discordant results. The full article was reviewed if eligibility was clearly met or if there was uncertainty regarding a priori–defined eligibility criteria based on the abstract. We included all randomized controlled trials (RCTs) and meta-analyses assessing a systemic antimycotic relative to no intervention, placebo, or another antifungal agent as prophylaxis in afebrile adults age >18 years with AML undergoing intensive chemotherapy or allogeneic HCT.
Outcome measures and data extraction
The primary outcome of interest was the incidence of probable and/or proven IFIs as defined by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG).11 Secondary outcomes of interest included frequency of IFIs by organism, rates of adverse drug effects and toxicity-related drug discontinuation, overall survival, and IFI-attributable as well as all-cause mortality. A database of abstracted variables was created that included the above outcomes as well as years of enrollment, study design, number and age of study subjects, inclusion/exclusion criteria, type of malignancies and transplants, adverse events, and duration of follow-up. The strength of recommendations and the quality of evidence were evaluated on the basis of the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system (Table 1).12
Table 1.
Summary of GRADE recommendations on rating the strength of recommendations and quality of evidence
| Strength of recommendation | Quality of evidence | ||
|---|---|---|---|
| 1 (“Strong”) | Desirable effects of an intervention clearly outweigh (or clearly do not outweigh) the undesirable effects | A (“High”) | Further research is unlikely to change our confidence in the estimate of effect |
| 2 (“Weak”) | Tradeoffs between desirable and undesirable effects are less certain (eg, because of low-quality evidence or evidence suggesting closely balanced effects) | B (“Moderate”) | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate |
| C (“Low”) | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate | ||
| D (“Very low”) | Any estimate of effect is very uncertain | ||
Each recommendation consists of a numerical score denoting the strength of the recommendation and a letter denoting the quality of the evidence.12
Results
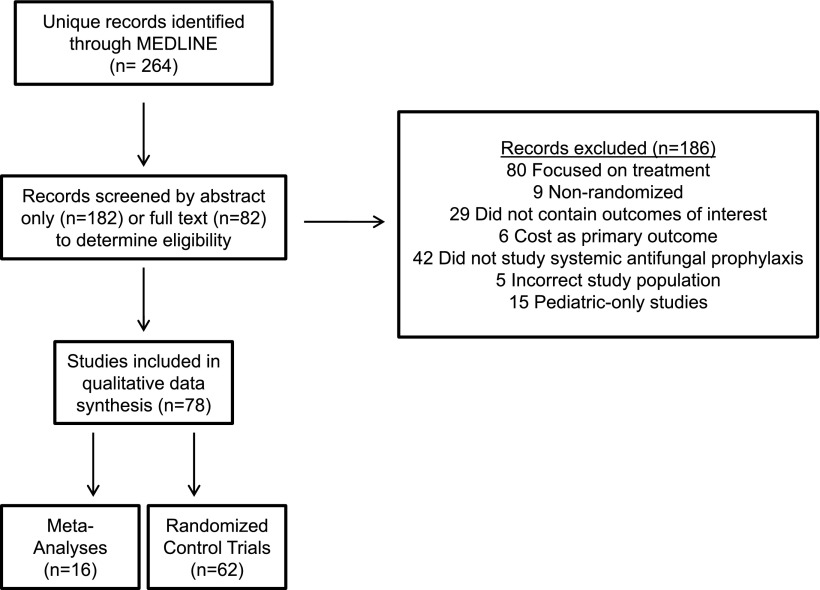
Our literature search yielded 264 records (Figure 1). Seventy-eight of these (62 RCTs [including 6 blinded, 20 double-blinded/placebo-controlled], 16 meta-analyses) were eligible for qualitative synthesis. We prioritized trials that exclusively or primarily involved patients with AML or high-risk myelodysplastic syndrome (MDS) receiving intensive chemotherapy to enhance the applicability of our findings. We excluded studies solely examining patients undergoing autologous HCT but included trials reporting on autologous and allogeneic (or allogeneic only) HCTs.
Figure 1.
PRISMA flow diagram.
Case 1: Antifungal prophylaxis in adults undergoing AML induction chemotherapy
Polyenes.
Regardless of the systemic formulation (deoxycholate or lipid formulation), amphotericin B is usually too toxic for antifungal prophylaxis in AML induction therapy.13-15 The oral formulation of amphotericin B is not widely available and has no activity against inhaled molds, as this drug is not absorbed systematically. Inhalations of aerosolized amphotericin B are better tolerated but are not well established as preventative measures in this setting and are not standardized for delivery to the alveoli.16,17
Fluconazole and itraconazole.
Fluconazole, available in IV and oral formulations, is well tolerated and has activity against many yeast (particularly Candida) species. However, the incidence of non-albicans Candida spp with intrinsic resistance or limited susceptibility to fluconazole is increasing,3 and the drug lacks activity against filamentous fungi such as Aspergillus spp. Fluconazole has been widely studied in RCTs and found to reduce the incidence and mortality of IFIs compared with placebo18 and to be equally effective, but better tolerated than, amphotericin B formulations.13,15,19 Itraconazole’s activity spectrum is wider than that of fluconazole and includes Aspergillus spp. Itraconazole is available in oral and IV formulations (in some countries) but has poor gastrointestinal (GI) tolerability when given orally as solution.20 Four independent meta-analyses of RCTs comparing these 2 triazoles in neutropenic patients with hematologic malignancies showed that itraconazole significantly reduced IFIs (but not IFI-attributable or all-cause mortality) compared with fluconazole at the expense of greater drug toxicity.8,9,21,22
Echinocandins.
Caspofungin, micafungin, and anidulafungin are available in IV form only. They are well tolerated and have activity against Candida and Aspergillus spp but not Mucorales or Fusarium spp. RCTs have only been conducted with caspofungin as prophylaxis in acute leukemia patients undergoing chemotherapy. Two studies in 175 patients with acute lymphoblastic leukemia or AML and 192 patients with AML or MDS found no differences in the incidence of probable/proven IFIs, IFI-attributable and all-cause mortality, and drug toxicities compared with itraconazole in 1 study or physician’s choice of either itraconazole (82%), fluconazole (12%), posaconazole (1%) or no prophylaxis (5%) in the other study.23,24
Voriconazole.
Available as IV and oral formulations, voriconazole is active against a broad range of fungi including Candida spp, Aspergillus spp, Scedosporium spp, and Fusarium spp.25 Its toxicity and safety profile is limited by visual hallucinations, cutaneous solar hypersensitivity, and hepatic transaminase elevation, whereas resistance of Aspergillus spp to voriconazole is increasingly recognized.26 RCT-level evidence supporting voriconazole prophylaxis in the nontransplant AML population is limited to 2 published studies, 1 of which was terminated after enrollment of only 25 patients because the use of placebo in the control arm was no longer deemed ethical.27 In the second study, 123 patients with AML/high-risk MDS receiving induction or salvage chemotherapy were randomized to oral voriconazole or IV itraconazole.28 This study failed to reach its target accrual and found no statistically significant differences in probable/proven IFIs, all-cause mortality, or toxicity-related drug discontinuation rates, suggesting equal value for prophylaxis in this patient population.
Posaconazole.
Posaconazole has activity against a diverse array of fungi including Candida spp, Aspergillus spp, Scedosporium spp, Fusarium spp, and several species of the Mucorales. Posaconazole has been compared with fluconazole and itraconazole (2 RCTs) but not to voriconazole or echinocandins in non-HCT AML patients. In a landmark study of AML/MDS patients undergoing induction chemotherapy, patients who received oral suspension posaconazole (n = 304) were less likely than those receiving oral fluconazole (n = 240) or oral itraconazole (n = 58) to develop probable/proven IFIs (total IFIs: 2% vs 8%, P < .001; invasive aspergillosis [IA]: 1% vs 7%, P < .001), and had lower 100-day mortality (14% vs 21%, P = .04) and lower IFI-attributable deaths (2% vs 5%, P = .01).20 Serious triazole-related adverse events were more frequent in the posaconazole arm (6% vs 2%, P = .01). An estimated 16 and 14 patients needed to be treated with posaconazole to prevent 1 IFI and 1 death, respectively. Partly consistent with these findings, a second RCT of 252 AML patients found that oral suspension posaconazole was associated with a lower rate of probable/proven IFIs than oral fluconazole (4% vs 9%, P = .026) and insignificantly lower all-cause mortality (2.6% vs 6%, P = .22), whereas drug-related adverse events were similar.29
A major limitation of oral suspension posaconazole is variable bioavailability, including in patients who develop diarrhea (eg, due to gut graft-versus-host disease [GVHD]), with subtherapeutic drug levels linked to increased risk of breakthrough IFIs in AML patients.30-33 Thus, monitoring plasma drug levels is recommended in patients with risk factors for poor absorption who are receiving the suspension.34 This limitation has been largely overcome by the recently introduced extended-release tablet form.35 Unlike the oral suspension formulation, absorption of the tablet is minimally affected by food, mucositis, and GI pH, resulting in increased serum drug levels in leukemia patients.34,36,37 The recommendation for monitoring of plasma drug levels therefore does not pertain to patients receiving the tablet form of posaconazole. Other important limitations of posaconazole include interactions with cytochrome P450 enzymes and P-glycoprotein,38 complicating its use in patients requiring multiple concomitant transplant-related medications or newer targeted antileukemic agents such as isocitrate dehydrogenase inhibitors. New-generation, broad-spectrum triazoles such as isavuconazole may overcome some of these limitations,39,40 but testing as prophylaxis in AML patients has just begun.41
Recommendation.
Fluconazole is better than placebo in preventing invasive candidiasis during AML remission induction therapy. However, fluconazole lacks activity against molds. Although itraconazole and voriconazole are used for prophylaxis against infections caused by Candida spp and molds, posaconazole is the only agent that has demonstrated a significant survival and outcome benefit in prophylaxis against these mycoses during AML induction therapy (GRADE 1A). Despite the lack of RCT data in AML, use of the tablet, if tolerated, rather than the oral suspension of posaconazole is recommended based on phase 1 findings in hematologic malignancies,35 retrospective studies,36,42 and early results from phase 3 studies43 demonstrating superior bioavailability of the tablet without worsening of adverse effects (GRADE 1C). As there is a good relationship between serum concentrations of posaconazole and therapeutic outcome of IA, use of the tablet formulation in lieu of the suspension is tenable. For patients who are not able to swallow the tablet, the oral suspension is recommended. Coadministration of posaconazole and drugs that strongly interfere with the 3A4 isoform of cytochrome P450 should be avoided. For patients who are not able to tolerate oral posaconazole and in cases where the IV formulation is not available, administration of an echinocandin is a reasonable substitute for antifungal prophylaxis (GRADE 1B). If there is a high risk for IA, IV voriconazole should be considered (GRADE 2B).
Case 2: Antifungal prophylaxis in adults with AML undergoing HCT
The incidence of IFIs after allogeneic HCT is as high as 10% to 20%, and associated mortality ranges from 30% to 80% depending on the organism.4,5 Although antifungal prophylaxis after HCT has long been considered standard,44 issues specific to this setting include concerns for drug-drug interactions with conditioning and immunosuppressive agents, poor oral absorption secondary to treatment-related gut toxicity, as well as GVHD and associated use of corticosteroids.45 Several factors, including donor source, history of IFIs, active hematologic cancer, and GVHD can identify patients at particularly high risk of IFIs throughout the post-HCT period and could help tailor prevention strategies.46
Polyenes.
Amphotericin B lacks significant drug-drug interactions, but nephrotoxicity and poor tolerability limit its usefulness as prophylaxis in HCT.14,47-50
Fluconazole and itraconazole.
Fluconazole is well suited for posttransplant use due to good tolerability and minimal drug-drug interactions, and is as effective as amphotericin B with regard to incidence of IFIs and mortality.49 In 2 placebo-controlled RCTs, conducted at a time when the majority of IFIs were due to Candida spp, fluconazole was associated with lower incidence of IFIs and reduced IFI-related mortality.51,52 In 1 of the studies, fluconazole given until day 75 after allogeneic HCT also led to improved overall survival.52,53 These findings established this use of fluconazole as standard prophylaxis at that time54 and as benchmark for novel antifungal agents. Several RCTs have exclusively compared itraconazole with fluconazole in patients undergoing HCT.55-58 In a study of 300 patients undergoing allogeneic HCT who received either itraconazole or fluconazole for 120 to 180 days posttransplant, rates of probable/proven IFIs or survival were similar but fewer mold infections were noted with itraconazole (5% vs 12%, P = .03).56 In a similar trial of 140 patients, oral itraconazole was associated with a lower rate of IFIs (9% vs 25%, P = .01), but no statistically significant difference in IFI-related or all-cause mortality.57 In contrast, in a third RCT of 195 patients with acute leukemia undergoing HCT who received either itraconazole or fluconazole until engraftment, there was no difference in IFIs, IA, or mortality.58 In all of these studies, there was significantly more GI toxicity with itraconazole. Although these studies largely supported the superiority of itraconazole over fluconazole in prevention of IFIs in allogeneic HCT, the toxicity profile of itraconazole has precluded its wide acceptance for prophylaxis in this setting. Increased renal and hepatic toxicities were observed when itraconazole was administered concurrently with cyclophosphamide and busulfan due to inhibition of their metabolism,59,60 highlighting the need for avoiding simultaneous exposures of antineoplastic agents and triazoles that inhibit hepatic microsomal enzymes. This toxicity may also increase the risk of sinusoidal obstruction syndrome and, consequently, nonrelapse mortality.61 Because of poor tolerance, itraconazole is therefore not recommended over fluconazole in HCT patients despite potentially higher efficacy.62
Echinocandins.
Because of their tolerability, echinocandins are good candidates for prophylaxis in HCT. Three RCTs have compared micafungin with fluconazole63,64 or itraconazole65 in this setting. In a study of 882 patients randomized to either micafungin or IV fluconazole in the neutropenic phase of HCT, micafungin was associated with a lower incidence of suspected or probable/proven IFIs (P = .03) and a trend toward lower IA (0.2% vs 1.5%, P = .07) but similar IFI-related and all-cause mortality and adverse events.63 On the other hand, in a similarly designed but underpowered trial of 106 patients, no differences in probable/proven IFIs or adverse events were found between micafungin and fluconazole.64 As an important limitation for our purpose, these 2 trials included a large proportion of non-AML patients and individuals undergoing autologous HCT. In the third RCT, there was no difference in probable/proven IFIs and IFI-related or all-cause mortality among 287 HCT patients (83% allogeneic) assigned to micafungin or itraconazole, but significantly more patients discontinued itraconazole early due to toxicity (19.7% vs 0.7%, P < .01).65 Thus, micafungin is noninferior to fluconazole and itraconazole and is less toxic than itraconazole.
Voriconazole.
Two large RCTs of 600 and 489 patients (>50% with AML or MDS) undergoing allogeneic HCT have compared voriconazole with fluconazole or itraconazole given until day 100 posttransplant.25,66 There were no differences in IFIs or survival between the treatment arms in either study, but in the study comparing voriconazole with fluconazole, a protocol-driven, structured empirical antifungal therapy and diagnostic screening program was used and evaluations for IFIs were mandated if a positive serum galactomannan assay or suggestive signs of fungal infection were noted.66 In the second study, better tolerance of voriconazole than itraconazole was demonstrated, with a higher proportion of patients completing the study (54% vs 39%, P < .01) although hepatotoxicity occurred more commonly with voriconazole (13% vs 5%, P < .01), requiring frequent adjustment of calcineurin inhibitors.25 Indeed, unpredictable interactions between voriconazole and commonly used immunosuppressants (tacrolimus, cyclosporine A) are well documented.67,68 Thus, voriconazole has no clear benefit over fluconazole or itraconazole in terms of efficacy, but is preferred over itraconazole due to better tolerability.
Posaconazole.
Two RCTs have evaluated posaconazole as prophylaxis in AML patients undergoing HCT. In a study of 40 patients randomized to either posaconazole oral solution or weekly IV amphotericin B lipid complex, rates of IFIs were similar (0% vs 5%, P = .48) but the median number of days on fungal prophylaxis was longer in the posaconazole group (42 vs 20 days, P = .01) due to amphotericin intolerance, particularly renal toxicity, which led to early trial termination.50 In the second RCT, 600 patients (30% with AML or MDS) with active GVHD requiring immunosuppressive therapy after allogeneic HCT were allocated to either posaconazole or fluconazole. Posaconazole was associated with a trend toward lower incidence of probable/proven IFIs (5% vs 9%, P = .07), a lower incidence of probable/proven IA (2.3% vs 7%, P = .006), and lower IFI-attributable mortality (1% vs 4%, P = .046), whereas all-cause mortality and rates of treatment-related adverse events were similar.69
Recommendations.
Following allogeneic HCT, fluconazole, voriconazole, posaconazole, and micafungin are all equally recommended over no antifungal prophylaxis in low-risk patients (GRADE 1B). For the quality of evidence, the distinction is made between use of posaconazole for prophylaxis in AML during induction therapy and for preengraftment and postengraftment prophylaxis after HCT, where matters of study design and interpretation influence the recommendations. Additional high-quality studies are needed to refine the recommendations regarding the optimal antifungal agent for the pre- and post-engraftment period following allogeneic HCT. For high-risk patients, especially those with GVHD, posaconazole is recommended over other antifungal strategies (GRADE 1A); if poor oral absorption of posaconazole is anticipated, voriconazole is an acceptable substitute (GRADE 2B).
Discussion
We found high-quality evidence to recommend posaconazole for IFI prophylaxis in patients with AML undergoing induction chemotherapy and those with GVHD following allogeneic HCT, whereas there is no strong evidence to guide the selection of antifungal prophylaxis following allogeneic HCT in patients who have no GVHD or other high-risk factors. Although costly, several studies have demonstrated cost-effectiveness of posaconazole in patients at high risk for fungal infections compared with other triazoles due to the reduction of IFIs and shortened hospital stays.70-74
As an important caveat for recommendations based on RCTs, studied subjects are typically highly selected based on stringent inclusion/exclusion criteria. Therefore, RCT findings may not be generalizable to all patients with AML.75 However, retrospective and prospective observational studies support the use of posaconazole rather than itraconazole or fluconazole in AML patients undergoing intensive chemotherapy76,77 or allogeneic HCT78 and complement the RCT data. Still, strategies for antifungal prophylaxis in a broader range of patients with AML are needed such as those with end-organ toxicity for whom individual assessments should be made. For example, in patients with preexisting hepatic transaminase elevation, an echinocandin may be the best choice for antifungal prophylaxis as it provides protection from most Candida spp and possibly Aspergillus spp. Furthermore, few studies of antifungal prophylaxis have been conducted in pediatric AML. Antifungal prophylaxis in pediatric patients has largely focused on high-risk acute lymphoblastic leukemia. In the absence of large robust randomized trials in the pediatric population, extrapolation with appropriate pharmacokinetic/pharmacodynamic bridging provides for rational dosing of antifungal agents in pediatric patients during chemotherapy-induced neutropenia.79,80
A number of questions remain unanswered, including the efficacy of the many novel antifungal agents that are under preclinical and/or clinical development (eg, isavuconazole, ravuconazole, albaconazole, aminocandin, among others), the efficacy and tolerability of newer formulations of established drugs, and the role and reliability of monitoring strategies for early detection of IA.81 Serum galactomannan testing is useful in the early detection of IA in neutropenic patients who are not receiving antimold prophylaxis. The presence of circulating levels of antimold agents diminishes the galactomannan index signal and utility of this assay as most positive galactomannan tests during surveillance in the setting of mold-active prophylaxis represent false-positive results.81 Open questions also relate to the best strategy for the treatment of breakthrough fungal infections in patients receiving prophylaxis with broad-spectrum antifungal drugs, the role of therapeutic drug monitoring, and resistance development.82-85 As each new antifungal agent has been introduced and accepted into wider use, the pattern of IFIs has shifted from susceptible invasive Candida spp to resistant Candida spp and IA. Now, with increased use of mold-active triazoles, breakthrough IFIs with resistant Aspergillus spp and non-Aspergillus molds such as Fusarium spp are being seen. Additionally, although the recommendations throughout this article are broad and population based, there are many subtleties in decision-making when choosing prophylaxis for individual patients, and other factors beyond type of therapy, engraftment stage, and GVHD (Table 2)86-104 are increasingly recognized as being important for risk stratification and choice of antifungal agent. As we move toward personalized medicine and targeted therapy in oncology, efforts to allow for more individualized choices in fungal prophylactic therapy are ongoing, with work being done on risk prediction scores for IFI development, immunogenetic profiling to determine host-specific risk factors for IFI infection, and individual mycobiome monitoring.86,87,100,105
Table 2.
Factors that may affect risk stratification for antifungal prophylaxis
| Factor | Comments |
|---|---|
| Genetic markers86-91 | • SNPs in Toll-like receptor genes |
| • SNPs in plasminogen genes | |
| • IL-1 gene polymorphisms and haplotype | |
| • Polymorphisms in the chemotactic cytokine CXC10 | |
| • Dectin-1 deficiency | |
| • Mannose-binding lectin deficiency | |
| Geographic factors92 | • US Ohio/Mississippi River Valley: Histoplasma capsulatum |
| • US Midwest and South Central: Blastomyces dermatitidis; Southwest: Coccidioides immitis | |
| • South America: Paracoccidioides brasiliensis | |
| • Southeast Asia: Penicillium marneffei | |
| Climate92 | • Incidence of IA associated with climate/season |
| Environmental exposure93-95 | • Soil contact, food safety, water safety |
| • Construction increases risk IFIs, especially IA | |
| • Increased in-hospital transfers out of hematology ward increased risk for filamentous IFIs | |
| Metabolic factors96,97 | • Increased bone marrow and peripheral markers of iron stores associated with an increased risk of fungal infections, including mucormycosis |
| Viral and bacterial coinfections98,99 | • CMV and respiratory virus coinfections increase risk of IA |
| • Bacteremia increases risk of IFIs | |
| Mycobiome98,100-104 | • Baseline colonization important risk for invasive candidiasis and predictive of subsequent infection |
| • More than 1 site of colonization/heavy colonization at single site increases risk IFIs | |
| • Rectal fungal colonization increases IFI risk | |
| • Nasal cultures for Aspergillus spp predict increased risk of IA | |
| • PCR and sequencing showed that the majority of fungi from lung infections in patients matched fungi from mouth and throat |
CMV, cytomegalovirus; IL, interleukin; PCR, polymerase chain reaction; SNP, single nucleotide polymorphism.
Acknowledgments
A.B.H. is supported by a fellowship training grant from the National Heart, Lung, and Blood Institute/National Institutes of Health (NHLBI/NIH: T32-HL007093).
T.J.W. is a Scholar of the Sharp Family Foundation in Emerging Pediatric Infectious Diseases and a Scholar of the Henry Schueler Foundation in Mucormycosis.
D.P.K. acknowledges the Francis King Black Endowment for Cancer Research.
R.B.W. is a Leukemia & Lymphoma Society Clinical Scholar in Clinical Research.
Footnotes
The online version of this article contains a data supplement.
Authorship
Contribution: A.B.H. and R.B.W. were responsible for the concept of this review and contributed to the literature search and data collection quality assessment, analyzed and interpreted data, and wrote the manuscript; G.H.L. was responsible for the concept of the review, contributed to the literature search, and critically revised the manuscript; and T.J.W. and D.P.K. contributed to the concept of the review, assessed the literature, and critically revised the manuscript.
Conflict-of-interest disclosure: T.J.W. has received research grants for experimental and clinical antimicrobial pharmacotherapeutics from Astellas, Novartis, Merck, ContraFect, Pfizer, Cubist, and Theravance, and has served as consultant to Astellas, ContraFect, Cubist, Drais, iCo, Novartis, Pfizer, Methylgene, SigmaTau, and Trius. D.P.K. has received research support from Merck, Pfizer, Astellas, and T2 Biosystems, and has received honoraria from Merck, Astellas, Gilead, Mylan, F2G, iNc, and T2 Biosystems. The remaining authors declare no competing financial interests.
Correspondence: Anna B. Halpern, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: halpern2@uw.edu.
References
- 1.Pechlivanoglou P, Le HH, Daenen S, Snowden JA, Postma MJ. Mixed treatment comparison of prophylaxis against invasive fungal infections in neutropenic patients receiving therapy for haematological malignancies: a systematic review. J Antimicrob Chemother. 2014;69(1):1–11. doi: 10.1093/jac/dkt329. [DOI] [PubMed] [Google Scholar]
- 2.Leventakos K, Lewis RE, Kontoyiannis DP. Fungal infections in leukemia patients: how do we prevent and treat them? Clin Infect Dis. 2010;50(3):405–415. doi: 10.1086/649879. [DOI] [PubMed] [Google Scholar]
- 3.Tacke D, Buchheidt D, Karthaus M, et al. Primary prophylaxis of invasive fungal infections in patients with haematologic malignancies. 2014 update of the recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Ann Hematol. 2014;93(9):1449–1456. doi: 10.1007/s00277-014-2108-y. [DOI] [PubMed] [Google Scholar]
- 4.Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48(3):265–273. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 5.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 6.Even C, Bastuji-Garin S, Hicheri Y, et al. Impact of invasive fungal disease on the chemotherapy schedule and event-free survival in acute leukemia patients who survived fungal disease: a case-control study. Haematologica. 2011;96(2):337–341. doi: 10.3324/haematol.2010.030825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis RE, Cahyame-Zuniga L, Leventakos K, et al. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: a 20-year autopsy study. Mycoses. 2013;56(6):638–645. doi: 10.1111/myc.12081. [DOI] [PubMed] [Google Scholar]
- 8.Ethier MC, Science M, Beyene J, Briel M, Lehrnbecher T, Sung L. Mould-active compared with fluconazole prophylaxis to prevent invasive fungal diseases in cancer patients receiving chemotherapy or haematopoietic stem-cell transplantation: a systematic review and meta-analysis of randomised controlled trials. Br J Cancer. 2012;106(10):1626–1637. doi: 10.1038/bjc.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25(34):5471–5489. doi: 10.1200/JCO.2007.12.3851. [DOI] [PubMed] [Google Scholar]
- 10.Uzun O, Anaissie EJ. Antifungal prophylaxis in patients with hematologic malignancies: a reappraisal. Blood. 1995;86(6):2063–2072. [PubMed] [Google Scholar]
- 11.De Pauw B, Walsh TJ, Donnelly JP, et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodey GP, Anaissie EJ, Elting LS, Estey E, O’Brien S, Kantarjian H. Antifungal prophylaxis during remission induction therapy for acute leukemia fluconazole versus intravenous amphotericin B. Cancer. 1994;73(8):2099–2106. doi: 10.1002/1097-0142(19940415)73:8<2099::aid-cncr2820730814>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Timmers GJ, Zweegman S, Simoons-Smit AM, van Loenen AC, Touw D, Huijgens PC. Amphotericin B colloidal dispersion (Amphocil) vs fluconazole for the prevention of fungal infections in neutropenic patients: data of a prematurely stopped clinical trial. Bone Marrow Transplant. 2000;25(8):879–884. doi: 10.1038/sj.bmt.1702243. [DOI] [PubMed] [Google Scholar]
- 15.Mattiuzzi GN, Estey E, Raad I, et al. Liposomal amphotericin B versus the combination of fluconazole and itraconazole as prophylaxis for invasive fungal infections during induction chemotherapy for patients with acute myelogenous leukemia and myelodysplastic syndrome. Cancer. 2003;97(2):450–456. doi: 10.1002/cncr.11094. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz S, Behre G, Heinemann V, et al. Aerosolized amphotericin B inhalations as prophylaxis of invasive aspergillus infections during prolonged neutropenia: results of a prospective randomized multicenter trial. Blood. 1999;93(11):3654–3661. [PubMed] [Google Scholar]
- 17.Rijnders BJ, Cornelissen JJ, Slobbe L, et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clin Infect Dis. 2008;46(9):1401–1408. doi: 10.1086/586739. [DOI] [PubMed] [Google Scholar]
- 18.Rotstein C, Bow EJ, Laverdiere M, Ioannou S, Carr D, Moghaddam N The Canadian Fluconazole Prophylaxis Study Group. Randomized placebo-controlled trial of fluconazole prophylaxis for neutropenic cancer patients: benefit based on purpose and intensity of cytotoxic therapy. Clin Infect Dis. 1999;28(2):331–340. doi: 10.1086/515128. [DOI] [PubMed] [Google Scholar]
- 19.Menichetti F, Del Favero A, Martino P, et al. GIMEMA Infection Program. Preventing fungal infection in neutropenic patients with acute leukemia: fluconazole compared with oral amphotericin B. Ann Intern Med. 1994;120(11):913–918. doi: 10.7326/0003-4819-120-11-199406010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 21.Vardakas KZ, Michalopoulos A, Falagas ME. Fluconazole versus itraconazole for antifungal prophylaxis in neutropenic patients with haematological malignancies: a meta-analysis of randomised-controlled trials. Br J Haematol. 2005;131(1):22–28. doi: 10.1111/j.1365-2141.2005.05727.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Zhan P, Zhou R, et al. Prophylaxis with itraconazole is more effective than prophylaxis with fluconazole in neutropenic patients with hematological malignancies: a meta-analysis of randomized-controlled trials. Med Oncol. 2010;27(4):1082–1088. doi: 10.1007/s12032-009-9339-0. [DOI] [PubMed] [Google Scholar]
- 23.Cattaneo C, Monte S, Algarotti A, et al. A randomized comparison of caspofungin versus antifungal prophylaxis according to investigator policy in acute leukaemia patients undergoing induction chemotherapy (PROFIL-C study). J Antimicrob Chemother. 2011;66(9):2140–2145. doi: 10.1093/jac/dkr271. [DOI] [PubMed] [Google Scholar]
- 24.Mattiuzzi GN, Alvarado G, Giles FJ, et al. Open-label, randomized comparison of itraconazole versus caspofungin for prophylaxis in patients with hematologic malignancies. Antimicrob Agents Chemother. 2006;50(1):143–147. doi: 10.1128/AAC.50.1.143-147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marks DI, Pagliuca A, Kibbler CC, et al. IMPROVIT Study Group. Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. Br J Haematol. 2011;155(3):318–327. doi: 10.1111/j.1365-2141.2011.08838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verweij PE, Ananda-Rajah M, Andes D, et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat. 2015;21-22:30–40. doi: 10.1016/j.drup.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Vehreschild JJ, Böhme A, Buchheidt D, et al. A double-blind trial on prophylactic voriconazole (VRC) or placebo during induction chemotherapy for acute myelogenous leukaemia (AML). J Infect. 2007;55(5):445–449. doi: 10.1016/j.jinf.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Mattiuzzi GN, Cortes J, Alvarado G, et al. Efficacy and safety of intravenous voriconazole and intravenous itraconazole for antifungal prophylaxis in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Support Care Cancer. 2011;19(1):19–26. doi: 10.1007/s00520-009-0783-3. [DOI] [PubMed] [Google Scholar]
- 29.Shen Y, Huang XJ, Wang JX, et al. Posaconazole vs. fluconazole as invasive fungal infection prophylaxis in China: a multicenter, randomized, open-label study. Int J Clin Pharmacol Ther. 2013;51(9):738–745. doi: 10.5414/CP201880. [DOI] [PubMed] [Google Scholar]
- 30.Dolton MJ, Ray JE, Chen SC, Ng K, Pont L, McLachlan AJ. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob Agents Chemother. 2012;56(11):5503–5510. doi: 10.1128/AAC.00802-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gubbins PO, Krishna G, Sansone-Parsons A, et al. Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob Agents Chemother. 2006;50(6):1993–1999. doi: 10.1128/AAC.00157-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cattaneo C, Panzali A, Passi A, et al. Serum posaconazole levels during acute myeloid leukaemia induction therapy: correlations with breakthrough invasive fungal infections. Mycoses. 2015;58(6):362–367. doi: 10.1111/myc.12326. [DOI] [PubMed] [Google Scholar]
- 33.Tonini J, Thiébaut A, Jourdil JF, et al. Therapeutic drug monitoring of posaconazole in allogeneic hematopoietic stem cell transplantation patients who develop gastrointestinal graft-versus-host disease. Antimicrob Agents Chemother. 2012;56(10):5247–5252. doi: 10.1128/AAC.00815-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miceli MH, Perissinotti AJ, Kauffman CA, Couriel DR. Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses. 2015;58(7):432–436. doi: 10.1111/myc.12339. [DOI] [PubMed] [Google Scholar]
- 35.Duarte RF, López-Jiménez J, Cornely OA, et al. Phase 1b study of new posaconazole tablet for prevention of invasive fungal infections in high-risk patients with neutropenia. Antimicrob Agents Chemother. 2014;58(10):5758–5765. doi: 10.1128/AAC.03050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung DS, Tverdek FP, Kontoyiannis DP. Switching from posaconazole suspension to tablets increases serum drug levels in leukemia patients without clinically relevant hepatotoxicity. Antimicrob Agents Chemother. 2014;58(11):6993–6995. doi: 10.1128/AAC.04035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cumpston A, Caddell R, Shillingburg A, et al. Superior serum concentrations with posaconazole delayed-release tablets compared to suspension formulation in hematological malignancies. Antimicrob Agents Chemother. 2015;59(8):4424–4428. doi: 10.1128/AAC.00581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagappan V, Deresinski S. Reviews of anti-infective agents: posaconazole: a broad-spectrum triazole antifungal agent. Clin Infect Dis. 2007;45(12):1610–1617. doi: 10.1086/523576. [DOI] [PubMed] [Google Scholar]
- 39.Ananda-Rajah MR, Kontoyiannis D. Isavuconazole: a new extended spectrum triazole for invasive mold diseases. Future Microbiol. 2015;10(5):693–708. doi: 10.2217/fmb.15.34. [DOI] [PubMed] [Google Scholar]
- 40.Chitasombat MN, Kontoyiannis DP. The ‘cephalosporin era’ of triazole therapy: isavuconazole, a welcomed newcomer for the treatment of invasive fungal infections. Expert Opin Pharmacother. 2015;16(10):1543–1558. doi: 10.1517/14656566.2015.1057500. [DOI] [PubMed] [Google Scholar]
- 41.Cornely OA, Böhme A, Schmitt-Hoffmann A, Ullmann AJ. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: results of a phase 2, dose escalation study. Antimicrob Agents Chemother. 2015;59(4):2078–2085. doi: 10.1128/AAC.04569-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durani U, Tosh PK, Barreto JN, Estes LL, Jannetto PJ, Tande AJ. Retrospective comparison of posaconazole levels in patients taking the delayed-release tablet versus the oral suspension. Antimicrob Agents Chemother. 2015;59(8):4914–4918. doi: 10.1128/AAC.00496-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cornely OA, Duarte RF, Haider S, et al. Berlin, Germany: European Society of Clinical Microbiology and Infectious Diseases; 2013. Phase 3 pharmacokinetics (PK) and safety study of posaconazole (POS) tablet in patients at risk for invasive fungal infection [abstract]. [Google Scholar]
- 44.Tomblyn M, Chiller T, Einsele H, et al. Center for International Blood and Marrow Research; National Marrow Donor program; European Blood and MarrowTransplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Disease Canada; Centers for Disease Control and Prevention. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective [published correction appears in Biol Blood Marrow Transplant. 2010;16(2):294]. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girmenia C, Barosi G, Piciocchi A, et al. Primary prophylaxis of invasive fungal diseases in allogeneic stem cell transplantation: revised recommendations from a consensus process by Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant. 2014;20(8):1080–1088. doi: 10.1016/j.bbmt.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 46.Girmenia C, Raiola AM, Piciocchi A, et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant. 2014;20(6):872–880. doi: 10.1016/j.bbmt.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Kelsey SM, Goldman JM, McCann S, et al. Liposomal amphotericin (AmBisome) in the prophylaxis of fungal infections in neutropenic patients: a randomised, double-blind, placebo-controlled study. Bone Marrow Transplant. 1999;23(2):163–168. doi: 10.1038/sj.bmt.1701543. [DOI] [PubMed] [Google Scholar]
- 48.Wolff SN, Fay J, Stevens D, et al. Fluconazole vs low-dose amphotericin B for the prevention of fungal infections in patients undergoing bone marrow transplantation: a study of the North American Marrow Transplant Group. Bone Marrow Transplant. 2000;25(8):853–859. doi: 10.1038/sj.bmt.1702233. [DOI] [PubMed] [Google Scholar]
- 49.Koh LP, Kurup A, Goh YT, Fook-Chong SM, Tan PH. Randomized trial of fluconazole versus low-dose amphotericin B in prophylaxis against fungal infections in patients undergoing hematopoietic stem cell transplantation. Am J Hematol. 2002;71(4):260–267. doi: 10.1002/ajh.10234. [DOI] [PubMed] [Google Scholar]
- 50.Chaftari AM, Hachem RY, Ramos E, et al. Comparison of posaconazole versus weekly amphotericin B lipid complex for the prevention of invasive fungal infections in hematopoietic stem-cell transplantation. Transplantation. 2012;94(3):302–308. doi: 10.1097/TP.0b013e3182577485. [DOI] [PubMed] [Google Scholar]
- 51.Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326(13):845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 52.Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation--a prospective, randomized, double-blind study. J Infect Dis. 1995;171(6):1545–1552. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 53.Marr KA, Seidel K, Slavin MA, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000;96(6):2055–2061. [PubMed] [Google Scholar]
- 54.Pagano L, Caira M. The role of primary antifungal prophylaxis in patients with haematological malignancies. Clin Microbiol Infect. 2014 doi: 10.1111/1469-0691.12464. 20(suppl 6):19-26. [DOI] [PubMed] [Google Scholar]
- 55.Annaloro C, Oriana A, Tagliaferri E, et al. Efficacy of different prophylactic antifungal regimens in bone marrow transplantation. Haematologica. 1995;80(6):512–517. [PubMed] [Google Scholar]
- 56.Marr KA, Crippa F, Leisenring W, et al. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood. 2004;103(4):1527–1533. doi: 10.1182/blood-2003-08-2644. [DOI] [PubMed] [Google Scholar]
- 57.Winston DJ, Maziarz RT, Chandrasekar PH, et al. Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients. A multicenter, randomized trial. Ann Intern Med. 2003;138(9):705–713. doi: 10.7326/0003-4819-138-9-200305060-00006. [DOI] [PubMed] [Google Scholar]
- 58.Oren I, Rowe JM, Sprecher H, et al. A prospective randomized trial of itraconazole vs fluconazole for the prevention of fungal infections in patients with acute leukemia and hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2006;38(2):127–134. doi: 10.1038/sj.bmt.1705418. [DOI] [PubMed] [Google Scholar]
- 59.Buggia I, Zecca M, Alessandrino EP, et al. Itraconazole can increase systemic exposure to busulfan in patients given bone marrow transplantation. GITMO (Gruppo Italiano Trapianto di Midollo Osseo). Anticancer Res. 1996;16(4A):2083–2088. [PubMed] [Google Scholar]
- 60.Marr KA, Leisenring W, Crippa F, et al. Cyclophosphamide metabolism is affected by azole antifungals. Blood. 2004;103(4):1557–1559. doi: 10.1182/blood-2003-07-2512. [DOI] [PubMed] [Google Scholar]
- 61.McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101(5):2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 62.Akan H, Antia VP, Kouba M, et al. Preventing invasive fungal disease in patients with haematological malignancies and the recipients of haematopoietic stem cell transplantation: practical aspects. J Antimicrob Chemother. 2013 doi: 10.1093/jac/dkt389. 68(suppl 3):iii5-iii16. [DOI] [PubMed] [Google Scholar]
- 63.van Burik JA, Ratanatharathorn V, Stepan DE, et al. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39(10):1407–1416. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 64.Hiramatsu Y, Maeda Y, Fujii N, et al. West-Japan Hematology and Oncology Group. Use of micafungin versus fluconazole for antifungal prophylaxis in neutropenic patients receiving hematopoietic stem cell transplantation. Int J Hematol. 2008;88(5):588–595. doi: 10.1007/s12185-008-0196-y. [DOI] [PubMed] [Google Scholar]
- 65.Huang X, Chen H, Han M, et al. Multicenter, randomized, open-label study comparing the efficacy and safety of micafungin versus itraconazole for prophylaxis of invasive fungal infections in patients undergoing hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2012;18(10):1509–1516. doi: 10.1016/j.bbmt.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 66.Wingard JR, Carter SL, Walsh TJ, et al. Blood and Marrow Transplant Clinical Trials Network. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116(24):5111–5118. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mori T, Kato J, Yamane A, et al. Drug interaction between voriconazole and tacrolimus and its association with the bioavailability of oral voriconazole in recipients of allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2012;95(5):564–569. doi: 10.1007/s12185-012-1057-2. [DOI] [PubMed] [Google Scholar]
- 68.Kikuchi T, Mori T, Yamane A, Kato J, Kohashi S, Okamoto S. Variable magnitude of drug interaction between oral voriconazole and cyclosporine A in recipients of allogeneic hematopoietic stem cell transplantation. Clin Transplant. 2012;26(5):E544–E548. doi: 10.1111/ctr.12016. [DOI] [PubMed] [Google Scholar]
- 69.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356(4):335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 70.Sung AH, Marcella SW, Xie Y. An update to the cost-effectiveness of posaconazole vs fluconazole or itraconazole in the prevention of invasive fungal disease among neutropenic patients in the United States. J Med Econ. 2015;18(5):341–348. doi: 10.3111/13696998.2014.1000460. [DOI] [PubMed] [Google Scholar]
- 71.Grau S, de la Cámara R, Sabater FJ, et al. Cost-effectiveness of posaconazole versus fluconazole or itraconazole in the prevention of invasive fungal infections among high-risk neutropenic patients in Spain. BMC Infect Dis. 2012 doi: 10.1186/1471-2334-12-83. 12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tahami Monfared AA, O’Sullivan AK, Rotstein C, Papadopoulos G. Economic evaluation of posaconazole versus standard azole therapy as prophylaxis against invasive fungal infections in patients with prolonged neutropenia in Canada. Can J Infect Dis Med Microbiol. 2012;23(2):59–64. doi: 10.1155/2012/583630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de la Cámara R, Jarque I, Sanz MA, et al. Economic evaluation of posaconazole vs fluconazole in the prevention of invasive fungal infections in patients with GVHD following haematopoietic SCT. Bone Marrow Transplant. 2010;45(5):925–932. doi: 10.1038/bmt.2009.272. [DOI] [PubMed] [Google Scholar]
- 74.Sánchez-Ortega I, Patiño B, Muñoz C, et al. Cost-effectiveness of primary antifungal prophylaxis with posaconazole versus itraconazole in allogeneic hematopoietic stem cell transplantation. J Med Econ. 2013;16(6):736–743. doi: 10.3111/13696998.2013.791301. [DOI] [PubMed] [Google Scholar]
- 75.Menichetti F. How to improve the design of trials of antifungal prophylaxis among neutropenic adults with acute leukemia. Clin Infect Dis. 2004;39(suppl 4):S181–S184. doi: 10.1086/421954. [DOI] [PubMed] [Google Scholar]
- 76.Pagano L, Caira M, Candoni A, et al. SEIFEM Group. Evaluation of the practice of antifungal prophylaxis use in patients with newly diagnosed acute myeloid leukemia: results from the SEIFEM 2010-B registry. Clin Infect Dis. 2012;55(11):1515–1521. doi: 10.1093/cid/cis773. [DOI] [PubMed] [Google Scholar]
- 77.Dahlén T, Kalin M, Cederlund K, et al. Decreased invasive fungal disease but no impact on overall survival by posaconazole compared to fluconazole prophylaxis: a retrospective cohort study in patients receiving induction therapy for acute myeloid leukaemia/myelodysplastic syndromes [published online ahead of print April 16, 2015]. Eur J Haematol. doi: 10.1111/ejh.12565. doi:10.1111/ejh.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sánchez-Ortega I, Patiño B, Arnan M, et al. Clinical efficacy and safety of primary antifungal prophylaxis with posaconazole vs itraconazole in allogeneic blood and marrow transplantation. Bone Marrow Transplant. 2011;46(5):733–739. doi: 10.1038/bmt.2010.185. [DOI] [PubMed] [Google Scholar]
- 79.Groll AH, Castagnola E, Cesaro S, et al. Fourth European Conference on Infections in Leukaemia; Infectious Diseases Working Party of the European Group for Blood Marrow Transplantation (EBMT-IDWP); Infectious Diseases Group of the European Organisation for Research and Treatment of Cancer (EORTC-IDG); International Immunocompromised Host Society (ICHS); European Leukaemia Net (ELN) Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol. 2014;15(8):e327–e340. doi: 10.1016/S1470-2045(14)70017-8. [DOI] [PubMed] [Google Scholar]
- 80.Stergiopoulou T, Walsh TJ. Clinical pharmacology of antifungal agents to overcome drug resistance in pediatric patients. Expert Opin Pharmacother. 2015;16(2):213–226. doi: 10.1517/14656566.2015.1000302. [DOI] [PubMed] [Google Scholar]
- 81.Duarte RF, Sánchez-Ortega I, Cuesta I, et al. Serum galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective antimold prophylaxis. Clin Infect Dis. 2014;59(12):1696–1702. doi: 10.1093/cid/ciu673. [DOI] [PubMed] [Google Scholar]
- 82.van der Linden JW, Camps SM, Kampinga GA, et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis. 2013;57(4):513–520. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
- 83.Gamaletsou MN, Walsh TJ, Zaoutis T, et al. A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin Microbiol Infect. 2014;20(1):O50–O57. doi: 10.1111/1469-0691.12312. [DOI] [PubMed] [Google Scholar]
- 84.Pagano L, Verga L, Busca A, et al. Systemic antifungal treatment after posaconazole prophylaxis: results from the SEIFEM 2010-C survey. J Antimicrob Chemother. 2014;69(11):3142–3147. doi: 10.1093/jac/dku227. [DOI] [PubMed] [Google Scholar]
- 85.Cornely OA, Gachot B, Akan H, et al. EORTC Infectious Diseases Group. Epidemiology and outcome of fungemia in a cancer Cohort of the Infectious Diseases Group (IDG) of the European Organization for Research and Treatment of Cancer (EORTC 65031). Clin Infect Dis. 2015;61(3):324–331. doi: 10.1093/cid/civ293. [DOI] [PubMed] [Google Scholar]
- 86.Bochud PY, Chien JW, Marr KA, et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med. 2008;359(17):1766–1777. doi: 10.1056/NEJMoa0802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zaas AK, Liao G, Chien JW, et al. Plasminogen alleles influence susceptibility to invasive aspergillosis. PLoS Genet. 2008;4(6):e1000101. doi: 10.1371/journal.pgen.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kesh S, Mensah NY, Peterlongo P, et al. TLR1 and TLR6 polymorphisms are associated with susceptibility to invasive aspergillosis after allogeneic stem cell transplantation. Ann N Y Acad Sci. 2005 doi: 10.1196/annals.1358.012. 1062:95-103. [DOI] [PubMed] [Google Scholar]
- 89.Sainz J, Hassan L, Perez E, et al. Interleukin-10 promoter polymorphism as risk factor to develop invasive pulmonary aspergillosis. Immunol Lett. 2007;109(1):76–82. doi: 10.1016/j.imlet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 90.Plantinga TS, van der Velden WJ, Ferwerda B, et al. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49(5):724–732. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- 91.Neth OW, Bacher U, Das P, et al. Influence of mannose-binding lectin genotypes and serostatus in allo-SCT: analysis of 131 recipients and donors. Bone Marrow Transplant. 2010;45(1):13–19. doi: 10.1038/bmt.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Panackal AA, Li H, Kontoyiannis DP, et al. Geoclimatic influences on invasive aspergillosis after hematopoietic stem cell transplantation. Clin Infect Dis. 2010;50(12):1588–1597. doi: 10.1086/652761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ariza-Heredia EJ, Kontoyiannis DP. Our recommendations for avoiding exposure to fungi outside the hospital for patients with haematological cancers. Mycoses. 2014;57(6):336–341. doi: 10.1111/myc.12167. [DOI] [PubMed] [Google Scholar]
- 94.Gayet-Ageron A, Iten A, van Delden C, et al. In-hospital transfer is a risk factor for invasive filamentous fungal infection among hospitalized patients with hematological malignancies: a matched case-control study. Infect Control Hosp Epidemiol. 2015;36(3):320–328. doi: 10.1017/ice.2014.69. [DOI] [PubMed] [Google Scholar]
- 95.Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis. 2015;61(3):433–444. doi: 10.1093/cid/civ297. [DOI] [PubMed] [Google Scholar]
- 96.Kontoyiannis DP, Chamilos G, Lewis RE, et al. Increased bone marrow iron stores is an independent risk factor for invasive aspergillosis in patients with high-risk hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation. Cancer. 2007;110(6):1303–1306. doi: 10.1002/cncr.22909. [DOI] [PubMed] [Google Scholar]
- 97.Maertens J, Demuynck H, Verbeken EK, et al. Mucormycosis in allogeneic bone marrow transplant recipients: report of five cases and review of the role of iron overload in the pathogenesis. Bone Marrow Transplant. 1999;24(3):307–312. doi: 10.1038/sj.bmt.1701885. [DOI] [PubMed] [Google Scholar]
- 98.Guiot HF, Fibbe WE, van ’t Wout JW. Risk factors for fungal infection in patients with malignant hematologic disorders: implications for empirical therapy and prophylaxis. Clin Infect Dis. 1994;18(4):525–532. doi: 10.1093/clinids/18.4.525. [DOI] [PubMed] [Google Scholar]
- 99.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100(13):4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 100.Hu R, Jiang X, Wu Y. Prospective trial finds nystatin mouthwash effective prophylaxis for pulmonary invasive fungal infections that originate in the throat of patients with hematologic malignancies. Neoplasma. 2013;60(3):315–321. doi: 10.4149/neo_2013_042. [DOI] [PubMed] [Google Scholar]
- 101.Aisner J, Murillo J, Schimpff SC, Steere AC. Invasive aspergillosis in acute leukemia: correlation with nose cultures and antibiotic use. Ann Intern Med. 1979;90(1):4–9. doi: 10.7326/0003-4819-90-1-4. [DOI] [PubMed] [Google Scholar]
- 102.Sandford GR, Merz WG, Wingard JR, Charache P, Saral R. The value of fungal surveillance cultures as predictors of systemic fungal infections. J Infect Dis. 1980;142(4):503–509. doi: 10.1093/infdis/142.4.503. [DOI] [PubMed] [Google Scholar]
- 103.Schwartz RS, Mackintosh FR, Schrier SL, Greenberg PL. Multivariate analysis of factors associated with invasive fungal disease during remission induction therapy for acute myelogenous leukemia. Cancer. 1984;53(3):411–419. doi: 10.1002/1097-0142(19840201)53:3<411::aid-cncr2820530308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 104.Bow EJ, Loewen R, Cheang MS, Schacter B. Invasive fungal disease in adults undergoing remission-induction therapy for acute myeloid leukemia: the pathogenetic role of the antileukemic regimen. Clin Infect Dis. 1995;21(2):361–369. doi: 10.1093/clinids/21.2.361. [DOI] [PubMed] [Google Scholar]
- 105.Stanzani M, Lewis RE, Fiacchini M, et al. A risk prediction score for invasive mold disease in patients with hematological malignancies. PLoS One. 2013;8(9):e75531. doi: 10.1371/journal.pone.0075531. [DOI] [PMC free article] [PubMed] [Google Scholar]