Abstract
In recent clinical practice guidelines for risk assessment for a first atherosclerotic cardiovascular disease (ASCVD) event, it is not routinely recommended to measure carotid intima-media thickness (CIMT) or the coronary calcium score (CACS). The aim of this study was to elucidate the effect of combining carotid artery evaluation and CACS as surrogate markers or predictive values. A total of 938 patients (562 male (59.9%), mean age 61.5±11.6 years) with ASCVD (n=690) or without (n=248) were enrolled in this study. The diagnosis of ASCVD was established with CT angiography. These patients had undergone carotid scanning (HP Sonos-5500; Philips, Bothell, WA, USA) at St. Mary’s Hospital between September 2003 and March 2009. ASCVD outcomes were evaluated with a median follow-up of 1451 days. Thirty participants experienced initial ASCVD events during this study. Another 118 patients suffered secondary ASCVD events. After propensity score matching, multivariate analysis revealed that CACS was associated with ASCVD [Odds ratio 1.002, 95% confidence interval (CI) 1.002-1.003, P<0.001]. For primary prevention in patients without ASCVD, we found that carotid plaques [Hazard ratio (HR) 2.409, 95% CI 1.093-5.309, P=0.029] are also associated with ASCVD events. Carotid plaques are also associated with ASCVD events with regard to secondary prevention [HR 1.723, 95% CI 1.188-2.499, P=0.004] in patients with ASCVD. We propose that CACS assessment is useful in the diagnosis of, and as a surrogate marker of ASCVD in patients with risk factors. Our results also suggest that carotid artery evaluation may have a valuable predictive method in primary and secondary ASCVD prevention and risk assessment. Therefore, although there are no synergic effects of combining carotid artery evaluation and CACS, carotid ultrasound seems to be a better predictive method for assessing ASCVD events than CACS.
Keywords: Carotid plaque, coronary calcium score, atherosclerotic cardiovascular event, primary or secondary prevention
Introduction
Atherosclerosis is a complex pathological process. The development and healing of atherosclerotic lesions involve lipoprotein deposition, an inflammatory response, apoptosis and necrosis, and, finally, calcification and fibrosis [1]. The presence of calcium indicates intimal atherosclerosis; therefore, detecting calcium may have prognostic significance [2]. Multi-slice spiral computer tomography (MDCT) is relatively accurate, and allows the quantification of calcification in coronary arteries. Similarly, coronary artery calcium score (CACS) calculated using automatic software is well correlated to atherosclerosis of the coronary arteries. A previous study showed that the use of CACS data in addition to traditional risk factors significantly improves the classification of a multiethnic cohort of asymptomatic individuals without known cardiovascular (CV) disease [3]. However, in 2013, the ACC/AHA guidelines for cardiovascular risk assessment indicated that if a risk-based treatment decision is uncertain after quantitative risk assessment, CACS assessment might be considered in treatment decision making [4].
The Multi-Ethnic Study of Atherosclerosis revealed that the presence of carotid plaques not only independently predicts CV events, but also improves the risk prediction for coronary heart disease when used in combination with Framingham risk factors [5]. Another prior study found that carotid plaques are related to CV death or non-fatal acute myocardial infarction (MI) in patients with stable angina; however, carotid intima-media thickness (CIMT) is a weak predictor of CV events [6]. Accordingly, in 2013, the ACC/AHA guidelines for CV risk assessment indicated that CIMT is not recommended as a routine measure of risk assessment in first atherosclerotic cardiovascular disease (ASCVD) [4].
It is unclear whether carotid artery evaluation and CACS could improve the methods of diagnosis, or of primary and secondary ASCVD prevention in asymptomatic high-risk patients. Furthermore, there have been no direct comparisons between carotid ultrasound and CACS. Therefore, the aim of this study was to elucidate the effect of the diagnostic and predictive performance using the combination of carotid artery evaluation and CACS for diagnosis and primary and secondary ASCVD prevention.
Methods
Study population
A total of 938 consecutive patients who underwent carotid scanning and coronary CT angiography (CCTA) in St Mary’s Hospital between September 2003 and March 2009 were included from the CIMT and CCTA registry. Inclusion criteria for primary prevention of ASCVD were age 18 years or older with one or more cardio-cerebrovascular risk factors. And also, inclusion criteria for secondary prevention were known ASCVD. ASCVD was defined as acute coronary syndromes (including ST elevation MI, non-ST elevation MI, and unstable angina), or a history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or PAD (defined as ankle-brachial index <0.9, using VP-1000; Omron Healthcare, Kyoto, Japan) presumed to be of atherosclerotic origin.
A CCTA (n=938, 100%) was performed in every patient to evaluate cardiac risk or non-cardiac perioperative risk. Coronary angiography (CAG) was also performed when necessary. 752 (76.9%) patients underwent CAG. Exclusion criteria included (1) significant valvular heart disease (i.e., greater than mild valvular insufficiency or stenosis); (2) pregnancy or lactating; and (3) major systemic illness such as chronic inflammatory disease, active malignancy, and other illnesses.
There was no industry involvement in the design, execution, or analysis of this study. The use of clinical data was approved by the ethics committee and all patients provided written informed consent.
Clinical and biochemical assessment
Trained research technicians collected baseline demographic and clinical data. Baseline patient characteristics included a complete history and physical examination with information regarding hypertension (HTN, defined as systolic pressure ≥140 mmHg and/or diastolic pressure ≥90 mmHg, based on more than three measurements or current use of antihypertensive drugs), smoking habits (both current and former use), body mass index (BMI, calculated by weight divided by height in meters squared), and DM (controlled with diet, oral hypoglycemic agents, or insulin; or fasting glucose level ≥126 mg/dl or 2-h oral glucose tolerance test ≥200 mg/dl). Fasting glucose levels were enzymatically calculated using the hexokinase method [7]. A blood sample was collected from every patient and centrifuged within 30 minutes. Blood serum samples were kept at -80°C. High sensitivity C-reactive protein (hs-CRP) was assessed using an immunoturbidity assay system (Liatest; Stago, Asnières-sur-Seine, France), with an interassay variability coefficient of variation of 6.25% [8]. In order to minimize the influence of circadian variations, blood specimens were obtained after 12- to 14-hour fast (8:00 p.m. to 9:30 a.m.). Total cholesterol (TC) and triglyceride (TG) concentrations were assessed using standard enzyme methods. High-density lipoprotein-cholesterol (HDL) was measured after very-low-density lipoprotein LDL was measured with phosphotungstic acid precipitation. LDL values were calculated using the Friedewald formula.
Coronary CT angiography
Patients were instructed not to eat for at least 4 hours before the examination and to avoid coffee, tea and tobacco. Patients with DM were instructed to stop taking metformin for 3 days prior to the examination. Contrast-enhanced CCTA was quantified on retrospective electrocardiography-gated cardiac CT scans using a 64-slice MDCT (Lightspeed VCT; GE Healthcare, Milwaukee, WI, USA). Patient heart rates were measured 1 hour before the examination. The CACS was measured using the scoring system (in units) described by Agatston et al. [2]. Our CCTA protocol was as follows: slice collimation, 64×0.625 mm; gantry rotation time, 0.5 seconds; pitch, 0.2; scan time, 0.4 seconds; table feed, 6 mm/second; tube voltage, 120 kV; and tube current, 596 mAs. Patients received 80 mL of contrast agent (Iopromide, Ultravist 300; Schering, Berlin, Germany) at 5.0 mL/s for 16 seconds at the time of the scan. Next, they received 50 mL of saline solution intravenously at a rate of 5.0 mL/s for 10 seconds. Injections were performed through an antecubital vein with an 18-gauge catheter.
Coronary angiography
Coronary angiogram was performed via the femoral or radial approach according to the American College of Cardiology/American Heart Association (ACC/AHA) recommendations. Coronary lesions were assessed with multiple orthogonal views. Lesions were visually evaluated for morphologic features similar to those reported by the ACC/AHA. Significant coronary artery stenosis was defined as >50% reduction of the internal diameter of major epicardial coronary arteries and side branches with a diameter ≥2.5 mm.
CIMT measurement
The carotid arteries were measured using high-resolution B-mode ultrasound with a 15-MHz linear array transducer (HP Sonos-5500; Philips, Bothell, WA, USA). Two certified sonographers who were blinded to all clinical information performed carotid arterial scanning. Patients were placed in the supine position with slight hyperextension and rotation of the neck to the contralateral side. The ultrasound probe was placed immediately proximal to the carotid bifurcation. CIMT measurements were gathered at 10-mm intervals of the far wall of the right common carotid arteries. To improve image quality, depth control was fixed at 4 cm. The transducer frequency was set to 15 MHz during the entire analysis with an axial resolution of 0.2 mm. Since systolic expansion of the lumen causes CIMT thinning, assessments were acquired during end-diastole (defined as the R wave of an electrocardiogram). CIMT was defined as the distance between the luminal border of the intima and the outer border of the media. The CIMT was measured by manually examining the thickness of every free plaque lesion. Calipers were placed for six individual measurements, and the average value was calculated. The average CIMT value of each segment was assessed to determine the mean CIMT per patient. A carotid plaque is a focal lesion that is at least 50% thicker than the surrounding vessel wall, or is a region (with a distinct boundary) with CIMT >1.5 mm that protrudes into the lumen [9].
Clinical follow-up
The primary and secondary prevention of the 938 patients are shown in Figure 1. For primary prevention, in patients without ASCVD, primary end points included Hard ASCVD (or the first occurrence of nonfatal MI or coronary heart disease), death, fatal/nonfatal stroke, and PAD presumed to be of atherosclerotic origin. Among 248 patients without ASCVD, 25 patients were excluded either because they were lost to follow-up (n=18), or because of in-hospital events (non-cardiac death, n=7). A total of 223 patients (121 male (54.2%), mean age 59.1±11.4), were enrolled for the primary prevention study. Clinical follow-up was performed for a median follow-up time of 1451 days (113-2858 days). Follow-up data were collected from all patients. All ASCVD events were reviewed by a panel of three physicians. These physicians reviewed data that had been collected from inpatient hospitalizations, hospital records (131 patients, 58.7%), and telephone interviews (92 patients, 41.3%) by a trained researcher. When a patient could not be reached, data were obtained via a telephone interview with the patient’s family members.
Figure 1.
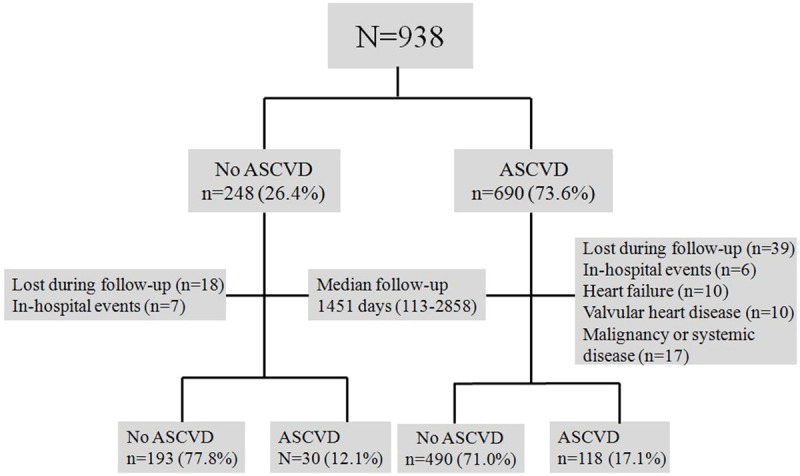
Flow chart of study population.
The secondary prevention defined as occurring new-onset ASCVD events in patients with ASCVD. Among 690 patients with ASCVD, a total of 82 patients were excluded. Of these, 39 were lost to follow-up, 6 experienced in-hospital events, 10 experienced heart failure, 10 had significant valvular heart disease, and 17 experienced active malignancy or systemic illness. A total of 608 patients (387 male (63.7%), mean age 61.5±11.4) were ultimately enrolled for secondary prevention. In these patients, follow-up data were obtained from all patients. A panel of three physicians reviewed all ASCVD events. These physicians reviewed data collected from inpatient hospitalization, hospital records by a trained researcher.
Statistical analysis
Continuous variables are presented as mean ± SD, and categorical variables are described as absolute and relative frequencies (%). Welch’s t-test was used to compare ASCVD event data between groups for continuous variables. In contrast, the Chi-squared test with Yates’ correction was used to compare categorical variables. The intraclass correlation coefficient (ICC) is a statistical method to measure intra- and inter-observer reliability. Reliability was measured by replicating measurements for 50 patients. Two certified sonographers performed carotid arterial scanning and 1 hour between the first and second measurement. The ICC assesses the consistency of multiple measurements of the same quantity by measuring the proportion of the variance that is attributed to different observers. The 95% confidence interval was calculated for each ICC to estimate the precision and the range of the correlation. Univariate Cox regression analysis was used to assess the relationship between the ASCVD events and several risk factors. Multivariate Cox regression analysis was performed to evaluate the association between ASCVD events and several risk factors. To reduce the impact of selection bias and potential confounding, we used propensity score matching to adjust for significant differences in patients’ baseline characteristics [10,11]. A receiver operator characteristic (ROC) curve was generated to determine the diagnostic power of CACS for ASCVD. All statistical analyses were conducted using R language ver. 3.01 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients characteristics
The mean age of the study participants was 61.5±11.6 years, and 562 subjects (59.9%) were men. The baseline patient characteristics of the 938 patients are shown in Table 1. CCTA was performed in 938 patients, and 752 (76.9%) patients underwent CAG. Patient findings included ASCVD (n=690, 73.6%), HTN (n=527, 56.2%), DM (n=285, 30.4%), smoking (n=227, 24.2%), and atrial fibrillation (n=22, 2.3%). Patients with ASCVD events had higher mean CIMT value and an increased incidence of carotid artery plaques than did those without ASCVD events. Additionally, patients with ASCVD events had higher CACS values than did those without ASCVD events.
Table 1.
Baseline Characteristics of the 938 patients according to Atherosclerotic Cardiovascular disease
| Variables | No ASCVD n=248 | ASCVD n=690 | p value |
|---|---|---|---|
| Age. yrs | 59.4±11.6 | 62.3±11.6 | <0.001 |
| Male, n (%) | 135 (54.4) | 427 (61.9) | 0.048 |
| Cerebrovascular history, n (%) | 0 (0) | 40 (5.8) | <0.001 |
| Myocardial Infarction history, n (%) | 0 (0) | 81 (11.7) | <0.001 |
| Coronary Revascularization history, n (%) | 0 (0) | 135 (19.6) | <0.001 |
| Body Mass Index, kg/m2 | 25.6±3.1 | 24.9±3.3 | 0.002 |
| Fasting plasma glucose, mg/dl | 119±40 | 119±39 | 0.982 |
| Hemoglobin A1c, % | 6.3±1.5 | 6.3±1.4 | 0.992 |
| Total Cholesterol, mg/dL | 191±42 | 164±39 | <0.001 |
| Triglyceride, mg/dL | 143±87 | 134±91 | 0.146 |
| High Density Lipoprotein cholesterol, mg/dL | 44.9±10.4 | 44.2±12.2 | 0.348 |
| Low Density Lipoprotein cholesterol, mg/dL | 117.2±33.3 | 93.5±33.1 | <0.001 |
| hsCRP, mg/dL | 7.1±21 | 8.6±20.8 | 0.356 |
| Systolic blood pressure, mmHg | 120±18 | 122±17 | 0.186 |
| Diastolic blood pressure, mmHg | 73.1±10.7 | 74.1±11 | 0.230 |
| Left ventricle Ejection Fraction, % | 61.8±5.9 | 59.1±9.5 | <0.001 |
| Smoking, n (%) | 59 (25.2) | 168 (25.5) | 1.000 |
| Diabetes mellitus, n (%) | 70 (28.2) | 215 (31.2) | 0.427 |
| Hypertension, n (%) | 127 (51.2) | 400 (58.1) | 0.074 |
| Atrial fibrilation, n (%) | 9 (3.6) | 13 (1.9) | 0.189 |
| Medication | |||
| Aspirin, n (%) | 30 (16.2) | 207 (34.3) | <0.001 |
| ACE inhibitor or ARB, n (%) | 123 (53) | 417 (62.4) | 0.015 |
| Beta-blocker, n (%) | 84 (36.1) | 293 (44) | 0.042 |
| Calcium channel blocker, n (%) | 60 (25.8) | 191 (28.6) | 0.447 |
Measurements of intra- or inter-observer reliability of CIMT Assessment
Intra- and inter-observer reliability values were calculated (using ICC) to determine the reliability of the CIMT measurements. The intra- and inter-observer reliabilities for the CIMT measurements were 0.965 and 0.900, respectively. In general, ICC values range from 0.00 (no agreement) to 1.00 (perfect agreement). An ICC value of 0.7-0.8 indicates strong agreement, and an ICC value >0.8 indicates excellent agreement.
Carotid measurement and coronary calcium score comparison according to ASCVD
The carotid artery measurements and CACS of 938 patients are shown in Table 2. Patients with ASCVD had greater mean CIMT values, and increased CACS than did those without ASCVD. Additionally, patients with ASCVD had an increased incidence of carotid artery plaques than did those without ASCVD. Multiple regression analysis after matching by propensity score reveals that CACS is associated with ASCVD (ROC curve, Figure 2). We used multiple regression analysis (after propensity score matching) to compare the diagnostic power of carotid artery measurements and CACS for ASCVD (Table 3). CACS has superior diagnostic power compared to that of carotid artery measurements.
Table 2.
Measurement of carotid artery and Cornary calcium score of the 938 patients according to Atherosclerotic Cardiovascular disease
| Variables | No ASCVD n=248 | ASCVD n=690 | p value |
|---|---|---|---|
| Intima-Media Thickness | |||
| maximum CCA thickness, mm | 0.963±0.262 | 0.997±0.334 | 0.103 |
| mean CCA thickness, mm | 0.819±0.197 | 0.854±0.244 | 0.027 |
| Plaque, n (%) | 93 (37.5) | 318 (46.1) | 0.024 |
| Calcium Score | 107 (0-1770) | 536 (0-4262) | <0.001 |
Figure 2.
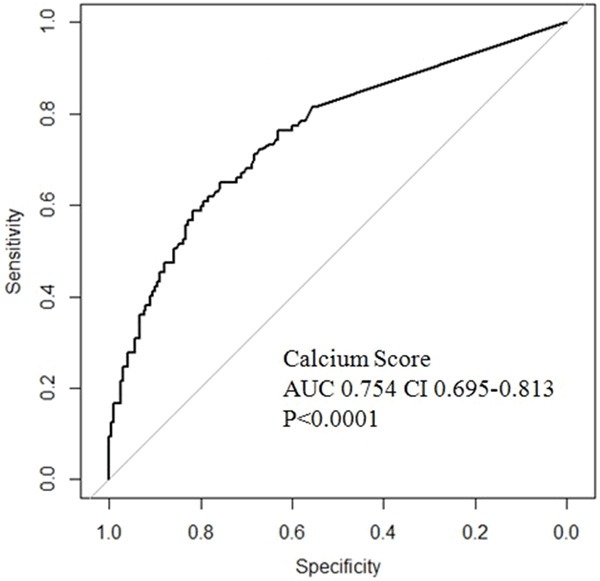
A receiver operator characteristic (ROC) curve demonstrating that coronary artery calcium score is associated with atherosclerotic cardiovascular disease after propensity score matching.
Table 3.
The relative risk of diagnosis for ASCVD using multiple regression analysis after sample matching by propensity scores
| OR | 95% CI | p value | C-index | |
|---|---|---|---|---|
| maximum CCA thickness, mm | 3.018 | 1.274-7.151 | 0.012 | 0.568 |
| mean CCA thickness, mm | 8.470 | 2.551-28.121 | <0.001 | 0.601 |
| Plaque, n (%) | 1.980 | 1.268-3.090 | 0.003 | 0.584 |
| Calcium Score | 1.003 | 1.002-1.004 | <0.001 | 0.817 |
Prognostic evaluation for primary and secondary prevention
Clinical follow-up was performed for a median 1451 days (113-2858). During follow-up, there were 30 (13.5%) new-onset ASCVD events. Among these 30 patients, 29 (96.7%) patients suffered CAD, and 1 (3.3%) had cerebrovascular disease. We evaluated the predictive power of carotid artery measurements and CACS for ASCVD using multiple regression analysis after propensity score matching (Table 4). Carotid plaque (HR 2.713, 95% CI 1.234-5.964, P=0.013) had superior predictive power for primary prevention of ASCVD compared to that of CACS.
Table 4.
The Prognosis Prediction indices of different measurements for Primary prevention ASCVD using multiple regression analysis after sample matching by propensity scores
| HR | 95% CI | p value | C-index | |
|---|---|---|---|---|
| maximum CCA thickness, mm | 0.546 | 0.122-2.442 | 0.429 | 0.554 |
| mean CCA thickness, mm | 1.109 | 0.163-7.566 | 0.916 | 0.523 |
| Plaque, n (%) | 2.713 | 1.234-5.964 | 0.013 | 0.621 |
| Calcium Score | 0.999 | 0.996-1.002 | 0.307 | 0.506 |
For secondary prevention of ASCVD events, during the follow-up, there were 118 (19.4%) new-onset ASCVD events. Among these 118 patients, 112 (18.4%) patients suffered CAD, MI or restenosis, 5 (0.8%) had cerebrovascular disease and 1 (0.2%) had PAD. We evaluated the predictive power of carotid artery measurements and CACS for ASCVD using multiple regression analysis after propensity score matching (Table 5). Carotid plaque (HR 1.723, 95% CI 1.188-2.494, P=0.004) had superior predictive power for secondary prevention of ASCVD as compared to that of CACS.
Table 5.
The Prognosis Prediction indices of different measurements for Secondary prevention ASCVD using multiple regression analysis after sample matching by propensity scores
| HR | 95% CI | p value | C-index | |
|---|---|---|---|---|
| maximum CCA thickness, mm | 1.315 | 0.770-2.243 | 0.316 | 0.534 |
| mean CCA thickness, mm | 1.149 | 0.529-2.494 | 0.725 | 0.524 |
| Plaque, n (%) | 1.723 | 1.188-2.499 | 0.004 | 0.556 |
| Calcium Score | 1.453 | 0.817-2.583 | 0.203 | 0.538 |
Finally, to evaluate the predictive performance of the combination of these imaging modalities, we performed the additive or synergic effect of carotid parameters and CACS for ASCVD prevention using multiple regression analysis after propensity score matching. However, there was no additive effect of the combination of these imaging modalities (HR 1.747, 95% CI 0.982-3.110, P=0.058).
Discussion
Diagnostic role of carotid artery evaluation and CACS
This study examined the diagnostic role of carotid artery evaluation and CACS for the diagnosis of ASCVD in asymptomatic high risk patients. Direct comparisons have not been made between carotid ultrasound and CACS, or between the diagnostic effects of the combination of these imaging modalities. We used the new concept of ASCVD in this study because risk estimates for ASCVD outcomes are more relevant to contemporary populations [4]. The roles of carotid artery evaluation and CACS for ASCVD risk assessment are controversial. Current guidelines for cardiovascular risk assessment do not recommend CIMT for routine assessment. Furthermore, if a risk-based treatment decision is uncertain, CACS assessment may be considered to inform treatment decision making [4]. This study uses ASCVD outcome as a more broadly definitive end point. It is also important to mention that many of the patients enrolled in this study had other comorbidities (example, HTN 56.2%, DM 30.4%). After propensity score matching (to limit confounding), the present study demonstrates that CACS is a more important diagnostic factor for ASCVD than is carotid artery evaluation.
These findings are similar to those of a previous study, which found that CACS improves the diagnosis, risk stratification, discrimination, and reclassification of ASCVD above that of traditional risk factor categories [2,12]. Our findings regarding carotid artery assessment are similar to those of a previous study, which reported that carotid plaque is associated with cardiovascular events in the general population [5]. However, our results differ from those of an earlier report, which indicated that CIMT (a surrogate marker of subclinical atherosclerosis) is consistently linked to cardiovascular and cerebrovascular disease [13-15].
Prognostic role of carotid artery evaluation and CACS
This study examined the prognostic role of carotid artery evaluation and CACS for the primary or secondary prevention of ASCVD events. In this study, carotid plaque was associated with primary and secondary prevention of ASCVD events. However, after propensity score matching, CIMT was not associated with ASCVD events. We only evaluated CIMT from the common carotid artery, which is less sensitive to local atherosclerosis than are carotid bifurcation and internal carotid segments [16]. The prognostic value of CIMT measurement to predict future cardiovascular events may improve when data from all three segments are combined. Therefore, these results may underestimate the relationship between ASCVD and carotid atherosclerosis.
Using established statistical methods [10], we found that CACS was not correlated to the prevention of ASCVD events. Our results differ from those of an earlier report, which indicated that CACS adds independent information to the traditional risk factors in the prediction of all-cause mortality [17]. The previous large observational study showed that coronary calcium adds independent information to the traditional risk factors in the prediction of all-cause mortality. When CACS was added to the risk factors in the previous study, risk estimation increased significantly according to the C-index (P<0.001) [18]. In another multi-ethnic cohort, adding CACS to a prediction model (based on traditional risk factors) significantly improved risk classification and placed more individuals in the most extreme risk categories [3]. Cho et al. showed that in asymptomatic individuals with moderately elevated CACS, CCTA improves risk prediction and reclassification of future fatal and non-fatal events beyond clinical risk assessment. CCTA does not reliably improve risk stratification for individuals with lower or higher CACS [19]. In contrast, carotid artery evaluation has a superior prognostic effect for primary and secondary prevention of ASCVD events than does CACS.
Combination of carotid assessment and CACS
Our aim was to evaluate whether using the combination of carotid artery evaluation and CACS would be useful in detecting or preventing ASCVD in high-risk patients. There are also no prior studies that evaluate the predictive ability of the combination of carotid ultrasound and CACS, especially in asymptomatic high-risk patients. In this study, there does not appear to be an additive effect of carotid artery evaluation and CACS; however, individually, each one is useful in the diagnosis and prognosis of ASCVD.
A prior study found that not only was CIMT significantly associated with coronary artery plaque in individuals with a CACS of zero, but also that CIMT can be a remarkable predictor of CAD regardless of CACS [20]. Two procedures can identify subclinical atherosclerosis. These measures can characterize disease and provide information predictive of graded risk for adverse vascular events. Another study found that CIMT evaluation can detect subclinical vascular disease in young to middle-aged patients with a low risk score and a CACS of zero. These findings have important implications regarding vascular disease screening and the implementation of primary-prevention strategies [21]. Risk scores with a CACS of zero were associated with evidence of subclinical atherosclerosis in the carotid artery. In addition, only 15% of those with a low CIMT had detectable CACS. Therefore, carotid ultrasound may be a better screening tool to assess cardiovascular risk in low-risk populations than is CACS [22]. Carotid ultrasound is feasible in all individuals, is relatively inexpensive and does not expose patients to radiation. Therefore, carotid ultrasound may be a better screening method to assess ASCVD events in high-risk populations than CACS.
Study limitations
This study has several limitations. It was a prospective, observational, longitudinal study that was not randomized. However, the sample size was relatively larger, and high quality statistical methods were used. The propensity score is the conditional probability of being treated given the covariates. It can be used to balance the covariates in the two groups, and therefore reduces this bias. In order to estimate the propensity score, one must model the distribution of the treatment indicator variable given the observed covariates [10]. Another limitation was that the CIMT assessments were manually performed, and that ultrasound performance is operator-dependent. In order to reduce operate-dependent variation, we calculated ICC, which is a statistical method for intra- and inter-observer reliability measurement. Carotid arterial scanning was performed by two certified sonographers who were blinded to all clinical information. Furthermore, in 2013 the ACC/AHA guidelines for blood cholesterol treatment identified 4 major statin benefit groups to reduce ASCVD in adults [23]. Therefore, a third limitation to this study is that we treated high blood cholesterol in patients according to Adult Treatment Panel III recommendations [24] rather than the ACC/AHA guidelines because data were collected from September 2003 to March 2009. Justification for the Atherosclerosis Regression Treatment Extension Study reported that two-year treatment with rosuvastatin inhibits the progression of CIMT and also improves carotid plaque composition [25]. Next, we used statistical analyses to reduce the treatment bias. A fourth limitation is that the CIMT of enrolled patients was thicker than that observed in the general Korean population [26]. We hypothesized that patients with risk factors may be more likely to visit the tertiary hospital for screening, explaining this discrepancy. Also, our CIMT-CCTA registry was designed to evaluate real-world outcomes in consecutive all-comers at high risk of cardiovascular disease.
Conclusion
We propose that CACS assessment is useful in the diagnosis of, and as a surrogate marker of ASCVD in asymptomatic high-risk patients. Our results also suggest that carotid artery evaluation may have a valuable predictive method in primary and secondary ASCVD prevention and risk assessment. Therefore, although there are no synergic effects of combining carotid artery evaluation and CACS, carotid ultrasound seems to be a better predictive method for assessing ASCVD events in high-risk populations than CACS.
Acknowledgements
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclosure of conflict of interest
None.
References
- 1.Li H, Horke S, Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237:208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 3.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AE, O’Leary DH. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2:e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Held C, Hjemdahl P, Eriksson SV, Bjorkander I, Forslund L, Rehnqvist N. Prognostic implications of intima-media thickness and plaques in the carotid and femoral arteries in patients with stable angina pectoris. Eur Heart J. 2001;22:62–72. doi: 10.1053/euhj.1999.2006. [DOI] [PubMed] [Google Scholar]
- 7.Tzou WS, Douglas PS, Srinivasan SR, Bond MG, Tang R, Chen W, Berenson GS, Stein JH. Increased subclinical atherosclerosis in young adults with metabolic syndrome: the Bogalusa Heart Study. J Am Coll Cardiol. 2005;46:457–463. doi: 10.1016/j.jacc.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 8.Mattsson N, Ronnemaa T, Juonala M, Viikari JS, Jokinen E, Hutri-Kahonen N, Kahonen M, Laitinen T, Raitakari OT. Arterial structure and function in young adults with the metabolic syndrome: the Cardiovascular Risk in Young Finns Study. Eur Heart J. 2008;29:784–791. doi: 10.1093/eurheartj/ehm576. [DOI] [PubMed] [Google Scholar]
- 9.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189-190. [DOI] [PubMed] [Google Scholar]
- 10.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Sjolander A. Propensity scores and M-structures. Stat Med. 2009;28:1416–1420. doi: 10.1002/sim.3532. author reply 1420-1413. [DOI] [PubMed] [Google Scholar]
- 12.Erbel R, Mohlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, Dragano N, Gronemeyer D, Seibel R, Kalsch H, Brocker-Preuss M, Mann K, Siegrist J, Jockel KH. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–1406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB Sr. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30:177–181. doi: 10.1161/ATVBAHA.108.173609. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Duncan BB, Metcalf PA, Crouse JR 3rd, Sharrett AR, Tyroler HA, Barnes R, Heiss G. B-mode-detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:2377–2383. doi: 10.1161/01.str.25.12.2377. [DOI] [PubMed] [Google Scholar]
- 16.Solberg LA, Eggen DA. Localization and Sequence of Development of Atherosclerotic Lesions in the Carotid and Vertebral Arteries. Circulation. 1971;43:711–724. doi: 10.1161/01.cir.43.5.711. [DOI] [PubMed] [Google Scholar]
- 17.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 18.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 19.Cho I, Chang HJ, Ó Hartaigh B, Shin S, Sung JM, Lin FY, Achenbach S, Heo R, Berman DS, Budoff MJ, Callister TQ, Al-Mallah MH, Cademartiri F, Chinnaiyan K, Chow BJ, Dunning AM, DeLago A, Villines TC, Hadamitzky M, Hausleiter J, Leipsic J, Shaw LJ, Kaufmann PA, Cury RC, Feuchtner G, Kim YJ, Maffei E, Raff G, Pontone G, Andreini D, Min JK. Incremental prognostic utility of coronary CT angiography for asymptomatic patients based upon extent and severity of coronary artery calcium: results from the Coronary CT Angiography Evaluation For Clinical Outcomes International Multicenter (CONFIRM) Study. Eur Heart J. 2015;36:501–8. doi: 10.1093/eurheartj/ehu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inohara T, Niinuma H, Nishihara S, Makita Z, Sanoyama K, Niwa K. Carotid intima-media thickness is a useful screening tool to detect coronary artery plaque in type 2 diabetic patients with zero calcium score. Int J Cardiol. 2014;172:e132–134. doi: 10.1016/j.ijcard.2013.12.110. [DOI] [PubMed] [Google Scholar]
- 21.Lester SJ, Eleid MF, Khandheria BK, Hurst RT. Carotid intima-media thickness and coronary artery calcium score as indications of subclinical atherosclerosis. Mayo Clin Proc. 2009;84:229–233. doi: 10.4065/84.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters SA, den Ruijter HM, Bots ML, Moons KG. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart. 2012;98:177–184. doi: 10.1136/heartjnl-2011-300747. [DOI] [PubMed] [Google Scholar]
- 23.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 24.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 25.Nohara R, Daida H, Hata M, Kaku K, Kawamori R, Kishimoto J, Kurabayashi M, Masuda I, Sakuma I, Yamazaki T, Yokoi H, Yoshida M Justification For Atherosclerosis Regression Treatment (JART) Investigators. Effect of long-term intensive lipid-lowering therapy with rosuvastatin on progression of carotid intima-media thickness--Justification for Atherosclerosis Regression Treatment (JART) extension study. Circ J. 2013;77:1526–1533. doi: 10.1253/circj.cj-12-1149. [DOI] [PubMed] [Google Scholar]
- 26.Bae JH, Seung KB, Jung HO, Kim KY, Yoo KD, Kim CM, Cho SW, Cho SK, Kim YK, Rhee MY, Cho MC, Kim KS, Jin SW, Lee JM, Kim KS, Hyun DW, Cho YK, Seong IW, Jeong JO, Park SC, Jeong JY, Woo JT, Koh G, Lim SW. Analysis of Korean Carotid Intima-Media Thickness in Korean Healthy Subjects and Patients with Risk Factors: Korea Multi-Center Epidemiological Study. Korean Circ J. 2005;35:513–524. [Google Scholar]


