Abstract
Neutrophils have been shown to efficiently kill Cryptococcus neoformans, a causative agent of meningoencephalitis. Here, using live-cell imaging, we characterize the dynamic interactions of neutrophils with C. neoformans and the underlying mechanisms in real time. Neutrophils were directly seen to chase C. neoformans cells and then rapidly internalize them. Complement C5a-C5aR signaling guided neutrophils to migrate to the yeast cells, resulting in optimal phagocytosis and subsequent killing of the organisms. The addition of recombinant complement C5a enhanced neutrophil movement but did not induce chemotaxis, suggesting that the C5a gradient is crucial. Incubation with C. neoformans resulted in enhanced activation of Erk and p38 mitogen-activated protein (MAP) kinases (MAPKs) in neutrophils. Inhibition of the p38 MAPK pathway, but not the Erk pathway, significantly impaired neutrophil migration and its subsequent killing of C. neoformans. Deficiency of CD11b or blocking of CD11b did not affect the migration of neutrophils toward C. neoformans but almost completely abolished phagocytosis and killing of the organisms by neutrophils. C5a-C5aR signaling induced enhanced surface expression of CD11b. Interestingly, the original surface expression of CD11b was essential and sufficient for neutrophils to attach to C. neoformans but was unable to mediate phagocytosis. In contrast, the enhanced surface expression of CD11b induced by C5a-C5aR signaling was essential for neutrophil phagocytosis and subsequent killing of yeast cells. Collectively, this is the first report of the dynamic interactions of neutrophils with C. neoformans, demonstrating a crucial role of C5a-C5aR signaling in neutrophil killing of C. neoformans in real time.
INTRODUCTION
The study of neutrophil interactions with Cryptococcus neoformans started decades ago (1, 2). So far, it has been shown that neutrophils are able to kill C. neoformans (3, 4) and that the antifungal capability of neutrophils is usually greater than that of monocytes (1, 5). In vitro, neutrophils have been shown to kill C. neoformans via intracellular (1, 2) or extracellular (6) killing mechanisms. However, recent data demonstrated that C. neoformans may negatively regulate the extracellular killing activity of neutrophils (7, 8). Both oxidative killing (1, 5, 9, 10) and nonoxidative killing (11) of C. neoformans by neutrophils have been described.
Although the important role of neutrophils in killing of C. neoformans is concluded based mainly on data from in vitro studies, recent results suggested that neutrophils may also exert anticryptococcal activity in vivo (12, 13). Augmentation of neutrophil defenses by administration of granulocyte colony-stimulating factor (G-CSF) remarkably reduced brain fugal burden and significantly enhanced the survival of infected mice, indicating that neutrophils play an important role in the clearance of C. neoformans in the brain in vivo (13). A deficiency of myeloperoxidase (MPO) significantly decreased the rate of survival of mice infected with C. neoformans (12). As MPO is most abundantly expressed in neutrophils (14), this finding also suggested that neutrophils contributed to host defenses against C. neoformans in vivo (12). In clinical settings, administration of G-CSF to AIDS patients enhances the anticryptococcal activity of neutrophils (15, 16). However, it was also reported that depletion of neutrophils did not affect the fungal burden in the lung following intratracheal infection with a mutant strain of C. neoformans that secreted gamma interferon (IFN-γ) (17). Importantly, depletion of neutrophils significantly reduced the pulmonary fungal burden on day 1 and enhanced the survival time of mice infected intratracheally with a wild-type strain of C. neoformans (serotype D), which was associated with higher lung concentrations of interleukin-4 (IL-4), IL-10, IL-12, and tumor necrosis factor alpha (TNF-α) (18). These data suggest that neutrophils may play a detrimental role via modulation of cytokine production during pulmonary infection with C. neoformans (18). Nevertheless, C. neoformans was seen to be efficiently engulfed by neutrophils in the lung in a murine model of cryptococcosis (19). More recently, we have shown that neutrophils contribute to intravascular clearance of disseminating C. neoformans cells following intravenous infection with these yeast cells in a mouse model (20). These data should not be interpreted to exclude the detrimental effect of neutrophils as shown during pulmonary infection (18); instead, they reflect the complexity of roles of neutrophils during C. neoformans infection (4).
In vitro experiments have shown that host defense against C. neoformans by neutrophils can be mediated by complement (21, 22). Both complement C3 and complement C5 are involved in the clearance of C. neoformans in animal models (23–26). In general, the activation of complement leads to the formation of C3 convertase, which cleaves C3 into C3a and C3b. C3b facilitates pathogen opsonization and enables the cleavage of C5 into C5a and C5b (27). C5a plays an important role in the attraction of phagocytes to infectious sites, whereas C5b, together with C6 to C9, leads to the formation of a membrane-attacking complex (MAC) (27). Neutrophils express complement receptors (3). In particular, complement receptor 3 (CR3) (also known as CD11b/CD18) has been shown to play an important role in complement-mediated phagocytosis of C. neoformans (28). However, how C3, C5, and CR3 precisely regulate the dynamic interactions of neutrophils with C. neoformans (i.e., migration of neutrophils toward C. neoformans and subsequent phagocytosis and killing of yeast cells) still remains poorly understood. In this study, we took advantage of live-cell imaging as well as the availability of gene knockout mice to directly characterize the transient and dynamic interactions of neutrophils with C. neoformans in the context of complement and the underlying mechanisms in real time. The results reveal multiple functions of C5a-C5aR signaling during the interactions of neutrophils with C. neoformans and highlight the important roles of this signal in neutrophil antifungal activities.
MATERIALS AND METHODS
Animals.
Inbred C57BL/6 wild-type mice were purchased from the National Cancer Institute (Frederick, MD). Breeding pairs of C3−/− (catalog no. JAX003641), C5−/− (catalog no. JAX000461), C3aR−/− (catalog no. JAX005712), C5aR−/− (catalog no. JAX006845), and CD11b−/− (catalog no. JAX003991) mice were purchased from the Jackson Laboratory and bred in the animal facilities of the University of Maryland. All experiments were performed by using 6- to 8-week-old mice. All experimental procedures were approved by the Institutional Animal Care and Animal Use Committee of the University of Maryland, College Park.
Fungal strains and culture.
Encapsulated Cryptococcus neoformans strain H99 (serotype A) (ATCC 208821) and C. neoformans H99 expressing green fluorescent protein (GFP) (a gift of Robin May, United Kingdom [29]) were used in the experiments. Yeast cells were obtained from glycerol stocks and maintained on Sabouraud dextrose agar (Becton Dickinson). A single colony of C. neoformans was inoculated into Sabouraud dextrose broth (Becton Dickinson) and cultured to exponential phase at 32°C with gentle rotation (180 rpm) for 24 h. The yeast cells were collected by centrifugation at 400 × g for 5 min and washed twice by using sterile phosphate-buffered saline (PBS) before use. The concentration of yeast cells was determined by using a hemocytometer.
Antibodies.
Rat anti-mouse CD11b (clone M1/70) and C5aR (clone 20/70) monoclonal antibodies (Abs) (MAbs) were purchased from Biolegend and used to block CD11b and C5a receptors on neutrophils, respectively. The rat anti-mouse C5a MAb (clone 295108; R&D systems) was used to neutralize C5a released into the medium. The goat anti-mouse CD18 polyclonal Ab (BD Pharmingen) was used for blockade of CD18. The rat anti-mouse CD16/32 MAb (clone 93; eBioscience) was used to block Fc receptors, and the rat anti-mouse CD35 MAb (clone 7E9; BD Science) was used to block complement receptor 1 (CR1). Isotype control antibodies, including rat IgG2a and rat IgG2b, were purchased from Bioxcell.
Chemicals.
Cytochalasin D and DIDS (4,4-diisothiocyanostilbene-2,2′-disulfonic acid) (an anion exchange inhibitor) were purchased from Sigma-Aldrich. SB202190 (a selective inhibitor of p38 mitogen-activated protein [MAP] kinase [MAPK]), PD98059 (a selective inhibitor of MAP/extracellular signal-regulated kinase [ERK] kinase), and DPI (diphenyleneiodonium) (an NADPH oxidase inhibitor) were purchased from Santa Cruz. Cytox orange (Life Technologies) was used as an indicator of cell viability.
Isolation of murine neutrophils from bone marrow.
Murine neutrophils were isolated as described previously (30). Briefly, mice were euthanized, the femur and the tibia from both hind legs were removed, and soft tissue was cut off by fine surgical scissors; the extreme distal tip of each extremity was cut off. Calcium-free Hanks' balanced saline solution (HBSS) was forced through the bone with a 23-gauge needle (BD Science) and filtered through a 40-μm nylon mesh. After cell clumps were dispersed, the cell suspension was centrifuged (400 × g for 10 min at 4°C) and resuspended in 1 ml HBSS. The cells were layered onto a three-layer Percoll gradient of 78%, 69%, and 52% Percoll (GE, USA). Neutrophils from the 69%-to-78% gradient interface and the upper part of the 78% gradient layer were harvested into 15-ml tubes, followed by lysis of red blood cells using ammonium chloride-potassium (ACK) lysis buffer (Life Technologies). The cells were washed twice with HBSS supplemented with 1% bovine serum albumin (BSA) and placed on ice until use. The purity of isolated cells was determined to be >90% neutrophils by flow cytometry following staining with anti-Ly6G MAb, and viability was >95% as determined by trypan blue staining.
In vitro killing assay.
Murine neutrophils were incubated with 2 × 103 C. neoformans cells in 96-well flat-bottomed plates containing 100 μl RPMI 1640 supplemented with 40% fresh wild-type, C3−/−, or C5−/− mouse serum. Control wells contained C. neoformans cells alone with 40% mouse serum. After 4 h of culture at 37°C in 5% CO2, 100 μl 0.1% Triton was added to each well to stop the reaction and lyse neutrophils. Dilutions from each well were plated onto Sabouraud dextrose agar plates and incubated at 30°C for 36 h for counting of CFU. An effector-to-target cell ratio of 10:1 was used throughout all experiments. No significant growth of C. neoformans was observed during the 4-h cultivation period.
Phagocytosis assay.
Quantification of phagocytosis was performed as described previously, with slight modifications (9). In brief, neutrophils (1 × 106) and C. neoformans cells expressing GFP (1 × 105 cells) were added to 12-mm-diameter glass coverslips in 24-well tissue culture plates (Costar, Cambridge, MA) containing 40% fresh mouse serum in RPMI 1640. After incubation of neutrophils with C. neoformans at 37°C for 60 min, cells were fixed in 1% paraformaldehyde at 4°C for 30 min, and neutrophils were stained by using phycoerythrin (PE)–anti-Ly6G MAb. Phagocytosis was quantified by laser scanning confocal microscopy (Zeiss LSM 510 system). For each sample, at least 100 yeast cells were counted, and the number of yeast cells ingested or bound was determined. The level of phagocytosis is presented as the percentage of C. neoformans cells within neutrophils out of the total number of yeast cells counted.
Live-cell imaging.
Live-cell imaging was performed by using the Zeiss LSM 510 system coupled with a CO2 module and a temperature control module (Pecon, Germany) connected to a transparent chamber to maintain 5% CO2 and 37°C. To visualize the neutrophil behaviors and the kinetics of phagocytosis, 200 μl RPMI 1640 supplemented with 40% fresh mouse serum was added to a 35-mm glass-bottom dish (thickness no. 1.5; MatTek, USA). Neutrophils and yeast cells were added to the system at the desired ratio sequentially. In some experiments, neutrophils were pretreated with different inhibitors or antibodies. After the addition of all designated cells and reagents, the glass-bottom dish was transferred to the incubation chamber and warmed to 37°C at the start of video recording. Videos were recorded at a speed of 4 frames per min or 1 frame per s, and recording lasted for at least 30 min. To ensure the best video quality, a 63× oil lens and the differential interference contrast (DIC) channel were used for all experiments. A laser was used only when it was necessary in order to minimize potential photocytotoxicity to neutrophils and C. neoformans. Videos obtained were analyzed by using imageJ with the MtrackJ plug-in for neutrophil movements. The coordinates generated by MtrackJ were further processed and plotted in Microsoft Excel. The distance traveled was defined as the total distance that each neutrophil migrated during the video-shooting period. The distance traveled toward C. neoformans was defined as the distance that the neutrophil migrated in the direction toward the nearest C. neoformans cell, ignoring nondirectional movements of neutrophils. Negative values here indicate that a neutrophil moved against its neighboring C. neoformans cell during the video-shooting period.
Flow cytometry.
After experiments were performed, cells were harvested and counted. Fc receptors were blocked by using anti-CD16/32 Abs (clone 93; eBioscience). The cells were stained by using anti-Ly6G-allophycocyanin (APC) (clone 1A8; eBioscience) which is specific for neutrophils. PE–anti-C5aR (clone 20/70; eBioscience) and PE–anti-CD11b (clone M1/70; eBioscience) were used for staining of C5aR and CD11b expressed on the neutrophil surface, respectively. For C3b deposition assays, yeast cells were cultured in RPMI 1640 supplemented with 40% fresh mouse serum for different time intervals; C3b/iC3b deposition was detected after staining with purified anti-mouse C3b/iC3b MAb (clone 6C9; Thermo) and Alexa Fluor 555-conjugated goat anti-mouse IgG secondary Abs. For all stains, isotype-matched Abs were used as controls. Flow cytometry was performed by using a FACSAria II flow cytometer (BD Biosciences); data obtained were subsequently analyzed by using FlowJo software.
Quantification of complement C5a release.
C5a release was measured by using a mouse C5a enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems according to the manufacturer's instructions.
Statistical analysis.
GraphPad 5.0 was used for all statistical analyses. For single comparisons, an unpaired two-sided Student t test was performed; for multiple comparisons, one-way analysis of variance (ANOVA) followed by Tukey's post hoc test was used. In both cases, a P value of <0.05 is considered to be significant.
RESULTS
Both complement C3 and complement C5 are required for phagocytosis and killing of C. neoformans by neutrophils.
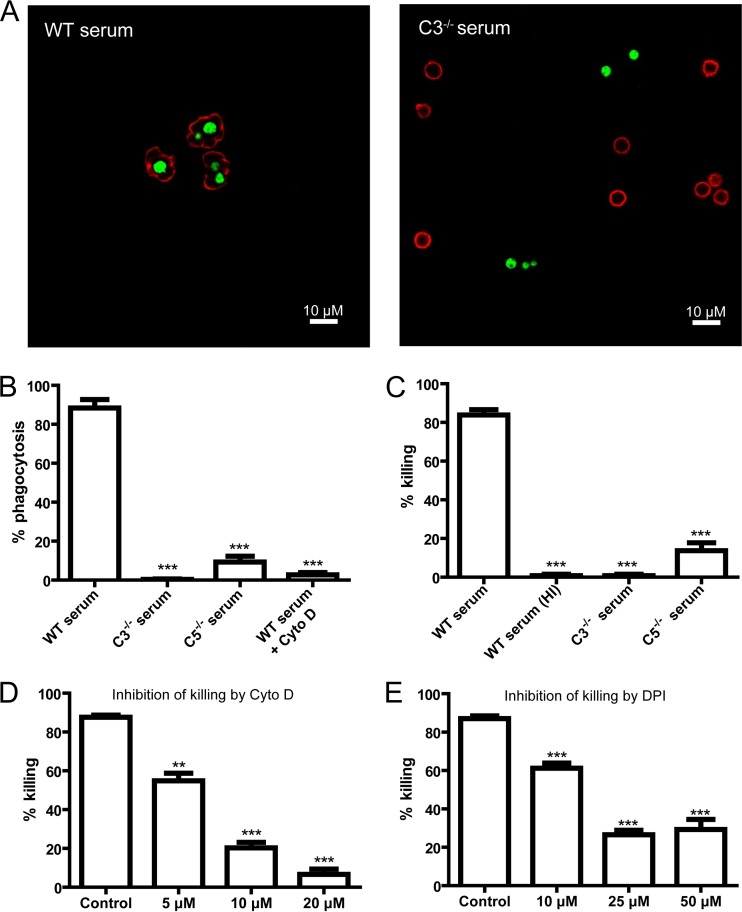
To evaluate the role of complement in phagocytosis of C. neoformans (H99 strain), yeast cells were incubated with neutrophils in the presence of mouse serum. As assessed by flow cytometry, the percentage of phagocytosis/binding of yeast cells by neutrophils increased with time in the presence of wild-type mouse serum (see Fig. S1A and S1B in the supplemental material). Next, we evaluated phagocytosis and killing of the organisms by confocal microscopy and CFU determination, respectively. In agreement with previously reported observations of binding of complement C3b/iC3b to cryptococcal capsules (22, 31–33), C. neoformans was ingested by neutrophils in the presence of wild-type mouse serum (Fig. 1A; see also Video S1 in the supplemental material) but not C3−/− mouse serum (Fig. 1A). Quantitative data indicated that phagocytosis and killing of C. neoformans by neutrophils were almost completely abolished in the presence of C3−/− mouse serum (Fig. 1B and C). In addition, phagocytosis and killing of the organisms by neutrophils were also dramatically reduced in the presence of C5−/− mouse serum compared to wild-type mouse serum (Fig. 1B and C). We also found that C3 and C5 played an important role in phagocytosis and killing of the other two strains of C. neoformans (B3501 and 52D) (see Fig. S2 in the supplemental material). Furthermore, the level of production of reactive oxygen intermediates (ROIs) by neutrophils following incubation with C. neoformans was significantly higher in the presence of wild-type mouse serum than in the presence of C3−/− or C5−/− mouse serum (see Fig. S3 in the supplemental material). Thus, both C3 and C5 play a crucial role in phagocytosis and killing of C. neoformans by neutrophils, consistent with previously reported findings showing that C3−/− (26) and C5−/− (25) mice are susceptible to infections.
FIG 1.
Essential role of C3 and C5 in phagocytosis and killing of C. neoformans by neutrophils. (A) Immunofluorescent confocal images showing C. neoformans (green) inside neutrophils (red) incubated in the presence of wild-type (WT) mouse serum (left) or outside neutrophils incubated with C3−/− mouse serum (right). (B) Percentage of yeast cells ingested by neutrophils in the presence of wild-type, C3−/−, or C5−/− mouse serum or wild-type mouse serum with cytochalasin D (Cyto D). (C) Percentage of yeast cells killed by neutrophils in the presence of wild-type, C3−/−, C5−/−, or heat-inactivated (HI) wild-type mouse serum. (D) Percentage of yeast cells killed by neutrophils in the presence of wild-type mouse serum and cytochalasin D. (E) Percentage of yeast cells killed by neutrophils in the presence of wild-type mouse serum and diphenyleneiodonium (DPI). Data are presented as means ± standard errors of the means. Data are representative of results from 3 independent experiments. **, P < 0.01; ***, P < 0.001.
To determine whether there is a link between phagocytosis/binding and killing, we separated C. neoformans cells associated with neutrophils from free yeast cells by flow cytometry and then determined their viability. We found that the majority of the yeast cells that were associated with neutrophils were killed by the phagocytes (see Fig. S4 in the supplemental material). In contrast, most of the free yeast cells survived (see Fig. S4 in the supplemental material). Phagocytosis of C. neoformans by neutrophils was abolished by the actin polymerization inhibitor cytochalasin D (20 μM) (Fig. 1B). Killing of the organisms was inhibited by cytochalasin D in a dose-dependent manner (Fig. 1D). Interestingly, the inhibition of killing by DPI treatment appears to be partial, suggesting a role for both oxidative and nonoxidative mechanisms (Fig. 1E). DPI treatment did not affect phagocytosis (data not shown).
Complement C5a-C5aR signaling and CD11b are crucial for killing of C. neoformans by neutrophils.
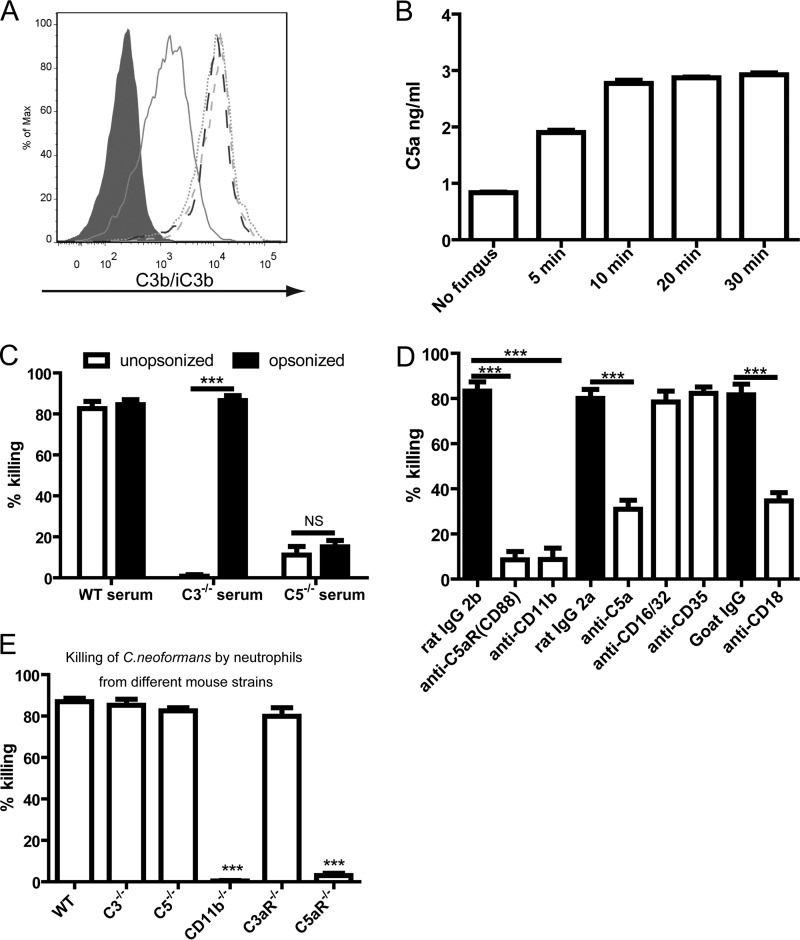
The above-described results demonstrated that C3 and C5 were required for killing of C. neoformans. Since both C3a and C5a are released during complement activation (3), we next evaluated the roles of these two cleavage fragments in killing of the fungus. We found that C3b/iC3b was rapidly deposited on the surface of the organism by as early as 5 min, and the deposition was saturated 10 min after incubation (Fig. 2A), which correlated with the release of C5a (Fig. 2B). Incubation of neutrophils with yeast cells preopsonized by wild-type mouse serum almost completely recovered the killing activity of C3−/− mouse serum (Fig. 2C). Since no C3a existed in the above-described system, we concluded that C3a was not essential for killing of yeast cells. This was further confirmed by the observation that neutrophils from C3aR−/− mice killed C. neoformans as efficiently as those from wild-type mice (Fig. 2E). In contrast to C3−/− mouse serum, the killing activity was not restored in C5−/− mouse serum (Fig. 2C), demonstrating that C5-related products were crucial for the killing of C. neoformans mediated by neutrophils. Among various C5-related products, we hypothesized that C5a played a crucial role. To address this hypothesis, we performed killing experiments using neutralizing and blocking Abs. Neutralization of C5a dramatically reduced the killing activity compared to control Abs (Fig. 2D). Blockade of C5aR (Fig. 2D) or deficiency of C5aR (Fig. 2E) almost completely abolished the killing of yeast cells. Blocking of Fc receptors (CD16/32) or complement receptor 1 (CD35) did not affect killing (Fig. 2D). Thus, C5a-C5aR signaling played a crucial role in the killing of C. neoformans by neutrophils.
FIG 2.
Crucial role of complement C5a-C5aR signaling and CD11b in killing of C. neoformans by neutrophils. (A) Deposition of C3b/iC3b on the surface of C. neoformans cells incubated in the presence of wild-type mouse serum at various time points. Gray-filled histogram, 0 min; solid line, 5 min; dotted line, 10 min; dashed line, 20 min; long dashed line, 30 min. (B) Release of C5a into the medium when C. neoformans is incubated in the presence of wild-type mouse serum at various time points. (C) Percentage of yeast cells killed by neutrophils in the presence of wild-type, C3−/−, or C5−/− mouse serum following preopsonization by wild-type mouse serum. (D) Percentage of C. neoformans cells killed by neutrophils in the presence of wild-type mouse serum and various Abs. (E) Percentage of C. neoformans cells killed by neutrophils derived from wild-type and knockout mice in the presence of wild-type mouse serum. Data are presented as means ± standard errors of the means. Data are representative of results from 3 independent experiments. ***, P < 0.001; NS, not significant.
Previously reported work showed that C5a-C5aR signaling can enhance the surface expression of CD11b on neutrophils (34, 35). We therefore explored the role of CD11b in the killing of C. neoformans. Blockade of CD11b almost completely abolished the killing activity of neutrophils (Fig. 2D). In addition, blockade of CD18 also significantly reduced the killing activity of neutrophils (Fig. 2D). The killing capability of neutrophils from C3−/− or C5−/− mice was not impaired (Fig. 2E). In contrast, neutrophils deficient in CD11b were unable to kill C. neoformans at all (Fig. 2E). These data suggested that CD11b is essential for neutrophils to kill C. neoformans.
Complement C5a-C5aR signaling navigates neutrophils toward C. neoformans.
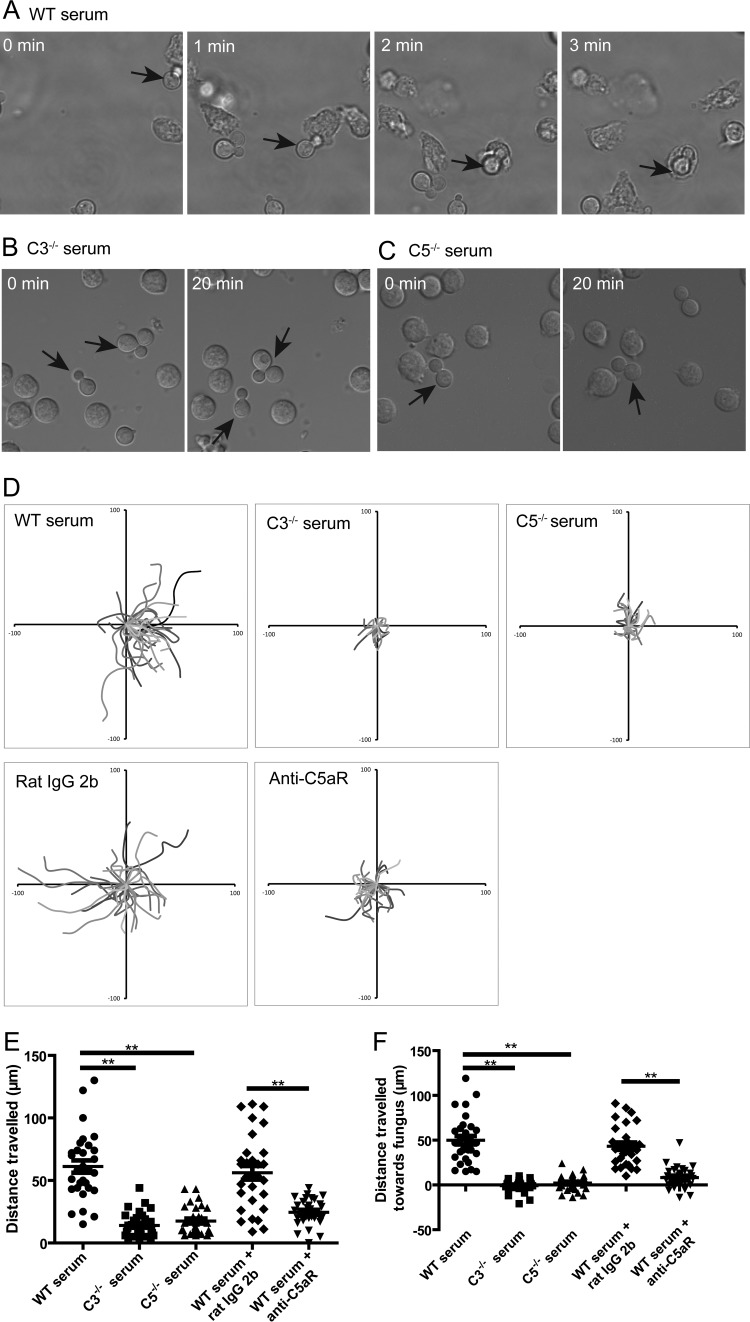
Next, we sought to dissect the mechanism(s) underlying the critical involvements of C5a-C5aR signaling in the killing of C. neoformans. Recent advances in live-cell imaging enabled us to directly visualize the dynamics of interactions of neutrophils with C. neoformans. As shown in Fig. 3A and in Video S2 in the supplemental material, a neutrophil chased a yeast cell in the presence of wild-type mouse serum. The neutrophil eventually caught the yeast cell and rapidly internalized it following long-distance travel (Fig. 3A; see also Video S2 in the supplemental material). During the movements, neutrophils adjusted their directions continuously in order to follow the yeast cells (see Video S3 in the supplemental material). In contrast, neutrophils were not observed to chase and ingest C. neoformans in the presence of C3−/− or C5−/− mouse serum (Fig. 3B and C; see also Videos S4 and S5 in the supplemental material). We measured the movements of individual neutrophils under various conditions. Neutrophils often made long-distance travel when incubated with C. neoformans in the presence of wild-type mouse serum but not C3−/− or C5−/− mouse serum (Fig. 3D). Moreover, this neutrophil behavior of long-distance travel was affected by blocking of C5aR (Fig. 3D). Quantitative analysis suggested that neutrophils incubated with C. neoformans traveled a significantly longer distance in the presence of wild-type mouse serum than in the presence of C3−/− or C5−/− mouse serum and that blockade of C5aR significantly shortened the travel distances of neutrophils (Fig. 3E). Finally, we quantified the distance traveled by neutrophils toward C. neoformans. As shown in Fig. 3F, the distances traveled by neutrophils toward C. neoformans were significantly shorter in the presence of C3−/− or C5−/− mouse serum or wild-type mouse serum with anti-C5aR Abs than in the presence of wild-type mouse serum or control Abs. Taken together, these results suggested that C5a-C5aR signaling was crucial for neutrophils to migrate toward C. neoformans.
FIG 3.
Complement C5a-C5aR signaling is crucial for neutrophils to migrate to C. neoformans. (A) Series of images showing a neutrophil chasing a yeast cell (arrow) followed by phagocytosis in the presence of wild-type mouse serum. (B and C) No chasing and phagocytosis of C. neoformans (arrows) by adjacent neutrophils during the observation period in the presence of C3−/− (B) or C5−/− (C) mouse serum. (D) Migration paths of neutrophils incubated with C. neoformans in the presence of wild-type, C3−/−, or C5−/− mouse serum or in the presence of wild-type mouse serum and anti-C5aR or control Abs. Paths are normalized for their origins. (E) Travel distances of neutrophils incubated with C. neoformans in the presence of wild-type, C3−/−, or C5−/− mouse serum or in the presence of wild-type serum and anti-C5aR or control Abs. (F) Travel distances of neutrophils toward C. neoformans in the presence of wild-type, C3−/−, or C5−/− mouse serum or in the presence of wild-type serum and anti-C5aR or control Abs. Data are presented as means ± standard errors of the means. Data are representative of results from 3 independent experiments. **, P < 0.01.
A concentration gradient of complement C5a around C. neoformans is required for optimal killing of yeast cells.
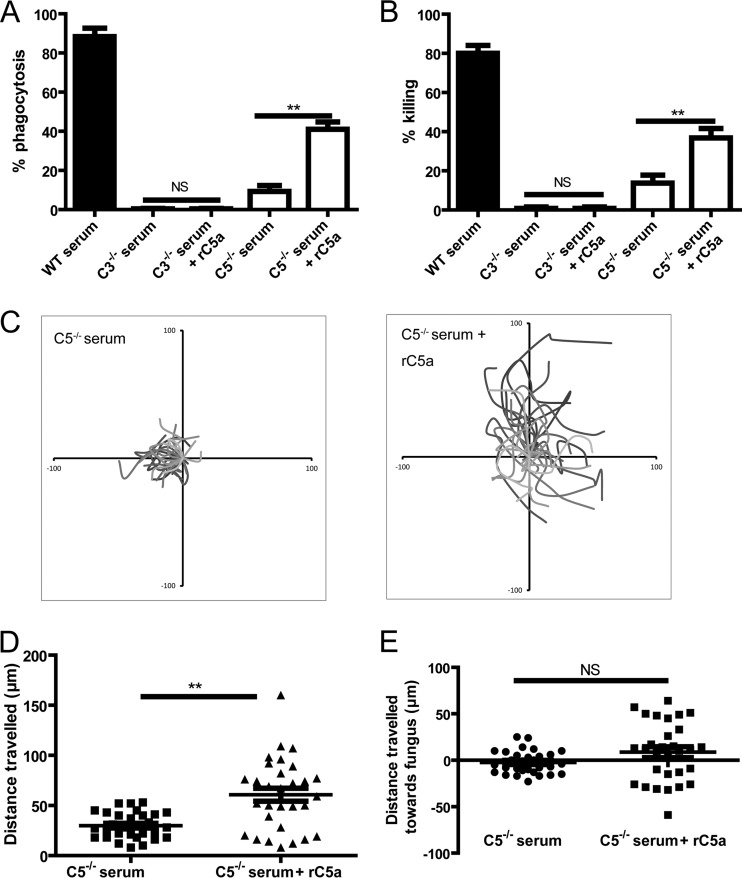
Having shown that C5a-C5aR signaling played a crucial role in killing of C. neoformans by neutrophils via guiding the migration of neutrophils toward the organism, we next set out to determine whether killing activity could be restored in the presence of C5−/− mouse serum through the addition of recombinant C5a (rC5a) in the environment. As a control, the addition of rC5a did not restore the phagocytosis and killing of C. neoformans at all in the presence of C3−/− mouse serum (Fig. 4A and B). In contrast, the addition of rC5a could partially, but not fully, restore the phagocytosis and killing of the organisms in the presence of C5−/− mouse serum (Fig. 4A and B). Interestingly, the addition of rC5a significantly enhanced the movements and travel distances of neutrophils (Fig. 4C and D; see also Video S6 in the supplemental material). However, the addition of rC5a did not induce the chasing of C. neoformans by neutrophils (see Video S6 in the supplemental material). Quantitative analysis suggested that the addition of rC5a did not affect the distances traveled by neutrophils toward C. neoformans (Fig. 4E). Obviously, the increased movements of neutrophils induced by the addition of rC5a were random and not specific for C. neoformans. These results suggested that a concentration gradient of C5a around C. neoformans was crucial for neutrophils to efficiently target organisms and exert their optimal killing activity.
FIG 4.
Addition of rC5a is unable to restore chemotaxis of neutrophils to C. neoformans in the presence of C5−/− mouse serum. (A) Percentage of yeast cells ingested by neutrophils in the presence of C3−/− or C5−/− mouse serum with or without the addition of rC5a. (B) Percentage of yeast cells killed by neutrophils in the presence of C3−/− or C5−/− mouse serum with or without the addition of rC5a. (C) Migration paths of neutrophils incubated with C. neoformans in the presence of C5−/− mouse serum with or without the addition of rC5a. Paths are normalized for their origins. (D) Travel distances of neutrophils incubated with C. neoformans in the presence of C5−/− mouse serum with or without the addition of rC5a. (E) Travel distances of neutrophils toward C. neoformans in the presence of C5−/− mouse serum with or without the addition of rC5a. Data are presented as means ± standard errors of the means. Data are representative of results from 3 independent experiments. **, P < 0.01.
The p38 MAPK pathway is critically involved in complement C5a-C5aR-mediated neutrophil migration.
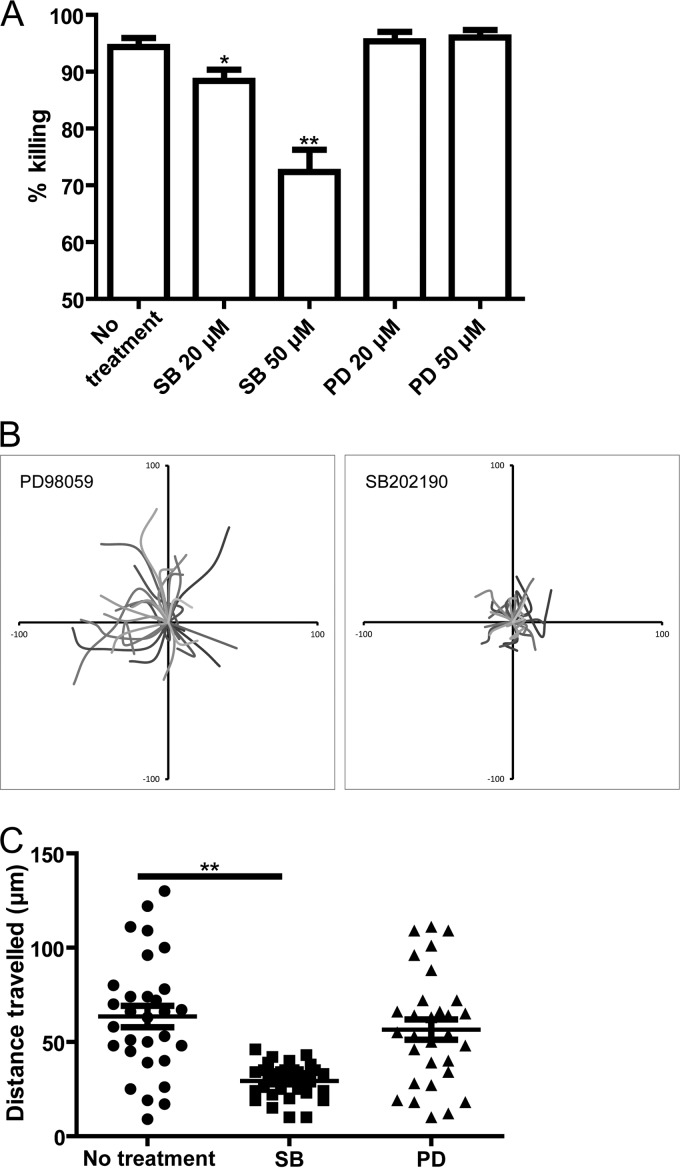
Next, we sought to determine the signal pathway(s) involved in the chemotaxis of neutrophils incubated with C. neoformans. Previously reported work suggested that MAPKs are involved in the regulation of neutrophil migration (36–38). The p38- and Erk-mediated signals controlled the chemotactic “go” and “stop” activities of G-protein-coupled-receptor-mediated chemotaxis, respectively (38). Using Western blot analysis, we showed that coincubation of neutrophils with C. neoformans led to the activation of the Erk and p38 pathways (see Fig. S5 in the supplemental material). In addition, pretreatment of neutrophils with SB202190, a selective inhibitor of p38, for 30 min significantly inhibited the killing of C. neoformans in a dose-dependent manner (Fig. 5A). In contrast, pretreatment of neutrophils with PD98059, a selective inhibitor of Erk, for 30 min did not affect the killing activity of neutrophils (Fig. 5A). Accordingly, SB202190, but not PD98059, significantly reduced the distances traveled by neutrophils incubated with yeast cells in the presence of wild-type mouse serum (Fig. 5B and C). These results suggested that the p38 MAPK pathway was critically involved in C5a-C5aR-mediated chemotaxis of neutrophils during their killing of C. neoformans.
FIG 5.
Complement C5a-C5aR mediated neutrophil migration involves the p38 MAPK pathway. (A) Percentage of C. neoformans cells killed by neutrophils pretreated with SB202190 (SB) or PD98059 (PD) in the presence of wild-type mouse serum. (B) Migration paths of neutrophils pretreated with SB202190 or PD98059 and incubated with C. neoformans in the presence of wild-type mouse serum. Paths are normalized for their origins. (C) Travel distances of neutrophils pretreated with SB202190 or PD98059 and incubated with C. neoformans in the presence of wild-type mouse serum. Data are presented as means ± standard errors of the means. Data are representative of results from 3 independent experiments. *, P < 0.05; **, P < 0.01.
Complement C5a-C5aR signaling enhances expression of CD11b on the surface of neutrophils.
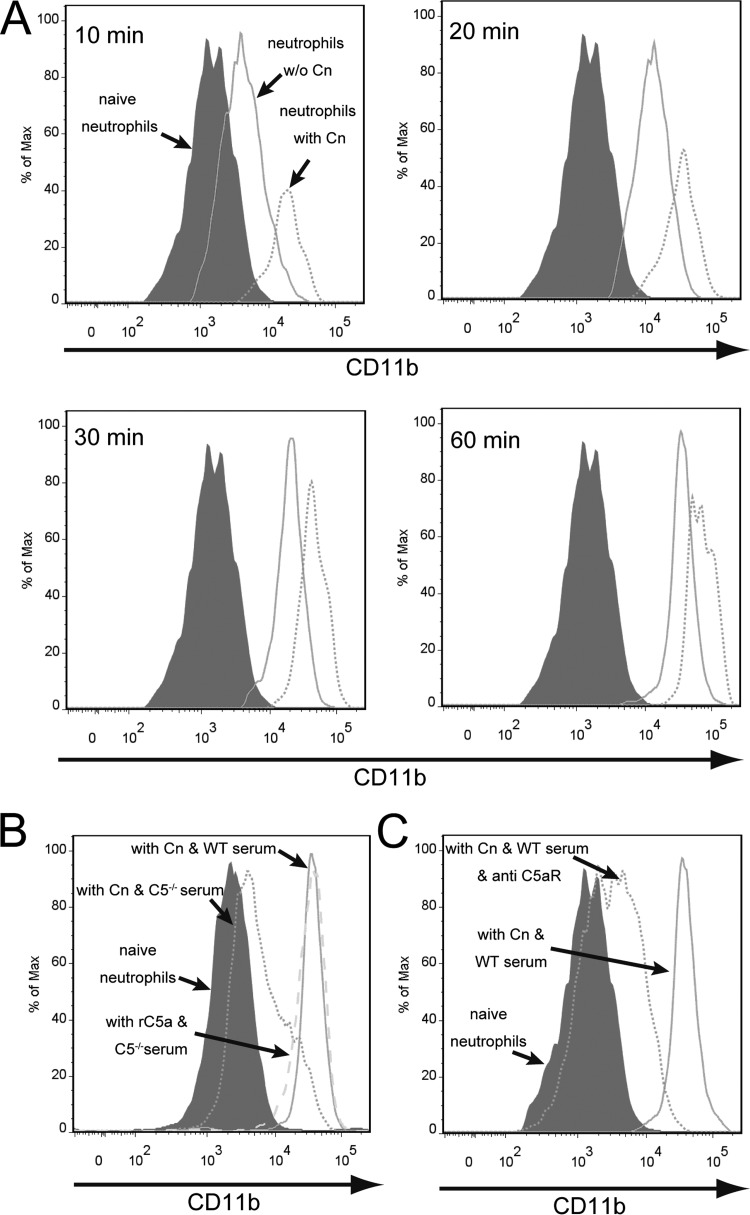
Neutrophil activation is usually associated with CD11b upregulation. As shown in Fig. 6A, enhanced expression of CD11b on the neutrophil surface was detected as early as 10 min after incubation with C. neoformans. Interestingly, neutrophils harboring or associated with yeast cells had an even higher level of surface expression of this molecule than neutrophils alone (Fig. 6A). The surface expression of CD11b on neutrophils increased continuously with increasing time of incubation (Fig. 6A). Next, we assessed whether C5a was involved in the enhanced expression of CD11b on the neutrophil surface. Neutrophils incubated with C. neoformans in the presence of C5−/− mouse serum displayed higher surface expression levels of CD11b when rC5a was added to the medium and had almost the same expression level of this molecule as those incubated with yeast cells in the presence of wild-type mouse serum (Fig. 6B). More importantly, blockade of C5aR dramatically reduced the elevation of CD11b expression on the surface of neutrophils incubated with C. neoformans in the presence of wild-type mouse serum (Fig. 6C). Collectively, these results suggested that C5a-C5aR signaling resulted in enhanced surface expression of CD11b on neutrophils.
FIG 6.
Complement C5a-C5aR signaling induces enhanced expression of CD11b on the surface of neutrophils. (A) Purified neutrophils (1 × 106) were incubated with C. neoformans (Cn) H99 expressing GFP (1 × 105 cells) for 10, 20, 30, or 60 min in 1 ml RPMI 1640 containing 40% wild-type mouse serum and then placed on ice. The cells were then fixed in 1% paraformaldehyde and stained with APC–anti-Ly6G and PE–anti-CD11b. The surface expression of CD11b on neutrophils was analyzed by using flow cytometry. Gray-filled histogram, naive neutrophils; solid line, stimulated neutrophils (without phagocytosis/binding of the fungus); dotted line, stimulated neutrophils (with phagocytosis/binding of the fungus). (B) Surface expression of CD11b on neutrophils incubated with C. neoformans in the presence of wild-type or C5−/− mouse serum with or without the addition of rC5a. Gray-filled histogram, naive neutrophils; dotted line, C. neoformans with C5−/− serum; solid line, C. neoformans with wild-type serum; dashed line, C5−/− serum with the addition of rC5a. (C) Surface expression of CD11b on neutrophils incubated with C. neoformans in the presence of wild-type serum with or without the addition of anti-C5aR MAb. Gray filled histogram, naive neutrophils; dotted line, C. neoformans with wild-type serum with the addition of anti-C5aR MAb; solid line, C. neoformans with wild-type serum. Data are representative of results from 3 independent experiments.
Elevated expression of CD11b on neutrophils is essential for phagocytosis and killing of C. neoformans.
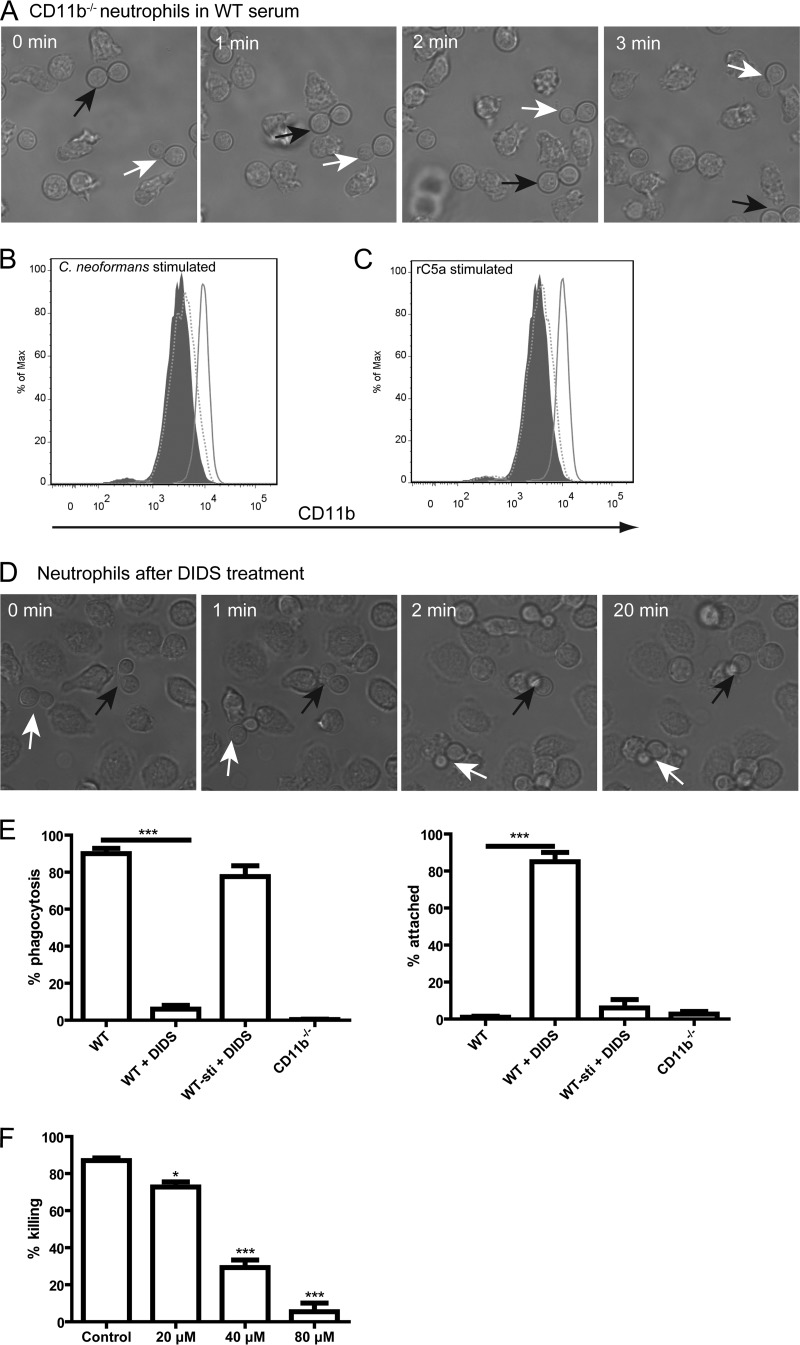
As described above, CD11b−/− neutrophils were unable to kill C. neoformans (Fig. 2E). We further demonstrated that the inability of CD11b−/− neutrophils to kill yeast cells was not attributed to impaired chemotaxis, because CD11b−/− neutrophils actively chased the yeast cells (Fig. 7A; see also Video S7 in the supplemental material). These results raised the possibility that the enhanced surface expression of CD11b on neutrophils induced by C5a-C5aR signaling significantly contributed to phagocytosis of C. neoformans. To this end, we pretreated neutrophils with the anion channel-blocking agent 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS), which has been shown to be capable of inhibiting increased surface expression of CD11b stimulated by N-formyl-methionyl-leucyl-phenylalanine (fMLP) (39). DIDS is also known not to interfere with other neutrophil functions, including superoxide generation and non-complement-mediated phagocytosis (40–42). As expected, DIDS (200 μM) almost completely inhibited the enhanced surface expression of CD11b on neutrophils induced by C. neoformans or rC5a but did not affect the original expression of this molecule on the surface of neutrophils (Fig. 7B and C). Live-cell imaging revealed that neutrophils pretreated with DIDS were able to stick to but were unable to engulf C. neoformans (Fig. 7D; see also Video S8 in the supplemental material). Quantitative analysis demonstrated that DIDS treatment resulted in a nearly complete inhibition of phagocytosis of C. neoformans by neutrophils, although it did not affect the binding of neutrophils to the organisms (Fig. 7E). As a positive control, pretreatment of neutrophils whose surface CD11b expression was upregulated by rC5a did not affect their ability to phagocytose C. neoformans (Fig. 7E). In addition, CD11b−/− neutrophils were unable to bind or engulf C. neoformans cells at all (Fig. 7E). Thus, the original surface expression of CD11b on neutrophils was essential and sufficient for neutrophils to bind C. neoformans but was unable to mediate phagocytosis. Furthermore, treatment of neutrophils with DIDS significantly reduced their killing activity against C. neoformans in a dose-dependent manner (Fig. 7F). Taken together, these results suggested that the increased surface expression of CD11b on neutrophils, which was induced by C5a-C5aR signaling, was essential for neutrophils to internalize and subsequently kill C. neoformans cells.
FIG 7.
Essential role of enhanced surface expression of CD11b on neutrophils in phagocytosis and subsequent killing of C. neoformans. (A) Series of images showing CD11b−/− neutrophils chasing C. neoformans cells (arrows) in the presence of wild-type mouse serum. (B) Surface expression of CD11b on neutrophils with or without pretreatment with DIDS after incubation with C. neoformans in the presence of wild-type mouse serum. Gray-filled histogram, naive neutrophils; solid line, neutrophils without pretreatment with DIDS; dotted line, neutrophils with pretreatment with DIDS. (C) Surface expression of CD11b on neutrophils with or without pretreatment with DIDS after incubation in the presence of C5−/− mouse serum with the addition of rC5a. Gray filled histogram, naive neutrophils; solid line, neutrophils without pretreatment with DIDS and with the addition of rC5a; dotted line, neutrophils with pretreatment with DIDS and with the addition of rC5a. (D) Series of images showing that neutrophils pretreated with DIDS are able to attach to C. neoformans (arrows) but are unable to engulf yeast cells in the presence of wild-type mouse serum. (E) Percentage of yeast cells ingested by (left) or attached to (right) CD11b−/− neutrophils and wild-type neutrophils with or without pretreatment with DIDS in the presence of wild-type mouse serum. Stimulated wild-type neutrophils pretreated with DIDS (WT-sti + DIDS) were first treated with rC5a (10 nM) for 10 min at 37°C to allow CD11b upregulation and then pretreated with DIDS. (F) Percentage of yeast cells killed by neutrophils pretreated with DIDS at various doses in the presence of wild-type mouse serum. Data are presented as means ± standard errors of the means. Data are representative of results from 3 independent experiments. *, P < 0.05; ***, P < 0.001.
DISCUSSION
Dissimilar to most infectious agents, C. neoformans is equipped with a polysaccharide capsule. Although the capsule is antiphagocytic and poorly immunogenic, serving as the major virulence factor, it can activate the complement system, resulting in phagocytosis of the organisms by neutrophils and macrophages (3). Neutrophils have been shown to kill C. neoformans more efficiently than macrophages (1, 5). The complement system plays an important role in defense against C. neoformans, as demonstrated both in vitro (21, 22) and in vivo (23, 25, 26), although it was also reported that some isolates of C. neoformans were resistant to phagocytosis despite binding of C3 fragments (43). In this study, we used live-cell imaging to directly characterize how the complement system precisely drives neutrophils to eliminate the encapsulated fungal pathogen in real time.
In the presence of wild-type mouse serum, neutrophils effectively engulfed and killed C. neoformans. In contrast, neutrophils were almost unable to ingest and kill C. neoformans in C3−/− or C5−/− mouse serum, suggesting that both complement C3 and complement C5 were critically involved in host defense against the organism. This is consistent with previously reported findings showing that both C3−/− (26) and C5−/− (24, 25) mice are more susceptible to C. neoformans infection. Additionally, the complete abrogation of phagocytosis in the presence of C3−/− mouse serum excluded C3-independent phagocytosis of C. neoformans by neutrophils in our system. This is in contrast to phagocytosis of C. neoformans by macrophages, as macrophages can also internalize the organism via a C3-independent mechanism (33), probably through direct interactions of cryptococcal glucuronoxylomannan (GXM) with complement receptor 3 (44, 45).
We found that C5a-C5aR signaling was crucial for killing of C. neoformans by neutrophils and further demonstrated that one of the underlying mechanisms is the requirement of C5a-C5aR signaling to guide neutrophils to target organisms. Although chemotaxis of neutrophils was previously suggested by studies using Boyden chamber assays (46, 47), we were able to directly visualize the migration of neutrophils toward C. neoformans in real time. Blocking of C5aR almost completely abolished the killing of C. neoformans via inhibiting the migration of neutrophils to the organism. In contrast, the addition of rC5a to C5−/− mouse serum in fungicidal assays only partially restored killing, which is in agreement with findings reported previously by Lovchik and Lipscomb (24). To explain the failure of the addition of rC5a to fully restore killing, Lovchik and Lipscomb suggested that a role for C5b cannot be ruled out (24). In this study, we further demonstrated that the addition of rC5a to C5−/− mouse serum enhanced the random movements of neutrophils but could not navigate neutrophils toward C. neoformans. In addition, we showed that the addition of rC5a to C5−/− mouse serum enhanced the surface expression of CD11b on neutrophils to the same level as that induced by C5a-C5aR signaling in the presence of C. neoformans and wild-type mouse serum, excluding the possibility of impaired phagocytosis due to less expression of CD11b. Obviously, C5a needs to be generated at the surface of C. neoformans by a C5 convertase and released into the surrounding environment. As a result, a concentration gradient of C5a will be generated around the yeast cells, which will attract neutrophils to migrate to the organisms. Apparently, the addition of rC5a cannot create such an environment for neutrophils. We suggested that the partial restoration of the neutrophil killing activity following the addition of rC5a to C5−/− mouse serum can be attributed to an accidental encounter of neutrophils with C. neoformans due to the enhanced random movements of the phagocytes. Therefore, a concentration gradient of C5a around encapsulated C. neoformans cells is required for optimal killing of the organisms by neutrophils. Interestingly, cryptococcal GXM has been shown to suppress C5aR expression on human neutrophils, which was accompanied by a decreased chemotactic response to C5a (48). In addition, purified cryptococcal mannoprotein is able to inhibit neutrophil migration, probably via a mechanism involving chemoattractant receptor cross-desensitization (49). Thus, how neutrophils migrate toward C. neoformans in vivo deserves further investigation.
Our data suggested that CD11b was not required for neutrophils to migrate toward C. neoformans. However, blockade of CD11b or deficiency of CD11b almost completely abolished the phagocytosis and killing of C. neoformans by neutrophils, suggesting that CD11b is essential for phagocytosis and killing of the organisms. We further demonstrated that C5a-C5aR signaling enhanced the surface expression of CD11b on neutrophils. These data are consistent with previously reported findings showing that blocking C5aR or neutralizing C5a inhibits phagocytosis of Escherichia coli or Candida albicans by neutrophils, associated with the abrogation of the enhanced surface expression of CD11b on neutrophils (34, 35). Although phagocytosis of these organisms was almost completely abolished by blocking of CD11b (34, 35), evidence that there is a direct link between enhanced CD11b expression and phagocytosis of the organisms has not been provided. This is because treatment of neutrophils with anti-CD11b Abs not only blocks the enhanced expression of CD11b but also blocks the original expression of CD11b on the surface of neutrophils. It is still unknown whether the enhanced CD11b expression or the original CD11b expression is essential for neutrophil phagocytosis. To further address this question, we used DIDS to inhibit the increased surface expression of CD11b by C5a-C5aR signaling, retaining the original expression of this molecule, as reported previously (39). Our results demonstrated that the original surface expression of CD11b was essential and sufficient for neutrophils to attach to C. neoformans but was unable to mediate phagocytosis and subsequent killing of the organisms, suggesting that C5a-C5aR signaling was critically involved in the neutrophil phagocytosis of encapsulated C. neoformans cells through inducing enhanced surface expression of CD11b on the phagocytes. We are aware that DIDS has not been proven to be a specific inhibitor of CD11b upregulation. Unfortunately, the pathways of CD11b upregulation have not been well characterized, and a specific inhibitor of CD11b upregulation is currently not available. Nevertheless, DIDS was used previously to inhibit CD11b upregulation stimulated by fMLP (39) and was proven not to interfere with other neutrophil functions, including superoxide generation and non-complement-mediated phagocytosis (40–42). Importantly, the present study showed that treatment of neutrophils with elevated expression of CD11b with DIDS did not affect their ability to phagocytose C. neoformans, further suggesting that the inability of DIDS-treated neutrophils to ingest yeast cells can be attributed to a failure to enhance the surface expression of CD11b. It is noteworthy that phagocytosis of C. albicans by neutrophils is dependent on C5a-C5aR signaling 10 min after infection but is independent of this signaling 60 min after infection (34). This is in contrast to the phagocytosis of C. neoformans; this discrepancy may reflect a differences in surface components and structures between the organisms. In contrast to C. albicans, the capsule of C. neoformans covers potential components that could be recognized by phagocytes in other pathways, as occurred in C. albicans.
CD11b and CD18 form CR3. Obviously, phagocytosis mediated by CR3 requires the activation of CR3. In this respect, staurosporine (a protein kinase C inhibitor) inhibits neutrophil phagocytosis but not iC3b binding mediated by CR3 (50). It is conceivable that the activation of CR3 involves protein kinase C signaling. We further speculate that CR3 activation triggers actin polymerization, leading to the phagocytosis of C. neoformans.
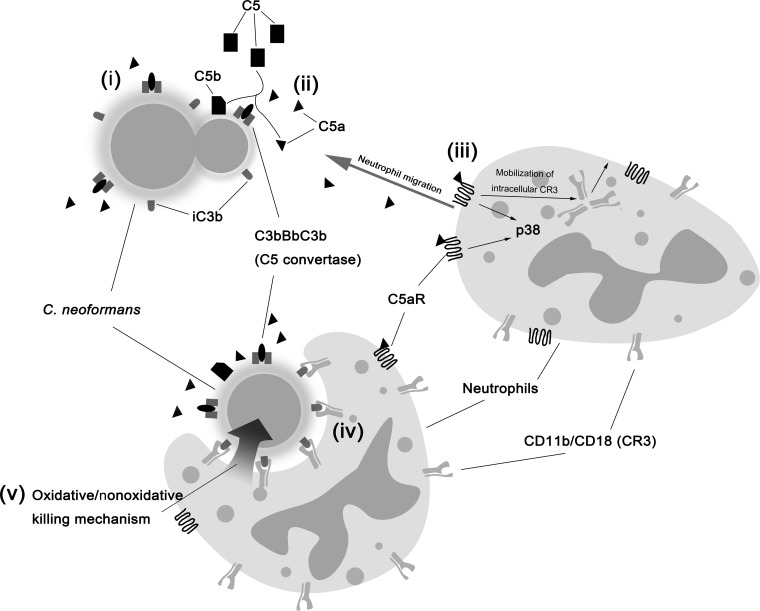
In conclusion, taking advantage of live-cell imaging and the availability of knockout mice, we have, for the first time, directly visualized the dynamic interactions of neutrophils with C. neoformans in real time and revealed the critical role of complement C5a-C5aR signaling in their interactions and subsequent killing of encapsulated yeast cells by neutrophils through guiding the migration of neutrophils toward the organisms and triggering phagocytosis via upregulation of surface CD11b expression. We have summarized our findings in Fig. 8. These findings contribute significantly to our understanding of the complicated interactions between phagocytes and pathogens.
FIG 8.
Schematic illustration of interactions of neutrophils with C. neoformans. (i) Activation of the complement system by fungal capsule results in deposition of C3b, which leads to the formation of complement C5 convertase or is quickly converted to iC3b. (ii) C5a is released from cleavage of C5 by C5 convertase, resulting in a C5a gradient around yeast cells. (iii) C5a binds to C5aR on the neutrophil surface, which induces p38-dependent chemotaxis toward yeast cells and, in the meantime, the surface translocation of intracellular CR3. (iv) CR3 upregulation is required for phagocytosis of C. neoformans. (v) Killing of yeast cells by neutrophils via oxidative and nonoxidative pathways.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to Yunsheng Wang for his technical assistance with confocal microscopy and FACS analysis.
Funding Statement
University of Maryland (UMD) provided startup funds to Meiqing Shi. National Institutes of Health (NIH) provided funding to Meiqing Shi under grant number AI115086A. National Science Foundation of Jiangsu Province provided funding to Hong Zhou under grant number BK2011769.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01197-15.
REFERENCES
- 1.Diamond RD, Root RK, Bennett JE. 1972. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis 125:367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- 2.Kozel TR, Highison B, Stratton CJ. 1984. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun 43:574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voelz K, May RC. 2010. Cryptococcal interactions with the host immune system. Eukaryot Cell 9:835–846. doi: 10.1128/EC.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang M, Sun D, Shi M. 2015. Dancing cheek to cheek: Cryptococcus neoformans and phagocytes. Springer Plus 4:410. doi: 10.1186/s40064-015-1192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller MF, Mitchell TG. 1991. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun 59:24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qureshi A, Subathra M, Grey A, Schey K, Del Poeta M, Luberto C. 2010. Role of sphingomyelin synthase in controlling the antimicrobial activity of neutrophils against Cryptococcus neoformans. PLoS One 5:e15587. doi: 10.1371/journal.pone.0015587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qureshi A, Grey A, Rose KL, Schey KL, Del Poeta M. 2011. Cryptococcus neoformans modulates extracellular killing by neutrophils. Front Microbiol 2:193. doi: 10.3389/fmicb.2011.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha JD, Nascimento MT, Decote-Ricardo D, Corte-Real S, Morrot A, Heise N, Nunes MP, Previato JO, Mendonca-Previato L, DosReis GA, Saraiva EM, Freire-de-Lima CG. 2015. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci Rep 5:8008. doi: 10.1038/srep08008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaturvedi V, Wong B, Newman SL. 1996. Oxidative killing of Cryptococcus neoformans by human neutrophils. Evidence that fungal mannitol protects by scavenging reactive oxygen intermediates. J Immunol 156:3836–3840. [PubMed] [Google Scholar]
- 10.Tacker JR, Farhi F, Bulmer GS. 1972. Intracellular fate of Cryptococcus neoformans. Infect Immun 6:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mambula SS, Simons ER, Hastey R, Selsted ME, Levitz SM. 2000. Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infect Immun 68:6257–6264. doi: 10.1128/IAI.68.11.6257-6264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Ishida-Okawara A, Suzuki K, Maeda N, Koyama H. 2006. Contribution of the myeloperoxidase-dependent oxidative system to host defence against Cryptococcus neoformans. J Med Microbiol 55:1291–1299. doi: 10.1099/jmm.0.46620-0. [DOI] [PubMed] [Google Scholar]
- 13.Graybill JR, Bocanegra R, Lambros C, Luther MF. 1997. Granulocyte colony stimulating factor therapy of experimental cryptococcal meningitis. J Med Vet Mycol 35:243–247. doi: 10.1080/02681219780001221. [DOI] [PubMed] [Google Scholar]
- 14.Klebanoff SJ. 2005. Myeloperoxidase: friend and foe. J Leukoc Biol 77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 15.Coffey MJ, Phare SM, George S, Peters-Golden M, Kazanjian PH. 1998. Granulocyte colony-stimulating factor administration to HIV-infected subjects augments reduced leukotriene synthesis and anticryptococcal activity in neutrophils. J Clin Invest 102:663–670. doi: 10.1172/JCI2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vecchiarelli A, Monari C, Baldelli F, Pietrella D, Retini C, Tascini C, Francisci D, Bistoni F. 1995. Beneficial effect of recombinant human granulocyte colony-stimulating factor on fungicidal activity of polymorphonuclear leukocytes from patients with AIDS. J Infect Dis 171:1448–1454. doi: 10.1093/infdis/171.6.1448. [DOI] [PubMed] [Google Scholar]
- 17.Wozniak KL, Kolls JK, Wormley FL Jr. 2012. Depletion of neutrophils in a protective model of pulmonary cryptococcosis results in increased IL-17A production by gammadelta T cells. BMC Immunol 13:65. doi: 10.1186/1471-2172-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mednick AJ, Feldmesser M, Rivera J, Casadevall A. 2003. Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur J Immunol 33:1744–1753. doi: 10.1002/eji.200323626. [DOI] [PubMed] [Google Scholar]
- 19.Feldmesser M, Kress Y, Novikoff P, Casadevall A. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun 68:4225–4237. doi: 10.1128/IAI.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M, Sun D, Liu G, Wu H, Zhou H, Shi M. 1 October 2015 Real-time in vivo imaging reveals the ability of neutrophils to remove Cryptococcus neoformans directly from the brain vasculature. J Leukoc Biol doi: 10.1189/jlb.4AB0715-281R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond RD, May JE, Kane MA, Frank MM, Bennett JE. 1974. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol 112:2260–2270. [PubMed] [Google Scholar]
- 22.Kozel TR, Pfrommer GS. 1986. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun 52:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond RD, May JE, Kane M, Frank MM, Bennett JE. 1973. The role of late complement components and the alternate complement pathway in experimental cryptococcosis. Proc Soc Exp Biol Med 144:312–315. doi: 10.3181/00379727-144-37580. [DOI] [PubMed] [Google Scholar]
- 24.Lovchik JA, Lipscomb MF. 1993. Role for C5 and neutrophils in the pulmonary intravascular clearance of circulating Cryptococcus neoformans. Am J Respir Cell Mol Biol 9:617–627. doi: 10.1165/ajrcmb/9.6.617. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes JC, Wicker LS, Urba WJ. 1980. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect Immun 29:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shapiro S, Beenhouwer DO, Feldmesser M, Taborda C, Carroll MC, Casadevall A, Scharff MD. 2002. Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect Immun 70:2598–2604. doi: 10.1128/IAI.70.5.2598-2604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolev M, Le Friec G, Kemper C. 2014. Complement—tapping into new sites and effector systems. Nat Rev Immunol 14:811–820. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- 28.Kozel TR, Pfrommer GS, Redelman D. 1987. Activated neutrophils exhibit enhanced phagocytosis of Cryptococcus neoformans opsonized with normal human serum. Clin Exp Immunol 70:238–246. [PMC free article] [PubMed] [Google Scholar]
- 29.Voelz K, Johnston SA, Rutherford JC, May RC. 2010. Automated analysis of cryptococcal macrophage parasitism using GFP-tagged cryptococci. PLoS One 5:e15968. doi: 10.1371/journal.pone.0015968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. 2004. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol 75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 31.Kozel TR, Wilson MA, Pfrommer GS, Schlageter AM. 1989. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect Immun 57:1922–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truelsen K, Young T, Kozel TR. 1992. In vivo complement activation and binding of C3 to encapsulated Cryptococcus neoformans. Infect Immun 60:3937–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaragoza O, Taborda CP, Casadevall A. 2003. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur J Immunol 33:1957–1967. doi: 10.1002/eji.200323848. [DOI] [PubMed] [Google Scholar]
- 34.Hunniger K, Bieber K, Martin R, Lehnert T, Figge MT, Loffler J, Guo RF, Riedemann NC, Kurzai O. 2015. A second stimulus required for enhanced antifungal activity of human neutrophils in blood is provided by anaphylatoxin C5a. J Immunol 194:1199–1210. doi: 10.4049/jimmunol.1401845. [DOI] [PubMed] [Google Scholar]
- 35.Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegard KT, Kohl J, Lambris JD. 2002. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 100:1869–1877. [PubMed] [Google Scholar]
- 36.Cara DC, Kaur J, Forster M, McCafferty DM, Kubes P. 2001. Role of p38 mitogen-activated protein kinase in chemokine-induced emigration and chemotaxis in vivo. J Immunol 167:6552–6558. doi: 10.4049/jimmunol.167.11.6552. [DOI] [PubMed] [Google Scholar]
- 37.Kim D, Haynes CL. 2013. The role of p38 MAPK in neutrophil functions: single cell chemotaxis and surface marker expression. Analyst 138:6826–6833. doi: 10.1039/c3an01076g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Ma B, Malik AB, Tang H, Yang T, Sun B, Wang G, Minshall RD, Li Y, Zhao Y, Ye RD, Xu J. 2012. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat Immunol 13:457–464. doi: 10.1038/ni.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vedder NB, Harlan JM. 1988. Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest 81:676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korchak HM, Eisenstat BA, Hoffstein ST, Dunham PB, Weissmann G. 1980. Anion channel blockers inhibit lysosomal enzyme secretion from human neutrophils without affecting generation of superoxide anion. Proc Natl Acad Sci U S A 77:2721–2725. doi: 10.1073/pnas.77.5.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith RJ, Bowman BJ, Iden SS. 1984. Effects of an anion channel blocker, 4,4′-diisothiocyano-2,2′-disulfonic acid stilbene (DIDS), on human neutrophil function. Biochem Biophys Res Commun 120:964–972. doi: 10.1016/S0006-291X(84)80201-6. [DOI] [PubMed] [Google Scholar]
- 42.Tauber AI, Goetzl EJ. 1981. Inhibition of complement-mediated functions of human neutrophils by impermeant stilbene disulfonic acids. J Immunol 126:1786–1789. [PubMed] [Google Scholar]
- 43.Kozel TR, Pfrommer GS, Guerlain AS, Highison BA, Highison GJ. 1988. Strain variation in phagocytosis of Cryptococcus neoformans: dissociation of susceptibility to phagocytosis from activation and binding of opsonic fragments of C3. Infect Immun 56:2794–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong ZM, Murphy JW. 1997. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect Immun 65:557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taborda CP, Casadevall A. 2002. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16:791–802. doi: 10.1016/S1074-7613(02)00328-X. [DOI] [PubMed] [Google Scholar]
- 46.Diamond RD, Erickson NF III. 1982. Chemotaxis of human neutrophils and monocytes induced by Cryptococcus neoformans. Infect Immun 38:380–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laxalt KA, Kozel TR. 1979. Chemotaxigenesis and activation of the alternative complement pathway by encapsulated and non-encapsulated Cryptococcus neoformans. Infect Immun 26:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monari C, Kozel TR, Bistoni F, Vecchiarelli A. 2002. Modulation of C5aR expression on human neutrophils by encapsulated and acapsular Cryptococcus neoformans. Infect Immun 70:3363–3370. doi: 10.1128/IAI.70.7.3363-3370.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coenjaerts FE, Walenkamp AM, Mwinzi PN, Scharringa J, Dekker HA, van Strijp JA, Cherniak R, Hoepelman AI. 2001. Potent inhibition of neutrophil migration by cryptococcal mannoprotein-4-induced desensitization. J Immunol 167:3988–3995. doi: 10.4049/jimmunol.167.7.3988. [DOI] [PubMed] [Google Scholar]
- 50.Roubey RA, Ross GD, Merrill JT, Walton F, Reed W, Winchester RJ, Buyon JP. 1991. Staurosporine inhibits neutrophil phagocytosis but not iC3b binding mediated by CR3 (CD11b/CD18). J Immunol 146:3557–3562. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.