Abstract
Purpose
To quantify changes in ocular dimensions associated with age, refractive error, and accommodative response, in vivo, in 30- to 50-year-old human subjects.
Methods
The right eyes of 91 adults were examined using ultrasonography, phakometry, keratometry, pachymetry, interferometry, anterior segment optical coherence tomography, and high resolution magnetic resonance imaging. Accommodation was measured subjectively with a push-up test and objectively using open-field autorefraction. Regression analyses were used to assess differences in ocular parameters with age, refractive error and accommodation.
Results
With age, crystalline lens thickness increased (0.03 mm/yr), anterior lens curvature steepened (0.11 mm/yr), anterior chamber depth decreased (0.02 mm/y) and lens equivalent refractive index decreased (0.001 /y) (all p < 0.01). With increasing myopia, there were significant increases in axial length (0.37 mm/D), vitreous chamber depth (0.34 mm/D), vitreous chamber height (0.09 mm/D) and ciliary muscle ring diameter (0.10 mm/D) (all p < 0.05). Increasing myopia was also associated with steepening of both the cornea (0.16 mm/D) and anterior lens surface (0.011 mm/D) (both p < 0.04). With accommodation, the ciliary muscle ring diameter decreased (0.08 mm/D), and the muscle thinned posteriorly (0.008 mm/D), allowing the lens to shorten equatorially (0.07 mm/D) and thicken axially (0.06 mm/D) (all p < 0.03).
Conclusions
Refractive error is significantly correlated with not only the axial dimensions, but the anterior equatorial dimension of the adult eye. Further testing and development of accommodating intraocular lenses should account for differences in patients’ preoperative refractive error.
Keywords: presbyopia, accommodation, refractive error, crystalline lens, ciliary muscle
Despite decades of research, no optical correction of presbyopia has achieved widespread acceptance in the way single vision eyeglasses and contact lenses have in the non-presbyopic population. In addition to improving multifocal lens designs, much of the current research in presbyopia is aimed at developing an intraocular lens (IOL) that can dynamically change like the natural crystalline lens.
Previous research has demonstrated that the ciliary muscle continues its ability to contract beyond the onset of clinical presbyopia.1, 2 Thus it is feasible that an implantable lens could harness ciliary muscle force to move optical components and/or fluid to generate a change in focus. While there is currently only one “accommodating IOL” approved for use by the United States Food and Drug Administration (FDA), many others have been approved in other countries or are currently in clinical trials.3–5 There have been mixed reports regarding the ability of the current devices to produce usable changes in focal distance, with none achieving wide acceptance to date.5 Improvements in these technologies will require an accurate knowledge of both the baseline ocular dimensions and the expected effect of accommodative effort.
The refractive error profile of the United States adult population is less than 5% hyperopic, about 30–40% myopic, and slightly more than half are emmetropic.6 The individual effects of age,1, 2, 7 refractive error8 and accommodative effort1, 2 on the ocular dimensions in adults have been reported but, to date, no study assessed the relative effects of all three variables in adult human subjects. Our previous research quantified the effects of age and accommodation on the lens and ciliary muscle in emmetropic eyes.2 This study is an extension of that work. It aims to quantify changes in ocular dimensions with age, cycloplegic refractive error, and objectively measured accommodative response in hyperopic, emmetropic and myopic adult eyes.
METHODS
The study was approved by the Biomedical Sciences Institutional Review Board at the Ohio State University and was conducted in accordance with the tenets of the Declaration of Helsinki. Subjects were recruited from the campus and general community. Anyone meeting the entry criteria was invited to participate. A total of 91 subjects were enrolled, and 85 completed both study visits. Eligible patients were 30 to 50 years of age and phakic with less than a Grade 1 cataract on the Lens Opacities Classification System (LOCS) scale in both eyes.9 Subjects could not be strabismic, amblyopic, nor have a history of vision therapy. In order to allow clear viewing of targets in the MRI scanner, subjects had to be emmetropic or able to wear soft contact lenses, and had to be correctable to at least 20/25 in each eye. Subjects could not have more than 1.25 D of refractive astigmatism. Those who were pregnant or breastfeeding, or who had a systemic disease that could compromise ocular health (e.g., diabetes) were not eligible. Subjects must also have met all 7 Tesla (T) magnetic resonance imaging (MRI) safety restrictions (e.g., no metal implant, tattoo, pacemaker, etc.).
Study Procedures
Data were collected on the right eye (with the left eye occluded, unless otherwise described). Accommodative amplitude was measured using the push-up to blur technique with the Royal Air Force (RAF) near point rule (Haag-Streit England, Essex, UK).10, 11 The subject was instructed to “pick a letter on the target and carefully focus on it” while the card was moved closer.12 If the subject could not clear the card at the most remote position, positive trial lenses were added. If used, the trial lens power was subtracted from the subject’s accommodative amplitude. Three measurements were made and averaged.
Subjects between the age of 30 and 50 years should have between 0 and 6 D of objectively measured accommodative ability,13 so objective accommodative response was measured using stimulus demands of 0, 2, 4, and 6D with an open field auto-refractor (Grand Seiko WV 500,Grand Seiko Ltd., Hiroshima, Japan) and a Badal lens track.2, 14 Subjects were asked to attempt to keep the target clear and five measures were recorded at each stimulus demand. The spherical equivalents of the five measures were averaged to quanitfy the objective accommodative response.
Central corneal curvatures (Ks) were measured using the Keratron Scout (Optikon, Rome, Italy). Three measurements were captured and averaged. Due to the low cylinder amounts in this study, the average of the two principal meridians’ curvature (mid-K) is reported. Five measures of axial length (AL) were taken and averaged using the IOLMaster (Carl Zeiss Meditec, Dublin, CA, USA). Three measures of central corneal thickness (pachymetry) were recorded and averaged using the Visante Anterior Segment Optical Coherence Tomography (OCT; Software version 3.0, Carl Zeiss Meditec, Dublin, CA, USA). The Visante OCT was also used to capture images of the crystalline lens and ciliary muscle as previously described.2, 15, 16 Briefly, for crystalline lens images, the subject was asked to focus on the Maltese cross target within the instrument. The internal minus lens system of the OCT was used to increase the accommodative stimulus demand. To image the ciliary muscle, the subject was instructed to look at an external Maltese cross target. The distant target was presented on the far wall of the room through a mirror system and the 2-, 4-, and 6-D targets were presented on an adjustable rod affixed to the instrument. Four crystalline lens and ciliary muscle images were recorded at each accommodative stimulus demand.
Following accommodative measures, one drop of 1% tropicamide was instilled in the right eye, and testing resumed 20 minutes after drop instillation. The subjects’ cycloplegic refractive error was recorded using the open field auto-refractor (described above). The reported refractive error (RE) was the average of five spherical equivalent measurements.
Anterior and posterior lens radii of curvature and equivalent refractive index were calculated using video phakometry-obtained data, and values from A-scan ultrasonography (US) and cycloplegic autorefractor measurements as previously described.17 Finally, a topical anesthetic (0.5% proparacaine) was instilled, and five US measures (Allergan-Humphrey Model 820, San Leandro, CA, USA) were recorded and averaged to determine anterior chamber depth, lens thickness, vitreous chamber depth, and axial length under cycloplegia.
On a separate visit that was between one day and one month after the primary visit, subjects were scanned in a 7 Tesla (T) MRI magnet (Phillips Achieva, Cleveland, OH, USA), using a volume head coil (Nova Medical, Inc., Wilmington, MA, USA) for transmission and a custom-built, single-loop, 2 × 2.3 cm radiofrequency surface coil for reception. The methods have been reported previously2, 18 but, briefly, the imaged (right) eye was taped closed, and the left eye fixated the targets in order to minimize motion artifacts. Subjects were instructed to focus through a mirror system on the same targets that were used during OCT imaging (Maltese cross at 0-, 2-, 4-, and 6-D stimulus demands). Inversion-recovery turbo field echo (IR-TFE) sequences were acquired with shot interval and inversion times of 1800 and 900 ms, respectively, a repetition time between the TFE read-outs of 6.8 ms, echo time of 2.3 ms, flip angle of 8°, TFE-factor of 260, field of view of 65 × 65 × 8 mm, and a scan time of 34 sec. At least eight three dimensional (3-D) scans were acquired for each accommodative stimulus. Images had acquired voxel dimensions of 0.25 × 0.25 × 1.0 mm and were interpolated by the system software to 0.10 × 0.10 by 0.50 mm for analysis. Due to time and cost considerations, MRI was not performed after cycloplegia.
Image Analysis
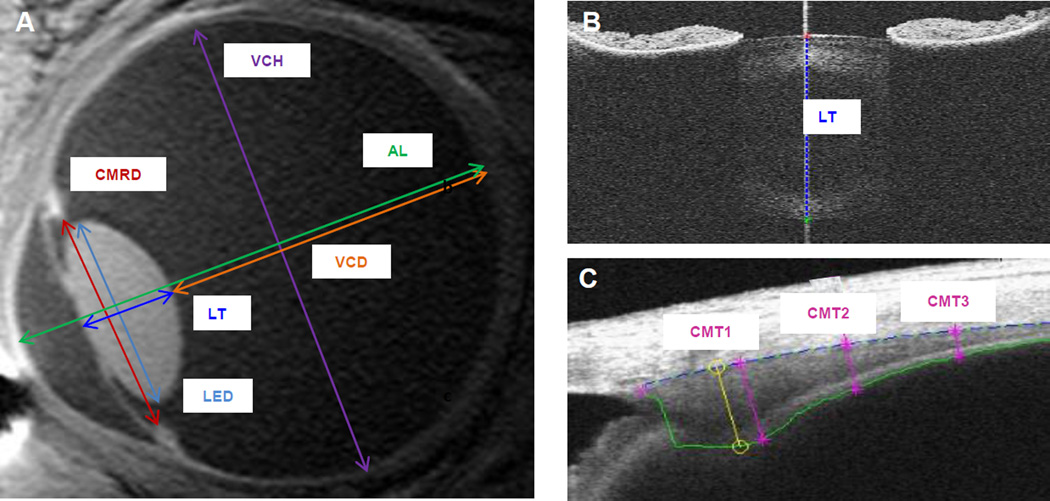
Example MRI and OCT images with reported ocular dimension measurements are illustrated in Figure 1. Custom MATLAB (MathWorks, Natick MA, USA) programs were used to determine the ciliary muscle and crystalline lens thickness from exported Visante OCT raw image files.19, 20 As described in previous work,19 the cross-sectional (transverse section) ciliary muscle thickness (CMT) at 1, 2, and 3 mm posterior to the scleral spur were calculated using a refractive index of 1.38 (ciliary muscle at 1310 nm) and 128 pixels/mm (high resolution corneal mode) conversion factors (Figure 1c). For OCT crystalline lens images (transverse section), an algorithm detected the anterior and posterior lens surfaces and computed the maximum distance (lens thickness) along the scan acquisition line (Figure 1b).20 A refractive index of 1.39 (estimate of equivalent refractive index at 1310 nm) and 64 pixel/mm (raw image mode images) conversions were applied. All algorithm-marked images were visually inspected prior to inclusion in the data set. These techniques have been shown to be reliable, repeatable, and able to detect changes between and within subjects.15, 19–21
Figure 1.
Example MRI (A) and OCT (B, C) images illustrating ocular dimensions studied.
A central sagittal slice of the 3D MRI images was analyzed using the Philips DICOM viewer (R2.5 Version 1). An examiner masked to subject age and accommodative ability manually scrolled through the 3-D dataset and visually identified the central slice. Measurements of the ciliary muscle ring diameter, lens thickness, lens equatorial diameter, vitreous chamber depth, vitreous chamber width, and axial length were made by the masked reader using straight line calipers (Figure 1a). The pixel resolution of the digital calipers was equal to the interpolated voxel size in that plane (0.1 mm). Measurements from all usable scans were averaged.
Statistical Methods
Descriptive statistics (mean, standard deviation, and range) were calculated for demographic, biometric, and accommodative data. To ensure robustness and validity of the data across imaging techniques, certain measurements (e.g., axial length) were compared across imaging methods (e.g., MRI, OCT, US) using Pearson correlations and Bland Altman analyses.22 Linear regression was used to describe the relationship between ocular parameters as a function of age and refractive error. It should be noted that while the actual value recorded for accommodative response by autorefraction is increasingly negative, per longstanding conventional notation, accommodative response is more positive with increasing accommodation.13, 23–25 Myopic refractive error is specified as minus and hyperopic as plus. For ocular parameters that were expected to change with accommodation (ciliary muscle and crystalline lens), repeated measured regression models were used to study the relative effects of age, refractive error and accommodation. Parsimonious models were developed by sequentially removing predictors that were not statistically significant.
RESULTS
The majority of participants were Caucasian (Caucasian: 76%, Asian: 15%, African American: 7%, other: 2%) and female (57%). The average enrolled subject was 40.5 ± 6.1 years old. There was a broad distribution of refractive errors (range: −10.90 to +1.75 D) with slightly more than half the sample being myopic (myope: 54%, emmetrope [+0.50 D to −0.50 D, inclusive] 29%, hyperope 18%). There was no significant difference in subject age across the three refractive error groups (p = 0.57). Ocular dimensions, by refractive error, are shown in Table 1.
Table 1.
Baseline ocular dimensions by refractive error (mean ± SD).
| Structure | Method | Myope (n = 49) |
Emmetrope (n = 26) |
Hyperope (n = 16) |
|
|---|---|---|---|---|---|
| Corneal thickness (µm) | OCT | 545.5 ± 35.3 | 543.0 ± 27.8 | 543.0 ± 32.7 | |
| Corneal curvature (D) | keratometry | 43.9 ± 1.5 | 43.7 ± 1.4 | 43.0 ± 1.8 | |
| Anterior chamber depth (mm) | US | 3.75 ± 0.32 | 3.58 ± 0.36 | 3.49 ± 0.36 | |
| Vitreous chamber depth (mm) | US | 17.4 ± 1.1 | 16.0 ± 0.7 | 16.0 ± 0.9 | |
| Vitreous chamber height (mm) | MRI | 23.8 ± 1.1 | 23.3 ± 0.8 | 23.3 ± 0.9 | |
| US | 25.1 ± 1.0 | 23.5 ± 0.7 | 23.6 ± 1.0 | ||
| Axial length (mm) | MRI | 25.0 ± 1.1 | 23.4 ± 0.7 | 23.6 ± 0.9 | |
| IOLMaster | 25.2 ± 1.1 | 23.6 ± 0.7 | 23.7 ± 1.0 | ||
| OCT | 3.93 ± 0.29 | 3.98 ± 0.29 | 4.17 ± 0.26 | ||
| Lens thickness (mm) | MRI | 3.95 ± 0.28 | 3.93 ± 0.30 | 4.15 ± 0.26 | |
| US | 3.91 ± 0.29 | 3.95 ± 0.30 | 4.12 ± 0.25 | ||
| Lens equatorial diameter (mm) | MRI | 9.60 ± 0.29 | 9.42 ± 0.24 | 9.46 ± 0.31 | |
| Lens equivalent refractive index | phakometry | 1.45 ± 0.01 | 1.45 ± 0.01 | 1.44 ± 0.01 | |
| Anterior lens curvature (mm) | phakometry | 10.3 ± 1.2 | 10.8 ± 1.3 | 10.2 ± 1.2 | |
| Posterior lens curvature (mm) | phakometry | 7.43 ± 0.55 | 7.43 ± 0.46 | 7.14 ± 0.41 | |
| Ciliary muscle ring diameter (mm) | MRI | 12.24 ± 0.57 | 11.84 ± 0.55 | 11.81 ± 0.63 | |
| Ciliary muscle thickness (mm): | CMT1 | OCT | 0.80 ± 0.07 | 0.79 ± 0.06 | 0.76 ± 0.07 |
| CMT2 | 0.53 ± 0.11 | 0.52 ± 0.07 | 0.46 ± 0.10 | ||
| CMT3 | 0.31 ± 0.08 | 0.31 ± 0.06 | 0.27 ± 0.08 | ||
Bland Altman analyses of the different imaging techniques indicated some small, but statistically significant, differences between absolute values for some techniques [e.g. axial length: MRI and US: mean difference = +0.07 (p = 0.08), 95% limits of agreement −0.61 to +0.75 mm; MRI and IOL: mean difference = −0.20 (p < 0.001), 95% limits of agreement −0.88 to +0.48 mm; lens thickness: MRI and US: mean difference = +0.01 (p =0.43), 95% limits of agreement −0.19 to +0.21 mm; MRI and OCT: mean difference = −0.04 (p = 0.002), 95% limits of agreement −0.25 to +0.17 mm]. As reported previously, the small differences are likely due to assumptions of speed of sound (US) and refractive index (IOL, OCT), as well as differences in resolution (MRI) of the techniques.8, 18, 26 Where multiple measures of the same parameter were available, the one with the higher resolution was used for further analyses and are indicated in the results.
Effect of Age
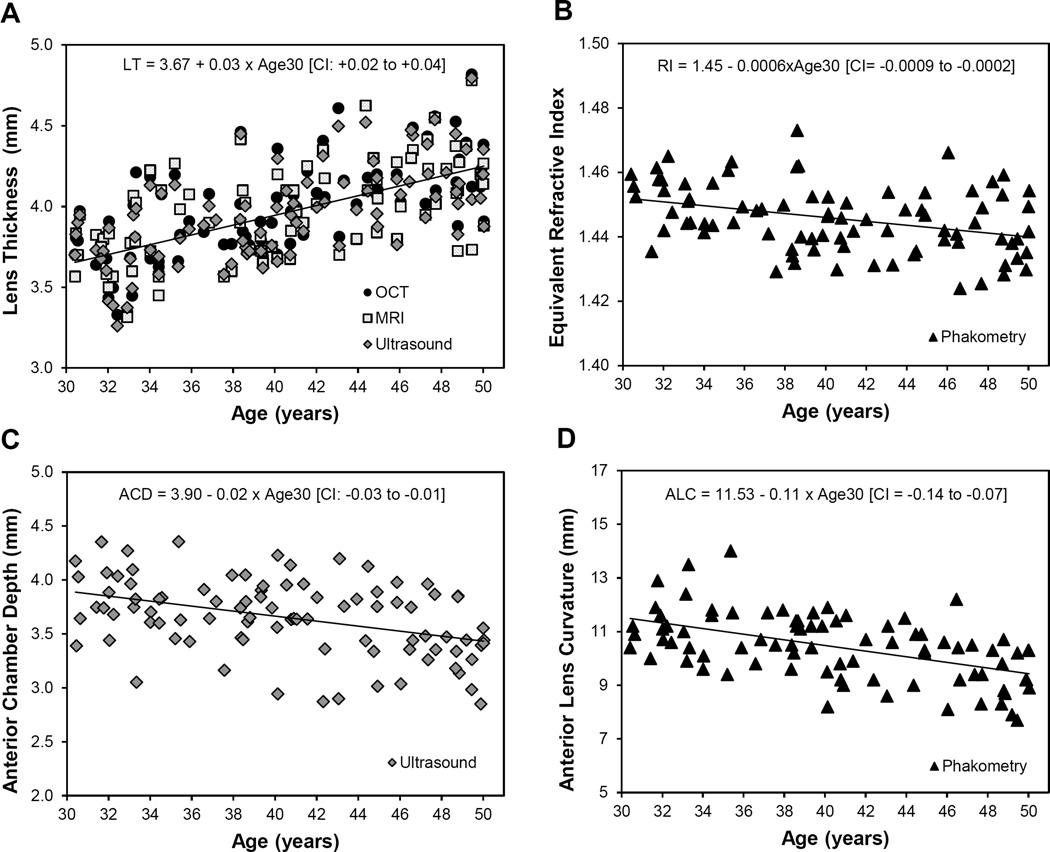
Univariate linear regressions were conducted to determine the relationship between each variable and age, with age centered at 30 years (designated as “Age30”). The maximum subjectively-measured accommodative amplitude declined about 0.6 D per year of age (Accommodation (D) = 12.6 – 0.57×[Age30], 95% confidence intervals (CI) for slope: 0.65 to −0.49, p < 0.001). Increased age was also related to a thicker crystalline lens (p < 0.001, Figure 2a), lower lens equivalent refractive index (p = 0.001, Figure 2b), shallower anterior chamber depth (p = 0.001, Figure 2c), and steeper anterior lens radius of curvature (p = 0.001, Figure 2D).
Figure 2.
Statistically significant changes in ocular dimension with age. Regression lines fitted with age centered at 30 years (Age30) and 95% confidence interval (CI) for slope in brackets. Only regression line for OCT shown for lens thickness.
Age was not related to corneal curvature (p = 0.81, 95% CI: −0.05 to +0.06 mm), central corneal thickness (p = 0.15, 95% CI: −0.41 to +2.49 µm), axial length (p = 0.75, 95% CI: −0.054 to +0.074 mm), vitreous chamber depth (p = 0.60, 95% CI: −0.051 to +0.030 mm), posterior lens curvature (p = 0.07, 95% CI: −0.035 to +0.001), ciliary muscle thickness at 1, 2, or 3 mm posterior to the scleral spur (CMT1, p = 0.38, 95% CI: −0.017 to +0.006 mm; CMT2, p = 0.14, 95% CI: −0.007 to +0.001; CMT3, p = 0.39, 95% CI: −0.004 to +0.002), ciliary muscle ring diameter (p = 0.12, 95% CI: −0.037 to +0.026 mm), lens equatorial diameter (p = 0.52, 95% CI: −0.007 to +0.014) or vitreous chamber height (p = 0.09, 95% CI: −0.067 to +0.006 mm).
Effect of Refractive Error
There was no relationship between refractive error and accommodative amplitude (p = 0.89). Increasing myopic refractive error was related to longer axial length (p < 0.001, Figure 3A), larger vitreous chamber depth (p < 0.001, Figure 3C), larger vitreous chamber height (p = 0.04, Figure 3E), larger ciliary muscle ring diameter (p < 0.001, Figure 3B), steeper corneal curvature (p = 0.01, Figure 3D), and steeper anterior lens radius of curvature (p = 0.03, Figure 3F).
Figure 3.
Statistically significant changes in ocular dimensions with refractive error (RE). The 95% confidence interval (CI) for slope is reported in brackets. Only regression line for ultrasound shown for axial length and vitreous chamber depth.
In this sample, refractive error was not related to central corneal thickness (p = 0.29, 95% CI = −3.94 to 1.21 µm), anterior chamber depth (p = 0.06, 95% CI = −0.05 to +0.00), lens thickness (p = 0.07, 95% CI = −0.002 to +0.045), posterior lens curvature (p = 0.68, 95% CI = −0.050 to +0.033), lens equivalent refractive index (p = 0.98, 95% CI = −0.001 to +0.001), lens equatorial diameter (p = 0.07, 95% CI = −0.047 to +0.002) or ciliary muscle thickness at any location (CMT1, p = 0.37, 95% CI = −0.015 to +0.040 mm; CMT2, p = 0.16, 95% CI = −0.016 to +0.003; CMT3, p = 0.24, 95% CI = −0.012 to +0.003,).
Effect of Accommodation
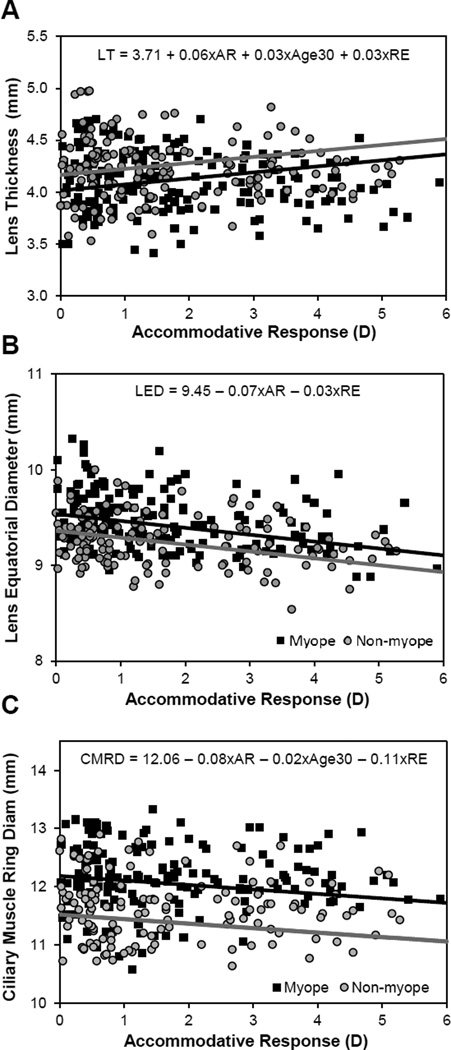
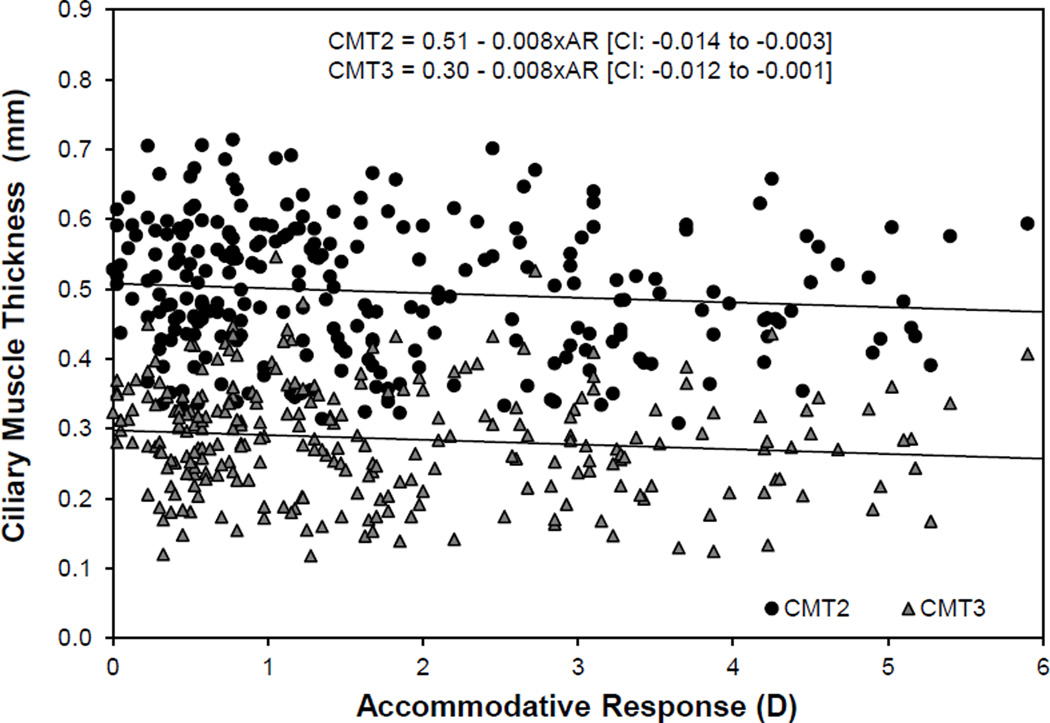
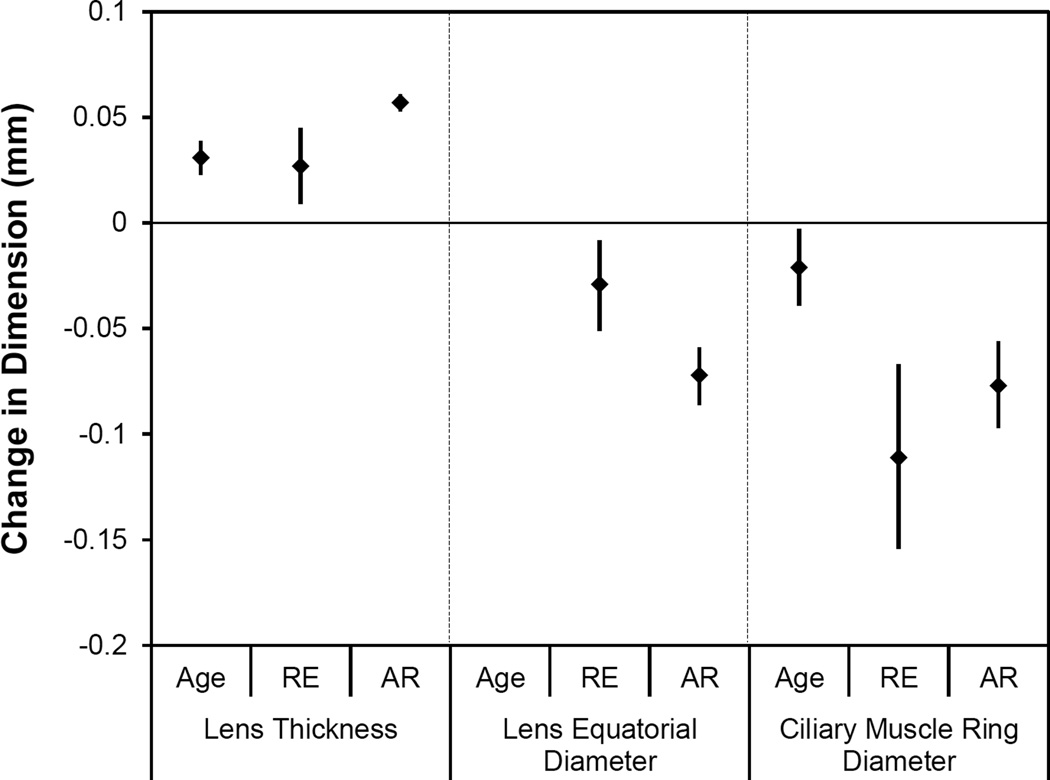
Only the structures of the accommodative system (lens and ciliary muscle) were expected to change with accommodative effort. Given the established relationships with refractive error and age, ciliary muscle and lens changes were modeled in a multivariate setting with refractive error, age and accommodation (with increased accommodation being a positive value per traditional notation) as predictors. Lens thickness increased with accommodation and age, and decreased with myopic refractive error (pAR < 0.001, pAge < 0.001, pRE = 0.004; Figure 4a). In a multivariate model, lens equatorial diameter was not related to age, and thus age was removed from the model. Lens equatorial diameter significantly decreased with accommodation and increased with myopic refractive error (pAR < 0.001, pRE = 0.009; Figure 4b). In the full model, ciliary muscle ring diameter was found to decrease with age, accommodation and hyperopic refractive error (pAR < 0.001, pAge = 0.026, pRE < 0.001; Figure 4c). Ciliary muscle thickness was not related to refractive error or age, and these parameters were removed from the model. The posterior ciliary muscle thinned with accommodation (CMT2 p = 0.003; CMT3 p = 0.002; Figure 5). There was no significant change in the anterior ciliary muscle with accommodation (p = 0.52, 95% CI = −0.006 to +0.003). An overview of the magnitudes of statistically significant changes in the accommodative structures associated with age, refractive error and accommodation is illustrated in Figure 6.
Figure 4.
Multivariate models of lens and ciliary muscle changes with accommodative response (AR), refractive error (RE) and age. In each plot, two regression lines were fitted holding age constant at age 40 years to visualize differences with refractive error and accommodation. The black lines illustrate a −3D myope and the grey line a +3D hyperope).
Figure 5.
Statistically significant changes in ciliary muscle thickness at 2 and 3 mm posterior to the scleral spur (CMT2, 3) with accommodative response (AR). The 95% confidence interval (CI) for slope is reported in brackets.
Figure 6.
Comparative magnitude of changes (parameter estimate with 95%CI) in lens thickness, lens equatorial diameter and ciliary muscle ring diameter per one year of age, one diopter of refractive error (RE) or one diopter of accommodative response (AR).
DISCUSSION
In agreement with previous adult human studies, we report that the crystalline lens steepens2, 7, 27 and thickens2, 7, 16, 28–30 and that the equivalent refractive index of the lens decreases with age.2, 7, 31 The anterior chamber shallows by about two-thirds of the amount of thickening in the crystalline lens.2, 7, 27, 28 Age was not a significant factor in a univariate model of ciliary muscle ring diameter, probably due to the strong influence of refractive error on ciliary muscle ring diameter. In our multivariate model, ciliary muscle ring diameter decreased by 0.02 mm per year of age. This finding agrees with previous reports suggesting decreases in the ciliary muscle ring diameter of about 0.015 to 0.025 mm per year of age.30, 32 One group reported an increase in lens equatorial diameter with age7, 33 but this finding has not been replicated in other in vivo human studies,29, 32 nor was it found in this study. While the lens continues to grow in all dimensions with age, it is likely that the equatorial diameter growth is cancelled by the inward movement of the ciliary muscle, which allows the lens to further shorten in diameter and thicken with age.
This study also provided estimates of important biometric differences associated with refractive error in the adult human eye. The distribution of refractive error in our participants is representative of the current United States refractive error distribution.6 Consistent with previous MRI and ultrasound studies, myopic eyes were larger (longer axial length and deeper vitreous chamber).8, 34, 35 For each diopter of myopic refractive error, vitreous chamber height increased by about one-quarter of the increase in vitreous chamber depth, demonstrating that myopia is associated with axial elongation rather than global expansion. Also consistent with previous reports, myopic eyes showed steeper corneal and anterior lens curvatures.34–36 This is the first study to demonstrate the association of larger ciliary muscle ring diameter with myopic refractive error.
As illustrated in Figure 6, the effect size of one diopter of refractive error was comparable to that of one year of age on lens thickness, but one diopter of accommodative response produced twice the increase in lens thickness. Similarly, accommodation produced more than twice the decrease in lens equatorial diameter compared to refractive error, but refractive error had a much larger effect on ciliary muscle ring diameter than either age or accommodation. The unaccommodated ciliary muscle model suggests that a 40-year-old 3D myope would be predicted to have about a 12 mm ciliary muscle ring diameter, while a 60-year-old 3D hyperope would have about an 11 mm ciliary muscle ring diameter. Current accommodating IOLs range in diameter from about 9.5 to 12.0 mm.3–5 Based on these projections, a younger and/or myopic patient would have to exert more accommodative effort to achieve contact with the IOL to elicit the same change in focal power compared to an older and/or hyperopic patient.
Previous reports of per diopter changes in lens and ciliary muscle with accommodation are somewhat variable due to the fact that many studies calculated changes based on the accommodative demand rather than the accommodative response.1, 29, 30, 32 It is well established that most subjects do not fully accommodate to near demands and that there are differences in lag as a function of refractive error.2, 14, 16 With that in mind, it is not surprising that our parameter estimates are slightly higher than those reported previously.
While measurement of true accommodative response and cross-referencing of imaging methods strengthened this study, there are some limitations to consider. The most marked limitations are the relatively small hyperopic refractive error group and the tight age range. Further research is needed in older patients with a wider range of refractive errors to further inform the development of accommodating IOLs.
CONCLUSIONS
This study confirmed age- and refractive error-related differences in many ocular parameters. The data presented here are of value not only to the study of presbyopia, but also to the field of refractive error development. More importantly, we have provided the first human data on refractive error-related differences in ciliary muscle ring diameter and quantified the expected per diopter accommodative changes that can be elicited with accommodation. The results of this study suggest that accommodating IOLs may need to be available in more than one diameter to allow for maximum benefit. Studies of accommodating IOLs could use pre-operative refractive error, as a surrogate for ciliary muscle ring diameter, to control for differences in accommodating IOL performance. Moving forward, device manufacturers may want to develop a non-invasive, cost-effective method of measuring ciliary muscle ring diameter which could be used pre-operatively to provide a “customized” IOL fit.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health National Eye Institute K23 and LRP awards (K23-EY019097, L30-EY016018), and American Optometric Foundation Bausch + Lomb and Presidents Circle Ezell Fellowships. We would like to thank the following people for their assistance with imaging development and analysis for this project: Chiu-Yen Kao, PhD; Petra Schmalbrock, PhD; Peter Wassenaar, MS; Yuanjie Zheng, PhD; James Gee, PhD; Benjamin Backus, PhD; Melissa Bailey, OD PhD; Nidhi Satiani OD, MS; Samuel Patz, PhD, and Adrian Glasser, PhD. Portions of this work were presented at the 2013 American Academy of Optometry meeting.
REFERENCES
- 1.Strenk SA, Strenk LM, Guo S. Magnetic resonance imaging of aging, accommodating, phakic, and pseudophakic ciliary muscle diameters. J Cataract Refract Surg. 2006;32:1792–1798. doi: 10.1016/j.jcrs.2006.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richdale K, Sinnott LT, Bullimore MA, Wassenaar PA, Schmalbrock P, Kao CY, Patz S, Mutti DO, Glasser A, Zadnik K. Quantification of age-related and per diopter accommodative changes of the lens and ciliary muscle in the emmetropic human eye. Invest Ophthalmol Vis Sci. 2013;54:1095–1105. doi: 10.1167/iovs.12-10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtinger A, Rootman DS. Intraocular lenses for presbyopia correction: past, present, and future. Curr Opin Ophthalmol. 2012;23:40–46. doi: 10.1097/ICU.0b013e32834cd5be. [DOI] [PubMed] [Google Scholar]
- 4.Sheppard AL, Bashir A, Wolffsohn JS, Davies LN. Accommodating intraocular lenses: a review of design concepts, usage and assessment methods. Clin Exp Optom. 2010;93:441–452. doi: 10.1111/j.1444-0938.2010.00532.x. [DOI] [PubMed] [Google Scholar]
- 5.Charman WN. Developments in the correction of presbyopia II: surgical approaches. Ophthalmic Physiol Opt. 2014;34:397–426. doi: 10.1111/opo.12129. [DOI] [PubMed] [Google Scholar]
- 6.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127:1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 7.Atchison DA, Markwell EL, Kasthurirangan S, Pope JM, Smith G, Swann PG. Age-related changes in optical and biometric characteristics of emmetropic eyes. J Vis. 2008;8:29. doi: 10.1167/8.4.29. 1-0. [DOI] [PubMed] [Google Scholar]
- 8.Atchison DA, Jones CE, Schmid KL, Pritchard N, Pope JM, Strugnell WE, Riley RA. Eye shape in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2004;45:3380–3386. doi: 10.1167/iovs.04-0292. [DOI] [PubMed] [Google Scholar]
- 9.Chylack LT, Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111:831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfield M, Cohen AS. Repeatability of clinical measurements of the amplitude of accommodation. Ophthalmic Physiol Opt. 1996;16:247–249. [PubMed] [Google Scholar]
- 11.Antona B, Sanchez I, Barrio A, Barra F, Gonzalez E. Intra-examiner repeatability and agreement in accommodative response measurements. Ophthalmic Physiol Opt. 2009;29:606–614. doi: 10.1111/j.1475-1313.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- 12.Stark LR, Atchison DA. Subject instructions and methods of target presentation in accommodation research. Invest Ophthalmol Vis Sci. 1994;35:528–537. [PubMed] [Google Scholar]
- 13.Anderson HA, Stuebing KK. Subjective versus objective accommodative amplitude: preschool to presbyopia. Optom Vis Sci. 2014;91:1290–1301. doi: 10.1097/OPX.0000000000000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutti DO, Mitchell GL, Hayes JR, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. Accommodative lag before and after the onset of myopia. The CLEERE Study Group. Invest Ophthalmol Vis Sci. 2006;47:837–846. doi: 10.1167/iovs.05-0888. [DOI] [PubMed] [Google Scholar]
- 15.Lossing LA, Sinnott LT, Kao CY, Richdale K, Bailey MD. Measuring changes in ciliary muscle thickness with accommodation in young adults. Optom Vis Sci. 2012;89:719–726. doi: 10.1097/OPX.0b013e318252cadc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richdale K, Bullimore MA, Zadnik K. Lens thickness with age and accommodation by optical coherence tomography. Ophthalmic Physiol Opt. 2008;28:441–447. doi: 10.1111/j.1475-1313.2008.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutti DO, Zadnik K, Adams AJ. A video technique for phakometry of the human crystalline lens. Invest Ophthalmol Vis Sci. 1992;33:1771–1782. [PubMed] [Google Scholar]
- 18.Richdale K, Wassenaar P, Teal Bluestein K, Abduljalil A, Christoforidis JA, Lanz T, Knopp MV, Schmalbrock P. 7 Tesla MR imaging of the human eye in vivo. J Magn Reson Imaging. 2009;30:924–932. doi: 10.1002/jmri.21959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kao CY, Richdale K, Sinnott LT, Grillott LE, Bailey MD. Semiautomatic extraction algorithm for images of the ciliary muscle. Optom Vis Sci. 2011;88:275–289. doi: 10.1097/OPX.0b013e3182044b94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y, Gee J, Backus B, Richdale K. Automated measurement of lens thickness using optical coherence tomography. Optom Vis Sci. 2013;92 E-abstract 135880. [Google Scholar]
- 21.Lossing LA, Richdale K, Sinnott LT, Bailey MD. Changes in ciliary body thickness with accommodation. Optom Vis Sci. 2009;86 doi: 10.1097/OPX.0b013e318252cadc. E-abstract 95815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 23.Ostrin L, Kasthurirangan S, Win-Hall D, Glasser A. Simultaneous measurements of refraction and A-scan biometry during accommodation in humans. Optom Vis Sci. 2006;83:657–665. doi: 10.1097/01.opx.0000232810.61191.02. [DOI] [PubMed] [Google Scholar]
- 24.Hofstetter HW. Useful age-amplitude formula. Optom World. 1950;38:42–45. [Google Scholar]
- 25.Duane A. Normal values of the accommodation of all ages. JAMA. 1912;59:1010–1013. [Google Scholar]
- 26.Tehrani M, Krummenauer F, Kumar R, Dick HB. Comparison of biometric measurements using partial coherence interferometry and applanation ultrasound. J Cataract Refract Surg. 2003;29:747–752. doi: 10.1016/s0886-3350(02)01739-x. [DOI] [PubMed] [Google Scholar]
- 27.Koretz JE, Strenk SA, Strenk LM, Semmlow JL. Scheimpflug and high-resolution magnetic resonance imaging of the anterior segment: a comparative study. J Opt Soc Am (A) 2004;21:346–354. doi: 10.1364/josaa.21.000346. [DOI] [PubMed] [Google Scholar]
- 28.Koretz JF, Kaufman PL, Neider MW, Goeckner PA. Accommodation and presbyopia in the human eye--aging of the anterior segment. Vision Res. 1989;29:1685–1692. doi: 10.1016/0042-6989(89)90150-8. [DOI] [PubMed] [Google Scholar]
- 29.Jones CE, Atchison DA, Pope JM. Changes in lens dimensions and refractive index with age and accommodation. Optom Vis Sci. 2007;84:990–995. doi: 10.1097/OPX.0b013e318157c6b5. [DOI] [PubMed] [Google Scholar]
- 30.Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. MRI study of the changes in crystalline lens shape with accommodation and aging in humans. J Vis. 2011;11:19, 1–6. doi: 10.1167/11.3.19. [DOI] [PubMed] [Google Scholar]
- 31.Dubbelman M, Van der Heijde GL. The shape of the aging human lens: curvature, equivalent refractive index and the lens paradox. Vision Res. 2001;41:1867–1877. doi: 10.1016/s0042-6989(01)00057-8. [DOI] [PubMed] [Google Scholar]
- 32.Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest Ophthalmol Vis Sci. 1999;40:1162–1169. [PubMed] [Google Scholar]
- 33.Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation. Invest Ophthalmol Vis Sci. 2008;49:2531–2540. doi: 10.1167/iovs.07-1443. [DOI] [PubMed] [Google Scholar]
- 34.Goss DA, Van Veen HG, Rainey BB, Feng B. Ocular components measured by keratometry, phakometry, and ultrasonography in emmetropic and myopic optometry students. Optom Vis Sci. 1997;74:489–495. doi: 10.1097/00006324-199707000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Olsen T, Arnarsson A, Sasaki H, Sasaki K, Jonasson F. On the ocular refractive components: the Reykjavik Eye Study. Acta Ophthalmol Scan. 2007;85:361–366. doi: 10.1111/j.1600-0420.2006.00847.x. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez Blanco F, Sanz Fernandez JC, Munoz Sanz MA. Axial length, corneal radius, and age of myopia onset. Optom Vis Sci. 2008;85:89–96. doi: 10.1097/OPX.0b013e3181622602. [DOI] [PubMed] [Google Scholar]