Abstract
Coronary artery disease (CAD) is a disease in which a waxy substance called plaque builds up inside the coronary arteries. Apelin is a novel endogenous peptide with inotropic and vasodilatory properties and is the ligand for the angiotensin receptor-like 1 (APJ) receptor. We aimed to determine genotype and allele frequencies of APJ receptor A445C gene polymorphism in Turkish patients with CAD and healthy controls by RFLP-PCR. This study was performed on 159 unrelated CAD patients and 62 healthy controls. We obtained AA, AC and CC genotype frequencies in CAD patients as 41.5%, 49.1% and 9.4%, respectively. In the control group, frequencies of genotypes were found as 35.5% for AA, 48.4% for AC and 16.1% for CC. We did not observe difference in APJ receptor A445C polymorphism between CAD patients and healthy controls (χ2 = 2.178; df = 2; P = 0.336). The A allele was encountered in 66% (210) of the CAD and 59.7% (74) of the controls. The C allele was seen in 34% (108) of the CAD and 40.3% (50) of the controls. Allele frequencies of interested genes were not significantly different between groups (χ2 = 1.57; df = 1; p = 0.225). The frequencies of APJ receptor A445C genotype were not significantly different between control and patients. None of the three APJ receptor A445C genotypes, AA, AC and CC displayed significant difference in CAD patients. We did not find any difference in the clinical parameters except for weight and diastolic blood pressure levels in the AA, AC and CC genotypes of patients. Individuals with CC genotypes had significantly higher weight, systolic and diastolic blood pressure levels and systolic blood pressure than other genotypes, P ≤ 0.05. In addition, HDL-C level was found decreased, but this reduction was not statistically significant. Contrarily, the low levels of weight, SBP, DBP and TC were statistically significant in the subjects with AA genotype in CAD. In conclusion, CC genotype carriers may have more risk than other genotypes in the development of hypertension in CAD, but not AAgenotype carriers. We suggest that this polymorphism may not be a marker of CAD, but it may cause useful in function of the apelin/APJ system and may be a genetic predisposing factor for diagnostic processes and can be helpfull in finding new treatment strategies. We think that it is required to further comprehensive studies in order to make clear this situation in CAD.
Keywords: APJ receptor A445C gene, coronary artery disease, polymorphism
Introduction
Coronary artery disease (CAD) is a common disease and among the leading cause of death in the general population. It is the most common form of heart disease known that as a complex disease. It occurs when arteries in the heart are blocked, due to both environmental and genetic aetiological determinants [1]. CAD has a number of well determined risk factors. A number of risk factors have been recognised as contributing to the disease, including obesity, hypertension, hyperlipidemia, diabetes, smoking, and family history of premature CAD, lack of exercise, stress [2].
The apelinergic system, consisting of apelin and APJ receptor pathway has emerged as an essential novel mediator of cardiovascular disease [3,4]. The APJ receptorwhich was identified as the receptor for the adipokine apelin is a 7-transmembrane domain receptor that was first cloned in 1993 [5,6]. The APJ receptor has been shown to counteract damaging effects of angiotensin II [7]. In the human cardiovascular system, apelin and APJ receptor are both expressed in the heart. APJ receptor is localized to endothelial and smooth muscle cells, and cardiomyocytes [8]. It plays an important role in the regulationfluid and glucose homeostasis, vessel formation, inhibition of vasopressin release, regulation of vascular tone, decreases blood pressure and increases diuresis as well as playing a role in diabetes and obesity [9].
APJ receptor A445C gene polymorphism can provide benefits to improve clinical treatments and help us understand CAD pathophysiology and development. Therefore, we aimed to determine the genotype and allele frequencies of APJ receptor A445C gene polymorphism in Turkish patient with CAD and healthy controls.
Methods and methods
The study was conducted with the collaboration of Department of Cardiovascular Surgery of Namık Kemal University in Tekirdağ, Turkey and the Department of Physiology of Dumlupınar University in Kütahya, Turkey. CAD was diagnosed on the basis of the patients’ clinical history, a physical examination and coronary angiography, according to the World Health Organization criteria [10]. Individuals with valvular heart disease, cardiomyopathy, chronic kidney disease and inflammatory disease were excluded. Clinical data of the patients are documented in Table 1. The control group consisted of 62 healthy age-matched subjects. The CAD group consisted of 42 females and 117 males, and the control group consisted of 31 females and 31 males. Participants of each group were chosen among the Turkish population. The procedures were explained to all subjects, and a written informed consent was obtained from each individual. The study protocol conformed to the ethical guidelines of Declaration of Helsinki and was approved by the Ethics Committee of Uludağ University (Bursa, Turkey).
Table 1.
Basic characteristics of control and CAD
| Control (n = 62) | CAD (n = 159) | P-value | |
|---|---|---|---|
| Age (years) | 61.8 ± 1.43 | 64.0 ± 0.96 | 0.497 |
| Height (cm) | 170.1 ± 0.89 | 169.1 ± 0.57 | 0.427 |
| Weight (kg) | 75.2 ± 1.23 | 77.3 ± 0.72 | 0.808 |
| BMI (kg/m2) | 25.9 ± 0.30 | 27.0 ± 0.24 | 0.024 |
| SBP (mmHg) | 128.0 ± 2.01 | 131.8 ± 1.18 | 0.530 |
| DBP (mmHg) | 79.8 ± 2.95 | 78.4 ± 0.81 | 0.209 |
| TC (mmol/L) | 186.4 ± 4.52 | 172.3 ± 3.66 | 0.046 |
| LDL-C (mmol/L) | 103.6 ± 4.17 | 104.1 ± 2.87 | 0.272 |
| HDL-C (mmol/L) | 48.4 ± 1.60 | 43.3 ± 1.47 | 0.251 |
| TG (mg/dL) | 165.3 ± 12.0 | 155.7 ± 8.04 | 0.863 |
| FBG (mmol/L) | 108.4 ± 2.75 | 128.6 ± 5.30 | 0.000 |
| BUN (mmol/L) | 39.0 ± 2.95 | 37.78 ± 1.75 | 0.757 |
| Creatinine (mg/L) | 0.88 ± 0.04 | 0.94 ± 0.04 | 0.456 |
Values are presented as mean ± SEM. Abbreviations: CAD, coronary artery disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; FBG, fasting blood glucose, BUN, blood urea nitrogen.
Serum levels of total cholesterol (TC) low density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), fasting blood glucose (FBG), blood urea nitrogen. (BUN) and creatinine were analyzed with commercial kits (Beckman-Coulter, USA) in an autoanalyser (BeckmanCoulter LX-20, USA). The weight sand heights were obtained to calculate body mass index (BMI) and obesity was defined as BMI > 30 kg /m2. The average of two measurements of blood pressure (BP) with the subject in the sitting position was taken at a 2-3 min interval after resting for at least 15 min.
Genetic analysis of APJ A445C gene polymorphism
DNA was isolated from peripheral blood leukocytes by GeneJET RNA Purification Kit (Thermo, Cat No: # K0722) according to the manufacturer’s protocol and genotyped by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. Single nucleotide polymorphism in the promoter region of APJ receptor gene A445C area was analyzed by the method of Falcone et al. [11]. The region obtained from the genomic DNAs was amplified with proper primers in PCR (method). PCR mixture included 30 ng genomic DNA, 0.5 nmol/L forward primer (5’-CAG CAT GGA GGA AGG TGG-3’), 0.5 nmol/L reverse primer (5’-GAC CCG CAG CCT CAG CCG-3’), 0.2 mmol/L dNTP, 1.5 mmol/L MgCl2, 10X PCR buffer, 0.025 U/mL Taq DNA polymerase; 50 μL of total PCR volume was studied. PCR conditions were hot start at 94°C for 3 min and 94°C for 30 sec, 62°C for 30 sec, 72°C for 1 min; the procedure was repeated 35 times. Duration of waiting time was 5 min at 72°C. The products obtained were visualized in 2% agarose gel and then the 436 bp of PCR products were digested with MwoI (New England Biolabs, Beverly, MA, USA) restriction enzyme at 37°C during the night. The restricted products were electrophoresed in 3% of agarosegel containing ethidiumbromide. Gels were visualized with the UV gel documentation system, bands were evaluated, and the genotyping was done. Evaluation of allele naming was 215 bp AA, 135 bp CC, 135-215 bp AC (Figure 1).
Figure 1.
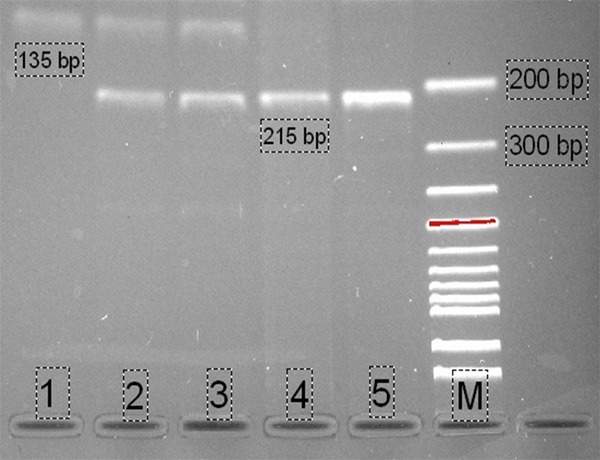
PCR-RFLP analysis of APJ A445C gene polymorphism: AA (4, 5, 215 bp), AC (2, 3, 135-215 bp), CC (1, 135 bp), M (Marker, 100 bp). The PCR products were separated by means of agarose gel electrophoresis and stained with ethidium bromide.
Statistical analysis
Statistical analyses were done by SPSS (Statistical Package for Social Sciences, Chicago, IL, USA) 16.0 package program. All data are given as mean ± standard error of the mean (SEM). Independent sample t test were used to analyse differences in continuous variables between control and CAD patients. Mann-Withney U test were used to analyze differences in continues variables between two genotypes. Kruskal-Wallis test were used to analyze differences in continues variables among three genotypes. Genotype frequencies were compared with values predicted by Hardy-Weinberg (HW) equilibrium equation (p2 + 2pq + q2 = 1) by the Chi-Square test, where p is frequency of dominant allele A and q is frequency of recessive allele C. The Chi-square test was used for comparison of genotype and allele frequencies between groups. All P values ≤ 0.05 were accepted as statistically significant.
Results
The comparison of basic characteristics data of the controls and CAD groups is given in Table 1. There were statistically significant differences between the groups for BMI, TC, and FBG. No significant differences between groups were detected for age, height, weight, SBP, DBP, LDL-C,HDL-C, TG, BUN, and creatinine.
The frequency of the genotype for the APJ receptor A445C gene polymorphism in the control and CAD groups did not show a significant deviation from the HW equilibrium (P > 0.05) (Table 2). Three different genotypes and allele frequencies of the APJ receptor A445C gene polymorphism were AA, AC and CC genotypes and A and C alleles, respectively. Allele frequencies and genotypes of the APJ receptor A445C gene polymorphism in CAD patients and healthy controls are shown in Table 3. The frequencies for each of the APJ receptor genotype were found as 41.5% for AA (66), 49.1% for AC (78) and 9.4% for CC (15) in the CAD group; 35.5% for AA (22), 48.4% for AC (30) and 16.1% for CC (10) in the control group. The distribution of APJ receptor genotypes was not found significantly different between groups (χ2 = 2.178; df = 2; P = 0.336) (Table 3). The A allele was encountered in 66% (210) of the CAD and 59.7% (74) of the controls. The C allele was seen in 34% (108) of the CAD and 40.3% (50) of the controls. Distribution of the alleles was similar in both groups (χ2 = 1.57; df = 1; P = 0.225) (Table 3).
Table 2.
Allele/Genotype frequencies and test of Hardy-Weinberg (HW) equilibrium
| Controls | CAD | |||
|
| ||||
| f (A) | 0.596 | 0.660 | ||
| f (C) | 0.404 | 0.340 | ||
|
| ||||
| O | E | O | E | |
|
| ||||
| AA | 22 | 22.08 | 66 | 69.34 |
| AC | 30 | 29.84 | 78 | 71.32 |
| CC | 10 | 10.08 | 15 | 18.34 |
| x2 = 0.001, df = 2, P = 0.99 | x2 = 1.37, df = 2, P = 0.50 | |||
f = observed frequency of each allele (A or C); O = observed genotype numbers; E = expected genotype numbers under a Hardy-Weinberg (HW) equilibrium assumption; X2 = Chi-square values; P = probability of difference.
Table 3.
Distribution of APJ A445C gene polymorphism in control and CAD
| Genotypes | Alleles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| AA | AC | CC | A | C | ||||||
|
|
|
|
|
|
||||||
| n | % | n | % | n | % | n | % | n | % | |
| CAD (n = 159) | 66 | 41.5 | 78 | 49.1 | 15 | 9.4 | 210 | 66 | 108 | 34 |
| Controls (n = 62) | 22 | 35.5 | 30 | 48.4 | 10 | 16.1 | 74 | 59.7 | 50 | 40.3 |
| χ2 = 2.178 df = 2 P = 0.336 | χ2 = 1.57 df = 1 P = 0.225 | |||||||||
In CAD patients, clinical characteristics as weight and DBP among the three APJ receptor A445C genotype, AA, AC and CC, were observed a significant differences (according to Kruskal-Wallis test, Table 4). Weight, SBP, DBP of patients with AA genotypes was found to be significantly lower than CC genotypes individuals in CAD group, P ≤ 0.05 (Table 4). In CC genotypes individuals DBP levels was found significantly higher than AA and AC genotypes with subjects (Table 4). In addition, TC levels between AA and AC genotypes differed significantly and the high level of TC was observed in CAD group with AC genotypes individuals P ≤ 0.05 (Table 4).
Table 4.
The comparisons of characteristics among the genotypes in CAD patients
| Apelin receptor (APJ) A445C gene polymorphism | ||||
|---|---|---|---|---|
|
|
||||
| AA | AC | CC | P-value | |
| Age (years) | 64.8 ± 1.50 | 62.8 ± 1.35 | 66.8 ± 3.29 | 0.325 |
| Height (cm) | 167.6 ± 0.89 | 169.9 ± 0.79 | 171.9 ± 1.96 | 0.090 |
| Weight (kg) | 75.3 ± 1.96a | 78.5 ± 1.14 | 80.3 ± 1.99a | 0.05 |
| BMI (kg/m2) | 26.9 ± 0.37 | 27.1 ± 0.35 | 27.2 ± 0.77 | 0.685 |
| SBP (mmHg) | 130.4± 1.93a | 132.0 ± 1.59 | 137.3 ± 3.92a | 0.115 |
| DBP (mmHg) | 77.0 ± 1.35a | 78.0 ± 1.04b | 86.6 ± 2.42a,b | 0.008 |
| TC (mmol/L) | 163.9 ± 5.24a | 181.0 ± 5.17a | 164.3 ± 14.9 | 0.097 |
| LDL-C (mmol/L) | 97.9 ± 4.29 | 109.9 ± 4.17 | 100.8 ± 9.37 | 0.206 |
| HDL-C (mmol/L) | 41.8 ± 1.31 | 42.7 ± 1.35 | 40.4 ± 3.98 | 0.401 |
| TG (mg/dL) | 160.2 ± 14.8 | 151.7 ± 9.00 | 157.3 ± 30.3 | 0.889 |
| FBG (mmol/L) | 122.7 ± 6.67 | 136.3 ± 9.05 | 114.7 ± 8.08 | 0.383 |
| BUN (mmol/L) | 37.8 ± 2.36 | 38.1 ± 2.91 | 35.5 ± 3.34 | 0.683 |
| Creatinine (mg/L) | 0.97 ± 0.08 | 0.91 ± 0.05 | 0.97 ± 0.05 | 0.375 |
P: Shows the differences between all groups (Kruskal Wallis test).
In each line, the difference between the means with same letters are significant, P ≤ 0.05 (Mann-Whitney U test).
In each line, the difference between the means with same letters are significant, P ≤ 0.05 (Mann-Whitney U test).
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; FBG, fasting blood glucose, BUN, blood urea nitrogen.
No significant difference was found between the three APJ receptor A445C genotypes of control group in terms of the clinical parameters except for HDL-C. HDL-C on AA genotypes individuals was found lower than subjects with CC genotypes in control group, P = 0.008 (Figure 2).
Figure 2.
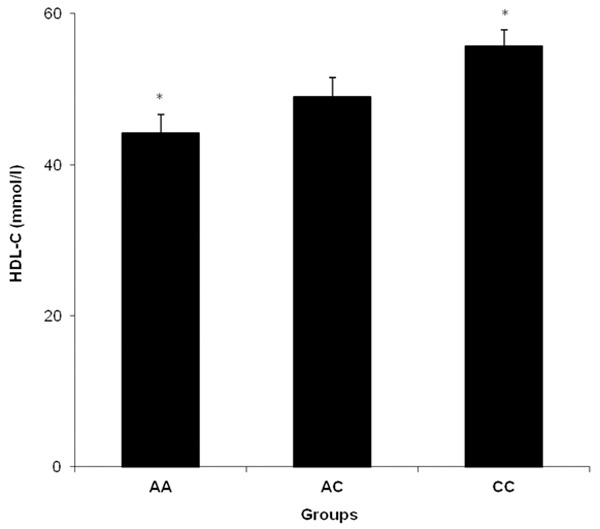
The comparisons of HDL-C among the APJ A445C genotypes in control. *Shows significance between AA and CC genotypes, *P ≤ 0.05.
Discussion
Coronary artery disease (CAD) is the predominant cause of death and morbidity worldwide which caused by both individual’s genetic makeup and various environmental factors such as obesity, hypercholesterolemia, alcohol intake, smoking, diabetes and hypertension [12,13]. APJ receptor occurs not only in human cardiomyocytes and endothelial cells, but also in vascular smooth muscle cells [14]. At the subcellular level, APJreceptor is localized in the T tubules and intercalated disks [15]. Within the myocardium itself, Apelin-APJ receptor activation increases contractility of myocardiocytes and is one of the most powerful positive inotropes known [3]. The apelin-APJ receptor signaling pathway has emerged as an important novel mediator of cardiovascular control and blood pressure homeostasis [16]. To our knowledge, this is the first study to investigate a common genotypic and allelic variant in the APJ receptor A445C gene and its association with CAD in Turkish subjects.
Falcone et al. [7] investigated the role of the G212A and A445C apelin-APJ polymorphisms in hypertensive Italian subjects with CAD and found no difference between patient and control groups in terms of allele and genotype frequencies [11]. Jin et al. [17] sought to investigate the association of 5 promising polymorphisms (rs3761581, rs56204867, rs7119375, rs10501367, rs9943582) in the apelin/APJ pathway with CAD among 1702 hypertensive Chinese patients. Single-locus analyses exhibited no significant differences in the genotype/allele frequencies of the examined polymorphisms between CAD patients and controls [17]. Moreover, Hinohara et al. [18] in the Japanese and Korean populations also failed to observe a positive association between rs9943582 in APJ receptor gene and CAD [18], in agreement with the results of our single-locus analysis. Our study revealed that there was not statistically significant difference between CAD patients and control group for APJ receptor A445C genotypes in Turkish patients. We obtained AA genotype in 66 (41.5%) cases, AC genotype in 78 (49.1%) cases and CC genotype was found in 15 (9.4%) cases of CAD in Tekirdağ province. There is a lack of information about the relationship between variants in the APJ receptor A445C gene and CAD in the literature.
APJ receptor A445C gene appears to be complex and is currently poorly understood. Genotype and allele functional role of APJ receptor A445C gene has not yet been identified.In present study, age, heart, BMI, SBP, TC, LDL-C, HDL-C, TG, FBG, BUN and creatinine levelswere not significantly different between the patients with CAD in the three different genotypes of APJ receptor A445C gene (P > 0.05) (Table 4). Weight, SBP and DBP levels were found significantly higher in individuals with CC geno types compared to the other genotypes in the patients with CAD. In addition, HDL-C level was found decreased, but this reduction was not statistically significant. At the same time HDL-C level of subjects with CC genotype in controls was higher than those of subjects with AA genotype. These findings suggest that CC genotype carriers may be under more risk for development of hypertension in CAD, due to the increase of weight, SBP and DBP and decrease HDL-C levels. Previous studies suggested that decreased HDL-C [19] and raised blood pressure levels are well-known risk factors for the pathogenesis of the disease [20]. It is generally accepted that HDL-C levels are inversely associated with risk of CAD [21]. In addition, in this study, weight, SBP, DBP and TC levels of subjects with AA genotype in CAD was lower than those ofsubjects with other genotypes.Therefore, AA genotype may be protective for hypertension, CAD and may not be a risk factor.
In conclusion, the results of the present study demonstrated that CAD patients did not differ from the healthy subjects for APJ receptor A445C gene polymorphism. In this study, weight, SBP, DBPand HDL-C were found to be significantly different in the genotypes distributions of APJ receptor A445C gene within CAD patients. We estimate that this polymorphism may not be a marker of CAD, but it may cause useful in function of the apelin/APJ system and may be a genetic predisposing factor for diagnostic processes. In particular, the APJ receptor gene appears to be a regulatory gene involved in the assessment of risk of hypertension in the CAD and can be helpfull in finding new treatment strategies. Our results could serve as a basis for a large-scale study in CAD between the APJ receptor A445C genotypes. We think that it is required to further comprehensive studies in order to make clear this situation in CAD.
Acknowledgements
The authors are greatful to Dumlupınar Research Center (DPU-ILTEM) for using the laboratory and equipments, to Didem Ocak and Arif Soylu for their help in experimental research.
Disclosure of conflict of interest
None.
References
- 1.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, König IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Kees Hovingh G, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Döring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson PW. Established risk factors and coronary artery disease: The Framingham Study. Am J Hypertens. 1994;7:7S–12S. doi: 10.1093/ajh/7.7.7s. [DOI] [PubMed] [Google Scholar]
- 3.Chen MM, Ashley EA, Deng DX, Tsalenko A, Deng A, Tabibiazar R, Ben-Dor A, Fenster B, Yang E, King JY, Fowler M, Robbins R, Johnson FL, Bruhn L, McDonagh T, Dargie H, Yakhini Z, Tsao PS, Quertermous T. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation. 2003;108:1432–1439. doi: 10.1161/01.CIR.0000091235.94914.75. [DOI] [PubMed] [Google Scholar]
- 4.Pitkin SL, Maguire JJ, Kuc RE, Davenport AP. Modulation of the apelin/APJ system in heart failure and atherosclerosis in man. Br J Pharmacol. 2010;160:1785–1795. doi: 10.1111/j.1476-5381.2010.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 6.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 7.Siddiquee K, Hampton J, McAnally D, May L, Smith L. The apelin receptor inhibits the angiotensin II type 1 receptor via allosteric trans-inhibition. Br J Pharmacol. 1998;168:1104–1117. doi: 10.1111/j.1476-5381.2012.02192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localization of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept. 2005;126:233–240. doi: 10.1016/j.regpep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Castan-Laurell I, Dray C, Knauf C, Kunduzova O, Valet P. Apelin, a promising target for type 2 diabetes treatment? Trends Endocrinol Metab. 2012;23:234–241. doi: 10.1016/j.tem.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–2. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 11.Falcone C, Bozzini S, Schirinzi S, Buzzi MP, Boiocchi C, Totaro R, Bondesan M, Pelissero G. APJ polymorphisms in coronary artery disease patients with and without hypertension. Mol Med Rep. 2012;5:321–5. doi: 10.3892/mmr.2011.685. [DOI] [PubMed] [Google Scholar]
- 12.Prins BP, Lagou V, Asselbergs FW, Snieder H, Fu J. Genetics of coronary artery disease: genomewide association studies and beyond. Atherosclerosis. 2012;225:1–10. doi: 10.1016/j.atherosclerosis.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Zhang XH, Lu ZL, Liu L. Coronary heart disease in China. Heart. 2008;94:1126–31. doi: 10.1136/hrt.2007.132423. [DOI] [PubMed] [Google Scholar]
- 14.Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107:198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Farkasfalvi K, Stagg MA, Coppen SR, Siedlecka U, Lee J, Soppa GK, Marczin N, Szokodi I, Yacoub MH, Terracciano CM. Direct effects of apelin on cardiomyocyte contractility and electrophysiology. Biochem Biophys Res Commun. 2007;357:889–895. doi: 10.1016/j.bbrc.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, Sugaya T, Matsuzaki H, Yamamoto R, Shiota N, Okunishi H, Kihara M, Umemura S, Sugiyama F, Yagami K, Kasuya Y, Mochizuki N, Fukamizu A. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279:26274–26279. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 17.Jin W, Su X, Xu M, Liu Y, Shi J, Lu L, Niu W. Interactive association of five candidate polymorphisms in Apelin/APJ pathway with coronary artery disease among Chinese hypertensive patients. PLoS One. 2012;7:e51123. doi: 10.1371/journal.pone.0051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinohara K, Nakajima T, Sasaoka T, Sawabe M, Lee BS, Ban JM, Park JE, Izumi T, Kimura A. Validation of the association between AGTRL1 polymorphism and coronary artery disease in the Japanese and Korean populations. J Hum Genet. 2009;54:554–556. doi: 10.1038/jhg.2009.78. [DOI] [PubMed] [Google Scholar]
- 19.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, König IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Kees Hovingh G, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Döring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verdecchia P, Reboldi G, Angeli F, Trimarco B, Mancia G, Pogue J, Gao P, Sleight P, Teo K, Yusuf S. Systolic and diastolic blood pressure changes in relation with myocardial infarction and stroke in patients with coronary artery disease. Hypertension. 2015;65:108–14. doi: 10.1161/HYPERTENSIONAHA.114.04310. [DOI] [PubMed] [Google Scholar]
- 21.Gordon DJ, Rifkind BM. High-density lipoprotein-the clinical implications of recent studies. N Engl J Med. 1989;321:1311–16. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]


