Abstract
Consequent to the 2010 Deepwater Horizon oil spill in the Gulf of Mexico, there is an emergent concern about the short- and long-term adverse health effects of exposure to crude oil, weathered-oil products, and oil dispersants among the workforce employed to contain and clean up the spill. Oil dispersants typically comprise of a mixture of solvents and surfactants that break down floating oil to micrometer-sized droplets within the water column, thus preventing it from reaching the shorelines. As dispersants are generally sprayed from the air, workers are at risk for exposure primarily via inhalation. Such inhaled fractions might potentially permeate or translocate to the brain via olfactory or systemic circulation, producing central nervous system (CNS) abnormalities. To determine whether oil dispersants pose a neurological risk, male Sprague-Dawley rats were exposed by whole-body inhalation exposure to a model oil dispersant, COREXIT EC9500A (CE; approximately 27 mg/m3 × 5 h/d × 1 d), and various molecular indices of neural dysfunction were evaluated in discrete brain areas, at 1 or 7 d postexposure. Exposure to CE produced partial loss of olfactory marker protein in the olfactory bulb. CE also reduced tyrosine hydroxylase protein content in the striatum. Further, CE altered the levels of various synaptic and neuronal intermediate filament proteins in specific brain areas. Reactive astrogliosis, as evidenced by increased expression of glial fibrillary acidic protein, was observed in the hippocampus and frontal cortex following exposure to CE. Collectively, these findings are suggestive of disruptions in olfactory signal transduction, axonal function, and synaptic vesicle fusion, events that potentially result in an imbalance in neurotransmitter signaling. Whether such acute molecular aberrations might persist and produce chronic neurological deficits remains to be ascertained.
The recent Gulf of Mexico Deepwater Horizon oil spill enlisted a rapid-response workforce to prevent an ecological and environmental disaster. A large number of workers were involved in various response activities, including in situ burning of surface oil, application of dispersants, and oil skimming and booming, as well as decontamination of booms, boats, and vessels. The oil spill response workers were exposed to a variety of chemical hazards that might potentially increase the risk of short- and long-term adverse health outcomes. These include exposures to chemical components of the crude oil, such as volatile organic compounds (VOC), polycylic aromatic hydrocarbons (PAH), and heavy metals, as well as components of oil dispersants employed to disperse the oil spill.
Oil dispersants are detergent-like surfactants comprising anionic and nonionic molecules dissolved or suspended in solvent that render both hydrophilic and hydrophobic properties to the dispersant. Dispersants enhance the dispersion of oil by reducing the oil/water interfacial tension, thereby orienting the oil–water interface and facilitating the formation of small mixed oil–surfactant micelles (Canevari 1978) within the water column, thus preventing the oil slick from reaching the shorelines. Natural forces such as waves and currents further promote the dissolution of oil droplets. As dispersants are normally sprayed aerially or from ships/boats, workers are at risk for exposure primarily via inhalation. Dispersant aerosols might potentially permeate or translocate to the brain via olfactory or systemic circulation, thereby eliciting central nervous system (CNS) abnormalities.
Health hazard evaluation surveys among response workers identified a variety of adverse health effects, including neurological symptoms (NIOSH 2010a). Unfortunately, as a significant number of response workers who experienced health symptoms were exposed to both crude oil and dispersant, the health effects of dispersant alone were difficult to discern from these surveys. In this study, well-controlled laboratory-based studies were undertaken to obtain health risk information to establish whether a toxicological potential of dispersants exists. To that end, acute inhalation exposure to the oil dispersant COREXIT EC9500A (CE) was conducted to elicit neurotoxicity in an experimental animal model. Such efforts should eventually contribute toward development of appropriate exposure assessment procedures, personnel protective equipment, pre-job planning protocols, and possible establishment of occupational exposure limits for oil dispersants.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley [Hla:(SD) CVF] rats (approximately 2 mo old; weighing 250–300 g) were purchased from Hilltop Lab Animals (Scottdale, PA). Rats were acclimated for at least 6 d after arrival and housed in ventilated polycarbonate cages with Alpha-Dri cellulose chips and hardwood Beta-chips as bedding, with provision for HEPA-filtered air, irradiated Teklad 2918 diet, and tap water ad libitum. The National Institute for Occupational Safety and Health (NIOSH) animal facility is specific pathogen free, environmentally controlled, and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). All animal procedures used during the study were reviewed and approved by the institution’s animal care and use committee.
Whole-Body Inhalation Exposure to CE
CE was provided by NALCO (Naperville, IL). An automated whole-body inhalation exposure system was developed to expose animals to CE (Goldsmith et al. 2011, this issue). Briefly, CE aerosol was generated using a collision type atomizer. Chamber aerosol concentration and pressure were regulated with software feedback loops to ensure that constant aerosol concentrations of CE (27 mg/m3 × 5 h/d × 1 d) were maintained throughout the duration of the exposure. Control animals were exposed to filtered air in an identical exposure system. Animals were euthanized either 1 d or 7 d following CE exposure. Brain tissue samples were collected from the same animals that were exposed for pulmonary toxicity studies (Roberts et al. 2011, this issue). Immediately after euthanasia, brains were excised and specific brain areas—olfactory bulb (OB), striatum (STR), midbrain (MB), hippocampus (HIP), and frontal cortex (FCT)—from the left and right hemispheres were dissected freehand. Brain tissues from the left hemisphere were placed in RNALater (Applied Biosystems, Foster City, CA) for mRNA expression analysis, and tissues from the right hemisphere were frozen for analysis of proteins. Due to the cellular heterogeneity of the CNS, as well as the progressive nature of neural injury, the timelines of expression of various neural proteins are distinct. Through a careful understanding of the time course of expression of various neural markers, based on previous studies (Sriram et al. 1997; 1998; 2004), changes were monitored at relevant time points. As calcium-mediated signaling events occur early following adverse insults, the mRNA expression of Cacna1d was monitored at 1 and 7 d postexposure. Changes in neuronal and glial proteins were monitored at 7 d postexposure, to determine whether these proteins exhibit more persistent alterations and thereby reveal neural injury.
RNA Isolation, cDNA Synthesis, and Real-Time PCR
The brain tissues (OB, HIP, STR, FCT, and MB) were homogenized in Tri Reagent (Molecular Research Center, Inc., Cincinnati, OH) and the aqueous phase was separated with MaXtract high-density gel (Qiagen, Valencia, CA). Total RNA from the aqueous phase was then isolated using RNeasy mini spin columns (Qiagen, Valencia, CA), and concentrations were determined with a NanoDrop ND-1000 ultraviolet–visible (UV-Vis) spectrophotometer (NanoDrop Technologies, Wilmington, DE). The isolated RNA was stored at −75°C until use.
First-strand cDNA synthesis was carried out using total RNA (1 μg), random hex-amers, and MultiScribe reverse transcriptase (high capacity cDNA reverse transcription kit, Applied Biosystems, Foster City, CA) in a 20-μl reaction. Real-time plymerase chain reaction (PCR) amplification was performed using the 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) in combination with TaqMan chemistry. Gene-specific primers and FAM dye-labeled TaqMan MGB probe sets (TaqMan gene expression assays) were procured from Applied Biosystems (Foster City, CA) and used according to the manufacturer’s recommendation. All PCR amplifications (40 cycles) were performed in a total volume of 25 μl, containing 1 μl cDNA, 1.25 μl of the specific TaqMan gene expression assay, and 12.5 μl of TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, CA), respectively. Sequence detection software (version 1.7; Applied Biosystems, Foster City, CA) results were exported as tab-delimited text files and imported into Microsoft Excel for further analysis. Following normalization to the endogenous control Actb (β-actin), relative quantification of gene expression was performed using the comparative threshold (CT) method as described by the manufacturer (Applied Biosystems, Foster City, CA; User Bulletin 2). The values are expressed as fold change over saline-treated controls.
Preparation of Brain Tissues for Protein Analysis
Brain tissue homogenates were prepared in T-PER tissue protein extraction reagent (Thermo Fisher Scientific, Inc., Rockford, IL) containing protease inhibitors and ethylene-diamine tetraacetic acid (EDTA). The samples were then centrifuged at 10,000 × g for 3 min and supernatant was collected. Total protein in supernatant was determined according to the micro-bicinchoninic acid (BCA) method (Thermo Fisher Scientific, Inc., Rockford, IL) using bovine serum albumin as a standard. The protein extracts were stored at −75°C until use.
Western Immunoblotting
Aliquots of brain homogenates (10 μg total protein) were diluted in Laemmli sample buffer, boiled, and loaded on 10% sodium dodecyl sulfate (SDS) polyacrylamide gels. Proteins then were electrophoretically resolved and transferred to 0.45-μm Immobilon-FL polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA). Following transfer, immunoblot analysis was performed. Briefly, membranes were blocked using Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) for 1 h at room temperature, washed (1 × 5 min; 2 × 10 min) with PBST, and incubated overnight at 4°C with appropriate primary antibody. Following incubation with antibodies to either olfactory marker protein (Omp; goat polyclonal, Wako Chemicals USA, Inc., Richmond, VA), tyrosine hydroxylase (Th; rabbit polyclonal, EMD Chemicals Inc., Gibbstown, NJ; or mouse monoclonal, Sigma-Aldrich Corp., St. Louis, MO), dopamine D2 receptor (Drd2; goat polyclonal, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), phosphosynapsin 1 S553 (pSyn1; rabbit monoclonal, Abcam, Cambridge, MA; detects S551 in rat), synaptotagmin I (Syt1; chicken polyclonal, Aves Labs, Inc., Tigard, OR), synaptosomal associated protein 25 (Snap25; rabbit polyclonal, EMD Chemicals Inc., Gibbstown, NJ), vesicle associated membrane protein 1/2 (Vamp1/2; mouse monoclonal, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), beta III tubulin (Tubb3; mouse monoclonal, Abcam, Cambridge, MA), neurofilament L (Nefl; mouse monoclonal, Millipore, Billerica, MA), glial fibrillary acidic protein (Gfap; mouse monoclonal, Cell Signaling Technology, Danvers, MA), or beta-actin (Actb; rabbit polyclonal, Abcam, Cambridge, MA), blots were washed with PBST (1 × 5 min; 3 × 10 min) and incubated for 1 h at room temperature with appropriate IRDye 680 or 800 secondary antibodies (LI-COR Biosciences, Lincoln, NE). The membranes were protected from light to minimize any photobleaching of the fluorescent dyes. Membranes were washed (1 × 5 min; 4 × 10 min) in PBST, followed by washes (2 × 3 min) in PBS. Near-infrared fluorescence detection was performed on the Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE) and the fluorescent signal intensities (k counts) of the individual bands were determined and normalized to an endogenous control, Actb.
Statistical Analysis
Data were analyzed by Student’s t-test using the SigmaPlot (Systat Software, Inc., San Jose, CA) statistical package. Values are expressed as mean ± SE. Asterisks indicate values significantly different from air-exposed controls (p < .05).
RESULTS
Relevance of Animal Exposure Dose to Potential Work-Zone Exposures
To relate our animal exposure dose to potential exposures that workers may have experienced during the 3-mo course of the oil spill cleanup, a mathematical calculation was utilized to determine potential exposure concentrations. The calculations conducted in this study do not account for clearance or influence of any other human confounding factors, but provide an estimate of the plausible worker exposure concentrations that our experimental paradigm mimics. The mass median aerodynamic diameter (MMAD) for CE aerosol was determined to be 0.9 μm, indicating that it is in the fine particle range. At this particle size, the deposition efficiency is estimated to be 15–20% or lower (ICRP 1994). Incorporating factors such as CE aerosol concentration (27 mg/m3), rat minute ventilation volume (0.16 L/min × 10−3 m3/ml), exposure duration (5 h/d × 60 min/h), and a predicted deposition efficiency of fine particles of 20% (ICRP 1994), it was determined that the total lung burden is about 260 μg/rat. Next, to relate the rat dosing paradigm employed in this study to workplace exposures, the total equivalent lung burden in humans was determined using surface area of alveolar epithelium (rat = 0.4 m2; human = 102 m2) as dose metric (Stone et al. 1992). From this, the equivalent total lung burden in humans was estimated to be approximately 66 mg. Assuming a worker ventilation rate of 9.6 m3/8-h d (ICRP 1994) and a deposition fraction of 20%, a total lung burden of 66 mg would be achieved in a worker over the 3-mo time frame of the oil spill at an exposure concentration of 0.38 mg/m3. To the best of our knowledge no workplace or human exposure data for CE are available.
Loss of Olfactory Marker Protein (Omp) Following Acute Exposure to CE
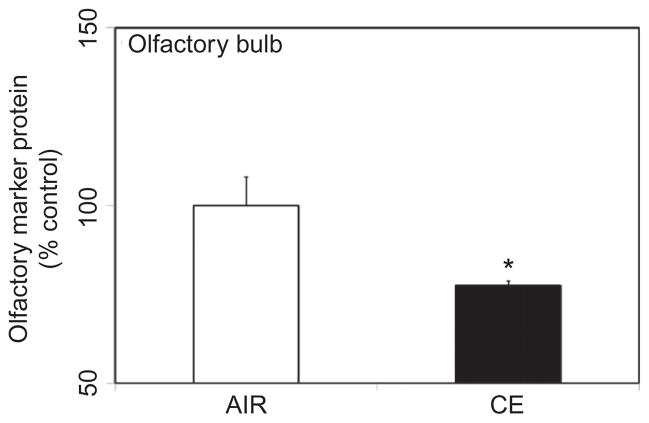
Omp is selectively expressed by mature olfactory sensory neurons whose axons terminate in the olfactory bulb (Buiakova et al. 1994). Omp plays an important role in neural signaling directed toward the olfactory bulb (Buiakova et al. 1996), as part of the odor stimulation process. Loss of Omp in CE-exposed animals may therefore be indicative of compromised odor stimuli. As dispersants contain surfactants and hydro-treated light petroleum distillates (HLPD) that potentially disrupt neuronal membranes, studies were undertaken to examine whether inhalation exposure to CE affected the olfactory neurons. Acute exposure to CE resulted in a significant loss (22% decrease) of Omp in the olfactory bulb at 7 d following exposure (Figure 1).
FIGURE 1.
CE exposure effect on olfactory marker protein (Omp). Rats were exposed by whole-body inhalation to CE (~27 mg/m3 × 5 h/d × 1 d). At 7 d postexposure, Omp protein content in the olfactory bulb was measured by Western immunoblot analysis. Following normalization to endogenous control (Actb), the levels of Omp are expressed as percent air-exposed controls. Graphical representations are mean ± SE (n = 5/group). Asterisk indicates significant change from air-exposed control (p < 0.05).
Tyrosine Hydroxylase (Th) Protein is Reduced in the Striatum Following Acute Exposure to CE
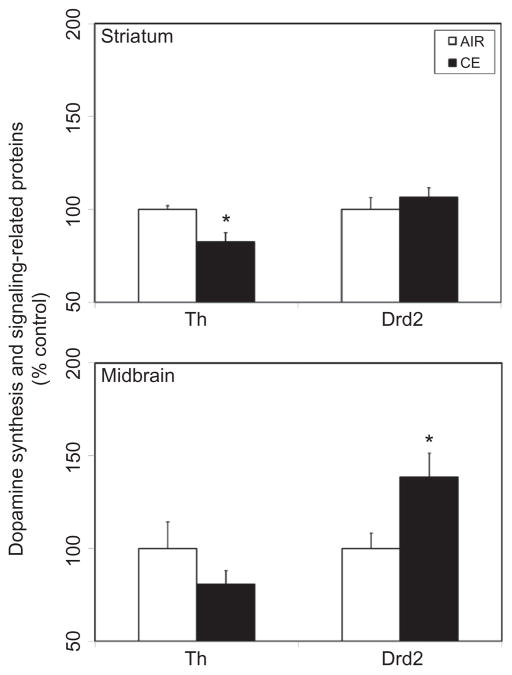
Th, a marker of dopaminergic neurons, is the rate-limiting enzyme in dopamine synthesis, and loss of Th enzyme activity or protein content is an index of injury to the dopaminergic neurons. Acute exposure to CE produced a significant 18% fall in striatal Th protein content, 7 d following exposure to CE (Figure 2). A similar quantitative decrease (19%) was also apparent in the midbrain (Figure 2). Exposure to CE also induced upregulation of Drd2 expression in the midbrain, but not in the striatum (Figure 2).
FIGURE 2.
Exposure to CE on expression of dopaminergic markers in the striatum and midbrain. Rats were exposed by whole-body inhalation to CE (~27 mg/m3 × 5 h/d × 1 d). At 7 d postexposure, the expression of tyrosine hydroxylase (Th) and dopamine D2 receptor (Drd2) proteins was measured by Western immunoblot analysis. Following normalization to endogenous control (Actb), the levels of Th and Drd2 are expressed as percent air-exposed controls. Graphical representations are mean ± SE (n = 5–6/group). Asterisk indicates significant change from air-exposed control (p < 0.05).
Modulation of Synaptic Protein Expression in Discrete Brain Areas Following Acute Exposure to CE
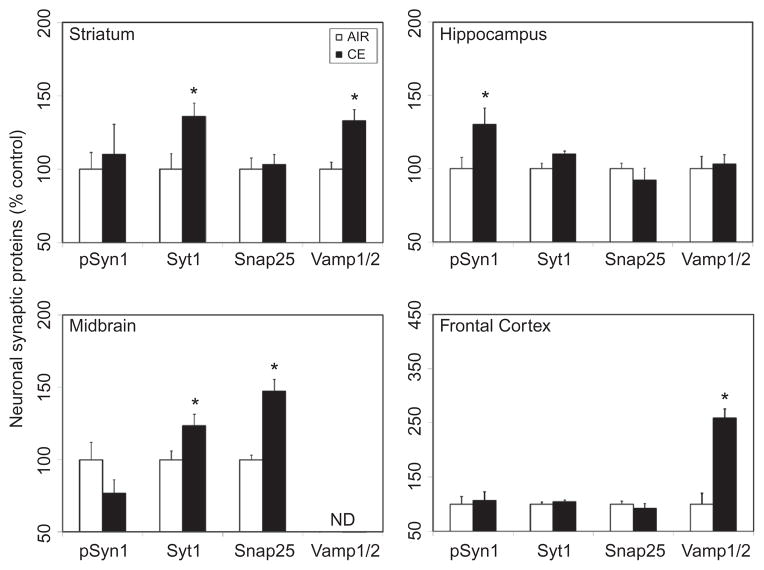
Since CE altered the expression of markers associated with olfactory and dopaminergic pathways, the influence of CE was examined on proteins involved in synaptic neurotrans-mission. Specifically, changes in the pSyn1, a major phosphoprotein in the nerve terminals that is involved in regulating neurotransmitter release; Syt1, a critical calcium sensor associated with neurotransmitter release; and Snap25 and Vamp1/2, main components of the soluble N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE) complex involved in the docking and/or fusion of synaptic vesicles with the presynaptic membrane, were determined. Exposure to CE resulted in differential expression of these synaptic markers in a brain-region-specific manner, as observed 7 d following exposure. CE induced significant increase in the expression of Syt1 (36%) and Vamp1/2 (33%) in the striatum (Figure 3). Significant elevation in the expression of Syt1 (23%) and Snap25 (47%) was observed in the midbrain (Figure 3). Neither Syt1 nor Snap25 was induced by CE in the hippocampus or frontal cortex (Figure 3). CE induced a significant rise (30%) in serine phosphorylation of synapsin 1 (S553; detects S551 in rat) in the hippocampus, but not in other brain regions examined (Figure 3). Exposure to CE produced a significant increase in the expression of Vamp1/2 (159%) in the frontal cortex (Figure 3). CE exposure did not markedly alter the expression of any of these synaptic proteins in the olfactory bulb (data not shown).
FIGURE 3.
Exposure to CE on heterogeneous expression of neuronal synaptic proteins in discrete brain regions. Rats were exposed by whole-body inhalation to CE (~27 mg/m3 × 5 h/d × 1 d). At 7 d postexposure, the expression of various synaptic proteins (pSyn1, Syt1, Snap25, Vamp1/2) was measured by Western immunoblot analysis. Following normalization to endogenous control (Actb), the levels of synaptic proteins are expressed as percent air-exposed controls. Graphical representations are mean ± SE (n = 5–6/group). Asterisk indicates significant change from air-exposed control (p < .05).
Modulation of Neuronal Intermediate Filament Proteins in Discrete Brain Areas Following Acute Exposure to CE
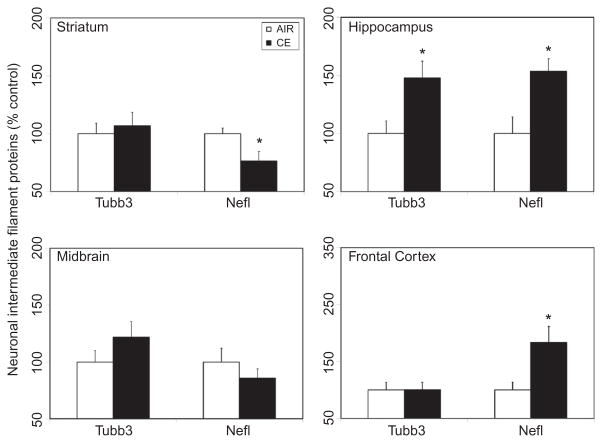
To examine whether exposure to CE altered axonal function, changes in the expression of the intermediate filaments proteins Tubb3, a protein involved in axonal guidance and maintenance, and Nefl, a type IV neuronal intermediate filament that plays a role in maintaining neuronal caliber and cellular transport to axons and dendrites, were determined. Exposure to CE resulted in differential expression of these neuronal intermediate filament markers in a brain-region-specific manner. While exposure to CE resulted in a significant 23% decrease of Nefl protein in the striatum (Figure 4), significant increases in the expression of this protein were observed in the hippocampus (54%) and frontal cortex (84%) 7 d postexposure (Figure 4). CE also induced significant Tubb3 protein expression in the hippocampus (48%), but not in other brain areas examined (Figure 4).
FIGURE 4.
Exposure to CE on heterogeneous expression of neuronal intermediate filament proteins in discrete brain regions. Rats were exposed by whole-body inhalation to CE (~27 mg/m3 × 5 h/d × 1 d). At 7 d postexposure, the expression of axonal proteins (Tubb3, Nefl) was measured by Western immunoblot analysis. Following normalization to endogenous control (Actb), the levels of axonal proteins are expressed as percent air-exposed controls. Graphical representations are mean ± SE (n = 5–6/group). Asterisk indicates significant change from air-exposed control (p < .05).
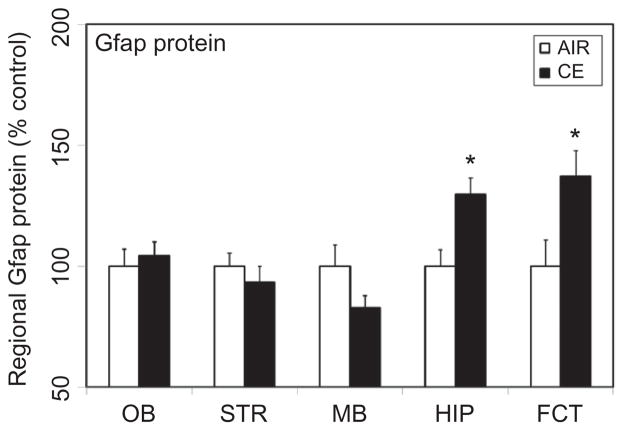
Induction of Glial Fibrillary Acidic Protein (Gfap) in Hippocampus and Frontal Cortex Following Acute Exposure to CE
Glial cells function as microsensors to detect subtle changes in neuronal milieu in response to neuronal injury (Kreutzburg 1996; Streit et al. 1999). Assessment of glial activation may therefore serve as an indicator of an underlying neuronal injury. The astrocyte marker Gfap is well known to be altered in response to a variety of brain insults (Sriram et al. 2004; 2005). Following exposure to CE, increased expression of this marker was observed in the hippocampus and frontal cortex (Figure 5). In the hippocampus, a significant increase (30%) in Gfap protein was seen at 7 d postexposure. A similar rise (37%) was also observed in frontal cortex. CE did not markedly alter the expression of Gfap in any of the other brain areas examined (Figure 5).
FIGURE 5.
Exposure to CE on astrogliosis in discrete brain areas. Rats were exposed by whole-body inhalation to CE (~27 mg/m3 × 5 h/d × 1 d). At 7 d postexposure, the expression of Gfap protein was measured by Western immunoblot analysis. Following normalization to endogenous control (Actb), the levels of Gfap are expressed as percent air-exposed controls. Graphical representations are mean ± SE (n = 5–6/group). Asterisk indicates significant change from air-exposed control (p < .05).
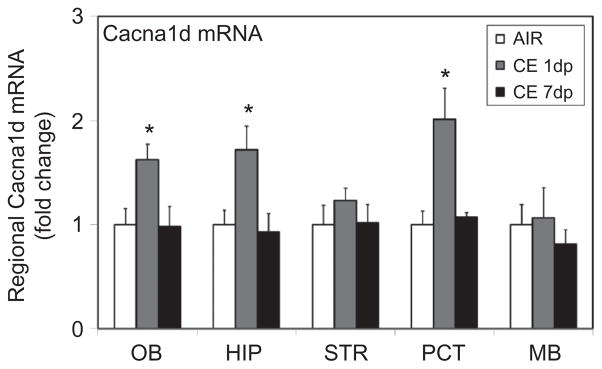
Region-Specific Expression of the Voltage-Gated Calcium Channel Cacna1d Following Acute Exposure to CE
Voltage-dependent calcium channels are known to regulate synaptic neurotransmission function in the CNS (Rusakov 2006). Since exposure to CE altered synaptic function, it was of interest to examine whether this was mediated by calcium-dependent mechanisms. Particularly, expression of Cacna1d (Cav1.3), a major L-type calcium channel expressed in the brain, was determined. Exposure to CE produced transient upregulation of Cacna1d mRNA in specific brain regions. At 1 d pos-texposure, a 1.6- to 2-fold marked increase (Figure 6) in Cacna1d was seen in olfactory bulb, hippocampus, and frontal cortex, but not in dopaminergic brain areas, striatum, and midbrain (Figure 6). Following 7 d of exposure, the expression of Cacna1d in the brain areas examined was not significantly different from that in air-exposed controls.
FIGURE 6.
CE effect on upregulation of the voltage-gated calcium channel, Cacna1d. Rats were exposed by whole-body inhalation to CE (~27 mg/m3 × 5 h/d × 1 d). At 1 and 7 d postexposure, the mRNA expression of Cacna1d was measured by real-time PCR analysis. Following normalization to endogenous control (Actb), the levels of Cacna1d mRNA are expressed as fold change over air-exposed controls. Expression levels of Cacna1d in air-exposed controls were not significantly different at 1 d and 7 d postexposure time points. For clarity of graphical representation, Cacna1d mRNA levels in CE-exposed animals (1 d and 7 d postexposure) are compared to air-exposed controls from 1 d postexposure. Values are mean ± SE (n = 5–6/group). Asterisk indicates significant change from air-exposed control (p < .05).
DISCUSSION
Explosion of the oil-drilling rig Deepwater Horizon in April 2010 resulted in the largest offshore oil spill catastrophe recorded in history. Although it was not the first oil spill disaster, the Deepwater Horizon oil spill in the Gulf of Mexico gained immense attention due to the enormity and duration of the oil spill. An estimated 4.9 million barrels (approximately 205 million gal) of crude oil was reported to have been discharged into the Gulf of Mexico over the course of the entire spill, which lasted nearly 3 mo (McNutt et al. 2011). A rapid-response action that resulted involved recruitment of nearly 48,000 personnel to contain and clean up the spill (BP 2010). The response action also resulted in the application of surface and subsurface chemical dispersants to prevent oil impacting the shoreline environment and ecosystems. Approximately 1.84 million gal of chemical dispersants was applied to contain the spill (BP 2010). Of this, nearly 58% was applied on the surface and about 42% was on the subsurface near the leaking well-head (Kujawinski et al. 2011). CE was the predominant oil dispersant used to enhance the oil biodegradation rate. As surface treatment with dispersants involved aerial application, there is a likelihood that the response workers may have been at risk for exposure, primarily via inhalation. Unfortunately, little toxicological information is available on oil dispersants, including those pertinent to human health effects. Thus, from an occupational safety perspective, it is of critical importance to evaluate the potential toxicological risks associated with oil dispersants. Such efforts will lead to a better understanding of the short-term and chronic adverse health effects of exposed worker populations. Besides, understanding the toxicological mechanisms may aid in the development of appropriate exposure assessment, exposure control and emergency preparedness protocols to prevent future exposures and human health risks.
The chemical ingredients in CE as disclosed by the manufacturer (NALCO 2010) include sorbitan, mono-(9Z)-9-octadecenoate (CAS number 1338-43-8, sorbitan monooleate); sorbitan, mono-(9Z)-9-octadecenoate, poly(oxy-1,2-ethanediyl) derivatives (CAS number 9005-65-6, polysor-bate 800; sorbitan, tri-(9Z)-9-octadecenoate, poly(oxy-1,2-ethanediyl) derivatives (CAS number 9005-70-3, polysorbate 85); butane-dioic acid, 2-sulfo-1,4-bis(2-ethylhexyl) ester, sodium salt (1:1) (CAS number 577-11-7, docusate sodium/dioctyl sodium sulfosuccinate), containing 2-propanediol (CAS number 57-55-6, propylene glycol); propanol, 1-(2-butoxy-1-methylethoxy) (CAS number 29911-28-2, 2-propanol); and distillates (petroleum), hydrotreated light (CAS number 64742-47-8, deodorized kerosene). The percent composition of ingredients as disclosed in the MSDS (NALCO 2010) is (1) distillates (petroleum), hydrotreated light (10–30% w/w); (2) organic sulfonic acid salt (10–30% w/w); and (3) propylene glycol (1–5% w/w). Petroleum distillates are derived from crude oil and are commonly used in the manufacture of dispersants, pesticides, deodorizers, and air fresheners. Dioctyl sodium sulfosuccinate is used as a dispersing, wetting, or emulsifying agent and is a component of dispersants, pesticides, and certain laxatives. Polypropylene glycol is a branched diol commonly used as a solvent, emulsifier, lubricant, or moisturizer and is a component of antifreeze mixtures, coolants, and sanitizers.
Although the toxicological potential of this dispersant in humans has not been established, there is some experimental evidence to suggest that certain individual constituents of the dispersant may exhibit toxicological properties. For example, mice exposed to a single dose (20 ul) of kerosene by aspiration exhibited drowsiness, lack of coordination, and behavioral changes (Nouri et al. 1983). Similarly, rats exposed to 12 g/kg body weight of deodorized kerosene (Deobase) via oral gavage exhibited unsteady gait and drowsiness (Muralidhara et al. 1982). Hyperactivity and increased tactile stimuli responses were observed in mice following dermal exposure (100 μl/d × 7 d) to kerosene (Upreti et al. 1989). Anionic surfactants such as dioctyl sodium sulfosuccinate, sodium ricinoleate, or sodium lauryl sulfate were found to reduce the number of nerve ganglion cells in the myenteric plexus following repeated application on the serosal surface of the rat jejunum (Fox et al. 1983). However, the molecular basis for such neurological effects remains unknown, partly due to limited neurotoxicity investigations conducted with such agents or mixtures containing these agents.
Since hydrocarbons and surfactants are membrane-perturbing substances, it was postulated that dispersants containing petroleum-related aliphatic hydrocarbons and/or anionic surfactant molecules may affect neuronal membranes producing aberrant synaptic signaling and impaired neurotransmission, defects that culminate in neural damage. Exposure to CE resulted in a partial loss of Omp in the olfactory bulb. Omp is exclusively expressed by mature olfactory receptor neurons, which are localized in the olfactory epithelium and project directly into the olfactory bulb within the central nervous system. As olfactory sensory neurons occupy nearly two-thirds of the sensory or olfactory epithelium, these neurons experience direct access to odorant molecules, as well as, allergens, airborne pollutants, toxic chemicals and microorganisms. Some of these toxicants may be potentially harmful producing olfactory sensory deafferentation. Although these neurons have the unique ability to regenerate throughout the life span of an animal (Crews and Hunter 1994), repeated exposure to toxicants might render an irreversible injury and attendant decrease in Omp. Omp-null mice exhibit reduced odor response to various odorants and impaired neural signaling directed toward the olfactory bulb (Buiakova et al. 1996). Reduction of Omp in CE-exposed animals may therefore be indicative of injury to the olfactory sensory neurons and perhaps compromised odor stimuli. However, since no olfactory or neurobehavioral assessments were conducted in the current study, the functional relevance of these molecular changes cannot be proven conclusively. Nevertheless, these early molecular events are indicative of neural abnormality following acute exposure to CE. Additional studies are warranted to determine whether such changes contribute to persistent sensory neuron damage and olfactory deficits.
In the striatum and midbrain, CE produced a decrease of Th, the rate-limiting enzyme in the synthesis of the neurotransmitter dopamine and a marker of dopaminergic neurons. Exposure to CE also initiated an increase in Drd2 protein in the midbrain. Drd2 expression is prominent in midbrain dopaminergic neurons, particularly in the substantia nigra pars compacta and the ventral tegmental area (Meador-Woodruff et al. 1989). The large presence of Drd2 in this region signifies its role in regulation of dopamine release (Carlsson 1975), functioning as “autoreceptors” to modulate dopaminergic activity in the nigrostriatal and mesolimbic regions (Skirboll et al. 1979). The release of dopamine in the striatum is tightly regulated by mechanisms involving Drd2. Drd2 inhibits nerve terminal excitability by modulating potassium channels and thereby reduces dopamine release (Tepper et al. 1984; Bowyer and Weiner 1987; Lacey et al. 1987). In addition, overexpression and/or activation of Drd2 was shown to reduce dopamine synthesis (El Mestikawy et al. 1986; Onali et al. 1992) through regulation of Th enzyme activity and/or protein expression. Besides altering the expression of dopaminergic markers, CE also modulated the expression of specific synaptic SNARE complex proteins associated with synaptic neurotransmission in the striatum and midbrain. Particularly, increased expression of Syt1, Snap25, and/or Vamp1/2 is suggestive of alterations in dopamine release, observations that corroborate changes in Th and Drd2 expression in these brain regions. Such abnormal accumulation of presynaptic proteins may result in functional disturbances. For example, overexpression of Snap25 alters neuronal autaptic currents, perhaps a consequence of inhibiting neurotransmitter release (Owe-Larsson et al. 1999). Further, exposure to CE resulted in loss of Nefl in the striatum. Nefl is a prominent neuronal intermediate filament protein that plays a functional role in sustaining axonal and dendritic branching, besides promoting axonal growth and thickening. As Nefl can self-assemble, it is critical for formation of the neurofilament. By controlling the axonal caliber, neurofilaments regulate the speed of nerve conductance along the axon and thus influence neurotransmitter release. Loss of Nefl, therefore, can result in abnormal neurofilament formation, contributing to reduced axonal caliber and conduction velocity of the nerve impulse. Collectively, these findings are suggestive of aberrant nerve conduction, synaptic vesicle fusion, and neurotransmitter release. Although the levels of striatal dopamine and its metabolites were not quantified in this study, our observations of abnormal synaptic activity in the striatum, Drd2 auto-receptor up-regulation in the midbrain, and concomitant loss of Th in striatum are suggestive of deficiencies in dopaminergic signaling and function. Indeed, recent studies reported that dynamic changes in the expression of presynaptic and axonal transport proteins precede dopaminergic neuronal loss (Chung et al. 2009), findings consistent with our observations. Previous studies also suggested that axonal transport disruption and axonal degeneration precede neuronal death and may underlie disease progression in neurodegenerative diseases including Alzheimer’s disease, amyotrophic lateral sclerosis, and Huntington’s disease (Li et al. 2001; Stokin et al. 2005).
In contrast, in the hippocampus and frontal cortex, the expression of Nefl increased following exposure to CE. These observations suggest the likelihood of enhanced axonal caliber and nerve conduction in these regions that modulate neurotransmitter release. The overexpression of Vamp1/2 in the frontal cortex provides further evidence of elevated synaptic activity. Interestingly, increased expression of Vamp1/2 in the prefrontal cortex was observed following hippocampal lesions that resulted in loss of hip-pocampal input to the prefrontal cortex (Halim et al. 2003). Moreover, excessive synaptic firing activity was noted in pyramidal neurons of the prefrontal cortex when hippocampus-lesioned animals were subjected to dopaminergic stress (O’Donnell et al. 2002). Thus, a complex interplay and/or cross-talk between discrete brain areas appears to be involved in the neurotoxicity of CE. Reactive astrogliosis, as determined by the increased expression of Gfap protein in these two brain regions, is likely reflective of neural injury.
In humans, mood disorders are frequently associated with altered activity in neural circuits involving prefrontal cortex, subgenual cingulate cortex, cingulated gyrus, amygdala, striatum, and hippocampus (Konarski et al. 2006; Ressler and Mayberg 2007; Krishnan and Nestler 2010). The marked increases in Vamp1/2 expression in the frontal cortex induced by CE are suggestive of abnormal neurotransmission in this brain area. Health hazard evaluation surveys conducted by NIOSH concluded that oil-spill response workers experienced abnormal neurological symptoms (NIOSH 2010a). NIOSH (2010a) identified that approximately 16% of workers employed in decontamination and waste management operations across 4 Gulf Coast states, Alabama, Florida, Louisiana, and Mississippi, reported one or more psychosocial symptoms that included depressed feeling, worries, stress, short temper, and frequent alterations in mood. Similar surveys of workers in other cleanup operation areas also revealed psychosocial symptoms in about 3–6% of the respondents (NIOSH 2010b). Unfortunately, whether such health effects were produced by exposure to crude oil or dispersant has been difficult to discern, as exposures to both agents were likely to have occurred. It remains to be investigated if the molecular changes seen following CE exposure are the underpinnings for some of the psychosocial symptoms reported by the oil-spill response workers.
While there is no direct evidence to demonstrate the presence of CE or its constituents in the brain, it is likely that permeation or systemic translocation of CE through the olfactory or pulmonary targets may be the basis for the observed neuronal abnormalities. Alternatively, stimulation or deafferentation of the olfactory sensory neurons may itself suffice to induce perturbations in deeper brain areas. Olfactory signals are relayed from the olfactory bulb to olfactory cortical areas, including piriform cortex, and then directly to the entorhinal cortex, a major afferent to the hippocampal dentate gyrus (Hjorth-Simonsen and Jeune 1972; Kosel et al. 1981). The close anatomical association between these regions and the critical role played by hippocampus in odor memory (Kosel et al. 1981; Eichenbaum et al. 1988; Staubli et al. 1984) suggest that stimulation and/or disruption of olfactory sensory neurons may contribute, at least partially, to abnormal hippocampal function. The loss of olfactory marker protein in the olfactory bulb and hippocampal dysfunction following CE exposure is perhaps a consequence of such a mechanism.
A major route for calcium entry in to neurons is via voltage-dependent calcium channels (VDCC). These are multi-subunit pore-forming and auxiliary protein complexes with variable gating properties. In the CNS, calcium currents are mediated predominantly by (1) L-type VDCC like Cacna1c (Cav1.2) and Cacna1d (Cav1.3), (2) P/Q-, N-, and R-type VDCC like Cacna1a (Cav2.1), Cacna1b (Cav2.2), and Cacna1e (Cav2.3), and (3) T-type VDCC like Cacna1g (Cav3.1; Vinet and Sik 2006). Cacna1c and Cacna1d are the predominant brain L-type VDCC and play a role in the regulation of neuronal synaptic plasticity (Kavalali and Plummer 1994), protein phosphorylation and gene regulation (Westenbroek et al. 1990; Murphy et al. 1991; Dolmetsch et al. 2001), spike-induced calcium entry (Fisher et al. 1990), and fear memory extinction and depression-like behavior (Striessnig et al. 2006; Busquet et al. 2010). The region-specific expression of Cacna1d mRNA following acute inhalation exposure to CE suggests calcium entry following membrane perturbation may likely contribute to modulation of neuronal protein phosphorylation, synaptic function, and gene expression in discrete brain areas. Further, through such differential signaling, calcium may regulate neurotransmitter release in discrete neuronal pathways. A combination of one or more such events may ultimately underlie depression-like behavior.
Taken together, our findings suggest that an imbalance in dopaminergic and/or cholinergic signaling, as a consequence of axonal and synaptic dysfunction, may underlie the neurotoxicity associated with CE exposure. It is possible that such alterations in neurotransmitter signaling may contribute to functional neurological abnormalities such as depression, lack of coordination, and short-term memory loss. Such functional deficits were not observed in the current study due to the acute exposure paradigm; nevertheless, our findings call for further evaluation of the neurotoxic potential of CE, including comprehensive long-term neurochemical, neurological, and neurobehavioral assessments.
Footnotes
The findings and conclusions of this article have not been formally disseminated by NIOSH and should not be construed to represent any agency determination or policy.
KS conceived the neurotoxicology studies, performed brain dissections, analyzed the data, and wrote this article. GXL and AMJ conducted all neurotoxicity-related assays. WTG, MJ, WM, and DGF designed and built the exposure system. WTG and MJ conducted whole-body inhalation exposures. VAR and VC were instrumental in procuring the test compound COREXIT EC9500A and obtaining approval for animal studies. All authors reviewed and approved the final article.
This article is not subject to U.S. copyright.
References
- Bowyer JF, Weiner N. Modulation of the Ca++-evoked release of [3H]dopamine from striatal synaptosomes by dopamine (D2) agonists and antagonists. J Pharmacol Exp Ther. 1987;241:27–33. [PubMed] [Google Scholar]
- BP. [accessed May 1, 2011];Offshore and onshore clean-up response information. 2010 http://www.bp.com/sectiongenericarticle800.do?categoryId=9036585&contentId=7067606.
- Buiakova OI, Baker H, Scott JW, Farbman A, Kream R, Grillo M, Franzen L, Richman M, Davis LM, Abbondanzo S, Stewart CL, Margolis FL. Olfactory marker protein (OMP) gene deletion causes altered physiological activity of olfactory sensory neurons. Proc Natl Acad Sci USA. 1996;93:9858–63. doi: 10.1073/pnas.93.18.9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiakova OI, Krishna NS, Getchell TV, Margolis FL. Human and rodent OMP genes: conservation of structural and regulatory motifs and cellular localization. Genomics. 1994;20:452–62. doi: 10.1006/geno.1994.1200. [DOI] [PubMed] [Google Scholar]
- Busquet P, Nguyen NK, Schmid E, Tanimoto N, Seeliger MW, Ben-Yosef T, Mizuno F, Akopian A, Striessnig J, Singewald N. CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int J Neuropsychopharmacol. 2010;13:499–513. doi: 10.1017/S1461145709990368. [DOI] [PubMed] [Google Scholar]
- Canevari GP. Some observations on the mechanism and chemistry aspects of chemical dispersion. In: McCarthy LT, editor. Chemical dispersants for the control of oil spills. Philadelphia, PA: American Society for Testing and Materials; 1978. pp. 2–5. [Google Scholar]
- Carlsson A. Receptor-mediated control of dopamine metabolism. In: Usdin E, Bunney WE, editors. Pre- and post-synaptic receptors. New York: Marcel Dekker; 1975. pp. 49–65. [Google Scholar]
- Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci. 2009;29:3365–73. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Hunter D. Neurogenesis in the olfactory epithelium. Perspect Dev Neurobiol. 1994;2:151–61. [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–39. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Fagan A, Mathews P, Cohen NJ. Hippocampal system dysfunction and odor discrimination learning in rats: impairment or facilitation depending on representational demands. Behav Neurosci. 1988;102:331–39. doi: 10.1037//0735-7044.102.3.331. [DOI] [PubMed] [Google Scholar]
- el Mestikawy S, Glowinski J, Hamon M. Presynaptic dopamine autoreceptors control tyrosine hydroxylase activation in depolarized striatal dopaminergic terminals. J Neurochem. 1986;46:12–22. doi: 10.1111/j.1471-4159.1986.tb12919.x. [DOI] [PubMed] [Google Scholar]
- Fisher RE, Gray R, Johnston D. Properties and distribution of single voltage-gated calcium channels in adult hippocampal neurons. J Neurophysiol. 1990;64:91–104. doi: 10.1152/jn.1990.64.1.91. [DOI] [PubMed] [Google Scholar]
- Fox DA, Epstein ML, Bass P. Surfactants selectively ablate enteric neurons of the rat jejunum. J Pharmacol Exp Ther. 1983;227:538–44. [PubMed] [Google Scholar]
- Goldsmith WT, McKinney W, Jackson M, Law B, Bledsoe T, Siegel P, Cumpston J, Frazer D. A computer-controlled whole-body inhalation exposure system for the oil dispersant COREXIT EC5900A. J Toxicol Environ Health. 2011;74:1368–1380. doi: 10.1080/15287394.2011.606793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim ND, Weickert CS, McClintock BW, Hyde TM, Weinberger DR, Kleinman JE, Lipska BK. Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol Psychiatry. 2003;8:797–810. doi: 10.1038/sj.mp.4001319. [DOI] [PubMed] [Google Scholar]
- Hjorth-Simonsen A, Jeune B. Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J Comp Neurol. 1972;144:215–32. doi: 10.1002/cne.901440206. [DOI] [PubMed] [Google Scholar]
- ICRP. Human respiratory tract model for radiological protection: A report of a task group of the international commission on radiological protection. Ann ICRP. 1994;24:267–72. [PubMed] [Google Scholar]
- Kavalali ET, Plummer MR. Selective potentiation of a novel calcium channel in rat hippocampal neurons. J Physiol. 1994;480:475–84. doi: 10.1113/jphysiol.1994.sp020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Soczynska JK, Bottas A, Kennedy SH. Clinical translation of neuroimaging research in mood disorders. Psychiatry (Edgmont) 2006;3:46–57. [PMC free article] [PubMed] [Google Scholar]
- Kosel KC, Van Hoesen GW, West JR. Olfactory bulb projections to the parahippocampal area of the rat. J Comp Neurol. 1981;198:467–82. doi: 10.1002/cne.901980307. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–18. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Linking molecules to mood: New insight into the biology of depression. Am J Psychiatry. 2010;167:1305–20. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawinski EB, Kido Soule MC, Valentine DL, Boysen AK, Longnecker K, Redmond MC. Fate of dispersants associated with the Deepwater Horizon oil spill. Environ Sci Technol. 2011;45:1298–306. doi: 10.1021/es103838p. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li SH, Yu ZX, Shelbourne P, Li XJ. Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington’s disease mice. J Neurosci. 2001;21:8473–81. doi: 10.1523/JNEUROSCI.21-21-08473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt M, Camilli R, Guthrie G, Hsieh P, Labson V, Lehr B, Maclay D, Ratzel A, Sogge M. Flow Rate Technical Group report to the National Incident Command. Interagency Solutions Group; 2011. Mar 10, [accessed 01 May 2011]. Assessment of flow rate estimates for the Deepwater Horizon/Macondo Well oil spill. http://on.doi.gov/hZU3Xf. [Google Scholar]
- Meador-Woodruff JH, Mansour A, Bunzow JR, Van Tol HHM, Watson SJ, Civelli O. Distribution of D2 receptor mRNA in rat brain. Proc Natl Acad Sci USA. 1989;86:7625–28. doi: 10.1073/pnas.86.19.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidhara KMK, Krishnakumari HP, Ramesh HP, Majumder SK. Toxicity of some petroleum fractions used in pesticidal emulsions to albino rats. J Food Sci Technol. 1982;19:260–62. [Google Scholar]
- Murphy TH, Worley PF, Baraban JM. L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron. 1991;7:625–35. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- NALCO. [accessed May 1, 2011];COREXIT 9500A MSDS. 2010 http://www.nalco.com/documents/Annual-Reports/9500A_MSDS.pdf.
- NIOSH. Health hazard evaluation of Deepwater Horizon response workers. [accessed May 1, 2011];Interim report number 8, 2010. 2010a http://www.cdc.gov/niosh/topics/oilspillresponse/gulfspillhhe.html.
- NIOSH. Health hazard evaluation of Deepwater Horizon response workers. [accessed May 1, 2011];Interim report number 9, 2010. 2010b http://www.cdc.gov/niosh/topics/oilspillresponse/gulfspillhhe.html.
- Nouri LA, Sordelli DO, Cerquetti MC, Saavedra JM, Hooke AM, Bellanti JA. Pulmonary clearance of Staphylococcus aureus and plasma angiotensin-converting enzyme activity in hydrocarbon pneumonitis. Pediatr Res. 1983;17:657–61. doi: 10.1203/00006450-198308000-00010. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hip-pocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–82. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- Onali P, Mosca E, Olianas MC. Presynaptic dopamine autoreceptors and second messengers controlling tyrosine hydroxylase activity in rat brain. Neurochem Int. 1992;20:89S–93S. doi: 10.1016/0197-0186(92)90217-f. [DOI] [PubMed] [Google Scholar]
- Owe-Larsson B, Berglund M, Kristensson K, Garoff H, Larhammar D, Brodin L, Low P. Perturbation of the synaptic release machinery in hippocampal neurons by overexpression of SNAP-25 with the Semliki Forest virus vector. Eur J Neurosci. 1999;11:1981–87. doi: 10.1046/j.1460-9568.1999.00614.x. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat Neurosci. 2007;10:1116–24. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JR, Reynolds JS, Thompson JA, Zaccone EJ, Shimko MJ, Goldsmith WT, Jackson M, McKinney W, Frazer DG, Kenyon A, Kashon ML, Piedimonte G, Castranova V, Fedan JS. Pulmonary effects after acute inhalation of oil dispersant (COREXIT EC9500A) in rats. J Toxicol Environ Health A. 2011;74:1381–1396. doi: 10.1080/15287394.2011.606794. [DOI] [PubMed] [Google Scholar]
- Rusakov DA. Ca2+-dependent mechanisms of presynaptic control at central synapses. Neuroscientist. 2006;12:317–26. doi: 10.1177/1073858405284672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirboll LR, Grace AA, Bunney BS. Dopamine auto- and postsynaptic receptors: electrophysiological evidence for differential sensitivity to dopamine agonists. Science. 1979;206:80–82. doi: 10.1126/science.482929. [DOI] [PubMed] [Google Scholar]
- Sriram K, Pai KS, Boyd MR, Ravindranath V. Evidence for generation of oxidative stress in brain by MPTP: in vitro and in vivo studies in mice. Brain Res. 1997;749:44–52. doi: 10.1016/s0006-8993(96)01271-1. [DOI] [PubMed] [Google Scholar]
- Sriram K, Shankar SK, Boyd MR, Ravindranath V. Thiol oxidation and loss of mitochondrial complex I precede excitatory amino acid-mediated neurodegeneration. J Neurosci. 1998;18:10287–96. doi: 10.1523/JNEUROSCI.18-24-10287.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Benkovic SA, Hebert MA, Miller DB, O’Callaghan JP. Induction of gp130-related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration: Key signaling pathway for astrogliosis in vivo? J Biol Chem. 2004;279:19936–47. doi: 10.1074/jbc.M309304200. [DOI] [PubMed] [Google Scholar]
- Sriram K, O’Callaghan JP. Signaling mechanisms underlying toxicant-induced gliosis. In: Aschner M, Costa LG, editors. The role of glia in neurotoxicity. 2. Boca Raton, FL: CRC Press; 2005. pp. 141–71. [Google Scholar]
- Staubli U, Ivy G, Lynch G. Hippocampal denervation causes rapid forgetting of olfactory information in rats. Proc Natl Acad Sci USA. 1984;81:5885–87. doi: 10.1073/pnas.81.18.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, Goldstein LS. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–88. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6:235–43. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–81. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Striessnig J, Koschak A, Sinnegger-Brauns MJ, Hetzenauer A, Nguyen NK, Busquet P, Pelster G, Singewald N. Role of voltage-gated L-type Ca2+ channel isoforms for brain function. Biochem Soc Trans. 2006;34:903–9. doi: 10.1042/BST0340903. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Young SJ, Groves PM. Autoreceptor-mediated changes in dopaminergic terminal excitability: Effects of increases in impulse flow. Brain Res. 1984;309:309–16. doi: 10.1016/0006-8993(84)90598-5. [DOI] [PubMed] [Google Scholar]
- Upreti RK, Das M, Shanker R. Dermal exposure to kerosene. Vet Hum Toxicol. 1989;31:16–20. [PubMed] [Google Scholar]
- Vinet J, Sík A. Expression pattern of voltage-dependent calcium channel subunits in hippocampal inhibitory neurons in mice. Neuroscience. 2006;143:189–212. doi: 10.1016/j.neuroscience.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Ahlijanian MK, Catterall WA. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990;347:281–84. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]