Abstract
Activation of a cellular stress response and the transcription factor XBP1 in dendritic cells has now been shown to limit the cells' ability to stimulate antitumour immune responses in a mouse model of ovarian cancer.
Cells in the microenvironment of a tumour are exposed to stressful events. This is due in part to a lack of oxygen and nutrients that leads to malfunction of the endoplasmic reticulum (ER), the cellular organelle responsible for protein folding. The result is an accumulation of unfolded proteins — a state referred to as ER stress. Cancer cells use a signalling pathway known as the ER stress response to survive in this environment. Whether this pathway also affects non-cancer cells in the tumour microenvironment, which are exposed to similar conditions, was not known. Writing in Cell, Cubillos-Ruiz et al.1 show, in a model of ovarian cancer, that induction of the ER stress response in tumour-associated dendritic cells disrupts the cells' ability to promote adaptive antitumour immunity.
Dendritic cells (DCs) are a type of immune cell found in all tissues. DCs are classed as antigen-presenting cells (APCs) because they present characteristic molecular structures (antigens) expressed by tissue cells to the immune systems T cells and so elicit tissue-specific T-cell responses2. In the tumour environment, DCs sample, process and present tumour-cell-associated antigens and are thus potent inducers of T-cell responses that can eliminate target tumour cells. Other APCs include tissue-resident macrophages and circulating monocytes. Although these are less potent than DCs, they can also modulate T-cell effector responses2.
The key role of T-cell responses in cancer treatment has been firmly established in recent years through the remarkable clinical success of strategies aimed at increasing the activation of tumour-targeting T cells. These therapies operate through the engineering of T cells to carry receptors that bind strongly to tumour antigens or by blocking inhibitory molecules on T cells to increase T-cell function. However, only subsets of patients respond to these treatments, and there is an urgent need to identify other brakes on antitumour immunity.
Although interactions between T cells and DCs typically occur in tissue-draining lymph nodes, it is now clear that DC presentation of tumour antigens also occurs in the tumour itself, suggesting that DCs in the tumour microenvironment can substantially influence the functions of antitumour T cells. Thus, it is crucial to assess the characteristics of tumour-associated DCs and to identify molecules for maximizing local antigen presentation that might also be potential drug targets. Altered DC function is common in tumours, and different mechanisms that underlie DC dysregulation in the tumour microenvironment have been identified, including reduced DC accumulation at the tumour site3 and increased immuno suppression induced by soluble factors (cytokines) that are produced by tumour cells or their surrounding tissue4.
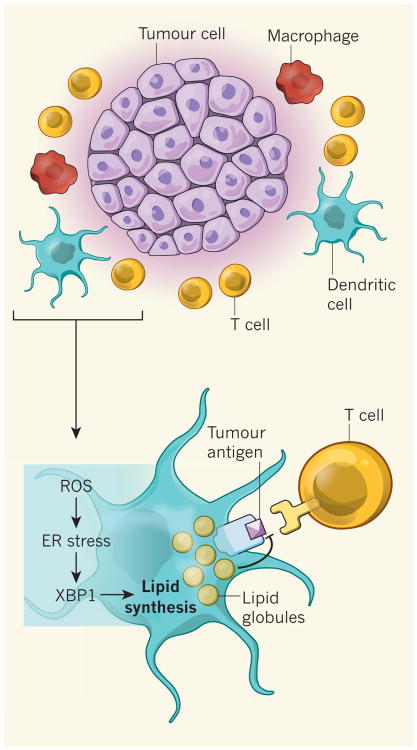
The ER stress response is mediated by several signalling molecules, among which the enzyme IRE 1α and its target transcription factor XBP1 are the most evolutionarily conserved. XBP1 has been shown to promote tumour growth when expressed in cancer cells5. Using a mouse model of ovarian cancer, Cubillos-Ruiz et al. show that damaging molecules called reactive oxygen species (ROS) accumulate in tumour-associated DCs, and that ROS cause lipid degradation (by peroxidation) and the accumulation of lipid by-products, which in turn leads to the ER stress response and XBP1 activation (Fig. 1). Analysis of the genes expressed by these DCs revealed that a lipid-synthesis program is induced in XBP1-expressing cells but not in those lacking XBP1.
Figure 1. XBP1 activation inhibits presentation of tumour antigens.
Antitumour immune responses rely on dendritic cells (DCs) to present molecular structures (antigens) from tumour cells to T cells, which can then kill the antigen-bearing tumour cells. Cubillos-Ruiz et al.1 show that damaging molecules called reactive oxygen species (ROS), which are produced in tumour-associated DCs, induce an endoplasmic reticulum (ER) stress response. The ER stress pathway leads to expression of the transcription factor XBP1, which induces lipid synthesis. The resulting accumulation of lipids in the DCs reduces their ability to present antigens and thus impairs antitumour T-cell responses. Macrophage cells may also contribute to the ER stress response.
The authors went on to show that deletion of the gene that encodes XBP1 in cells expressing CD11c (an integrin protein expressed at high levels on DCs), or delivery of short inhibitory RNA molecules that inhibit XBP1 expression, led to reduced lipid accumulation in DCs, increased DC antigen-presentation capacity, greater accumulation of T cells in the ovarian tumours and reduced tumour growth. Scavenger molecules that eliminate lipids and reduce ROS levels similarly improved DC antigen-presentation ability, suggesting that XBP1's function in enhancing tumour formation is at least partly due to the induction of lipid biosynthesis in DCs.
Although DCs express high levels of CD11c in the steady state, other APCs such as macrophages and monocyte-derived cells can also express CD11c (ref. 2), and so the therapeutic benefit of XBP1 deletion in CD11c-express-ing (CD11c+) cells might not solely reflect its effect on DCs. XBP1 has an important role in the physiology of APCs under homeostatic conditions: deletion of XBP1 in non-tumour DCs compromises their survival6 and antigen-presentation ability7, and loss of XBP1 in activated macrophages reduces cytokine release in response to microbial stimuli8. These findings contrast with Cubillos-Ruiz and colleagues' demonstration that XBP1 deletion in tumour-associated CD11c+ cells does not compromise DC survival and improves their antigen-presentation function. Such surprising results suggest that the pro-immunogenic homeostatic function of XBP1 is subverted in tumour-associated DCs. The extent to which the tumour or its environment promotes XBP1 activation or subverts its function should be explored further.
Increased lipid accumulation has been observed in tumour-associated CD11c+ cells in tumour-bearing mice and in patients with cancer9. Whether the lipid-laden CD11c+ cells described in these studies represent bona fide DCs, monocyte-derived cells or macrophages will need to be addressed by cell-specific assays. Lipid accumulation in macrophages has also been linked to neurodegenerative diseases and atherosclerosis, and it will be interesting to explore whether XBP1 contri butes to the progression of these conditions.
Cubillos-Ruiz and colleagues' results extend previous studies10,11 showing that metabolic changes, for example increased breakdown of the amino acids arginine and tryptophan in tumour-associated DCs, alter T-cell effector function. Greater understanding of the extent of metabolic alterations in tumour-associated DCs should help to identify yet other pathways of immune dysregulation in tumours. In the meantime, the authors' finding that XBP1 deletion in DCs increases T-cell infiltration of tumours and reduces tumour growth is a timely addition to the pressing search for target that increase antitumour T-cell function.
References
- 1.Cubillos-Ruiz JR, et al. Cell. 2015;161:1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merad M, Sathe P, Helft J, Miller J, Mortha A. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spranger S, Bao R, Gajewski TF. Nature. 2015 doi: 10.1038/nature14404. http://dx.doi.org/10.1038/nature14404. [DOI] [PubMed]
- 4.Chaput N, Conforti R, Viaud S, Spatz A, Zitvogel L. Oncogene. 2008;27:5920–5931. doi: 10.1038/onc.2008.270. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, et al. Nature. 2014;508:103–107. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwakoshi NN, Pypaert M, Glimcher LH. J Exp Med. 2007;204:2267–2275. doi: 10.1084/jem.20070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osorio F, et al. Nature Immunol. 2014;15:248–257. doi: 10.1038/ni.2808. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F, Chen X, Lee AH, Glimcher LH. Nature Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herber DL, et al. Nature Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norian LA, et al. Cancer Res. 2009;69:3086–3094. doi: 10.1158/0008-5472.CAN-08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellor AL, Munn DH. Nature Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]