Abstract
Welding generates complex metal aerosols, inhalation of which is linked to adverse health effects among welders. An important health concern of welding fume (WF) exposure is neurological dysfunction akin to Parkinson’s disease (PD). Some applications in manufacturing industry employ a variant welding technology known as “weld-bonding” that utilizes resistance spot welding, in combination with adhesives, for metal-to-metal welding. The presence of adhesives raises additional concerns about worker exposure to potentially toxic components like Methyl Methacrylate, Bisphenol A and volatile organic compounds (VOCs). Here, we investigated the potential neurotoxicological effects of exposure to welding aerosols generated during weld-bonding. Male Sprague–Dawley rats were exposed (25 mg/m3 targeted concentration; 4 h/day × 13 days) by whole-body inhalation to filtered air or aerosols generated by either weld-bonding with sparking (high metal, low VOCs; HM) or without sparking (low metal; high VOCs; LM). Fumes generated under these conditions exhibited complex aerosols that contained both metal oxide particulates and VOCs. LM aerosols contained a greater fraction of VOCs than HM, which comprised largely metal particulates of ultrafine morphology. Short-term exposure to LM aerosols caused distinct changes in the levels of the neurotransmitters, dopamine (DA) and serotonin (5-HT), in various brain areas examined. LM aerosols also specifically decreased the mRNA expression of the olfactory marker protein (Omp) and tyrosine hydroxylase (Th) in the olfactory bulb. Consistent with the decrease in Th, LM also reduced the expression of dopamine transporter (Slc6a3; Dat), as well as, dopamine D2 receptor (Drd2) in the olfactory bulb. In contrast, HM aerosols induced the expression of Th and dopamine D5 receptor (Drd5) mRNAs, elicited neuroinflammation and blood–brain barrier-related changes in the olfactory bulb, but did not alter the expression of Omp. Our findings divulge the differential effects of LM and HM aerosols in the brain and suggest that exposure to weld-bonding aerosols can potentially elicit neurotoxicity following a short-term exposure. However, further investigations are warranted to determine if the aerosols generated by weld-bonding can contribute to persistent long-term neurological deficits and/or neurodegeneration.
Keywords: Aerosols, manganese, neurotoxicity, occupational exposure, Parkinson’s disease, volatile organic compounds, welding, welding fume
Introduction
Weld-bonding is a hybrid welding process that combines the advantages of resistance spot welding and adhesive bonding to enhance weld quality and joint performance, while welding metal-to-metal components or structures. In resistance welding, the weld is made by a combination of heat, pressure and time (Miller Electric, 2012). The weld is accomplished when current is initiated to flow through the electrode tips on to a sandwich of two metal sheets to form a joint. The resistance of the base metal to the electrical current flow causes localized heating in the joint and the weld is made. The pressure exerted by the tongs and electrode tips, through which the current flows, holds the parts to be welded together. Resistance spot welds are unique in that the actual weld nugget is formed on the internal surface of the base metal. The time or duration of current flow in the joint is determined by the nature of the material (metal type, thickness) to be welded, the extent of current flow, as well as, the cross-sectional surface area that the welding tip is in contact with.
A major problem with resistance spot welding is material stress, fatigue and corrosion particularly at the periphery of spot welds and joints. By applying an adhesive to bond and seal the spot weld joints, these deficiencies can be significantly improved. Such weld-bonded joints exhibit superior tearing strength and form reliable joints as compared to bonding with adhesive alone (Chang et al., 1999). Generally, weld-bonding is used in fabricating sheet metal products from light gauge metals up to 3 mm in thickness that is sandwiched with a layer of an adhesive. Due to the excellent mechanical properties of weld-bonded joints, and the ability to perform high speed, repetitive welds on relatively thin sheets of metal, weld bonding gained popularity in the automotive, aviation and space-flight and appliance industries.
A Health Hazard Evaluation (HHE) conducted by the National Institute for Occupational Safety and Health (NIOSH), at an automotive assembly plant where robotic resistance spot welding was predominantly performed, reported respiratory health issues among some workers (Kanwal & Boylstein, 2006). Air sampling measurements at various locations in the plant and from the headspace of the adhesive bulk samples revealed the presence of several chemicals in the air. Additional chemicals were detected in the headspace after heating the adhesive bulk samples, indicating increased volatilization of the chemicals or formation of newer chemicals due to the heating of the samples. Some of the chemicals detected included Methyl Methacrylate, Acetic acid, Phthalic anhydride, Formaldehyde, Styrene, Benzene and Toluene that can potentially cause adverse health effects (Kanwal & Boylstein, 2006). While the HHE and a few other studies (Hammond et al., 2005; Loukzadeh et al., 2009; Luo et al., 2006) have reported respiratory irritation, asthma, bronchitis and sinusitis among workers exposed to aerosols generated during resistance spot welding using adhesives, no information on the potential neurological effects exists.
There is a growing concern that occupational exposure to welding fumes may be associated with the development of neurological and psychological/psychiatric disturbances that have Parkinson’s disease (PD)-like manifestations (Bowler et al., 2006, 2007a,b; Josephs et al., 2005). Neuroepidemiological findings, albeit limited, also suggest that a potential relationship between welding and Parkinsonism (Racette et al., 2001, 2005). Occupational exposure to manganese, a component of WF, has been linked to neurotoxicity (Donaldson, 1987; Mergler & Baldwin, 1997), deficits in the ability to perform rapid hand movements, loss of coordination and balance and symptoms such as forgetfulness, anxiety or insomnia (Bowler et al., 2007a,b). Manganese in occupational settings such as mining and dry battery industries has been shown to cause a Parkinsonian syndrome referred to as manganism (Emara et al., 1971; Rodier, 1955). Cumulative manganese exposure in workers at foundries and ferroalloy industries has been shown to be associated with preclinical signs of motor and cognitive function (Lucchini et al., 1999; Wennberg et al., 1991). High prevalence of Parkinsonian disorders has also been reported in populations living in the vicinities of ferroalloy industries, which has been linked to high manganese dust levels (Lucchini et al., 2007). Environmental manganese pollution from fuel additives has also been linked to increased risk of PD-like disorders (Finkelstein & Jerrett, 2007).
Given the complex nature of the aerosols generated during weld-bonding, which are comprised of ultrafine metal oxide particulates and VOCs, and the fact that inhaled ultrafine particles and/or VOCs can translocate or permeate across the olfactory pathway to the brain, raises concerns about the potential neurological effects following exposure to such aerosols. In light of this, it is critical to evaluate the neurotoxicological effects of exposure to weld-bond aerosols.
Materials and methods
Resistance spot welding and inhalation exposure system
A novel resistance spot welder and inhalation exposure system was designed and constructed by NIOSH (Afshari et al., 2014). For resistance spot welding, two rolls of low alloy, carbon steel sheet metal strips [thickness: 1/40″ (0.64 mm), 24 gauge; width: 1 inch] were directed by a set of rollers to copper-tipped electrodes of the welder. An adhesive (Terokal® 2300; Henkel Surface Technologies, Madison Heights, MI) was injected between the two strips of metal and the sandwich was spot-welded at equidistant sites (3/4″; 20 mm) by an automated, computer-controlled resistance spot welding gun (C-type chassis Trans-gun 136 kVA-AC; Milco Manufacturing, Warren, MI). The number of welds made per interval of time was dependent on the desired aerosol concentration within the animal exposure chamber. The spot welder was set at 7.5–10 kA with a welding time of 140 ms, a post-weld holding time of 50 ms, and a clamping force of 2.6 KiloNewton (kN; equivalent to about 600 pounds force).
Aerosols were generated either by weld-bonding with sparking (high metal, low VOCs; HM) or without sparking (low metal; high VOCs; LM). In brief, by varying weld current and lag-time between weld, aerosols with different characteristics were generated (Table 1). Aerosols generated at a weld current of 7.5–9.0 kA or 10.5 kA produced good quality spot welds. At a weld current of 7.5–9.0 kA, there was no sparking or expulsion of molten metal, which produced aerosols with low metal content (LM). At a weld current of 10.5 kA, sparking and expulsion of molten metal was observed, which produced aerosols with higher metal content (high metal, HM).
Table 1.
Parameters for low metal and high metal resistance spot-welding.
| Low metal (LM) | High metal (HM) | |
|---|---|---|
| Weld process | Resistance spot welding | Resistance spot welding |
| Fabrication metal | Low alloy carbon steel strip (24 gauge; 1/40″ thick; 1″ width) | Low alloy carbon steel strip (24 gauge; 1/40″ thick; 1″ width) |
| Weld current | 7.5–9.0 kA | 10.5 kA |
| Weld time | 140 ms | 140 ms |
| Post-weld hold time | 50 ms | 50 ms |
| Lag-time between weld | 5 s | 20 s |
| Spot-weld distance | 3/4″ apart | 3/4″ apart |
| Bonding adhesive | Terokal® 2300 | Terokal® 2300 |
By varying weld current and lag-time between weld, aerosols with different characteristics were generated. Aerosols generated at a weld current of 7.5–9.0 kA or 10.5 kA produced good quality spot welds. At a weld current of 7.5–9.0 kA, there was no sparking or expulsion of molten metal, which produced aerosols with low metal content (LM). At a weld current of 10.5 kA, sparking and expulsion of molten metal was observed, which produced aerosols with higher metal content (high metal, HM).
To achieve a chamber aerosol concentration of 25 mg/m3, the average lag-time between welds was 5 s when operating in the no spark mode (weld current of 7.5–9.0 kA), whereas an average lag-time of 20 s was needed to generate the same chamber concentration of 25 mg/m3 when operating at the spark mode (weld current of 10.5 kA).
To achieve a chamber aerosol concentration of 25 mg/m3, the average lag-time between welds was 5 s when operating in the no spark mode (weld current of 7.5–9.0 kA), whereas an average lag-time of 20 s was needed to generate the same chamber concentration of 25 mg/m3 when operating at the spark mode (weld current of 10.5 kA).
Characterization of the fume samples (Table 2) from the two welding methods revealed that the size distribution of the generated particulates was multi-modal. A majority of particulates were chain-like agglomerates of ultrafine primary particles. The submicron mode of agglomerated particles accounted for a greater portion of particles in terms of particle number. Metal expulsion during spot welding caused the formation of larger, more spherical particles (spatter). These spatter particles appeared in the micron size mode and accounted for the greatest amount of particles in terms of mass.
Table 2.
Characteristics of welding fume particulates generated by low and high metal resistance spot-welding.
| Low metal (LM) | High metal (HM) | |
|---|---|---|
| Chamber aerosol concentration | 25 mg/m3 | 25 mg/m3 |
| Chamber metal concentration | 6.44 mg/m3 | 24.5 mg/m3 |
| Total metals/filter | 184 μg | 699 μg |
| Metal:VOC content (%) | ~25% Metals; 75% VOCs | ~98% Metals; 2% VOCs |
| Particle morphology | ||
| Micron sized mode | Spherical particles | Spherical particles |
| Submicron sized mode | Chain-like aggregates | Chain-like aggregates |
| Nanosized/ultrafine mode | Chain-like aggregates | Chain-like aggregates |
| Particle size, MMAD | ||
| Micron sized mode | 1.66 μm (67% of particle mass) | 3.04 μm (61% of particle mass) |
| Submicron sized mode | 0.30 μm (33% of particle mass) | 0.25 μm (36% of particle mass) |
| Nano sized/ultrafine mode | 0.01–0.05 μm (~1% of particle mass) | 0.01–0.1 μm (~3% of particle mass) |
| Particle diameter | 0.18–1.8 μm (less particles) | 0.18–1.8 μm (more particles) |
| Major Elements (Wt%) | ||
| Fe | 99.0% | 99.2% |
| Mn | 0.534% | 0.539% |
| Cu | 0.431% | 0.280% |
| Volatile organic compounds (VOCs), Major peaks | Benzene | Benzene |
| Toluene | Toluene | |
| Isopropanol | Isopropanol | |
| 2-butoxyethanol | 2-butoxyethanol | |
| Volatile organic compounds (VOCs), Minor peaks | Butanol | Butanol |
| Ethylene glycol | Ethylene glycol | |
Particle morphology was determined from SEM photomicrographs of aerosols collected on 47 mm Nuclepore polycarbonate filters. The samples were examined under a Hitachi S4800 field emission scanning electron microscope (FESEM) and a Bruker X-ray system.
Particle size distribution was determined in the exposure chamber by using Micro-Orifice Uniform Deposit Impactor (MOUDI) and Nano-MOUDI. Three modes of particle sizes were measured when spot welding parameters were to set to generate aerosols that contained either low (no sparking) or high (sparking) metal particulates. The mass median aerodynamic diameter (MMAD) was determined for each particle size mode.
Spot welding aerosols were collected inside the animal exposure chamber onto 5 μm polyvinyl chloride membrane filters in 37 mm cassette. The particle samples were digested and the metals analyzed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) by Bureau Veritas North America, Inc. according to NIOSH method 7303 modified for hot block/HCl/HNO3 digestion.
During the spot-welding process, gas samples were collected in thermal desorption tubes for qualitative analysis of volatile organic compounds (VOCs) by thermal desorption gas chromatography/mass spectrometry (TD-GC-MS).
The aerosols generated were delivered to a custom-built stainless steel (22″ × 22″ × 20″) animal exposure chamber that was designed for holding laboratory rodents and homogenously exposing them to the spot welding aerosol (Afshari et al., 2014). The temperature and relative humidity (Vaisala Temperature-Humidity Probe, model# HMP233; Woburn, MA) inside the exposure chamber were measured and continuously recorded. The mass concentration of the aerosols in the chamber was monitored in real time with a DataRAM (DataRAM, MIE, Inc. DR-2000, Bedford, MA) that provided feedback to the computer software to control and regulate the aerosol concentration in the exposure chamber.
Control animals were housed in an identical chamber located adjacent to the spot welding exposure chamber and received filtered air. Airflow into the chamber was manually set with a Rotameter to 25 L/min. The temperature and relative humidity of the chamber were continuously monitored.
Animal exposures
Male Sprague–Dawley [Hla:(SD) CVF] rats (250–300 g) were procured from Hilltop Lab Animals (Scottdale, PA). The rats were acclimated for at least 6 days after arrival and were housed in ventilated polycarbonate cages with Alpha-Dri cellulose chips as bedding, with provision for HEPA-filtered air, irradiated Teklad 2918 diet and water ad libitum. The NIOSH animal facility is pathogen-free, environmentally controlled and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). All animal procedures used during the study have been reviewed and approved by the institution’s Animal Care and Use Committee.
Rats were exposed by whole-body inhalation to aerosols (25 mg/m3 targeted concentration; 4 h/day × 13 days) generated by weld-bonding with sparking (high metal, low VOCs; HM) or without sparking (low metal; high VOCs; LM). The achieved concentrations were as follows: HM aerosols (21.8±2.2 mg/m3; 4 h/day × 13 days), LM aerosols (26.9±4.7 mg/m3; 4 h/day × 13 days). During exposures, food and water were withheld from the animals. Body weight was monitored before and after each exposure. No significant changes in body weight were observed. No animal exhibited any outward signs of labored breathing or respiratory distress. Respiratory rate was not significantly different from air-exposed controls (data not shown).
Animals were euthanized 1 day after the last exposure. Euthanasia was performed by administration of an intraperitoneal injection of sodium pentobarbital (Sleepaway®;>100 mg/kg body weight, Fort Dodge Animal Health, Division of Pfizer, Fort Dodge, IA), and the animals were exsanguinated prior to collection of tissues. Immediately after euthanasia, the brains were excised and discrete brain regions, olfactory bulb (OB), striatum (STR), mid-brain (MB), frontal cortex (FCT), hippocampus (HIP) and hypothalamus (HYP) from the left and right hemispheres were dissected free-hand. Tissues from right hemisphere were frozen at −75 ºC for High Performance Liquid Chromatography (HPLC) analysis of neurotransmitters. Tissues from the left hemisphere were stored in RNALater® for isolation of ribonucleic acids (RNA) for real-time Polymerase Chain Reaction (PCR) studies.
High performance liquid chromatography with electrochemical detection
Biogenic amines (norepinephrine, dopamine, and serotonin) and some of their metabolites (DOPAC, HVA and 5-HIAA) were measured by HPLC-EC. Brain tissues were homogenized in 0.1M perchloric acid containing isoproterenol (as internal standard) and centrifuged for 10 min at 12 000×g. The supernatant was filtered through a 0.2 μm filter and 10 μl aliquots were injected onto a C-18 reverse phase HPLC column (Agilent Technologies, Santa Clara, CA) using an Ultrafast Liquid Chromatography (UFLC) system (Shimadzu Instruments, Columbia, MD) attached to an autosampler. A BAS-LC4B amperometric detector (BASi Inc, West Lafayette, IN) with a glassy carbon oxidative flow cell was used for detection at an electrode potential of 0.8V. The mobile phase consisted of 0.15M monochloroacetic acid, 0.115M sodium hydroxide, 0.015% sodium octyl sulfate, 0.1mM EDTA, 1.5% methanol and 3% acetonitrile at a pH of 3.2. Under these conditions, norepinephrine, epinephrine, dopamine and its metabolites (DOPAC and HVA), and serotonin and its metabolite (5-HIAA) were chromatographically separated and detected simultaneously. Analytes were quantified by comparing peak area detector responses in the sample with those produced by a series of standards similarly prepared in 0.1M perchloric acid. The pellet from each of the processed sample was solubilized with 1N NaOH and the protein content was estimated. The neurotransmitter content in each sample was then normalized to the amount of protein. The values were calculated as ng/mg total protein and are graphically represented as percent of air-exposed controls.
RNA isolation, cDNA synthesis and real-time PCR
The brain tissues were homogenized in Tri Reagent® (Molecular Research Center, Inc., Cincinnati, OH) and the aqueous phase separated with MaXtract High Density gel (Qiagen, Valencia, CA). Total RNA from the aqueous phase was then isolated using RNeasy mini spin columns (Qiagen, Valencia, CA) and concentrations were determined with a NanoDrop® ND-1000 UV–Vis Spectrophotometer (NanoDrop Technologies, Wilmington, DE). The isolated RNA was stored at −75 ºC until use.
First strand cDNA synthesis was carried out using total RNA (1 μg), random hexamers and MultiScribe™ reverse transcriptase (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems, Foster City, CA) in a 20 μl reaction. Real-time PCR amplification was performed using the 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) in combination with TaqMan® chemistry. Specific primers and FAM™ dye-labeled TaqMan® MGB probe sets (TaqMan® Gene Expression Assays) were procured from Applied Biosystems (Foster City, CA) and used according to the manufacturer’s recommendation. The list of Gene Expression Assays (assay IDs in parenthesis) used are as follows: Ifng (Rn00594078_m1); Tnfa (Rn99999017_m1); Nos2 (Rn00561646_m1); Cldn1 (Rn00581740_m1); Cldn3 (Rn00581751_s1); Cldn5 (Rn01753146_s1); Edn2 (Rn00 561135_m1); Omp (Rn00561696_s1); Th (Rn00562 500_m1); Gfap (Rn00566603_m1); Drd1a (Drd1, Rn03062 203_s1); Drd2 (Rn01418275_m1); Drd3 (Rn00567568_m1); Drd5 (Rn01640412_m1); Slc6a3 (Rn00562224_m1); Slc18a2 (Rn00564688_m1); Cacna1d (Rn00568820_m1). All PCR amplifications (40 cycles) were performed in a total volume of 25 μl, containing 1 μl cDNA, 1.25 μl of the specific TaqMan® Gene Expression Assay and 12.5 μl of TaqMan® Gene Expression Master mix (Applied Biosystems, Foster City, CA), respectively. Sequence detection software, version 1.7 (Applied Biosystems, Foster City, CA) results were exported as tab-delimited text files and imported into Microsoft Excel for further analysis. Following normalization to beta actin (Actb), relative quantification of gene expression was performed using the comparative threshold (CT) method as described by the manufacturer (Applied Biosystems, Foster City, CA; User Bulletin 2). The values are expressed as fold difference from air-exposed controls.
Statistical analysis
The samples generated were from separate experiments that were run for low and high metal exposures. Each experiment had separate control (filtered Air) groups. For statistical analysis, all the control and exposed groups from the two welding methods were analyzed together for each independent variable. Data were analyzed by one-way ANOVA followed by Student’s–Newman–Keuls (SNK) multiple-comparison test, using SigmaStat 3.1 statistical software (Systat Software Inc., San Jose, CA). Where data failed equal variance or normality tests, they were analyzed by one-way ANOVA on Ranks followed by SNK multiple-comparison tests. Results were considered significant at p<0.05. The values are expressed as percent of air-exposed controls and graphical representations are Mean±SE. The sample sizes (n) for the low metal exposure experiments were 4/group and that for the high metal exposure experiments were 6/group. For RT-PCR assays, four samples from each group of the two experiments (low metal and high metal exposures) were analyzed. The data are graphed together for comparison.
Results
Exposure to LM aerosols generated by weld-bonding altered the levels of dopamine and norepinephrine in discrete brain areas
Neurotransmitters play an important role in neuronal communication and maintenance of brain function. Abnormality or dysregulation of the physiological levels of neurotransmitters can potentially result in neuronal dysfunction and pathogenesis. As VOCs and metals have been shown to interact with and/or affect various neurotransmitter systems, such as dopaminergic, serotonergic, cholinergic and GABAergic (Kiriu et al., 1990; Long, 2006), we investigated if exposure to aerosols generated during weld-bonding affected the biogenic amine neurotransmitters in the brain.
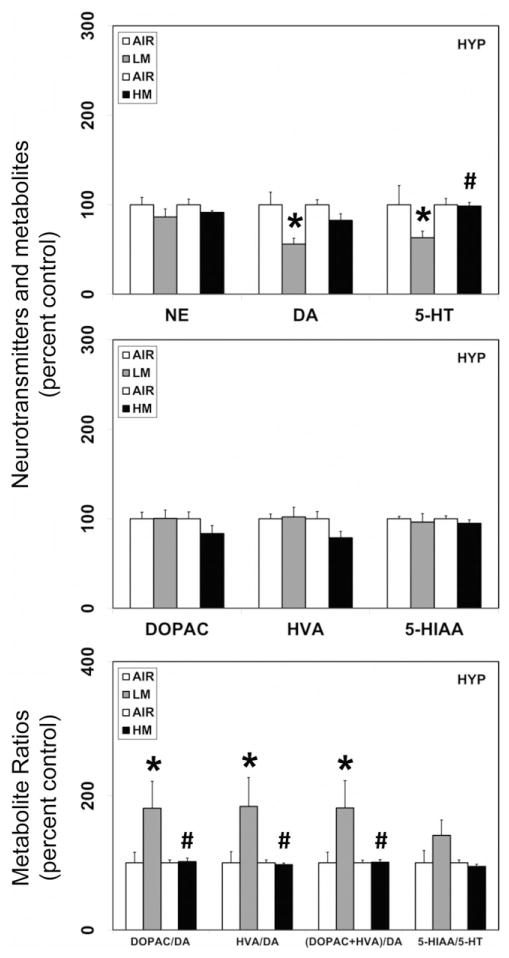
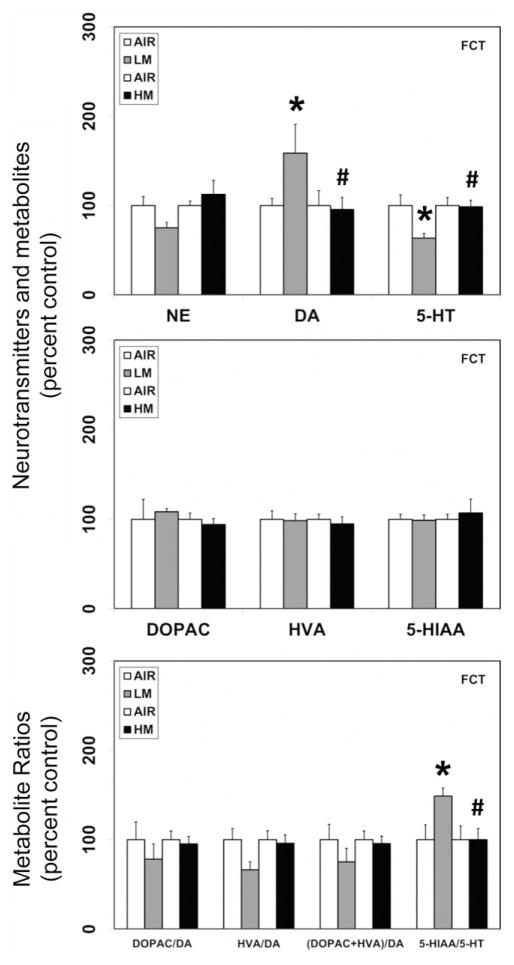
In rats exposed to LM aerosols for 13 days, significant reductions in the levels of dopamine were observed in the OB (18% decrease from air-exposed controls, p<0.05; Figure 1), MB (33.6% decrease from air-exposed controls, p<0.05; Figure 2) and HYP (44% decrease from air-exposed controls, p<0.05; Figure 3). However, dopamine levels increased in the FCT (58.5% increase over air-exposed controls, p<0.05; Figure 4) and HIP (72% increase over air-exposed controls, p<0.05; Figure 5). Dopamine levels remained unaltered in the STR following LM exposure (data not shown). The metabolite of dopamine, DOPAC, decreased following LM exposure only in the OB (41.7% decrease from air-exposed controls, p<0.05; Figure 1). The dopamine metabolite, HVA was unaffected by exposure to LM. The dopamine-metabolite ratios, DOPAC/DA, HVA/DA and (DOPAC+HVA)/DA increased (82–84% increase over air-exposed controls; p<0.05) in the HYP following LM exposure (Figure 3). At the same time, LM aerosols caused a decrease in HVA/DA ratio in the FCT (33.7% decrease from air-exposed controls; p<0.05; Figure 4). The ratios of DOPAC/DA, HVA/DA and (DOPAC+HVA)/DA also decreased (36–48% decrease from air-exposed controls; p<0.05; Figure 5) in the HIP after LM exposure. Exposure to LM aerosols, also decreased norepinephrine (24.8% decrease from air-exposed controls, p<0.05; Figure 4) levels in the FCT, but not other brain areas.
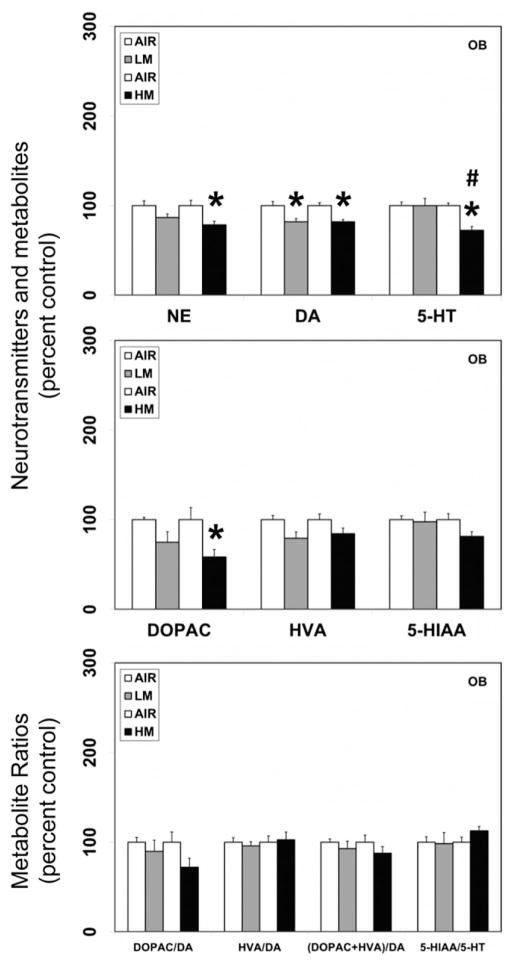
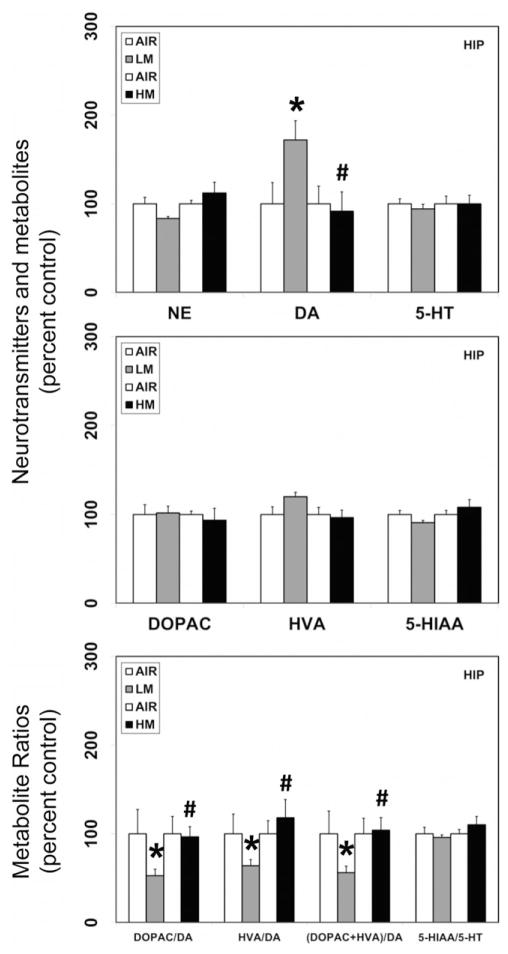
Figure 1.
Resistance spot welding aerosols alter the levels of neurotransmitters and their metabolites in the olfactory bulb (OB). Rats were exposed to LM or HM aerosols (25 mg/m3 × 4 h/day) by whole-body inhalation for 13 days. At 1 day post-exposure, the levels of norepinephrine (NE), dopamine (DA), serotonin (5-HT), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA) and 5-hydroxyindoleacetic acid (5-HIAA) were measured by HPLC-EC. Following normalization to an internal standard (isoproterenol), the concentration of each compound was calculated as ng/mg total protein. The metabolite ratios, DOPAC/DA, HVA/DA (DOPAC+HVA/DA) and 5-HIAA/DA were then calculated from the individual measures. The values are expressed as percent of air-exposed controls and graphical representations are mean±SE (n=4/group) for the low metal exposure experiments and (n=6/group) for the high metal exposure experiments. *Indicates significant change from corresponding air-exposed control (p<0.05). #Indicates significantly different from LM-exposed group.
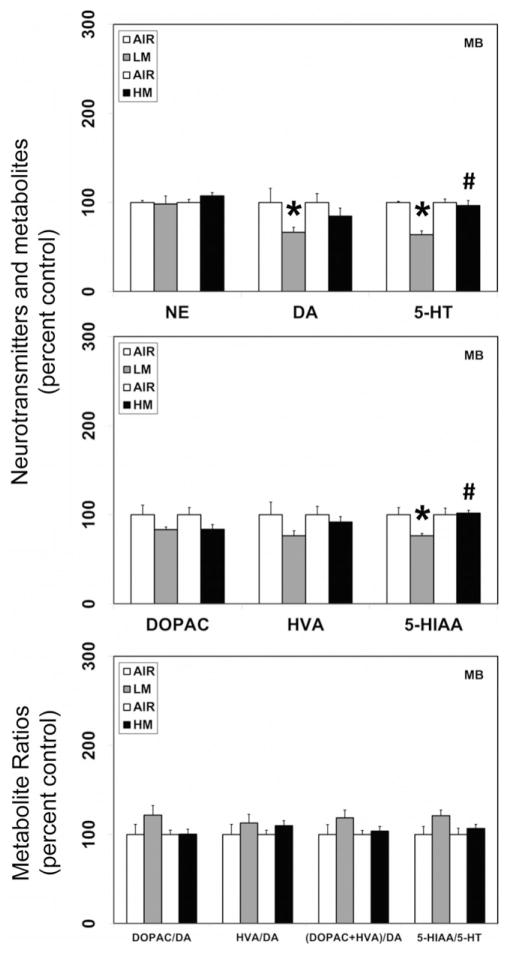
Figure 2.
Resistance spot welding aerosols alter the levels of neurotransmitters and their metabolites in the midbrain (MB). Rats were exposed to LM or HM aerosols (25 mg/m3 × 4 h/day) by whole-body inhalation for 13 days. At 1 day post-exposure, the levels of NE, DA, 5-HT, DOPAC, HVA and 5-HIAA were measured by HPLC-EC. Following normalization to an internal standard (isoproterenol), the concentration of each compound was calculated as ng/mg total protein. The metabolite ratios, DOPAC/DA, HVA/DA, (DOPAC+HVA/DA) and 5-HIAA/DA were then calculated from the individual measures. The values are expressed as percent of air-exposed controls and graphical representations are mean±SE (n=4/group) for the low metal exposure experiments and (n=6/group) for the high metal exposure experiments. *Indicates significant change from corresponding air-exposed control (p<0.05). #Indicates significantly different from LM-exposed group.
Figure 3.
Resistance spot welding aerosols alter the levels of neurotransmitters and their metabolites in the hypothalamus (HYP). Rats were exposed to LM or HM aerosols (25 mg/m3 × 4 h/day) by whole-body inhalation for 13 days. At 1 day post-exposure, the levels of NE, DA, 5-HT, DOPAC, HVA and 5-HIAA were measured by HPLC-EC. Following normalization to an internal standard (isoproterenol), the concentration of each compound was calculated as ng/mg total protein. The metabolite ratios, DOPAC/DA, HVA/DA, (DOPAC+HVA/DA) and 5-HIAA/DA were then calculated from the individual measures. The values are expressed as percent of air-exposed controls and graphical representations are Mean±SE (n=4/group) for the low metal exposure experiments and (n=6/group) for the high metal exposure experiments. *Indicates significant change from corresponding air-exposed control (p<0.05). #Indicates significantly different from LM-exposed group.
Figure 4.
Resistance spot welding aerosols alter the levels of neurotransmitters and their metabolites in the frontal cortex (FCT). Rats were exposed to LM or HM aerosols (25 mg/m3 × 4 h/day) by whole-body inhalation for 13 days. At 1 day post-exposure, the levels of NE, DA, 5-HT, DOPAC, HVA and 5-HIAA were measured by HPLC-EC. Following normalization to an internal standard (isoproterenol), the concentration of each compound was calculated as ng/mg total protein. The metabolite ratios, DOPAC/DA, HVA/DA, (DOPAC+HVA/DA) and 5-HIAA/DA were then calculated from the individual measures. The values are expressed as percent of air-exposed controls and graphical representations are Mean±SE (n=4/group) for the low metal exposure experiments and (n=6/group) for the high metal exposure experiments. *Indicates significant change from corresponding air-exposed control (p<0.05). #Indicates significantly different from LM-exposed group.
Figure 5.
Resistance spot welding aerosols alter the levels of neurotransmitters and their metabolites in the hippocampus (HIP). Rats were exposed to LM or HM aerosols (25 mg/m3 × 4 h/day) by whole-body inhalation for 13 days. At 1-day post-exposure, the levels of NE, DA, 5-HT, DOPAC, HVA and 5-HIAA were measured by HPLC-EC. Following normalization to an internal standard (isoproterenol), the concentration of each compound was calculated as ng/mg total protein. The metabolite ratios, DOPAC/DA, HVA/DA, (DOPAC+HVA/DA) and 5-HIAA/DA were then calculated from the individual measures. The values are expressed as percent of air-exposed controls and graphical representations are mean±SE (n=4/group) for the low metal exposure experiments and (n=6/group) for the high metal exposure experiments. *Indicates significant change from corresponding air-exposed control (p<0.05). #Indicates significantly different from LM-exposed group.
In contrast to the neurotransmitter changes seen in various brain areas following LM exposure, a similar exposure regimen of HM aerosols, decreased dopamine (18% decrease from air-exposed controls, p<0.05) and its metabolite, DOPAC (41.7% decrease from air-exposed controls, p<0.05) only in the OB (Figure 1). The levels of dopamine or its metabolites were unaltered in other brain areas (MB, HYP, FCT, HYP and STR) following exposure to HM aerosols. Likewise, dopamine–metabolite ratios in all brain areas were unaffected by HM aerosols. Exposure to HM aerosols, also caused a decrease in norepinephrine (21.4% decrease from air-exposed controls, p<0.05; Figure 1) levels in the OB, but not other brain areas.
Exposure to LM aerosols generated by weld-bonding altered the levels of serotonin in discrete brain areas
In addition to its effects on dopamine, LM aerosols also caused significant reductions in the levels of serotonin in the MB (36.3% decrease from air-exposed controls, p<0.05; Figure 2), HYP (36.8% decrease from air-exposed controls, p<0.05; Figure 3) and FCT (36.7% decrease from air-exposed controls, p<0.05; Figure 4). Serotonin levels remained unaltered in the OB (Figure 1), HIP (Figure 5) and STR (data not shown) following LM exposure. The levels of the serotonin metabolite (HIAA) decreased only in the MB (23.5% decrease from air-exposed controls, p<0.05; Figure 2) following LM exposure, but not in other brain areas examined. An increase in the serotonin–metabolite ratio (5-HT/5-HIAA) was seen only in FCT (48.8% increase over air-exposed controls, p<0.05; Figure 4).
A similar exposure regimen of HM aerosols, significantly decreased the levels of serotonin in the OB (27.6% decrease from air-exposed controls, p<0.05; Figure 1) but not in other brain regions that were evaluated. The levels of 5-HIAA or the 5-HIAA/5-HT ratio were unaffected by exposure to HM aerosols in all brain areas examined.
Exposure to LM aerosols generated by weld-bonding decreased the levels of olfactory marker protein (Omp) and tyrosine hydroxylase (Th) mRNA expression in the olfactory bulb
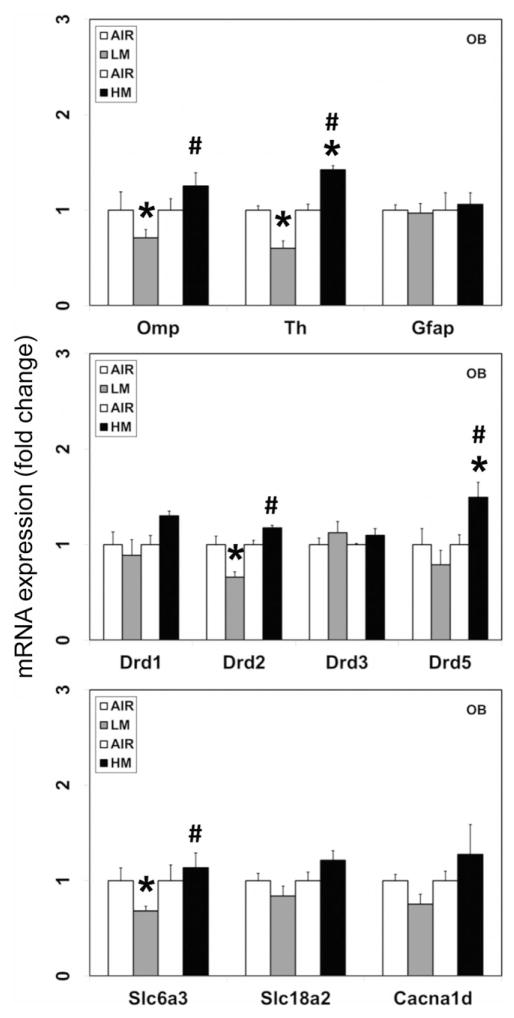
Omp is a cytoplasmic protein that is expressed almost exclusively by mature olfactory neurons (Buiakova et al., 1994) and can thus serve as a marker of olfactory cytotoxicity and/or injury. In rats exposed to LM, but not HM aerosols, a small but significant decrease in the mRNA expression of Omp (−1.4-fold change from air-exposed controls; p<0.05; Figure 6) was seen in the OB. Omp expression was not detected in any other brain area examined (data not shown), confirming its specific expression in the olfactory neurons.
Figure 6.
Effect of resistance spot welding aerosols on the mRNA expression of olfactory marker protein and dopaminergic markers in the olfactory bulb (OB). Rats were exposed to LM or HM aerosols (25 mg/m3 × 4 h/day) by whole-body inhalation for 13 days. At 1-day post-exposure, the mRNA expression of olfactory marker protein (Omp), tyrosine hydroxylase (Th), glial fibrillary acidic protein (Gfap), dopamine receptors 1, 2, 3 and 5 (Drd1, Drd2, Drd3 and Drd5), dopamine transporter (Slc6a3/Dat), vesicular monoamine transporter 2 (Slc18a2/Vmat2) and voltage-gated calcium channel member 1D (Cacna1d/Cav1.3) were assayed by TaqMan real-time PCR. Following normalization to the endogenous control beta actin (Actb), the values are expressed as fold change from air-exposed controls. Graphical representations are mean ±SE (n=4/group). *Indicates significant change from corresponding air-exposed control (p<0.05). #Indicates significantly different from LM-exposed group.
As deficiency of Omp has been shown to cause downstream gene expression changes, including decreased expression of Th, the rate-limiting enzyme in catecholamine synthesis (Buiakova et al., 1996), we examined the expression of Th mRNA in the OB. Exposure to LM aerosols, caused a small but significant decrease in the mRNA expression of Th (−1.7-fold change from air-exposed controls; p<0.05; Figure 6) in the OB. On the other hand, exposure to HM aerosols caused a small increase in Th mRNA expression in the OB (1.4-fold change over air-exposed controls; p<0.05; Figure 6).
Exposure of rats to LM or HM aerosols did not elicit glial activation in the OB, as assessed by the mRNA expression of the astroglial marker, glial fibrillary acidic protein (Gfap; Figure 6).
Exposure to LM aerosols generated by weld-bonding decreased the levels of dopamine transporter (Slc6a3, Dat) and dopamine D2 receptor (Drd2) mRNA expression in the olfactory bulb
Consistent with the reduction in dopamine and Th mRNA in the OB, exposure to LM but not HM aerosols, also caused small but significant decreases in the mRNA expression of the dopamine D2 receptor (Drd2; −1.5-fold change from air-exposed controls; p<0.05; Figure 6) and the solute carrier family 6 (neurotransmitter transporter) member 3 (Slc6a3; Dat; dopamine transporter; −1.5-fold change from air-exposed controls; p<0.05; Figure 6) in the OB.
On the other hand, exposure to HM aerosols caused small increases in the expression of the dopamine D1 receptor (Drd1; 1.3-fold change over air-exposed controls; not statistically significant) and dopamine D5 receptor (Drd5; 1.5-fold change over air-exposed controls; p<0.05; Figure 6) in the OB. Neither LM nor HM altered the expression of the solute carrier family 18 (vesicular monoamine transporter) member 2 (Slc18a2; Vmat2) that is involved in transport of monoamines to synaptic vesicles or the voltage-dependent L-type alpha 1D calcium channel (Cacna1d; Cav 1.3), a voltage-gated calcium channel involved in synaptic neurotransmitter release (Figure 6).
Exposure to HM but not LM aerosols elicited neuroinflammation in the olfactory bulb
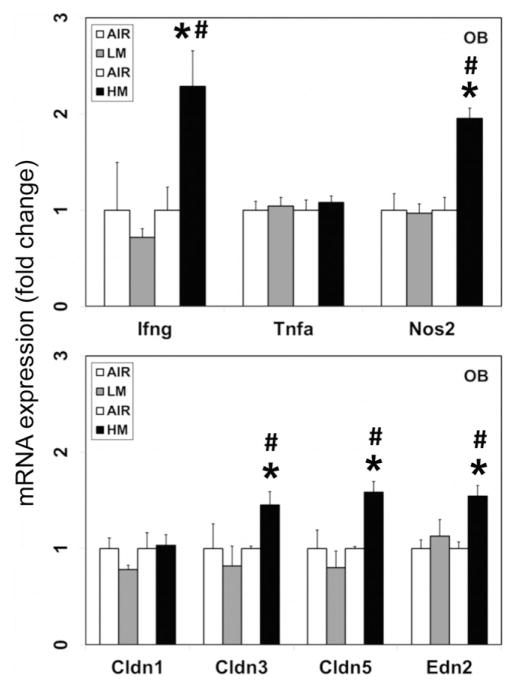
Neuroinflammation has been linked to the pathogenesis of PD (Hunot et al., 2001; McGeer & McGeer, 2004; Mogi et al., 1994) and dopaminergic neurotoxicity in experimental animals (Sriram et al., 2002, 2006a,b). We have also shown that neuroinflammation can contribute to the dopaminergic neurotoxicity seen following exposure to welding fumes (Sriram et al., 2010a,b). We therefore analyzed the expression of specific proinflammatory mediators like, interferon (Ifng), tumor necrosis factor α (Tnfα) and the inducible form of nitric oxide synthase (Nos2; iNos) in the OB after exposure to the two types of weld-bonding aerosols. Exposure to HM but not LM aerosols, induced the mRNA expression of Ifng (2.3-fold change over air-exposed controls; p<0.05; Figure 7) and Nos2 (2.0-fold change over air-exposed controls; p<0.05; Figure 7) in the OB, 1 day after the last exposure. Neither of the aerosols affected the levels of these mediators in other brain areas examined (data not shown).
Figure 7.
High metal containing resistance spot welding aerosols induce inflammation and blood–brain barrier changes in the olfactory bulb (OB). Rats were exposed to LM or HM aerosols (25 mg/m3 × 4 h/day) by whole-body inhalation for 13 days. At 1-day post-exposure, the mRNA expression of interferon gamma (Ifng), tumor necrosis factor alpha (Tnfa), inducible nitric oxide synthase (Nos2), claudins 1, 3 and 5 (Cldn1, Cldn3 and Cldn5) and endothelin 2 (Edn2) were assayed by TaqMan real-time PCR. Following normalization to the endogenous control beta actin (Actb), the values are expressed as fold change from air-exposed controls. Graphical representations are mean±SE (n=4/group). *Indicates significant change from corresponding air-exposed control (p<0.05). #Indicates significantly different from LM-exposed group.
Exposure to HM but not LM aerosols altered the markers of blood–brain barrier integrity in the olfactory bulb
Systemic inflammatory and oxidative stress events have been shown to disrupt the blood–brain barrier (BBB) (Huber et al., 2001; Thiel & Audus 2001). As neuronal injury is often associated with cerebrovascular dysfunction and modification of BBB function, we examined if exposure to weld-bonding aerosols affected the integrity of BBB. For this, we analyzed the expression of specific markers of BBB endothelium like, Claudin 1 (Cldn1), Claudin 3 (Cldn3), Claudin 5 (Cldn5) and Endothelin 2 (Edn2) in the OB after exposure to the two types of weld-bonding aerosols. Exposure to HM but not LM aerosols, induced the mRNA expression of Cldn3 (1.5-fold change over air-exposed controls; p<0.05; Figure 7), Cldn5 (1.6-fold change over air-exposed controls; p<0.05; Figure 7) and Edn2 (1.5-fold change over air-exposed controls; p<0.05; Figure 7) in the OB, 1 day after the last exposure. Neither of the aerosols affected the levels of these mediators in other brain areas examined (data not shown).
Discussion
Welding is an important industrial process employed to coalesce metals. An unavoidable consequence of welding is the generation of fumes that can be potentially hazardous to the welder’s health. Due to the high temperatures associated with welding, metal aerosols originate from the molten tip of the welding electrode and rapidly condense into fine and nano-sized particulates. The aerodynamic diameter of WF aerosols in the welder’s breathing zone is reported to range from 100 nm to 1 μm (Jenkins et al., 2005; Zimmer & Biswas, 2001), which are easily respirable and can deposit in the olfactory and lower respiratory tracts. As ultrafine particulates or their soluble metal fractions can translocate and accumulate in the brain (Elder et al., 2006; Hunter & Undem 1999; Oberdörster et al., 2002, 2004), there is a likelihood of a causal association between WF exposure and the neurological effects seen in welders. Particularly, the presence of Mn in welding consumables is thought to be associated with the appearance of PD-like neurological manifestations.
A popular variant of the welding process that is particularly employed in the manufacturing industry is weld-bonding; a hybrid welding technology that combines resistance spot welding and adhesive bonding to weld metal-to-metal components or structures. While there are several advantages to weld-bonding, in terms of weld quality, superior tearing strength and joint reliability, there are emerging concerns of adverse health effects following exposure to the aerosols generated by weld-bonding. The fumes generated during weld-bonding contains complex aerosols of gases, metals and VOCs, the composition and morphology of which are influenced by the type and elemental content of the metal sheets to be welded, the adhesive used to bond the metal sheets and the welding process conditions (voltage, current and temperature).
Information about the potential health effects of exposure to aerosols generated by weld-bonding is limited. While a few studies have reported respiratory irritation, asthma, bronchitis and sinusitis among workers exposed to aerosols generated during resistance spot welding using adhesives (Hammond et al., 2005; Kanwal & Boylstein, 2006; Luo et al., 2006; Loukzadeh et al., 2009), there is a lack of information about the potential neurological effects of exposure to aerosols generated during weld-bonding. Assessment of the neurotoxicological potential of weld-bonding aerosols is critical because of the presence of chemicals like methyl methacrylate, formaldehyde, styrene, benzene and toluene that constitute the adhesive and are individually known to be neurotoxic. In addition, some of these chemicals have been reported to occur in the air samples collected from an automotive plant where robotic weld-bonding was performed (Kanwal & Boylstein, 2006), suggesting the possibility of worker exposure. Thus, from an occupational safety perspective, it is of critical importance to evaluate the potential toxicological risks associated with weld-bond aerosols. Such efforts will help in better understanding of the short-term and long-term adverse health effects of exposed worker populations. Besides, understanding the toxicological mechanisms can aid in the development of appropriate exposure assessment paradigms, exposure control and preventive practices to avert adverse exposures and human health risks.
Resistance spot welding with adhesive bonding produced complex aerosols that consisted of metal particulates and VOCs (Afshari et al., 2014). Given the complex nature of the aerosols generated during weld-bonding, and the fact that inhaled ultrafine particles and/or VOCs can translocate or permeate across the olfactory pathway to the brain, raises concerns about the potential neurological effects following exposure to such aerosols. The metal particulates derived from welding strips of mild-steel sheet metal comprised largely of iron (99%) and manganese (0.5%), with other metals like copper, zinc and chromium making up the remainder. We have previously shown that repeated pulmonary exposure of rats to fume particulates derived from gas metal arc welding with mild-steel (GMA-MS) caused neuroinflammation and dopaminergic neurotoxicity (Sriram et al., 2010a). Consistent with this, exposure to HM aerosols, which contained predominantly metal particulates (Afshari et al., 2014), elicited neuroinflammation, as evidenced by increased expression of mRNAs for Ifng and Nos2 (iNos) in the olfactory bulb. Ifng pathway has been shown to be associated with manganese toxicity (Sengupta et al., 2007). Likewise, Nos2 has also been shown to be induced by manganese (Chang & Liu, 1999; Filipov et al., 2005) and gene deletion of Nos2 is reported to be protective against manganese neurotoxicity (Streifel et al., 2012). Our own findings with manganese-containing fume particulates generated by GMA-MS or manual metal arc-hard surfacing (MMAHS) welding show induction of Nos2 in various brain areas (Sriram et al., 2010a). As manganese, but not other metals, was selectively taken up by the brain following exposure to GMA-MS or MMA-HS (Sriram et al., 2010b), this suggests that the induction of Nos2 may be mediated by manganese. Concurrent with neuroinflammation, HM aerosols also altered the expression of Cldn3, Cldn5 and Edn2, markers that are associated with BBB integrity and vascular blood flow. Claudins are cell adhesion molecules of tight junctions that are critical for the formation of the BBB. The expression of Cldn 1, Cldn3, and Cldn5 at the BBB has been reported (Bazzoni & Dejana, 2004). Cldn5, in particular, is a major cell adhesion molecule of tight junctions in the brain endothelium (Nitta et al., 2003). Reductions in Cldn3 and Cldn5 expression are known to be associated with a reduction in BBB permeability (Kniesel & Wolburg, 2000; Ueno et al., 2004). Conversely, our findings showing increased expression of Cldn3 and Cldn5 are suggestive of increased permeability of the BBB. Indeed, inflammation with release of Tnfa or Ifng has been associated with increased permeability and breakdown of BBB (Lopez-Ramirez et al., 2012). Endothelins are widely expressed in the brain and are potent vasoconstrictors known to decrease cerebral blood flow. In the olfactory bulb, endothelins appear to play an additional role in regulating catecholaminergic function. Particularly, endothelins have been shown to stimulate the expression and activity of the rate-limiting enzyme in dopamine synthesis, Th (Nabhen et al., 2009, 2013), suggesting an interaction between the endothelin and catecholaminergic systems. Our findings of increased mRNA expression of endothelin 2 (Edn2) and a concordant increase in Th mRNA expression in the olfactory bulb following exposure to HM aerosols are consistent with this notion. Apart from neuroinflammation and BBB changes, HM aerosols also decreased norepinephrine, dopamine and serotonin content, and caused a small increase in the expression of Th mRNA in the olfactory bulb. However, such changes were not observed in the striatum or midbrain following HM exposure, perhaps due to the short duration of exposure employed in this study.
Importantly, the short-term study was undertaken to determine if spot welding using an adhesive, which has the potential to generate VOCs in the resulting aerosols, can cause neurotoxicity that is distinct from that observed after exposure to aerosols with high metal (HM aerosols) and other standard gas metal arc welding fume particulates. During weld-bonding, several VOCs were generated that are characteristic of the adhesive Terokal® 2300 used for bonding the sheet metals. The chemical composition of Terokal® 2300 as disclosed by the manufacturer (Henkel Surface Technologies) in their Material Safety Data Sheet (MSDS ID: IDH No. 887651) includes: bisphenol A-epichlorohydrin polymer (CAS# 25068-38-6); flexibilized DGEBA (proprietary; flexibilized bisphenol A diglycidyl ether); 1,2,3-propanetriyl ester of 12-(oxiranylmethoxy)-9-octadecanoic acid (CAS# 74398-71-3); epoxy diluent (proprietary) and dimethyl silicone polymer with silica (CAS# 67762-90-7; dimethyl siloxane, reaction product with silica). The percent compositions of the ingredients as disclosed in the MSDS are: bisphenol A-epichlorohydrin polymer (30–60%), flexibilized DGEBA (10–30%), 1,2,3-propanetriyl ester of 12-(oxiranylmethoxy)-9-octadecanoic acid (1–10%); epoxy diluent (1–10%) and dimethyl silicone polymer with silica (1–10%). As some of the components were proprietary, it was difficult to discern their exact chemical composition from the MSDS. Analysis of the aerosols generated during weld-bonding with Terokal® 2300 revealed the presence of benzene, toluene, 2-propanol (isopropanol, isopropyl alcohol), 2-butoxyethanol, butanol, ethylene glycol and siloxanes (Afshari et al., 2014). Although bisphenol A was a major component of the adhesive, it was not detected in the aerosols under the conditions of this study. It is likely that thermal decomposition of Bisphenol A may have occurred at the high temperatures that are typically encountered during resistance spot welding.
Many of the chemicals identified in the weld-bond aerosols, including the VOCs, benzene, toluene and 2-propanol, were also detected in the animal exposure chamber and were consistent with that reported in air samples from the automotive processing plant where NIOSH conducted a HHE (Kanwal & Boylstein, 2006). Acute central nervous system (CNS) health effects of VOCs include headache, dizziness, unconsciousness and seizures (White & Procter, 1997). Persisting chronic and/or high-dose exposures can lead to mood and personality changes, loss of coordination and memory impairment (Dick, 2006). Short-term exposure to high concentrations of Benzene affects the CNS and can cause paralysis, coma, convulsions, dizziness, sleepiness and tremor. Toluene is highly lipophilic and can readily cross the BBB after inhalation (Balster, 1998). Toluene mainly affects the CNS and elicits sleepiness, headache, dizziness, depression, cerebellar dysfunction, hippocampal atrophy and memory impairment (Deleu & Hanssens, 2000; Echeverria et al., 1991; Kamran & Bakshi, 1998; Kishi et al., 1993; Wang & Chen, 1993; Yamanouchi et al., 1995; ). Inhalation of low concentrations of toluene has been shown to induce a persistent deficit in spatial learning and memory, both in humans and in animals (Chouaniere et al., 2002; Lee et al., 2003; von Euler et al., 1993, 2000; Yu et al., 2004). As organic hydrocarbons are membrane-perturbing substances, they can affect neuronal membranes causing aberrant synaptic signaling and impaired neurotransmission. Hence, we examined the effects of LM aerosols on biogenic amine neurotransmitters and their receptors. Exposure to LM aerosols caused brain-region specific alterations in levels of the neurotransmitters, dopamine and serotonin. While a small reduction in dopamine was seen in olfactory bulb, dopamine and serotonin were significantly reduced in the midbrain and hypothalamus. However, in the frontal cortex and hippocampus, an increase in dopamine was observed. Such brain area specific alterations in neurotransmitters have been reported to occur following exposure to Toluene (Kiriu et al., 1990). Our findings are in agreement with the observation of Kiriu et al. (1990), who reported that inhalation exposure to Toluene caused a reduction in the levels of dopamine in the midbrain and hypothalamus. Increased dopamine activity in the frontal cortex, particularly in the prefrontal cortex, has been shown to cause memory impairment by affecting spatial memory performance (Murphy et al., 1996), consistent with that reported to occur following Toluene exposure (Chouaniere et al., 2002; von Euler et al., 1993, 2000).
In addition, exposure to LM aerosols also caused a small reduction in the mRNA expression of Omp and dopaminergic markers (Th, Drd2 and Slc6a3) in the olfactory bulb. The expression of these markers in other brain areas was unaffected. Omp is exclusively expressed by mature olfactory receptor neurons, which are localized in the olfactory epithelium and project directly into the olfactory bulb within the CNS. Th is expressed predominantly in the glomerular layer of the olfactory bulb, specifically by the periglomerular and external tufted cells, which are collectively known as juxtaglomerular cells (Halász et al., 1977; Kosaka et al., 1985). As olfactory sensory neurons occupy nearly two-thirds of the sensory or olfactory epithelium, they experience direct access to odorant molecules, as well as, allergens, airborne pollutants, toxic chemicals and microorganisms. Some of these toxicants, including metallic and organic hydrocarbon compounds, can be potentially harmful causing olfactory sensory deafferentation and olfactory dysfunction (Doty & Hastings, 2000). Indeed, mice deficient in Omp exhibit reduced odor response to various odorants and impaired neural signaling directed towards the olfactory bulb (Buiakova et al., 1996). Reduction of Omp in LM aerosol-exposed animals may therefore be indicative of injury to the olfactory sensory neurons and perhaps compromised odor stimuli. Likewise, Drd2 signaling has also been shown to be important for odor discrimination. Mice deficient in Drd2 and Slc6a3 are capable of olfaction but exhibit impairment in acquisition of odor-driven reinforcement and odor discrimination (Kruzich & Grandy, 2004; Tillerson et al., 2006). Loss of olfactory bulb dopamine or Drd2 antagonism has been shown to impair olfactory discrimination (Jin et al., 1996; Johnson et al., 1996; Wilson & Sullivan, 1995). These findings suggest the association of dopamine with odor processing. This appears to be consistent with the reports that patients with PD exhibit olfactory abnormalities several years before the diagnosis of the disorder (Berendse et al., 2001). However, olfactory or neurobehavioral assessments were not conducted in this study, therefore, the functional relevance of these molecular changes cannot be proven conclusively. Nevertheless, the early molecular changes in the expression of Omp and the dopaminergic markers are suggestive of potential neural abnormality following acute exposure to LM aerosols that is likely mediated by the dopamine-signaling pathway.
Taken together, our findings demonstrate that short-term exposure to weld-bonding aerosols can potentially elicit neurotoxicity; importantly, our study divulges the differential neurotoxic effects of LM and HM aerosols. Further investigations are warranted to determine if the aerosols generated by weld-bonding can contribute to persistent long-term neurological deficits and/or neurodegeneration.
Footnotes
Declaration of interest
The authors declare they have no proprietary, financial or personal interest of any kind or nature in any samples, products, supplies, service or company that could be construed as being a conflict of interest.
References
- Afshari A, Zeidler-Erdely PC, McKinney M, et al. Development and characterization of a resistance spot welding aerosol generator and inhalation exposure system. Inhal Toxicol. 2014 doi: 10.3109/08958378.2014.941118. [DOI] [PubMed] [Google Scholar]
- Balster RL. Neural basis of inhalant abuse. Drug Alcohol Depend. 1998;51:207–14. doi: 10.1016/s0376-8716(98)00078-7. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Booij J, Francot CM, et al. Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann Neurol. 2001;50:34–41. doi: 10.1002/ana.1049. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, et al. Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–26. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Nakagawa S, Drezgic M, et al. Sequelae of fume exposure in confined space welding: a neurological and neuropsychological case series. Neurotoxicology. 2007a;28:298–311. doi: 10.1016/j.neuro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, et al. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med. 2007b;64:167–77. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiakova OI, Krishna NS, Getchell TV, Margolis FL. Human and rodent OMP genes: conservation of structural and regulatory motifs and cellular localization. Genomics. 1994;20:452–62. doi: 10.1006/geno.1994.1200. [DOI] [PubMed] [Google Scholar]
- Buiakova OI, Baker H, Scott JW, et al. Olfactory marker protein (OMP) gene deletion causes altered physiological activity of olfactory sensory neurons. Proc Natl Acad Sci USA. 1996;93:9858–63. doi: 10.1073/pnas.93.18.9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BH, Shi YW, Dong SJ. A study on the role of adhesives in weld-bonded joints. Welding J. 1999;78:275s–9s. [Google Scholar]
- Chang JY, Liu LZ. Manganese potentiates nitric oxide production by microglia. Brain Res Mol Brain Res. 1999;68:22–8. doi: 10.1016/s0169-328x(99)00082-0. [DOI] [PubMed] [Google Scholar]
- Chouaniere D, Wild P, Fontana JM, et al. Neurobehavioral disturbances arising from occupational toluene exposure. Am J Ind Med. 2002;41:77–88. doi: 10.1002/ajim.10030. [DOI] [PubMed] [Google Scholar]
- Deleu D, Hanssens Y. Cerebellar dysfunction in chronic toluene abuse: beneficial response to amantadine hydrochloride. J Toxicol Clin Toxicol. 2000;38:37–41. doi: 10.1081/clt-100100913. [DOI] [PubMed] [Google Scholar]
- Dick FD. Solvent neurotoxicity. Occup Environ Med. 2006;63:221–6. doi: 10.1136/oem.2005.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. The physiopathologic significance of manganese in brain: its relation to schizophrenia and neurodegenerative disorders. Neurotoxicology. 1987;8:451–62. [PubMed] [Google Scholar]
- Doty RL, Hastings L. Neurotoxic exposure and olfactory impairment. Clin Occup Environ Med. 2000;1:547–75. [Google Scholar]
- Echeverria D, Fine L, Langolf G, et al. Acute behavioural comparisons of toluene and ethanol in human subjects. Br J Ind Med. 1991;48:750–61. doi: 10.1136/oem.48.11.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder A, Gelein R, Silva V, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114:1172–8. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara AM, el-Ghawabi SH, Madkour OI, el-Samra GH. Chronic manganese poisoning in the dry battery industry. Br J Ind Med. 1971;28:78–82. doi: 10.1136/oem.28.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipov NM, Seegal RF, Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol Sci. 2005;84:139–48. doi: 10.1093/toxsci/kfi055. [DOI] [PubMed] [Google Scholar]
- Finkelstein MM, Jerrett M. A study of the relationships between Parkinson’s disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ Res. 2007;104:420–32. doi: 10.1016/j.envres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Halász N, Ljungdahl A, Hökfelt T, et al. Transmitter histochemistry of the rat olfactory bulb. I. Immunohistochemical localization of monoamine synthesizing enzymes. Support for intrabulbar, periglomerular dopamine neurons. Brain Res. 1977;126:455–74. doi: 10.1016/0006-8993(77)90597-2. [DOI] [PubMed] [Google Scholar]
- Hammond SK, Gold E, Baker R, et al. Respiratory health effects related to occupational spray painting and welding. J Occup Environ Med. 2005;47:728–39. doi: 10.1097/01.jom.0000165748.31326.e8. [DOI] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–25. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Hunot S, Hartmann A, Hirsch EC. The inflammatory response in the Parkinson brain. Clin Neurosci Res. 2001;1:434–43. [Google Scholar]
- Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med. 1999;159:1943–8. doi: 10.1164/ajrccm.159.6.9808078. [DOI] [PubMed] [Google Scholar]
- Jenkins NT, Pierce WM-G, Eagar TW. Particle size distribution of gas metal and flux cored arc welding fumes. Welding J. 2005;84:156s–63s. [Google Scholar]
- Jin BK, Franzen L, Baker H. Regulation of c-Fos mRNA and fos protein expression in olfactory bulbs from unilaterally odor-deprived adult mice. Int J Dev Neurosci. 1996;14:971–82. doi: 10.1016/s0736-5748(96)00044-5. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Ninomiya-Tsuboi K, Leon M. Synaptophysin-like immunoreactivity in the rat olfactory bulb during postnatal development and after restricted early olfactory experience. Brain Res Dev Brain Res. 1996;92:24–30. doi: 10.1016/0165-3806(95)00197-2. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, et al. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–9. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- Kamran S, Bakshi R. MRI in chronic toluene abuse: low signal in the cerebral cortex on T2-weighted images. Neuroradiology. 1998;40:519–21. doi: 10.1007/s002340050637. [DOI] [PubMed] [Google Scholar]
- Kanwal R, Boylstein RJ. Daimler-Chrysler Jefferson North Assembly Plant. Detroit, MI. Washington, DC: DHHS/Center for Disease Control and Prevention; 2006. NIOSH Health Hazard Evaluation Report: HETA# 2006-0059-3009. [Google Scholar]
- Kiriu T, Ameno K, Fuke C, et al. The distribution of toluene in the brain and its effects on the brain catecholamines in acute toluene poisoning. Nihon Hoigaku Zasshi. 1990;44:25–33. [PubMed] [Google Scholar]
- Kishi R, Harabuchi I, Katakura Y, et al. Neurobehavioral effects of chronic occupational exposure to organic solvents among Japanese industrial painters. Environ Res. 1993;62:303–13. doi: 10.1006/enrs.1993.1115. [DOI] [PubMed] [Google Scholar]
- Kniesel U, Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20:57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Hataguchi Y, Hama K, et al. Coexistence of immunoreactivities for glutamate decarboxylase and tyrosine hydroxylase in some neurons in the periglomerular region of the rat main olfactory bulb: possible coexistence of gamma-aminobutyric acid (GABA) and dopamine. Brain Res. 1985;343:166–71. doi: 10.1016/0006-8993(85)91172-2. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Grandy DK. Dopamine D2 receptors mediate two-odor discrimination and reversal learning in C57BL/6 mice. BMC Neurosci. 2004;5:12. doi: 10.1186/1471-2202-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YL, Pai MC, Chen JH, Guo YL. Central neurological abnormalities and multiple chemical sensitivity caused by chronic toluene exposure. Occup Med. 2003;53:479–82. doi: 10.1093/occmed/kqg095. [DOI] [PubMed] [Google Scholar]
- Long H. Inhalants. In: Goldfrank LR, Flomenbaum N, editors. Goldfranks’ toxicologic emergencies. New York: McGraw-Hill; 2006. pp. 1192–201. [Google Scholar]
- Lopez-Ramirez MA, Fischer R, Torres-Badillo CC, et al. Role of caspases in cytokine-induced barrier breakdown in human brain endothelial cells. J Immunol. 2012;189:3130–9. doi: 10.4049/jimmunol.1103460. [DOI] [PubMed] [Google Scholar]
- Loukzadeh Z, Sharifian SA, Aminian O, Shojaoddiny-Ardekani A. Pulmonary effects of spot welding in automobile assembly. Occup Med. 2009;59:267–9. doi: 10.1093/occmed/kqp033. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, et al. Long-term exposure to “low levels” of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology. 1999;20:287–97. [PubMed] [Google Scholar]
- Lucchini RG, Albini E, Benedetti L, et al. High prevalence of Parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am J Ind Med. 2007;50:788–800. doi: 10.1002/ajim.20494. [DOI] [PubMed] [Google Scholar]
- Luo JCJ, Hsu K-H, Shen W-S. Pulmonary function abnormalities and airway irritation symptoms of metal fumes exposure on automotive spot welders. Am J Ind Med. 2006;49:407–16. doi: 10.1002/ajim.20320. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10:S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Environ Res. 1997;73:92–100. doi: 10.1006/enrs.1997.3710. [DOI] [PubMed] [Google Scholar]
- Miller Electric. Handbook for resistance spot welding. Appleton, WI: Miller Electric; 2012. pp. 1–10. [Google Scholar]
- Mogi M, Harada M, Riederer P, et al. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–10. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996;93:1325–9. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhen SL, Perfume G, Battistone MA, et al. Short-term effects of endothelins on tyrosine hydroxylase activity and expression in the olfactory bulb of normotensive rats. Neurochem Res. 2009;34:953–63. doi: 10.1007/s11064-008-9859-6. [DOI] [PubMed] [Google Scholar]
- Nabhen SL, Guil MJ, Saffioti N, et al. Calcium-dependent mechanisms involved in the modulation of tyrosine hydroxylase by endothelins in the olfactory bulb of normotensive rats. Neurochem Int. 2013;62:389–98. doi: 10.1016/j.neuint.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–60. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, et al. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65:1531–43. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–45. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, et al. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- Racette BA, Tabbal SD, Jennings D, et al. Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology. 2005;64:230–5. doi: 10.1212/01.WNL.0000149511.19487.44. [DOI] [PubMed] [Google Scholar]
- Rodier J. Manganese poisoning in Moroccan miners. Br J Ind Med. 1955;12:21–35. doi: 10.1136/oem.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Mense SM, Lan C, et al. Gene expression profiling of human primary astrocytes exposed to manganese chloride indicates selective effects on several functions of the cells. Neurotoxicology. 2007;28:478–89. doi: 10.1016/j.neuro.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, et al. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson’s disease. FASEB J. 2002;16:1474–6. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, et al. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-alpha. FASEB J. 2006a;20:670–82. doi: 10.1096/fj.05-5106com. [DOI] [PubMed] [Google Scholar]
- Sriram K, Miller DB, O’Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem. 2006b;96:706–18. doi: 10.1111/j.1471-4159.2005.03566.x. [DOI] [PubMed] [Google Scholar]
- Sriram K, Lin GX, Jefferson AM, et al. Dopaminergic neurotoxicity following pulmonary exposure to manganese-containing welding fumes. Arch Toxicol. 2010a;84:521–40. doi: 10.1007/s00204-010-0525-9. [DOI] [PubMed] [Google Scholar]
- Sriram K, Lin GX, Jefferson AM, et al. Mitochondrial dysfunction and loss of Parkinson’s disease-linked proteins contribute to neurotoxicity of manganese-containing welding fumes. FASEB J. 2010b;24:4989–5002. doi: 10.1096/fj.10-163964. [DOI] [PubMed] [Google Scholar]
- Streifel KM, Moreno JA, Hanneman WH, et al. Gene deletion of nos2 protects against manganese-induced neurological dysfunction in juvenile mice. Toxicol Sci. 2012;126:183–92. doi: 10.1093/toxsci/kfr335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel VE, Audus KL. Nitric oxide and blood-brain barrier integrity. Antioxid Redox Signal. 2001;3:273–8. doi: 10.1089/152308601300185223. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Parent JM, et al. Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behav Brain Res. 2006;172:97–105. doi: 10.1016/j.bbr.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Ueno M, Sakamoto H, Liao YJ, et al. Blood-brain barrier disruption in the hypothalamus of young adult spontaneously hypertensive rats. Histochem Cell Biol. 2004;122:131–7. doi: 10.1007/s00418-004-0684-y. [DOI] [PubMed] [Google Scholar]
- von Euler G, Ogren SO, Li XM, et al. Persistent effects of subchronic toluene exposure on spatial learning and memory, dopamine mediated locomotor activity and dopamine D2 agonist binding in the rat. Toxicology. 1993;77:223–32. doi: 10.1016/0300-483x(93)90162-l. [DOI] [PubMed] [Google Scholar]
- von Euler M, Pham TM, Hillefors M, et al. Inhalation of low concentrations of toluene induces persistent effects on a learning retention task, beam-walk performance, and cerebrocortical size in the rat. Exp Neurol. 2000;163:1–8. doi: 10.1006/exnr.1999.7288. [DOI] [PubMed] [Google Scholar]
- Wang JD, Chen JD. Acute and chronic neurological symptoms among paint workers exposed to mixtures of organic solvents. Environ Res. 1993;61:107–16. doi: 10.1006/enrs.1993.1054. [DOI] [PubMed] [Google Scholar]
- Wennberg A, Iregren A, Struwe G, et al. Manganese exposure in steel smelters a health hazard to the nervous system. Scand J Work Environ Health. 1991;17:255–62. doi: 10.5271/sjweh.1705. [DOI] [PubMed] [Google Scholar]
- White RF, Proctor SP. Solvents and neurotoxicity. Lancet. 1997;349:1239–43. doi: 10.1016/S0140-6736(96)07218-2. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. The D2 antagonist spiperone mimics the effects of olfactory deprivation on mitral/tufted cell odor response patterns. J Neurosci. 1995;15:5574–81. doi: 10.1523/JNEUROSCI.15-08-05574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi N, Okada S, Kodama K, et al. White matter changes caused by chronic solvent abuse. Am J Neuroradiol. 1995;16:1643–9. [PMC free article] [PubMed] [Google Scholar]
- Yu IT, Lee NL, Zhang XH, et al. Occupational exposure to mixtures of organic solvents increases the risk of neurological symptoms among printing workers in Hong Kong. J Occup Environ Med. 2004;46:323–30. doi: 10.1097/01.jom.0000121367.69269.07. [DOI] [PubMed] [Google Scholar]
- Zimmer AT, Biswas P. Characterization of the aerosols resulting from arc welding processes. J Aerosol Sci. 2001;32:993–1008. [Google Scholar]