Abstract
Rapid-response systems (RRSs) are a popular intervention in U.S. hospitals and are supported by accreditors and quality improvement organizations. The purpose of this review is to evaluate the effectiveness and implementation of these systems in acute care settings. A literature search was performed between 1 January 2000 through 30 October 2012 using PubMed, PsycINFO, CINAHL, and the Cochrane Central Register of Controlled Trials. Studies published in any language evaluating outcome changes that occurred after implementing an RRS and differences between groups using and not using an RRS (effectiveness) or describing methods used by RRSs (implementation) were reviewed.
A single reviewer (checked by a second reviewer) abstracted data and rated study quality and strength of evidence. Moderate-strength evidence from a high-quality meta-analysis of 18 studies and 26 lower-quality before-and-after studies published after that meta-analysis showed that RRSs are associated with reduced rates of cardiorespiratory arrest outside of the intensive care unit and reduced mortality. Eighteen studies examining facilitators of and barriers to implementation suggested that the rate of use of RRSs could be improved.
The Problem
Patients in the general ward often experience unrecognized deterioration that may progress to cardiorespiratory arrest. Patients commonly show signs and symptoms of deterioration for hours or days before cardiorespiratory arrest (median time, 6 hours) (1). Such arrests are associated with a poor prognosis (mortality up to 80%).
Almost all cardiorespiratory arrests have a common set of antecedents that are often poorly recognized secondary to the low sensitivity and fidelity of periodic assessments by staff. Improving this process should lead to earlier recognition and intervention. Many approaches have been devised (for example, single- and multiple-track and trigger systems and weighted early warning scoring systems), but none has been shown to have a clear advantage.
Even when recognition of deterioration is prompt, intervention may lag because of such barriers as a physician-centric medical culture that discourages speaking up or bypassing the chain of command, and imbalances between patient and clinician needs and resources. Improving recognition and overcoming the barriers to an effective and timely response should reveal problems before they become life-threatening.
Patient Safety Strategy
Rapid-response systems (RRSs) were created to improve recognition of and response to deterioration of patients on general hospital wards, with the goal of reducing the incidence of cardiorespiratory arrest and hospital mortality. An RRS generally has 3 components.
1) Criteria and a system for notifying and activating the response team (known as an “afferent limb,” the mechanism by which team responses are triggered)
Activation criteria usually include vital signs (single-trigger criteria vs. aggregate and weighted early warning scoring) or general concern expressed by a clinician or family member. The afferent limb defines the variables that indicate deterioration and democratizes that knowledge to all clinicians. It also empowers bedside clinicians to trigger the response team (or “efferent limb,” the team of clinicians that respond to an event) when the clinician has a suspicion that a patient is deteriorating (2). As such, most RRSs rely on clinicians to proactively identify deteriorating patients rather than solely on continuous monitoring technology, which is common in the intensive care unit (ICU).
2) The response team (efferent limb)
The response team most frequently comprises ICU-trained personnel and equipment. Team composition varies on the basis of local needs and resources but generally uses one of the following models: medical emergency teams (METs), which include a physician; rapid-response teams, which do not include a physician; and critical care outreach teams, which follow up on patients discharged from an ICU but also respond to all ward patients.
3) An administrative and quality improvement component
This team collects and analyzes event data and provides feedback, coordinates resources, and ensures improvement or maintenance over time.
Many hospitals have implemented RRSs to remedy the failure of our current system to adequately monitor patients in the general ward, recognize the signs and symptoms of deterioration, rescue deteriorating patients, and deliver optimal care rapidly through escalation and triage. That RRSs should be able to improve outcomes has strong face validity. Given the rapid pace of RRS literature since the last systematic review on the subject, done in 2010, we conducted this systematic review to update the current state of the evidence for RRS effectiveness and implementation.
Review Processes
PubMed, PsycINFO, CINAHL, and the Cochrane Central Register of Controlled Trials were searched between January 2000 through October 2012. The Supplement (available at www.annals.org) describes the search strategies and includes the summary of evidence search and selection. Effectiveness articles were restricted to studies that used a MET, rapid-response team, or critical care outreach team model; had a comparison group; and were published after November 2008 (the end date for the high-quality systematic review described later) (3).
Studies of RRS implementation selected for inclusion could be either qualitative (that is, studies using interviews, focus groups, or ethnographic methods) or quantitative (that is, studies examining implementation strategy on use of the RRS or patient outcomes that provided numerical outcome data). There were no exclusions based on country or language.
Two reviewers independently screened all abstracts. Full articles identified for inclusion had outcome data abstracted by a single reviewer and checked by a second reviewer. We did not abstract data on nursing satisfaction, which was rarely reported. The strength of evidence, including risk of bias, was evaluated using the Grading of Recommendations Assessment, Development and Evaluation Working Group criteria adapted by the Agency for Healthcare Research and Quality (4). We evaluated the quality of systematic reviews by using the assessment of multiple systematic reviews criteria (5).
Of 2560 abstracts captured by the search strategy, 2321 were excluded during screening and 232 articles were excluded in 2 rounds of article screening. Forty-three articles met the inclusion criteria (26 studies for effectiveness; 17 studies for implementation).
For effectiveness, the main outcome variables were adult and pediatric non-ICU cardiorespiratory arrest and adult and pediatric total hospital mortality. Studies that provided complete raw data (sample sizes and number of events both before and after the intervention periods) or relative risk (RR) estimate and its 95% CI or RR estimate with its associated accurate P value for any of these main outcomes were included. Studies that did not provide sufficient quantitative data were excluded.
Using data from each study, we were able to present or calculate a risk ratio estimate, its logarithm, and the associated SE. The results are summarized as risk ratios with 95% CIs and are shown in Figures 1 to 4, which include the data from the high-quality systematic review (3) The 95% CIs were computed and plotted in the log scale but labeled in the original scale. We used R, version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria), for these analyses. For implementation, Table 2 of the Supplement shows qualitative summaries of individual studies.
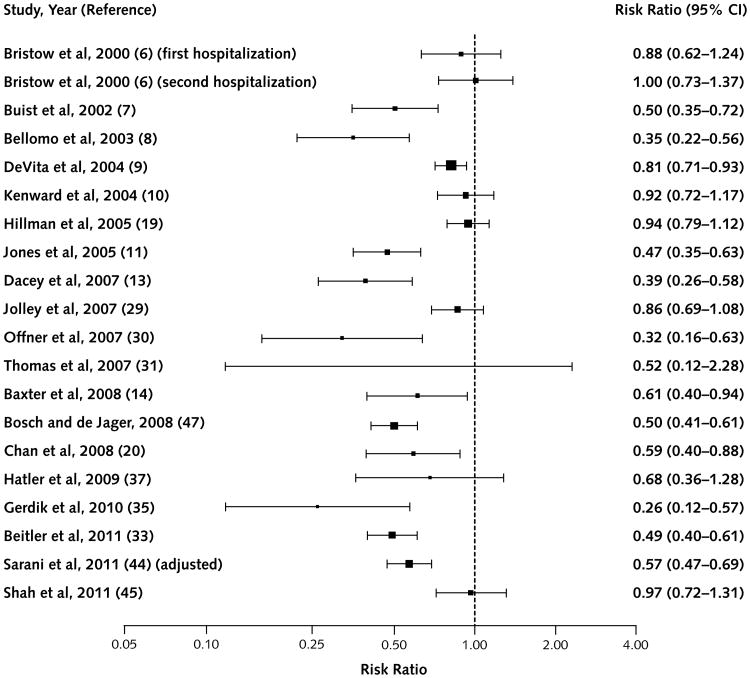
Figure 1. Studies that reported the outcome of non–intensive care unit adult cardiorespiratory arrest.
Figure 4. Studies that reported the outcome of total hospital pediatric mortality.
This review was supported by the Agency for Healthcare Research and Quality, which had no role in the selection or review of the evidence or the decision to submit this manuscript for publication.
Benefits and Harms
Benefits
We identified 7 systematic reviews of RRSs; however, only 1 was rated as high quality and is described in detail here (3). A second review addressed implementation and is discussed in the next section. The highest-quality systematic review (3) (assessment of multiple systematic reviews criteria score, 10 of 11) identified 16 studies (6–21) through November 2008 involving nearly 1.3 million hospital admissions.
The meta-analysis concluded that, among adults, implementation of an RRS was associated with a statistical reduction in non-ICU cardiorespiratory arrest (RR, 0.66 [95% CI, 0.54 to 0.80]) but not with lower total hospital mortality (RR, 0.96 [CI, 0.84 to 1.09]). In children, implementation of an RRS was associated with statistical reductions in both non-ICU cardiorespiratory arrest (RR, 0.62 [CI, 0.46 to 0.84]) and total hospital mortality (RR, 0.79 [CI, 0.63 to 0.98]).
The review rated studies as high quality if they adjusted for confounders and for time trends by using either concurrent control groups or an interrupted time series design. Studies were rated as fair quality if they adjusted only for confounding. Five of the 18 studies were rated as high quality, 2 as fair quality, and the rest as low quality.
In this updated review, we identified 26 additional effectiveness studies (22– 47) that met our inclusion criteria and were published since the previous high-quality systematic review. None used randomization in its methodology or had a concurrent control group; 2 studies included multiple centers. Three studies were done in pediatric hospitals. Most took place in the United States, Australia, or Canada, with only a few in Europe or Asia. Most studies were conducted in teaching hospitals.
Almost no studies included any information on context, and no studies reported a theoretical or logic model. The number of included hospital admissions or discharges in the studies ranged from 1920 to 277 717. All were rated as low or moderate quality.
Most studies reported the main outcomes of non-ICU cardiorespiratory arrest and total hospital mortality; some studies also reported variations on these outcomes, such as unexpected cardiorespiratory arrest or unexpected mortality. From our included studies, Figures 1 to 4 show studies providing adequate data (29–47) along with the studies included in the high-quality review.
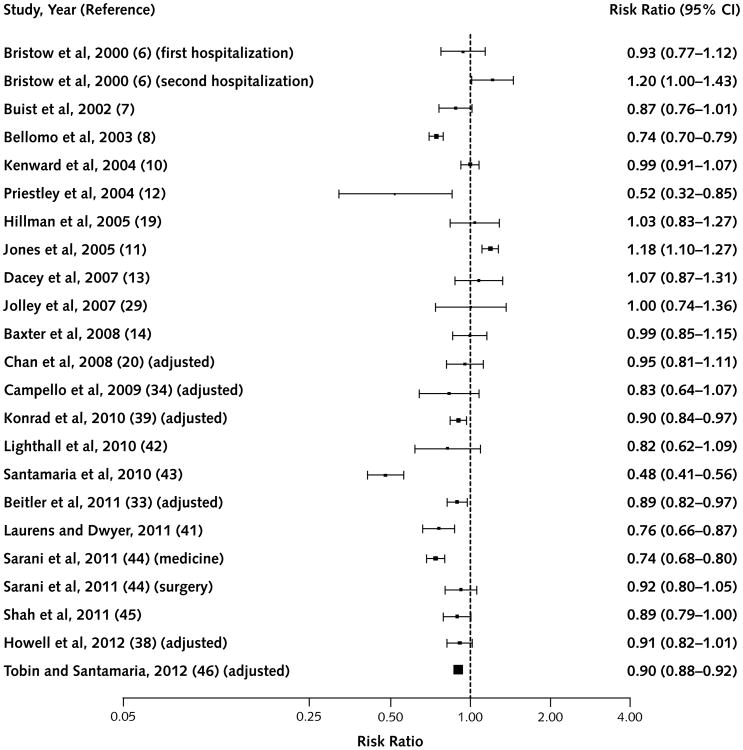
Figure 1 shows studies describing adult non-ICU car-diorespiratory arrest. Nineteen of 20 studies reporting this outcome had point estimates favoring the intervention, 12 of which reached statistical significance. Figure 2 shows adult total hospital mortality. Eighteen of 23 studies showed favorable point estimates, 7 of which were significant.
Figure 2. Studies that reported the outcome of total hospital adult mortality.
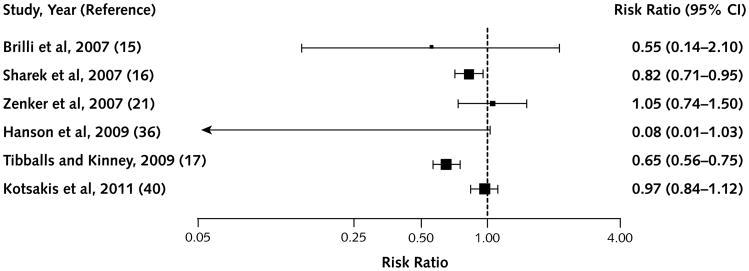
Figure 3 shows pediatric non-ICU cardiorespiratory arrest; all point estimates favored the RRS, and 3 of 7 were significant. Figure 4 shows pediatric total hospital mortality, where all but 1 study (21) had point estimates favoring RRSs; however, only 2 of these findings were significant.
Figure 3. Studies that reported the outcome of non–intensive care unit pediatric cardiorespiratory arrest.
More recent studies more often showed positive results. Although outcomes were heterogeneous—number of hospital discharges during the study period, type of hospital (size and teaching vs. nonteaching status), and RRS model—there was no clear correlation between intervention effectiveness and these characteristics (data not shown).
The overall strength of evidence was moderate when the high-quality systematic review (3) was included, but strength of evidence in the additional studies identified since 2008 was low to moderate. Risk of bias was high for all additional studies because of the before-and-after design. Only a few studies accounted for differences in patient populations over time or reported characteristics of providers in the 2 periods.
Few studies attempted to control for secular trends over time that could have affected outcomes. The 1 study that accounted for such trends found that benefits in mortality and cardiorespiratory arrest rate remained after they were adjusted for (33). No studies reported on or accounted for other safety initiatives that may have influenced the outcomes.
No studies conducted blinded outcome assessment. Although mortality is an objective outcome, the other key outcome, incidence of cardiorespiratory arrest, can be defined in numerous ways (for example, calling the code team vs. documented use of cardiopulmonary resuscitation) and is subject to bias, as are some of the other outcome variations reported (for example, unexpected mortality).
Studies ideally should not report rates for the ICU and emergency departments because these patient populations are rarely part of the exposure group; however, hospital-wide rates were often reported. One study (20) included ICU cardiorespiratory arrest in the analysis (total hospital cardiorespiratory arrest); this study concluded that there was no effect, although data presented on the non-ICU cardio-respiratory arrest rate showed a statistical improvement.
Such arrest rates are also affected by changes in patient case-mix over time, frequency of do-not-resuscitate orders, and terminal illness. However, most studies did not account for these potential confounders. Other outcomes reported, such as unanticipated ICU admissions, are indirect outcomes. In addition, no studies compared RRSs with other interventions that may affect these outcomes, such as enhanced nurse–patient ratios or hospitalists. An unexpected beneficial consequence was improved frequency and quality of end-of-life discussions with patients and their families.
Harms
Potential harms included “deskilling” of ward staff because of dependence on the RRS, inappropriate patient care for other patients (decreased responsiveness of the usual team), staff conflict, diversion of ICU staff from usual care in the ICU, and communication errors caused by introducing additional providers (2). Despite several articles discussing potential harms and unexpected consequences, neither the high-quality systematic review nor any of the additional studies reported any quantitative data for these variables.
Implementation Considerations and Costs
Seventeen studies (10, 48–63) met our inclusion criteria for studies of the implementation processes surrounding RRSs. Eleven of these used quantitative methods primarily for evaluating the effect of a change in the implementation process for an RRS program and 7 used primarily qualitative methods. Most implementation studies were conducted in academic hospitals; however, several studies specifically detailed implementation in community hospitals (10, 13, 14, 23).
Rapid-response systems have been implemented in several contexts and vary in composition, activation criteria, and implementation process. Strong external factors have driven the implementation of RRSs in U.S. hospitals: The 2009 Joint Commission's National Patient Safety Goal 16 (64) and the Institute for Healthcare Improvement (65), as well as numerous other organizations, have created toolkits to help implement RRS interventions. Despite these attempts to reduce variability in the implementation process, our review found that implementation processes differed widely and that local needs and resources tended to dominate the processes.
Education and promotion of the new service was often a factor in preparing for implementation. For staff training and education, several studies introduced new staff, such as a nurse educator. Most studies indicated that implementation processes explicitly included educational activities; however, such activities varied in the degree to which they were strictly information-based or included dedicated training and practice opportunities for RRS members or staff. Such activities as simulation education and training were uncommon. Most studies explicitly noted that cognitive aids, such as posters listing activation criteria, were included.
During development of the afferent limb, various objective criteria were used for calling the team and many interventions depended on nurses' clinical judgment to activate the team on the basis of subjective “worry” or “general concern” (58, 59). One study found that MET hospitals in the MERIT (Medical Emergency Response Intervention and Therapy) trial were 35 times more likely to activate their emergency response team based on the “worried” criteria than control (activated by vital signs) hospitals (P < 0.001) (58).
A few studies also included systems for family or patient initiation of the RRS team. One study showed improved outcomes only after family-initiated activation was implemented (35). No studies reported specific use of technology (such as computerized alerts) to enhance RRS implementation. Some studies used single vital sign triggers, whereas others used early warning scoring systems. In most reports, activation of the efferent limb was voluntary, although 1 study changed its program to mandatory activation on the basis of alert criteria (59).
Most studies used METs as the efferent limb model, but several studies examined rapid-response teams or critical care outreach teams (23); however, none were directly compared. One of the very few studies to compare these models studied a resident-led team and an attending-led MET and found no difference in outcomes (24).
Many RRS implementation efforts have low utilization rates; that is, ward staff do not activate the team despite criteria for activation being met. Although the systematic reviews that we identified did not address this issue, several did so individually. Jones and colleagues (66) examined the barriers and facilitators that affecting nurses' activation of the RRS. The following 5 major themes emerged: adequate education on the RRSs' purpose and role, clinical expertise, support by medical and nursing staff, nurses' familiarity with and advocacy for the patient, and nurses' workload.
Other studies found changes in culture (that is, development of strong support for calling for help and lack of criticism or punishment for activating the team), knowledge of activation criteria, communication, teamwork, and perceptions about the team's helpfulness to nurses and patients to be important influences on utilization. Another factor affecting utilization is the time since initial implementation or duration of the RRS. For example, 1 study (67) specifically examined RRS processes over time and found that the proportion of patients with delayed RRS activation decreased as the RRS matured (40.3% vs. 22.0%; P < 0.001). Other programs have tried various strategies to improve utilization (education, mandatory activation, and changing the activation criteria) (59 – 63).
Team structure may also influence utilization. For example, 1 study reported the effects of separating the overall emergency response system into 2 teams with different activation criteria and processes. Utilization increased sharply (15.7 to 24.7 activations per 1000 admissions; P < 0.001) after the changes were implemented (61).
Patient populations may also benefit differently from RRSs. The high-quality systematic review concluded that RRSs were associated with significantly reduced hospital mortality in pediatric patients but not in adults (3). One study that included 2 separate RRS teams showed an effect in a medical but not a surgical population (44). Most studies to date were conducted in academic centers, although nonacademic hospitals also frequently reported RRS success. Earlier studies that the high-quality systematic review reported were mainly from Australia and the United States; 2 were in England, and 1 was in Canada (3). Since then, the number of countries reporting effectiveness data for RRSs has increased, but how differing national medical cultures affect implementation and effectiveness of the intervention is unclear.
Finally, cost was not evaluated in the high-quality review (3) or in the additional articles that we reviewed.
Discussion
The previous high-quality systematic review and meta-analysis found that, although RRSs were associated with a statistical reduction in rates of cardiorespiratory arrest outside of the ICU among pediatric and adult patients, total hospital mortality was not reduced in adults (3). Our update supports the previous conclusions, although the most recent studies were more likely to show positive results.
The high-quality review found the opposite in its cumulative analysis: Early studies tended to have more positive results. In fact, in 7 sequentially published studies, starting with Kenward and associates' study (10) in 2004 and continuing to Chan and coworkers' study (20) in 2008, the point estimate of effect did not decrease below 0.95.
After Chan and coworkers' study (20) in 2008, all point estimates were less than 0.95. Potential explanations for this result include maturation of the intervention and improved implementation strategies that may have led to improved results in and across institutions. In addition, secular trends of total hospital mortality may have decreased over time unrelated to the intervention, and few studies controlled for this, although 1 study that did found it to not be the case (33).
Although the beneficial effects of RRSs are becoming clearer as the intervention is more universally applied, not all RRS programs realize these benefits. There are several potential explanations for this. An archaic model of patient monitoring on general wards limits the afferent limb (the low sensitivity of periodic visits by clinicians to identify deteriorating patients) (1). Automating the identification of a deteriorating patient through continuous monitoring and a directly activated response team potentially would both improve sensitivity and fidelity and mitigate cultural barriers.
Optimal team composition and structure are unknown. Restricted financial resources may also affect the RRS's ability to self-audit, evaluate events, and improve systematically. Utilization rates are often reported to be low. Creativity and maturation of the intervention are necessary to achieve ultimate long-term goals. Other factors may affect commonly measured outcomes, and several metrics (that is, total cardiorespiratory arrest) count patients who are not exposed to the intervention. Staff and education themes mainly focus on information rather than training. Barriers to effective recognition and response ingrained in the culture of medicine persist.
Given that 80% of patients who have an inpatient cardiorespiratory arrest die, several potential reasons may explain why results for mortality are less robust than those for arrests. For example, the measurement of cardiorespi-ratory arrest can be subjective. In addition, many arrests occur in terminally ill patients, and some studies of RRSs have found evidence for increased rates of do-not-resuscitate orders after RRS implementation. Some studies have tried to account for this factor by measuring rates of cardiorespiratory arrests that are “unexpected” or that occur in patients without do-not-resuscitate orders, but accurately defining terminally ill patients is challenging and may be subject to bias or measurement error.
Our review and the literature have limitations. The updated literature since 2008 includes low- to moderate-quality studies, and several studies have inconsistent findings across outcomes. The elements of the RRS, sample size, and reporting of outcomes varied among these studies. All of the most recent studies have used a before-and-after historically controlled design, which needs to be considered carefully because a recent evaluation of a multifaceted patient safety program in the United Kingdom found statistical improvements in the before-and-after comparison but not in the concurrent cohort controlled comparison (68), as the MERIT study (19) did.
In addition, we reviewed only “effectiveness” studies that reported raw data for mortality and cardiorespiratory arrest. Also, the possibility of selective reporting and publication bias cannot be excluded. For implementation studies, few used formal qualitative methods, and these also addressed various RRS types and study populations. Finally, the relative effectiveness of RRSs compared with other interventions to identify and treat deteriorating patients is unknown.
In summary, we found moderate strength of evidence that RRSs improve outcomes from both a high-quality systematic review through November 2008 and the additional literature published through October 2012. Our review also identified key barriers and facilitators of effective RRS implementation, which included staff acceptance and leadership of the RRS, rates of calling the RRS, and trigger mechanisms.
Rapid-response systems have been described as a “band-aid” for a failed model of managing patients in the general ward in hospitals (69). Although this intervention is beginning to help many hospitals increase recognition of patient deterioration and reduce preventable deaths, they are unlikely to more universally improve these outcomes until we address the culture and system defects that contribute to the root of the problem. For now, RRSs seem to be the best option.
Key Summary Points.
Many hospitals have implemented rapid-response systems (RRSs) over the past 15 years to improve recognition of and response to deteriorating patients in the general ward.
Moderate-strength evidence suggests that RRSs are associated with reduced rates of cardiorespiratory arrest and mortality.
Important components of successful RRSs include criteria and a system for notifying and activating the response team; a response team; and an administrative and quality improvement component to train staff, collect and analyze event data, provide feedback, coordinate resources, and ensure improvement or maintenance over time.
Implementation issues are critical in RRSs because rates of use are often suboptimal secondary to various barriers that could be improved.
Acknowledgments
Financial Support: From the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services (contract HHSA-290- 2007-10062I).
Dr. Winters: Grant (money to institution): Agency for Healthcare Research and Quality; Employment: Johns Hopkins University; Expert testimony: several law firms; Payment for lectures including service on speakers bureaus: 3M; Royalties: Lippincott. Dr. Weaver: Grant (money to institution): Agency for Healthcare Research and Quality; Travel/accommodations/meeting expenses unrelated to activities listed: Improvement Science Research Network. Ms. Pfoh: Grant (money to institution): Agency for Healthcare Research and Quality. Dr. Dy: Grant (money to institution): Agency for Healthcare Research and Quality.
Footnotes
Note: The Agency for Healthcare Research and Quality reviewed contract deliverables to ensure adherence to contract requirements and quality, and a copyright release was obtained from the Agency for Healthcare Research and Quality before submission of the manuscript.
Disclaimer: All statements expressed in this work are those of the authors and should not in any way be construed as official opinions or positions of the Johns Hopkins University, Agency for Healthcare Research and Quality, or U.S. Department of Health and Human Services.
Potential Conflicts of Interest: All other authors have no disclosures. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M12-2568.
Author Contributions: Conception and design: B.D. Winters, S.J. Weaver, J.C. Pham, S.M. Dy.
Analysis and interpretation of the data: B.D. Winters, T. Yang, J.C. Pham, S.M. Dy.
Drafting of the article: B.D. Winters, S.J. Weaver, E.R. Pfoh, J.C. Pham, S.M. Dy.
Critical revision of the article for important intellectual content: B.D. Winters, J.C. Pham, S.M. Dy.
Final approval of the article: B.D. Winters, S.J. Weaver, E.R. Pfoh, J.C. Pham, S.M. Dy.
Provision of study materials or patients: B.D. Winters, Statistical expertise: T. Yang, J.C. Pham.
Obtaining of funding: S.M. Dy.
Administrative, technical, or logistic support: E.R. Pfoh.
Collection and assembly of data: B.D. Winters, S.J. Weaver, E.R. Pfoh, S.M. Dy.
Current author addresses and author contributions are available at www.annals.org.
References
- 1.Buist MD, Jarmolowski E, Burton PR, Bernard SA, Waxman BP, Anderson J. Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary-care hospital. Med J Aust. 1999;171:22–5. doi: 10.5694/j.1326-5377.1999.tb123492.x. [DOI] [PubMed] [Google Scholar]
- 2.Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011;365:139–46. doi: 10.1056/NEJMra0910926. [DOI] [PubMed] [Google Scholar]
- 3.Chan PS, Jain R, Nallmothu BK, Berg RA, Sasson C. Rapid Response Teams: A Systematic Review and Meta-analysis. Arch Intern Med. 2010;170:18–26. doi: 10.1001/archinternmed.2009.424. [DOI] [PubMed] [Google Scholar]
- 4.Owens DK, Lohr KN, Atkins D, Treadwell JR, Reston JT, Bass EB, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—Agency for Healthcare Research and Quality and the Effective Health-Care Program. J Clin Epidemiol. 2010;63:513–23. doi: 10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bristow PJ, Hillman KM, Chey T, Daffurn K, Jacques TC, Norman SL, et al. Rates of in-hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173:236–40. doi: 10.5694/j.1326-5377.2000.tb125627.x. [DOI] [PubMed] [Google Scholar]
- 7.Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324:387–90. doi: 10.1136/bmj.324.7334.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellomo R, Goldsmith D, Uchino S, Buckmaster J, Hart GK, Opdam H, et al. A prospective before-and-after trial of a medical emergency team. Med J Aust. 2003;179:283–7. doi: 10.5694/j.1326-5377.2003.tb05548.x. [DOI] [PubMed] [Google Scholar]
- 9.DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL Medical Emergency Response Improvement Team (MERIT) Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13:251–4. doi: 10.1136/qshc.2003.006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenward G, Castle N, Hodgetts T, Shaikh L. Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61:257–63. doi: 10.1016/j.resuscitation.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Jones D, Bellomo R, Bates S, Warrillow S, Goldsmith D, Hart G, et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Crit Care. 2005;9:R808–15. doi: 10.1186/cc3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priestley G, Watson W, Rashidian A, Mozley C, Russell D, Wilson J, et al. Introducing Critical Care Outreach: a ward-randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30:1398–404. doi: 10.1007/s00134-004-2268-7. [DOI] [PubMed] [Google Scholar]
- 13.Dacey MJ, Mirza ER, Wilcox V, Doherty M, Mello J, Boyer A, et al. The effect of a rapid response team on major clinical outcome measures in a community hospital. Crit Care Med. 2007;35:2076–82. doi: 10.1097/01.ccm.0000281518.17482.ee. [DOI] [PubMed] [Google Scholar]
- 14.Baxter AD, Cardinal P, Hooper J, Patel R. Medical emergency teams at The Ottawa Hospital: the first two years. Can J Anaesth. 2008;55:223–31. doi: 10.1007/BF03021506. [DOI] [PubMed] [Google Scholar]
- 15.Brilli RJ, Gibson R, Luria JW, Wheeler TA, Shaw J, Linam M, et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatr Crit Care Med. 2007;8:236–46. doi: 10.1097/01.PCC.0000262947.72442.EA. [DOI] [PubMed] [Google Scholar]
- 16.Sharek PJ, Parast LM, Leong K, Coombs J, Earnest K, Sullivan J, et al. Effect of a rapid response team on hospital-wide mortality and code rates outside the ICU in a children's hospital. JAMA. 2007;298:2267–74. doi: 10.1001/jama.298.19.2267. [DOI] [PubMed] [Google Scholar]
- 17.Tibballs J, Kinney S. Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Pediatr Crit Care Med. 2009;10:306–12. doi: 10.1097/PCC.0b013e318198b02c. [DOI] [PubMed] [Google Scholar]
- 18.Hunt EA, Zimmer KP, Rinke ML, Shilkofski NA, Matlin C, Garger C, et al. Transition from a traditional code team to a medical emergency team and categorization of cardiopulmonary arrests in a children's center. Arch Pediatr Adolesc Med. 2008;162:117–22. doi: 10.1001/archpediatrics.2007.33. [DOI] [PubMed] [Google Scholar]
- 19.Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, Doig G, et al. MERIT study investigators. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365:2091–7. doi: 10.1016/S0140-6736(05)66733-5. [DOI] [PubMed] [Google Scholar]
- 20.Chan PS, Khalid A, Longmore LS, Berg RA, Kosiborod M, Spertus JA. Hospital-wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–13. doi: 10.1001/jama.2008.715. [DOI] [PubMed] [Google Scholar]
- 21.Zenker P, Schlesinger A, Hauck M, Spencer S, Hellmich T, Finkelstein M, et al. Implementation and impact of a rapid response team in a children's hospital. Jt Comm J Qual Patient Saf. 2007;33:418–25. doi: 10.1016/s1553-7250(07)33048-1. [DOI] [PubMed] [Google Scholar]
- 22.Benson L, Mitchell C, Link M, Carlson G, Fisher J. Using an advanced practice nursing model for a rapid response team. Jt Comm J Qual Patient Saf. 2008;34:743–7. doi: 10.1016/s1553-7250(08)34097-5. [DOI] [PubMed] [Google Scholar]
- 23.Bader MK, Neal B, Johnson L, Pyle K, Brewer J, Luna M, et al. Rescue me: saving the vulnerable non-ICU patient population. Jt Comm J Qual Patient Saf. 2009;35:199–205. doi: 10.1016/s1553-7250(09)35027-8. [DOI] [PubMed] [Google Scholar]
- 24.Karvellas CJ, de Souza IA, Gibney RT, Bagshaw SM. Association between implementation of an intensivist-led medical emergency team and mortality. BMJ Qual Saf. 2012;21:152–9. doi: 10.1136/bmjqs-2011-000393. [DOI] [PubMed] [Google Scholar]
- 25.Medina-Rivera B, Campos-Santiago Z, Palacios AT, Rodriguez-Cintron W. The effect of the medical emergency team on unexpected cardiac arrest and death at the VA Caribbean Healthcare System: a retrospective study. Critical Care and Shock. 2010;13:98–105. [Google Scholar]
- 26.Rothberg MB, Belforti R, Fitzgerald J, Friderici J, Keyes M. Four years' experience with a hospitalist-led medical emergency team: an interrupted time series. J Hosp Med. 2012;7:98–103. doi: 10.1002/jhm.953. [DOI] [PubMed] [Google Scholar]
- 27.Scherr K, Wilson DM, Wagner J, Haughian M. Evaluating a new rapid response team: NP-led versus intensivist-led comparisons. AACN Adv Crit Care. 2012;23:32–42. doi: 10.1097/NCI.0b013e318240e2f9. [DOI] [PubMed] [Google Scholar]
- 28.Scott SS, Elliott S. Implementation of a rapid response team: a success story. Crit Care Nurse. 2009;29:66–75. doi: 10.4037/ccn2009802. [DOI] [PubMed] [Google Scholar]
- 29.Jolley J, Bendyk H, Holaday B, Lombardozzi KA, Harmon C. Rapid response teams: do they make a difference? Dimens Crit Care Nurs. 2007;26:253–60. doi: 10.1097/01.DCC.0000297401.67854.78. [DOI] [PubMed] [Google Scholar]
- 30.Offner PJ, Heit J, Roberts R. Implementation of a rapid response team decreases cardiac arrest outside of the intensive care unit. J Trauma. 2007;62:1223–7. doi: 10.1097/TA.0b013e31804d4968. [DOI] [PubMed] [Google Scholar]
- 31.Thomas K, VanOyen Force M, Rasmussen D, Dodd D, Whildin S. Rapid response team: challenges, solutions, benefits. Crit Care Nurse. 2007;27:20–7. [PubMed] [Google Scholar]
- 32.Anwar-ul-Haque, Saleem AF, Zaidi S, Haider SR. Experience of pediatric rapid response team in a tertiary care hospital in Pakistan. Indian J Pediatr. 2010;77:273–6. doi: 10.1007/s12098-010-0032-2. [DOI] [PubMed] [Google Scholar]
- 33.Beitler JR, Link N, Bails DB, Hurdle K, Chong DH. Reduction in hospital-wide mortality after implementation of a rapid response team: a long-term cohort study. Crit Care. 2011;15:R269. doi: 10.1186/cc10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campello G, Granja C, Carvalho F, Dias C, Azevedo LF, Costa-Pereira A. Immediate and long-term impact of medical emergency teams on cardiac arrest prevalence and mortality: a plea for periodic basic life-support training programs. Crit Care Med. 2009;37:3054–61. doi: 10.1097/CCM.0b013e3181b02183. [DOI] [PubMed] [Google Scholar]
- 35.Gerdik C, Vallish RO, Miles K, Godwin SA, Wludyka PS, Panni MK. Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2010;81:1676–81. doi: 10.1016/j.resuscitation.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Hanson CC, Randolph GD, Erickson JA, Mayer CM, Bruckel JT, Harris BD, et al. A reduction in cardiac arrests and duration of clinical instability after implementation of a paediatric rapid response system. Qual Saf Health Care. 2009;18:500–4. doi: 10.1136/qshc.2007.026054. [DOI] [PubMed] [Google Scholar]
- 37.Hatler C, Mast D, Bedker D, Johnson R, Corderella J, Torres J, et al. Implementing a rapid response team to decrease emergencies outside the ICU: one hospital's experience. Medsurg Nurs. 2009;18:84–90. [PubMed] [Google Scholar]
- 38.Howell MD, Ngo L, Folcarelli P, Yang J, Mottley L, Marcantonio ER, et al. Sustained effectiveness of a primary-team-based rapid response system. Crit Care Med. 2012;40:2562–8. doi: 10.1097/CCM.0b013e318259007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konrad D, Jäderling G, Bell M, Granath F, Ekbom A, Martling CR. Reducing in-hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36:100–6. doi: 10.1007/s00134-009-1634-x. [DOI] [PubMed] [Google Scholar]
- 40.Kotsakis A, Lobos AT, Parshuram C, Gilleland J, Gaiteiro R, Mohseni-Bod H, et al. Ontario Pediatric Critical Care Response Team Collaborative. Implementation of a multicenter rapid response system in pediatric academic hospitals is effective. Pediatrics. 2011;128:72–8. doi: 10.1542/peds.2010-0756. [DOI] [PubMed] [Google Scholar]
- 41.Laurens N, Dwyer T. The impact of medical emergency teams on ICU admission rates, cardiopulmonary arrests and mortality in a regional hospital. Resuscitation. 2011;82:707–12. doi: 10.1016/j.resuscitation.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 42.Lighthall GK, Parast LM, Rapoport L, Wagner TH. Introduction of a rapid response system at a United States veterans affairs hospital reduced cardiac arrests. Anesth Analg. 2010;111:679–86. doi: 10.1213/ANE.0b013e3181e9c3f3. [DOI] [PubMed] [Google Scholar]
- 43.Santamaria J, Tobin A, Holmes J. Changing cardiac arrest and hospital mortality rates through a medical emergency team takes time and constant review. Crit Care Med. 2010;38:445–50. doi: 10.1097/CCM.0b013e3181cb0ff1. [DOI] [PubMed] [Google Scholar]
- 44.Sarani B, Palilonis E, Sonnad S, Bergey M, Sims C, Pascual JL, et al. Clinical emergencies and outcomes in patients admitted to a surgical versus medical service. Resuscitation. 2011;82:415–8. doi: 10.1016/j.resuscitation.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Shah SK, Cardenas VJ, Jr, Kuo YF, Sharma G. Rapid response team in an academic institution: does it make a difference? Chest. 2011;139:1361–7. doi: 10.1378/chest.10-0556. [DOI] [PubMed] [Google Scholar]
- 46.Tobin AE, Santamaria JD. Medical emergency teams are associated with reduced mortality across a major metropolitan health network after two years service: a retrospective study using government administrative data. Crit Care. 2012;16:R210. doi: 10.1186/cc11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosch FH, de Jager CPC. Number of resuscitations for in-hospital cardio-pulmonary arrests decreases after introduction of a medical emergency team. “The Arnhem experience”. Netherlands Journal of Critical Care. 2008;12:256–9. [Google Scholar]
- 48.Calzavacca P, Licari E, Tee A, Egi M, Downey A, Quach J, et al. The impact of Rapid Response System on delayed emergency team activation patient characteristics and outcomes—a follow-up study. Resuscitation. 2010;81:31–5. doi: 10.1016/j.resuscitation.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 49.Williams DJ, Newman A, Jones C, Woodard B. Nurses' perceptions of how rapid response teams affect the nurse, team, and system. J Nurs Care Qual. 2011;26:265–72. doi: 10.1097/NCQ.0b013e318209f135. [DOI] [PubMed] [Google Scholar]
- 50.Shapiro SE, Donaldson NE, Scott MB. Rapid response teams seen through the eyes of the nurse. Am J Nurs. 2010;110:28–34. doi: 10.1097/01.NAJ.0000377686.64479.84. [DOI] [PubMed] [Google Scholar]
- 51.Donaldson N, Shapiro S, Scott M, Foley M, Spetz J. Leading successful rapid response teams: A multisite implementation evaluation. J Nurs Adm. 2009;39:176–81. doi: 10.1097/NNA.0b013e31819c9ce9. [DOI] [PubMed] [Google Scholar]
- 52.Adelstein BA, Piza MA, Nayyar V, Mudaliar Y, Klineberg PL, Rubin G. Rapid response systems: a prospective study of response times. J Crit Care. 2011;26:635.e11–8. doi: 10.1016/j.jcrc.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Cretikos MA, Chen J, Hillman KM, Bellomo R, Finfer SR, Flabouris A MERIT Study Investigators. The effectiveness of implementation of the medical emergency team (MET) system and factors associated with use during the MERIT study. Crit Care Resusc. 2007;9:206–12. [PubMed] [Google Scholar]
- 54.Mackintosh N, Rainey H, Sandall J. Understanding how rapid response systems may improve safety for the acutely ill patient: learning from the frontline. BMJ Qual Saf. 2012;21:135–44. doi: 10.1136/bmjqs-2011-000147. [DOI] [PubMed] [Google Scholar]
- 55.Shearer B, Marshall S, Buist MD, Finnigan M, Kitto S, Hore T, et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi-campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21:569–75. doi: 10.1136/bmjqs-2011-000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soo S, Berta W, Baker GR. Role of champions in the implementation of patient safety practice change. Healthc Q. 2009:123–8. doi: 10.12927/hcq.2009.20979. 12 Spec No Patient. [DOI] [PubMed] [Google Scholar]
- 57.Chen J, Bellomo R, Hillman K, Flabouris A, Finfer S MERIT Study Investigators for the Simpson Centre and the ANZICS Clinical Trials Group. Triggers for emergency team activation: a multicenter assessment. J Crit Care. 2010;25:359.e1–7. doi: 10.1016/j.jcrc.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Genardi ME, Cronin SN, Thomas L. Revitalizing an established rapid response team. Dimens Crit Care Nurs. 2008;27:104–9. doi: 10.1097/01.DCC.0000286837.95720.8c. [DOI] [PubMed] [Google Scholar]
- 59.Jones CM, Bleyer AJ, Petree B. Evolution of a rapid response system from voluntary to mandatory activation. Jt Comm J Qual Patient Saf. 2010;36:266–70. 241. doi: 10.1016/s1553-7250(10)36042-9. [DOI] [PubMed] [Google Scholar]
- 60.Peebles E, Subbe CP, Hughes P, Gemmell L. Timing and teamwork—an observational pilot study of patients referred to a rapid response team with the aim of identifying factors amenable to re-design of a rapid response system. Resuscitation. 2012;83:782–7. doi: 10.1016/j.resuscitation.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 61.Jones DA, Mitra B, Barbetti J, Choate K, Leong T, Bellomo R. Increasing the use of an existing medical emergency team in a teaching hospital. Anaesth Intensive Care. 2006;34:731–5. doi: 10.1177/0310057X0603400606. [DOI] [PubMed] [Google Scholar]
- 62.Foraida MI, DeVita MA, Braithwaite RS, Stuart SA, Brooks MM, Simmons RL. Improving the utilization of medical crisis teams (Condition C) at an urban tertiary care hospital. J Crit Care. 2003;18:87–94. doi: 10.1053/jcrc.2003.50002. [DOI] [PubMed] [Google Scholar]
- 63.Jones D, Bates S, Warrillow S, Goldsmith D, Kattula A, Way M, et al. Effect of an education programme on the utilization of a medical emergency team in a teaching hospital. Intern Med J. 2006;36:231–6. doi: 10.1111/j.1445-5994.2006.01045.x. [DOI] [PubMed] [Google Scholar]
- 64.Joint Commission on Accreditation of Healthcare Organizations. 2008 National Patient Safety Goals. Joint Commission Perspectives. 2007;27:10–22. [PubMed] [Google Scholar]
- 65.Institute for Healthcare Improvement. 5 Million Lives Campaign: Overview. Accessed at www.ihi.org/offerings/Initiatives/PastStrategicInitiatives/5MillionLivesCampaign/Pages/default.aspx on 28 November 2012.
- 66.Jones L, King L, Wilson C. A literature review: factors that impact on nurses' effective use of the Medical Emergency Team (MET) J Clin Nurs. 2009;18:3379–90. doi: 10.1111/j.1365-2702.2009.02944.x. [DOI] [PubMed] [Google Scholar]
- 67.Buist M, Harrison J, Abaloz E, Van Dyke S. Six year audit of cardiac arrests and medical emergency team calls in an Australian outer metropolitan teaching hospital. BMJ. 2007;335:1210–2. doi: 10.1136/bmj.39385.534236.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benning A, Dixon-Woods M, Nwulu U, Ghaleb M, Dawson J, Barber N, et al. Multiple component patient safety intervention in English hospitals: controlled evaluation of second phase. BMJ. 2011;342:d199. doi: 10.1136/bmj.d199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Litvak E, Pronovost PJ. Rethinking rapid response teams. JAMA. 2010;304:1375–6. doi: 10.1001/jama.2010.1385. [DOI] [PubMed] [Google Scholar]