Abstract
Recent advances in neurodegenerative diseases point to novel mechanisms of protein aggregation. RNA binding proteins are abundant in the nucleus, where they carry out processes such as RNA splicing. Neurons also express RNA binding proteins in the cytoplasm and processes to enable functions such as mRNA transport and local protein synthesis. The biology of RNA binding proteins turns out to have important features that appear to promote the pathophysiology of amyotrophic lateral sclerosis and might contribute to other neurodegenerative disease. RNA binding proteins consolidate transcripts to form complexes, termed RNA granules, through a process of physiological aggregation mediated by glycine rich domains that exhibit low protein complexity and in some cases share homology to similar domains in known prion proteins. Under conditions of cell stress these RNA granules expand, leading to form stress granules, which function in part to sequester specialized transcript and promote translation of protective proteins. Studies in humans show that pathological aggregates occurring in ALS, Alzheimer’s disease, and other dementias co-localize with stress granules. One increasingly appealing hypothesis is that mutations in RNA binding proteins or prolonged periods of stress cause formation of very stable, pathological stress granules. The consolidation of RNA binding proteins away from the nucleus and neuronal arbors into pathological stress granules might impair the normal physiological activities of these RNA binding proteins causing the neurodegeneration associated with these diseases. Conversely, therapeutic strategies focusing on reducing formation of pathological stress granules might be neuroprotective.
Introduction
I begin this essay by posing a question: What is the most common cause of death in the elderly? You might imagine that heart disease, Alzheimer’s disease, stroke, cancer, diabetes, or other diseases would be at the top of the list. Indeed, by age 85 almost half of individuals suffer from dementia, which emphasizes the importance of dementia in aging. Today, the answer to this question would clearly highlight these diseases. However, in 1970, the answer would have also unequivocally put pneumonia high up on the list. Why the difference? Was pneumonia so much more prevalent then? The answer lies not in the prevalence of pneumonia, but rather in a change made in the way the health care system codes for death. This seemingly bureaucratic change had profound implications for modern neurological science.
In the late 1970’s, Robert Terry, Robert Katzman, and David Drachman were all faculty at the Albert Einstein College of Medicine, and were all working on Alzheimer’s disease. This disease had first been described by Alois Alzheimer in 1906, in Munich, Germany. Despite the well-known association of dementia with the presence of neuritic plaques and neurofibrillary tangles, most death certificates did not note the presence of dementia as a primary contributor to death. Consequently, heart disease and stroke were well known as important contributors to death in the elderly, but Alzheimer’s disease was not on people’s minds. Robert Terry, though, was studying the neuropathology of AD and was keenly aware of the strong prevalence of AD. Robert Katzman and David Drachman were neurologists, and were also keenly aware of the prevalence of dementia in the elderly, affecting about 10% of people at age 65 and steadily rising to capture more than 50% of elderly in its relentless talons by age 85.
The question that they pondered together was how to raise awareness of this important public health issue. These three prescient brain researchers formulated a plan of action. The key action item was that they began lobbying governments and health care system to change the coding of death from the immediate cause of death to the underlying disease that precipitated death. Once the administrative change was accomplished, the coding of death was changed for the large fraction of elderly patients who had gotten pneumonia because they were lying for months on end, curled up in their beds, moving rarely until they finally developed a bacterial infection in their lungs and succumbed to inevitable claws of pneumonia. These patients were given the correct diagnosis of Alzheimer’s disease, a relentless degeneration of the brain characterized by the accumulation of two types of aggregated proteins, β-amyloid, which forms extracellular neuritic plaques and micro-tubule associated protein tau, which forms intracellular neurofibrillary tangles. Spurred on by this seemingly minor administrative change, Katzman, Terry, and Drachman began a publicity campaign to raise public awareness of the importance of Alzheimer’s disease as a major public health issue (McKhann et al., 1984; Terry and Katzman, 1983).
Less than a decade after Katzman, Terry, and Drachman began their efforts, the Alzheimer Association had been established, amyloid precursor protein discovered, and the role of tau in neurofibrillary tangle formation established. Several years after that, John Hardy and Dennis Selkoe formulated the amyloid cascade hypothesis, which has been the driving paradigm in neurodegenerative disease research since (Hardy, 1992; Hardy and Selkoe, 2002). The amyloid cascade hypothesis begins with a very simple proposition, which is that the β-amyloid peptide is aggregation prone, forms non-functional aggregates that accumulate with time, and causes death of surrounding neurons. As they die, these neurons accumulate aggregates of tau protein through another non-physiological process. The essence of the model is that the protein aggregation that occurs in the brains of Alzheimer patients, occurs almost as a biological mistake for which the only regulatory solution is to degrade it through autophagy and/or the ubiquitin proteasomal system. This model works fairly well for an explanation of how the extracellular accumulations of β-amyloid build up to form neuritic plaques, but the model might well be fundamentally misguided as an explanation for the etiology of the intracellular protein aggregates that occur in Alzheimer’s disease, as well as many other neurodegenerative diseases.
The Discovery of RNA Binding Proteins as a Basis for Neurodegeneration
Let us fast forward to 2006, when Virginia Lee, John Trojanowski, Manuella Neumann, and colleagues isolated the predominant protein that accumulates in most cases of sporadic amyotrophic lateral sclerosis (ALS) (Neumann et al., 2006). The protein that they discovered was Tar DNA Binding protein-43 (TDP-43), which turns out to be an RNA binding protein. TDP-43 contains two striking types of sequence motifs. The first is two domains that bind mRNA, and are known as RNA recognition motifs (Dormann and Haass, 2011). These motifs place TDP-43 into a family of about 800 other RNA binding proteins. The second type of domain is a glycine rich domain that has low sequence complexity and a very strong tendency to aggregate. Many members of the RNA binding protein family also contain this type of motif, and computational analysis indicates that this motif is common among prion proteins, hence this glycine rich domain that has low sequence complexity domain is referred to as a prion domain (Han et al., 2012). Within a year of the association of TDP-43 with pathology in ALS, geneticists identified mutations in TDP-43 that were associated with familial ALS (Sreedharan et al., 2008). After that, mutations in other RNA binding proteins were also found associated with ALS, including FUS, Angiogenin, Optineurin, EWAS, and recently hnRNPA1 and A2B1. The repertoire of diseases that RNA binding proteins are associated with extends well beyond neurodegenerative diseases, including diseases such as myopathies and cancers.
The overwhelming majority of disease-linked mutations occur in the prion domains; one exception is FUS, where mutations occur in a nuclear localization signal (increasing cytoplasmic localization), as well as in the prion domain (Dormann and Haass, 2011); I will explain later how this fits into the new model of regulated protein aggregation. Each of these mutations appears to lead to the same result, which is to increase the tendency of the RNA binding protein to aggregate.
RNA Binding Proteins: Physiological Aggregation to Form Stress Granules
The biology of RNA binding proteins provides an important model for an alternative mechanism of protein aggregation, which is a model with potentially profound implications for neurodegenerative diseases. The fundamental point of focus in RNA binding protein biology is their ability to sequester transcripts. To gain perspective on this unusual biology, it is helpful to first understand how the cell typically keeps molecules in a particular domain. Surrounding molecules with lipid membranes is the most common mechanism used by any cell to sequester essential cellular components. For instance, the nucleus is delimited by a membrane that acts to sequester DNA and its regulatory proteins. Lipid membranes also delineate other organelles, including the lysosome, autophagosome, the peroxisome, the synaptic vesicle, the endosome, the mitochondria, and, indeed, the entire cell.
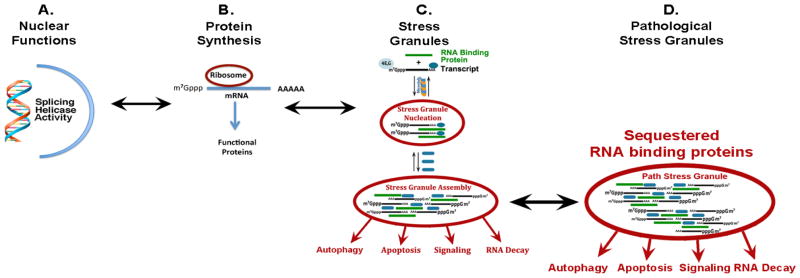
One striking exception to this strategy is the mechanism by which RNA binding proteins bind up, and sequester, transcripts. RNA binding proteins are a group of about 800 proteins that “manage” RNA metabolism in the cell. Many RNA binding proteins perform key functions in the nucleus where they facilitate transcription of RNA (through helicase activity or keeping track of polymerase progression) and regulate RNA splicing, controlling which exons remain in the mature transcript (Figure 1) (Lagier-Tourenne et al., 2012; Polymenidou et al., 2011). RNA binding proteins exhibit other important functions, though. One function that is critical for the biology of neurodegenerative diseases is the generation of stress granules. Upon exposure to stress, a cell needs to shift its protein production to focus on making protective, “housekeeping” proteins, while correspondingly reducing production of proteins required for specialized functions. This shift in protein synthesis is accomplished by having RNA binding proteins, such as TIA-1, translocate from the nucleus to the cytoplasm where they bind 7-methyl-guanosine capped mRNA through their RNA recognition motifs, forming large complexes termed, stress granules (Figure 1). Most transcripts in stress granules are translationally silent, which allows the protein synthesis machinery to focus on the remaining transcripts, which tend to be housekeeping proteins and either do not have a cap or use internal ribosomal entry sites (Anderson and Kedersha, 2008).
Figure 1.
Pathological stress granule formation in neurodegenerative disease. RNA binding proteins carry out many critical cellular functions under basal conditions. A) In the nucleus they regulate RNA splicing and contribute to RNA polymerase elongation by providing helicase activity and position marking. B) In the cytoplasm, they regulate RNA translation and transport transcripts. C) Under stressful conditions, RNA binding proteins translocate to the cytoplasm, where they transiently sequester unnecessary transcripts in stress granules. D) Mutations that enhance aggregation of RNA binding proteins and possibly chronic stress and disease lead to formation of pathological stress granules. Stable, pathological stress granules might cause or exacerbate disease by sequestering RNA binding proteins away from their principle site of action.
Binding of RNA binding proteins creates a large macro-molecular complex because these proteins contain domains that have low sequence complexity, a high content of glycines, and a strong ability to aggregate (Han et al., 2012). For many RNA binding proteins these low complexity domains share homology with prion-like domains, such as that in yeast Sup35 (Gilks et al., 2004). These proteins exhibit a strong tendency to aggregate, which is also like Sup35. Binding of a core group of nucleating RNA binding proteins, such as TIA-1, TIAR and G3BP, is thought to initiate formation of the stress granule. Other RNA binding proteins bind to the transcripts at other sequence motifs. These proteins also cross-link to each other through binding of the low complexity, prion-like domains, and the cross-linking creates the stress granule, which sequesters the unnecessary specialized transcripts away from the translational machinery (the ribosome) during the stress. These multiply cross-linked proteins form protein aggregates that in some cases are detergent insoluble, but the degree of insolubility is less than that seen with the insoluble protein aggregates that form in neurodegenerative diseases (Collier et al., 1988; Liu-Yesucevitz et al., 2010; Wolozin, 2012).
Stress is commonly a transient phenomenon, and stress granules are designed to be transient structures. Despite forming large intermolecular complexes with properties of protein aggregates, stress granules are designed to rapidly disassemble upon removal of the stress. For instance, within 30 minutes after removal of heat shock, stress granules disassemble, protein synthesis returns to normal, and RNA binding proteins return to the nucleus, where they carry on with normal functions, such as RNA splicing (Gilks et al., 2004).
Pathological Stress Granules Form in Neurodegenerative Disease
Discovery that mutations in RNA binding proteins are associated with motor neuron disease focused attention on this group of proteins. In 2006, Tar DNA binding protein-43 (TDP-43, TARDBP) was discovered to be the major protein that aggregates to form inclusions in amyotrophic lateral sclerosis (ALS) and in frontotemporal dementia (FTLD) (Neumann et al., 2006). Mutations in TDP-43 were soon shown to be associated with familial ALS (Rothstein, 2009; Sreedharan et al., 2008). These mutations occur largely in the low complexity, glycine rich domain, and were shown to increase the tendency of TDP-43 to aggregate forming stress granules (Colombrita et al., 2009; Dewey et al., 2011; Johnson et al., 2009; Liu-Yesucevitz et al., 2010). The direct link between stress granules and disease came from studying spinal cords from subjects with ALS. We demonstrated that the TDP-43 pathology occurring in ALS and FTLD co-localizes with other stress granule markers (Liu-Yesucevitz et al., 2010).
Further studies identified families with ALS and FTLD that had mutations in other RNA binding proteins, including FUS, Angiogenin, Optineurin, EWAS, and recently hnRNPA1 and A1B2. In addition, mutations in other RNA binding proteins were shown to increase the risk of ALS or to be associated with other motor neuron diseases (Elden et al., 2010; Lorson et al., 1998). Inclusions containing FUS, huntingtin, and PrP have all been shown to co-localize with stress granule markers (Bosco et al., 2011; Goggin et al., 2008; Waelter et al., 2001). Most recently, we demonstrated that brains of patients with Alzheimer’s disease show large amounts of stress granule formation (Vanderweyde et al., 2012). Interestingly, the stress granule proteins that accumulate in AD generally co-localize with tau pathology, and induction of stress granules in cells over-expressing tau and TIA-1 appears to be able to induce tau pathology (Vanderweyde et al., 2012). Thus, stress granule formation appears to be a general feature of neurodegenerative diseases associated with protein aggregates.
Neurodegenerative Disease: Stress Granules Run Amuck
The intrinsic biology of RNA binding proteins and RNA granule formation provides a cogent model for the pathophysiology of many neurodegenerative diseases. As mentioned, most RNA binding proteins normally function in the nucleus, where they control functions such as RNA splicing. These are proteins that exhibit an intrinsic tendency to aggregate, and essentially exist in a reversible equilibrium between aggregated and disaggregated states. Upon exposure to stress they translocate to the cytoplasm, where they aggregate, but the equilibrium rapidly shifts when the stress is removed, whereupon RNA binding proteins return to the nucleus.
A key point in this model is that anything that alters the equilibrium will shift the amount of aggregation. Mutations that increase the tendency of these RNA binding proteins to aggregate provide one clear factor that will shift the equilibrium and tend to favor formation of stress granules. However, any type of chronic physiological stress might also promote stress granule formation. Alzheimer’s disease is known to be associated with the accumulation of β-amyloid, which causes neuronal stress and injury. Alzheimer’s disease is also associated with many types of cardiovascular disease and diabetes, both of which can lead to cerebral hypo-perfusion. Repetitive head trauma causes stress, and has been associated with diseases such as ALS and dementia among professional football players and military personnel (Goldstein et al., 2012). Aging also readily factors into this model, because with aging protein aggregation generally increases, in part due to a reduced ability to degrade the aggregates. Recent studies directly demonstrate that some disease-linked genes, such as valosin containing protein (VCP), function to remove stress granules (Kim et al., 2013). In each of these scenarios, we hypothesize that the biological stresses associated with chronic stress or aging shift the equilibrium of regulated protein aggregation towards aggregation of RNA binding proteins leading to formation of pathological stress granules (Figure 1).
Chronic stress granule formation poses many problems for a cell. Neurons in the brains of subjects with neurodegenerative diseases frequently show a striking loss of nuclear RNA binding proteins, such as TIA-1. Loss of RNA splicing for 10 minutes, such as might occur with a typical stress, might not be very problematic for a neuron. However, it seems possible that loss of RNA splicing for 10 months or 10 years, such as occurs in disease, might render neurons more prone to degeneration. Knockout and knockdown studies emphasize this because knockout of RNA binding proteins, such as TDP-43, leads to neurodegeneration (Wang et al., 2011; Wu et al., 2012).
Why Do Defects in RNA Granule Biology Cause Neurodegenerative Disease?
The unique role that RNA binding proteins play in the physiology of neurons provides a cogent explanation for the neurocentric effects of RNA binding protein dysfunction. Neurons are burdened with extreme physical requirements. A tiny neuronal cell body, measuring only 40 μm in diameter must service an axon that can be over a meter away and a dendritic arbor that can be millimeters in breadth. Transcripts must be transported to the synapse, and once at the synapse, protein synthesis is frequently activity dependent. These functions require cytoplasmic RNA binding proteins. This requirement highlights an important, unique feature of neurons, which is that neurons constitutively have high levels of RNA binding proteins and need to transport RNA granules over very large distances. Several studies have shown that RNA binding proteins, such as TDP-43 and ataxin-2, are present in the neuronal arbor and exhibit activity dependent transport. Our recent results indicate that disease-linked mutations produce TDP-43 granules that are larger and slower moving (Liu-Yesucevitz et al., 2013). The ability of mutations to enlarge RNA granules that are constitutively present in the neuronal soma and arbor provides a clear starting point for initiating pathology associated with RNA binding proteins. This mechanism also potentially explains the neurotropic nature of diseases associated with RNA binding proteins.
Implications
The biology of RNA granules, including stress granules, is based on the concept of reversible, regulated protein aggregation. The presence of a normal biological process that is based on protein aggregation provides a cogent model explaining why protein aggregation would play such an important role in aging and chronic diseases that affect the brain. Another key aspect of this biological system is that it is tightly regulated and reversible, which provides novel opportunities for therapeutic intervention by targeting the signaling cascades that regulate RNA granule biology. Interventions that dampen or reverse the stress granule response might be able to halt the progression, or even reduce, aspects of neurodegenerative diseases that were previously thought to be irreversible.
Footnotes
Disclosure
Dr. Wolozin is co-founder and a member of the Scientific Advisory Board for Aquinnah Pharmaceuticals LLC and a member of the scientific advisory board of CMD Bioscience LLC.
References
- Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008;33(3):141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Bosco DA, Lemay N, Ko HK, Zhou H, Burke C, Kwiatkowski TJ, Jr, Sapp P, Mckenna-Yasek D, Brown RH, Jr, Hayward LJ. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum Mol Genet. 2011;19(21):4160–4175. doi: 10.1093/hmg/ddq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier NC, Heuser J, Levy MA, Schlesinger MJ. Ultrastructural and biochemical analysis of the stress granule in chicken embryo fibroblasts. J Cell Biol. 1988;106(4):1131–1139. doi: 10.1083/jcb.106.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E, Silani V, Ratti A. TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem. 2009;111(4):1051–1061. doi: 10.1111/j.1471-4159.2009.06383.x. [DOI] [PubMed] [Google Scholar]
- Dewey CM, Cenik B, Sephton CF, Dries DR, Mayer P, 3rd, Good SK, Johnson BA, Herz J, Yu G. TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Mol Cell Biol. 2011;31(5):1098–1108. doi: 10.1128/MCB.01279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann D, Haass C. TDP-43 and FUS: a nuclear affair. Trends Neurosci. 2011;34(7):339–348. doi: 10.1016/j.tins.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, Padmanabhan A, Clay-Falcone D, Mccluskey L, Elman L, Juhr D, Gruber PJ, Rub U, Auburger G, Trojanowski JQ, Lee VM, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466(7310):1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15(12):5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggin K, Beaudoin S, Grenier C, Brown AA, Roucou X. Prion protein aggresomes are poly(A)+ ribonucleoprotein complexes that induce a PKR-mediated deficient cell stress response. Biochim Biophys Acta. 2008;1783(3):479–491. doi: 10.1016/j.bbamcr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4(134):134ra160. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, Allen J, Xiao G, Mcknight SL. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell. 2012;149(4):768–779. doi: 10.1016/j.cell.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Hardy J. Framing beta-amyloid. Nat Genet. 1992;1:233–234. doi: 10.1038/ng0792-233. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Johnson BS, Snead D, Lee JJ, Mccaffery JM, Shorter J, Gitler AD. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J Biol Chem. 2009;284(30):20329–20339. doi: 10.1074/jbc.M109.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NC, Tresse E, Kolaitis RM, Molliex A, Thomas RE, Alami NH, Wang B, Joshi A, Smith RB, Ritson GP, Winborn BJ, Moore J, Lee JY, Yao TP, Pallanck L, Kundu M, Taylor JP. VCP is essential for mitochondrial quality control by PINK1/Parkin and this function is impaired by VCP mutations. Neuron. 2013;78(1):65–80. doi: 10.1016/j.neuron.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Polymenidou M, Hutt KR, Vu AQ, Baughn M, Huelga SC, Clutario KM, Ling SC, Liang TY, Mazur C, Wancewicz E, Kim AS, Watt A, Freier S, Hicks GG, Donohue JP, Shiue L, Bennett CF, Ravits J, Cleveland DW, et al. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat Neurosci. 2012;15(11):1488–1497. doi: 10.1038/nn.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Bilgutay A, Zhang YJ, Vanderweyde T, Citro A, Mehta T, Zaarur N, Mckee A, Bowser R, Sherman M, Petrucelli L, Wolozin B. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS One. 2010;5(10):e13250. doi: 10.1371/journal.pone.0013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Lin A, Wolozin B. Disease-linked TDP-43 mutations reduce rapid transport of TDP-43 containing RNA granules. 2013 Submitted. [Google Scholar]
- Lorson CL, Strasswimmer J, Yao JM, Baleja JD, Hahnen E, Wirth B, Le T, Burghes AH, Androphy EJ. SMN oligomerization defect correlates with spinal muscular atrophy severity. Nat Genet. 1998;19(1):63–66. doi: 10.1038/ng0598-63. [DOI] [PubMed] [Google Scholar]
- Mckhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, Mccluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling SC, Sun E, Wancewicz E, Mazur C, Kordasiewicz H, Sedaghat Y, Donohue JP, Shiue L, Bennett CF, Yeo GW, Cleveland DW. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011;14(4):459–468. doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(Suppl 1):S3–S9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, De Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Katzman R. Senile dementia of the Alzheimer type. Ann Neurol. 1983;14(5):497–506. doi: 10.1002/ana.410140502. [DOI] [PubMed] [Google Scholar]
- Vanderweyde T, Yu H, Varnum M, Liu-Yesucevitz L, Citro A, Ikezu T, Duff K, Wolozin B. Contrasting Pathology of Stress Granule Proteins TIA-1 and G3BP in Tauopathies. J Neurosci. 2012;32(24):8270–8283. doi: 10.1523/JNEUROSCI.1592-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelter S, Boeddrich A, Lurz R, Scherzinger E, Lueder G, Lehrach H, Wanker EE. Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol Biol Cell. 2001;12(5):1393–1407. doi: 10.1091/mbc.12.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Brent JR, Tomlinson A, Shneider NA, Mccabe BD. The ALS-associated proteins FUS and TDP-43 function together to affect Drosophila locomotion and life span. J Clin Invest. 2011;121(10):4118–4126. doi: 10.1172/JCI57883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B. Regulated protein aggregation: stress granules and neurodegeneration. Mol Neurodegener. 2012;7:56. doi: 10.1186/1750-1326-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LS, Cheng WC, Shen CK. Targeted depletion of TDP-43 expression in the spinal cord motor neurons leads to the development of amyotrophic lateral sclerosis-like phenotypes in mice. J Biol Chem. 2012;287(33):27335–27344. doi: 10.1074/jbc.M112.359000. [DOI] [PMC free article] [PubMed] [Google Scholar]