Abstract
Aims
To test whether drinking onset moderates genetic and environmental contributions to individual differences in the etiology of alcohol expectancies across adolescence.
Design
Longitudinal twin design.
Setting
Community sample from Los Angeles, CA, USA.
Participants
A total of 1292 male and female twins, aged 11–18 years, were assessed at 1 (n = 440), 2 (n = 587) or 3 (n = 265) occasions as part of the risk factors for the Antisocial Behavior Twin Study.
Measurements
Social behavioral (SB) alcohol expectancies were measured using an abbreviated version of the Social Behavioral subscale from the Alcohol Expectancy Questionnaire for adolescents (AEQ-A). Drinking onset was defined as >1 full drink of alcohol.
Findings
Alcohol expectancies increased over age and the increase became more rapid following onset of drinking. The importance of genetic and environmental influences on SB scores varied with age and drinking status, such that variation prior to drinking onset was attributed solely to environmental influences, whereas all post-onset variation was attributed to genetic influences. Results did not differ significantly by sex.
Conclusion
Only environmental factors explain beliefs about the social and behavioral consequences of alcohol use prior to drinking onset, whereas genetic factors explain an increasing proportion of the variance in these beliefs after drinking onset.
Keywords: Adolescence, alcohol expectancies, drinking motives, genetic, drinking onset, longitudinal, twin
INTRODUCTION
Alcohol expectancies, or the anticipated behavioral, cognitive and emotional consequences of alcohol use, are associated strongly with adolescent drinking onset, level of consumption and alcohol-related problems both cross-sectionally and longitudinally [1]. Alcohol expectancies develop in early childhood, well before substantial personal experience with alcohol [2–4], and strengthen from childhood into adolescence [5,6] corresponding with age-related increases in drinking [7,8]. Expectations that alcohol will facilitate social interactions (social behavior expectancies) are especially predictive of the onset of alcohol use and problematic drinking among adolescents [8–13]. Although the importance of alcohol expectancies in the initiation and maintenance of drinking is evident, little is known about the mechanisms that contribute to the changes in these cognitive processes across development. Identifying the genetic and environmental etiology of alcohol expectancies across adolescence is a critical research priority, as this developmental period is associated with the onset of alcohol consumption, binge drinking and risk for the development of alcohol use disorders [14–16]. In the present study, we examine the role of genetic and environmental factors in initial level and change in social behavior alcohol expectancies from early to late adolescence, and test whether drinking onset moderates these processes.
Alcohol expectancies initially develop via an accumulation of environmental factors (e.g. family, peers, media) that provide a framework for drinking [17]. Family history of alcoholism is associated with positive alcohol expectancies [18–22], and at least some of the familial effect appears to be mediated via environmental factors (e.g. via social learning and modeling), as indicated by higher positive expectancies among adolescents who have both a family history of alcoholism and environmental exposure to an alcoholic family member [19]. Additionally, personal experience with alcohol has been shown to strengthen alcohol expectancies differentially [17,23], and biological influences may become more important predictors of individual differences in expectancies following drinking onset [9,24]. For example, genetically influenced individual differences in physiological responses (e.g. hedonic effects, ethanol sensitivity) might render certain individuals more reactive than others to the pharmacological effects of alcohol, altering future expectations about the consequences of drinking. Consistent with this hypothesis, alcohol metabolizing genes and levels of response to alcohol are associated with alcohol expectancies among drinkers [25,26], and their effects on risk for various drinking outcomes may be mediated partially by alcohol expectancies [27–30]. Further, while expectancies have been attributed predominantly to environmental factors among adolescents [31], twin studies of adult drinkers indicate that positive alcohol expectancies and drinking motives are also influenced by genetic factors [32–34].
Despite theoretical and practical reasons to hypothesize that the sources of familial influences that contribute to individual differences in alcohol expectancies vary with drinking status, few studies have evaluated this question. Using data from 2627 female twins aged 14–22 years from the Missouri Adolescent Female Twin Study (MOAFTS), Slutske et al. [31] found that positive alcohol expectancies for affect regulation and performance enhancement were influenced primarily by environmental factors, rather than genetic influences. However, among the minority of participants who were regular drinkers, there was some evidence for heritable influences on expectancies about performance enhancement. Drinking status analyses were restricted to twin pairs concordant for drinking or abstaining, which removes variance that is overlapping between becoming a drinker and alcohol expectancies and limits interpretation of drinking status results. Subsequently, a study of 3656 twins aged 18–29 years from the MOAFTS found no evidence for heritable influences on positive alcohol expectancies in the overall sample or in analyses stratified by drinking status [35].
In summary, despite consistent evidence for the importance of alcohol expectancies in drinking behaviors, relatively little attention has been paid to the genetic and environmental mechanisms underlying the development of alcohol expectancies across adolescence. If drinking onset potentiates the behavioral expression of genetic influences on alcohol expectancies, greater genetic variance and higher heritability estimates for alcohol expectancies are expected after the onset of alcohol use. Previous twin research that has examined heritability of alcohol expectancies by drinking status is limited to two cross-sectional examinations of females from the MOAFTS study that did not include males or take into account inter- or intra-individual changes in alcohol expectancies over time.
Addressing key gaps in the literature, the current study aims to: (a) test how genetic and environmental factors contribute to individual differences in both level and change in social behavior alcohol expectancies in a sample of 1292 monozygotic (MZ) and dizygotic (DZ) male and female twins aged 11–18 years; and (b) test whether sex and drinking experience moderates these effects. To our knowledge, this is the first study to examine the role of genetic and environmental factors in the trajectories of social behavior alcohol expectancies across adolescence.
METHOD
Participants
The participants in this study were drawn from the University of Southern California Risk Factors for Antisocial Behavior Twin Study (RFAB), a prospective study of the interplay of biological, genetic, environmental and social factors on the development of aggressive and antisocial behavior. To maximize generalizability, there were few inclusion criteria: age of the twins (9–10 years), English proficiency and availability to participate in a 6–8-hour laboratory assessment. Participating families were recruited from the Los Angeles community, and the sample is representative of the ethnic and socio-economic diversity of the greater Los Angeles area, with 28.6% Caucasian, 34.3% Hispanic, 13.1% African American, 4.1% Asian, 17.6% mixed and 2.3% other [36]. Of the participating youth, 22% are monozygotic male, 22% are monozygotic female, 15% are dizygotic males, 15% are dizygotic females and 26% are dizygotic male–female twins. Four waves of data collection have been completed to date: wave 1 (W1: age 9–10) 614 pairs; wave 2 (W2: age 11–13) 445 pairs; wave 3 (W3: age 14–15) 604 pairs; and wave 4 (W4: age 16–18) 504 pairs. The large variation in ns across waves occurred in part because not all subjects participated in all waves and new participants were recruited at W3 (166 new families) [36]. Of the 614 original W1 families, 72% (n = 445) returned for W2, 71% (n = 438) returned for W3 and 59% (n = 365) returned for W4. Of the original 614 families, 529 (86%) participated in ≥ one subsequent wave. Attrition analyses indicated that Caucasians were less likely to drop out of the study than other racial/ethnic groups [odds ratio (OR) = 0.70, 95% confidence interval (CI) = 0.50–0.99]; however, study dropout was not associated with substance use or other psychopathology of twins or parents. Complete details on the study protocol can be found elsewhere [36].
The present study used data from W2–4 as alcohol expectancies were not assessed at W1. Data on alcohol expectancies were available for 666 twins at W2 [mean age=11.79, standard deviation (SD) = 0.92], 990 twins at W3 (mean age = 14.87, SD = 0.87) and 753 twins at W4 (mean age = 17.28, SD = 0.77). The present study used all available data on expectancies for a total of 2409 observations from 1292 individuals (49% male). Of these unique participants, 440 participated in one wave, 587 participated in two waves and the remaining 265 participated in three waves. Twin pairs with data from only one wave contribute to the twin pair correlations at that wave, and the inclusion of individuals who have a single occasion of measurement reduces biases from only including people with complete data.
Measures
Alcohol expectancies
Participants completed an abbreviated version of the Alcohol Expectancy Questionnaire–Adolescent Version (AEQ-A) at W2–4, based on the original 100-item AEQ-A [17]. The abbreviated version of AEQ-A was developed for RFAB prior to W2 using factor analysis to select the five questions that loaded most strongly on each subscale. The AEQ-A has good validity [37–39], and is used commonly to assess alcohol expectancies among adolescents. The measure utilizes a five-point Likert scale (1=‘disagree strongly’ to 5=‘agree strongly’). Consistent with previous studies [40], we focused solely on the Changes in Social Behavior subscale (SB), as expectations about the social outcomes of alcohol use are associated most strongly with alcohol consumption [8–13]. The retained SB questions were: ‘People become harder to get along with after they have a few drinks of alcohol’ (reverse-coded), ‘Drinking alcohol is OK because it allows people to join in with others who are having fun’, ‘Alcoholic beverages make parties fun’, ‘Drinking alcohol makes people friendlier’ and ‘People feel more caring and giving after a few drinks of alcohol’. SB scores were calculated by averaging the scores of the five questions (internal consistency: W2 α = 0.56, W3 α = 0.53, W4 α = 0.54). Mean SB scores and standard deviations increased over time (Table 1).
Table 1.
Social behavior alcohol expectancies [mean (standard deviation)] by age, sex and drinking status in 1292 adolescent twins measured up to three times from ages 11 to 18 years.
| Males |
Females |
|||
|---|---|---|---|---|
| Non drinkers | Post-onset | Non drinkers | Post-onset | |
| Age 11 | 1.95 (0.80) n = 88 | 2.10 (0.66) n = 56 | 1.98 (0.63) n=113 | 2.25 (0.77) n = 39 |
| Age 12 | 2.02 (0.68) n = 68 | 2.09 (0.79) n = 37 | 1.85 (0.66) n = 80 | 2.09 (0.69) n = 44 |
| Age 13 | 2.20 (0.75) n = 23 | 2.17 (0.86) n = 24 | 2.20 (0.63) n = 13 | 2.04 (0.65) n = 30 |
| Age 14 | 2.11 (0.66) n = 135 | 2.49 (0.75) n = 113 | 1.97 (0.63) n = 129 | 2.45 (0.74) n = 147 |
| Age 15 | 1.98 (0.69) n = 57 | 2.52 (0.78) n = 102 | 2.10 (0.70) n = 61 | 2.52 (0.75) n = 107 |
| Age 16 | 2.34 (0.63) n = 38 | 2.65 (0.82) n = 91 | 2.09 (0.70) n = 30 | 2.92 (0.74) n = 88 |
| Age 17 | 2.39 (0.84) n = 48 | 2.80 (0.74) n = 118 | 1.87 (0.61) n = 57 | 2.82 (0.85) n =150 |
| Age 18+ | 2.22 (0.80) n = 34 | 2.86 (0.79) n = 104 | 2.51 (0.85) n = 28 | 2.85 (0.87) n =102 |
Drinking status
At each wave, twins answered three items asking whether they: (i) had ever consumed any alcohol, (ii) ever had more than just a couple of sips of alcohol at one time and (iii) ever had a whole drink or more of alcohol at once. Drinking status was classified based on item (iii), ever having had a full drink and participants who endorsed drinking were considered ever drinkers at all subsequent waves. The percentage of drinkers in the sample increased with age from 32% (age 11) to 77% (age 18+) (Table 1).
Data analysis
Multi-level regression analyses were conducted using PROC MIXED in SAS [41] to characterize the phenotypical (within-person) developmental trajectory of SB over age and reflecting changes in drinking status, while accounting for the nested structure of the data (i.e. occasions within twins within families). We used age at assessment rather than measurement occasion as the time basis, because the intervals between waves are not equal across individuals. This leads effectively to collective observation of all age points from 11 to 18 years. We initially fitted models allowing non-linear functions of age, but after including drinking status, we found age-related change was captured by a linear function.
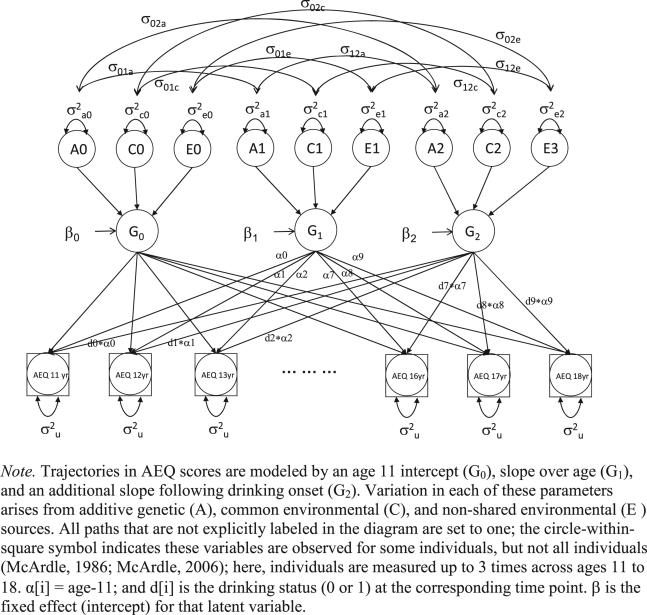
We estimated the genetic and environmental contributions to individual differences in SB trajectories over age using biometric latent growth curve analyses (LGCA) [42] based on the twin-pair data. We fitted two types of biometric LCGA following standard methodology for multivariate twin models. A standard, single-slope model represented the developmental trajectory of SB score ignoring drinking status. SB trajectories were represented by two latent factors: level (G0) and rate of change (G1), with their mean values denoted by intercept (β0) and age-based slope (β1) values, respectively. The basis coefficients α[t] are weights used to represent the function of time for the observations. A second slope factor (G2) was included in some models to represent the change in the slope of SB after drinking onset. The two-slope model is depicted in Fig. 1. The product term d[t]*α[t] is the basis coefficient for each specific age, where α[t] = age-11 and d[t] = 1 if the subject drank by the ith age but 0 if he/she did not. This effectively fits a spline model to each person's trajectory, placing the inflection point at the age of drinking onset. In addition to the parameters estimated in the one-slope biometric LGCA model, parameters associated with the second slope are the post-onset change in SB (denoted by β2, the intercept of G2), the slope of SB beyond that associated with age, and the partitioning of the covariance of the post-onset and pre-onset scores (represented by the covariance of G2 with G1 and G0) (see Appendix in the Supporting information for model estimation details).
Figure 1.
Two-slope biometric latent growth model for social behavior alcohol expectancies
Using the twin-pair information, variation and covariation among the latent factors (G0, G1, G2) is partitioned into three sources: additive genetic variation (A) from genes whose allelic effects combine additively; common environment (C), which includes all environments that make twin pairs similar (e.g. family socio-economic status); and non-shared environment (E), which includes environmental factors that make twins dissimilar (e.g. different friends) and measurement error. The model also includes time-specific residual variation, which we equated across age. MZ twins are assumed to share all their genetic and shared environmental factors, whereas DZ twins resemble each other because they share on average half their genetic factors and their entire shared environment. Comparing the resemblance of MZ and DZ twins allows for the estimation of each source's contribution to individual differences in SB scores.
We began with a least restrictive two-slope model that included ACE contributions to SB level and both slopes and allowed sex differences on all parameters. The fit of this model was compared to fits of more restrictive models to evaluate the strength of the evidence for the second slope, sex differences in the genetic and environmental parameters and alternative genetic and environmental structures for individual differences in the SB trajectory components (intercept and two slopes).
Alternative models were compared using the Bayesian information criterion (BIC) index and the difference in the log-likelihood of nested models, which is distributed as a χ2 statistic. The biometric latent growth models were fitted to raw data using maximum likelihood estimation with Mplus [43]. All available AEQ data were used, regardless of completeness across waves or members of the twin pair.
RESULTS
Phenotypical multi-level models
Descriptive statistics on SB scores by sex and drinking status are presented in Table 1. Overall, SB scores were higher at later waves and higher among drinkers than non-drinkers.
Results from three multi-level regression models fitted to the individual SB data are shown in Table 2: model A included age as the only predictor, model B added the main effect of drinking status and model C added the age × drinking status interaction. Including drinking status significantly improved the model fit, as did including the interaction, and much of the age effect observed in models A and B was due to older adolescents being more likely to drink. The predicted trajectory of SB score differed across drinkers and non-drinkers, with an immediate increase of 0.08 points associated with onset and a steeper increase annually among drinkers (0.13) than non-drinkers (0.06).
Table 2.
Results from multi-level regression analyses of social behavior alcohol expectancies on age and drinking status in 1292 adolescent twins measured up to three times from ages 11 to 18 years.
| Model A | Model B | Model C | |
|---|---|---|---|
| Intercept | 1.93 (0.033)* | 1.85 (0.034)* | 1.95 (0.041)* |
| Age (years) | 0.12 (0.007)* | 0.10 (0.008)* | 0.06 (0.011)* |
| Drinking status | (–) | 0.29 (0.034)* | 0.08 (0.056) |
| Age × drinking status | (–) | (–) | 0.07 (0.014)* |
| –2LL | 5157.4 | 4979.6 | 4952.8 |
| Δχ2/df | (–) | 177.8/1 | 26.8/1 |
Results based on 2350 observations from 1292 individuals. Drinking status is coded 1 if the subject reported drinking onset prior to that assessment and 0 otherwise. Age is centered around 11 years. – = parameter not included in model. Δχ2 is the difference in fit relative to the prior model.
Significance at P<0.05 level.
Multi-level regression was used to account for the nesting of multiple occasions within twins and of twins within pairs.
Twin models
Twin pairs with the same drinking status were more similar in their AEQ scores. Pooling across waves, correlations for AEQ SB scores were r = 0.56 for MZ pairs who were both drinkers, r = 0.39 for MZ pairs who were discordant for drinking status and r = 0.47 for MZ pairs who were both abstainers. The corresponding values in DZ twins were r = 0.39, r = 0.18 and r = 0.41, respectively. This pattern is consistent with family environmental (C) contributions to SB trajectories prior to drinking onset (i.e. similar correlations in MZ and DZ pairs), with genetic influences after onset (i.e. greater correlations in MZ than DZ pairs).
Table 3 shows results from the biometric LGCA models. The fit of the two-slope model (model 2) was significantly better than that of the one-slope model (model 1), indicating that the post-onset slope was needed to represent individual differences in SB trajectories, so this was retained in subsequent models. There was little evidence for sex differences (model 3 versus 1), and all parameters were equated over sex in remaining models. Model 4 tested whether new variation in SB associated with drinking onset was attributable to genetic and non-shared environmental sources (AE), and this model fitted well. Comparing model 5 versus 4 tested whether variation in SB prior to onset was attributable to shared and non-shared environmental but not genetic sources (CE), and this was also confirmed. A final model that evaluated whether all new post-onset variation was attributable to genetic effects did not worsen the fit (model 6 versus 5).
Table 3.
Comparison of models representing alternative structures for individual differences in social behavior alcohol expectancies.
| Estimated sources of variation |
Model fit |
χ2 Difference test |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Hypothesis tested | Level and pre-onset slope | Post-onset slope | –2LL | BIC | #free param | Compare to model | Δ χ 2 | Δdf | P |
| 1 Two slopes, sexes differ on score mean, variance and slopes | ACE (m,f) | ACE (m,f) | 2115.1 | 2350.8 | 44 | – | – | – | – |
| 2 One slope, sexes differ | ACE (m,f) | – | 2182.1 | 2310.6 | 24 | 1 | 67.0 | 20 | <0.01 |
| 3 Two slopes, no sex differences | ACE | ACE | 2137.4 | 2255.3 | 22 | 1 | 22.3 | 22 | 0.44 |
| 4 No common environment influence on post-onset slope | ACE | AE | 2138.7 | 2240.4 | 19 | 3 | 1.26 | 3 | 0.74 |
| 5 No genetic influences before onset | CE | AE | 2140.5 | 2215.5 | 14 | 4 | 1.84 | 5 | 0.87 |
| 6 All new variation post-onset is genetic | CE | A | 2140.5 | 2199.4 | 11 | 5 | <0.1 | 3 | 0.99 |
Level = intercept of expectancy score at age 11 years; pre-onset slope= expectancy slope over age prior to drinking onset; post-onset slope = expectancy slope over age after drinking onset; #free param= number of estimated parameters; A = additive genetic variance, C = shared environmental variance, E = non-shared environmental variance; (m,f) = parameters estimated separately for males and females; otherwise parameters are equated over sex. Analyses based on 2350 observations on 1292 individuals from 682 twin pairs. BIC = Bayesian information criterion.
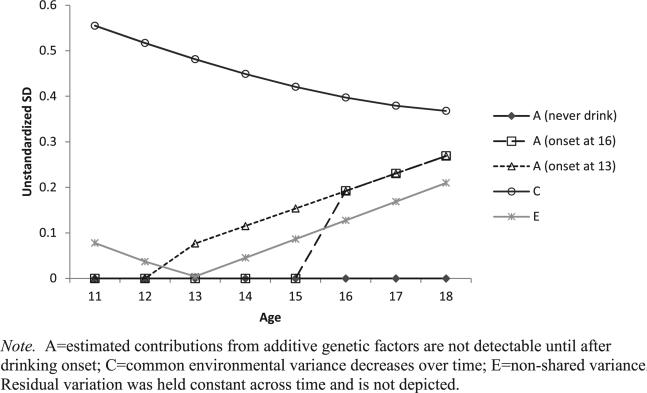
Parameter estimates for model 6 (Table 3) based on the Cholesky decomposition of variance–covariance matrix are provided in Appendix S1. The expected genetic, shared environmental and non-shared environmental contributions to individual differences in SB scores from ages 11 to 18 based on model 6 are plotted in Fig. 2. The predictions differed by component, age and drinking status, and we show separate curves for three scenarios: non-drinkers to age 18 and individuals with drinking onsets at ages 13 and 16. Shared environmental effects decreased with age whereas non-shared environmental effects increased with age, and neither depended on drinking status. In contrast, the predicted genetic influences on SB were negligible while individuals were non-drinkers, but increased more steeply following drinking onset.
Figure 2.
Sources of variation underlying age trajectories of social behavior alcohol expectancies by drinking onset age based on the two-slope biometric latent growth model
DISCUSSION
There were three key findings in the current study. First, both the mean and variation in SB alcohol expectancies increased with age, and there was an interaction between age and drinking onset, such that alcohol expectations increased more quickly following drinking onset. This finding builds on previous research indicating that alcohol expectancies strengthen from childhood into adolescence [5,6] and with drinking experience [17,23]. Secondly, there were no sex differences in level or change in SB alcohol expectancies over time. Thirdly, results from biometric growth curve models indicated that genetic and environmental influences on social behavior alcohol expectancies varied over time as a function of both age and drinking status.
Prior to drinking onset, variation in expectancies was attributable almost completely to environmental influences shared by twin pairs (96%). Across adolescence, environmental factors that were not shared by twins became more important, accounting for 39% of the variation associated with age-related trajectory. Individual differences associated with drinking onset were explained completely by genetic influences. Among drinkers, genetic influences explained an increasingly greater proportion of the variance in social behavior alcohol expectancies, such that by age 18 the estimated genetic influence is nearly equal to that of shared environmental influences (Fig. 2). This finding is consistent with the hypothesis that genetically influenced individual differences in physiological responses to alcohol (e.g. metabolism, ethanol sensitivity) come into play following drinking experience and alter future expectations about the expected effects of alcohol on social behavior.
Importantly, the contribution of the shared environmental influences to social behavior alcohol expectancies was strong regardless of drinking status. Thus, environmental factors that contribute to similarity of a twin pair for drinking (e.g. cultural beliefs, rearing influences, shared friends) continue to play a role in expectations about the effects of alcohol on social behavior across adolescence. Further, non-shared environmental factors were increasingly important predictors of individual differences in social behavior alcohol expectancies over time, indicating that twins are growing apart in their expectancies about alcohol, possibly reflecting a divergence in their drinking experiences and peer groups.
These results build on cross-sectional findings from the MOAFTS study which indicated that genetic factors may be a more important cause of individual differences in expectations that alcohol will enhance cognitive and general performance among drinkers than among non-drinkers in a sample of female twins aged 14–22 [31]. However, our results are inconsistent with a subsequent MOAFTS study of female twins aged 18–29, which found no evidence for heritable influences on positive alcohol expectancies overall or in analyses stratified by drinking status [35]. Our study included younger twins aged 11–18 (male and female) and took into account changes in alcohol expectancies and drinking status over time, possibly accounting for these differences in findings. Additional twin studies with older adolescents will help to understand further the mechanisms underlying the development of social behavior alcohol expectancies as adolescents enter adulthood and move away from their families.
Results from the current study are also parallel with findings from other behavior genetic studies indicating that the onset and course of drinking behaviors in early adolescence is largely attributable to environmental influences shared by siblings [44], whereas genetic factors explain an increasingly greater proportion of the variance as individuals grow older and drinking experience increases [45,46]. In the current study, increases in heritability of social behavior alcohol expectancies across development were due to new genetic variability, rather than a reduction in the influence of environmental factors over time. It is possible that genetic influences on social behavior alcohol expectancies following drinking onset may overlap with genetic influences on drinking onset, and it remains to be seen whether delaying age at first drink would alter the relative effects of genetic and environmental factors on alcohol expectancies.
Results from the current study should be interpreted within the context of several potential limitations. First, data on alcohol use and alcohol expectancies are based on self-report, which may limit their accuracy. However, confidential youth self-reports of alcohol use tend to be reliable and valid [47,48] and our data collection procedures are designed to ensure high confidentiality (e.g. twins are interviewed separately by different research staff away from their parents). Secondly, as is true for all longitudinal studies, we had some attrition across the three waves of data collection, but attrition was not associated with alcohol expectancy scores or drinking status. Thirdly, the abbreviated version of the AEQ-A utilized in the current study had somewhat low internal consistency, and expectancies prior to drinking onset are unknown for those who began drinking prior to their first assessment. Fourthly, we did not have a large enough sample to test for racial/ethnic differences in twin modeling analyses. Fifthly, there are several assumptions embedded in twin models, including the equal environment assumption, that monozygotic twins are no more likely than dizygotic twins to share environmental factors that are etiologically relevant to the phenotype being studied. To the extent that higher correlations among MZ than DZ pairs are due to more similar environments, genetic influence will be overestimated. Another assumption is random mating with respect to the outcome studied; failure of this assumption leads to underestimations of genetic influence. Finally, our study focused on social behavior alcohol expectancies and drinking onset, and we did not include other domains of positive alcohol expectancies (e.g. tension reduction), negative alcohol expectancies, variables representing parental or peer alcohol use or measures of drinking frequency/quantity. Additional studies of adolescent twins will help to elucidate further the mechanisms through which drinking behaviors moderate genetic influences on the development of additional alcohol expectancy domains.
In summary, results from this longitudinal twin study indicated that prior to drinking onset, individual differences in social behavioral alcohol expectancies from childhood to adolescence were accounted for primarily by social learning and environmental factors shared by siblings (e.g. home environment, cultural beliefs), and these declined in importance across time. In contrast, the influence of non-shared environmental factors that make siblings divergent in their alcohol expectancies (e.g. different friends) increased as adolescents aged and developed their own expectancies about the effects of alcohol on social behavior. Genetic influences were negligible until individuals had experience with alcohol, after which they explained an increasingly greater proportion of the variation in social behavior alcohol expectancies. Taken together, findings suggest that genetic variance in expectations about the positive effects of alcohol on social interactions may be uncovered as adolescents begin drinking alcohol and experience its physiological and subjective effects.
Supplementary Material
Acknowledgement
This study was funded by grants R01 MH58354, T32 HL007034-37 and F31 AA018611. Data collection was supported in part by a Dissertation Award to K.C.Y.W. from the Society for Multivariate Experimental Psychology. We thank the Southern California Twin Project staff for their assistance in collecting data, and the twins and their families for their participation.
Footnotes
Declaration of interests
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Appendix S1 Parameter estimates for model 6 in Table 3 based on Cholesky decomposition of variance–covariance matrix.
References
- 1.Goldman MS, Boca FK, Darkes J. Alcohol expectancy theory: the application of cognitive neuroscience. In: Leonard KE, Blaine HT, editors. Psychological Theories of Drinking and Alcoholism. 2nd edn. Guilford Press; New York, NY: 1999. pp. 203–46. [Google Scholar]
- 2.Lang A, Stritzke W. Young children's knowledge, attitudes, and expectations about alcohol. In: Galanter M, editor. Recent Developments on Alcoholism. Vol. 11. Plenum Press; New York: 1993. pp. 73–85. [DOI] [PubMed] [Google Scholar]
- 3.Miller PM, Smith GT, Goldman MS. Emergence of alcohol expectancies in childhood: a possible critical period. J Stud Alcohol. 1990;51:343–9. doi: 10.15288/jsa.1990.51.343. [DOI] [PubMed] [Google Scholar]
- 4.Zucker RA, Kincaid SB, Fitzgerald HE, Bingham RC. Alcohol schema acquisition in preschoolers: differences between children of alcoholics and children of nonalcoholics. Alcohol Clin Exp Res. 1996;19:1011–17. doi: 10.1111/j.1530-0277.1995.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 5.Christiansen BA, Goldman MS, Brown SA. The differential development of adolescent alcohol expectancies may predict adult alcoholism. Addict Behav. 1985;10:299–306. doi: 10.1016/0306-4603(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 6.Cumsille PE, Sayer AG, Graham JW. Perceived exposure to peer and adult drinking as predictors of growth in positive alcohol expectancies during adolescence. J Consult Clin Psychol. 2000;68:531–6. [PubMed] [Google Scholar]
- 7.Bauman KE, Fisher LA, Bryan EES, Chenoweth RL. Relationships between subjective expected utility and behavior: a longitudinal study of adolescent drinking behavior. J Stud Alcohol. 1985;46:32–8. doi: 10.15288/jsa.1985.46.32. [DOI] [PubMed] [Google Scholar]
- 8.Smith GT, Goldman MS, Greenbaum PE, Christiansen BA. Expectancy for social facilitation from drinking: the divergent paths of high-expectancy and low-expectancy adolescents. J Abnorm Psychol. 1995;104:32–40. doi: 10.1037//0021-843x.104.1.32. [DOI] [PubMed] [Google Scholar]
- 9.Christiansen BA, Goldman MS, Inn A. Development of alcohol-related expectancies in adolescents: separating pharmacological from social learning influences. J Consult Clin Psychol. 1982;50:336–44. doi: 10.1037//0022-006x.50.3.336. [DOI] [PubMed] [Google Scholar]
- 10.Christiansen BA, Smith GT, Roehling PV, Goldman MS. Using alcohol expectancies to predict adolescent drinking behavior after one year. J Consult Clin Psychol. 1989;57:93–9. doi: 10.1037//0022-006x.57.1.93. [DOI] [PubMed] [Google Scholar]
- 11.Kline RB. Eight-month predictive validity and covariance structure of the Alcohol Expectancy Questionnaire for Adolescents (AEQ-A) for junior high school students. J Stud Alcohol. 1996;57:396–405. doi: 10.15288/jsa.1996.57.396. [DOI] [PubMed] [Google Scholar]
- 12.Reese FL, Chassin L, Molina BSG. Alcohol expectancies in early adolescents: predicting drinking behavior from alcohol expectancies and parental alcoholism. J Stud Alcohol. 1994;55:276–84. doi: 10.15288/jsa.1994.55.276. [DOI] [PubMed] [Google Scholar]
- 13.Leigh BC, Stacy AW. Alcohol outcome expectancies: scale construction and predictive utility in higher order confirma-tory models. Psychol Assess. 1993;5:216–29. [Google Scholar]
- 14.Brown SA, McGue M, Magos J, Schulenberg J, Hingson R, Swartzwelder S, et al. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121:S290–310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–7. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masten AS, Faden VB, Zucker R, Spear LP. Underage drinking: a developmental framework. Pediatrics. 2008;121:S235–51. doi: 10.1542/peds.2007-2243A. [DOI] [PubMed] [Google Scholar]
- 17.Brown SA, Creamer VA, Stetson BA. Adolescent alcohol expectancies in relation to personal and parental drinking patterns. J Abnorm Psychol. 1987;96:117–21. doi: 10.1037//0021-843x.96.2.117. [DOI] [PubMed] [Google Scholar]
- 18.Brown SA, Tate SR, Vik PW, Haas AL, Aarons GA. Modeling of alcohol use mediates the effect of family history of alcoholism on adolescent alcohol expectancies. Exp Clin Psychopharmacol. 1999;7:20–7. doi: 10.1037//1064-1297.7.1.20. [DOI] [PubMed] [Google Scholar]
- 19.Mann LM, Chassin L, Sher KJ. Alcohol expectancies and the risk for alcoholism. J Consult Clin Psychol. 1987;55:411–17. doi: 10.1037//0022-006x.55.3.411. [DOI] [PubMed] [Google Scholar]
- 20.Lundahl LH, Davis TM, Adesso VJ, Lukas SE. Alcohol expectancies: effects of gender, age, and family history of alcoholism. Addict Behav. 1997;22:115–25. doi: 10.1016/s0306-4603(96)00022-6. [DOI] [PubMed] [Google Scholar]
- 21.Handley ED, Chassin L. Intergenerational transmission of alcohol expectancies in a high-risk sample. J Stud Alcohol Drugs. 2009;70:675–82. doi: 10.15288/jsad.2009.70.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sher KJ, Wood MD, Wood PK, Raskin G. Alcohol outcome expectancies and alcohol use: a latent variable cross-lagged panel study. J Abnorm Psychol. 1996;105:561–74. doi: 10.1037/0021-843X.105.4.561. [DOI] [PubMed] [Google Scholar]
- 23.Goldman MS, Brown SA, Christiansen BA. Expectancy theory: thinking about drinking. In: Blane HT, Leonard KE, editors. Psychological Theories of Drinking and Alcoholism. Guilford Press; New York: 1987. pp. 181–226. [Google Scholar]
- 24.Rohsenow DJ. Drinking habits and expectancies about alcohol's effects for self versus others. J Consult Clin Psychol. 1983;51:752–6. doi: 10.1037//0022-006x.51.5.752. [DOI] [PubMed] [Google Scholar]
- 25.Hahn CY, Huang SY, Ko HC, Hsieh CH, Lee IH, Yeh TL, et al. Acetaldehyde involvement in positive and negative alcohol expectancies in Han Chinese persons with alcoholism. Arch Gen Psychiatry. 2006;63:817–23. doi: 10.1001/archpsyc.63.7.817. [DOI] [PubMed] [Google Scholar]
- 26.Ehlers C, Carr L, Betancourt M, Montane-Jaime K. Association of the ADH2*3 allele with greater alcohol expectancies in African American young adults. J Stud Alcohol. 2003;64:176–81. doi: 10.15288/jsa.2003.64.176. [DOI] [PubMed] [Google Scholar]
- 27.Hendershot CS, Neighbors C, George WH, McCarthy DM, Wall TL, Liang T, et al. ALDH2, ADH1B and alcohol expectancies: integrating genetic and learning perspectives. Psychol Addict Behav. 2009;23:452–63. doi: 10.1037/a0016629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarthy DM, Brown SA, Carr LG, Wall TL. ALDH2 status, alcohol expectancies, and alcohol response: preliminary evidence for a mediation model. Alcohol Clin Exp Res. 2001;25:1558–63. [PubMed] [Google Scholar]
- 29.McCarthy DM, Wall TL, Brown SA, Carr LG. Integrating biological and behavioral factors in alcohol use risk: the role of ALDH2 status and alcohol expectancies in a sample of Asian Americans. Exp Clin Psychopharmacol. 2000;8:168–75. doi: 10.1037//1064-1297.8.2.168. [DOI] [PubMed] [Google Scholar]
- 30.Schuckit MA, Smith TL, Danko GP, Anderson KG, Brown SA, Kuperman S, et al. Evaluation of a level of response to alcohol-based structural equation model in adolescents. J Stud Alcohol. 2005;66:174–84. doi: 10.15288/jsa.2005.66.174. [DOI] [PubMed] [Google Scholar]
- 31.Slutske WS, Cronk NJ, Sher KJ, Madden PA, Bucholz KK, Heath AC. Genes, environment, and individual differences in alcohol expectancies among female adolescents and young adults. Psychol Addict Behav. 2002;16:308–17. [PubMed] [Google Scholar]
- 32.Perry A. The effect of heredity on attitudes toward alcohol, cigarettes and coffee. J Appl Psych. 1973;58:275–7. [Google Scholar]
- 33.Prescott CA, Cross RJ, Kuhn JW, Horn JL, Kendler KS. Is risk for alcoholism mediated by individual differences in drinking motivations? Alcohol Clin Exp Res. 2004;28:29–39. doi: 10.1097/01.ALC.0000106302.75766.F0. [DOI] [PubMed] [Google Scholar]
- 34.Vernon PA, Lee D, Harris JA, Jang KL. Genetic and environmental contributions to individual differences in alcohol expectancies. Pers Indiv Differ. 1996;21:183–7. [Google Scholar]
- 35.Agrawal A, Dick DM, Bucholz KK, Madden PAF, Cooper ML, Sher KJ, et al. Drinking expectancies and motives: a genetic study of young adult women. Addiction. 2008;103:194–204. doi: 10.1111/j.1360-0443.2007.02074.x. [DOI] [PubMed] [Google Scholar]
- 36.Baker LA, Tuvblad C, Wang P, Gomez K, Bezdjian S, Niv S, et al. The Southern California Twin Register at the University of Southern California: III. Twin Res Hum Genet. 2013;16:336–43. doi: 10.1017/thg.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aas HN, Leigh BC, Anderseen N, Jakobsen R. Two-year longitudinal study of alcohol expectancies and drinking among Norwegian adolescents. Addiction. 1998;93:373–84. doi: 10.1046/j.1360-0443.1998.9333736.x. [DOI] [PubMed] [Google Scholar]
- 38.Christiansen BA, Goldman MS. Alcohol-related expectancies versus demographic/background variables in the prediction of adolescent drinking. J Consult Clin Psychol. 1983;51:249–57. doi: 10.1037//0022-006x.51.2.249. [DOI] [PubMed] [Google Scholar]
- 39.Goldman MS, Brown SA, Christiansen BA, Smith GT. Alcoholism and memory: broadening the scope of alcohol expectancy research. Psychol Bull. 1991;110:137–46. doi: 10.1037/0033-2909.110.1.137. [DOI] [PubMed] [Google Scholar]
- 40.Tomlinson KL, Brown SA. Self-medication or social learning? A comparison of models to predict early adolescent drinking. Addict Behav. 2012;37:179–86. doi: 10.1016/j.addbeh.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 41.SAS Institute Inc. SAS/STAT 9.2 User's Guide. Institute Inc.; Cary, NC: 2008. [Google Scholar]
- 42.McArdle JJ. Latent curve analyses of longitudinal twin data using a mixed-effects biometric approach. Twin Res Hum Genet. 2006;9:343–59. doi: 10.1375/183242706777591263. [DOI] [PubMed] [Google Scholar]
- 43.Muthén LK, Muthén BO. Mplus User's Guide. 7th edn. Muthén & Muthén; Los Angeles, CA: 1998–2012. [Google Scholar]
- 44.Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. J Am Acad Child Adolesc Psychiatry. 2003;42:710–19. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- 45.Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritabilty of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Res Hum Genet. 2007;10:423–33. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- 46.Rose RJ, Dick DM. Gene–environment interplay in adolescent drinking behavior. Alcohol Res Health. 2005;28:222–9. [Google Scholar]
- 47.Ciesla JR, Spear SF, Skala SY. Reliability over time of self-reports given byadolescents andtheir parents in substance abuse outcome research. J Child Adolesc Subst Abuse. 1999;9:57–73. [Google Scholar]
- 48.Frissell KC, McCarthy DM, D'Amico EJ, Metrik J, Ellingstad TP, Brown SA. The impact of consent procedures on reported levels of adolescent alcohol use. Psychol Addict Behav. 2004;18:307–15. doi: 10.1037/0893-164X.18.4.307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.