Abstract
Cardiac function is required for blood circulation and systemic oxygen delivery. However, the heart has intrinsic oxygen demands that must be met to maintain effective contractility. Hypoxia-inducible factor 1 (HIF-1) is a transcription factor that functions as a master regulator of oxygen homeostasis in all metazoan species. HIF-1 controls oxygen delivery, by regulating angiogenesis and vascular remodeling, and oxygen utilization, by regulating glucose metabolism and redox homeostasis. Analysis of animal models suggests that by activation of these homeostatic mechanisms, HIF-1 plays a critical protective role in the pathophysiology of ischemic heart disease and pressure-overload heart failure.
Keywords: L heart failure, myocardial ischemia, preconditioning, pressure overload, vascular remodeling
INTRODUCTION: OXYGEN HOMEOSTASIS AND HYPOXIA-INDUCIBLE FACTOR 1
The increase in body mass during vertebrate evolution was made possible by the coevolution of complex respiratory and circulatory systems designed to efficiently capture and distribute sufficient O2 and nutrients to each cell within the organism. The first physiological system to become functional during mammalian development is the circulatory system, which must be established once the embryo becomes too large for O2 to be delivered simply by diffusion from uterine vessels.
In all metazoan species, hypoxia-inducible factor 1 (HIF-1) functions as a master regulator of oxygen homeostasis by controlling both the delivery and utilization of O2. HIF-1 is a heterodimer that is composed of an O2-regulated HIF-1α subunit and a constitutively expressed HIF-1β subunit (1, 2). Mouse embryos that are homozygous for a knockout allele at the Hif1a locus, which encodes HIF-1α, arrest in their development at embryonic day 8.5 and die by embryonic day 10.5 with cardiac and vascular defects as well as decreased red blood cell production, indicating that all three components of the circulatory system are dependent upon HIF-1 for proper development (3–5). Recent data suggest that partial HIF-1α deficiency may be associated with congenital heart defects in humans as well (6).
HIF-1 activity is induced by hypoxia through changes in HIF-1α mRNA and protein levels in brain (7, 8), heart (9–11), kidney (7, 12), lung (7, 13), and skeletal muscle (14). The regulation of HIF-1α mRNA levels is not well understood because it is not observed in most cell lines, whereas O2-dependent prolyl hydroxylation and asparaginyl hydroxylation of HIF-1α negatively regulate its protein stability and transcriptional activity, respectively (15).
HIF-1 activates gene transcription by binding to the core DNA sequence 5′-RCGTG-3′ (R = A or G), which is embedded within a hypoxia response element (HRE) (16). The HRE is defined functionally as a cis-acting regulatory element that—when inserted into a reporter gene, such as luciferase coding sequences driven by a basal SV40 promoter—mediates hypoxia-inducible and HIF-dependent gene expression (17). In many HREs, the HIF-1-binding site is followed after 0–8 nucleotides by the sequence 5′-CACA-3′ (Table 1). When either the 5′-RCGTG-3′ or the 5′-CACA-3′ sequence in the EPO gene HRE was mutated, the HRE no longer mediated hypoxia-inducible transcription (17). Whereas HIF-1 is known to bind to 5′-RCGTG-3′, the factor binding to 5′-CACA-3′ has not been determined.
Table 1.
Examples of bipartite sequence motifs within hypoxia response elements (HREs)a
| Gene | Nucleotide sequence | Reference |
|---|---|---|
| EPO |
|
17 |
| ALDOA |
|
16 |
| PKM2 |
|
74 |
| PDGFB |
|
75 |
| CXCR3 (HRE1)b |
|
76 |
| ANGPTL4 |
|
77 |
| CP |
|
78 |
| COX4I2 (HRE2)b |
|
54 |
| CXCR3 (HRE2)b |
|
76 |
| BNIP3 |
|
79 |
| ILK |
|
80 |
| MXI1 |
|
81 |
| PGF (HRE2)b |
|
76 |
| PGF (HRE1)b |
|
76 |
| COX4I2 (HRE1)b |
|
54 |
Blue font indicates the 5′-RCGTG-3′ sequence; HIF-1 binds to this site. Red font indicates the 5′-CACA-3′ sequence; the factor binding to this site has not been identified. Hyphens have been introduced to facilitate alignment of the sequences.
Genes may contain more than one HRE.
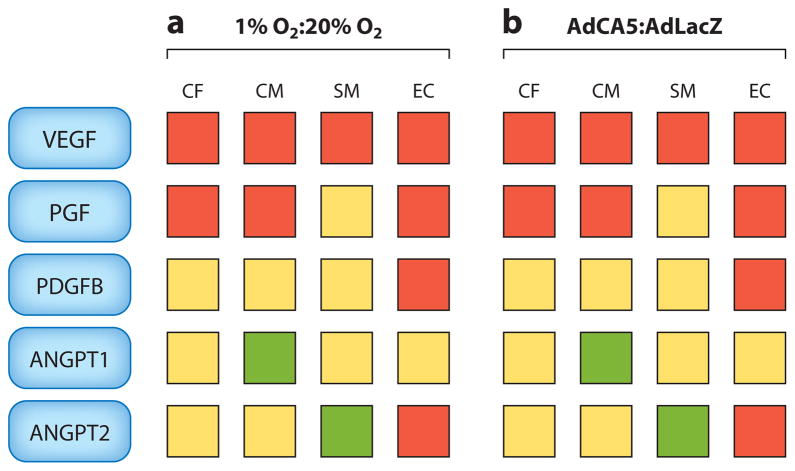
Genome-wide chromatin immunoprecipitation (ChIP) assays have been employed to comprehensively identify all sites of hypoxia-inducible binding of HIF-1 to genes whose mRNA expression is induced by hypoxia (18–20). When all methods of analysis are taken into consideration, HIF-1 directly regulates the expression of more than 1,000 human genes. Whereas the expression of a subset of HIF-1 target genes is induced by hypoxia in most or all cell types, the vast majority of these genes are induced by hypoxia in a cell type–specific manner. Indeed, the analysis of just five genes, those encoding vascular endothelial growth factor (VEGF), angiopoietin 1 (ANGPT1), ANGPT2, placental growth factor (PGF), and platelet-derived growth factor B, in four primary cell types (cardiomyocytes, cardiac fibroblasts, vascular endothelial cells, and vascular smooth muscle cells) revealed that each cell type showed a different pattern of gene expression in response to hypoxia (Figure 1). The same transcriptional response was induced under nonhypoxic conditions by transfecting the cells with AdCA5, an adenovirus encoding a constitutively active form of HIF-1α (21). Thus, HIF-1 mediates transcriptional responses to hypoxia that are context dependent.
Figure 1.
HIF-1 mediates cell type–specific responses to hypoxia. The relative expression levels of mRNAs encoding vascular endothelial growth factor (VEGF), placental growth factor (PGF), platelet-derived growth factor B (PDGFB), angiopoietin 1 (ANGPT1), and ANGPT2 were determined in primary cultures of cardiac fibroblasts (CF), cardiomyocytes (CM), vascular smooth muscle cells (SM), and vascular endothelial cells (EC) exposed for 24 h either to (a) 1% versus 20% O2 or to (b) an adenoviral vector encoding a constitutively active form of HIF-1α(AdCA5) versus Escherichia coli β-galactosidase (AdLacZ) at 20% O2. Red, yellow, and green squares indicate values for the indicated ratios (1% O2:20% O2 and AdCA5:AdLacZ) of >1, = 1, and < 1, respectively. HIF-1 target gene products are depicted as blue rectangles. Adapted from Reference 21.
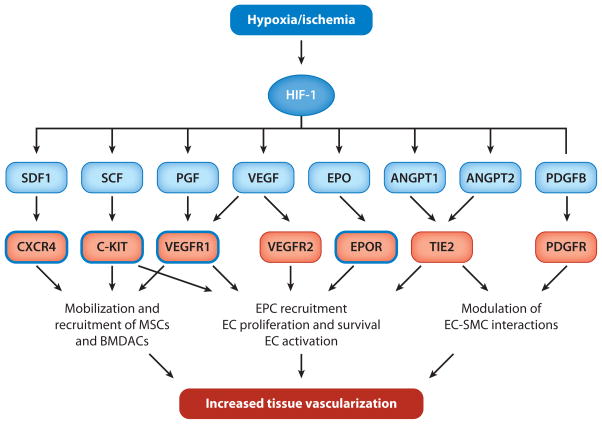
Among the HIF-1 target genes are those encoding secreted factors and cell surface receptors that control O2 delivery by regulating angiogenesis and vascular remodeling (Table 2). HIF-1 functions as a master regulator in this process because it coordinately regulates the expression of a large number of genes whose protein products play critical roles in mediating vascular responses to hypoxia and ischemia (Figure 2). In addition to promoting O2 delivery, HIF-1 also activates the transcription of genes encoding enzymes, transporters, and mitochondrial proteins that decrease O2 utilization, again functioning as a master regulator to switch cells from oxidative metabolism to glycolytic metabolism (Table 3). The protective role of these HIF-1-dependent homeostatic mechanisms in the pathophysiology of ischemic heart disease (IHD) and pressure-overload heart failure is described in greater detail below.
Table 2.
Examples of HIF-1 target genes encoding secreted factors (black text) and cell surface receptors (red text) involved in angiogenesis, vascular reactivity, and vascular remodeling
| Gene | Encoded protein | References demonstrating regulation by HIF-1 |
|---|---|---|
| ADM | Adrenomedullin | 82, 83 |
| ADM2 | Adrenomedullin 2 (intermedin) | 84 |
| ADORA2A | Adenosine A2A receptor | 83, 85 |
| ADORA2B | Adenosine A2B receptor | 86 |
| ADR1B | α1B-Adrenergic receptor | 87 |
| ANGPTL4 | Angiopoietin-like 4 | 77, 83 |
| ANGPT1 | Angiopoietin 1 | 14, 21 |
| ANGPT2 | Angiopoietin 2 | 21, 88 |
| APLN | Apelin | 89 |
| CCL2 | Macrophage chemotactic protein 1 | 90 |
| CTGF | Connective tissue growth factor | 91 |
| CXCR4 | CXC chemokine receptor 4 | 83, 92 |
| EDN1 | Endothelin 1 | 93, 94 |
| EDNRB | Endothelin receptor B | 95 |
| EFNA1 | Ephrin A1 | 96, 97 |
| EFNB2 | Ephrin B2 | 96 |
| ENG | Endoglin (CD105) | 98 |
| EPHB4 | Eph B4 | 96 |
| EPO | Erythropoietin | 17 |
| EPOR | Erythropoietin receptor | 5, 83 |
| FLT1 | VEGF receptor 1 | 99, 100 |
| KITLG | KIT ligand (stem cell factor) | 14, 101 |
| LEP | Leptin | 102 |
| MDK | Midkine | 103 |
| PDGFB | Platelet-derived growth factor B | 21, 75 |
| PGF | Placental growth factor | 21, 76 |
| PROK1 | Prokineticin 1 | 104 |
| CXCL12 | Stromal cell–derived factor 1 | 105 |
| VEGF | Vascular endothelial growth factor | 3, 106 |
Figure 2.
HIF-1 coordinately regulates vascular responses to hypoxia and ischemia. HIF-1 activates the transcription of multiple genes encoding angiogenic growth factors and cytokines (blue rectangles), which bind to cognate cell surface receptors (red rectangles) to mediate their biological effects on endothelial cells (ECs), vascular smooth muscle cells (SMCs), endothelial progenitor cells (EPCs), mesenchymal stem cells (MSCs), and other bone marrow–derived angiogenic cells (BMDACs). The blue outlines denote receptors whose expression is also regulated by HIF-1 in certain cell types.
Table 3.
Examples of HIF-1 target genes encoding metabolic enzymes (blue text), transporters (red text), and mitochondrial proteins (green text) involved in glucose metabolism
| Gene | Encoded protein | References supporting regulation by HIF-1 |
|---|---|---|
| ALDOA | Aldolase A | 3, 16, 107 |
| ALDOC | Aldolase C | 3, 108 |
| BNIP3 | BNIP3 | 79, 83, 109 |
| BNIP3L | BNIP3-like (NIX) | 83, 109 |
| CAR9 | Carbonic anhydrase 9 | 19, 29 |
| COX4I2 | Cytochrome oxidase subunit 4-2 | 54 |
| ENO1 | Enolase 1 | 3, 16 |
| ENO2 | Enolase 2 | 83 |
| GAPDH | Glyceraldehyde phosphate dehydrogenase | 3, 107, 110 |
| GPI | Glucose phosphate isomerase | 18, 19, 111 |
| HK1 | Hexokinase 1 | 3 |
| HK2 | Hexokinase 2 | 3, 18, 19 |
| LDHA | Lactate dehydrogenase A | 3, 16, 112 |
| miR210 | MicroRNA-210 | 58, 59 |
| PDK1 | Pyruvate dehydrogenase kinase 1 | 55, 56 |
| PDK3 | Pyruvate dehydrogenase kinase 3 | 57 |
| PFKFB3 | 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 | 113 |
| PFKFB4 | 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 | 113 |
| PFKL | Phosphofructokinase, liver | 3, 19 |
| PGAM1 | Phosphoglycerate mutase 1 | 19 |
| PGK1 | Phosphoglycerate kinase 1 | 3, 107, 114 |
| PGM1 | Phosphoglucomutase 1 | 3, 83 |
| PGM3 | Phosphoglucomutase 3 | 83 |
| SLC2A1 | Glucose transporter 1 | 3, 107, 115 |
| SLC2A3 | Glucose transporter 3 | 3, 83 |
| SLC9A1 | Sodium-hydrogen exchanger 1 | 116 |
| SLC16A3 | Monocarboxylate transporter 4 | 18, 29, 117 |
| TKT | Transketolase | 118 |
| TKTL2 | Transketolase-like 2 | 118 |
| TPI1 | Triosephosphate isomerase | 3, 119 |
ISCHEMIC HEART DISEASE
Clinical Overview: Coronary Artery Stenosis and Collateral Remodeling
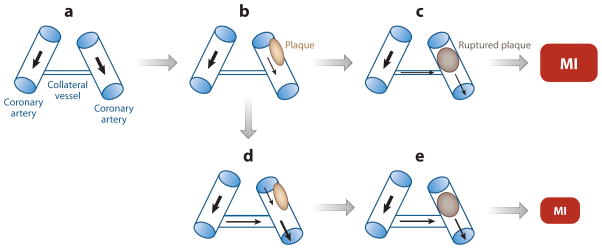
IHD is the leading cause of mortality in the US population, accounting for one in every six deaths at a rate of one death every minute (22). IHD is an age-dependent phenotype, with a prevalence of 3.1% in those less than 50 years old versus 12.3% in those over the age of 50 (23). The formation of an atherosclerotic plaque in the wall of a major coronary artery reduces the perfusion of myocardial tissue downstream of the stenosis (Figure 3a,b). Ischemia refers to the state in which perfusion is not adequate to supply adequate O2 and nutrients or to remove toxic metabolites. Ischemia is particularly likely to occur under conditions of increased heart work, such as that occurring in response to physical exertion or emotional stress, and the associated chest pain is referred to as stable (exertional) angina. Patients with severe stenosis that results in ischemia and chest pain at rest manifest unstable angina, which can lead to death of the ischemic tissue (myocardial infarction [MI]). Unstable angina and myocardial infarction are often precipitated by plaque rupture, which results in complete arterial occlusion (Figure 3c).
Figure 3.
Remodeling of a collateral blood vessel in response to coronary artery stenosis. (a) Two major coronary arteries, which are connected by an undeveloped collateral vessel, are shown. (b) Atherosclerotic plaque formation (tan oval ) results in stenosis of one artery. (c) Plaque rupture results in complete arterial obstruction (brown oval ), leading to a large myocardial infarction (MI). (d ) Remodeling of the collateral vessel to increase luminal diameter results in increased blood flow. (e) When plaque rupture occurs, the reduction in blood flow is mitigated, resulting in a smaller area of infarction. Black arrows denote the direction and relative magnitude of blood flow.
The normal physiological response to reduced tissue perfusion is that the resulting tissue hypoxia induces HIF-1 activity, which activates transcription of genes encoding angiogenic factors (Figure 2 and Table 2). These factors stimulate the remodeling of collateral blood vessels, leading to increased blood flow (Figure 3d). Among patients with IHD who have critical coronary artery stenosis (angiographic narrowing of at least 70% of luminal diameter), approximately two-thirds have a remodeled collateral vessel, and one-third do not; among patients with MI, those patients with collaterals have decreased infarct size (Figure 3e) and increased survival compared with patients without collaterals (Figure 3e) (24, 25). However, the factors that determine whether or when patients with critical coronary artery stenosis will develop collaterals have not been established.
Effects of Aging and HIF-1 on Ischemia-Induced Vascular Remodeling
Mice subjected to femoral artery ligation recover blood flow over the course of several weeks. The rate and extent of recovery are progressively impaired as the age of the mouse increases, leading to progressively more severe tissue damage (14, 26). Although Hif1a−/− mice were not viable, Hif1a+/− mice developed normally and were indistinguishable from their wild-type (WT) littermates (3). At every age, compared with their WT littermates, Hif1a+/− mice showed reduced recovery of blood flow and increased tissue damage after femoral artery ligation (14). Both aging and the Hif1a+/− genotype were associated with decreased expression of HIF-1α protein and of multiple mRNAs encoding angiogenic growth factors, including VEGF, ANGPT1, ANGPT2, PGF, stromal cell–derived factor 1 (also known as CXCL12), and stem cell factor (also known as KIT ligand) (14). Intramuscular injection of AdCA5 into the ischemic limb was sufficient to overcome the age-dependent impairment of ischemia-induced vascular remodeling in 8-month-old mice (14). In rabbits, AdCA5 therapy increased the luminal diameter of collateral vessels, as demonstrated by both angiography and immunohistochemistry (27)
In contrast to the beneficial effects of AdCA5 in 8-month-old mice, the same gene therapy had no beneficial effect in 13-month-old mice (28). However, intramuscular injection of AdCA5 into the ischemic limb, followed 24 h later by intravenous injection of bone marrow–derived angiogenic cells (BMDACs) that had been cultured for 4 days in the presence of angiogenic factors and a chemical inducer of HIF-1 activity, led to dramatically increased recovery of blood flow and protection against tissue damage (28). The local injection of AdCA5 induced expression of angiogenic factors that served as a homing signal for the BMDACs. HIF-1 induction in the BMDACs had two major effects: It induced the cell surface expression of β2 integrins that promoted adherence of the BMDACs to endothelial cells in the ischemic tissue (28), and it mediated a switch from oxidative metabolism to glycolytic metabolism that promoted BMDAC survival in the ischemic limb (29). These mouse studies suggest that aging impairs ischemia-induced vascular remodeling by inhibiting the induction of HIF-1 and its downstream target genes, thereby blocking both the production of angiogenic signals and the ability of BMDACs to respond to them.
In a porcine model of coronary artery stenosis, pressure-regulated retroinfusion of the great cardiac vein with an adenovirus that encoded a fusion protein consisting of the amino-terminal half of HIF-1α (which contains the dimerization and DNA-binding domains) fused to VP16 (a herpesvirus transactivator) was associated with an increased number of angiographically detectable collateral vessels and with increased myocardial function (30). However, a clinical trial failed to demonstrate increased myocardial perfusion in patients who were undergoing coronary artery bypass graft surgery and who received this gene therapy (31). Although the age of the pigs used in the animal study was not stated (30), it is likely that the researchers used young animals that did not appropriately model the age of the clinical population. Studies of combined AdCA5 and BMDAC therapy in aged animal models of myocardial ischemia are needed to inform the design of future clinical trials.
Genetic Data Implicate HIF-1 in Responses to Ischemic Heart Disease
Are the data from animal models of limb and myocardial ischemia relevant to IHD? Does genetic variation at the human HIF1A locus influence whether collateral vessels develop in response to coronary artery stenosis or whether patients present with stable angina or MI? In IHD patients undergoing coronary angiography, analysis of a single-nucleotide polymorphism (SNP) that changes the translated amino acid sequence of HIF-1α from proline to serine at codon 582 (P582S) revealed that the frequency of the variant allele was fivefold higher in patients who had collaterals compared with patients lacking collaterals (32), suggesting that IHD patients with the variant allele either have impaired collateral remodeling or present earlier in the disease process. A genome-wide SNP study to identify genetic markers that predicted presentation with stable angina versus MI revealed increased frequency of P582S and two other SNPs at the HIF1A locus in patients who presented with stable angina compared with patients who presented with MI (33). The interpretation of these results is unclear because the latter study did not include angiographic data regarding the severity of coronary artery stenosis in the patients. However, taken together, these two human studies and the mouse studies described above support the conclusion that genetic variation at the locus encoding HIF-1α influences the response to ischemic cardiovascular disease. Another angiographic study reported an association between the P582S allele and reduced coronary artery branching (34), providing further evidence that HIF-1 regulates the coronary vasculature.
HIF-1 Mediates Cardioprotection Induced by Ischemic Preconditioning
Plaque rupture is a catastrophic event that results in complete arterial occlusion and, within ~20 min, the onset of progressive death of cardiac cells (MI) due to O2 deprivation (35). Rapid reperfusion after ischemia (by thrombolytic therapy or percutaneous coronary intervention) is the single most important clinical factor that limits infarct size while at the same time reperfusion contributes to tissue injury by increasing intracellular reactive oxygen species and Ca2+ (36). Exposure of the heart to short (i.e., 5-min) episodes of ischemia and reperfusion protects the heart against injury caused by a subsequent prolonged (i.e., 30-min) episode of ischemia-reperfusion, a phenomenon known as ischemic preconditioning (IPC) (37). The protection afforded by IPC occurs in two phases: an early phase, which begins immediately after the IPC stimulus and lasts for several hours (37), and a late phase, which begins approximately 24 h after the IPC stimulus and lasts for several days (38, 39). Acute cardioprotection against ischemia-reperfusion injury can also be induced pharmacologically, e.g., by perfusing the heart with adenosine (40).
When hearts from WT mice were exposed to an IPC stimulus and were immediately subjected to prolonged ischemia-reperfusion, infarct size was dramatically decreased, whereas the IPC stimulus afforded no protection in hearts from Hif1a+/− mice (41). In contrast, adenosine perfusion induced acute cardioprotection in both WT and Hif1a+/− mice, indicating a specific defect in IPC (41). These results were surprising because early-phase cardioprotection was generally thought to involve posttranslational modification of existing proteins or metabolic alterations, whereas late-phase cardioprotection was thought to involve new protein synthesis.
Further studies revealed that conditional knockout of HIF-1α or HIF-1β expression in endothelial cells of the heart also resulted in a lack of acute cardioprotection following an IPC stimulus (42). This result was also surprising because IPC was generally thought to primarily involve responses in cardiomyocytes, leading some investigators to develop cell-based models (43). The requirement for both HIF-1α and HIF-1β strongly suggested a requirement for HIF-1 transcriptional activity, despite the rapidity of the protective response. Furthermore, when WT hearts were infused immediately prior to the IPC stimulus with acriflavine, a drug that inhibits the dimerization of HIF-1α and HIF-1β, cardioprotection was also blocked (42), indicating that acute induction of HIF-1 activity was required and thus ruling out a more trivial role for HIF-1 in the baseline expression of a protein that was subsequently modified in response to the IPC stimulus.
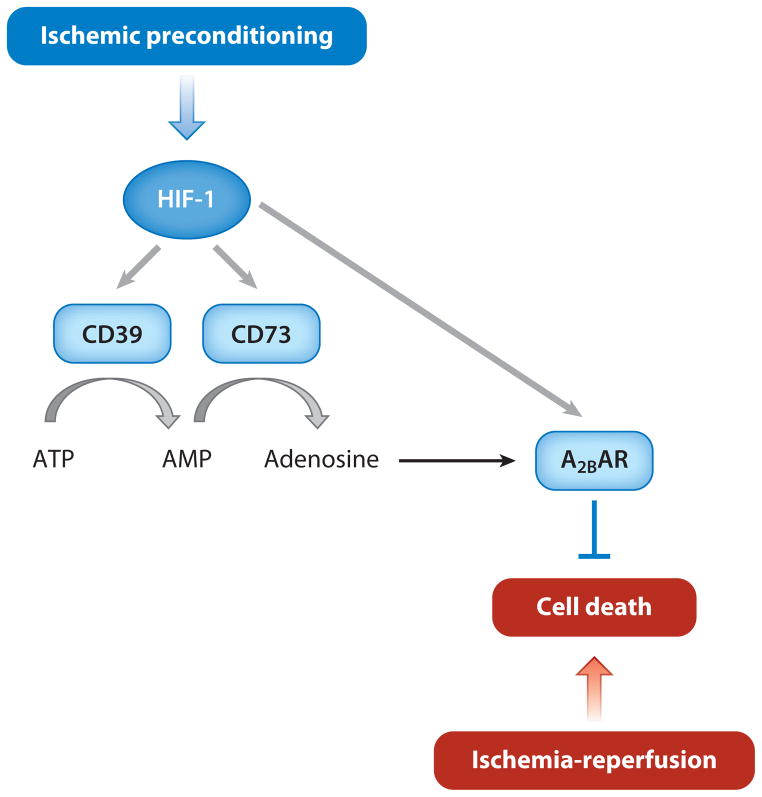
ATP is metabolized to adenosine in response to an IPC stimulus, adenosine receptor antagonists block cardioprotection induced by an IPC stimulus, and adenosine infusion is sufficient to induce cardioprotection (40). ATP is metabolized to adenosine through the activity of two extracellular enzymes: CD39 (also known as ectonucleoside triphosphate diphosphohydrolase), which hydrolyzes ATP to ADP and then to AMP, and CD73 (also known as ecto-5′-nucleotidase), which hydrolyzes AMP to adenosine (Figure 4). Genetic ablation or pharmacological inhibition of CD39 or CD73 activity in the mouse heart results in a loss of IPC-induced cardioprotection (44, 45). The increase in adenosine levels in the heart induced by IPC was blocked by pretreatment of the heart with short interfering RNA against HIF-1α (46). CD39 and CD73 are expressed in vascular endothelial cells (45, 47), and expression of both CD39 and CD73 mRNA was induced by an IPC stimulus in WT hearts, but not in Hif1a+/− hearts (42). IPC also induced cardiac expression of the adenosine A2B receptor in a HIF-1α-dependent manner (46). Taken together, the extensive body of data presented in this section indicates that IPC induces HIF-1-dependent CD39 and CD73 expression in vascular endothelial cells, leading to increased levels of adenosine, which is an obligatory mediator of acute cardioprotection. Both endothelial cells and cardiomyocytes express adenosine receptors, and adenosine binding may activate AKT signaling—which is impaired in Hif1a+/− hearts (41)—and other pathways that mediate the protective effects of IPC.
Figure 4.
Adenosine production mediated by HIF-1 contributes to the cardioprotective effect of ischemic preconditioning. A2BAR denotes the adenosine A2B receptor.
HIF-1 induces the expression of hundreds of gene products in response to hypoxia or ischemia, and other pathways activated by HIF-1 may therefore play a role in IPC, particularly in late-phase cardioprotection. Treatment of rodents with cobalt chloride, desferrioxamine, or dimethyloxalylglycine, which are chemical inducers of HIF-1 transcriptional activity (48), or exposure to cycles of ambient hypoxia and reoxygenation induced late-phase cardioprotection (49–51). The cardioprotective effect of cobalt chloride was lost in Nos2−/− mice lacking expression of inducible nitric oxide synthase, which is a known mediator of late-phase cardioprotection induced by IPC (50). Nos2 expression was induced in the hearts of rats exposed to hypoxia and in isolated cardiomyocytes in a HIF-1-dependent manner (52).
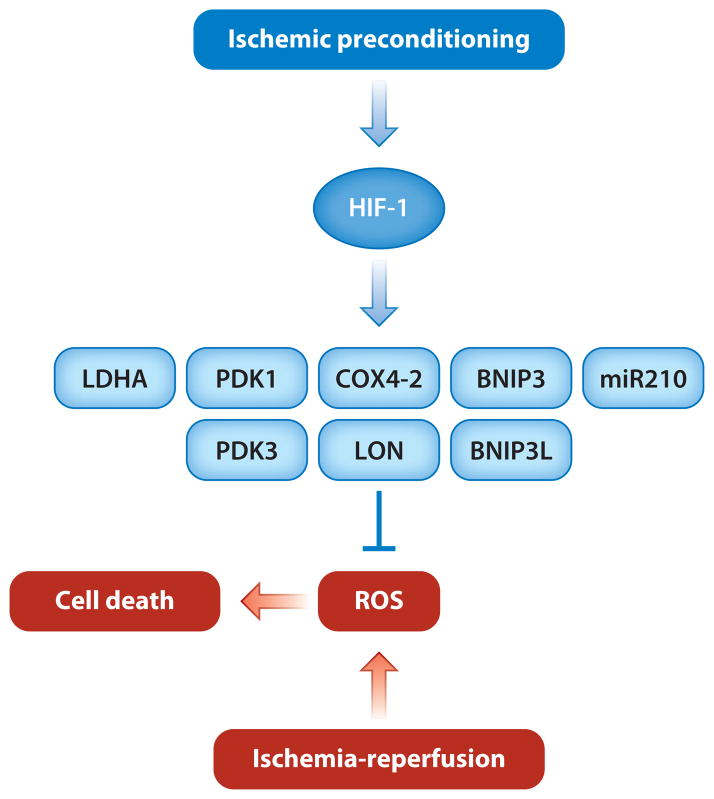
Another mechanism by which HIF-1 may mediate cardioprotection is by altering the balance between glycolytic metabolism and oxidative metabolism (53). HIF-1 coordinately activates the transcription of genes encoding glucose transporters and glycolytic enzymes (Table 3). Increased glycolytic flux allows cells to maintain ATP levels under hypoxic conditions. HIF-1 also inhibits mitochondrial oxidative metabolism, thereby reducing the generation of reactive oxygen species under conditions of hypoxia or hypoxia-reoxygenation, by multiple strategies. Such strategies include the following (Figure 5): (a) a regulatory subunit switch in cytochrome c oxidase, in which COX4-1 is degraded by LON protease and replaced by COX4-2 (54); (b) induction of lactate dehydrogenase A, which converts pyruvate to lactate (16); (c) induction of pyruvate dehydrogenase kinase 1 (PDK1) or PDK3, which inactivates pyruvate dehydrogenase and thereby shunts pyruvate away from the mitochondria (55–57); (d ) induction of microRNA-210, which inhibits the expression of ISCU, an iron-sulfur cluster assembly factor that is required for activity of aconitase and electron transport complex I (58, 59); and (e) induction of BNIP3 and BNIP3L, which trigger mitochondria-selective autophagy (60, 61). However, these responses to hypoxia have been studied primarily in tissue culture cells, and it is not known whether HIF-1 activates the expression of any of these genes in response to IPC and, if so, whether their protein products contribute to cardioprotection.
Figure 5.
Metabolic adaptations that are mediated by HIF-1 and that may contribute to the cardioprotective effect of ischemic preconditioning. HIF-1 activates the transcription of genes whose protein products (LDHA, PDK1, PDK3, COX4I2, LON, BNIP3, BNIP3L, and miR210) play key roles in reducing mitochondrial oxidative metabolism and thereby in reducing the generation of reactive oxygen species (ROS). However, it is not known whether HIF-1 activates any of these genes in the heart in response to ischemic preconditioning.
HIF-1 Protects Against Pressure-Overload Heart Failure
Heart failure is a major cause of morbidity and mortality in the US population, with a prevalence of 5.8 million individuals (62). Hypertension, by increasing systemic resistance, leads to a compensatory left ventricular hypertrophy, which allows the ejection fraction to be maintained, but eventually progresses to an uncompensated state characterized by decreased ejection fraction, increased left ventricular end-diastolic volume, and the clinical signs and symptoms of heart failure (63, 64). As in the case of IHD described above, investigation of the molecular pathophysiology of heart failure has focused largely on the response of cardiomyocytes to pressure overload, which is experimentally induced by transaortic constriction (TAC) in mice. Conditional knockout of HIF-1α expression in cardiomyocytes was associated with a failure to maintain VEGF expression and neovascularization, which are required to increase O2 delivery to the rapidly increasing cardiac muscle mass associated with compensatory hypertrophy, leading to the accelerated onset of heart failure beginning 3 weeks after TAC (65). Thus, HIF-1 activity in cardiomyocytes is required to maintain O2 homeostasis during compensatory myocardial hypertrophy in response to pressure overload.
In contrast to the phenotype associated with cardiomyocyte-specific knockouts, Hif1af /f ;Tie2-cre mice with conditional knockout of HIF-1α in both cardiomyocytes and endothelial cells manifested a much more severe phenotype of cardiac decompensation, with profoundly decreased ejection fraction and increased end-systolic ventricular diameter within 1 week after TAC (66). The loss of cardiac function was due to myocardial hypoxia because of decreased myocardial capillary density, which resulted from markedly increased endothelial cell apoptosis. Analysis of signal transduction pathways revealed that, compared with Hif1af /f mice and Tie2-cre mice, Hif1af /f ;Tie2-cre mice subjected to TAC manifested increased transforming growth factor β (TGF-β) signaling through both the canonical pathway leading to activation of SMAD2/3 and the noncanonical pathway leading to activation of the MAP kinases ERK1 and ERK2. Treatment of Hif1af /f ;Tie2-cre mice with a neutralizing antibody against TGF-β or an inhibitor of MEK-ERK signaling prevented TAC-induced loss of myocardial capillary density and contractile dysfunction (66). This effect was likely due to the inhibition of ERK activation in endothelial cells because the neutralizing antibody is unable to penetrate into the myocardium (67) and mice with cardiomyocyte-specific knockout of HIF-1α did not manifest rapid cardiac decompensation after TAC.
Further studies are required to address several unanswered questions. What is the mechanism by which excessive TGF-β signaling is induced in cardiac endothelial cells when Hif1af /f ;Tie2-cre mice are subjected to TAC? Is the mechanism cell autonomous, or does it involve signaling from cardiomyocytes? What is the mechanism by which HIF-1 prevents excessive TGF-β signaling in cardiac endothelial cells when WT mice are subjected to TAC?
Taken together, these studies point to a protective role of endothelial cells in the contexts of both IPC (42) and pressure-overload heart failure (66). In both cases, further study is required to investigate whether the findings obtained in mouse models are relevant to human cardiac physiology. In this regard, it was striking that treatment of WT mice with digoxin, which is a potent inhibitor of HIF-1 activity (68), also induced rapid cardiac decompensation after TAC (66), suggesting that the drug may have countertherapeutic effects. This may explain why, even though digoxin increases cardiac contractility, it does not increase survival in patients with heart failure (69).
In addition to the role of vascular physiology in the pathogenesis of heart failure, dramatic changes in glucose and lipid metabolism are also associated with heart failure. Whereas the healthy heart generates ATP by the oxidation of fatty acids, the failing heart shifts to the utilization of glucose as a substrate for glycolytic metabolism, and fatty acids are converted to lipids (70, 71). The outcome of this metabolic reprogramming is a failure to produce adequate ATP to maintain cardiac function. As discussed above, the switch from oxidative metabolism to glycolytic metabolism is one of the key intracellular adaptations to hypoxia that is mediated by HIF-1. In addition, HIF-1 also mediates the switch from fatty acid oxidation to lipid synthesis by activating the transcription of the peroxisome proliferator–activated receptor PPAR-γ (72).
These results suggest that, whereas HIF-1 may play a protective, proangiogenic role during cardiac hypertrophy, it may play a pathogenic role during end-stage failure by mediating metabolic reprogramming that leads to energetic failure. Hif1a+/− mice were reported to have impaired (65, 73) or improved (72) cardiac function after TAC relative to WT mice. These conflicting results may be a reflection of the complexity of adaptive responses mediated by HIF-1. In this regard, HIF-2α is also induced by myocardial hypoxia, dimerizes with HIF-1β, and activates the transcription of some but not all HIF-1 target genes (11). For example, several of the genes encoding angiogenic factors, including VEGF, are regulated by HIF-2, whereas the genes encoding LDHA and PDK1, which are responsible for the reprogramming of glucose metabolism (Figure 5), are regulated only by HIF-1. Thus, if it were possible to selectively increase HIF-2 activity, this strategy might provide therapeutic benefit in the failing heart.
SUMMARY POINTS.
HIF-1 functions as a master regulator of O2 homeostasis by controlling both O2 delivery and utilization.
Tissue hypoxia or ischemia resulting from arterial stenosis induces HIF-1 activity, which is required for the production of angiogenic growth factors that stimulate vascular remodeling to increase blood flow through collateral vessels. Aging and chronic disease impair this adaptive response.
HIF-1 is required for ischemic preconditioning, in which short episodes of ischemia and reperfusion protect the heart against injury following prolonged ischemia-reperfusion. HIF-1 is required for adenosine production in response to ischemic preconditioning, and increased adenosine levels in the heart are both necessary and sufficient for cardioprotection.
HIF-1 also mediates metabolic reprogramming that may protect the heart against injury following prolonged ischemia-reperfusion by reducing the production of reactive oxygen species.
HIF-1 plays a complex and multifactorial role in the pathophysiology of pressure-overload heart failure. It may be protective during the stage of compensatory hypertrophy by stimulating adaptive angiogenesis, whereas it may contribute to the pathology of end-stage failure by mediating maladaptive metabolic reprogramming.
Glossary
- HIF
hypoxia-inducible factor
- Hypoxia response element (HRE)
a DNA sequence containing a HIF-1-binding site that is required for hypoxia-induced gene expression
- Chromatin immunoprecipitation (ChIP) assay
a technique for demonstrating the binding of transcription factors to gene sequences within living cells
- VEGF
vascular endothelial growth factor
- ANGPT
angiopoietin
- PGF
placental growth factor
- AdCA5
a recombinant, replication-defective adenovirus engineered to express a constitutively active form of HIF-1α for gene therapy
- Ischemic heart disease (IHD)
heart disease due to atherosclerotic coronary artery stenosis
- Myocardial infarction (MI)
the death of heart tissue due to lack of perfusion
- WT
wild type
- BMDAC
bone marrow–derived angiogenic cell
- SNP
single-nucleotide polymorphism
- Ischemic preconditioning (IPC)
a phenomenon in which exposure of the heart to short episodes of ischemia and reperfusion protects the heart against injury caused by a subsequent prolonged episode of ischemia-reperfusion
- CD39
a cell surface protein that is also known as ectonucleoside triphosphate diphosphohydrolase and that hydrolyzes extracellular ATP to AMP
- CD73
a cell surface protein that is also known as ecto-5′-nucleotidase and that hydrolyzes extracellular AMP to adenosine
- Transaortic constriction (TAC)
an experimental technique in which a ligature is placed around the aorta to narrow its lumen and thereby induce pressure overload of the heart
- TGF
transforming growth factor
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–37. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 2.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–14. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–62. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compernolle V, Brusselmans K, Franco D, Moorman A, Dewerchin M, et al. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1α. Cardiovasc Res. 2003;60:569–79. doi: 10.1016/j.cardiores.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, et al. Hypoxia-inducible factor 1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006;281:25703–11. doi: 10.1074/jbc.M602329200. [DOI] [PubMed] [Google Scholar]
- 6.Lage K, Greenway SC, Rosenfeld JA, Wakimoto H, Gorham JM, et al. Genetic and environmental risk factors in congenital heart disease functionally converge in protein networks driving heart development. Proc Natl Acad Sci USA. 2012;109:14035–40. doi: 10.1073/pnas.1210730109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun. 1996;225:485–88. doi: 10.1006/bbrc.1996.1199. [DOI] [PubMed] [Google Scholar]
- 8.Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci. 1999;11:4159–70. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin C, Yu AY, Jiang BH, Davis L, Kimberly D, et al. Cardiac hypertrophy in chronically anemic fetal sheep: Increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia-inducible factor 1. Am J Obstet Gynecol. 1998;178:527–34. doi: 10.1016/s0002-9378(98)70433-8. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, et al. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–33. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 11.Jürgensen JS, Rosenberger C, Wiesener MS, Warnecke C, Hörstrup JH, et al. Persistent induction of HIF-1αand -2αin cardiomyocytes and stromal cells of ischemic myocardium. FASEB J. 2004;18:1415–17. doi: 10.1096/fj.04-1605fje. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, et al. Expression of hypoxia-inducible factor 1αand 2αin hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002;13:1721–32. doi: 10.1097/01.asn.0000017223.49823.2a. [DOI] [PubMed] [Google Scholar]
- 13.Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, et al. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor 1 in the lung. Am J Physiol Lung Cell Mol Physiol. 1998;275:L818–26. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]
- 14.Bosch-Marcé M, Okuyama H, Wesley JB, Sarkar K, Kimura H, et al. Effects of aging and hypoxia-inducible factor 1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101:1310–18. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 15.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–37. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 17.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–54. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia X, Lemieux ME, Li W, Carroll JS, Brown M, et al. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci USA. 2009;106:4260–65. doi: 10.1073/pnas.0810067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, et al. Genome-wide association of hypoxia-inducible factor (HIF)-1α and HIF-2α DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem. 2009;284:16767–75. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schödel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117:e207–17. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, et al. Cell type–specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–81. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 22.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–14. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 24.Habib GB, Heibig J, Forman SA, Brown BG, Roberts R, et al. Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. Circulation. 1991;83:739–46. doi: 10.1161/01.cir.83.3.739. [DOI] [PubMed] [Google Scholar]
- 25.Sabia PJ, Powers ER, Ragosta M, Sarembock IJ, Burwell LR, et al. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med. 1992;327:1825–31. doi: 10.1056/NEJM199212243272601. [DOI] [PubMed] [Google Scholar]
- 26.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–20. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 27.Patel TH, Kimura H, Weiss CR, Semenza GL, Hofmann LV. Constitutively active HIF-1α improves perfusion and arterial remodeling in an endovascular model of limb ischemia. Cardiovasc Res. 2005;68:144–54. doi: 10.1016/j.cardiores.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Rey S, Lee K, Wang CJ, Gupta K, Chen S, et al. Synergistic effect of HIF-1αgene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia. Proc Natl Acad Sci USA. 2009;106:20399–404. doi: 10.1073/pnas.0911921106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rey S, Luo W, Shimoda LA, Semenza GL. Metabolic reprogramming by HIF-1 promotes the survival of bone marrow–derived angiogenic cells in ischemic tissue. Blood. 2011;117:4988–98. doi: 10.1182/blood-2010-11-321190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinkel R, Lebherz C, Fydanaki M, Wuchrer A, El-Aouni C, et al. Angiogenetic potential of Ad2/Hif-1α/VP16 after regional application in a preclinical pig model of chronic ischemia. Curr Vasc Pharmacol. 2013;11:29–37. [PubMed] [Google Scholar]
- 31.Kilian EG, Sadoni S, Vicol C, Kelly R, van Hulst K, et al. Myocardial transfection of hypoxia-inducible factor 1αvia an adenoviral vector during coronary artery bypass grafting. A multicenter phase I and safety study. Circ J. 2010;74:916–24. doi: 10.1253/circj.cj-09-0594. [DOI] [PubMed] [Google Scholar]
- 32.Resar JR, Roguin A, Voner J, Nasir K, Hennebry TA, et al. Hypoxia-inducible factor 1α polymorphism and coronary collaterals in patients with ischemic heart disease. Chest. 2005;128:787–91. doi: 10.1378/chest.128.2.787. [DOI] [PubMed] [Google Scholar]
- 33.Hlatky MA, Quertermous T, Boothroyd DB, Priest JR, Glassford AJ, et al. Polymorphisms in hypoxia inducible factor 1 and the initial clinical presentation of coronary disease. Am Heart J. 2007;154:1035–42. doi: 10.1016/j.ahj.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 34.Duran J, Gotzens V, Carballo J, Martin E, Petit M, et al. The HIF1A C85T polymorphism influences the number of branches of the human coronary tree. Cardiology. 2012;121:156–59. doi: 10.1159/000336818. [DOI] [PubMed] [Google Scholar]
- 35.Nallamothu BK, Bradley EH, Krumholz HM. Time to treatment in primary percutaneous coronary intervention. N Engl J Med. 2007;357:1631–38. doi: 10.1056/NEJMra065985. [DOI] [PubMed] [Google Scholar]
- 36.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Investig. 1985;76:1713–19. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 38.Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, et al. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–99. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 39.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–72. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 40.Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, et al. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–56. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- 41.Cai Z, Zhong H, Bosch-Marcé M, Fox-Talbot K, Wang L, et al. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1α. Cardiovasc Res. 2008;77:463–70. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- 42.Sarkar K, Cai Z, Gupta R, Parajuli N, Fox-Talbot K, et al. Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning. Proc Natl Acad Sci USA. 2012;109:10504–9. doi: 10.1073/pnas.1208314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armstrong S, Ganote CE. Adenosine receptor specificity in preconditioning of isolated rabbit cardiomyocytes: evidence of A3 receptor involvement. Cardiovasc Res. 1994;28:1049–56. doi: 10.1093/cvr/28.7.1049. [DOI] [PubMed] [Google Scholar]
- 44.Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–90. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 45.Köhler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–94. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 46.Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor 1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–75. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 47.Koszalka P, Ozuyaman B, Huo Y, Zernecke A, Flögel U, et al. Targeted disruption of cd73/ecto-5′-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ Res. 2004;95:814–21. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
- 48.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1α. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253–60. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 49.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, et al. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation. 2003;108:79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- 50.Xi L, Taher M, Yin C, Salloum F, Kukreja RC. Cobalt chloride induces delayed cardiac preconditioning in mice through selective activation of HIF-1αand AP-1 and iNOS signaling. Am J Physiol Heart Circ Physiol. 2004;287:H2369–75. doi: 10.1152/ajpheart.00422.2004. [DOI] [PubMed] [Google Scholar]
- 51.Ockaili R, Natarajan R, Salloum F, Fisher BJ, Jones D, et al. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 2005;289:H542–48. doi: 10.1152/ajpheart.00089.2005. [DOI] [PubMed] [Google Scholar]
- 52.Jung F, Palmer LA, Zhou N, Johns RA. Hypoxic regulation of inducible nitric oxide synthase via hypoxia-inducible factor 1 in cardiac myocytes. Circ Res. 2000;86:319–25. doi: 10.1161/01.res.86.3.319. [DOI] [PubMed] [Google Scholar]
- 53.Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol. 2011;76:347–53. doi: 10.1101/sqb.2011.76.010678. [DOI] [PubMed] [Google Scholar]
- 54.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, et al. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–22. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 55.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate de-hydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–85. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–97. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 57.Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase 3 by hypoxia-inducible factor 1 promotes metabolic switch and drug resistance. J Biol Chem. 2008;283:28106–14. doi: 10.1074/jbc.M803508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, et al. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–84. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Favaro E, Ramachandran A, McCormick R, Gee H, Blancher C, et al. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and Krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS ONE. 2010;5:e10345. doi: 10.1371/journal.pone.0010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang H, Bosch-Marcé M, Shimoda LA, Tan YS, Baek JH, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–81. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 63.Levy D, Garrison RJ, Savage DD, Dannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–66. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 64.Frohlich ED, Apstein C, Chobanian AV, Devereaux RB, Dustan HP, et al. The heart in hypertension. N Engl J Med. 1992;327:998–1008. doi: 10.1056/NEJM199210013271406. [DOI] [PubMed] [Google Scholar]
- 65.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, et al. p53-induced inhibition of HIF-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–8. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 66.Wei H, Bedja D, Koitabashi N, Xing D, Chen J, et al. Endothelial expression of hypoxia-inducible factor 1 protects the murine heart and aorta from pressure overload by suppression of TGF-βsignaling. Proc Natl Acad Sci USA. 2012;109:E841–50. doi: 10.1073/pnas.1202081109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, et al. Pivotal role of cardiomyocyte TGF-β signaling in the murine pathological response to sustained pressure overload. J Clin Investig. 2011;121:2301–12. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, et al. Digoxin and other cardiac glycosides inhibit HIF-1αsynthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–86. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eade E, Cooper R, Mitchell AR. Digoxin—time to take the gloves off? Int J Cardiol. 2013;164:365–67. doi: 10.1016/j.ijcard.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 70.Lehman JJ, Kelly DP. Gene regulatory mechanisms governing energy metabolism during cardiac hypertrophic growth. Heart Fail Rev. 2002;7:175–85. doi: 10.1023/a:1015332726303. [DOI] [PubMed] [Google Scholar]
- 71.Taegtmeyer H, Golfman L, Sharma S, van Arsdall M. Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Ann NY Acad Sci. 2004;1015:202–13. doi: 10.1196/annals.1302.017. [DOI] [PubMed] [Google Scholar]
- 72.Krishnan J, Suter M, Windak R, Krebs T, Felley A, et al. Activation of a HIF-1α-PPARγ axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 2009;9:512–24. doi: 10.1016/j.cmet.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 73.Silter M, Kogler H, Zieseniss A, Wilting J, Schafer K, et al. Impaired Ca2+ handling in HIF-1α+/−mice as a consequence of pressure overload. Pflüg Arch. 2010;459:569–77. doi: 10.1007/s00424-009-0748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo W, Hu H, Chang R, Zhong J, Knabel M, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–44. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schito L, Rey S, Tafani M, Zhang H, Wong CC, et al. Hypoxia-inducible factor 1–dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. Proc Natl Acad Sci USA. 2012;109:E2707–16. doi: 10.1073/pnas.1214019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaturvedi P, Gilkes DM, Wong CC, Kshitiz, Luo W, et al. Hypoxia-inducible factor–dependent breast cancer–mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Investig. 2013;123:189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang H, Wong CC, Wei H, Gilkes DM, Korangath P, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31:1757–70. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Mukhopadhyay CK, Mazumder B, Fox PL. Role of hypoxia-inducible factor-1 in transcriptional activation of ceruloplasmin by iron deficiency. J Biol Chem. 2000;275:21048–54. doi: 10.1074/jbc.M000636200. [DOI] [PubMed] [Google Scholar]
- 79.Bruick RK. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA. 2000;97:9082–87. doi: 10.1073/pnas.97.16.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abboud ER, Coffelt SB, Figueroa YG, Zwezdaryk KJ, Nelson AB, et al. Integrin-linked kinase: a hypoxia-induced anti-apoptotic factor exploited by cancer cells. Int J Oncol. 2007;30:112–22. [PubMed] [Google Scholar]
- 81.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–20. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Cormier-Regard S, Nguyen SV, Claycomb WC. Adrenomedullin gene expression is developmentally regulated and induced by hypoxia in rat ventricular cardiac myocytes. J Biol Chem. 1998;273:17787–92. doi: 10.1074/jbc.273.28.17787. [DOI] [PubMed] [Google Scholar]
- 83.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–69. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 84.Pfeil U, Aslam M, Paddenberg R, Quanz K, Chang CL, et al. Intermedin/adrenomedullin-2 is a hypoxia-induced endothelial peptide that stabilizes pulmonary microvascular permeability. Am J Physiol Lung Cell Mol Physiol. 2009;297:L837–45. doi: 10.1152/ajplung.90608.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, et al. Adenosine A2A receptor is a unique angiogenic target of HIF-2αin pulmonary endothelial cells. Proc Natl Acad Sci USA. 2009;106:10684–89. doi: 10.1073/pnas.0901326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–50. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 87.Eckhart AD, Yang N, Xin X, Faber JE. Characterization of the α1B-adrenergic receptor gene promoter region and hypoxia regulatory elements in vascular smooth muscle. Proc Natl Acad Sci USA. 1997;94:9487–92. doi: 10.1073/pnas.94.17.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simon MP, Tournaire R, Pouyssegur J. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1. J Cell Physiol. 2008;217:809–18. doi: 10.1002/jcp.21558. [DOI] [PubMed] [Google Scholar]
- 89.Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, et al. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res. 2008;103:432–40. doi: 10.1161/CIRCRESAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 90.Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, et al. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflamm. 2007;4:12. doi: 10.1186/1742-2094-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, et al. Hypoxic induction of CTGF is directly mediated by HIF-1. Am J Physiol Ren Physiol. 2004;287:F1223–32. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- 92.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245:894–99. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- 94.Camenisch G, Stroka DM, Gassmann W, Wenger RH. Attenuation of HIF-1 DNA-binding activity limits hypoxia-inducible endothelin-1 expression. Pflüg Arch. 2001;443:240–49. doi: 10.1007/s004240100679. [DOI] [PubMed] [Google Scholar]
- 95.Patel N, Gonsalves CS, Malik P, Kalra VK. Placenta growth factor augments endothelin-1 and endothelin-B receptor expression via hypoxia-inducible factor-1α. Blood. 2008;112:856–65. doi: 10.1182/blood-2007-12-130567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vihanto MM, Plock J, Emi D, Frey BM, Frey FJ, et al. Hypoxia up-regulates expression of Eph receptors and ephrins in mouse skin. FASEB J. 2005;19:1689–91. doi: 10.1096/fj.04-3647fje. [DOI] [PubMed] [Google Scholar]
- 97.Yamashita T, Ohneda K, Nagano M, Miyoshi C, Kaneko N, et al. Hypoxia-inducible transcription factor 2αin endothelial cells regulates tumor neovascularization through activation of ephrin A1. J Biol Chem. 2008;283:18926–36. doi: 10.1074/jbc.M709133200. [DOI] [PubMed] [Google Scholar]
- 98.Sánchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabéu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-βpathways. J Biol Chem. 2002;277:43799–808. doi: 10.1074/jbc.M207160200. [DOI] [PubMed] [Google Scholar]
- 99.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. J Biol Chem. 1997;272:23659–67. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 100.Okuyama H, Krishnamachary B, Zhou YF, Nagasawa H, Bosch-Marcé M, et al. Expression of vascular endothelial growth factor receptor 1 in bone marrow–derived mesenchymal cells is dependent on hypoxia-inducible factor 1. J Biol Chem. 2006;281:15554–63. doi: 10.1074/jbc.M602003200. [DOI] [PubMed] [Google Scholar]
- 101.Han ZB, Ren H, Zhao H, Chi Y, Chen K, et al. Hypoxia-inducible factor (HIF)-1α directly enhances the transcriptional activity of stem cell factor (SCF) in response to hypoxia and epidermal growth factor (EGF) Carcinogenesis. 2008;29:1853–61. doi: 10.1093/carcin/bgn066. [DOI] [PubMed] [Google Scholar]
- 102.Grosfeld A, Andre J, Hauguel–De Mouzon S, Berra E, Pouyssegur J, et al. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J Biol Chem. 2002;277:42953–57. doi: 10.1074/jbc.M206775200. [DOI] [PubMed] [Google Scholar]
- 103.Reynolds PR, Mucenski ML, Le Cras TD, Nichols WC, Whitsett JA. Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J Biol Chem. 2004;279:37124–32. doi: 10.1074/jbc.M405254200. [DOI] [PubMed] [Google Scholar]
- 104.LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–84. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 105.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 106.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ryan HE, Lo J, Johnson RS. HIF-1αis required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–15. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jean JC, Rich CB, Joyce-Brady M. Hypoxia results in an HIF-1-dependent induction of brain-specific aldolase C in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L950–56. doi: 10.1152/ajplung.00087.2006. [DOI] [PubMed] [Google Scholar]
- 109.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001;61:6669–73. [PubMed] [Google Scholar]
- 110.Graven KK, Yu Q, Pan D, Roncarati JS, Farber HW. Identification of an oxygen responsive enhancer element in the glyceraldehyde-3-phosphate dehydrogenase gene. Biochim Biophys Acta. 1999;1447:208–18. doi: 10.1016/s0167-4781(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 111.Niizeki H, Kobayashi M, Horiuchi I, Akakura N, Chen J, et al. Hypoxia enhances the expression of autocrine motility factor and the motility of human pancreatic cancer cells. Br J Cancer. 2002;86:1914–19. doi: 10.1038/sj.bjc.6600331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc Natl Acad Sci USA. 1994;91:6496–500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Minchenko O, Opentanova I, Caro J. Hypoxic regulation of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene family (PFKFB-1–4) expression in vivo. FEBS Lett. 2003;554:264–70. doi: 10.1016/s0014-5793(03)01179-7. [DOI] [PubMed] [Google Scholar]
- 114.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, et al. Role of HIF-1αin hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 115.Wood SM, Wiesener MS, Yeates KM, Okada N, Pugh CW, et al. Selection and analysis of a mutant cell line defective in the hypoxia-inducible factor-1α-subunit (HIF-1α). Characterization of HIF-1α-dependent and -independent hypoxia-inducible gene expression. J Biol Chem. 1998;273:8360–68. doi: 10.1074/jbc.273.14.8360. [DOI] [PubMed] [Google Scholar]
- 116.Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L941–49. doi: 10.1152/ajplung.00528.2005. [DOI] [PubMed] [Google Scholar]
- 117.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is upregulated by hypoxia through a HIF-1α-dependent mechanism. J Biol Chem. 2006;281:9030–37. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 118.Zhao F, Mancuso A, Bui TV, Tong X, Gruber JJ, et al. Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1α-induced metabolic reprograming. Oncogene. 2010;29:2962–72. doi: 10.1038/onc.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gess B, Hofbauer KH, Deutzmann R, Kurtz A. Hypoxia upregulates triosephosphate isomerase expression via a HIF-dependent pathway. Pflüg Arch. 2004;448:175–80. doi: 10.1007/s00424-004-1241-1. [DOI] [PubMed] [Google Scholar]