Abstract
Population-based studies of atopic dermatitis (AD) in Korean children are lacking. Thus, the purpose of this study was to determine the prevalence, geographic distribution, and risk factors of AD in the Korean pediatric population. We examined AD prevalence using data from the 2008-2011 Korea National Health and Nutrition Examination Survey (KNHANES), which was a cross-sectional survey of 8,947 children up to age 18 throughout the country. Overall, 13.50% of children reported a diagnosis of AD. The age-standardized prevalence ranged from 9.13% to 17.67% between cities and provinces, with the highest prevalence-observed in many of the larger cities at low latitudes, as well as Jeju-do. After adjusting for confounders, high economic status was found to be a significant factor for predicting increased prevalence of AD, with an odds ratio of 1.35 (95% confidence interval of 1.02-1.79, P=0.0034). Urban living (odds ratio 1.24, 95% confidence interval of 1.00-1.53, P=0.0526) was also associated with a higher prevalence of AD. In this first large scale, nationwide study in Korean children, we found that the overall prevalence of AD depends on age, household income, and geographic distribution.
Keywords: Atopic dermatitis, Korean, child, prevalence
INTRODUCTION
Atopic dermatitis (AD) is a chronic relapsing pruritic inflammatory skin disease. AD is characterized by abnormalities in skin barrier function, allergen sensitization, and recurrent skin infections. Since the 1960s the prevalence of AD has increased more than 3-fold, and now affects between 10% and 20% of children in industrialized countries.1,2,3 The prevalence of AD in developing countries is also increasing.4 However, the reason for the increasing prevalence of AD remains unclear. Several reports have suggested that environmental factors are important for expressing and aggravating AD. In addition, small family size, increased income, education, migration from rural to urban environments, and increased use of antibiotics may also be associated with the rise in AD.5,6
While the pathogenesis of AD remains unclear, genetic and environmental factors have been suggested among many potential ones. Large-scale epidemiological studies are needed to identify potential AD-aggravating environmental factors. Many of the existing large-scale studies on AD have been performed in US and European populations, while only a few have specifically evaluated the prevalence of AD in the Korean population. However, these Korean studies used different methodologies compared to international studies and were limited because they utilize data from only certain provinces.7,8,9 Thus, the aim of this study was to determine the prevalence, geographic distribution, and risk factors of AD in Korean children based on data obtained from the Korea National Health and Nutrition Examination Survey (KNHANES), a nationwide study representing both 7 cities and 9 provinces in Korea.
MATERIALS AND METHODS
Data source
This study utilized data from KNHANES 2008-2011. The KNHANES was performed with an annual rolling sampling design including a complex, stratified, multistage probability cluster survey of a representative Korean population sample aged one year and above.10 KNHANES was organized by the Korean Ministry of Health and Welfare and consisted of a cross-sectional survey composed of a health interview survey, a health examination survey, and a nutrition survey. KNHANES was conducted by specially trained interviewers or examiners who were not provided with any prior information about the participants. To evaluate the prevalence of disease based on age standardization, we used data from the 2005 Population and Housing Census, which was performed by Statistics Korea.11 The survey results were weighted to represent the noninstitutionalized population nationally as well as in each province. A detailed description of the plan and operation of the survey is available on the KNHANES website (http://knhanes.cdc.go.kr/). The Institutional Review Board at the Korea Centers for Disease Control and Prevention approved the protocol, and all participants signed informed consent forms. This study was approved by the Institutional Review Board of the Catholic University of Korea (Approved No. KC13RISI0820).
A total of 9,308 (74.30%) out of 12,528 subjects, 10,078 (79.22%) out of 12,722 subjects, 8,473 (77.46%) out of 10,938 subjects, and 8,055 (76.07%) out of 10,589 subjects in 2008, 2009, 2010, and 2011, respectively, participated in KNHANES. In the present analysis, we limited the study population to children aged 1-18 years (8,947: 4,675 boys and 4,272 girls) among participants in KNHANES 2008-2011.
Study variables
We examined the prevalence of AD in the pediatric population-based on the KNHANES survey question, "Have you ever been diagnosed of AD by a physician?" or "Have you been told by a doctor that (your child) had AD?" According to the answer of these questions, we examined AD in two categories, AD group and non-AD group. KNHANES data were interpreted to calculate the national prevalence of AD for all Koreans and for each city or province. The region of residence for each participant was grouped as follows: urban (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, Ulsan, and Gyeonggi-do) and rural (Gangwon-do, Chungcheongbuk-do, Chungcheongnam-do, Jeollabuk-do, Jeollanam-do, Gyeongsangbuk-do, Gyeongsangnam-do, and Jeju-do). Further investigation into the influence of age, sex, geography, economic status, and place of residence were performed because these factors have previously been shown to correlate with the prevalence of AD in US and European populations.12,13 According to KNHANES, monthly income was standardized according to the number of family members (monthly income/number of family members) and was divided into 4 quartile groups: lowest, lower middle, higher middle, and highest. Children were categorized in the low income group if their parents' income belonged to the lowest quartile.
Statistical analysis
Statistical analyses were performed using SAS survey procedure (version 9.2; SAS Institute, Inc., Cary, NC, USA). Demographics were compared between participants with and without AD using Rao-Scott Chi-square tests for categorical variables and t tests for continuous variables. To adjust for stratified, clustered, and systematic data from the KNHANES sampling, all estimates were calculated based on sampling weights for sample area, sample household, and response rate of the subjects. The prevalence of AD was calculated using the PROC SURVEYFREQ procedure to obtain chi-square statistics. We also used PROC SURVEYREG procedure to perform a t test analyzing continuous variables.14 Data are presented as the mean±standard error (SE) and %±SE. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by logistic analysis. AD prevalence was also compared according to age, sex, income level, and place of residence using multiple logistic regression after adjusting for potentially confounding factors related to AD. A P value <0.05 was considered statistically significant.
RESULTS
Overall, 1,285 out of 8,947 children under 18 years old had a diagnosis of AD, which was translated into a 13.50% prevalence of AD in children nationwide. Table 1 shows the demographic characteristics of children between the AD and non-AD groups. The mean age of the patients was significantly younger in the AD group than in the non-AD group; however, there was no difference in mean body weight, height, body mass index (BMI), or waist circumference after adjusting for age. There was a significant difference in income levels (P=0.0120), with the higher income group having a higher prevalence of AD. In addition, the AD prevalence was higher in urban areas than in rural areas (86.60% vs 13.40%) (P=0.0380).
Table 1. Demographic comparison of AD and non-AD groups among Korean children under 18 years old.
| Characteristics | Groups | P value | |
|---|---|---|---|
| AD group | Non-AD group | ||
| Unweighted numbers | 1,285 | 7,662 | |
| Age (year) | 9.45±0.18 | 10.47±0.09 | <0.0001 |
| Sex n (%) | 0.1000 | ||
| Male | 672 (50.32) | 4,003 (53.14) | |
| Female | 613 (49.68) | 3,659 (46.86) | |
| Body weight (kg) | 37.33±0.71 | 40.95±0.34 | <0.0001 |
| Height (cm) | 136.31±0.96 | 141.18±0.48 | <0.0001 |
| BMI (kg/m2) | 18.77±0.14 | 19.28±0.06 | 0.0005 |
| Waist circumference (cm) | 60.71±0.43 | 62.41±0.21 | 0.0003 |
| Income level, n (%) | |||
| Lowest | 107 (10.45) | 779 (12.80) | 0.0120 |
| Lower middle | 308 (25.15) | 2,084 (29.02) | |
| Higher middle | 450 (34.12) | 2,528 (31.58) | |
| Highest | 408 (30.28) | 2,166 (26.60) | |
| Place of residence, n (%) | |||
| Rural | 175 (13.40) | 1,213 (16.24) | 0.0380 |
| Urban | 1,110 (86.60) | 6,449 (83.76) | |
Means±SE or percentages are weighted. P values for continuous variables are from t test, and for categorical variables from Rao-Scott chi-square test. AD, atopic dermatitis; BMI, body mass index; SE, standard error.
Age and household income in AD patients were significantly associated with AD prevalence after applying a multiple logistic regression model (Table 2). Younger age (OR 0.96, 95% CI of 0.95-0.98, P<0.0001) was also significantly associated with a higher prevalence of AD compared to older age. Children from families with a higher household income had a significantly higher prevalence of AD with an OR of 1.35 (95% CI of 1.02-1.79, P=0.0034) compared to those from lower income households. Urban children were more prone to AD than rural children, although the difference was not statistically significant.
Table 2. Subgroup comparison of variables included in the multiple models.
| Odds ratio (95% confidence interval) |
P value | |
|---|---|---|
| Age | 0.96 (0.95-0.98) | <0.0001 |
| Sex: male vs female | 0.90 (0.79-1.03) | 0.1298 |
| Income level | 0.0034 | |
| Lowest | Reference | |
| Lower middle vs lowest | 1.00 (0.75-1.32) | |
| Higher middle vs lowest | 1.25 (0.94-1.65) | |
| Highest vs lowest | 1.35 (1.02-1.79) | |
| Place of residence: urban vs rural | 1.24 (1.00-1.53) | 0.0526 |
P value represents significance of association from weighted logistic regression model in complex sample.
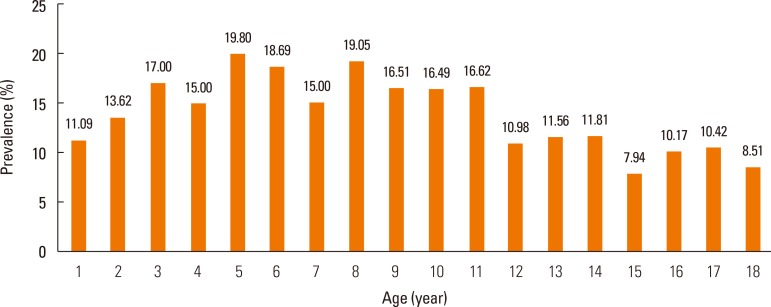
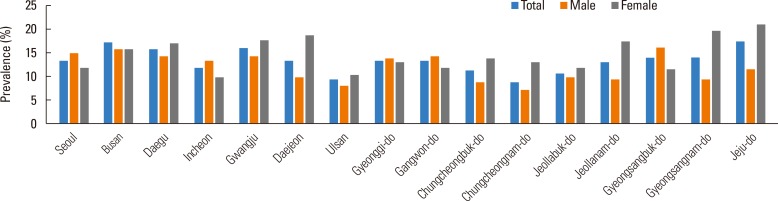
AD prevalence ranged from 7.94% to 19.80% according to age (Fig. 1), and age was a significant determinant of AD prevalence (P<0.0001) (Table. 1). The weighted frequencies (%) of patients at ages 3, 5, 6, and 8 years were 70,161 (17.00%), 93,695 (19.80%), 90,131 (18.69%), and 109,549 (19.05%), respectively. Together, these age groups comprised the highest prevalence of AD. Fig. 2 (Supplement Table 1) shows the AD prevalence estimates with respect to province for Korean children who were previously diagnosed with AD. We calculated that age standardized prevalence was calculated using the 2005 Census Korean population. Many large cities in the southern area of Koreas such as Daegu (16.08%), Gwangju (16.28%), Busan (17.24%), as well as Jeju-do (17.67%), showed a higher prevalence of AD. On the other hand, AD prevalence was lower in middle parts of Korea, notably Chungcheongnam-do (9.13%). Interestingly, province location was significantly associated with disease prevalence in females (P=0.0286) (Supplement Table 1). Specifically, the prevalence of female AD patients ranged from 21.14% (Jeju-do), 19.80% (Gyeongsangnam-do), 17.83% (Gwangju), and 17.50% (Jeollanam-do) (Fig. 2).
Fig. 1. Age-adjusted prevalence of AD in Korean children according to age. Data are from the 2008-2011 KNHANES.
Fig. 2. Age-adjusted prevalence of AD in Korean children according to place of residence. Data are from the 2008-2011 KNHANES.
DISCUSSION
This is the first large-scale population-based study to examine AD prevalence according to geographic distribution among Koreans aged 18 years or younger. We found the prevalence of AD to be 13.50% (7.94%-19.80%) in Korean children who participated in KNHANES 2008-2011. A previous study showed that the lifetime prevalence of AD in school-aged (12- to 15-year-old) Korean children was 7.2% in 1995 and 9.2% in 2000. Another study based on a 2005 survey showed that the lifetime and 1-year prevalence of AD in Korean adults were 10.9% and 8.8%, respectively.
AD usually begins in early infancy; however, the prevalence of AD after 2 years of age was unexpectedly higher than 1 year of age. Specifically, we found that children between 3 and 8 years of age had the highest prevalence of AD. The prevalence of AD over the previous 12 months in Korean children under 12 years of age is 14.4% according to the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire performed in Seoul (2009).7 This can be compared to a 12%-13% prevalence of childhood AD (6-13 years of age) in the Japan mainland.15 Likewise, the prevalence of eczema in US children at 0 to 17 years of age is 10.7%,13 while the point prevalence of flexural eczema is 14.2% in 8- to 12-year-old schoolchildren in Sweden.16
AD was found to be associated with income level, age, and place of residence. Specifically, younger age, urban living, and a high level of income were significantly associated with a higher prevalence of AD. The mean age of the AD group was 9.45, which was significantly younger than that of the non-AD group, which was consistent with previous studies showing a decreasing trend of AD with age.17,18 Our results also showed that there was no difference in the prevalence of AD between females and males, which was different from a previous report showing a female preponderance for AD, with an overall female/male ratio of 1.3:1.0.19 Several previous reports have shown that there is no significant relationship between AD and demographic data, such as gender, BMI, and height.13,20,21,22 Likewise, in our study, BMI, height, weight, and waist circumference were not associated with AD in Korean children after adjusting for age.
As expected, the AD group in the highest quartile of household income was more likely to have AD, compared to the AD group in the lower or lowest quartiles of household income. In addition, the prevalence of AD was higher in the urban living group than in the rural living group. We also assessed potential risk factors for AD by multiple logistic regression analysis. Age and high economic status were significantly associated with a higher prevalence of AD. Thus, risk factors for AD in Koreans were younger age and high household income.
Our study also revealed significant geographic variability in disease prevalence within Korea, with a higher prevalence in the lower latitudes and larger cities, namely, Daegu, Gwangju, Busan, and Jeju-do. Several previous reports suggested that the prevalence of AD in certain countries varies significantly between states and provinces.13,23,24 According to a previous report based in Korea, the prevalence of AD in Jeju-do was 18.6%-30.5% in school-aged children (6-17 years of age).25 Indeed, the prevalence of AD in Jeju-do in 2005 was the highest in the country based on treatment data from the National Health Insurance Corporation.25
We observed a significant correlation between place of residence and prevalence of AD in girls. Although the etiology underlying this difference is unclear, we assume that environmental factors, such as latitude, outdoor temperature, and humidity may play a key role in this observation.26 Indeed, previous reports have shown that AD symptoms correlate positively with latitude and negatively with annular outdoor temperature.27 These data may also suggest that females are susceptible to environmental factors with respect to AD. Our data also revealed a significant geographic variability in disease prevalence in Korea with a higher prevalence in lower latitude cities and province. The reason for this variability is not clear and likely to be multifactorial. One explanation may be the presence of a higher number of large cities in provinces at lower latitudes. In other words, urbanization may play an important role in AD prevalence. Several previous studies of AD have reported a similar increase in disease prevalence in metropolitan/urban areas compared to rural areas.28,29 Potential explanations for this phenomenon include metropolitan-related environmental factors, such as exposure to environmental pollution.4 Furthermore, use of modern fuels, low exposure to microorganisms in soil and vegetation, and an increased hygiene score have been reported to be associated with an increased prevalence of allergic diseases.30,31,32 Another explanation may be the presence of high humidity, which can increase sweating and in turn exacerbate AD symptoms.
There were some limitations in this study that should be considered, especially recall bias and differential access to medical care according to region. The effects of sampling error were excluded because the study data comprised the entire population of Korea. Importantly, our results suggest that analysis of statistics from national surveys may be useful for investigating the prevalence of AD. In conclusion, this is the first nationally representative, population-based study to examine children with AD. In Korean children, AD prevalence appeared to depend on age, household income, and geographic distribution.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
Supplementary Material
Prevalence of AD in Korean children according to place of residence in details
References
- 1.Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251–1258.e23. doi: 10.1016/j.jaci.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Williams H, Robertson C, Stewart A, Aït-Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103:125–138. doi: 10.1016/s0091-6749(99)70536-1. [DOI] [PubMed] [Google Scholar]
- 3.Williams HC. Is the prevalence of atopic dermatitis increasing? Clin Exp Dermatol. 1992;17:385–391. doi: 10.1111/j.1365-2230.1992.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 4.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 5.Eichenfield LF, Hanifin JM, Beck LA, Lemanske RF, Jr, Sampson HA, Weiss ST, et al. Atopic dermatitis and asthma: parallels in the evolution of treatment. Pediatrics. 2003;111:608–616. doi: 10.1542/peds.111.3.608. [DOI] [PubMed] [Google Scholar]
- 6.von Mutius E. Gene-environment interactions in asthma. J Allergy Clin Immunol. 2009;123:3–11. doi: 10.1016/j.jaci.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 7.Baek JO, Hong S, Son DK, Lee JR, Roh JY, Kwon HJ. Analysis of the prevalence of and risk factors for atopic dermatitis using an ISAAC questionnaire in 8,750 Korean children. Int Arch Allergy Immunol. 2013;162:79–85. doi: 10.1159/000351403. [DOI] [PubMed] [Google Scholar]
- 8.Yu JS, Lee CJ, Lee HS, Kim J, Han Y, Ahn K, et al. Prevalence of atopic dermatitis in Korea: analysis by using national statistics. J Korean Med Sci. 2012;27:681–685. doi: 10.3346/jkms.2012.27.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim CW, Park CJ, Kim JW, Koo DW, Kim KW, Kim TY. Prevalence of atopic dermatitis in Korea. Acta Derm Venereol. 2000;80:353–356. doi: 10.1080/000155500459295. [DOI] [PubMed] [Google Scholar]
- 10.Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35:561–566. doi: 10.4093/dmj.2011.35.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee SY, Park SW, Kim DJ, Woo J. Gender disparity in the secular trends for obesity prevalence in Korea: analyses based on the KNHANES 1998-2009. Korean J Intern Med. 2013;28:29–34. doi: 10.3904/kjim.2013.28.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320–322. doi: 10.1038/jid.2008.252. [DOI] [PubMed] [Google Scholar]
- 13.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. J Invest Dermatol. 2011;131:67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park HJ, Lee JH, Park KH, Ann HW, Jin MN, Choi SY, et al. A nationwide survey of inhalant allergens sensitization and levels of indoor major allergens in Korea. Allergy Asthma Immunol Res. 2014;6:222–227. doi: 10.4168/aair.2014.6.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furue M, Chiba T, Takeuchi S. Current status of atopic dermatitis in Japan. Asia Pac Allergy. 2011;1:64–72. doi: 10.5415/apallergy.2011.1.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flohr C, Weiland SK, Weinmayr G, Bjorksten B, Braback L, Brunekreef B, et al. The role of atopic sensitization in flexural eczema: findings from the International Study of Asthma and Allergies in Childhood Phase Two. J Allergy Clin Immunol. 2008;121:141–147.e4. doi: 10.1016/j.jaci.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 17.Sugiura H, Umemoto N, Deguchi H, Murata Y, Tanaka K, Sawai T, et al. Prevalence of childhood and adolescent atopic dermatitis in a Japanese population: comparison with the disease frequency examined 20 years ago. Acta Derm Venereol. 1998;78:293–294. doi: 10.1080/000155598441891. [DOI] [PubMed] [Google Scholar]
- 18.Guiote-Domínguez MV, Muñoz-Hoyos A, Gutiérrez-Salmerón MT. Prevalence of atopic dermatitis in schoolchildren in Granada, Spain. Actas Dermosifiliogr. 2008;99:628–638. [PubMed] [Google Scholar]
- 19.Tay YK, Kong KH, Khoo L, Goh CL, Giam YC. The prevalence and descriptive epidemiology of atopic dermatitis in Singapore school children. Br J Dermatol. 2002;146:101–106. doi: 10.1046/j.1365-2133.2002.04566.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Gysel D, Govaere E, Verhamme K, Doli E, De Baets F. Body mass index in Belgian schoolchildren and its relationship with sensitization and allergic symptoms. Pediatr Allergy Immunol. 2009;20:246–253. doi: 10.1111/j.1399-3038.2008.00774.x. [DOI] [PubMed] [Google Scholar]
- 21.Leung TF, Kong AP, Chan IH, Choi KC, Ho CS, Chan MH, et al. Association between obesity and atopy in Chinese schoolchildren. Int Arch Allergy Immunol. 2009;149:133–140. doi: 10.1159/000189196. [DOI] [PubMed] [Google Scholar]
- 22.Thomas MW, Panter AT, Morrell DS. Corticosteroids' effect on the height of atopic dermatitis patients: a controlled questionnaire study. Pediatr Dermatol. 2009;26:524–528. doi: 10.1111/j.1525-1470.2009.00865.x. [DOI] [PubMed] [Google Scholar]
- 23.Agata H, Kondo N, Fukutomi O, Hayashi T, Shinoda S, Nishida T, et al. Comparison of allergic diseases and specific IgE antibodies in different parts of Japan. Ann Allergy. 1994;72:447–451. [PubMed] [Google Scholar]
- 24.Pöysä L, Korppi M, Pietikinen M, Remes K, Juntunen-Backman K. Asthma, allergic rhinitis and atopic eczema in Finnish children and adolescents. Allergy. 1991;46:161–165. doi: 10.1111/j.1398-9995.1991.tb00564.x. [DOI] [PubMed] [Google Scholar]
- 25.Bae JM, Shin KS. Estimating the prevalence of atopic dermatitis in school students of jejudo, Korea. J Prev Med Public Health. 2009;42:171–176. doi: 10.3961/jpmph.2009.42.3.171. [DOI] [PubMed] [Google Scholar]
- 26.Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy. 2014;69:3–16. doi: 10.1111/all.12270. [DOI] [PubMed] [Google Scholar]
- 27.Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752–1759. doi: 10.1038/jid.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649–655. doi: 10.1067/mjd.2000.107773. [DOI] [PubMed] [Google Scholar]
- 29.Mercer MJ, Joubert G, Ehrlich RI, Nelson H, Poyser MA, Puterman A, et al. Socioeconomic status and prevalence of allergic rhinitis and atopic eczema symptoms in young adolescents. Pediatr Allergy Immunol. 2004;15:234–241. doi: 10.1111/j.1399-3038.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 30.Venn AJ, Yemaneberhan H, Bekele Z, Lewis SA, Parry E, Britton J. Increased risk of allergy associated with the use of kerosene fuel in the home. Am J Respir Crit Care Med. 2001;164:1660–1664. doi: 10.1164/ajrccm.164.9.2103101. [DOI] [PubMed] [Google Scholar]
- 31.von Hertzen L, Haahtela T. Disconnection of man and the soil: reason for the asthma and atopy epidemic? J Allergy Clin Immunol. 2006;117:334–344. doi: 10.1016/j.jaci.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Sherriff A, Golding J, Team AS Alspac Study Team. Hygiene levels in a contemporary population cohort are associated with wheezing and atopic eczema in preschool infants. Arch Dis Child. 2002;87:26–29. doi: 10.1136/adc.87.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prevalence of AD in Korean children according to place of residence in details