Abstract
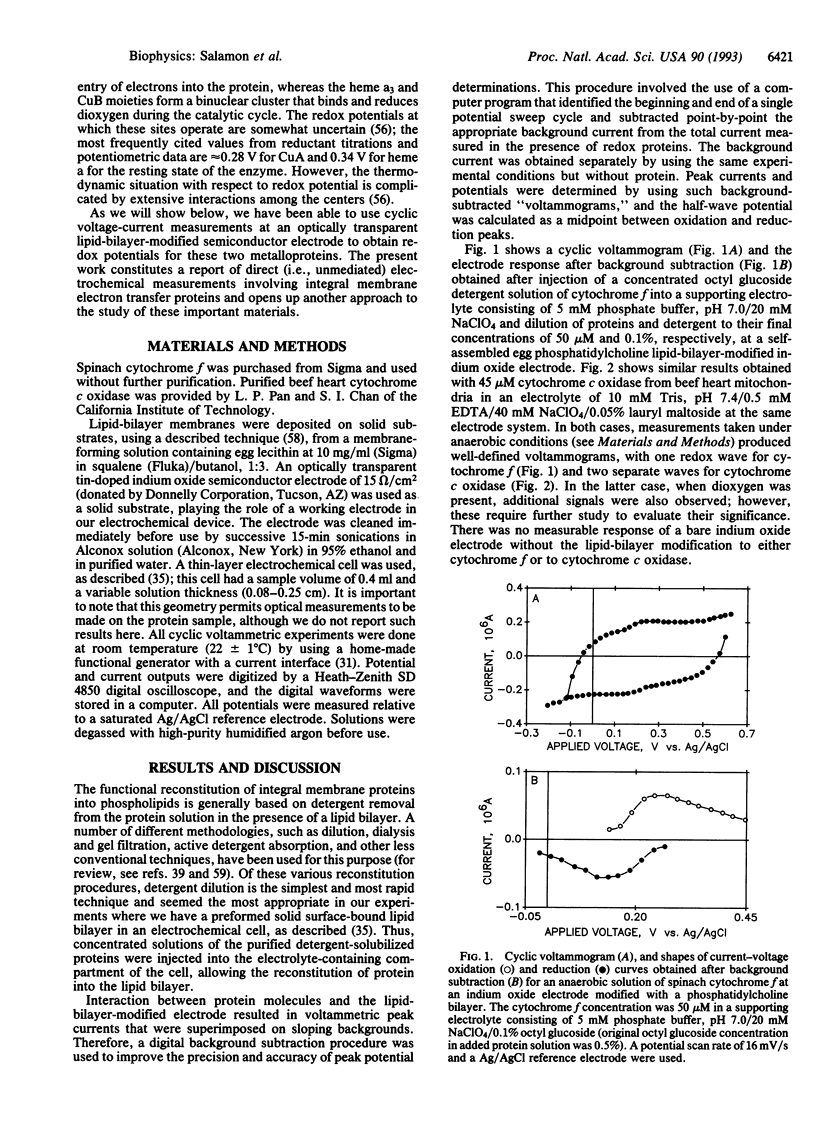
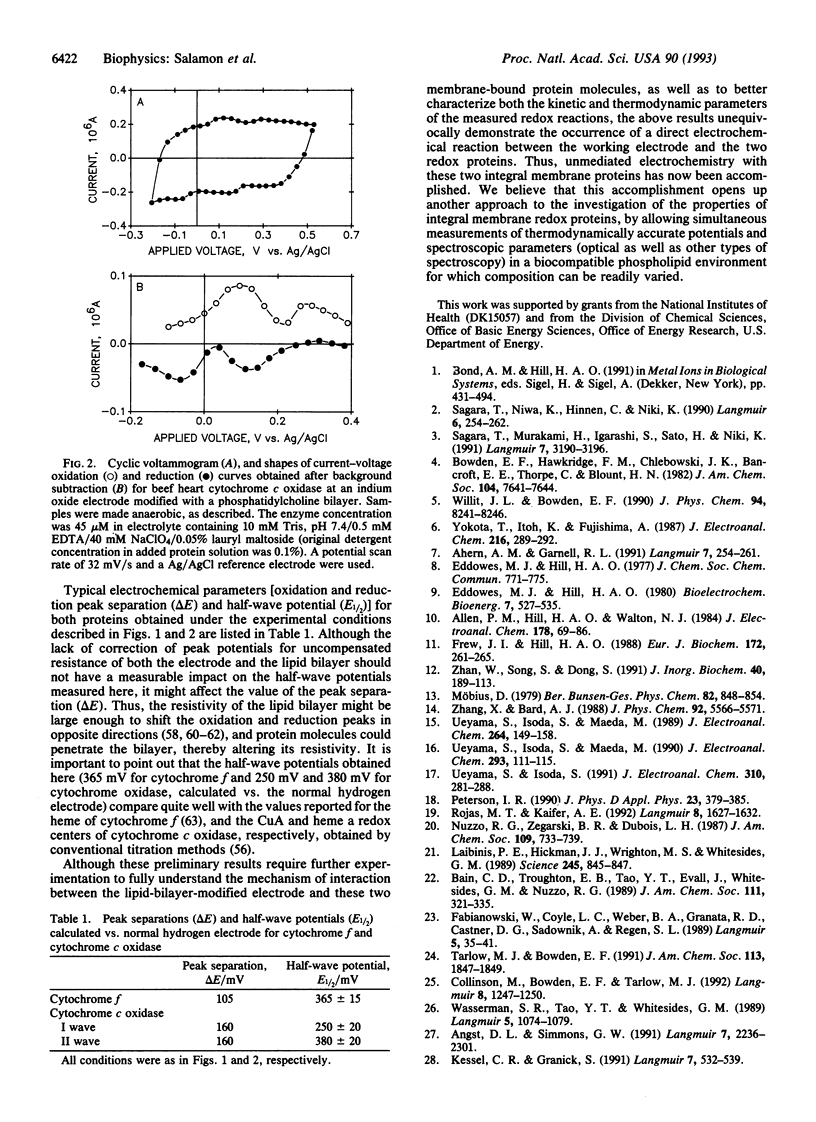
Direct cyclic voltage-current responses, produced in the absence of redox mediators, for two detergent-solubilized integral membrane proteins, spinach cytochrome f and beef heart cytochrome c oxidase, have been obtained at an optically transparent indium oxide electrode modified with a self-assembled lipid-bilayer membrane. The results indicate that both proteins interact with the lipid membrane so as to support quasi-reversible electron transfer redox reactions at the semiconductor electrode. The redox potentials that were obtained from analysis of the cyclic "voltammograms," 365 mV for cytochrome f and 250 and 380 mV for cytochrome c oxidase (vs. normal hydrogen electrode), compare quite well with the values reported by using conventional titration methods. The ability to obtain direct electrochemical measurements opens up another approach to the investigation of the properties of integral membrane redox proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capaldi R. A. Structure and function of cytochrome c oxidase. Annu Rev Biochem. 1990;59:569–596. doi: 10.1146/annurev.bi.59.070190.003033. [DOI] [PubMed] [Google Scholar]
- Cariello N. F., Keohavong P., Kat A. G., Thilly W. G. Molecular analysis of complex human cell populations: mutational spectra of MNNG and ICR-191. Mutat Res. 1990 Aug;231(2):165–176. doi: 10.1016/0027-5107(90)90023-w. [DOI] [PubMed] [Google Scholar]
- Downer N. W., Li J., Penniman E. M., DeLuca L. W., Smith H. G. Surface-bound biomembranes incorporating receptors: electrochemical and structural characterization. Biosens Bioelectron. 1992;7(6):429–440. doi: 10.1016/0956-5663(92)85042-9. [DOI] [PubMed] [Google Scholar]
- Eadie J. S., Conrad M., Toorchen D., Topal M. D. Mechanism of mutagenesis by O6-methylguanine. Nature. 1984 Mar 8;308(5955):201–203. doi: 10.1038/308201a0. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Feng W. Y., Lee E. H., Hays J. B. Recombinagenic processing of UV-light photoproducts in nonreplicating phage DNA by the Escherichia coli methyl-directed mismatch repair system. Genetics. 1991 Dec;129(4):1007–1020. doi: 10.1093/genetics/129.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew J. E., Hill H. A. Direct and indirect electron transfer between electrodes and redox proteins. Eur J Biochem. 1988 Mar 1;172(2):261–269. doi: 10.1111/j.1432-1033.1988.tb13882.x. [DOI] [PubMed] [Google Scholar]
- Goldmacher V. S., Cuzick R. A., Jr, Thilly W. G. Isolation and partial characterization of human cell mutants differing in sensitivity to killing and mutation by methylnitrosourea and N-methyl-N'-nitro-N-nitrosoguanidine. J Biol Chem. 1986 Sep 25;261(27):12462–12471. [PubMed] [Google Scholar]
- Gray J. C. Purification and properties of monomeric cytochrome f from charlock, Sinapis arvensis L. Eur J Biochem. 1978 Jan 2;82(1):133–141. doi: 10.1111/j.1432-1033.1978.tb12004.x. [DOI] [PubMed] [Google Scholar]
- Ho K. K., Krogmann D. W. Cytochrome f from spinach and cyanobacteria. Purification and characterization. J Biol Chem. 1980 May 10;255(9):3855–3861. [PubMed] [Google Scholar]
- Holmes J., Jr, Clark S., Modrich P. Strand-specific mismatch correction in nuclear extracts of human and Drosophila melanogaster cell lines. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5837–5841. doi: 10.1073/pnas.87.15.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran P., Bignami M. Self-destruction and tolerance in resistance of mammalian cells to alkylation damage. Nucleic Acids Res. 1992 Jun 25;20(12):2933–2940. doi: 10.1093/nar/20.12.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran P., Marinus M. G. Mismatch correction at O6-methylguanine residues in E. coli DNA. Nature. 1982 Apr 29;296(5860):868–869. doi: 10.1038/296868a0. [DOI] [PubMed] [Google Scholar]
- Keohavong P., Thilly W. G. Mutational spectrometry: a general approach for hot-spot point mutations in selectable genes. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4623–4627. doi: 10.1073/pnas.89.10.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahue R. S., Au K. G., Modrich P. DNA mismatch correction in a defined system. Science. 1989 Jul 14;245(4914):160–164. doi: 10.1126/science.2665076. [DOI] [PubMed] [Google Scholar]
- Laibinis P. E., Hickman J. J., Wrighton M. S., Whitesides G. M. Orthogonal self-assembled monolayers: alkanethiols on gold and alkane carboxylic acids on alumina. Science. 1989 Aug 25;245(4920):845–847. doi: 10.1126/science.245.4920.845. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Reconstitution of membrane receptor systems. Biochim Biophys Acta. 1985 Jun 12;822(1):127–153. doi: 10.1016/0304-4157(85)90005-x. [DOI] [PubMed] [Google Scholar]
- Längle-Rouault F., Maenhaut-Michel G., Radman M. GATC sequences, DNA nicks and the MutH function in Escherichia coli mismatch repair. EMBO J. 1987 Apr;6(4):1121–1127. doi: 10.1002/j.1460-2075.1987.tb04867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmström B. G. Cytochrome oxidase: some unsolved problems and controversial issues. Arch Biochem Biophys. 1990 Aug 1;280(2):233–241. doi: 10.1016/0003-9861(90)90325-s. [DOI] [PubMed] [Google Scholar]
- Modrich P. Mechanisms and biological effects of mismatch repair. Annu Rev Genet. 1991;25:229–253. doi: 10.1146/annurev.ge.25.120191.001305. [DOI] [PubMed] [Google Scholar]
- Oller A. R., Thilly W. G. Mutational spectra in human B-cells. Spontaneous, oxygen and hydrogen peroxide-induced mutations at the hprt gene. J Mol Biol. 1992 Dec 5;228(3):813–826. doi: 10.1016/0022-2836(92)90866-i. [DOI] [PubMed] [Google Scholar]
- Plant J. E., Roberts J. J. A novel mechanism for the inhibition of DNA synthesis following methylation: the effect of N-methyl-N-nitrosourea on HeLa cells. Chem Biol Interact. 1971 Oct;3(5):337–342. doi: 10.1016/0009-2797(71)90013-5. [DOI] [PubMed] [Google Scholar]
- Qin L., Kostić N. M. Electron-transfer reactions of cytochrome f with flavin semiquinones and with plastocyanin. Importance of protein-protein electrostatic interactions and of donor-acceptor coupling. Biochemistry. 1992 Jun 9;31(22):5145–5150. doi: 10.1021/bi00137a008. [DOI] [PubMed] [Google Scholar]
- Salamon Z., Gleason F. K., Tollin G. Direct electrochemistry of thioredoxins and glutathione at a lipid bilayer-modified electrode. Arch Biochem Biophys. 1992 Nov 15;299(1):193–198. doi: 10.1016/0003-9861(92)90262-u. [DOI] [PubMed] [Google Scholar]
- Salamon Z., Tollin G. Direct electrochemistry of spinach plastocyanin at a lipid bilayer-modified electrode: cyclic voltammetry as a probe of membrane-protein interactions. Arch Biochem Biophys. 1992 May 1;294(2):382–387. doi: 10.1016/0003-9861(92)90699-w. [DOI] [PubMed] [Google Scholar]
- Su S. S., Lahue R. S., Au K. G., Modrich P. Mispair specificity of methyl-directed DNA mismatch correction in vitro. J Biol Chem. 1988 May 15;263(14):6829–6835. [PubMed] [Google Scholar]
- Szczepaniak A., Black M. T., Cramer W. A. Topography of the chloroplast cytochrome b6: orientation of the cytochrome and accessibility of the lumen-side interhelix loops. Z Naturforsch C. 1989 May-Jun;44(5-6):453–461. doi: 10.1515/znc-1989-5-619. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Roberts J. D., Kunkel T. A. Heteroduplex repair in extracts of human HeLa cells. J Biol Chem. 1991 Feb 25;266(6):3744–3751. [PubMed] [Google Scholar]
- Tiede D. M. Incorporation of membrane proteins into interfacial films: model membranes for electrical and structural characterization. Biochim Biophys Acta. 1985 Dec;811(4):357–379. doi: 10.1016/0304-4173(85)90007-2. [DOI] [PubMed] [Google Scholar]
- Vaux D. L. Toward an understanding of the molecular mechanisms of physiological cell death. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):786–789. doi: 10.1073/pnas.90.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]


