Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
TULA-2 negatively regulates platelet FcγRIIA signaling by dephosphorylating Syk.
miR-148a targets TULA-2 and inhibition of miR-148a decreases FcγRIIA-mediated platelet activation and thrombosis in vivo.
Abstract
Fc receptor for IgG IIA (FcγRIIA)–mediated platelet activation is essential in heparin-induced thrombocytopenia (HIT) and other immune-mediated thrombocytopenia and thrombosis disorders. There is considerable interindividual variation in platelet FcγRIIA activation, the reasons for which remain unclear. We hypothesized that genetic variations between FcγRIIA hyper- and hyporesponders regulate FcγRIIA-mediated platelet reactivity and influence HIT susceptibility. Using unbiased genome-wide expression profiling, we observed that human hyporesponders to FcγRIIA activation showed higher platelet T-cell ubiquitin ligand-2 (TULA-2) mRNA expression than hyperresponders. Silent interfering RNA-mediated knockdown of TULA-2 resulted in hyperphosphorylation of spleen tyrosine kinase following FcγRIIA activation in HEL cells. Significantly, we found miR-148a-3p targeted and inhibited both human and mouse TULA-2 mRNA. Inhibition of miR-148a in FcγRIIA transgenic mice upregulated the TULA-2 level and reduced FcγRIIA- and glycoprotein VI–mediated platelet αIIbβ3 activation and calcium mobilization. Anti–miR-148a also reduced thrombus formation following intravascular platelet activation via FcγRIIA. These results show that TULA-2 is a target of miR-148a-3p, and TULA-2 serves as a negative regulator of FcγRIIA-mediated platelet activation. This is also the first study to show the effects of in vivo miRNA inhibition on platelet reactivity. Our work suggests that modulating miR-148a expression is a potential therapeutic approach for thrombosis.
Introduction
Heparin is one of the most effective and widely used anticoagulants in hospitalized patients with cardiovascular diseases. During or after exposure to heparin, 0.2% to 3% of patients develop heparin-induced thrombocytopenia (HIT), a disorder characterized by low platelet count and thrombosis.1 About 30% to 70% of untreated HIT patients develop venous or arterial thrombi that are life-/limb-threatening.2 HIT is a paradigm of the family of immune-mediated thrombocytopenia and thrombosis disorders3 and caused by the formation of immunoglobulin (Ig)G antibodies against the heparin-platelet factor 4 (PF4) complex. Subsequently, this immune complex activates platelets via Fc receptor for IgG IIA (FcγRIIA) receptors, resulting in thrombocytopenia and thrombosis.4
Multiple Fcγ receptors for IgG antibody are present in humans. Among them, FcγRIIA, encoded by the FCGR2A gene, is the only one present on human platelets.5 We first demonstrated that platelet FcγRIIA was necessary for HIT development in vivo with our human FcγRIIA/PF4 transgenic mouse model.5 Binding of the Fc portion of IgG in immune complexes or crosslinking FcγRIIA promotes phosphorylation of tyrosine residues in the immunoreceptor tyrosine-base activation motifs (ITAMs), which further provides binding sites for the Src homology 2 (SH2) domains in spleen tyrosine kinase (Syk). Multiple tyrosine phosphorylation events on Syk occur after FcγRIIA ITAM phosphorylation and Syk becomes an activated protein kinase. The observation that a Syk inhibitor is able to prevent HIT in our FcγRIIA/PF4 transgenic mouse model demonstrated the central role of Syk in the FcγRIIA pathway and HIT.6 The signaling is further transmitted by phosphorylation of phospholipase Cγ2 (PLCγ2), phosphatidylinositide 3-kinases (PI3Ks), and the linker for activation of T cells (LAT), followed by calcium mobilization and protein kinase C activation. These signals ultimately lead to platelet activation and thrombus formation.7 Recently, FcγRIIA was also identified as a key regulator in platelet integrin outside-in signaling.6,8,9
There is considerable interindividual variation in platelet activation via FcγRIIA among healthy donors and patients. The genetic mechanisms behind this phenotypic variation are incompletely understood. A His131Arg polymorphism of FcγRIIA has been shown to associate with receptor activity and further HIT pathophysiology.10 Rollin et al11 linked single nucleotide polymorphisms (SNPs) in CD148 with platelet reactivity. Another study correlated a combination of FcγRIIA SNP and platelet endothelial cell adhesion molecule-1 SNP genotypes with HIT thrombosis.12 Aiming to identify genetic variations that affect FcγRIIA and HIT, our Platelet RNA and eXpression-1 (PRAX-1) study13 was designed to find differentially expressed genes among hypo- and hyperresponders to FcγRIIA activation.
Although many recent studies11,14,15 have focused on the molecular mechanism by which FcγRIIA promotes platelet activation, less is known about negative regulators of the signaling pathway. T-cell ubiquitin ligand-2 (TULA-2), a protein tyrosine phosphatase identified as a negative effector of FcγRIIA in this study, is encoded by the UBASH3B (ubiquitin associated and SH3 domain-containing protein B) gene. It belongs to the TULA family of proteins, with TULA-2 as the sole family member detectable in platelets.16 TULA-2 functions as a tyrosine phosphatase, and deficiency of TULA-2 results in the hyperphosphorylation of Syk homolog ζ-chain-associated protein kinase 70 (ZAP70) in T cells.17-19 TULA-2 also associates with Syk and negatively regulates murine platelet activation via glycoprotein VI (GPVI)/Fc receptor γ-chain (FcRγ), another ITAM-containing receptor complex.20 GPVI/FcRγ is the primary receptor for platelet-collagen interaction.21 Patients with defective GPVI receptor show loss of collagen binding and higher tendency to bleed.22 The downstream signaling of GPVI is similar to the FcγRIIA-Syk pathway.23 However, the role of TULA-2 in the FcγRIIA pathway has not been reported.
MicroRNAs (miRNAs) have been found to inhibit protein expression by inhibiting translation or targeting mRNAs for degradation. Anti-miRNAs, like locked nucleic acids (LNAs), are emerging tools for delivering small, stable RNAs in vitro and in vivo.24 LNAs are modified nucleic acids containing 1 or more of the 2′-O,4′-C-methylene-β-d-ribofuranosyl nucleosides. LNAs are physiologically stable, resistant to nucleases, have low cytotoxicity, and have robust antisense efficacy and specificity in vivo.25 In cardiovascular diseases, miRNA inhibition has been used to regulate atherosclerosis, cardiac function, and vascular biology in animal models.26-30 Bhagat et al31 showed that anti–miR-21 treatment in mice elevated SMAD7 expression and stimulated hematopoiesis. Garchow et al32 identified anti–miR-21 effects in a mouse model of systemic lupus erythematosus. Janssen et al33 used anti–miR-122 (Miravirsen) to treat human chronic hepatitis C virus infection in a phase 2a clinical trial.33
The effect of in vivo inhibition of miRNAs on platelet reactivity has not been reported. In this study, we identified miR-148a-3p and TULA-2 as 2 mediators of the FcγRIIA pathway. Inhibition of miR-148a in our FcγRIIA transgenic mouse model for HIT increased TULA-2 expression and protected against thrombocytopenia and thrombus formation.
Material and methods
Antibodies and reagents
Antibodies against human CD9 (Beckman Coulter; clone Alb6, mIgG1), murine CD9 (BD Pharmingen; clone KMC8, rat IgG2a), human FcγRIIA (clone IV.3; StemCell Technologies), human total Syk (clone 4D10; Santa Cruz Biotech), human phospho-Syk Y323 (Cell Signaling), human phospho-Syk Y525/526 (murine Y519/520; Cell Signaling), murine phospho-LAT Y191 (Millipore), phycoerythrin (PE)-labeled anti-mouse integrin αIIbβ3 (clone JON/A; Emfret), goat anti-mouse IgG Fab’2 (Santa Cruz Biotech), Fluo-4-AM (Life Technologies), thrombin (Chrono-PAR), and collagen-related peptide (CRP; from Dr Richard Farndale) were purchased. Anti–TULA-2 antibody was described previously.34
Cell lines
HEL 92.1.7 human erythroleukemia cells (ATCC, Manassas, VA) were grown in RPMI-1640 (Gibco BRL, Rockville, MD) media supplemented with 10% fetal calf serum (Atlanta Biologicals, Norcross, GA), 100 U/mL penicillin, and 100 μg/mL streptomycin. HCT116-Dicer-KO 2 cells were previously described.35
Mouse model for HIT
FcγRIIA transgenic mice (B6IIA) were created as previously described.5 All mice are on the 100% C57BL/6 strain background. TULA-2 knockout (KO) mice were described previously.17 All animals were maintained at Thomas Jefferson University animal facility, which is approved by the Association for Assessment and Accreditation of Laboratory Animal Care International. All protocols for using experimental mice were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Human PRAX-1 study
Recruitment of the donors, whole blood collection, platelet isolation, aggregation by anti-CD9 antibody, and platelet RNA profiling were done as previously described.13,35 Briefly, platelet-rich plasma aggregation assay via FcγRIIA was conducted on 154 human healthy donors as part of the PRAX-1 study.
Murine platelet isolation
Murine platelet isolation was previously described.14
Small interfering RNA and miRNA overexpression
The 200 nM TULA-2 small interfering (si)RNA, scrambled control siRNA (GE Dharmacon), 60 nM hsa-miR-148a-3p mirVana Mimics, or control scrambled microRNA mimics (Life Technologies) were transfected into 2 × 106/mL HEL cells using the Amaxa Nucleofector II device (Lonza) and Nucleofector Kit V (Lonza) following the company’s protocol. Protein or RNA was extracted and analyzed 48 hours after transfection.
TULA-2 gene luciferase reporter assay
A region from TULA-2 3′ untranslated region (3′UTR) consisting of 113 bp upstream and 93 bp downstream of the potential binding site of miR148a-3p was cloned into the pMIR-REPORT luciferase construct (Life Technologies). The mutant construct of TULA-2 3′UTR was created by using a QuikChange Site-Directed Mutagenesis Kit (Agilent Technologies). In the TULA-2 3′UTR mutant, the nucleotide sequence of the seed region was mutated from 5′TGCACT3′ to 5′CCGCCC3′. The reporter plasmids (60 ng), β-gal vector (50 ng), and miRNA or scrambled control (Ambion; 60 nM final concentration) were transfected into HCT-116-Dicer KO 2 cells in triplicates with Lipofectamine LTX and PLUS reagent. Data were obtained and normalized by Luciferase Assay System and the β-galactosidase enzyme assay system (Promega) using the LUMIstar OPTIMA luminescence microplate reader (BMG Labtech).
Anti–miR-148a treatment in vivo
Custom-designed anti–miR-148a-3p LNA was purchased from Exiqon. Siblings of FcγRIIA transgenic mice at the same age were assigned into scrambled LNA or anti–miR-148a LNA treatment groups. Mice were treated with 25 mg/kg LNA in sterile saline on alternate days for 5 doses via intraperitoneal injection. Blood counts were measured by the Hemavet HV950 (Drew Scientific, Dallas, TX).
Integrin αIIbβ3 activation assay
Murine platelets were washed and resuspended in Tyrode’s buffer with 1 mM CaCl2 and 0.35% bovine serum albumin to 5 × 107 platelets/mL. Various concentrations of CRP or IV.3 + goat anti-mouse Fab’2 (GAM) were used to activate platelets in the presence of 2 µg/mL JON/A-PE, an antibody that binds to activated form of αIIbβ3. Fluorescent intensity was measured by a BD Accuri C6 flow cytometer after 10 minutes.
Calcium mobilization assay
A total of 1 × 106 washed murine platelets were labeled with 2.5 µg/mL Fluo-4-AM for 10 minutes at 37°C. Platelets were analyzed for fluorescence intensity with 1 mM CaCl2 for 1 minute to establish a baseline. At 60 seconds, indicated concentrations of CRP and IV.3 + GAM were added, and calcium mobilization was measured over a period of 5 minutes by a BD Accuri C6 flow cytometer. The calcium fold change data in the plot represent the calcium concentration at every second divided by basal calcium concentration.
FcγRIIA-mediated thrombosis model
Under anesthesia by inhaling isoflurane, mice were injected with the anti-mouse CD9 antibody into the retro-orbital sinus at a concentration of 2.5 mg/kg body weight. Livers, spleens, and lungs were obtained by laparotomy and thoracotomy and were stored immediately in RNAlater RNA stabilization reagent (Qiagen). Bone marrow was collected from the tibias. Total RNA was isolated from tissues by lysing with TRIzol reagent (Invitrogen) or by RNeasy mini RNA purification kit (Qiagen). Reverse transcription-polymerase chain reaction (RT-PCR) was performed, and target RNAs were quantified by quantitative RT-PCR.
Hematoxylin and eosin staining
Inflated lungs were extracted and fixed in 10% formalin for 24 hours. Cryosection and hematoxylin and eosin staining were prepared by the Veterinary Medical Diagnostic Laboratory at University of Missouri, College of Veterinary Medicine. Images were captured with Carl Zeiss Axio Observer Z1 microscope.
Statistics
Results were reported as mean ± standard error of the mean. Statistical significance was determined by a 2-tailed Student t test or 2-way analysis of variance. P < .05 was considered significant.
Results
TULA-2 is differentially expressed between hypo- and hyperresponders to platelet FcγRIIA stimulation
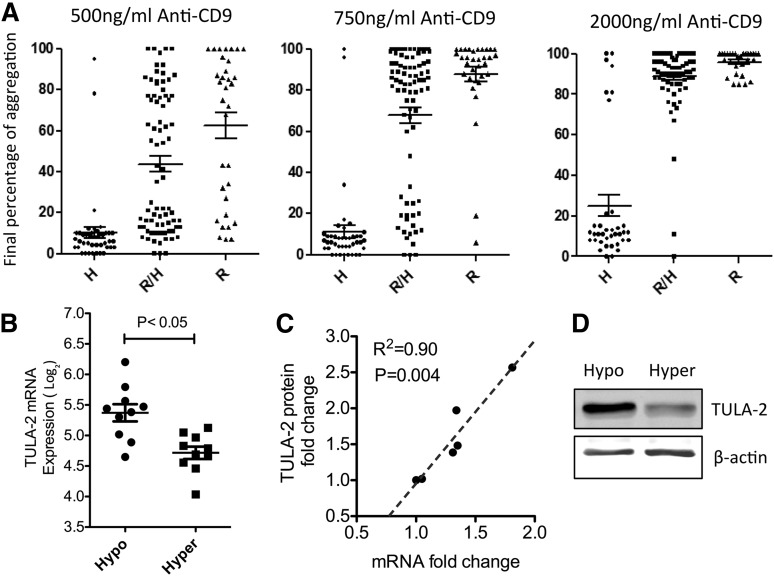
Three concentrations of an anti-CD9 antibody (mIgG1 isotype) were used in our PRAX-1 study as a model of FcγRIIA-mediated platelet activation relevant to HIT.13 Platelet FcγRIIA surface protein expression level and the H/R131 SNV genotype (rs1801274) were determined for each donor, because these variations have previously been reported as contributing to variation in platelet activation via FcγRIIA.10,36-38 We chose a mIgG1 agonist specifically to see if our work replicated the known dependence on the RH polymorphism. R/R131 homozygotes were highly responsive to the mIgG1 anti-CD9, and H/H homozygotes were weakly responsive (Figure 1), as expected.36 We used linear regression to calculate the dependence of the aggregation on the HR polymorphism and on the FcγRIIa level. Across all 154 donors, the HR polymorphism explains 47% of the observed variance. In contrast, we observed a bimodal pattern of platelet reactivity at the intermediate dose of anti-CD9 (750 ng/mL) among the 79 individuals with the R/H 131 heterozygous genotype. It is particularly striking then to see the wide variation in reactivity among donors identical for the RH heterozygous genotype. These individuals all had a platelet surface FcγRIIA expression level within 2 standard deviations of the mean for the PRAX-1 cohort. The FcγRIIA level accounts for 12% of the observed variance. After controlling for the previously recognized sources of variation in platelet FcγRIIA reactivity, the expression level and the H/R131 genotype, we observed a major, unexplained variation (88%) in reactivity.
Figure 1.
Hyperresponders to FcγRIIA-mediated platelet activation have reduced TULA-2 expression compared with hyporesponders. (A) Platelets from 154 healthy human donors were activated by indicated dose of anti-human CD9. Final percentage of aggregation was used as the readout for reactivity. This population was further divided by genetic variation at the codon 131 of FCGR2A gene. Hyperresponders were defined as having >75% final aggregation at 750 ng/mL anti-CD9, whereas hyporesponders were defined as having <25% aggregation. (B) TULA-2 mRNA (UBASH3B) is differentially expressed between 10 top hyperresponders and 10 bottom hyporesponders. All donors were ranked based on the final percentage of aggregation (Student t test, P < .05). (C) TULA-2 protein levels were measured by western blot. The correlation between TULA-2 protein level and TULA-2 mRNA level was determined by Pearson correlation (R2 = 0.90). (D) Representative western blot of platelet TULA-2 level in hypo- and hyperresponders.
To elucidate the molecular basis of the unexplained variation in reactivity, we defined hyperresponders and hyporesponders among the R/H131 heterozygotes as >75% final aggregation and <25% final aggregation in response to 750 ng/mL anti-CD9, respectively (Figure 1A). Gene expression profiling identified 76 differentially expressed (DE) mRNAs between FcγRIIA hyper- and hyporesponders, 54 up and 22 down in hyporesponders. We applied Gene Ontology (www.geneontology.org) and Kyoto Encyclopedia of Genes and Genomes analyses (www.genome.jp/kegg/) of the DE mRNAs, and genes that are involved in protein tyrosine phosphorylation and in ubiquitylation processes were enriched in these lists (data not shown), including TULA-2, which was upregulated in hyporesponders. Notably, TULA-2 was not DE with respect to adenosine 5′-diphosphate-, protease-activated receptor 1- or protease-activated receptor 4-mediated platelet reactivity (www.plateletomics.com13). The top 10 hyporesponders to FcγRIIA activation showed significantly higher TULA-2 platelet mRNA expression than the bottom 10 hyperresponders (Figure 1B). TULA-2 protein level measured by western blotting correlates well with the mRNA level (Figure 1C-D).
Biological validation of the function of differentially expressed TULA-2
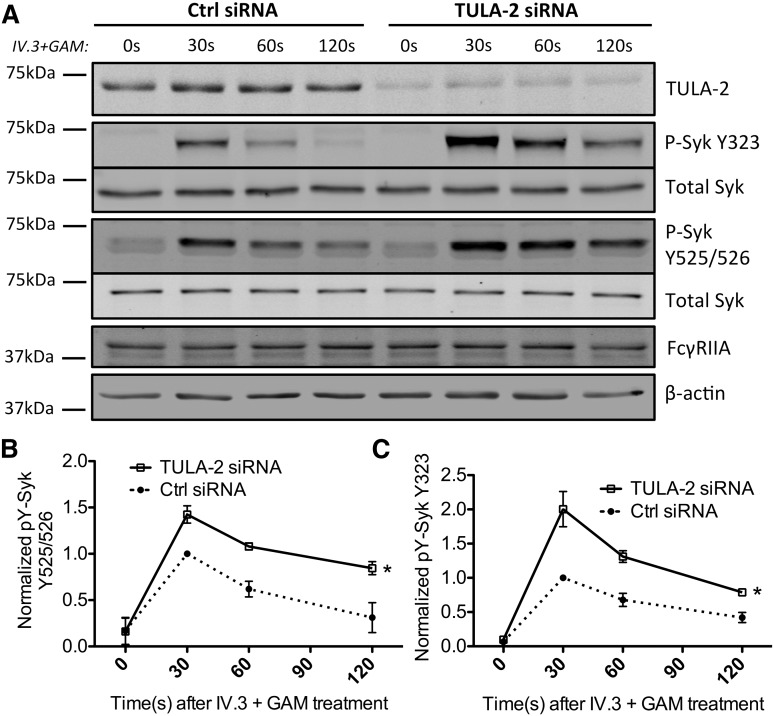
We next tested the hypothesis that TULA-2 serves as a negative regulator of FcγRIIA signaling by inactivating phospho-Syk. Syk is phosphorylated on activation at multiple tyrosine residues including Y323, Y352, and Y525/526. TULA-2 KO mice have been reported to have hyperphosphorylation of Syk at several sites, including Y323 and Y525/526 tyrosine residues.18,39 In this study we used Y525/526 and Y323 as the readout for Syk activation in HEL cells for platelet FcγRIIA signaling. Crosslinking FcγRIIA by an anti-FcγRIIA antibody (IV.3) and GAM induces receptor clustering and activation, which leads to phosphorylation of Syk in HEL cells. In scrambled control siRNA-transfected HEL cells, phosphorylation of Syk at both Y525/526 and Y323 peaked at 30 seconds after receptor crosslinking and slowly declined. In contrast, downregulation of TULA-2 showed a significantly higher level of phosphorylated Syk (P < .05; Figure 2A-B), consistent with TULA-2 dephosphorylating Syk in FcγRIIA signaling.
Figure 2.
TULA-2 dephosphorylates Syk in FcγRIIA pathway. The 50 nM anti–TULA-2 siRNA or scrambled siRNA was transiently transfected into HEL cells via nucleofection. Cells were then treated with anti-FcγRIIA clone IV.3 antibody and goat anti-mouse antibody Fab’2 (GAM) at 10 and 30 µg/mL, respectively, under stirring conditions at 37°C to crosslink and activate FcγRIIA for the indicated time. (A) At the 48-hour end point, cells were lysed, and protein was immunoblotted for TULA-2 and phosphorylated Syk at tyrosine 525/526 and 323, total Syk, FcγRIIA, and β-actin. (B-C) Phosphorylated Syk at tyrosine 525/526 and tyrosine 323 was normalized to total Syk and was plotted as mean ± standard deviation against time for the anti–TULA-2 siRNA and scrambled siRNA control groups (*P < .05, n = 5 for Y323, and n = 3 for Y525/526, 2-way analysis of variance).
miR-148a targets TULA-2 mRNA and downregulates TULA-2 protein expression
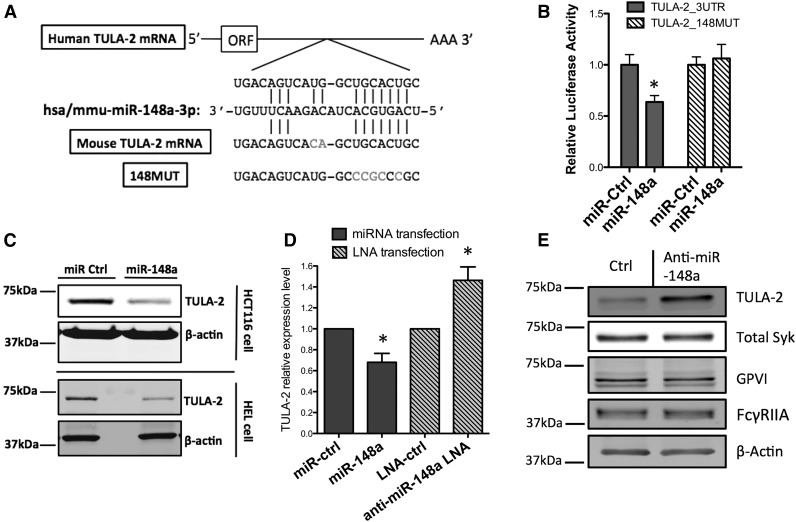
To investigate the mechanism of differential expression of TULA-2, we sought to identify regulators of TULA-2 in platelets that might influence platelet reactivity. We used TargetScan (www.targetscan.org) and RNA2240 programs to screen for microRNAs predicted to target TULA-2. miR-148a-3p was selected for further study for 3 reasons: (1) miR-148a-3p was predicted by RNA22 and TargetScan to bind at the seed position 1111-1118 of the TULA-2 3′UTR (Figure 3A); (2) it is highly expressed in the megakaryocyte-platelet lineage; and, (3) the miR-148a sequence and the potential binding site on TULA-2 are conserved between human and mouse.
Figure 3.
miR-148a targets 3′UTR of TULA-2 mRNA and downregulates protein expression. (A) Schematic representation of miR-148a and 3′UTR of TULA-2 mRNA interaction. TULA2_148MUT construct was made by mutating 5 of the 7 nucleotides in the miR-148a binding seed region on TULA-2 3′UTR. Mouse TULA-2 mRNA (Ubash3b) varies by 2 nucleotides outside of the seed region from the human ortholog. (B) Luciferase reporter plasmids containing wild-type 3′UTR of TULA-2 or 148MUT was cotransfected into HCT116-Dicer-KO cells with β-galactosidase expression vector and miR-148a-3p mimic or scrambled miRNA control for 24 hours. Bar graph was plotted as normalized. (n = 3, *P < .05). (C) The 60 nM miR-148a-3p or control miRNAs were transfected into HCT-116-Dicer-KO cells or HEL cells by lipofectamine 2000 for 48 hours. TULA-2 protein level was blotted by western blotting. (D) HCT-116-Dicer-KO cells were transfected with 60 nM miR-148a-3p mimic, scrambled miRNA control, anti-miR-148a-3p LNA, or scrambled LNA control by lipofectamine 2000. RNA was isolated 48 hours after transfection, and TULA-2 mRNA expression was determined by quantitative RT-PCR (For miRNA overexpression, n = 3, *P < .05. for LNA transfection, n = 4, *P < .05). (E) HEL cells were transfected with 100 nM anti–miR-148a or scrambled anti-miR for 48 hours. TULA-2, total Syk, GPVI, and FcγRIIA protein level was detected by immunoblotting.
miR-148a-3p is the predominant form over miR-148a-5p (http://www.mirbase.org/). To investigate whether miR-148a interacts directly with the putative binding site on TULA-2, we first cotransfected the luciferase reporter plasmid containing the 3′UTR of TULA-2 along with the miR-148a-3p in HCT cells. A mutant construct (TULA2_148MUT) was created as a control (Figure 3A). For the luciferase reporter containing wild-type TULA-2 3′UTR, miR-148a-3p overexpression significantly decreased luciferase activity compared with control miRNA mimic. In contrast, miR-148a-3p overexpression failed to downregulate the TULA-2_148MUT construct, suggesting that the 7-nucleotide seed match is responsible for the miR-148a and TULA-2 interaction (Figure 3B).
We then overexpressed miR-148a-3p in HEL cells and HCT cells for 48 hours. Overexpression of miR-148a-3p significantly decreased the TULA-2 mRNA level (Figure 3D). Consistent with the luciferase assay and the mRNA results, miR-148a-3p overexpression led to decreased TULA-2 protein expression (Figure 3C).
Downregulation of miR-148a led to de-repression of TULA-2 in vitro
The effect of endogenous miR-148a-3p on TULA-2 mRNA was further tested by overexpressing an anti–miR-148a-3p in HEL cells. Inhibition of endogenous miR-148a-3p led to increased TULA-2 mRNA expression (Figure 3D). The TULA-2 protein level was also increased by the anti–miR-148a, whereas Syk, GPVI, and FcγRIIA were unaffected by miR-148a inhibition (Figure 3E). Together these results identified miR-148a-3p as a regulator of TULA-2.
Anti–miR-148a-3p LNA represses endogenous murine mmu-miR-148a-3p, upregulates platelet TULA-2, and leads to hypophosphorylation of Syk
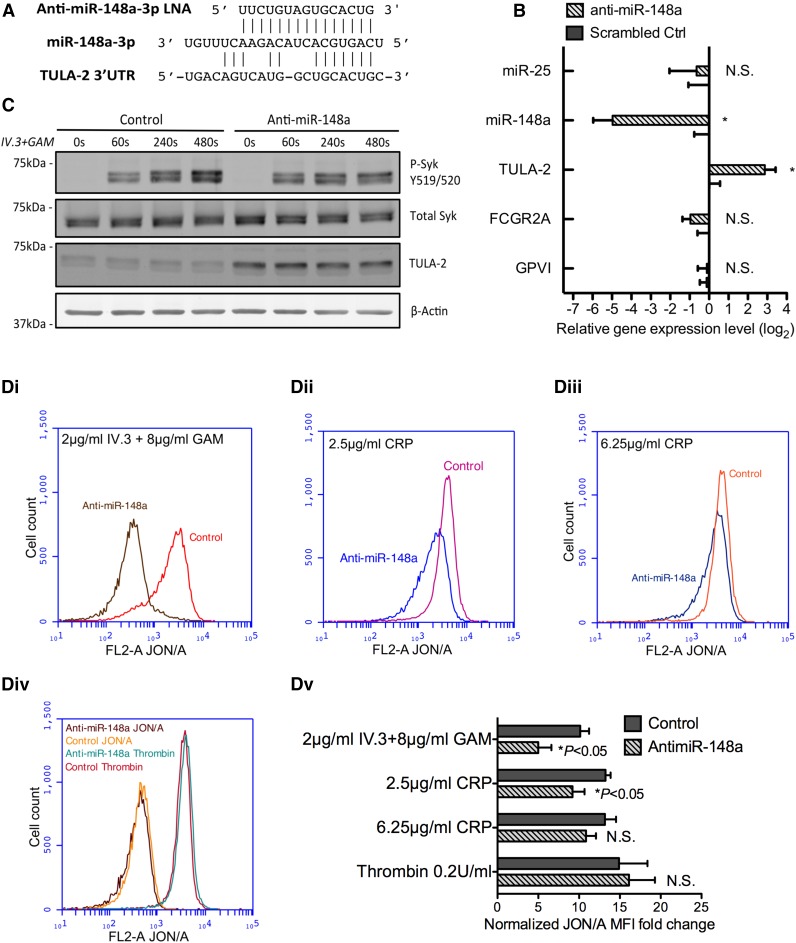
Based on our observations, we hypothesized that the inhibition of miR-148a would increase the TULA-2 protein level and attenuate FcγRIIA-mediated thrombosis in vivo. Mice lack the FCGR2A gene encoding the FcγRIIA receptor. We used mice transgenic for human FcγRIIA as previously described.4 Murine miR-148a-3p shares the same sequence with its human counterpart, and it is expressed in murine platelets and megakaryocytes (data not shown). FcγRIIA transgenic mice were treated with 25 mg/kg anti–miR-148a or scrambled anti-miR control for 5 times total on alternative days. We based our treatment on the protocol of Bhagat et al,31 who used this approach in modulating mouse hematopoietic cell miRNA. The sequence of the chosen 14-nucleotide anti-miR is complementary to mmu/hsa-miR-148a-3p sequence (Figure 4A).
Figure 4.
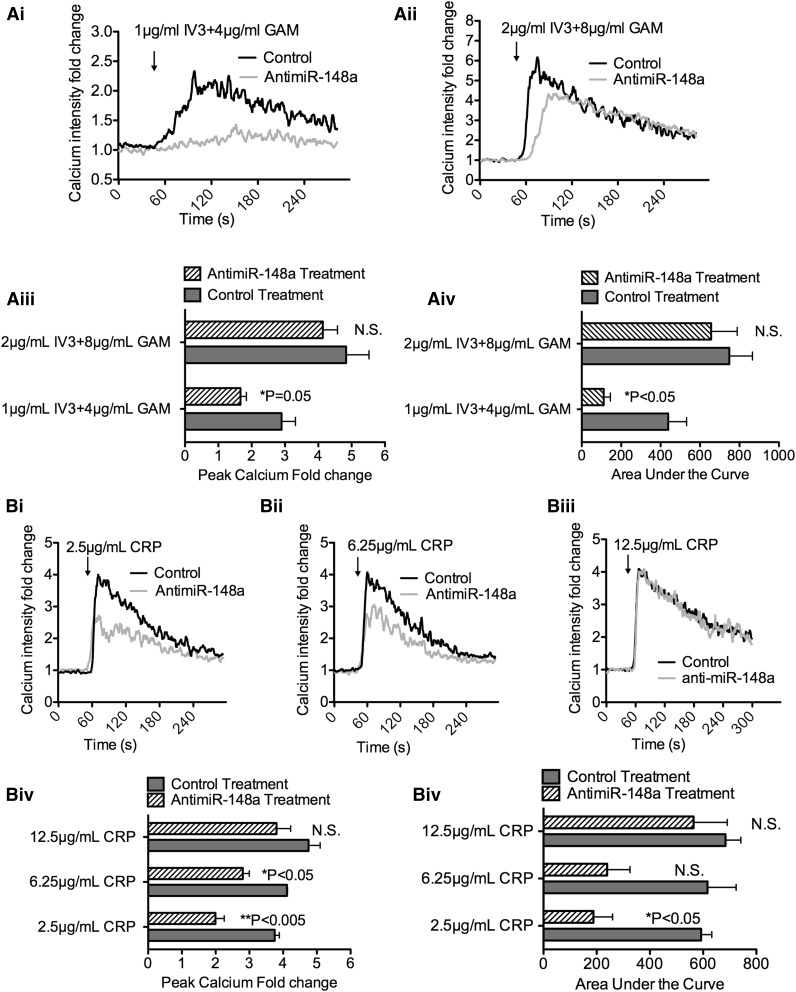
Anti–miR-148a LNA de-represses TULA-2 expression and inhibits ITAM-Syk signaling in vivo. (A) Sequence of the 15-nucleotide anti–miR-148a-3p LNA used to inhibit mmu-miR-148a-3p in vivo. (B) RNA expression analysis of bone marrow from B6.IIA mice treated with anti–miR-148a LNA or scrambled LNA control by quantitative RT-PCR. Plots represent 4 anti–miR-148a–treated mice and 4 scrambled LNA-treated mice. Fold change on the y-axis is plotted as log2 value. miRNA expression was normalized to mouse let-7 miRNA, and TULA-2, FcγRIIA, and GPVI mRNA was normalized to mouse β-actin. miR-148a was significantly decreased by LNA, whereas TULA-2 mRNA expression was upregulated significantly (n = 4, *P < .05 for TULA-2, **P < .005 for miR-148a). (C) P-Syk Y519/520, TULA-2, and mouse β-actin protein expression in murine platelet lysates was detected by immunoblotting (representative of 3 experiments). Mice were treated with control or anti–miR-148a. (D) Washed anti–miR-148a or control-treated platelets were incubated with JON/A alone or indicated concentration of agonists for 10 minutes followed by measurement of JON/A binding (integrin activation) by flow cytometry. All traces are representative of ≥3 independent experiments. Varying concentration of agonists include the following: (Di) 2 µg/mL IV.3 + 8 µg/mL GAM; (Dii) 2.5 µg/mL CRP; (Diii) 6.25 µg/mL CRP; (Div) JON/A only or JON/A plus 0.1 U/mL thrombin; and (Dv) quantification of mean fluorescent intensity fold change (Student t test, n = 5). Mean fluorescent intensity of JON/A-PE and agonists were normalized to background signal of platelets with JON/A-PE alone.
No mice showed any observable changes in behavior or gross pathologic abnormalities during the 14-day period of administration of either anti-miR. Platelet count, mean platelet volume, or other blood cell counts were not changed by LNA treatment (supplemental Figure 1B-D available on the Blood Web site). Compared with the scrambled control group, anti–miR-148a–treated mice showed a significant drop (97%) in miR-148a expression in mouse bone marrow (Figure 4B). As a negative control, miR-25 level remained unaffected (data not shown). TULA-2 mRNA level showed the opposite trend: anti–miR-148a treatment significantly increased TULA-2 expression level (Figure 4B). On the other hand, FCGR2A, as well as GPVI mRNA, that do not contain potential miR-148a binding sites, was not changed by anti-miR (Figure 4B). In platelets, TULA-2 protein level was elevated by anti–miR-148a to more than threefold, and phosphorylation of Syk after FcγRIIA activation was subsequently reduced by 50% at 480 seconds under nonstirring condition (Figure 4C; supplemental Figure 2B).
Inhibition of endogenous murine mmu-miR-148a-3p diminished platelet reactivity via FcγRIIA and GPVI
We tested the effect of the anti-miR on 2 Syk-mediated platelet functions: integrin activation and calcium mobilization. Murine platelets from anti–miR-148a–treated mice or control mice were washed and activated by different doses of IV.3 + GAM or CRP. Integrin αIIbβ3 activation is crucial in platelet-fibrinogen and von Willebrand factor binding, hemostasis, and thrombosis.41-43 We found that FcγRIIA activation by 2 µg/mL IV.3 and 8 µg/mL GAM in anti-miR-148a–treated murine platelets showed 50% reduction in integrin activation compared with the control group. CRP-induced integrin activation was also diminished at a concentration of 2.5 µg/mL by anti-miR-148a treatment (Figure 4Di-Dv). As a non-ITAM agonist control, thrombin did not induce differential integrin activation (Figure 5D).
Figure 5.
In vivo miR-148a inhibition diminished calcium mobilization via GPVI and FcγRIIA stimulation. A total of 1 × 106 platelets from anti–miR-148a– or control-treated mice were labeled with Fluo-4-AM. Baseline intracellular calcium was assessed for 60 seconds, and indicated doses of agonists were added to the platelets: (Ai-Aii) 1 µg/mL IV.3 + 4 µg/mL GAM and 2 µg/mL IV.3 + 8 µg/mL GAM were added, respectively, at the indicated time. (Aiii) Quantification of peak calcium fold change (n = 3). (Aiv) Quantification of area under the curve (AUC) for calcium mobilization curve (n = 3). AUC was calculated in Prism software. (Bi-Biii) 2.5 µg/mL CRP, 6.25 µg/mL CRP, and 12.5 µg/mL CRP were used to induce calcium influx, respectively. (Biv-Bv) Quantification of peak calcium fold change and AUC were performed as discussed above (n = 3).
Increased cytoplasmic calcium plays a critical role in platelet reactivity.44 In miR-148a knockdown platelets, 70% decreased calcium influx (area under the curve) was observed when induced by 2.5 µg/mL CRP (Figure 5B). The 1 µg/mL IV.3 and 4 µg/mL GAM treatment also showed impaired calcium influx in the anti–miR-148a–treated platelets (Figure 5A). Taken together, miR-148a inhibition attenuated FcγRIIA- and GPVI-mediated platelet reactivity.
Inhibition of miR-148a in vivo by an anti-miR protects FcγRIIA-mediated thrombosis
To test the hypothesis that inhibition of miR-148a could protect mice from thrombosis secondary to activation of platelets via FcγRIIA, we used anti-mouse CD9 antibody to induce HIT-like symptoms. Anti-CD9 Ab binds to platelet surface and lead to platelet activation by the interaction between its Fc part and FcγRIIA.45 Both the anti–miR-148a and scrambled anti-miR–treated groups showed a drop in platelet count 1 hour after anti-mCD9 antibody administration, due to the combination of intravascular platelet activation and splenic clearance.45 At 3 and 24 hours, the platelet count recovered. miR-148a inhibition resulted in significantly less severe thrombocytopenia in comparison with the control group (supplemental Figure 1A).
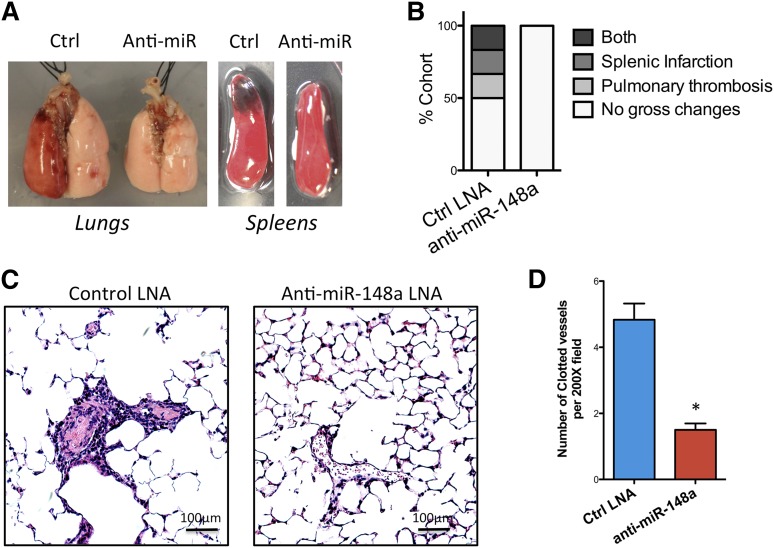
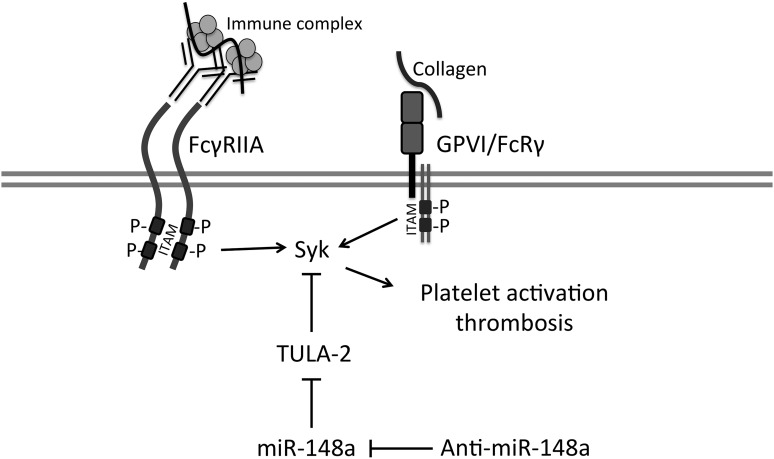
At the gross pathologic level, pulmonary thrombosis and spleen infarction are well-established features of this model.14 On examination, control anti-miR–treated mice showed more gross pathologic changes than the anti–miR-148a group. Specifically, 3 of 6 control mice had visibly evident thrombosis, whereas none of the 6 miR-148a–inhibited mice exhibited gross pathologic changes (Figure 6A-B). Additionally, histologic changes of the lungs were examined by light microscopy. Control anti-miR–treated mice manifested diffuse thrombosis with platelet/fibrin deposition in the lung vasculature. In comparison, anti–miR-148a–treated mice displayed significantly reduced thrombi, as measured by thrombus-per-200 × field (Figure 6C-D). All together, these data points to a protective effect of miR-148a inhibition on FcγRIIA-mediated thrombosis in vivo. A possible mechanism of action of anti–miR-148a is indicated (Figure 7).
Figure 6.
Inhibition of miR-148a in vivo is protective against FcγRIIA-mediated thrombosis. (A) Comparison of gross pathologic changes in mouse lungs and spleens between 2 experimental groups showing pulmonary thrombosis/hemorrhage, and splenic infarction. (B) Bar graph showing percentage of mice with pathologic changes (n = 6 for each group). (C) Microscopic examination of the mouse lungs after induction of thrombosis by anti-CD9 antibody treatment. (D) Quantification of total clotted vessels from lung histology. Images were captured with Carl Zeiss Axio Observer Z1 microscope and Leica Microsystems DFC 420 camera. Vessel count was conducted under light microscopy. Numbers of clotted vessels was recorded per 200× field. Three separate slides from each treatment group were analyzed (P < .01, n = 12).
Figure 7.
Schematic representation of the mechanism of the antithrombosis effect by miR-148a inhibition. Depicted is the ITAM-Syk pathway and the proposed role of TULA-2, miR-148a, and anti–miR-148a. Anti–miR-148a indirectly ameliorates ITAM-Syk–mediated platelet activation by upregulating TULA-2 expression, which further inactivates Syk and its downstream effectors.
Discussion
We identified anti–miR-148a as a negative regulator of platelet activation and thrombosis via FcγRIIA. Anti–miR-148a increases the levels of platelet TULA-2, a protein tyrosine phosphatase. TULA-2, which has higher expression level in FcγRIIA hyporesponders, was previously shown to regulate T-cell signaling, bone remodeling, and the GPVI pathway in murine platelets.18,20,46,47 Our PRAX-1 project is the first unbiased study to elucidate mRNA and miR determinants of the FcγRIIA pathway in human platelets. The underlying causes of differential expression of TULA-2 are unknown. Allelic variation of TULA-2 in human studies has been reported in Behçet disease,48 but not in platelet-related diseases. Unidentified SNPs, epigenetic changes, protein turnover, and miRNAs may impact the level of TULA-2 within individuals. Measuring TULA-2 level in platelets may serve as a biomarker to predict HIT susceptibility and severity.
LNA-based miRNA inhibition is proven to be safe, potent, and applicable in human therapy.25,33 We used a 14-nucleotide anti–miR-148a-3p LNA for our in vivo experiments for optimum specificity and uptake efficiency. Quantitative RT-PCR revealed that the anti-miR was effectively taken up by murine bone marrow and spleen, as demonstrated by >96% miR-148a inhibition (Figure 4B). In humans, miR-148a is highly expressed in megakaryocytes, platelets, granulocytes, and erythrocytes, but less well expressed in other cells.49 miR-148a has been associated with gastrointestinal cancers,50,51 HIV infection,41,52 and Th1-cell survival.53 However, its role in megakaryocyte/platelet function has not been reported. Our proposed 2-week treatment regimen will not likely have significant effects on tumorigenesis, giving that inhibition of miR-148a is not permanent. We did not observe any abnormalities in our anti-miR–treated mice. We also detect no effects of anti–miR-148a treatment on HEL cell proliferation compared with scrambled control. However, we cannot rule out the potential long-term effects of anti–miR-148a. Its potential side effects in mouse and human would need to be carefully investigated. Murine miR-148a KO model, which is currently unavailable, would be beneficial in answering some of these questions.
FcγRIIA and GPVI are the 2 ITAM-containing receptors in platelets. Using CRP, we showed anti–miR-148a attenuated platelet integrin activation and calcium mobilization via GPVI, which is consistent with the previous finding that TULA-2 KO mice showed increased platelet reactivity through the same pathway.20 Furthermore, FcγRIIA agonists demonstrated a similar reduction of platelet reactivity in anti–miR-148a–treated mice. Syk was demonstrated as an important player in C-type lectin-like receptor 2 (CLEC-2) signaling.54-56 CLEC-2, another tyrosine kinase pathway receptor containing hemITAM on platelets, is important in separation of the vascular system and lymphatic system57-59 and thrombotic stability.60 Neither TULA-2 nor miR-148a was indicated to affect CLEC-2 signaling by previous studies. Here we briefly investigated the role of TULA-2 and anti–miR-148a in CLEC-2 signaling via rhodocytin, a CLEC-2 ligand. First, anti–miR-148a treatment reduced Syk phosphorylation at 480 seconds after CLEC-2 activation without changing CLEC-2 protein level (supplemental figure 2A-B). Furthermore, platelets from TULA-2 double KO mice showed increased integrin activation by 3 nM rhodocytin treatment compared with wild-type mice (supplemental figure 2C). These data suggested that TULA-2 and miR-148a also regulate CLEC-2 signaling. Further comprehensive experiments on CLEC-2 signaling are needed in the future.
In contrast, thrombin-mediated calcium influx did not show significant difference between the 2 experimental conditions. The ITAM-specific differential activation indicates TULA-2 upregulation is the specific mediator for reduced thrombosis by anti–miR-148a. Syk plays a critical role in vascular injury response and thrombosis, as demonstrated by strong antithrombotic effects in vivo by Syk inhibitors.6,61 Upregulation of TULA-2 by anti–miR-148a acts as a Syk inhibitor. We speculate that with multiple phosphate groups to be removed on active Syk, the amount of Syk phosphatase, TULA-2, is rate limiting. Therefore, upregulation of TULA-2 by threefold (Figure 4C; supplemental Figure 2B) is plausible to have significant effects on platelet activation and thrombosis. However, it is also possible that anti–miR-148a alters the expression of additional genes beyond TULA-2 that could affect platelet function and thrombosis. Understanding whether multiple genes cooperatively mediate the antithrombotic effect of anti–miR-148a inhibition will be beneficial.
In conclusion, this is the first study to use the anti-miRNA approach to regulate platelet reactivity in vivo. Because the sequence of miR-148a and its binding site on TULA-2 are highly conserved between mice and humans, this finding is potentially translatable to human thrombotic diseases, such as HIT. Understanding the determinants underlying the HIT pathophysiologic process will give insights into novel diagnostic tests and therapies. The role of Syk in arterial thrombosis and as an adapter of outside-in integrin αIIbβ3 signaling9 makes the anti–miR-148a regimen of potentially greater utility. Further understanding of the comprehensive molecular and cellular mechanism of the anti–miR-148a’s effects in murine platelet reactivity and thrombosis would help to delineate platelet physiology and facilitate the development of novel prophylaxis and treatment of human thrombotic diseases.
Acknowledgments
The authors thank Drs Barry Garchow and Marianthi Kiriakidou for valuable suggestions.
This work was supported by National Institutes of Health, National Heart, Lung and Blood Institute grants R01 HL102482, P01 HL110860, and R01 HL106009, American Heart Association predoctoral fellowship 15PRE25690045, and the Cardeza Foundation for Hematologic Research.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.Z. designed and performed experiments, analyzed results, and wrote the manuscript; S.A., P.A., C.A.S., and C.A.D. performed experiments and analyzed data; L.C.E. and P.F.B. designed experiments and analyzed data; A.Y.T. and S.P.K. contributed reagents and antibodies; and S.E.M. provided overall direction, designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for P.A. is T2 Biosystems, Inc, Lexington, MA.
Correspondence: Steven E. McKenzie, 1020 Locust St, Philadelphia, PA, 19107; e-mail: steven.mckenzie@jefferson.edu.
References
- 1.Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106(8):2710–2715. doi: 10.1182/blood-2005-04-1546. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt BP, Adelman B. Heparin-associated thrombocytopenia: a critical review and pooled analysis. Am J Med Sci. 1993;305(4):208–215. doi: 10.1097/00000441-199304000-00003. [DOI] [PubMed] [Google Scholar]
- 3.McKenzie SE, Sachais BS. Advances in the pathophysiology and treatment of heparin-induced thrombocytopenia. Curr Opin Hematol. 2014;21(5):380–387. doi: 10.1097/MOH.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilly MP, Taylor SM, Hartman NK, et al. Heparin-induced thrombocytopenia/thrombosis in a transgenic mouse model requires human platelet factor 4 and platelet activation through FcgammaRIIA. Blood. 2001;98(8):2442–2447. doi: 10.1182/blood.v98.8.2442. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie SE, Taylor SM, Malladi P, et al. The role of the human Fc receptor Fc γ RIIA in the immune clearance of platelets: a transgenic mouse model. J Immunol. 1999;162(7):4311–4318. [PubMed] [Google Scholar]
- 6.Reilly MP, Sinha U, André P, et al. PRT-060318, a novel Syk inhibitor, prevents heparin-induced thrombocytopenia and thrombosis in a transgenic mouse model. Blood. 2011;117(7):2241–2246. doi: 10.1182/blood-2010-03-274969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanaga F, Poole A, Asselin J, et al. Syk interacts with tyrosine-phosphorylated proteins in human platelets activated by collagen and cross-linking of the Fc gamma-IIA receptor. Biochem J. 1995;311(Pt 2):471–478. doi: 10.1042/bj3110471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boylan B, Gao C, Rathore V, Gill JC, Newman DK, Newman PJ. Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets. Blood. 2008;112(7):2780–2786. doi: 10.1182/blood-2008-02-142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhi H, Rauova L, Hayes V, et al. Cooperative integrin/ITAM signaling in platelets enhances thrombus formation in vitro and in vivo. Blood. 2013;121(10):1858–1867. doi: 10.1182/blood-2012-07-443325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arepally G, McKenzie SE, Jiang X-M, Poncz M, Cines DB. Fc γ RIIA H/R 131 polymorphism, subclass-specific IgG anti-heparin/platelet factor 4 antibodies and clinical course in patients with heparin-induced thrombocytopenia and thrombosis. Blood. 1997;89(2):370–375. [PubMed] [Google Scholar]
- 11.Rollin J, Pouplard C, Gratacap MP, et al. Polymorphisms of protein tyrosine phosphatase CD148 influence FcγRIIA-dependent platelet activation and the risk of heparin-induced thrombocytopenia. Blood. 2012;120(6):1309–1316. doi: 10.1182/blood-2012-04-424044. [DOI] [PubMed] [Google Scholar]
- 12.Pamela S, Anna Maria L, Elena D, et al. Heparin-induced thrombocytopenia: the role of platelets genetic polymorphisms. Platelets. 2013;24(5):362–368. doi: 10.3109/09537104.2012.701026. [DOI] [PubMed] [Google Scholar]
- 13.Simon LM, Edelstein LC, Nagalla S, et al. Human platelet microRNA-mRNA networks associated with age and gender revealed by integrated plateletomics. Blood. 2014;123(16):e37–e45. doi: 10.1182/blood-2013-12-544692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stolla M, Stefanini L, André P, et al. CalDAG-GEFI deficiency protects mice in a novel model of Fcγ RIIA-mediated thrombosis and thrombocytopenia. Blood. 2011;118(4):1113–1120. doi: 10.1182/blood-2011-03-342352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeung J, Tourdot BE, Fernandez-Perez P, et al. Platelet 12-LOX is essential for FcγRIIa-mediated platelet activation. Blood. 2014;124(14):2271–2279. doi: 10.1182/blood-2014-05-575878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsygankov AY. TULA-family proteins: a new class of cellular regulators. J Cell Physiol. 2013;228(1):43–49. doi: 10.1002/jcp.24128. [DOI] [PubMed] [Google Scholar]
- 17.Carpino N, Turner S, Mekala D, et al. Regulation of ZAP-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity. 2004;20(1):37–46. doi: 10.1016/s1074-7613(03)00351-0. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Ren L, Kim S, et al. Determination of the substrate specificity of protein-tyrosine phosphatase TULA-2 and identification of Syk as a TULA-2 substrate. J Biol Chem. 2010;285(41):31268–31276. doi: 10.1074/jbc.M110.114181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman TN, Liverani E, Ivanova E, et al. Members of the novel UBASH3/STS/TULA family of cellular regulators suppress T-cell-driven inflammatory responses in vivo. Immunol Cell Biol. 2014;92(10):837–850. doi: 10.1038/icb.2014.60. [DOI] [PubMed] [Google Scholar]
- 20.Thomas DH, Getz TM, Newman TN, et al. A novel histidine tyrosine phosphatase, TULA-2, associates with Syk and negatively regulates GPVI signaling in platelets. Blood. 2010;116(14):2570–2578. doi: 10.1182/blood-2010-02-268136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102(2):449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 22.Dumont B, Lasne D, Rothschild C, et al. Absence of collagen-induced platelet activation caused by compound heterozygous GPVI mutations. Blood. 2009;114(9):1900–1903. doi: 10.1182/blood-2009-03-213504. [DOI] [PubMed] [Google Scholar]
- 23.Mócsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10(6):387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elmén J, Lindow M, Silahtaroglu A, et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008;36(4):1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vester B, Wengel J. LNA (locked nucleic acid): high-affinity targeting of complementary RNA and DNA. Biochemistry. 2004;43(42):13233–13241. doi: 10.1021/bi0485732. [DOI] [PubMed] [Google Scholar]
- 26.Rayner KJ, Esau CC, Hussain FN, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478(7369):404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marquart TJ, Wu J, Lusis AJ, Baldán Á. Anti-miR-33 therapy does not alter the progression of atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2013;33(3):455–458. doi: 10.1161/ATVBAHA.112.300639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rayner KJ, Sheedy FJ, Esau CC, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121(7):2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Zhu H, Zhang X, et al. Loss of the miR-144/451 cluster impairs ischaemic preconditioning-mediated cardioprotection by targeting Rac-1. Cardiovasc Res. 2012;94(2):379–390. doi: 10.1093/cvr/cvs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery RL, Hullinger TG, Semus HM, et al. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124(14):1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhagat TD, Zhou L, Sokol L, et al. miR-21 mediates hematopoietic suppression in MDS by activating TGF-β signaling. Blood. 2013;121(15):2875–2881. doi: 10.1182/blood-2011-12-397067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garchow BG, Bartulos Encinas O, Leung YT, et al. Silencing of microRNA-21 in vivo ameliorates autoimmune splenomegaly in lupus mice. EMBO Mol Med. 2011;3(10):605–615. doi: 10.1002/emmm.201100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 34.Smirnova EV, Collingwood TS, Bisbal C, et al. TULA proteins bind to ABCE-1, a host factor of HIV-1 assembly, and inhibit HIV-1 biogenesis in a UBA-dependent fashion. Virology. 2008;372(1):10–23. doi: 10.1016/j.virol.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Edelstein LC, Simon LM, Montoya RT, et al. Racial differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376c. Nat Med. 2013;19(12):1609–1616. doi: 10.1038/nm.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J, Dong JF, Sun C, et al. Platelet FcgammaRIIA His131Arg polymorphism and platelet function: antibodies to platelet-bound fibrinogen induce platelet activation. J Thromb Haemost. 2003;1(2):355–362. doi: 10.1046/j.1538-7836.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 37.Arepally G, McKenzie SE, Jiang XM, Poncz M, Cines DB. Fc gamma RIIA H/R 131 polymorphism, subclass-specific IgG anti-heparin/platelet factor 4 antibodies and clinical course in patients with heparin-induced thrombocytopenia and thrombosis. Blood. 1997;89(2):370–375. [PubMed] [Google Scholar]
- 38.Bachelot-Loza C, Saffroy R, Lasne D, Chatellier G, Aiach M, Rendu F. Importance of the FcgammaRIIa-Arg/His-131 polymorphism in heparin-induced thrombocytopenia diagnosis. Thromb Haemost. 1998;79(3):523–528. [PubMed] [Google Scholar]
- 39.Buitrago L, Bhavanasi D, Dangelmaier C, et al. Tyrosine phosphorylation on spleen tyrosine kinase (Syk) is differentially regulated in human and murine platelets by protein kinase C isoforms. J Biol Chem. 2013;288(40):29160–29169. doi: 10.1074/jbc.M113.464107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miranda KC, Huynh T, Tay Y, et al. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 41.Bennett JS. Structure and function of the platelet integrin alphaIIbbeta3. J Clin Invest. 2005;115(12):3363–3369. doi: 10.1172/JCI26989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shattil SJ, Kashiwagi H, Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91(8):2645–2657. [PubMed] [Google Scholar]
- 43.Calvete JJ. On the structure and function of platelet integrin α IIb β 3, the fibrinogen receptor. Proc Soc Exp Biol Med. 1995;208(4):346–360. doi: 10.3181/00379727-208-43863a. [DOI] [PubMed] [Google Scholar]
- 44.Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7(7):1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 45.Taylor SM, Reilly MP, Schreiber AD, Chien P, Tuckosh JR, McKenzie SE. Thrombosis and shock induced by activating antiplatelet antibodies in human Fc γ RIIA transgenic mice: the interplay among antibody, spleen, and Fc receptor. Blood. 2000;96(13):4254–4260. [PubMed] [Google Scholar]
- 46.Back SH, Adapala NS, Barbe MF, Carpino NC, Tsygankov AY, Sanjay A. TULA-2, a novel histidine phosphatase, regulates bone remodeling by modulating osteoclast function. Cell Mol Life Sci. 2013;70(7):1269–1284. doi: 10.1007/s00018-012-1203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agrawal R, Carpino N, Tsygankov A. TULA proteins regulate activity of the protein tyrosine kinase Syk. J Cell Biochem. 2008;104(3):953–964. doi: 10.1002/jcb.21678. [DOI] [PubMed] [Google Scholar]
- 48.Fei Y, Webb R, Cobb BL, Direskeneli H, Saruhan-Direskeneli G, Sawalha AH. Identification of novel genetic susceptibility loci for Behçet’s disease using a genome-wide association study. Arthritis Res Ther. 2009;11(3):R66. doi: 10.1186/ar2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teruel-Montoya R, Kong X, Abraham S, et al. MicroRNA expression differences in human hematopoietic cell lineages enable regulated transgene expression. PLoS One. 2014;9(7):e102259. doi: 10.1371/journal.pone.0102259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Song Y, Wang Z, et al. Altered expression of MiR-148a and MiR-152 in gastrointestinal cancers and its clinical significance. J Gastrointest Surg. 2010;14(7):1170–1179. doi: 10.1007/s11605-010-1202-2. [DOI] [PubMed] [Google Scholar]
- 51.Zheng B, Liang L, Wang C, et al. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17(24):7574–7583. doi: 10.1158/1078-0432.CCR-11-1714. [DOI] [PubMed] [Google Scholar]
- 52.Kulkarni S, Savan R, Qi Y, et al. Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature. 2011;472(7344):495–498. doi: 10.1038/nature09914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haftmann C, Stittrich AB, Zimmermann J, et al. miR-148a is upregulated by Twist1 and T-bet and promotes Th1-cell survival by regulating the proapoptotic gene Bim. Eur J Immunol. 2015;45(4):1192–1205. doi: 10.1002/eji.201444633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki-Inoue K, Fuller GL, García A, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107(2):542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 55.Hughes CE, Pollitt AY, Mori J, et al. CLEC-2 activates Syk through dimerization. Blood. 2010;115(14):2947–2955. doi: 10.1182/blood-2009-08-237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lorenz V, Stegner D, Stritt S, et al. doi: 10.1182/blood-2014-11-611905. Targeted downregulation of platelet CLEC-2 occurs through Syk-independent internalization. Blood. 2015;125(26):4069-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki-Inoue K, Inoue O, Ding G, et al. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010;285(32):24494–24507. doi: 10.1074/jbc.M110.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navarro-Núñez L, Langan SA, Nash GB, Watson SP. The physiological and pathophysiological roles of platelet CLEC-2. Thromb Haemost. 2013;109(6):991–998. doi: 10.1160/TH13-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pollitt AY, Poulter NS, Gitz E, et al. Syk and Src family kinases regulate C-type lectin receptor 2 (CLEC-2)-mediated clustering of podoplanin and platelet adhesion to lymphatic endothelial cells. J Biol Chem. 2014;289(52):35695–35710. doi: 10.1074/jbc.M114.584284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bender M, May F, Lorenz V, et al. Combined in vivo depletion of glycoprotein VI and C-type lectin-like receptor 2 severely compromises hemostasis and abrogates arterial thrombosis in mice. Arterioscler Thromb Vasc Biol. 2013;33(5):926–934. doi: 10.1161/ATVBAHA.112.300672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andre P, Morooka T, Sim D, et al. Critical role for Syk in responses to vascular injury. Blood. 2011;118(18):5000–5010. doi: 10.1182/blood-2011-06-360743. [DOI] [PMC free article] [PubMed] [Google Scholar]