Abstract
Background:
This guideline is intended to provide an evidence-based approach to the initial evaluation of patients with known or suspected lung cancer. It also includes an assessment of the impact of timeliness of care and multidisciplinary teams on outcome.
Methods:
The applicable current medical literature was identified by a computerized search and evaluated using standardized methods. Recommendations were framed using the approach described by the Guidelines Oversight Committee of the American College of Chest Physicians. Data sources included MEDLINE and the Cochrane Database of Systematic Reviews.
Results:
Initial evaluation should include a thorough history and physical examination; CT imaging; pulmonary function tests; and hemoglobin, electrolyte, liver function, and calcium levels. Additional testing for distant metastases and paraneoplastic syndromes should be determined on the basis of these results. Paraneoplastic syndromes may have an adverse impact on cancer treatment, so they should be controlled rapidly with the goal of proceeding with definitive cancer treatment in a timely manner. Although the relationship between timeliness of care and survival is difficult to quantify, efforts to deliver timely care are reasonable and should be balanced with the need to attend to other dimensions of health-care quality (eg, safety, effectiveness, efficiency, equality, consistency with patient values and preferences). Quality care will require multiple disciplines. Although it is difficult to assess the impact, we suggest that a multidisciplinary team approach to care be used, particularly for patients requiring multimodality therapy.
Conclusions:
The initial evaluation of patients with lung cancer should include a thorough history and physical examination, pulmonary function tests, CT imaging, basic laboratory tests, and selective testing for distant metastases and paraneoplastic syndromes.
Summary of Recommendations
4.4.1. For patients with known or suspected lung cancer, we suggest that the delivery of care be timely and efficient (Grade 2C).
Remark: Interventions to improve timeliness should be developed locally by addressing barriers to providing timely care that are specific to the local setting.
Remark: Efforts to improve timeliness should be balanced with the need to attend to other dimensions of health-care quality (such as safety, effectiveness, efficiency, equality, and consistency with patient values and preferences).
5.1.1. For patients with lung cancer who require multimodality therapy, we suggest using a multidisciplinary team approach (Grade 2C).
Remark: We suggest that multidisciplinary teams have representatives from pulmonary medicine, thoracic surgery, medical oncology, radiation oncology, palliative care, radiology, and pathology.
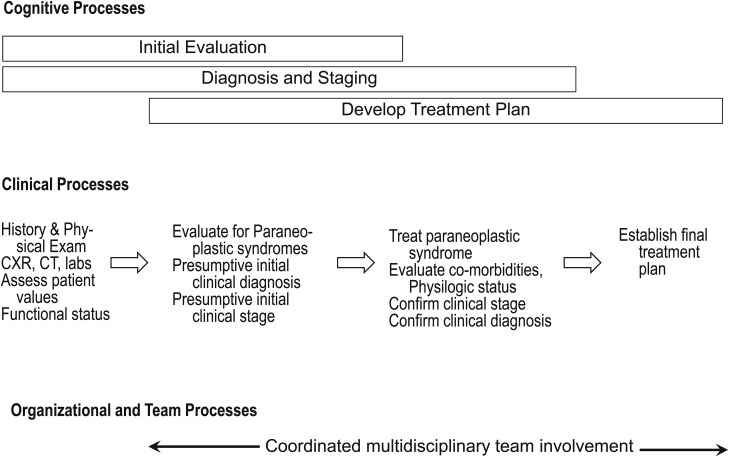
The goal of the initial evaluation of patients with known or suspected lung cancer is to acquire sufficient information such that a definitive diagnostic and treatment plan can be developed in the most cost-effective manner possible, balancing the considerations of efficiency, safety, and effectiveness with patient values and preferences. The large number of factors that need to be considered; the number of disciplines involved; and the need to diagnose, stage, and treat patients in a timely fashion present a real challenge. Factors such as comorbidities, functional status, patient preferences, probability of lung cancer, probability of metastatic disease, symptom control, and presence of paraneoplastic syndromes all need to be considered. Given this context, it is useful to start with a broad overview of the evaluation, diagnosis, staging, and treatment process (Fig 1). This guideline is intended to provide an evidence-based approach to the initial evaluation of patients with suspected or known lung cancer. It focuses on patients who present with a relatively high probability of cancer; it does not necessarily apply to patients with a very low probability of cancer, such as those identified with a lung nodule during CT screening, although the principles are still relevant to that problem. The specifics of the other steps in the diagnosis, staging, and treatment process are handled in more detail in other articles of these lung cancer guidelines.
Figure 1.
[Introduction, Section 2.3] Overview of initial evaluation, diagnosis, staging, and treatment processes. There is significant overlap between cognitive processes. Developing a clinical diagnosis and assessment of the probable stage begins during the initial evaluation. This clinical assessment is subsequently refined on the basis of biopsy specimen findings that are part of formal staging and diagnosis. Similarly, information regarding the patient’s functional status, comorbid conditions, and preferences may have an impact on treatment alternatives, and this in turn may have an impact on the type of diagnostic testing strategies chosen. CXR = chest radiograph.
The initial evaluation must set the stage for all subsequent steps by identifying and clarifying key issues related to the patient’s overall health, the probability of cancer, and the probability of metastatic disease. These issues, in turn, have an impact on every other step of the diagnosis, staging, and treatment process. The fundamental principle we highlight is that choosing the best strategy for initial evaluation requires consideration of many elements that often are only thought about much later in the process of care (Fig 1). Although some of these elements cannot be known precisely during the early stages of a workup, in many cases, certain options can be eliminated and the initial diagnostic evaluation streamlined and made more efficient with lower cost and less risk to the patient if a few simple questions are asked up front.
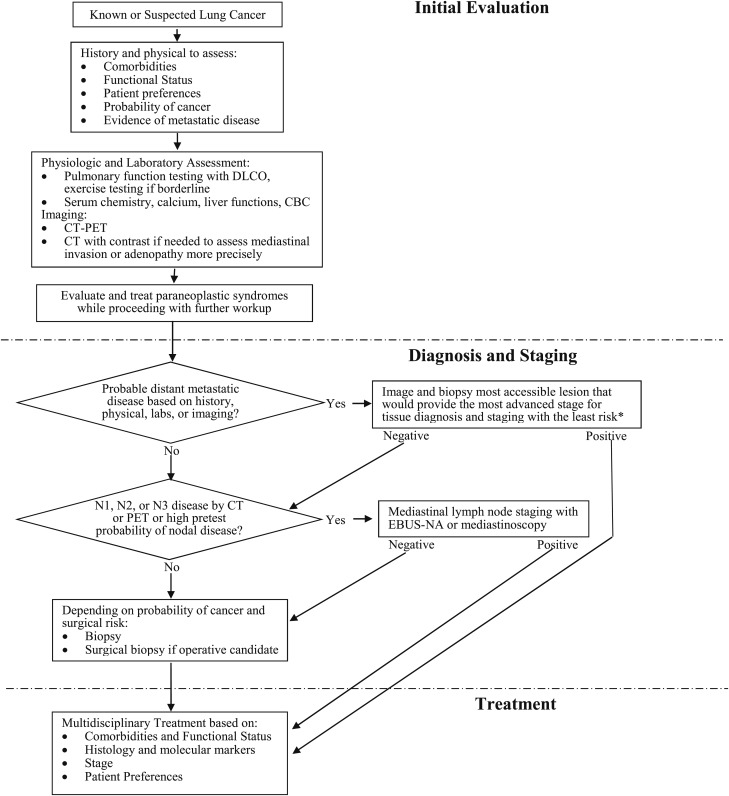
On the basis of this framework, the initial evaluation must provide information with regard to three basic questions: (1) What is the extent of disease, and is there evidence of metastases? (2) Are there comorbidities (ie, COPD) present that might limit treatment options? (3) Are there symptoms or paraneoplastic syndromes present that need to be identified and treated early? Once these questions have been addressed, tissue will be needed to stage and diagnose the patient (Fig 2). The fundamental principle is that staging and diagnosis should be achieved as efficiently as possible. As shown in Figure 2, this usually means biopsy of the most advanced site of disease. If there is evidence of distant metastatic disease, that will be the first target if tissue acquisition is feasible from the distant site. If there is no clinical evidence of distant metastatic disease but there is evidence of mediastinal nodal disease, then mediastinal sampling will be the first target. If neither is present, then lung biopsy or surgery is warranted. Note that efficient diagnosis and staging is contingent on a thorough initial evaluation to identify the first biopsy targets.
Figure 2.
[Introduction] Diagnostic algorithm for patients with suspected non-small cell lung cancer.The initial evaluation provides information on comorbidities, functional status, preferences, and probable extent of disease. The results of the initial evaluation determine the optimal site and sequencing of additional diagnostic and staging tests. Staging and diagnosis will be most efficient if the most advanced site of disease is targeted first. *When there is overwhelming imaging evidence of distant metastases, biopsy of the most accessible site is sufficient. DLCO = diffusing capacity of lung for carbon monoxide; EBUS-NA = endobronchial ultrasound-guided needle aspiration.
In addition to these clinical considerations, quality care requires that the initial evaluation process be executed in a timely, well-coordinated manner so that all the necessary disciplines can be integrated and brought to bear on the problem. A variety of organizational and system-level variables that affect patient outcomes must be considered. Among the most important of these variables are the timeliness of care and the role of multidisciplinary teams. The remainder of this article, therefore, will focus on the following:
Presenting signs, symptoms, laboratory evaluation, and initial imaging tests
Evaluation of paraneoplastic syndromes
Impact of the timeliness of care on patient outcomes
Impact of multidisciplinary teams on patient survival
1.0 Methods
To update previous recommendations on the initial evaluation of the patient with lung cancer and the role of the practice organization, we used systematic methods to identify studies, synthesize evidence, assess study methods, formulate recommendations, and rate the strength of recommendations as described by Lewis et al1 the American College of Chest Physicians (ACCP) Lung Cancer Guidelines. We used the population, intervention, comparator, and outcome (PICO) format to generate questions (Table S1 (359.9KB, pdf) ). Search criteria, references identified, relevant articles reviewed, flow diagrams, and final selections are detailed in Appendix S1 (359.9KB, pdf) .
After a systematic search, articles were selected for inclusion according to the selection criteria listed. The data were abstracted, and the writing committee drafted recommendations. These were discussed, revised, and ultimately approved by the entire ACCP Lung Cancer Guidelines panel, as described elsewhere.1
2.0 Presenting Symptoms, Signs, Laboratory Evaluation, and Imaging
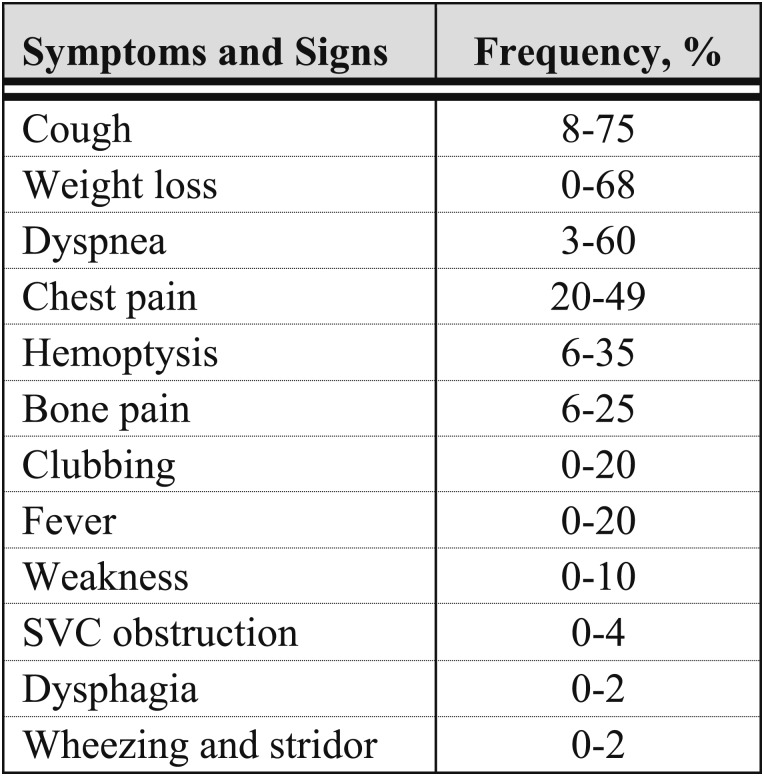
An appreciation of the varying ways in which patients with lung cancer initially present is important because it facilitates identifying patients who are more likely to have either intrathoracic spread or distant metastases. This distinction in turn guides the diagnostic evaluation. Approximately one-fourth of patients are asymptomatic at the time lung cancer is identified, and these patients are more likely to have less-advanced disease. Unfortunately, the majority of patients present because of cancer-related symptoms and have more-advanced disease. The initial evaluation, therefore, should focus on symptoms and signs that reflect local effects from the primary tumor, extension of disease in the thoracic cavity, radiographic correlates, distant metastatic disease, and findings suggestive of paraneoplastic syndromes (Fig 3).2‐10
Figure 3.
[Section 2.0] Range of frequencies of initial symptoms and signs of lung cancer.
2.1 Symptoms Related to the Primary Tumor
Small pulmonary nodules are usually asymptomatic. Larger pulmonary lesions, central tumors, or tumors with an endobronchial component are more likely to result in pulmonary symptoms, including cough, dyspnea, chest pain, and hemoptysis.2‐7 Of these, cough is the most common presentation and may result from endobronchial irritation, parenchymal infiltration, or postobstructive pneumonia. Recurrent pneumonia in the same anatomic distribution or relapsing acute exacerbations of COPD should raise concern for neoplasm. Dyspnea may accompany these scenarios. Localized or unilateral wheezing may reflect endobronchial obstruction. Patients often describe nonspecific chest discomfort, and pleuritic chest pain should raise concern for invasion of the pleura. Hemoptysis accompanying lung cancer is rarely massive. Patients may dismiss small amounts of blood streaking in sputum as related to bronchitis and cough. However, hemoptysis may be the presenting symptom of lung cancer even in the setting of a normal or nonlocalizing chest radiograph.12 Persistent hemoptysis, even in scant amounts, in patients with a history of smoking and COPD should raise concern about the possibility of endobronchial tumor.
2.2 Symptoms and Signs of Intrathoracic Spread
A number of structures within the thorax are susceptible to tumor invasion either by local extension or by lymphatic spread. The most common sites of spread are the hilar and mediastinal lymph nodes. Although nodal metastases rarely cause symptoms, bulky adenopathy in the hila can cause extrinsic airway compression with airway symptoms, and in the subcarinal area, they can compress the midesophagus with resultant dysphagia. More commonly, symptoms relate to invasion or compression of other structures, including the pleura, nerves, blood vessels, and chest wall.
Many patients may have nonspecific chest discomfort with lung cancer. Extension of the primary tumor into the pleura or chest wall causes more localized pain, which may be severe. Invasion of the chest wall can cause painful soft tissue masses or rib destruction. Pleural effusion may be related to direct extension of the primary tumor, to implantation of tumor metastasis, or from mediastinal lymphatic obstruction and is typically heralded by dyspnea or chest pain.
A number of nerves are susceptible to compromise by local extension of lung cancer. Recurrent laryngeal nerve palsy typically is seen in left-sided tumors because of the circuitous route of the left recurrent laryngeal nerve deep into the thorax and under the aortic arch, which renders it susceptible to compression by adjacent tumor or malignant nodes. The resultant vocal cord paresis causes hoarseness and may predispose to coughing and aspiration. The right-side recurrent laryngeal nerve is less commonly involved because its course does not traverse extensively into the chest. Phrenic nerve dysfunction, demonstrated by an elevated hemidiaphragm, may also be caused by tumor extension into the mediastinum. Superior sulcus tumors are classically associated with some or all the manifestations of Pancoast syndrome.13 The entire syndrome consists of shoulder and arm pain related to invasion of the brachial plexus and the adjacent soft tissues, ribs, and vertebrae; Horner syndrome (unilateral ptosis, meiosis, and lack of facial sweating) from infiltration of the sympathetic chain and stellate ganglion; and weakness, pain, and paresthesias of the arm and hand in the distribution of the eighth cervical and first and second thoracic nerve roots. The distribution of symptoms, particularly pain, outside the chest may delay consideration of lung cancer as the primary etiology.
Lung cancer is the most common cause of the superior vena cava syndrome.14 Patients typically experience facial and neck swelling and, less commonly, may describe dysphagia, cough, headache, dizziness, and blurred vision. Physical examination findings include facial edema and plethora, dilated neck veins, and a prominent venous pattern on the chest. Chest radiographs typically show a widened mediastinum or right hilar mass but may appear normal.
Metastases to other vascular structures in the mediastinum need to be considered. The pericardium is the most common site of cardiac involvement by lung cancer either by direct extension or by lymphatic spread. Pericardial effusion, occasionally presenting as pericardial tamponade and arrhythmias, may result.
2.3 Radiographic Presentations of Lung Cancer
All patients undergoing initial evaluation for lung cancer will require noninvasive imaging studies, which in conjunction with tissue confirmation of disease, will ultimately yield the clinical stage. Pulmonary physiologic evaluation is also required because this will help to stratify the patient’s risk, which in turn will affect both diagnostic testing and treatment strategy. In terms of the proper timing and sequencing of tests, it is important to recognize that these tests should be performed early during the initial evaluation phase of the workup because they provide pivotal information that will have an impact on all subsequent decisions (Fig 1). This discussion focuses on the clinicoradiographic presentation of patients with lung cancer. The utility and indications for noninvasive testing, invasive diagnostic modalities, and pulmonary physiologic testing are discussed in more detail in the articles By Detterbeck et al15 on lung cancer staging and Brunelli et al16 on physiologic evaluation in the ACCP Lung Cancer Guidelines.
2.3.1 Clinically Asymptomatic Presentation:
Only a minority of lung cancers are diagnosed while the patient is asymptomatic. Typically, these individuals are discovered with stage I or II non-small cell lung cancer (NSCLC), with tumors appearing as solitary lesions on chest radiographs or chest CT scans performed for reasons unrelated to the tumors themselves. Pulmonary nodules are common incidental radiographic findings; most patients with pulmonary nodules will have no symptoms related to the nodules per se. By definition, a pulmonary nodule is a lesion ≤ 3 cm in diameter surrounded by normal lung tissue and not associated with other radiographic abnormalities, such as lymphadenopathy, atelectasis, or pleural effusion. Larger lesions are defined as masses. Pulmonary nodules may be solid, completely ground glass opacities, or partially solid and partially ground glass in density. The decision to pursue further evaluation of an asymptomatic pulmonary nodule will be influenced by the pretest probability of malignancy based on clinical risk factors and the radiographic appearance of the nodule. Several radiographic features of the nodule are particularly pertinent. Size is an important factor. The larger the nodule, the more likely it is to be malignant, with the majority of asymptomatic pulmonary nodules > 20 mm being malignant.17 Location within the lung is worth noting, with a higher risk of malignancy for upper lobe than for lower lobe lesions.18 Densely calcified nodules typically reflect healed prior inflammatory processes, although heterogeneous or eccentric distribution of calcium within a nodule does not exclude the possibility of malignancy. Nodule edge characteristics also contribute to the evaluation, with higher risk for malignancy associated with spiculated edges compared with smooth borders.18 The topic of pulmonary nodule evaluation is addressed more comprehensively by Gould et al19 in the ACCP Lung Cancer Guidelines.
The National Lung Screening Trial (NLST) demonstrated a 20% relative reduction in mortality from lung cancer in patients undergoing annual screening with low-dose chest CT scan compared with chest radiography.20 The NLST also demonstrated a stage distribution shift toward the less advanced stages in patients whose lung cancers were diagnosed because of a positive screening test. In the NLST, 63% of subjects with lung cancer diagnosed during the 2 years of active screening had stage IA or IB disease, a dramatic contrast with the distribution of current symptom-driven lung cancer evaluation in which most patients have advanced stage disease at diagnosis. If low-dose CT screening for lung cancer is incorporated into medical practice for the patient population identified by the NLST, more asymptomatic stage I and II lung cancers will be identified.
2.3.2 Clinically Symptomatic Presentation:
The majority of patients with lung cancer present because of symptoms related to their tumors. In patients with nonspecific systemic symptoms, such as weight loss, the chest radiograph will be helpful in focusing attention quickly on the lungs as the most likely primary site. In patients with symptoms related to the primary tumor itself, certain chest radiographic features should heighten concern for lung cancer. Unexplained pleural effusion should always raise concern for malignancy. Central tumors may cause mechanical airway obstruction with associated atelectasis or parenchymal consolidation.21,22 Classically, squamous cell carcinomas are believed to arise in the central bronchi, with local extension into the hilum and mediastinum, and because of this, may predispose to symptoms related to bronchial obstruction.23,24 However, peripheral squamous cell carcinomas with or without cavitation are not uncommon. Similarly, adenocarcinomas, which now comprise the majority of bronchogenic carcinomas, have been classically described as presenting as nodules or masses in the lung periphery; however, these cancers may also present as central lesions with large airway obstruction. Small cell carcinomas, accounting for about 15% of all lung cancers, rarely present as isolated pulmonary nodules and are typically associated with bulky hilar or mediastinal adenopathy and distant metastasis.
2.4 Symptoms, Signs, and Laboratory Tests Indicating Extrathoracic Metastases
Lung cancer can metastasize to any organ. Patients with distant metastases often have nonspecific systemic symptoms of anorexia, weight loss, or fatigue.25 The most common sites of metastasis are the lymph nodes, liver, adrenal glands, bone, brain, and pleura. As noted, symptoms related to lymph node metastasis are uncommon except in the situation of very bulky adenopathy. Liver metastases often are accompanied by symptoms of weakness and weight loss, which are typically not associated with abnormal liver function tests until liver involvement is very advanced. Adrenal metastases typically are asymptomatic and rarely cause adrenal insufficiency; these must be distinguished from benign adrenal adenomas, which are common incidental findings. Although metastases to the lymph nodes, liver, and adrenal glands often are clinically silent, these sites often are readily amenable to biopsy. Patients with systemic symptoms who are suspected of having metastatic lung cancer should be evaluated with this in mind because biopsy of a metastatic site has the potential to most efficiently establish both diagnosis and stage.
Bone pain is a common symptom of metastatic lung cancer, being evident in 6% to 25% of patients at presentation. Although the vertebral bodies are the most common site, any bone may be involved. Pain, bony tenderness, and elevation of serum calcium or alkaline phosphatase should heighten concern for skeletal metastasis. Malignant involvement of the nervous system by lung cancer most commonly presents as intracranial metastases, although neurologic symptoms may also be related to paraneoplastic syndromes. The lung is the primary site of about 70% of cancers that initially present with symptomatic brain metastases.26 Symptoms of brain metastasis include headache, nausea, vomiting, seizures, or mental status changes, but patients may also be asymptomatic. As discussed in the article on diagnosis in these guidelines,15 any symptoms or focal neurologic signs should prompt an appropriate brain imaging study.
2.4.1 Standardized Evaluation for Systemic Metastasis:
Readily available clinical factors are useful for assessing the likelihood of systemic metastasis. Feinstein and Wells25 performed an illuminating analysis of the effect of the presence or absence of symptoms on prognosis in a large cohort of patients with lung cancer. This effort built on 2 decades of previous work investigating the relationship between symptoms of lung cancer at diagnosis and eventual outcomes.10,25,27‐29 In their cohort of 1,266 lung cancer cases at two institutions, patients demonstrating the best prognosis were either asymptomatic or had symptoms referable only to the primary tumor.25 In contrast, the presence of systemic symptoms, such as anorexia, weight loss, and fatigue, or localized symptoms attributable to metastatic sites was associated with poor prognosis.25 The relationship between systemic symptoms and prognosis was also demonstrable within individual stages, with worse prognosis observed in patients within a given stage if systemic symptoms were evident (these data come from an era that predates many of the imaging tests available today).25
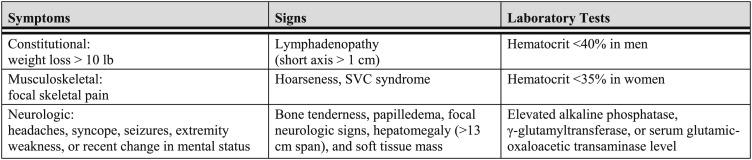
Hooper and colleagues30,31 also investigated this concept, using a panel of clinical factors incorporating symptoms, signs, and laboratory tests to screen for metastatic disease. They examined the correlation between the presence and absence of clinical factors suggesting metastatic disease and the likelihood of identifying abnormalities on liver, brain, and bone imaging studies.30,31 Their initial work demonstrated that clinical abnormalities were frequently associated with abnormalities on radionuclide imaging, whereas routine scanning in asymptomatic patients did not identify a significant number with unsuspected metastatic disease. Extending this work to brain imaging by CT scan in a relatively small retrospective study, 16 of 89 patients with lung cancer undergoing routine brain CT scanning were found to have abnormal studies.30 Of the 16 patients with CT scan findings, only nine had evidence of CNS disease on history or physical examination, but all 16 had significant abnormalities identified on the clinical evaluation panel for metastatic disease. Conversely, no abnormal CT scans were identified in asymptomatic patients with a negative clinical screen. Silvestri et al32 adapted the criteria of Hooper and colleagues and retrospectively asked whether they would be a useful screen for detecting adrenal metastases. As was noted by Feinstein and Wells25 and Hooper and colleagues, if the clinical screen was unremarkable, adrenal metastases were not identified on CT scan; conversely, the more clinical abnormalities present, the more likely it was that adrenal metastases would be found. These and other studies reinforce the observation that abnormalities identified on a fairly simple clinical panel can strongly suggest the presence of metastases, although they may not necessarily be helpful in identifying the metastatic sites.25,30‐35 This body of work is important when considering the question of whether routine brain, abdominal, and bone imaging studies looking for metastatic disease should be performed in all patients with lung cancer because it suggests that this evaluation should be influenced by the results of a simple clinical screening panel. In a meta-analysis of all studies in patients with lung cancer that provided data on both radiographic studies and the clinical factors adapted from the criteria of Hooper and colleagues, Silvestri et al35 demonstrated that patients with metastatic disease commonly show abnormalities on a clinical screen (Fig 4).However, in the absence of abnormalities in the clinical assessment, identification of asymptomatic metastatic disease on routine imaging is unlikely.
Figure 4.
[Section 2.4.1] Features of a standardized evaluation for systemic metastases.
Possible exceptions to this general approach would include patients with more-advanced clinical stage disease (IIIA and IIIB), particularly those with involvement of the mediastinal (N2) nodes. Limited evidence suggests that the presence of distant metastatic disease is higher in this group, even when there are no associated clinical symptoms.32,36 Another important caveat is that patients who are subsequently proven to have small cell lung cancer (SCLC) after the initial evaluation will require brain imaging even if there are no focal neurologic deficits, although this can be done after a tissue diagnosis is established. In general, for clinical stage I and II lung cancer, the important conclusion is that performing an assessment of readily available clinical factors obtained through a thorough history and physical examination and standard laboratory tests (including hemoglobin, electrolyte, liver function, and calcium levels) is a useful approach both in avoiding unnecessary evaluation in patients without clinical evidence of metastatic disease and in lowering the threshold for a metastatic evaluation in patients with an abnormal clinical screen.
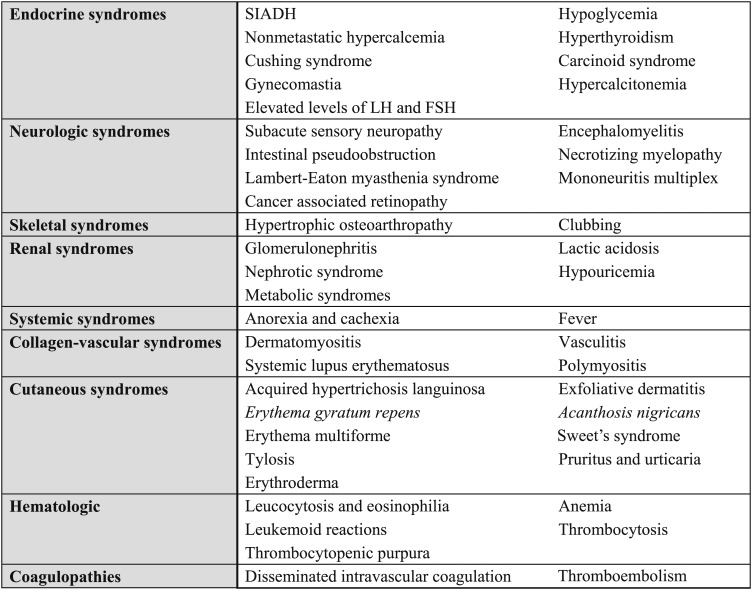
3.0 Evaluation of Paraneoplastic Syndromes
The presentation of patients with lung cancer may also be complicated by the presence of paraneoplastic syndromes. A wide variety of paraneoplastic syndromes have been reported in lung cancer (Fig 5). Some of these syndromes are relevant to the initial evaluation of lung cancer because they are associated with significant morbidity and mortality that may limit effective cancer treatment if they are undiagnosed. Conversely, in many of these syndromes, early recognition and intervention can limit the associated morbidity and mortality, facilitating more effective cancer treatment. It is therefore important that these syndromes be identified early in the evaluation of the patient with lung cancer.
Figure 5.
[Section 3.0] Paraneoplastic syndromes in patients with lung cancer.
Many paraneoplastic syndromes have previously been described and characterized on the basis of clinical manifestations. However, it is now recognized that many of these syndromes share similar underlying mechanisms. Broadly speaking, they can be categorized as being either hormonally based or immunologically based.
3.1 Hormonal Syndromes
3.1.1 Ectopic Cushing Syndrome:
Paraneoplastic Cushing syndrome (CS) typically is caused by ectopic production of adrenocorticotropin (ACTH)37; ectopic production of corticotropin-releasing hormone is rare. Bronchial carcinoid and SCLC are commonly associated with ectopic ACTH.38‐40 Ectopic CS is associated with poor prognosis in SCLC.41,42 CS is clinically apparent in 1.6% to 4.5% of SCLCs,43‐45 whereas biochemical abnormalities suggestive of ectopic CS may be found in 30% to 50% of SCLCs.46‐48
Paraneoplastic CS should be suspected in patients with lung cancer if concurrent clinical features of CS are present. In most cases, clinical features include moon faces, acne, purple striae, proximal muscle weakness, peripheral edema, hypertension, and metabolic alkalosis with hypokalemia. Skin hyperpigmentation usually is more prominent with ectopic ACTH. Weight gain is common in CS, but weight loss occurs in about 10% of CS associated with SCLC.49
If these clinical features of CS are present in a patient with lung cancer, the next step is to exclude iatrogenic CS. According to the Endocrine Society clinical practice guidelines for CS, it is important to exclude iatrogenic CS before biochemical testing.50‐53 The Endocrine Society indicated that this strong recommendation is based on high-quality evidence that failure to exclude exogenous glucocorticoid use is associated with unnecessary testing and resulting consequences without any benefit to the patient.
Once iatrogenic disease is excluded, the Endocrine Society clinical practice guidelines recommend initial testing for CS with one of the following: 24-h urinary free cortisol test (more than one measurement), late-night salivary cortisol test (more than one measurement), or dexamethasone suppression test (1 mg overnight or 2 mg/d for 2 days).50‐53 The Endocrine Society indicated that these strong recommendations are based on very-low-quality evidence because data that link specific diagnostic strategies to patient outcomes are limited and most of the work has focused on developing, validating, and assessing diagnostic test performance.
If initial testing is normal and the pretest probability of disease is low or moderate, CS is unlikely. If one of these tests is abnormal and if patients have normal test results but a high clinical suspicion of CS, the Endocrine Society clinical practice guidelines recommend further evaluation by an endocrinologist. The Endocrine Society indicated that these strong recommendations are based on very-low-quality evidence because the guidelines panel observed that subsequent testing in this context requires considerable expertise both in the clinic and in the laboratory such that referral was likely to be associated with better outcomes.50‐53
It is important to diagnose paraneoplastic CS as part of the initial evaluation of patients with SCLC or bronchial carcinoid tumors so that appropriate treatment of hypercortisolism can be accomplished prior to chemotherapy or surgery. Although surgical removal or chemotherapy for the cancer is the primary therapy that will remove or suppress the ectopic source of ACTH, the hypercortisolism represents a significant barrier to effective cancer treatment because it increases the risk for therapy-induced complications. For example, the mortality of patients with SCLC and CS is high after chemotherapy induction.41,42 Severe opportunistic infections soon after starting chemotherapy cause severe morbidity and mortality before any potential anticancer benefit can be realized. Similarly, patients with CS also have increased clotting factors II, V, VIII, IX, XI, and XII, which increase the risk for VTE.54,55 The VTE risk in patients with CS is about 2% without surgery and 4% after surgery56; thus, it is expected that patients with lung cancer and ectopic CS have a higher VTE risk than patients without ectopic CS. The goal, therefore, is to recognize and diagnose CS during the initial evaluation so that treatment of hypercortisolism can be initiated, thereby permitting more-effective cancer treatment with less risk. Effective cancer treatment in turn will be the primary treatment of the ectopic CS.
Consensus statements and guidelines51,57 are available to guide the management of hypercortisolism in paraneoplastic CS in patients with lung cancer. Metyrapone, ketoconazole, etomidate, mitotane, and mifepristone can be used to decrease circulating glucocorticoids. Laparoscopic bilateral adrenalectomy can rapidly decrease circulating cortisol levels to allow patients with rapidly progressive SCLC and ectopic CS to begin chemotherapy in a timely manner. To prevent adrenal insufficiency from developing, replacement glucocorticoid therapy may be needed, and prophylaxis for Pneumocystis carinii and fungi during chemotherapy may be considered.
3.1.2 Syndrome of Inappropriate Antidiuretic Hormone:
Approximately 10% to 45% of SCLC and 1% of other lung cancer cases (squamous cell carcinoma58 and adenocarcinoma59,60) produce arginine vasopressin (ie, antidiuretic hormone [ADH]), but only 1% to 5% of patients with lung cancer have symptomatic syndromes of inappropriate ADH (SIADH).11 Excess ADH activates vasopressin 2 receptors in renal tubules, resulting in increased aquaporins and impaired free water clearance. Hyponatremia in patients with SCLC is associated with shortened survival.61 Early detection and appropriate management can prevent severe hyponatremia, which can lead to seizures, coma, and death.
Signs and symptoms are determined by the degree of hyponatremia and the acuity of hypoosmolality. At serum sodium concentrations of 125 to 130 mEq/L, patients usually experience general weakness, confusion, headache, and nausea. When serum sodium levels drop to < 120 mEq/L, life-threatening manifestations may ensue.
SIADH manifests as euvolemic hypoosmolar hyponatremia characterized by low serum osmolality and inappropriately high urine osmolality in the absence of diuretic treatment, adrenal insufficiency, heart failure, cirrhosis, and hypothyroidism as follows:
Hyponatremia (serum sodium < 134 mEq/L)
Hypoosmolality (plasma osmolality, < 275 mosm/kg)
Inappropriately high urine osmolality (> 500 mosm/kg)
Inappropriately high urinary sodium concentration (> 20 mEq/L)
Absence of hypothyroidism or adrenal insufficiency or volume depletion
Hyponatremia should be further investigated by clinical assessment of intravascular volume status and biochemical measurements in blood and urine. By assessing the effective arterial blood volume with the fractional excretion of urate, the accuracy of a diagnostic algorithm for SIADH can approach 95%.62 After exclusion of hypoadrenalism and hypothyroidism, hypoosmolar hyponatremia in the absence of renal salt wasting, dehydration, and intravascular volume depletion will be consistent with SIADH. Laboratory findings in SIADH include urine osmolality of > 300 mosm/kg, urinary sodium level of > 40 mEq/L, serum osmolality of < 275 mosm/kg, and serum uric acid concentration of < 4 mg/dL.
Paraneoplastic hyponatremia secondary to elevated atrial natriuretic peptide has also been described.63 This etiology, along with other non-ADH-mediated causes of hyponatremia (sodium wasting because of drug nephrotoxicity, iatrogenic IV infusion of hypotonic fluid, etc) should be distinguished from SIADH in the differential diagnosis of hyponatremia.
There are no evidence-based guidelines for managing SIADH. Recommended management is based on expert opinion.64,65 Free water restriction (< 1 L/d) is a first-line treatment of asymptomatic mild SIADH and a recommended adjunct to other therapy for severe cases. Hypertonic 3% saline IV is given in life-threatening or acute symptomatic and severe (< 120 mEq/L) hyponatremia. Demeclocycline, lithium, and vasopressin 2 receptor antagonists (conivaptan, lixivaptan, tolvaptan, and satavaptan) may also be used to correct hyponatremia.
3.1.3 Hypercalcemia of Malignancy:
Hypercalcemia occurs in 10% to 25% of patients with lung cancer66 and is most commonly seen in patients with squamous cell lung cancer.67 The common etiologic mechanisms of hypercalcemia of malignancy are parathyroid hormone-related protein (PTHrP) production, increased active metabolite of vitamin D (calcitriol, also called 1,25-dihydroxyvitamin D3), and localized osteolytic hypercalcemia.68,69 PTHrP-mediated hypercalcemia is characterized by a suppressed intact parathyroid hormone (iPTH) level and a low or normal calcitriol level, which contrasts with the findings of elevated iPTH and calcitriol levels in primary hyperparathyroidism. The median survival after discovery of hypercalcemia of malignancy in patients with lung cancer is about 1 month.70
Clinical symptoms of hypercalcemia depend on severity and acuity of onset. For mild or moderate hypercalcemia, symptoms include polyuria, polydipsia, nausea, confusion, vomiting, abdominal pain, and myalgia. Patients may present with severe dehydration and acute renal failure. When hypercalcemia is severe (> 14.0 mg/dL), patients may develop mental status changes, bradycardia, and hypotension. The diagnostic evaluation includes measuring serum concentrations of iPTH, PTHrP, 1,25-dihydroxyvitamin D3, 25-hydroxyvitamin D, calcium, albumin, magnesium, and phosphorus.
There are guidelines for the management of hypercalcemia,71,72 but they are not specifically for patients with lung cancer. Oral hydration may be effective in mild hypercalcemia. In moderate to severe hypercalcemia, management includes rehydrating with IV crystalloid fluids not containing calcium and giving loop diuretics (eg, furosemide) as needed after correction of intravascular volume. Bisphosphonates (clodronate, pamidronate, and zoledronic acid) usually are effective. Additional therapeutic options such as glucocorticoids, gallium nitrate, and salmon calcitonin may be considered.68,73
3.1.4 Carcinoid Syndrome:
Bronchopulmonary neuroendocrine tumors (NETs) comprise about 20% of all lung neoplasms.74 They are classified as low-grade typical carcinoid, intermediate-grade atypical carcinoid, large cell NET, and SCLC.75
Ectopic serotonin production can produce the carcinoid syndrome in about 1% to 5% of these patients.76 This syndrome is characterized by skin flushing of the upper thorax, secretory diarrhea, and bronchoconstriction. Fibrosis of the valves of the right side of the heart may also develop in chronic cases, although this is extremely rare in carcinoids of lung origin.
Acute carcinoid crisis can occur as a result of a massive release of serotonin, causing bronchospasm, hypotension, arrhythmias, and cardiopulmonary failure. The crisis may be precipitated by chemotherapy, biopsy, anesthesia, surgery, or adrenergic drugs (eg, dopamine, epinephrine). The risk of carcinoid crisis associated with an invasive procedure is difficult to predict, and perioperative management emphasizes prevention. Because patients with lung cancer often will require invasive diagnostic procedures or surgical interventions, recognition of carcinoid syndrome in the initial evaluation can facilitate proper prevention and treatment of carcinoid crisis. Carcinoid crisis can be prevented, aborted, or treated with IV octreotide acetate.77
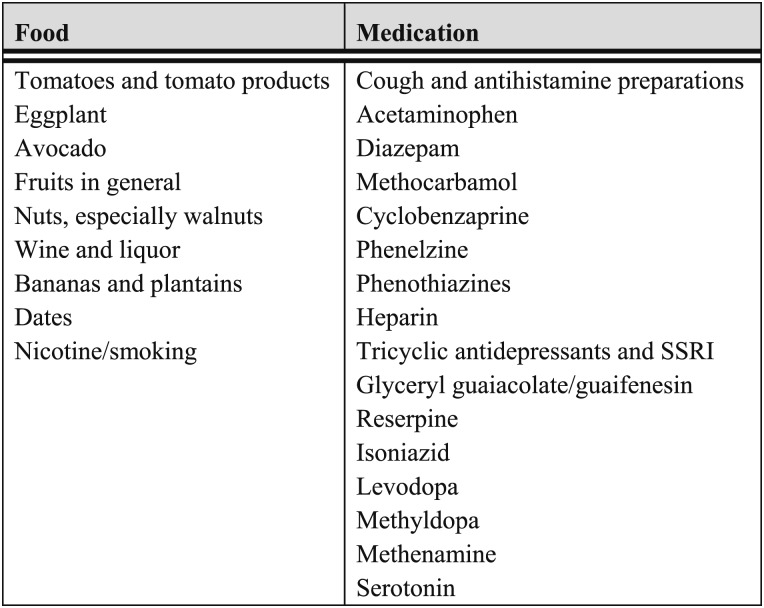
To diagnose carcinoid syndrome, the main metabolite of serotonin, that is, 5-hydroxyindoleacetic acid (5-HIAA), is measured in 24-h urine collections with precautions taken for interference by certain foods and medications78 (Fig 6).The specificity of the urinary 5-HIAA test is close to 90%.81 Serum NET biomarkers, such as neuron-specific enolase82 and chromogranin A, should be measured. An elevated serum level of chromogranin A, which occurs in 75% of carcinoid tumors and 60% of SCLCs,83 has the highest reliability and accuracy among the biomarkers of NETs.82 Neuron-specific enolase and 5-HIAA are highly specific but not very sensitive (sensitivity, 32.9% and 35.1%, respectively).82 Because up to 80% of bronchopulmonary NETs express somatostatin receptors, radionuclide-labeled octreotide scintigraphy is helpful in detecting tumors that may be missed by other diagnostic studies.
Figure 6.
[Section 3.1.4] Food and medications to avoid during urine collection for 5-HIAA measurement.
Whenever feasible, surgery is the first-line treatment of bronchial carcinoids. Metastatic disease is not curable. In such cases, control of symptoms from carcinoid syndrome is important to improve quality of life. Somatostatin analogs are effective. Other therapies include serotonin receptor blockers, interferon, and antidiarrheal medications.
3.2 Immunologic Syndromes
3.2.1 Paraneoplastic Neurologic Syndromes:
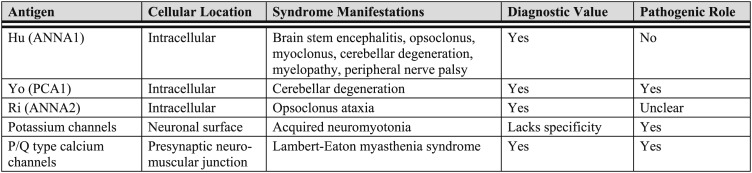
Paraneoplastic neurologic syndromes (PNSs) are nervous system dysfunctions caused by an autoimmune mechanism but not by local effects of tumors. Many neuronal autoantibodies play diagnostic and pathogenic roles (Fig 7).84 Most PNSs in adults are associated with lung cancer of neuroendocrine origin (ie, SCLC, carcinoid tumors).85 Most of the PNSs damaging the CNS are T-cell mediated.86‐89 The prevalence of onconeural antibodies in SCLC for Hu, CRMP5, amphiphysin, Yo, Ri, and Ma2 are 22.5%, 5.0%, 2.5%, 0.5%, 1.5%, and 1.0%, respectively.90 Therefore, the anti-Hu syndrome is the PNS most relevant to lung cancer.
Figure 7.
[Section 3.2.1] Autoimmune paraneoplastic syndromes associated with lung cancer.
3.2.2 Anti-Hu Syndrome:
Hu (neuronal embryonic lethal, abnormal vision [n-ELAV]-like proteins) is an RNA-binding protein that regulates RNA processing and function from splicing to translation91 and plays important roles in neuronal differentiation and plasticity.92 Most SCLCs express Hu, but only a minority of patients have anti-Hu antibodies. Expression of major histocompatibility complex class 1 in these tumors may play a role in autoimmunity.93 Anti-Hu antibodies have a sensitivity of 82% and a specificity of 99% in the diagnosis of PNS.94 Although helpful in diagnosis, these antibodies are not pathogenic; T-cell-mediated autoimmunity is the cause of neurologic damage.86,89,95,96
SCLC accounts for > 90% of cases of paraneoplastic anti-Hu syndrome. The clinical manifestation may include limbic encephalitis, brainstem encephalitis, cerebellar degeneration, opsoclonus myoclonus, myelopathy, cranial nerve palsy,97 and sensory neuropathy.98 Limbic encephalitis usually presents with rapidly progressive loss of short-term memory, seizures, and psychosis. Cerebellar degeneration causes ataxia. Opsoclonus myoclonus is the myoclonus of head and limbs and truncal ataxia accompanied by continual conjugated chaotic saccades of the eyes. Epilepsy and status epilepticus are potential complications.99 Peripheral nerve palsy leads to distal symmetric sensorimotor deficits.
Response to therapy for SCLC favorably affects the course of PNS.100 Concomitant immunotherapy does not adversely affect the malignancy outcome.101 Limited experience suggests that immunosuppressive therapy with a combination of IV immunoglobulin (IVIg), methylprednisolone, and cyclophosphamide may stabilize the PNS transiently, but these treatments cannot improve PNS over the long term.102 Guidelines on using IVIg to treat PNS have been proposed.103
3.2.3 Anti-Yo Syndrome:
Yo (CDR2) is a cytoplasmic c-Myc-interacting protein expressed in Purkinje cells of the cerebellum and brain stem neurons. Anti-Yo antibodies disrupt the regulation of c-Myc by Yo, thereby causing apoptosis. Therefore, anti-Yo antibodies are directly involved, at least initially, in the pathogenic mechanism for paraneoplastic cerebellar degeneration,104 although T-cell-mediated autoimmunity is the major cause of neuronal degeneration.
The anti-Yo syndrome is more commonly associated with ovarian and breast cancers than with SCLC.90 The clinical manifestation is primarily brain stem abnormalities and cerebellar degeneration. Administered within 1 month of the onset, IVIg may induce a good response with stabilization of neurologic symptoms. The benefit of adding high-dose IV glucocorticoid to IVIg therapy is unclear.105 Plasmapheresis106 and plasma exchange107 have also been attempted, with variable success.
3.2.4 Anti-Ri Syndrome:
Ri (Nova) is a highly conserved protein108 that regulates RNA splicing and RNA metabolism in certain neurons in the ventral brainstem and spinal cord; several alternative forms are expressed in the CNS and tumor cells because of alternative mRNA splicing. Ri is involved in PNS manifesting as opsoclonus ataxia.108 Ri antibodies are present in 1.5% to 4.5% of patients with SCLC.90,109
3.2.5 Autoimmune Neuromuscular Disease:
These paraneoplastic syndromes are mediated by autoantibodies directed against components of the neuromuscular junction. The best characterized is Lambert-Eaton myasthenic syndrome (LEMS) in which the autoantibodies are directed against voltage-gated calcium channels (VGCCs).110,111 Similarly, antibodies against voltage-gated potassium channels cause acquired neuromyotonia.
More than 90% of LEMS have antibodies against VGCC type P/Q, which is the etiology for LEMS.112,113 The prevalence of LEMS in SCLC is 1% to 1.6%.114,115 Anti-VGCC antibodies directly block ion channel function111 and decrease calcium influx into the presynaptic nerve terminal to prevent release of acetylcholine secretory vesicles, thereby resulting in decreased synaptic transmission at the neuromuscular junction.111,116
Diagnosis of LEMS generally is by clinical characteristics, electromyography, and anti-VGCC antibodies. LEMS is clinically characterized by craniocaudally progressive proximal muscle weakness that occurs predominantly at the hip girdle. In the absence of clinical features of LEMS, routine measurement of VGCC antibodies is not recommended.117 IVIg is a potential treatment of LEMS.118 Guidelines for using IVIg for LEMS have been proposed.103
Paraneoplastic neuromyotonia (Isaacs syndrome) is associated with antibodies against the voltage-gated potassium channel, leading to an increased release of acetylcholine and prolonged muscle fiber action potential. Results from electromyography show fibrillation, fasciculation, and high frequency of discharge, which are abolished by curariform anticholinergic drugs but not by sleep induction or general anesthesia.119,120 Symptoms are primarily muscle cramping, weakness, and stiffness. The syndrome usually is caused by lung cancer and thymoma.
3.2.6 Autoimmune Autonomic Ganglionopathy:
The syndrome of autoimmune autonomic ganglionopathy (paraneoplastic dysautonomia) is associated with SCLC. Autoantibodies against the neuronal acetylcholine receptors in autonomic ganglia can cause dysautonomia. Antibodies that bind to and block ganglionic acetylcholine receptors are pathogenic and diagnostic for this syndrome.121 Paraneoplastic autonomic neuropathy can lead to symptoms that include hypothermia, hypoventilation, dry mouth, and sleep apnea; esophageal and GI dysmotility; intestinal pseudoobstruction; impotence; sphincter dysfunction; orthostatic hypotension; and cardiac arrhythmia. Symptoms may be improved by immunosuppressive therapy.122 Because dysautonomia can also result from paraneoplastic encephalomyelitis, the differential diagnosis should include anti-Hu syndrome.
3.2.7 Dermatomyositis:
Dermatomyositis is an inflammatory myopathy accompanied by characteristic dermatologic abnormalities. The diagnostic criteria are progressive symmetrical proximal muscle weakness, elevated serum levels of muscle enzymes, abnormal electromyographs, abnormal muscle biopsy results, Gottron papules, and photodistributed heliotrope eruption with poikiloderma.123,124 Other features, such as interstitial lung disease, Raynaud phenomenon, and inflammatory arthritis, may also be present. Electromyographic changes are typical of muscle fiber necrosis. The diagnosis of dermatomyositis can be confirmed by muscle and skin biopsy tests. In addition to breast, ovarian, and GI cancers, dermatomyositis is associated with lung cancer,125‐127 and it is often diagnosed within 1 year of the cancer diagnosis.126 The precise etiologic link between malignancy and dermatomyositis is elusive but is likely to be autoimmune.128‐130 The responsiveness to immunosuppressive therapy is consistent with an autoimmune etiology.130 Medications include corticosteroids,131 methotrexate, cyclophosphamide, azathioprine, mycophenolate, rituximab, and IVIg.103,132
3.3 Importance of Paraneoplastic Syndromes in the Initial Evaluation
Prompt recognition and treatment of paraneoplastic syndromes is important for patients with lung cancer. Delayed diagnosis of paraneoplastic syndromes may lead to increased complications that limit or delay cancer treatment, highlighting the need for rapid recognition, proper evaluation, and timely intervention. However, proper evaluation requires time that in turn may affect or delay other steps in the cancer care process. Therefore, it is reasonable to do as much of the workup for paraneoplastic syndromes as possible in parallel with the main diagnosis and staging workup so that delays can be minimized. Sometimes, treatment and control of paraneoplastic syndromes will require a delay in the initiation of treatment. In such instances, physicians need to consider and balance the benefits of treating the paraneoplastic syndrome with the potential harms associated with delays in treatment of the cancer itself. In such cases, it is useful to consider the available evidence on the impact of the timeliness of care and how best to organize medical systems to deliver the needed care efficiently.
4.0 Impact of the Timeliness of Care on Patient Outcomes
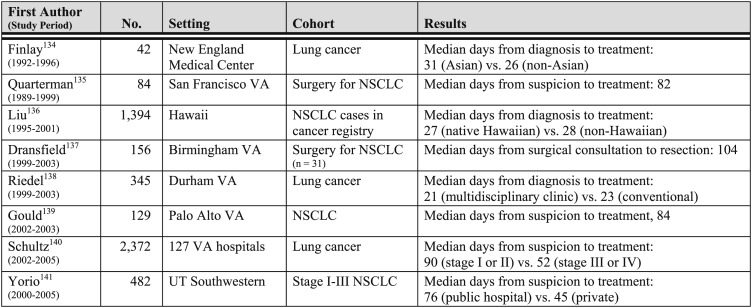
The Institute of Medicine has identified timeliness of care as one of six dimensions of health-care quality.133 In lung cancer, less timely care may be associated with missed opportunities for cure or effective palliation, and it probably contributes to the emotional distress patients and family members experience. The relatively few studies that have been performed in the United States indicate that times to diagnosis and treatment often are longer than recommended (Fig 8). Factors found to be associated with less timely care include atypical symptoms,143,144 less advanced disease stage,145 the presence of at least one comorbidity,146,147 initial referral to a nonrespiratory clinician,148 the need for multiple diagnostic tests,149,150 and care provided at a public hospital141 or in a teaching hospital setting.140,151,152
Figure 8.
[Section 4.0] Time to treatment in US studies of timeliness of care in lung cancer.
4.1 Timeliness of Lung Cancer Care and Outcomes
The relationship between timeliness of care and lung cancer outcomes is difficult to sort out. Several studies reported seemingly paradoxical results in which more timely care was associated with worse outcomes (Tables S2, S3 (359.9KB, pdf) ).139,146,153‐155 One of these studies examined timeliness of care and survival in a mixed sample of 432 patients with NSCLC in Sweden.155 In this study, the median time from symptom onset to treatment was 4.6 months, and median time from hospitalization to treatment was 1.6 months. After adjustment for age, sex, tumor histology, TNM stage, and surgical treatment, longer time to treatment was associated with a reduced risk of death. Such paradoxical results are almost surely explained by residual confounding by indication. Essentially, patients with more-advanced or aggressive disease are both more likely to receive timely care and less likely to survive than patients with less-advanced disease.
In contrast, several studies of patients with surgically treated lung cancer reported no association between timeliness and survival.135,156,157 However, results of these studies might have been biased toward the null by the exclusion of patients who did not undergo surgery because they had more-advanced disease caused by longer delays in care.
Support for an association between less-timely care and worse survival comes from studies of patients with lung cancer identified through population-based mass screening in Japan. In one such study, median survival was worse in patients with screening-detected lung cancer who did not follow up promptly and correlated with increased tumor size.158 Similarly, a study of screening with chest radiography found that survival was worse among patients who received a lung cancer diagnosis > 4 months after the initial radiographic abnormality was found compared with those who received a diagnosis more promptly.159 Of course, the main finding of the NLST that screening with low-dose CT scan reduced lung cancer-specific mortality by 20% also supports a link between timeliness and survival, at least in extreme cases.20
Additional support for this link comes from a small study in 29 patients with NSCLC from the United Kingdom that examined the relationship between tumor growth and delays in initiation of radiotherapy.160 Comparing measurements obtained from diagnostic vs planning CT scans separated by a median interval of 54 days, the median increase in tumor cross-sectional area was 19%, but tumor size increased by as much as 373%, and six patients became ineligible for curative treatment. This finding is consistent with previous studies of NSCLC that showed a doubling of times, ranging from 20 to 300 days.161‐166
4.2 Improving Timeliness of Lung Cancer Care
Few studies have demonstrated the effectiveness of interventions to improve timeliness of care. In one study, shorter times to surgery were observed following implementation of a nurse-led redesign of thoracic surgical care services at a single center in London, England.167 The redesign included a detailed analysis of the local care process, restructuring of referral patterns, and a hospital-wide educational initiative. Similarly, a telemedicine-based intervention improved access to surgery and time from referral to operation in another study from the United Kingdom.168 Two other studies from the United Kingdom reported reductions in time to treatment using a two-stop diagnostic process for patients with suspected lung cancer. In an uncontrolled prospective study, 275 patients treated at a single tertiary-care facility underwent imaging tests, biopsy, or both at the initial visit, and a treatment plan was developed during a multidisciplinary meeting within 3 days.169 The median time from first specialist visit to surgery was reduced by 50%, and the overall successful surgical resection rate improved from 10% in historical controls to 25%. In a subsequent randomized controlled trial, the two-stop process reduced time from first presentation to treatment by > 50%.170
Studies of other interventions have yielded disappointing results. A retrospective study of 345 US veterans that compared usual care with care provided in a multidisciplinary clinic found no differences in times to diagnosis or survival.138 A larger retrospective study of 1,044 patients with suspected lung cancer referred to a single facility during the 12 months prior and 24 months after introduction of the British Department of Health urgent referral guidelines found that the median time from referral to first visit with a respiratory specialist actually increased from 7 to 9 days, and the frequency of false-positive referrals for findings not related to lung cancer increased from 22% to 54%.162‐165,171
4.3 Recommendations From Other Sources
Other organizations have published specific recommendations for timeliness. The British Thoracic Society recommends that all patients with suspected lung cancer be evaluated by a respiratory specialist within 7 days and that the results of diagnostic tests be communicated to the patient within 2 weeks.172 In a quality indicator published for lung cancer care, the RAND Corporation specifies that a diagnosis of lung cancer should be established within 2 months of presentation and that treatment should begin within 6 weeks of diagnosis.173 Although efforts should be made to provide timely care, these should be balanced by the need to attend to other dimensions of quality, including safety, effectiveness, and consistency with patient values and preferences. Analysis of practice patterns and identification of local barriers to the delivery of timely care should be undertaken so that local interventions can be developed and deployed.
4.4 Recommendation
4.4.1. For patients with known or suspected lung cancer, we suggest that the delivery of care be timely and efficient (Grade 2C).
Remark: Interventions to improve timeliness should be developed locally by addressing barriers to providing timely care that are specific to the local setting.
Remark: Efforts to improve timeliness should be balanced with the need to attend to other dimensions of health-care quality.
5.0 Impact of Multidisciplinary Groups on Patient Outcomes
After the initial evaluation is complete, staging and diagnosis need to proceed, often in parallel. Optimal diagnostic, staging, and treatment strategies will depend on the patient’s comorbid conditions, physiology, extent of disease, and preferences. State-of-the-art care will therefore require the input of a wide variety of disciplines, including pulmonology, thoracic surgery, medical oncology, radiation oncology, pathology, and radiology. Coordination of care in this complex context may be difficult, resulting in delays in treatment, fragmented care, poor care coordination, and patient frustration.
Therefore, one key component of the initial evaluation must be to rapidly and efficiently bring patients with lung cancer into a system of care that integrates all the relevant disciplines while not being excessively slow or costly. Some of these disciplines are more related to diagnosis, whereas others are related to treatment and still others to palliation. However, it is important to consider them together and up front because future treatment options often have an impact on the optimal diagnostic strategy.174 Conversely, the quality of the information obtained from diagnostic procedures often affects or limits future treatment decisions. How the system of care is organized may therefore be a significant determinant of outcome. Indeed, practice guidelines for lung cancer in several countries recommend that multidisciplinary teams be used to plan the management of all patients with lung cancer.172,175‐177
However, although multidisciplinary care is widely advocated, there is limited evidence about the efficacy of a multidisciplinary approach. We conducted a literature search to address the issue of whether multidisciplinary team management compared with conventional care improved survival in patients with known or suspected lung cancer. Detailed search criteria and the results are presented in Figure S1 (359.9KB, pdf) .
Only one randomized trial was identified.170 This was a pilot study of 88 patients in the United Kingdom that compared standard of care with rapid assessment. Eligible patients were defined as those with possible lung cancer who could tolerate a CT scan and biopsy procedure. Patients were randomized to standard of care at one of three local district general hospitals or to a rapid assessment at a centralized hospital. The standard of care was itself a multidisciplinary team consisting of the lead lung cancer and chest physicians at the local hospital. In the standard of care arm, patients had a bronchoscopy at week 1, a CT scan in 2 weeks, a discussion of the case the following week, 1 more week for referral, and then 1 week for scheduling of surgical outpatient procedures or referral. Optimal timing was considered 6 weeks. Patients in the rapid assessment arm were admitted as day cases for CT scan and tissue biopsy, with results reviewed 3 days later at a meeting that included oncologists, radiologists, thoracic surgeons, a palliative care team representative, and a study coordinator. If the patient was found to have lung cancer, referral for treatment was to be initiated, with optimal timing being considered 2 weeks maximum. There was no difference in survival (P = .7). Patients in the rapid assessment arm were more likely to receive chemotherapy (P = .03), and there was a significant improvement in time from presentation to treatment (3 vs 7 weeks, P = .0025). However, there was no significant difference in time from diagnosis to the start of radical treatment.
Three other single-center studies used a before-and-after design to evaluate the use of multidisciplinary teams relative to survival.178‐180 A single-center study in the United Kingdom evaluated survival before and after the appointment of a specialist thoracic surgeon.180 A weekly multidisciplinary meeting was established at the same time. Although 2,891 patients received a diagnosis of lung cancer during the 6-year period of the study, only the 240 patients who underwent surgery were included in the analysis. Surgical resection rates increased (P < .001), and the types of surgeries performed changed from predominantly pneumonectomies to lobectomies (P < .001). There was no difference in hospital mortality and 1- and 5-year survival rates. A single-center UK study evaluated survival preintroduction and postintroduction of a multidisciplinary team in patients with inoperable NSCLC.179 Complete staging was performed in 70% of patients in the preteam period and 81% of patients in the postteam period (P = .035). Only patients who had complete staging were included in the study. Median survival improved from 3.4 to 6.6 months. A single-center US study evaluated survival before and after opening a cancer center connected to a community hospital.178 Whether a multidisciplinary team approach was used in the period preceding the opening of the cancer center was not reported. Lung cancer survival improved once the cancer center was opened (5-year survival, 16%-19%; median survival, 11-13 months; P = .12).
Overall, our systematic review of the literature identified few studies assessing the impact of multidisciplinary care on lung cancer survival. Those studies identified were of limited quality, the populations and problems studied varied widely, and the strength of inference for many of the studies was suspect. The definition of multidisciplinary teams varied significantly between studies, as did the comparators. The problem context also varied significantly, with some studies evaluating the impact of teams on patients undergoing a diagnostic workup170 and others focusing primarily on the impact of teams on patients undergoing treatment.178‐180 Studies of patients undergoing treatment also varied, with some focused on surgery180 and others on nonsurgical options.179 In addition, although some reports inferred that it was the multidisciplinary team that accounted for differences in outcome, the impact of the team often could not be separated from other confounders, such as organizational and system-level changes that were made as a result of the intervention or changes in treatment protocols over time.170,178 For example, in the one randomized trial, most of the benefits in terms of time to treatment seemed to result simply from changes in scheduling and a new care pathway rather than from the multidisciplinary discussion itself.170 In other reports, the training of the health-care providers was not the same, possibly accounting for differences in types of surgery and outcome.180 For secondary outcomes, such as waiting times, patient satisfaction, and quality of life, the evidence was similarly limited.181
Given the heterogeneity of definitions, patient populations, clinical contexts, and confounders, it is not possible at this time to make a definitive evidence-based statement on the impact of multidisciplinary teams on outcomes. To do so would require a more precise definition of the problem context (diagnosis, staging, or treatment), the patients being treated, the makeup of the team, and the comparators.
It is reasonable to recommend that providers look at practice organization and evaluate how it affects outcomes locally. Variables related to practice organization can be thought of as system-level variables. One of these system-level variables is whether a multidisciplinary team is used for various tasks. As the previous discussion highlights, there are many other system-level variables that also are probably important, such as availability of specialty services, resourcing for vital functions, care pathways in place, and the makeup of the team. At a minimum, we suggest that multidisciplinary teams comprise representatives from pulmonary, thoracic surgery, medical oncology, radiation oncology, palliative care, radiology, and pathology. It is highly probable that the impact of multidisciplinary teams is greatly affected by these other variables.
The impact of multidisciplinary teams when used for direct patient management will also change depending on the problem. For example, multidisciplinary team management may be very useful in patients with complex conditions who require multimodality treatment. However, in cases that are more straightforward and in cases that have not yet been completely staged, multidisciplinary team management may not be as beneficial and may only result in more delays. As such, it is probably prudent to organize practices in such a fashion that multidisciplinary team approaches are used when they can be of maximum benefit, specifically in complex management cases. In relatively straightforward cases and in those without a clear cancer diagnosis or when staging is incomplete, other methods of practice organization may be more suitable (eg, care pathways). Input from multiple disciplines will still play a vital role in developing these pathways, but that is distinct from the role of multidisciplinary teams in direct patient management.
5.1 Recommendation
5.1.1. For patients with lung cancer who require multimodality therapy, we suggest using a multidisciplinary team approach (Grade 2C).
Remark: We suggest that multidisciplinary teams have representatives from pulmonary, thoracic surgery, medical oncology, radiation oncology, palliative care, radiology, and pathology.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Ost had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Ost: contributed to the writing and editing and overall guideline coordination and revisions and was the multidisciplinary care section leader.
Dr Yeung: contributed to the writing and editing and was the paraneoplastic syndrome section leader.
Dr Tanoue: contributed to the writing and editing and was the presenting symptoms, signs, laboratory evaluation, and imaging section leader.
Dr Gould: contributed to the writing and editing and was the timeliness of care section leader.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of Sponsors: The American College of Chest Physicians was solely responsible for the development of these guidelines. The remaining supporters played no role in the development process. External supporting organizations cannot recommend panelists or topics, nor are they allowed prepublication access to the manuscripts and recommendations. Further details on the Conflict of Interest Policy are available online at http://chestnet.org.
Endorsements: This guideline is endorsed by the European Society of Thoracic Surgeons, Oncology Nursing Society, American Association for Bronchology and Interventional Pulmonology, and the Society of Thoracic Surgeons.
Other contributions: The authors thank Sandra Zelman Lewis, PhD, and Rebecca Diekemper, MPH, for their contributions to the discussion on how to incorporate other professional society evidence-based guidelines. Adelina Fuentes provided administrative support and coordination.
Additional information: The supplement appendix, figures, and tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- 5-HIAA
5-hydroxyindoleacetic acid
- ACCP
American College of Chest Physicians
- ACTH
adrenocorticotropin
- ADH
antidiuretic hormone
- CS
Cushing syndrome
- iPTH
intact parathyroid hormone
- IVIg
IV immunoglobulin
- LEMS
Lambert-Eaton myasthenic syndrome
- NET
neuroendocrine tumor
- NLST
National Lung Screening Trial
- NSCLC
non-small cell lung cancer
- PNS
paraneoplastic neurologic syndrome
- PTHrP
parathyroid hormone-related protein
- SCLC
small cell lung cancer
- SIADH
syndromes of inappropriate antidiuretic hormone
- VGCC
voltage-gated calcium channel
Footnotes
Funding/Sponsors: The overall process for the development of these guidelines, including matters pertaining to funding and conflicts of interest, are described in the methodology article.1 The development of this guideline was supported primarily by the American College of Chest Physicians. The lung cancer guidelines conference was supported in part by a grant from the Lung Cancer Research Foundation. The publication and dissemination of the guidelines was supported in part by a 2009 independent educational grant from Boehringer Ingelheim Pharmaceuticals, Inc.
COI grids reflecting the conflicts of interest that were current as of the date of the conference and voting are posted in the online supplementary materials.
Disclaimer: American College of Chest Physicians guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at http://dx.doi.org/10.1378/chest.1435S1.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Modified from Andersen et al,2 Grippi,3 Hyde and Hyde,4 Cromartie et al,5 Karsell and McDougall,6 and the American Thoracic Society and European Respiratory Society.7 SVC = superior vena cava. Reproduced with permission from Spiro et al.11
Modified from Silvestri et al.35 See Figure 3 legend for expansion of abbreviation. Reproduced and modified with permission from Spiro et al.11
FSH = follicle-stimulating hormone; LH = luteinizing hormone; SIADH = syndromes of inappropriate antidiuretic hormone. From Spiro et al.11
From Mashige et al79 and Aggarwal.80 5-HIAA = 5-hydroxyindoleacetic acid; SSRI = selective serotonin reuptake inhibitor.
From Kernstine and Reckamp.142 NSCLC = non-small cell lung cancer; VA = Veterans Administration.
References
- 1.Lewis SZ, Diekemper R, Addrizzo-Harris DJ. Methodology for development of guidelines for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5)(suppl):41S-50S. [DOI] [PubMed] [Google Scholar]
- 2.Andersen H, Prakash U. Diagnosis of symptomatic lung cancer. Semin Respir Med. 1982;3(3):165-175. [Google Scholar]
- 3.Grippi MA. Clinical aspects of lung cancer. Semin Roentgenol. 1990;25(1):12-24. [DOI] [PubMed] [Google Scholar]
- 4.Hyde L, Hyde CI. Clinical manifestations of lung cancer. Chest. 1974;65(3):299-306. [DOI] [PubMed] [Google Scholar]
- 5.Cromartie RS, III, Parker EF, May JE, Metcalf JS, Bartles DM. Carcinoma of the lung: a clinical review. Ann Thorac Surg. 1980;30(1):30-35. [DOI] [PubMed] [Google Scholar]
- 6.Karsell PR, McDougall JC. Diagnostic tests for lung cancer. Mayo Clin Proc. 1993;68(3):288-296. [DOI] [PubMed] [Google Scholar]
- 7.The American Thoracic Society, The European Respiratory Society. Pretreatment evaluation of non-small-cell lung cancer. Am J Respir Crit Care Med. 1997;156(1):320-332. [DOI] [PubMed] [Google Scholar]
- 8.Koyi H, Hillerdal G, Brandén E. Patient’s and doctors’ delays in the diagnosis of chest tumors. Lung Cancer. 2002;35(1):53-57. [DOI] [PubMed] [Google Scholar]
- 9.Buccheri G, Ferrigno D. Lung cancer: clinical presentation and specialist referral time. Eur Respir J. 2004;24(6):898-904. [DOI] [PubMed] [Google Scholar]
- 10.Carbone P, Frost J, Feinstein A. Lung cancer: perspectives and prospects. Ann Intern Med. 1970;73:1002-1024. [DOI] [PubMed] [Google Scholar]
- 11.Spiro SG, Gould MK, Colice GL; American College of Chest Physicians. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007;132(suppl 3):149S-160S. [DOI] [PubMed] [Google Scholar]
- 12.Colice GL. Detecting lung cancer as a cause of hemoptysis in patients with a normal chest radiograph: bronchoscopy vs CT. Chest. 1997;111(4):877-884. [DOI] [PubMed] [Google Scholar]
- 13.Arcasoy SM, Jett JR. Superior pulmonary sulcus tumors and Pancoast’s syndrome. N Engl J Med. 1997;337(19):1370-1376. [DOI] [PubMed] [Google Scholar]
- 14.Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med. 2007;356(18):1862-1869. [DOI] [PubMed] [Google Scholar]
- 15.Detterbeck FC, Postmus PE, Tanoue LT.The stage classification of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5)(suppl):e191S-e210S. [DOI] [PubMed] [Google Scholar]
- 16.Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5)(suppl):e166S-e190S. [DOI] [PubMed] [Google Scholar]
- 17.Midthun DE, Swensen SJ, Jett JR, Hartman TE. Evaluation of nodules detected by screening for lung cancer with low dose spiral computed tomography. Lung Cancer. 2003;41(suppl 2):S40 [Google Scholar]
- 18.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157(8):849-855. [PubMed] [Google Scholar]
- 19.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5)(suppl):e93S-e120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn D, Gianlupi A, Broste S. The changing radiographic presentation of bronchogenic carcinoma with reference to cell types. Chest. 1996;110(6):1474-1479. [DOI] [PubMed] [Google Scholar]
- 22.Byrd RB, Carr DT, Miller WE, Payne WS, Woolner LB. Radiographic abnormalities in carcinoma of the lung as related to histological cell type. Thorax. 1969;24(5):573-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sider L. Radiographic manifestations of primary bronchogenic carcinoma. Radiol Clin North Am. 1990;28(3):583-597. [PubMed] [Google Scholar]
- 24.Theros EG. 1976 Caldwell Lecture: varying manifestation of peripheral pulmonary neoplasms: a radiologic-pathologic correlative study. AJR Am J Roentgenol. 1977;128(6):893-914. [DOI] [PubMed] [Google Scholar]
- 25.Feinstein AR, Wells CK. A clinical-severity staging system for patients with lung cancer. Medicine (Baltimore). 1990;69(1):1-33. [DOI] [PubMed] [Google Scholar]
- 26.Merchut MP. Brain metastases from undiagnosed systemic neoplasms. Arch Intern Med. 1989;149(5):1076-1080. [PubMed] [Google Scholar]
- 27.Feinstein AR, Wells CK. Lung cancer staging. A critical evaluation. Clin Chest Med. 1982;3(2):291-305. [PubMed] [Google Scholar]
- 28.Feinstein AR. Symptomatic patterns, biologic behavior, and prognosis in cancer of the lung. Practical application of Boolean algebra and clinical taxonomy. Ann Intern Med. 1964;61:27-43. [DOI] [PubMed] [Google Scholar]
- 29.Feinstein AR. Symptoms as an index of biological behaviour and prognosis in human cancer. Nature. 1966;209(5020):241-245. [DOI] [PubMed] [Google Scholar]
- 30.Hooper RG, Tenholder MF, Underwood GH, Beechler CR, Spratling L. Computed tomographic scanning of the brain in initial staging of bronchogenic carcinoma. Chest. 1984;85(6):774-776. [DOI] [PubMed] [Google Scholar]
- 31.Hooper RG, Beechler CR, Johnson MC. Radioisotope scanning in the initial staging of bronchogenic carcinoma. Am Rev Respir Dis. 1978;118(2):279-286. [DOI] [PubMed] [Google Scholar]
- 32.Silvestri GA, Lenz JE, Harper SN, Morse RA, Colice GL. The relationship of clinical findings to CT scan evidence of adrenal gland metastases in the staging of bronchogenic carcinoma. Chest. 1992;102(6):1748-1751. [DOI] [PubMed] [Google Scholar]
- 33.Quinn DL, Ostrow LB, Porter DK, Shelton DK, Jr, Jackson DE., Jr Staging of non-small cell bronchogenic carcinoma. Relationship of the clinical evaluation to organ scans. Chest. 1986;89(2):270-275. [DOI] [PubMed] [Google Scholar]
- 34.Colice GL, Birkmeyer JD, Black WC, Littenberg B, Silvestri G. Cost-effectiveness of head CT in patients with lung cancer without clinical evidence of metastases. Chest. 1995;108(5):1264-1271. [DOI] [PubMed] [Google Scholar]
- 35.Silvestri GA, Littenberg B, Colice GL. The clinical evaluation for detecting metastatic lung cancer. A meta-analysis. Am J Respir Crit Care Med. 1995;152(1):225-230. [DOI] [PubMed] [Google Scholar]
- 36.Grant D, Edwards D, Goldstraw P. Computed tomography of the brain, chest, and abdomen in the preoperative assessment of non-small cell lung cancer. Thorax. 1988;43(11):883-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meador CK, Liddle GW, Island DP, et al. Cause of Cushing’s syndrome in patients with tumors arising from “nonendocrine” tissue. J Clin Endocrinol Metab. 1962;22:693-703. [DOI] [PubMed] [Google Scholar]
- 38.Oldfield EH, Doppman JL, Nieman LK, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med. 1991;325(13):897-905. [DOI] [PubMed] [Google Scholar]
- 39.Wajchenberg BL, Mendonça B, Liberman B, Adelaide M, Pereira A, Kirschner MA. Ectopic ACTH syndrome. J Steroid Biochem Mol Biol. 1995;53(1-6):139-151. [DOI] [PubMed] [Google Scholar]
- 40.Howlett TA, Drury PL, Perry L, Doniach I, Rees LH, Besser GM. Diagnosis and management of ACTH-dependent Cushing’s syndrome: comparison of the features in ectopic and pituitary ACTH production. Clin Endocrinol (Oxf). 1986;24(6):699-713. [DOI] [PubMed] [Google Scholar]
- 41.Dimopoulos MA, Fernandez JF, Samaan NA, Holoye PY, Vassilopoulou-Sellin R. Paraneoplastic Cushing’s syndrome as an adverse prognostic factor in patients who die early with small cell lung cancer. Cancer. 1992;69(1):66-71. [DOI] [PubMed] [Google Scholar]
- 42.Sarlis NJ, Chanock SJ, Nieman LK. Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin. J Clin Endocrinol Metab. 2000;85(1):42-47. [DOI] [PubMed] [Google Scholar]
- 43.Kato Y, Ferguson TB, Bennett DE, Burford TH. Oat cell carcinoma of the lung. A review of 138 cases. Cancer. 1969;23(3):517-524. [DOI] [PubMed] [Google Scholar]
- 44.Shepherd FA, Laskey J, Evans WK, Goss PE, Johansen E, Khamsi F. Cushing’s syndrome associated with ectopic corticotropin production and small-cell lung cancer. J Clin Oncol. 1992;10(1):21-27. [DOI] [PubMed] [Google Scholar]
- 45.Delisle L, Boyer MJ, Warr D, et al. Ectopic corticotropin syndrome and small-cell carcinoma of the lung. Clinical features, outcome, and complications. Arch Intern Med. 1993;153(6):746-752. [PubMed] [Google Scholar]
- 46.Gewirtz G, Yalow RS. Ectopic ACTH production in carcinoma of the lung. J Clin Invest. 1974;53(4):1022-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gropp C, Havemann K, Scheuer A. Ectopic hormones in lung cancer patients at diagnosis and during therapy. Cancer. 1980;46(2):347-354. [DOI] [PubMed] [Google Scholar]
- 48.Bondy PK, Gilby ED. Endocrine function in small cell undifferentiated carcinoma of the lung. Cancer. 1982;50(10):2147-2153. [DOI] [PubMed] [Google Scholar]
- 49.Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90(8):4955-4962. [DOI] [PubMed] [Google Scholar]
- 50.Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biller BM, Grossman AB, Stewart PM, et al. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93(7):2454-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elamin MB, Murad MH, Mullan R, et al. Accuracy of diagnostic tests for Cushing’s syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab. 2008;93(5):1553-1562. [DOI] [PubMed] [Google Scholar]
- 53.Guignat L, Bertherat J. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline: commentary from a European perspective. Eur J Endocrinol. 2010;163(1):9-13. [DOI] [PubMed] [Google Scholar]
- 54.Small M, Lowe GD, Forbes CD, Thomson JA. Thromboembolic complications in Cushing’s syndrome. Clin Endocrinol (Oxf). 1983;19(4):503-511. [DOI] [PubMed] [Google Scholar]
- 55.Kastelan D, Dusek T, Kraljevic I, et al. Hypercoagulability in Cushing’s syndrome: the role of specific haemostatic and fibrinolytic markers. Endocrine. 2009;36(1):70-74. [DOI] [PubMed] [Google Scholar]
- 56.Van Zaane B, Nur E, Squizzato A, et al. Hypercoagulable state in Cushing’s syndrome: a systematic review. J Clin Endocrinol Metab. 2009;94(8):2743-2750. [DOI] [PubMed] [Google Scholar]
- 57.Phan AT, Oberg K, Choi J, et al. ; North American Neuroendocrine Tumor Society (NANETS). NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus). Pancreas. 2010;39(6):784-798. [DOI] [PubMed] [Google Scholar]
- 58.Katsuragi N, Shiraishi Y, Nakajima Y, Kurai M, Takahashi N, Tanaka S. Squamous cell bronchogenic carcinoma with syndrome of inappropriate secretion of antidiuretic hormone [in Japanese]. Kyobu Geka. 2004;57(9):847-850. [PubMed] [Google Scholar]
- 59.Kagawa K, Fujitaka K, Isobe T, et al. Syndrome of inappropriate secretion of ADH (SIADH) following cisplatin administration in a pulmonary adenocarcinoma patient with a malignant pleural effusion. Intern Med. 2001;40(10):1020-1023. [DOI] [PubMed] [Google Scholar]
- 60.Moses AM, Scheinman SJ. Ectopic secretion of neurohypophyseal peptides in patients with malignancy. Endocrinol Metab Clin North Am. 1991;20(3):489-506. [PubMed] [Google Scholar]
- 61.Gross AJ, Steinberg SM, Reilly JG, et al. Atrial natriuretic factor and arginine vasopressin production in tumor cell lines from patients with lung cancer and their relationship to serum sodium. Cancer Res. 1993;53(1):67-74. [PubMed] [Google Scholar]
- 62.Fenske W, Maier SK, Blechschmidt A, Allolio B, Störk S. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med. 2010;123(7):652-657. [DOI] [PubMed] [Google Scholar]
- 63.Kamoi K, Ebe T, Hasegawa A, et al. Hyponatremia in small cell lung cancer. Mechanisms not involving inappropriate ADH secretion. Cancer. 1987;60(5):1089-1093. [DOI] [PubMed] [Google Scholar]
- 64.Raftopoulos H. Diagnosis and management of hyponatremia in cancer patients. Support Care Cancer. 2007;15(12):1341-1347. [DOI] [PubMed] [Google Scholar]
- 65.Vaidya C, Ho W, Freda BJ. Management of hyponatremia: providing treatment and avoiding harm. Cleve Clin J Med. 2010;77(10):715-726. [DOI] [PubMed] [Google Scholar]
- 66.Hiraki A, Ueoka H, Bessho A, et al. Parathyroid hormone-related protein measured at the time of first visit is an indicator of bone metastases and survival in lung carcinoma patients with hypercalcemia. Cancer. 2002;95(8):1706-1713. [DOI] [PubMed] [Google Scholar]
- 67.Lazaretti-Castro M, Kayath M, Jamnik S, Santoro IL, Tadokoru H, Vieira JG. Prevalence of hypercalcemia in patients with lung cancer [in Portuguese]. Rev Assoc Med Bras. 1993;39(2):83-87. [PubMed] [Google Scholar]
- 68.Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373-379. [DOI] [PubMed] [Google Scholar]
- 69.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655-1664. [DOI] [PubMed] [Google Scholar]
- 70.Coggeshall J, Merrill W, Hande K, Des Prez R. Implications of hypercalcemia with respect to diagnosis and treatment of lung cancer. Am J Med. 1986;80(2):325-328. [DOI] [PubMed] [Google Scholar]
- 71.Smith A, Wisloff F, Samson D; UK Myeloma Forum; Nordic Myeloma Study Group; British Committee for Standards in Haematology. Guidelines on the diagnosis and management of multiple myeloma 2005. Br J Haematol. 2006;132(4):410-451. [DOI] [PubMed] [Google Scholar]
- 72.Body JJ, Coleman R, Clezardin P, Ripamonti C, Rizzoli R, Aapro M; International Society of Geriatric Oncology. International Society of Geriatric Oncology (SIOG) clinical practice recommendations for the use of bisphosphonates in elderly patients. Eur J Cancer. 2007;43(5):852-858. [DOI] [PubMed] [Google Scholar]
- 73.Lumachi F, Brunello A, Roma A, Basso U. Cancer-induced hypercalcemia. Anticancer Res. 2009;29(5):1551-1555. [PubMed] [Google Scholar]
- 74.Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113(1):5-21. [DOI] [PubMed] [Google Scholar]
- 75.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40(2):90-97. [DOI] [PubMed] [Google Scholar]
- 76.Lim E, Goldstraw P, Nicholson AG, et al. Proceedings of the IASLC International Workshop on Advances in Pulmonary Neuroendocrine Tumors 2007. J Thorac Oncol. 2008;3(10):1194-1201. [DOI] [PubMed] [Google Scholar]
- 77.Kvols LK. Therapy of the malignant carcinoid syndrome. Endocrinol Metab Clin North Am. 1989;18(2):557-568. [PubMed] [Google Scholar]
- 78.Feldman JM, Lee EM. Serotonin content of foods: effect on urinary excretion of 5-hydroxyindoleacetic acid. Am J Clin Nutr. 1985;42(4):639-643. [DOI] [PubMed] [Google Scholar]
- 79.Mashige F, Matsushima Y, Kanazawa H, et al. Acidic catecholamine metabolites and 5-hydroxyindoleacetic acid in urine: the influence of diet. Ann Clin Biochem. 1996;33(pt 1):43-49. [DOI] [PubMed] [Google Scholar]
- 80.Aggarwal G, Obideen K, Wehbi M. Carcinoid tumors: what should increase our suspicion?. Cleve Clin J Med. 2008;75(12):849-855. [DOI] [PubMed] [Google Scholar]
- 81.Tormey WP, FitzGerald RJ. The clinical and laboratory correlates of an increased urinary 5-hydroxyindoleacetic acid. Postgrad Med J. 1995;71(839):542-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bajetta E, Ferrari L, Martinetti A, et al. Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumors. Cancer. 1999;86(5):858-865. [DOI] [PubMed] [Google Scholar]
- 83.Seregni E, Ferrari L, Bajetta E, Martinetti A, Bombardieri E. Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol. 2001;12(suppl 2):S69-S72. [DOI] [PubMed] [Google Scholar]
- 84.Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. 2010;257(4):509-517. [DOI] [PubMed] [Google Scholar]
- 85.Tschernatsch M, Dierkes C, Gerriets T, et al. Paraneoplastic neurological syndromes in patients with carcinoid. Eur J Neurol. 2008;15(12):1390-1394. [DOI] [PubMed] [Google Scholar]
- 86.Roberts WK, Deluca IJ, Thomas A, et al. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8+ T cells. J Clin Invest. 2009;119(7):2042-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Graaf M, de Beukelaar J, Bergsma J, et al. B and T cell imbalances in CSF of patients with Hu-antibody associated PNS. J Neuroimmunol. 2008;195(1-2):164-170. [DOI] [PubMed] [Google Scholar]
- 88.Koyama K, Kagamu H, Miura S, et al. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14(21):6770-6779. [DOI] [PubMed] [Google Scholar]
- 89.Plonquet A, Gherardi RK, Créange A, et al. Oligoclonal T-cells in blood and target tissues of patients with anti-Hu syndrome. J Neuroimmunol. 2002;122(1-2):100-105. [DOI] [PubMed] [Google Scholar]
- 90.Monstad SE, Knudsen A, Salvesen HB, Aarseth JH, Vedeler CA. Onconeural antibodies in sera from patients with various types of tumours. Cancer Immunol Immunother. 2009;58(11):1795-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci. 2008;65(20):3168-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fornaro M, Raimondo S, Lee JM, Giacobini-Robecchi MG. Neuron-specific Hu proteins sub-cellular localization in primary sensory neurons. Ann Anat. 2007;189(3):223-228. [DOI] [PubMed] [Google Scholar]
- 93.Dalmau J, Graus F, Cheung NK, et al. Major histocompatibility proteins, anti-Hu antibodies, and paraneoplastic encephalomyelitis in neuroblastoma and small cell lung cancer. Cancer. 1995;75(1):99-109. [DOI] [PubMed] [Google Scholar]
- 94.Senties-Madrid H, Vega-Boada F. Paraneoplastic syndromes associated with anti-Hu antibodies. Isr Med Assoc J. 2001;3(2):94-103. [PubMed] [Google Scholar]
- 95.Rousseau A, Benyahia B, Dalmau J, et al. T cell response to Hu-D peptides in patients with anti-Hu syndrome. J Neurooncol. 2005;71(3):231-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Graaf MT, de Beukelaar JW, Haasnoot GW, et al. HLA-DQ2+ individuals are susceptible to Hu-Ab associated paraneoplastic neurological syndromes. J Neuroimmunol. 2010;226(1-2):147-149. [DOI] [PubMed] [Google Scholar]
- 97.Hammam T, McFadzean RM, Ironside JW. Anti-hu paraneoplastic syndrome presenting as bilateral sixth cranial nerve palsies. J Neuroophthalmol. 2005;25(2):101-104. [DOI] [PubMed] [Google Scholar]
- 98.Eggers C, Hagel C, Pfeiffer G. Anti-Hu-associated paraneoplastic sensory neuropathy with peripheral nerve demyelination and microvasculitis. J Neurol Sci. 1998;155(2):178-181. [DOI] [PubMed] [Google Scholar]
- 99.Espay AJ, Kumar V, Sarpel G. Anti-Hu-associated paraneoplastic limbic encephalitis presenting as rapidly progressive non-convulsive status epilepticus. J Neurol Sci. 2006;246(1-2):149-152. [DOI] [PubMed] [Google Scholar]
- 100.Suzuki M, Kimura H, Tachibana I, et al. Improvement of anti-Hu-associated paraneoplastic sensory neuropathy after chemoradiotherapy in a small cell lung cancer patient. Intern Med. 2001;40(11):1140-1143. [DOI] [PubMed] [Google Scholar]
- 101.Keime-Guibert F, Graus F, Broët P, et al. Clinical outcome of patients with anti-Hu-associated encephalomyelitis after treatment of the tumor. Neurology. 1999;53(8):1719-1723. [DOI] [PubMed] [Google Scholar]
- 102.Keime-Guibert F, Graus F, Fleury A, et al. Treatment of paraneoplastic neurological syndromes with antineuronal antibodies (Anti-Hu, anti-Yo) with a combination of immunoglobulins, cyclophosphamide, and methylprednisolone. J Neurol Neurosurg Psychiatry. 2000;68(4):479-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feasby T, Banwell B, Benstead T, et al. Guidelines on the use of intravenous immune globulin for neurologic conditions. Transfus Med Rev. 2007;21(suppl 1):S57-S107. [DOI] [PubMed] [Google Scholar]
- 104.Greenlee JE, Clawson SA, Hill KE, Wood BL, Tsunoda I, Carlson NG. Purkinje cell death after uptake of anti-Yo antibodies in cerebellar slice cultures. J Neuropathol Exp Neurol. 2010;69(10):997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Widdess-Walsh P, Tavee JO, Schuele S, Stevens GH. Response to intravenous immunoglobulin in anti-Yo associated paraneoplastic cerebellar degeneration: case report and review of the literature. J Neurooncol. 2003;63(2):187-190. [DOI] [PubMed] [Google Scholar]
- 106.Landtblom AM, Lindvall B, Ledin T, Berlin G. A case report of plasmapheresis treatment in a patient with paraneoplastic cerebellar degeneration and high anti-yo antibody titers. Ther Apher Dial. 2008;12(1):82-85. [DOI] [PubMed] [Google Scholar]
- 107.Meloni C, Iani C, Dominijanni S, et al. A case report of plasma exchange therapy in non-paraneoplastic cerebellar ataxia associated with anti-Yo antibody. Ther Apher Dial. 2004;8(6):500-502. [DOI] [PubMed] [Google Scholar]
- 108.Buckanovich RJ, Posner JB, Darnell RB. Nova, the paraneoplastic Ri antigen, is homologous to an RNA-binding protein and is specifically expressed in the developing motor system. Neuron. 1993;11(4):657-672. [DOI] [PubMed] [Google Scholar]
- 109.Knudsen A, Monstad SE, Dørum A, et al. Ri antibodies in patients with breast, ovarian or small cell lung cancer determined by a sensitive immunoprecipitation technique. Cancer Immunol Immunother. 2006;55(10):1280-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prommer E. Neuromuscular paraneoplastic syndromes: the Lambert-Eaton myasthenic syndrome. J Palliat Med. 2010;13(9):1159-1162. [DOI] [PubMed] [Google Scholar]
- 111.Vincent A. Autoimmune channelopathies: well-established and emerging immunotherapy-responsive diseases of the peripheral and central nervous systems. J Clin Immunol. 2010;30(suppl 1):S97-S102. [DOI] [PubMed] [Google Scholar]
- 112.Voltz R, Carpentier AF, Rosenfeld MR, Posner JB, Dalmau J. P/Q-type voltage-gated calcium channel antibodies in paraneoplastic disorders of the central nervous system. Muscle Nerve. 1999;22(1):119-122. [DOI] [PubMed] [Google Scholar]
- 113.Lennon VA, Kryzer TJ, Griesmann GE, et al. Calcium-channel antibodies in the Lambert-Eaton syndrome and other paraneoplastic syndromes. N Engl J Med. 1995;332(22):1467-1474. [DOI] [PubMed] [Google Scholar]
- 114.Elrington GM, Murray NM, Spiro SG, Newsom-Davis J. Neurological paraneoplastic syndromes in patients with small cell lung cancer. A prospective survey of 150 patients. J Neurol Neurosurg Psychiatry. 1991;54(9):764-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seute T, Leffers P, ten Velde GP, Twijnstra A. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer. 2004;100(4):801-806. [DOI] [PubMed] [Google Scholar]
- 116.Mareska M, Gutmann L. Lambert-Eaton myasthenic syndrome. Semin Neurol. 2004;24(2):149-153. [DOI] [PubMed] [Google Scholar]
- 117.Payne M, Bradbury P, Lang B, et al. Prospective study into the incidence of Lambert Eaton myasthenic syndrome in small cell lung cancer. J Thorac Oncol. 2010;5(1):34-38. [DOI] [PubMed] [Google Scholar]
- 118.Maddison P, Newsom-Davis J. Treatment for Lambert-Eaton myasthenic syndrome. Cochrane Database Syst Rev. 2005;(2):CD003279. [DOI] [PubMed] [Google Scholar]
- 119.Newsom-Davis J, Mills KR. Immunological associations of acquired neuromyotonia (Isaacs’ syndrome). Report of five cases and literature review. Brain. 1993;116(pt 2):453-469. [DOI] [PubMed] [Google Scholar]
- 120.Vernino S. Autoimmune and paraneoplastic channelopathies. Neurotherapeutics. 2007;4(2):305-314. [DOI] [PubMed] [Google Scholar]
- 121.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343(12):847-855. [DOI] [PubMed] [Google Scholar]
- 122.Winston N, Vernino S. Autoimmune autonomic ganglionopathy. Front Neurol Neurosci. 2009;26:85-93. [DOI] [PubMed] [Google Scholar]
- 123.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292(8):403-407. [DOI] [PubMed] [Google Scholar]
- 124.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292(7):344-347. [DOI] [PubMed] [Google Scholar]
- 125.Chen YJ, Wu CY, Huang YL, Wang CB, Shen JL, Chang YT. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12(2):R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang W, Jiang SP, Huang L. Dermatomyositis and malignancy: a retrospective study of 115 cases. Eur Rev Med Pharmacol Sci. 2009;13(2):77-80. [PubMed] [Google Scholar]
- 127.Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357(9250):96-100. [DOI] [PubMed] [Google Scholar]
- 128.Levine SM. Cancer and myositis: new insights into an old association. Curr Opin Rheumatol. 2006;18(6):620-624. [DOI] [PubMed] [Google Scholar]
- 129.Krathen MS, Fiorentino D, Werth VP. Dermatomyositis. Curr Dir Autoimmun. 2008;10:313-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Antonioli CM, Airò P. Dermatomyositis associated with lymphoproliferative disorder of NK cells and occult small cell lung carcinoma. Clin Rheumatol. 2004;23(3):239-241. [DOI] [PubMed] [Google Scholar]
- 131.Iorizzo LJ, III, Jorizzo JL. The treatment and prognosis of dermatomyositis: an updated review. J Am Acad Dermatol. 2008;59(1):99-112. [DOI] [PubMed] [Google Scholar]
- 132.Elovaara I, Apostolski S, van Doorn P, et al. ; EFNS. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur J Neurol. 2008;15(9):893-908. [DOI] [PubMed] [Google Scholar]
- 133.Institute of Medicine (Committee on Quality of Health Care in America). Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press;2001. [Google Scholar]
- 134.Finlay GA, Joseph B, Rodrigues CR, Griffith J, White AC. Advanced presentation of lung cancer in Asian immigrants: a case-control study. Chest. 2002;122(6):1938-1943. [DOI] [PubMed] [Google Scholar]
- 135.Quarterman RL, McMillan A, Ratcliffe MB, Block MI. Effect of preoperative delay on prognosis for patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;125(1):108-113. [DOI] [PubMed] [Google Scholar]
- 136.Liu DM, Kwee SA. Demographic, treatment, and survival patterns for Native Hawaiians with lung cancer treated at a community medical center from 1995 to 2001. Pac Health Dialog. 2004;11(2):139-145. [PubMed]
- 137.Dransfield MT, Lock BJ, Garver RI, Jr. Improving the lung cancer resection rate in the US Department of Veterans Affairs Health System. Clin Lung Cancer. 2006;7(4):268-272. [DOI] [PubMed]
- 138.Riedel RF, Wang X, McCormack M, et al. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care. J Thorac Oncol. 2006;1(7):692-696. [PubMed] [Google Scholar]
- 139.Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133(5):1167-1173. [DOI] [PubMed] [Google Scholar]
- 140.Schultz EM, Powell AA, McMillan A, et al. Hospital characteristics associated with timeliness of care in veterans with lung cancer. Am J Respir Crit Care Med. 2009;179(7):595-600. [DOI] [PubMed] [Google Scholar]
- 141.Yorio JT, Xie Y, Yan J, Gerber DE. Lung cancer diagnostic and treatment intervals in the United States: a health care disparity?. J Thorac Oncol. 2009;4(11):1322-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kernstine KH, Reckamp KL. eds. Lung Cancer: A Multidisciplinary Approach to Diagnosis and Management. New York, NY: Demos Medical; 2011.
- 143.Bjerager M, Palshof T, Dahl R, Vedsted P, Olesen F. Delay in diagnosis of lung cancer in general practice. Br J Gen Pract. 2006;56(532):863-868. [PMC free article] [PubMed] [Google Scholar]
- 144.Strojan P, Debevec M, Kovac V. Superior sulcus tumor (SST): management at the Institute of Oncology in Ljubljana, Slovenia, 1981-1994. Lung Cancer. 1997;17(2-3):249-259. [DOI] [PubMed] [Google Scholar]
- 145.Johnston GM, MacGarvie VL, Elliott D, Dewar RA, MacIntyre MM, Nolan MC. Radiotherapy wait times for patients with a diagnosis of invasive cancer, 1992-2000. Clin Invest Med. 2004;27(3):142-156. [PubMed] [Google Scholar]
- 146.Annakkaya AN, Arbak P, Balbay O, Bilgin C, Erbas M, Bulut I. Effect of symptom-to-treatment interval on prognosis in lung cancer. Tumori. 2007;93(1):61-67. [DOI] [PubMed] [Google Scholar]
- 147.Simunovic M, Thériault ME, Paszat L, et al. Using administrative databases to measure waiting times for patients undergoing major cancer surgery in Ontario, 1993-2000. Can J Surg. 2005;48(2):137-142. [PMC free article] [PubMed] [Google Scholar]
- 148.Kesson E, Bucknall CE, McAlpine LG, et al. Lung cancer—management and outcome in Glasgow, 1991-92. Br J Cancer. 1998;78(10):1391-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Devbhandari MP, Soon SY, Quennell P, et al. UK waiting time targets in lung cancer treatment: are they achievable? Results of a prospective tracking study. J Cardiothorac Surg. 2007;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ringbaek T, Borgeskov S, Lange P, Viskum K. Diagnostic and therapeutic process and prognosis in suspected lung cancer. Scand Cardiovasc J. 1999;33(6):337-343. [DOI] [PubMed] [Google Scholar]
- 151.Bardell T, Belliveau P, Kong W, Mackillop WJ. Waiting times for cancer surgery in Ontario: 1984-2000. Clin Oncol (R Coll Radiol). 2006;18(5):401-409. [DOI] [PubMed] [Google Scholar]
- 152.Simunovic M, Rempel E, Thériault ME, et al. Influence of hospital characteristics on operative death and survival of patients after major cancer surgery in Ontario. Can J Surg. 2006;49(4):251-258. [PMC free article] [PubMed] [Google Scholar]
- 153.Berthelet E, Truong PT, Lesperance M, et al. Examining time intervals between diagnosis and treatment in the management of patients with limited stage small cell lung cancer. Am J Clin Oncol. 2006;29(1):21-26. [DOI] [PubMed] [Google Scholar]
- 154.Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98(8):238-239. [PubMed] [Google Scholar]
- 155.Myrdal G, Lambe M, Hillerdal G, Lamberg K, Agustsson T, Ståhle E. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59(1):45-49. [PMC free article] [PubMed] [Google Scholar]
- 156.Aragoneses FG, Moreno N, Leon P, Fontan EG, Folque E; Bronchogenic Carcinoma Cooperative Group of the Spanish Society of Pneumology and Thoracic Surgery (GCCB-S). Influence of delays on survival in the surgical treatment of bronchogenic carcinoma. Lung Cancer. 2002;36(1):59-63. [DOI] [PubMed] [Google Scholar]
- 157.Pita-Fernández S, Montero-Martinez C, Pértega-Diaz S, Verea-Hernando H. Relationship between delayed diagnosis and the degree of invasion and survival in lung cancer. J Clin Epidemiol. 2003;56(9):820-825. [DOI] [PubMed] [Google Scholar]
- 158.Kashiwabara K, Koshi S, Itonaga K, Nakahara O, Tanaka M, Toyonaga M. Outcome in patients with lung cancer found on lung cancer mass screening roentgenograms, but who did not subsequently consult a doctor. Lung Cancer. 2003;40(1):67-72. [DOI] [PubMed] [Google Scholar]
- 159.Kanashiki M, Satoh H, Ishikawa H, Yamashita YT, Ohtsuka M, Sekizawa K. Time from finding abnormality on mass-screening to final diagnosis of lung cancer. Oncol Rep. 2003;10(3):649-652. [PubMed] [Google Scholar]
- 160.O’Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol). 2000;12(3):141-144. [DOI] [PubMed] [Google Scholar]
- 161.Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med. 2012;185(4):363-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aoki T, Nakata H, Watanabe H, et al. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time. AJR Am J Roentgenol. 2000;174(3):763-768. [DOI] [PubMed] [Google Scholar]
- 163.Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol. 2000;73(876):1252-1259. [DOI] [PubMed] [Google Scholar]
- 164.Takashima S, Sone S, Li F, et al. Small solitary pulmonary nodules (< or =1 cm) detected at population-based CT screening for lung cancer: Reliable high-resolution CT features of benign lesions. AJR Am J Roentgenol. 2003;180(4):955-964. [DOI] [PubMed] [Google Scholar]
- 165.Yankelevitz DF, Henschke CI. Does 2-year stability imply that pulmonary nodules are benign?. AJR Am J Roentgenol. 1997;168(2):325-328. [DOI] [PubMed] [Google Scholar]
- 166.Yankelevitz DF, Reeves AP, Kostis WJ, Zhao B, Henschke CI. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology. 2000;217(1):251-256. [DOI] [PubMed] [Google Scholar]
- 167.Leary A, Corrigan P.Redesign of thoracic surgical services within a cancer network-using an oncology focus to inform change. Eur J Oncol Nurs 2005;9(1):74-78. [DOI] [PubMed] [Google Scholar]
- 168.Davison AG, Eraut CD, Haque AS, et al. Telemedicine for multidisciplinary lung cancer meetings. J Telemed Telecare. 2004;10(3):140-143. [DOI] [PubMed] [Google Scholar]
- 169.Laroche C, Wells F, Coulden R, et al. Improving surgical resection rate in lung cancer. Thorax. 1998;53(6):445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Murray PV, O’Brien ME, Sayer R, et al. The pathway study: results of a pilot feasibility study in patients suspected of having lung carcinoma investigated in a conventional chest clinic setting compared to a centralised two-stop pathway. Lung Cancer. 2003;42(3):283-290. [DOI] [PubMed] [Google Scholar]
- 171.Lewis NR, Le Jeune I, Baldwin DR. Under utilisation of the 2-week wait initiative for lung cancer by primary care and its effect on the urgent referral pathway. Br J Cancer. 2005;93(8):905-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. Thorax. 1998;53(suppl 1):S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reifel J. Lung Cancer. In: Asch S, Kerr E, Hamilton E, eds. Quality of Care for Oncologic Conditions and HIV: A Review of the Literature and Quality Indicators RAND Corporation; 2000;133-172. [Google Scholar]
- 174.Ost DE, Gould MK. Decision making in the patient with pulmonary nodules. Am J Respir Crit Care Med. 2012;185(4):363-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Crinò L, Weder W, van Meerbeeck J, Felip E; ESMO Guidelines Working Group. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v103-v115. [DOI] [PubMed] [Google Scholar]
- 176.Alberts WM, Bepler G, Hazelton T, Ruckdeschel JC, Williams JH, Jr; American College of Chest Physicians. Lung cancer. Practice organization. Chest. 2003;1231):332S-337S. [DOI] [PubMed] [Google Scholar]
- 177.British Thoracic Society; Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56(2):89-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dillman RO, Chico SD. Cancer patient survival improvement is correlated with the opening of a community cancer center: comparisons with intramural and extramural benchmarks. J Oncol Pract. 2005;1(3):84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Forrest LM, McMillan DC, McArdle CS, Dunlop DJ. An evaluation of the impact of a multidisciplinary team, in a single centre, on treatment and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;93(9):977-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Martin-Ucar AE, Waller DA, Atkins JL, Swinson D, O’Byrne KJ, Peake MD. The beneficial effects of specialist thoracic surgery on the resection rate for non-small-cell lung cancer. Lung Cancer. 2004;46(2):227-232. [DOI] [PubMed] [Google Scholar]
- 181.Coory M, Gkolia P, Yang IA, Bowman RV, Fong KM. Systematic review of multidisciplinary teams in the management of lung cancer. Lung Cancer. 2008;60(1):14-21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement