Abstract
Chromobacterium violaceum is a Gram-negative organism found in water and soil. C. violaceum is not usually pathogenic in humans; only approximately 150 human cases have been reported worldwide. C. violaceum bacteraemia progresses rapidly, leading to fatal sepsis on dissemination to multiple organs within a short time. We describe two cases of fatal septicaemia caused by C. violaceum in siblings. Our initial impression was that these cases were associated with an undiagnosed immunodeficiency in the siblings. However, detailed patient histories revealed a potential underlying immunodeficiency in only one patient. These findings prompted us to investigate possible environmental exposure. We identified C. violaceum in filtered water that was sold to the family at a nearby store. This discovery led to a public health alert and closer scrutiny of similar stores by the Ministry of Health.
Background
Chromobacterium violaceum is a rare cause of bacteraemia and death. However, previous reports have linked C. violaceum deaths to underlying immunodeficiencies.1 2 In this report, we present two cases of siblings who died due to septic shock. Blood cultures from the patients confirmed C. violaceum bacteraemia. Our initial assumption that both patients were immunodeficient was not correct, which prompted a search for an environmental source of infection. We isolated C. violaceum from the family's source of drinking water.
Cases of C. violaceum bacteraemia have been reported in 20 countries, all located between the tropical and subtropical latitudes.3 In the largest case series,3 the most common sources of infection were natural bodies of water, and water and soil in puddles.
To the best of our knowledge, C. violaceum sepsis has never been reported in the arid climates of the Middle East and North Africa. In this report, C. violaceum was traced to treated drinking water that became contaminated due to poor storage conditions and poor hygiene. A search of the medical literature showed only one reported case of C. violaceum found in drinking water; that case involved spring water in Uganda.4
This case study emphasises the importance of obtaining a thorough patient history and following up the differential diagnosis to make a final diagnosis. Moreover, this report highlights the danger of unhygienic water storage conditions in regions that depend on desalinised water.
Case presentation
Case 1
An 11-year-old Jordanian boy with cerebellar atrophy, midbrain atrophy and seizure disorder presented with convulsions to the accident and emergency department at our hospital. The patient had been sick for a few days prior to presentation, with fever, vomiting, cough and rhinorrhoea. A previous convulsive episode occurred 5 years prior, and antiepileptic medication had been discontinued 3 years prior to the current presentation.
The patient had moved to Bahrain with his family a year earlier after his father, who was in the Jordanian military, accepted a contract position in the Bahrain Defence Force. The boy's medical history was significant for repeat infections (according to his father) involving the throat and chest; these episodes were usually treated with oral antibiotics. He was hospitalised in Jordan at 2 years of age with invasive salmonellosis, which was confirmed by blood culture. There was no history of oral thrush, chronic skin inflammation, recurrent skin infections or chronic diarrhoea. The boy's parents were non-consanguineous and there was no family history of immunodeficiency or childhood death.
Despite his congenital brain malformation and seizure disorder, the boy had normal motor function and development. His expressive language development was delayed (his first words were at 3 years of age), but his receptive language development was normal. He had a significant learning disability, mainly in reading and math; therefore, he was enrolled in an individualised education programme at school. A formal assessment was not conducted. The boy independently performed all activities of daily living, including changing clothes, eating and bathing.
The boy was admitted to the ward, and treated with phenytoin and ceftriaxone. He was hospitalised for 3 days, during which he remained asymptomatic and stable. He was discharged home in good condition and advised to continue oral cefdinir for 5 days.
Meanwhile, blood culture results were negative at 120 h. A complete blood study showed a white cell count of 7×109/L, haemoglobin of 13.3 g/L, platelet count of 322×109/L and neutrophilia (83.1%). Electrolytes, urea and creatinine were within normal; however, liver enzyme levels deviated slightly from normal (alkaline phosphatase, 201 IU/L; alanine aminotransferase, 85.6 IU/L; aspartate aminotransferase, 113.7 IU/L; and γ-glutamyl transpeptidase, 24 IU/L).
On 1 January 2015, 2 days after discharge, the boy returned to the emergency department with a 2-day history of throat pain and fever. He registered a high-grade fever (up to 40°C). He had been experiencing loss of appetite and poor oral intake for 2 days, as well as a dry cough (which had persisted since the last admission). He experienced no changes in bowel habits and had no abdominal pain, headache or photophobia. He did not have a history of trauma or recent travel.
On presentation, the boy was stable and afebrile. Physical examination revealed findings similar to those at first presentation (congested throat, brisk reflexes and abnormal heel-to-shin test). Venous blood gas (VBG) results were normal, white cell count was 22.23×109/L, Hg was 13.7 g/L, platelet count was 396×109/L and neutrophilia was present (88.20%). Electrolyte analysis revealed a total carbon dioxide level of 15.2 mmol/L, and urea and creatinine levels were normal. The boy was kept in the emergency short-stay unit for intravenous hydration and intravenous ceftriaxone, and he was to be transferred to the ward once a bed became available.
On day 2, the boy experienced emesis approximately 15–17 times in the accident and emergency department; the past six episodes of vomiting were coffee-coloured. The coagulation profile of the patient was normal. During the next assessment, he was lethargic and appeared sick, with rapid deep breathing. On auscultation of the chest, bilateral rhonchi were observed at the bases. A central nervous system examination revealed sluggish pupils and no meningeal signs. Cardiovascular and gastrointestinal examinations were normal. VBG results revealed metabolic acidosis and hypoglycaemia was observed, which prompted the administration of a dextrose infusion. Because the patient showed irregular breathing and low Glasgow Coma Scale, he was intubated and ventilated. At this point, treatment with vancomycin, ceftriaxone, erythromycin and acyclovir was initiated.
A few hours later, the boy developed hypotension with hypoglycaemia that did not respond to corrective measures. Repeated electrolyte, renal function and liver function analyses revealed deterioration of renal function and further deviation of liver enzyme levels. Unfortunately, due to the rapid progression of his illness, we were unable to perform an abdominal ultrasound.
The boy suddenly developed bradycardia and went into cardiac arrest. Despite cardiopulmonary resuscitation and resuscitation efforts, he passed away.
A few days later, blood culture results revealed a pigmented C. violaceum strain that was sensitive to cefepime, amikacin, piperacillin/tazobactam, imipenem, gentamicin and trimethoprim/sulfamethoxazole, and resistant to ampicillin, amoxicillin, cefazolin, cephalexin, cefuroxime, co-amoxi-clavulanic acid and ertapenem.
Case 2
A previously healthy 5-year-old boy from Jordan presented to the accident and emergency department at the Bahrain Defence Force Hospital with a 1-week history of right-sided submandibular swelling with tenderness and fever. He was initially seen at his primary healthcare centre, where he was treated with amoxicillin/clavulanic acid. Although the fever resolved on day 3 of antibiotic treatment, the health centre referred him to the accident and emergency department because the swelling on the right side of the neck did not improve and remained tender.
On examination, the boy was afebrile, alert and well hydrated. The ear, nose and throat examination did not reveal a source of infection, as the bilateral oropharynx and tympanic membranes were normal. There was no evidence of trauma and no puncture or scratch marks were visible on the skin. Furthermore, we observed no evidence of chronic skin inflammation. The neck mass, approximately 4×1 cm, was tender on palpation and mobile. The overlying skin did not show evidence of fluctuance or erythaema. The boy had no history of chronic cough or recent weight loss.
The boy had moved with his family to Bahrain a year prior to presentation after his father accepted a position in the Bahrain Defence Force. He had no previous hospitalisations and had never undergone a surgical procedure. He had no history of recurrent sinopulmonary infections, skin infections, lymphadenitis, chronic skin rash or diarrhoea. He was current on all vaccinations; he did not receive a BCG vaccine. He had not travelled outside of Bahrain after relocating from Jordan. His neurodevelopment was normal.
A complete blood study performed 2 days before the boy presented to the accident and emergency department revealed a white cell count of 9.9×109/L with equal lymphocyte and neutrophil populations, a Hg level of 10.7 g/L, a platelet count of 389×109/L and an erythrocyte sedimentation rate (ESR) of 12. A neck ultrasound showed a swollen lymph node (4×2.5 cm) occupying the area between the tail of the right parotid gland, the posterior surface of the right sternocleidomastoid muscle and the right internal jugular vein/common carotid artery and its bifurcation.
At this point, a decision was made to continue oral antibiotic therapy and to have the boy examined by an otolaryngologist on an outpatient basis.
He was examined by an otolaryngologist a week after his visit to the accident and emergency department. He remained afebrile; however, no improvement was observed in the size of the right submandibular swelling, which remained tender. The otolaryngologist arranged for fine needle aspiration by an interventional radiologist and conducted a Mantoux test.
The Mantoux test results were read a few days later, and they showed no reaction. Fine needle aspiration recovered very little fluid, and another ultrasound examination revealed multiple matted hypoechoic lesions with poorly defined borders. Gram staining and acid fast bacillus staining of the aspirate were negative, and an aspirate culture did not grow any organisms. The pathological examination revealed evidence of reactive lymphadenitis with no malignant cells.
Four weeks after the boy's initial presentation, the otolaryngologist evaluated him again. The right submandibular swelling had not changed in size. The analyses had not revealed an aetiology and, consequently, the boy was admitted for incision and drainage (I&D) to reduce the swelling.
The admission was uneventful. On admission, the boy was treated with cefuroxime, and an I&D was performed the next day. The operation revealed a fluctuant 5 cm mass, which was incised and drained, displaying central liquefaction and pus. A pus sample was sent for microbiology and culture.
The pus Gram stain was negative, and the pathology report revealed granulation tissue with mixed acute and chronic inflammatory infiltration. There was no evidence of malignancy.
The wound was packed, and the boy was discharged, and advised to return daily for dressing changes and wound care. He regularly returned to the otolaryngology clinic for wound care, and on day 10 postoperative, the wound was almost completely healed. At this point, the packing was removed, and the wound was closed with Steri-Strips. Throughout his visits to the otolaryngology clinic, the boy was stable and afebrile.
A week after his last clinic visit, the boy presented to the accident and emergency department with a 2-day history of abdominal cramps, vomiting and fever. On arrival, he presented with hypotension with poor perfusion, drowsiness and confusion. The signs and symptoms were consistent with septic shock. Despite aggressive fluid resuscitation, placement of an advanced airway and early use of antibiotics, he continued to deteriorate and went into asystole in the accident and emergency department.
He was treated with meropenem and gentamicin based on the sensitivity pattern of the Chromobacterium isolate obtained from the blood of his sibling. Despite our best efforts, the boy passed away.
A few days later, the boy's blood cultures confirmed a pigmented strain of C. violaceum with the same resistance pattern as that in his sibling's blood.
Investigations
Looking back at the investigations carried out for the siblings while they were alive, most of the results were very non-specific. In fact, what triggered further investigation of these two cases is that sepsis was unexpected and developed very rapidly. This in the context of an uncommon organism found in the blood of the siblings.
The older brother's investigations on his first admission, when he presented after a seizure, showed an unexplained mild elevation in liver enzymes, as mentioned above. The elevated liver enzymes were attributed to his recent viral infection; however, in retrospect, they may have been due to early septicaemia.
The younger sibling's non-specific findings included thrombocytosis and elevated ESR. This was attributed to his lymphadenitis at the time of presentation. Again, in retrospect, it was clear that the lymphadenitis and laboratory findings of inflammation were due to exposure to C. violaceum.
If we look at the timeline of illness in the siblings, both developed the first symptoms during the last week of December 2014. The older boy died on 3 January 2015, and his younger brother died on 31 January 2015. This suggests an exposure from a source in common to both siblings.
Differential diagnosis
Given that two siblings were involved, our initial hypothesis was that both had an underlying undiagnosed immunodeficiency. The older sibling had a history of repeat sinopulmonary infections and at least one documented episode of invasive Salmonella. Although invasive Salmonella is more common in children with T-cell deficiency, there have been many reported cases in children with humoral immunodeficiency and in those with impaired phagocytic function.5 Unfortunately, we were unable to conduct a thorough work up for immunodeficiencies due to the rapid progression of symptoms and the subsequent death of the older boy. The recurrent sinopulmonary infections in this child suggested a humoral immunodeficiency.
On the other hand, the younger child's medical history did not suggest an immunodeficiency. He did not have repeat infections, chronic diarrhoea, eczema, thrush or failure to thrive. This fact pushed us towards an environmental survey to see if the siblings were exposed to a contaminated water source.
Outcome and follow-up
As mentioned previously, C. violaceum is mainly found in water and soil. Therefore, we searched for possible sources of C. violaceum contamination in the patients’ local environment. With consent from the father, we obtained samples of the family's home water source (not potable water), water from the local filtered water supplier that sold drinking water, and a water puddle at the 11-year-old patient's school.
The water samples were incubated in aerobic and anaerobic culture media (commonly used for blood cultures), and the soil sample was directly plated. MacConkey agar was used to for Gram-negative organisms. Of the three samples tested, the one from the filtered drinking water contained a pigmented strain of C. violaceum.
The drinking water was sold to the local distributer by a private water desalination plant. All the water processed at the desalination plant is from the sea. The local distributer stored the water in tanks and sold the water to the two boys’ family.
The identity of the organism (C. violaceum) was confirmed using an automated microdiffusion identification system (MicroScan) and an API 20E system. The isolated C. violaceum produced violacein and, therefore, it was classified as a pigmented strain. The organism was sensitive to cefepime, amikacin, piperacillin/tazobactam, imipenem, gentamicin and trimethoprim/sulfamethoxazole, and resistant to ampicillin, amoxicillin, cefazolin, cephalexin, cefuroxime, co-amoxi-clavulanic acid and ertapenem.
Ideally, 16s rRNA analysis should have been used to confirm C. violaceum; however, this was not available. To confirm that a single organism from one source was responsible for the disease in both siblings, we should have used pulsed-field gel electrophoresis or DNA fingerprinting to analyse the blood cultures from both siblings and the drinking water sample. Unfortunately, when C. violaceum was identified in the water sample, the blood culture samples were no longer available.
After the presence of C. violaceum was confirmed in the filtered water source, we alerted public health officials. In Bahrain, environmental and occupational healthcare is the responsibility of the Public Health Directorate, which plays a major role in raising the sanitation standards in Bahrain as well as controlling and eradicating infectious and communicable diseases.
The officials visited the store that supplied the water and found numerous health violations with respect to how the water was stored (figures 1 and 2) and how the tubing that delivered the water to the access point (figure 3) was connected to the main tank. Furthermore, the storeowner's licence had expired, and, thus, the store was not subjected to regular water sampling. The Public Heath Directorate repeated the culture on the water from the storage tanks. The sample taken directly from the water tank grew C. violaceum (pigmented), Bacillus cereus and Pseudomonas aeruginosa (figure 4).
Figure 1.

The two tanks used to store water from the desalination plant.
Figure 2.

An inspection revealed an unhygienic storage area with leaky connection points to the consumer access point. Arrows indicate water leaking from pipes.
Figure 3.

The water access point where customers fill water containers for home use.
Figure 4.
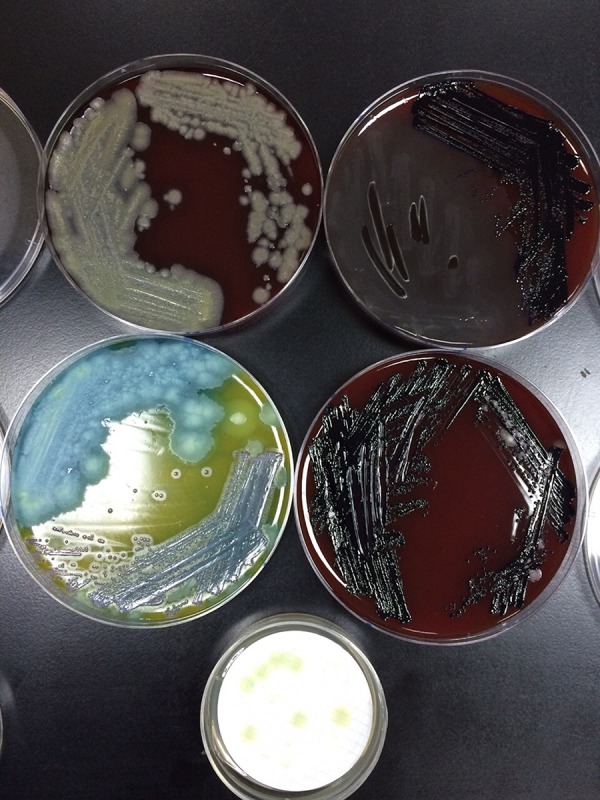
Cultures from the water tank grew Bacillus cereus (top left), Pseudomonas aeruginosa (bottom left) and Chromobacterium violaceum (top and bottom right).
Public health officials proceeded to screen all the water providers in possession of a valid licence. They also ensured that stores with expired licenses had in fact stopped selling water. Legal action was initiated against the storeowner.
Further actions taken by the Public Health Directorate included screening all the water tanks on delivery trucks that transport water from the desalination plant and screening the water at the desalination plant. All the cultures were negative.
Discussion
C. violaceum is a motile, Gram-negative, facultative anaerobic, oxidase-positive and saprophytic microorganism commonly found in water and soil.6–8 It is sensitive to extreme temperatures, and flourishes in tropical and subtropical areas.3
This microorganism grows well on MacConkey agar and produces a bright purple pigment, violacein. Approximately 9% of C. violaceum strains are non-pigmented. Pigmented and non-pigmented strains are equally pathogenic in mice, but only one case of infection with a non-pigmented strain has been reported in humans.8
Although C. violaceum is usually not pathogenic in humans, an increasing number of cases have been reported.3 C. violaceum bacteraemia results in rapidly progressing and often fatal sepsis, with dissemination to multiple organs within a short time.9–13 The first documented case of C. violaceum in humans occurred in Malaysia in 1952.14 Yang and Li3 reported 106 cases between 1952 and 2009; 42% of these patients were less than 10 years of age, and the mortality rate in children was 71.4%.
Previous studies have isolated C. violaceum from children with chronic granulomatous disorders;1 2 however, case reviews by Yang and Li3 in 2011 and Sirinavin et al6 in 2005, demonstrated that the majority of patients did not have a known immunodeficiency. In their case review, Yang and Li identified the most common risk factors for C. violaceum bacteraemia as exposure to contaminated water or soil, trauma followed by exposure to soil and swimming in contaminated water.
Yang and Li reported that the most common symptoms of C. violaceum infection are fever and abdominal pain as well as pain, erythaema and swelling at the infection site, with disseminated abscess formation; the infection rapidly progresses to sepsis.
Although we were unable to confirm by DNA fingerprinting that both siblings were infected by drinking contaminated water, the circumstantial evidence is very suggestive. In the cases described in the literature, the point of entry for this pathogen was the skin, oropharynx or lungs after inhalation of contaminated water.3 Neither sibling had a recent history of trauma and neither had swum in a potentially contaminated body of water. As mentioned above, the water tank in their apartment was not contaminated, so tap water was not a source. Therefore, the only entry point for C. violaceum was the nasopharynx/oropharynx.
Importantly, the antibiotics used to empirically treat sepsis are usually not effective against this organism. The first sibling was treated with vancomycin, ceftriaxone, erythromycin and acyclovir, and the second sibling was treated with meropenem and gentamicin based on the sensitivity pattern of the C. violaceum isolated from the blood of the first sibling.
In the first case, the antibiotics were most likely not effective; however, this patient was treated empirically for sepsis because the organism had not been isolated at the time. Even in the second case, the chosen antibiotics may not have been ideal. Although the blood samples from both brothers showed sensitivity to gentamicin and imipenem, most of the successfully treated cases in the literature received ciprofloxacin and a carbapenem.6
Bahrain is an oil-producing country with many free facilities available to the public. Although municipal water access is universal, the water is not treated appropriately for consumption. As an alternative, drinking water is sold throughout the country at a low price. The cheapest water is sold by distributors who buy water in bulk from private desalination water plants. Despite public health scrutiny of the water that is sold by these distributors, the water sold to the family of the two siblings was not subjected to this process. Because the owner of the water distributor did not renew his licence, the water sold by his company was not routinely tested.
This incident demonstrates the importance of strict water sanitation and the major public health risk of undersanitation. These two fatalities present a strong argument for the provision of clean drinking water via a municipal water supply in Bahrain.
Patients’ perspective.
The boys’ father found it very difficult to discuss his experience. I offered to facilitate the discussion by asking him a few questions in an interview. We spoke in Arabic; I have translated the interview to the best of my abilities. My questions are in bold.
Sir, thank you for taking the time to talk about your experience and the loss of your two sons. I know it must be very hard to relate the events of their illnesses. What thoughts were going through your mind as the events unfolded?
As you can imagine, it was a very difficult time for me and my family. We lost two sons within a period of 4 weeks. I had barely recovered from the shock of my older son's death when the same thing happened to my younger boy.
My older son had multiple throat and chest infections and was constantly in and out of clinics; he also had other problems, such as seizures and difficulties at school. Even with the problems he had, I never expected to lose him like this, or so quickly. On the day my younger son got very sick and was in the emergency room, he became worse very suddenly; the doctors said that his blood pressure was dangerously low. A group of doctors quickly came in to help, and they asked me to wait outside. Later, they told me that my son had died despite their best efforts. I was dumbfounded; I could not believe that this happened again. The boy was a completely healthy child before all this began. It was enough to drive a man crazy.
I was told that my first son died as a result of a very rare and resistant bacteria. I thought this was a rare uncommon infection, and I was shocked to learn that the same bacteria killed my other son. Did it come from the hospital or home? I did not know. I feared for the safety of my other children. I sold my car, bought airline tickets and moved my family back to Jordan. Because of my work, I had to remain in Bahrain, and so I moved into the army accommodations.
I can only imagine how hard it must have been to lose two children in such a short period of time. How are you and your family coping?
At first, I was gripped with panic. I feared for my other children. Once I managed to safely send them abroad and I was left alone, the grief took full force. I was paralysed; I felt that I would never recover. As the days passed, things got better with the support of my faith and friends. There was also a financial burden; I had spent a great deal of money to send my family abroad. The hospital put me in contact with local charities that helped me cope with this burden.
Was there anything that the medical team could have done differently?
My older son was admitted to hospital during the new year's break; because it was very busy, they kept us in the emergency observation unit until a bed became available. A nurse was present, but the doctors came in only twice a day. I know that it was a holiday and that it was busy, but maybe if they saw him more often, they could have seen signs that he was taking a turn for the worse. Also, the blood culture that revealed the lethal bacteria came back 3 days after he died. What good will it do at that point?
Thank you for your honesty. I just want to address these two issues. The observation unit has a senior nurse who is experienced in emergency care of our patients. Doctors are available by request to address any concerns that the nurse or the family may have. The physicians see the patients at least twice a day. This is similar to the wards, where each child is seen at least twice a day, and our nurses voice any concerns to the ward physician. Furthermore, the unit is in the emergency department, so our emergency physicians can provide immediate help if necessary. As for the blood culture delay, I wish there was a practical way to more rapidly identify this bacteria, but as this is a rare bacteria that we do not commonly see, the use of rapid DNA testing is not practical or easily available. The only other option is to allow the bacteria to grow and to identify it using staining techniques and a microscope.
In the future, telemetric monitoring of vital signs that are processed and relayed to physicians may provide more minute-by-minute monitoring. Also, DNA testing techniques may become more available and inclusive of common and rare bacteria.
Is there anything else you want to add?
I'm very grateful for the fact that the bacteria was found in the drinking water. Knowing this gave me peace of mind. On the other hand, I would urge closer monitoring of drinking water. Water that is sold to the public should be safe for use. Even though this particular seller did not renew his licence, the authorities should have checked that he stopped selling water. This should never happen again.
I completely agree with you; with the resources in Bahrain, we should not be dealing with this problem. In my opinion, instead of monitoring water that is for sale, potable water should be available for free through a municipal tap water system.
Again, thank you for sharing your story with us.
Learning points.
Always conduct a thorough patient history.
Do not be satisfied with the initial diagnosis if it does not fit your patient.
Maintain a broad differential diagnosis and follow-up on the possibilities.
Empiric antibiotic therapy for sepsis is not effective for Chromobacterium violaceum sepsis.
Access to clean water is a pillar of public health; even the smallest gap in water sanitation regulation present a major public health risk.
Footnotes
Twitter: Follow Salman Al Khalifa at @drsalkhalifa
Contributors: SMAK wrote the summary and background for case 2, made investigations, and wrote the sections on the differentials, follow-up and outcome, and discussion. He was also involved in patient care, and interviewed the father and surveyed the environmental sources. TAK wrote case 1. He was involved in patient care, interviewed the father and surveyed the environmental sources. In addition, he reviewed and improved the paper. MMA provided expertise on isolation and culture of the organism, and reviewed and improved the paper. AMAA provided advice on format and formulation, and also reviewed and improved the paper.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Macher AM, Casale TB, Gallin JI et al. Chromobacterium violaceum infectious and chronic granulomatous disease. Ann Intern Med 1983;98:259 10.7326/0003-4819-98-2-259_2 [DOI] [PubMed] [Google Scholar]
- 2.Macher AM, Casale TB, Fauci AS. Chronic granulomatous disease of childhood and Chromobacterium violaceum infections in the southeastern United States. Ann Intern Med 1982;97:51–5. 10.7326/0003-4819-97-1-51 [DOI] [PubMed] [Google Scholar]
- 3.Yang CH, Li YH. Chromobacterium violaceum infection: a clinical review of an important but neglected infection. J Chin Med Assoc 2011;74:435–41. 10.1016/j.jcma.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 4.Byamukama D, Farnleitner AH, Kansiime F et al. Contrasting occurrence of Chromobacterium violaceum in tropical drinking water springs of Uganda. J Water Health 2005;3:229–38. [DOI] [PubMed] [Google Scholar]
- 5.Haeusler GM, Curtis N. Non-typhoidal Salmonella in children: microbiology, epidemiology and treatment. Adv Exp Med Biol 2013;764:13–26. 10.1007/978-1-4614-4726-9_2 [DOI] [PubMed] [Google Scholar]
- 6.Sirinavin S, Techasaensiri C, Benjaponpitak S et al. Invasive Chromobacterium violaceum infection in children: case report and review. Pediatr Infect Dis J 2005;24:559–61. 10.1097/01.inf.0000164761.81491.3f [DOI] [PubMed] [Google Scholar]
- 7.Ponte R, Jenkins SG. Fatal Chromobacterium violaceum infections associated with exposure to stagnant waters. Pediatr Infect Dis J 1992;11:583–6. 10.1097/00006454-199207000-00013 [DOI] [PubMed] [Google Scholar]
- 8.Tucker RE, Winter WG Jr, Wilson HD. Osteomyelitis associated with Chromobacterium violaceum sepsis. A case report. J Bone Joint Surg Am 1979;61:949–51. [PubMed] [Google Scholar]
- 9.Sivendra R, Tan SH. Pathogenicity of nonpigmented cultures of Chromobacterium violaceum. J Clin Microbiol 1977;5:514–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ang YM. A very rare and rapidly fatal case of Chromobacterium violaceum septicemia. Med J Malaysia 2004;59:535–7. [PubMed] [Google Scholar]
- 11.Campbell JI, Lan NP, Qui PT et al. A successful antimicrobial regime for Chromobacterium violaceum induced bacteremia. BMC Infect Dis 2013;13:4 10.1186/1471-2334-13-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter E, Cain K, Rutland B. Chromobacterium violaceum cellulitis and sepsis following cutaneous marine trauma. Cutis 2008;81:269–72. [PubMed] [Google Scholar]
- 13.Chattopadhyay A, Kumar V, Bhat N et al. Chromobacterium violaceum infection: a rare but frequently fatal disease. J Pediatr Surg 2002;37:108–10. 10.1053/jpsu.2002.29439 [DOI] [PubMed] [Google Scholar]
- 14.Patterson RH Jr, Banister GB, Knight V. Chromobacterial infection in man. AMA Arch Intern Med 1952;90:79–86. 10.1001/archinte.1952.00240070085008 [DOI] [PubMed] [Google Scholar]


