Abstract
Cardiomyocytes derived from human induced pluripotent stem cells (iPSCs) show great promise as autologous donor cells to treat heart disease. A major technical obstacle to this approach is that available induction methods often produce heterogeneous cell population with low percentage of cardiomyocytes. Here we describe a cardiac enrichment approach using nonintegrating adeno-associated virus (AAV). We first examined several AAV serotypes for their ability to selectively transduce iPSC-derived cardiomyocytes. Results showed that AAV1 demonstrated the highest in vitro transduction efficiency among seven widely used serotypes. Next, differentiated iPSC derivatives were transduced with drug-selectable AAV1 expressing neomycin resistance gene. Selection with G418 enriched the cardiac cell fraction from 27% to 57% in 2 weeks. Compared with other enrichment strategies such as integrative genetic selection, mitochondria labeling, or surface marker cell sorting, this simple AAV method described herein bypasses antibody or dye labeling. These findings provide proof of concept for large-scale cardiomyocyte enrichment by exploiting AAV's intrinsic tissue tropism.
Introduction
A variety of gene delivery methods, such as liposomes, lentiviruses, and adenoviruses, have been evaluated in cardiomyocytes differentiated from stem cells. Adeno-associated viral (AAV) vectors have an established track record of efficient and safe transgene delivery. A recent report documented in total 92 registered clinical trials with AAV worldwide1 and the number continues to increase. Several unique properties distinguish AAV from other vectors for targeted gene delivery, including serotype-specific tropisms toward certain tissues and sustained epi-chromosomal expression with attenuated oncogenic risk.2,3 A comprehensive in vitro survey of AAV transduction efficiency on various mammalian cell types has been conducted.4 Though previous study has proven the feasibility of AAV to transduce stem cell differentiated cardiomyocytes on a small scale,5 an extensive optimization of AAV on stem cell-derived cardiomyocytes has not been reported. Here we compared the transduction efficiency of seven commonly used AAV serotypes in low-purity induced pluripotent stem cell (iPSC) differentiated cardiomyocytes, and all tested serotypes demonstrated preferential cardiomyocytes transduction in comparison to noncardiomyocytes, with AAV1 showing the highest cardiac transduction efficiency. This unique tropism was subsequently utilized to improve cardiomyocyte purity, by delivering a neomycin resistance gene to facilitate simple G418 selection. This study demonstrated that viral intrinsic tissue tropism could be exploited to enrich certain stem cell derivatives to benefit downstream applications.
Materials and Methods
iPSC maintenance and cardiac induction
The iPSC line designated UC3-4 was used for this study. The derivation and maintenance of iPSCs was described previously.6 Briefly, undifferentiated iPSCs were maintained under feeder-free condition with daily change of mTeSR-1 medium (Cat No. 05850; Stemcell Technologies, Vancouver, BC, Canada), following manufacturer's instructions. Every 4–5 days, cells were passaged by incubating with Versene solution (Cat No. 15040-066; Life Technologies, Grand Island, NY) for 7 min at room temperature and split at the ratio of 1:3–1:5. The cardiac induction method was described previously with modification.7 Briefly, after incubating with Versene solution, iPSCs were plated on Matrigel (Cat. No 354277; Corning, Tewksbury, MA)-coated, tissue culture-treated 24-well plates at the density of 250,000 cells/cm2, followed by daily mTeSR-1 medium changes. Three days postseeding, cells were treated with 10 μM of CHIR99021 (Cat No. S2924; Selleckchem, Houston, TX) in differentiation medium, consisting of RPMI1640 medium (Cat No: 21870-084), 2% of B27 minus insulin supplement (Cat No: A1895601), 1% L-glutamine (Cat No: 21051024), and 1% of penicillin/streptomycin (Cat No: 15140). All cell culture reagents were from Life Technologies. Differentiation medium was refreshed at 24 hr. Three days post-CHIR99021 treatment, differentiation medium was refreshed with the addition of 5 μM of IWP-4 (Cat No: 04-0036; Stemcells, Cambridge, MA). Two days post-IWP4 treatment, medium was switched to cardiac maintenance medium consisting of RPMI1640, B27 culture supplement (Cat. No: 17504; Life Technologies), 1% L-glutamine, and 1% penicillin/streptomycin. Maintenance medium was replaced every 48 hr.
AAV vector production
HEK293 cells (ATCC CRL-1573) were seeded in CellStack Cells 5 (CS5) chambers with vent caps (Cat No: CLS3330; Sigma-Aldrich, St. Louis, MO) cultured with DMEM (Cat No. 11965; Life Technologies) supplemented with 10% fetal bovine serum (Cat No: 16000; Life Technologies) and 1% penicillin/streptomycin. At approximately 80% confluency, cells were cotransfected with the vector plasmid and helper plasmid (containing helper genes from adenovirus and the rep cap genes according to the capsid serotype) using the CaPO4 precipitate technique.8 The culture medium was removed from the CS5 and exchanged with the transfection medium; cells were subsequently incubated 6–15 hr at 37±1°C and 5%±1% CO2. The transfection medium was removed from the CS5 and replaced by fresh exchange medium (DMEM, 1% penicillin/streptomycin) before a 3-day incubation at 37±1°C and 5%±1% CO2. The cells of the CS5 transfected were then harvested. Depending on serotype, the supernatant was precipitated at 5±3°C overnight with PEG and centrifuged. The supernatant was discarded and the PEG-pellet was resuspended in Tris-buffered saline before benzonase digestion. AAV particles were extracted from the cell pellet with Hank's balanced salt solution after benzonase digestion.
The viral suspension was centrifuged and the vector-containing supernatant was loaded on a step density CsCl gradient and centrifuged at 28,000 rpm for 24 hr at 15°C. The full particle band was collected and centrifuged at 38,000 rpm for 48 hr. The enriched full particle band was collected. The viral suspension was then subjected to four successive rounds of dialysis in a Slide-a Lyzer cassette against phosphatase buffered saline (PBS). The purified vector was collected, sampled for viral genome (vg) titer and purity assay, and stored at less than −70°C in polypropylene low-binding cryovials.
Cell transduction
For AAV transduction, cardiac differentiated iPSC cultures, a heterogeneous population containing 20%–40% of cardiomyocytes, were dissociated by 0.25% Trypsin–EDTA (Cat No. 15040-066; Life Technologies) and replated in 24-well plate in maintenance medium with AAV at designated multiplicity of infections (MOIs). The next day, 250 μl fresh medium was added to each well. Medium was changed every 48 hr.
To determine the optimal MOI for serotype comparison, a preliminary dose escalation experiment was conducted with AAV6 and 8, MOI ranging from 10, 100, 1000, and 10,000 vg/cell (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/humc). MOI at 1000 yielded a robust GFP transgene expression 6 days postinitial transduction, and the difference of mean fluorescence intensity (ΔMFI) between GFP+/cTnT+population and GFP−/cTnT− population was within 3 logs (16,425 and 6,201 units, AAV6 and 8, respectively), while GFP transgene signal at MOI 10,000 was too strong and saturated the detection range (ΔMFI 83,380 and 22,852 units, AAV6 and 8, respectively). Based on this result, MOI of 1000 vg/cell was chosen for serotype comparison experiment (Supplementary Fig. S1).
Immunocytochemical staining
Cells transduced with AAV GFP were seeded onto Matrigel-coated coverslips for imaging. Attached cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton-X (Cat No: X100; Sigma-Aldrich) in PBS. After blocking with DAKO serum-free protein block at room temperature for 2 hr, samples were probed with primary antibodies against alpha-sarcomeric actinin (Cat No: PA5-17308; Thermo Scientific, Rockland, IL) at 4°C overnight. On the second day, samples were developed with TxRed-conjugated anti-mouse IgG for visualization. All images were captured with a Nikon A1 confocal microscope (Nikon Instrument Inc., Melville, NY) and processed with Nikon Elements analysis software (advanced research, Version 3.2 64bit; Nikon Instrument Inc.).
Flow cytometry analysis
Cells were dissociated with 0.25% Trypsin–EDTA, fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton-X in PBS with 5% BSA. Cardiac troponin T antibody (Thermo Scientific) was prelabeled with Zenon Alexa Fluor 647 mouse IgG labeling reagent (Life Technologies) following the manufacturer's instructions. The prelabeled cardiac troponin antibody was used to probe the cells. After PBS wash, labeled cells were analyzed by FACS CantoII cell analyzer (BD Bioscience, San Jose, CA) equipped with BD FACSDiva software. All data were processed by FlowJo (V10; Flowjo LLC, Ashland, OR).
Statistics
Data were analyzed with GraphPad Prism (Version 6; GraphPad Software, La Jolla, CA). AAV serotype comparison was analyzed by one-way ANOVA with Bonferroni's post hoc analysis (significantly different at p<0.05). Cardiomyocyte enrichment data was analyzed by unpaired t-test (two-tailed, significantly different at p<0.05).
Results
Cardiomyocyte induction of human iPSCs
Human iPS cell line UC3-46 was differentiated into cardiac lineage as described previously.7 Spontaneously contracting cell clusters could be observed between days 10 and 14, and the beating area gradually increased over time. However, the efficiency of cardiac induction varied among different experiments, ranging from 20% to 80% as indicated by cardiac troponin T (cTnT) flow cytometry analysis (Supplementary Fig. S2).
Various AAV serotypes demonstrate robust cardiac tropism in vitro
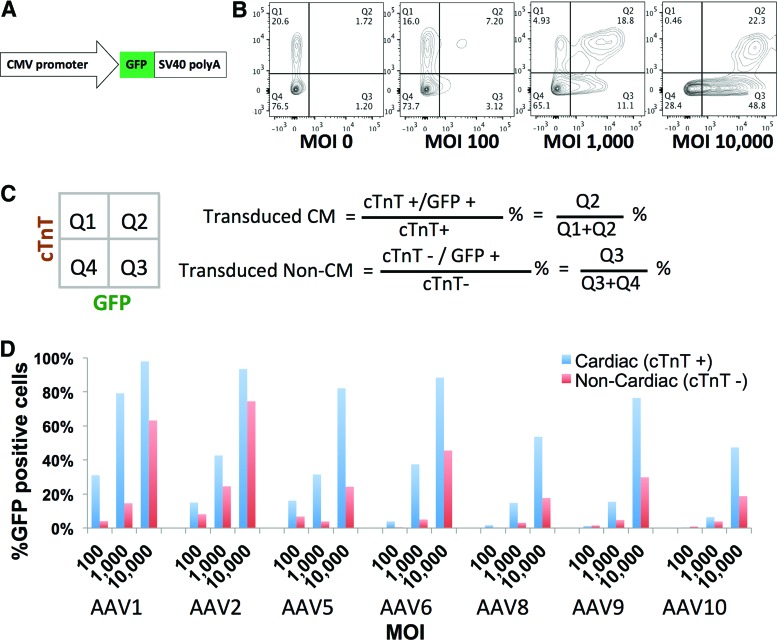
AAV's cardiac tropism is well documented in vivo9 but not in vitro. Here, the heterogeneous population differentiated from iPSCs provided an opportunity to assay AAV's tissue tropism for cardiomyocytes versus noncardiomyocytes. From 14 to 21 days postcardiac induction, the mixed iPSC derivatives were replated in multiwell plates to ensure uniform cellular composition for downstream comparison. Initially, cardiomyocytes constitute approximately 20% of total population (Fig. 1B). In order to determine the optimal experimental condition, a panel of AAV serotypes expressing GFP driven by CMV promoter (Fig. 1A) was added at various MOIs. Transduction efficiency, evident by GFP expression, was separately calculated within cardiac (cTnT+) and noncardiac (cTnT−) populations (Fig. 1B and C). Though higher MOIs resulted in increased transduction efficiency in both populations, AAV preferentially transduces cardiomyocytes, as evident by the higher percentage of GFP-positive cardiomyocytes in all tested conditions (Fig. 1D). The best separation between cardiac and noncardiac populations was with AAV1 at an MOI of 1000 vg/cell, which labeled 79.2% of cardiomyocytes and only 14.6% of noncardiomyocytes (Fig. 1D), recapitulating the cardiac tropism reported in vivo. Live cell imaging also confirmed that GFP expression was localized to spontaneously contracting myocyte foci (see Supplementary Videos S1 and S2).
Figure 1.
Cardiac tropism of multiple AAV serotypes. (A) Structure of the AAV vector expressing GFP. (B) Representative flow-cytometric analysis of cardiac differentiated iPSC cultures transduced with AAV serotype 1. Cardiomyocytes were identified by expression of cTnT, and transduction efficiency was evaluated by GFP expression. (C) GFP-positive cells were quantitatively assessed within cardiac (cTnT+) and noncardiac (cTnT−) populations. (D) AAV transduction screening of cardiac differentiated iPSC cultures with 7 AAV serotypes (1, 2, 5, 6, 8, 9, and 10) across 3 different MOIs (100, 1000, and 10,000 vg/cell). GFP% cells were calculated based on the formula in (B) (n=1 for each condition). AAV, adeno-associated virus; CMV, cytomegalovirus; cTnT, cardiac troponin T; GFP, green fluorescent protein; iPSC, induced pluripotent stem cell; MOI, multiplicity of infection; SV40, Simian Virus 40. Color images available online at www.liebertpub.com/humc
AAV1 demonstrates the highest transduction efficiency among a panel of AAV serotypes
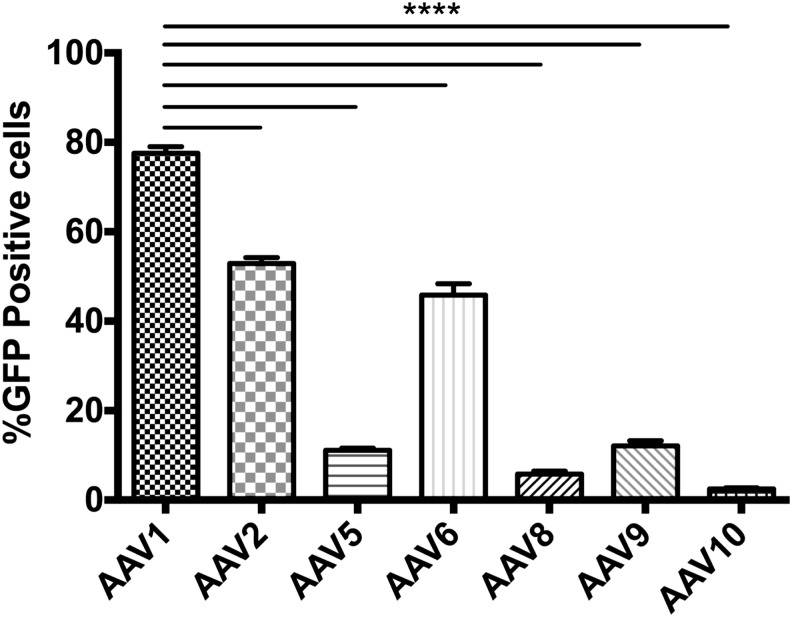
To closely monitor in vitro transduction efficiency and provide statistical power, the AAV transduction experiment was repeated, with an MOI of 1000 in triplicates instead of the entire range. The result confirmed AAV1 as the best serotype in transducing cardiomyocytes with an efficiency of 77.5%±1%, followed by AAV2 and AAV6 with efficiencies of 52.9%±1% and 45.8%±3%, respectively. AAV8 and AAV10 were the least efficient, with an efficiency of 5.82%±0.6% and 2.46%±0.2%, respectively (Fig. 2).
Figure 2.
Systematic evaluation of AAV transduction efficiency. Flow cytometry analysis of AAV serotype transduction efficiency on cardiac differentiated iPSC cultures at MOI of 1000, scored by the percentage of GFP-positive cardiomyocytes against total cardiomyocytes, based on formula in Fig. 1B (n=3 for each serotypes; ****p<0.0001).
Harnessing the cardiac tropism of AAV1 for targeted gene delivery
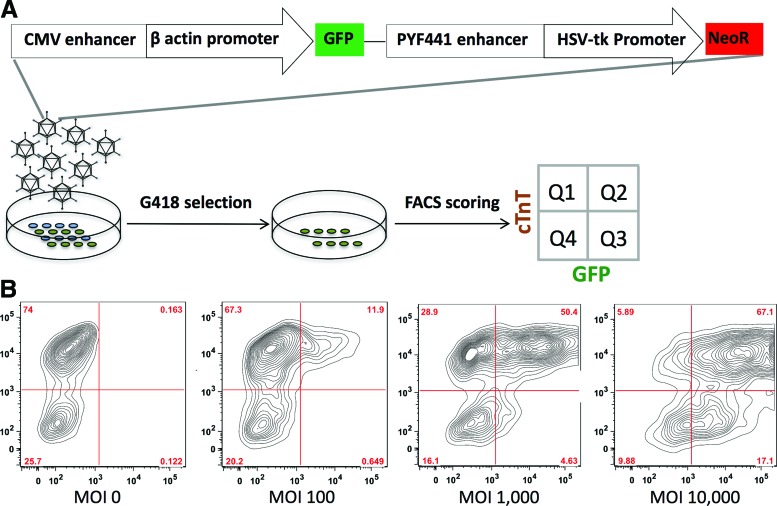
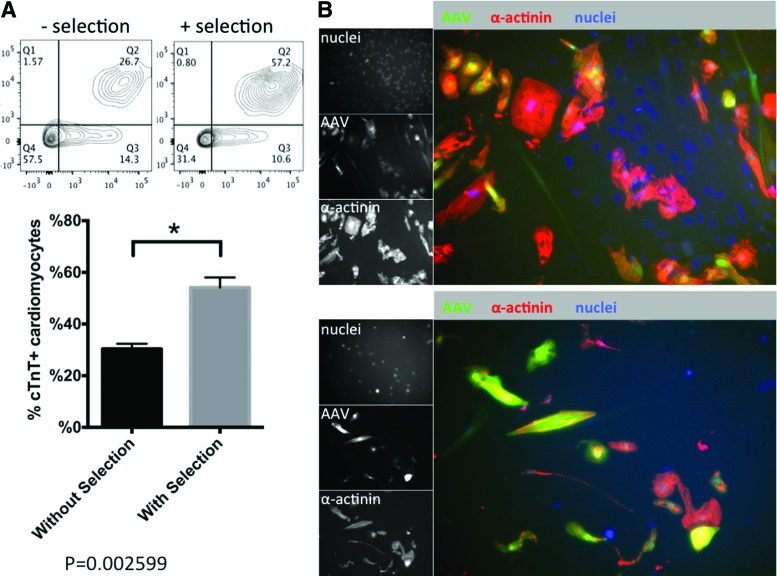
The fact that AAV preferentially transduces cardiomyocytes prompted us to hypothesize that the intrinsic tissue tropism of AAV could be exploited to enrich cardiomyocytes by specifically delivering a genetic selection marker. To test this hypothesis, a plasmid (pTR-UF-11) containing GFP and neomycin resistance genes was packaged into the most efficient AAV1 (Fig. 3A). A dose escalation experiment using AAV1-pTR-UF-11 confirmed that an MOI at 1000 vg/cell was optimal to separate the cardiac and noncardiac populations, transducing 63.6% of cardiomyocytes and 22.7% of noncardiac cells (Fig. 3B). Therefore, 1000 vg/cell AAV1-pTR-UF-11 was delivered to a heterogeneous population differentiated from iPSCs. After stabilizing transgene expression for 6 days, transduced cells were exposed to 250 μg/ml G418 (Fig. 3A). For an experiment with 26% cardiomyocytes, 2-fold enrichment was achieved (Fig. 4A; from 26.7% to 57.2%) after 2-week drug selection. Immunocytochemistry confirmed the enrichment, using co-localization of GFP expression and α-actinin staining (Fig. 4B).
Figure 3.
Harnessing AAV's tissue tropism for cardiac enrichment. (A) Schematic representation of the experimental design. AAV1 vector-packaged pTR-UF11 was employed to transduce cardiac differentiated iPSC cultures. After G418 selection, the remaining cells were subjected to FACS analysis. (B) Cardiac differentiated iPSC cultures transduced with various amounts of AAV1-pTR-UF11 vector were scored by FACS. CMV, cytomegalovirus; FACS, fluorescence-activated cell sorting; HSV-tk, herpes simplex virus-thymidine kinase; NeoR, neomycin-resistance gene. Color images available online at www.liebertpub.com/humc
Figure 4.
Evaluating AAV cardiac enrichment by neomycin-resistant AAV. (A) FACS analysis of AAV1-pTR-UF11-transduced iPSC derivatives with and without G418 selection. Cells were probed for cardiac-specific cTnT (n=3 for each conditions; *p=0.0007). (B) Immunofluorescent staining of AAV1-pTR-UF11-transduced iPSC derivatives with and without G418 selection. Cells were probed for cardiac-specific α-actinin. Color images available online at www.liebertpub.com/humc
Discussion
Intracoronary administration of AAV1 carrying the sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) was the first clinical study using AAV for cardiovascular diseases.10 Our data suggest that multiple AAV serotypes can efficiently transduce iPSC-derived cardiomyocytes in vitro, with AAV1, AAV2, and AAV6 showing good cardiomyocyte affinity. Though evidence collected in vitro cannot predict viral in vivo performance, it has been documented that both AAV1 and AAV6 are preferred strains for clinical and several preclinical large animal studies (see review11).
Cell transplantation has emerged as a promising treatment for a wide range of heart diseases, including bradycardia, ischemic heart disease, and dilated cardiomyopathy.12–15 Although cell transplantation alone has demonstrated encouraging clinical efficacy, genetic modification of donor cells may provide extra benefit. For example, genetic modification of cells for autologous transplantation confers long-term pacemaker function,16 boosts the secretion of paracrine factors,17 and augments post-transplantation cell engraftment and survival.18 In contrast to beneficial effects, transplanted cells could potentially inflict fatal adverse effects such as ventricular tachycardia.13 In that case, genetic modification in the form of a “suicide gene” might be employed as a safeguard to eliminate deleterious outcomes.19 The current study demonstrates that AAV is an effective gene delivery vehicle to target cardiomyocytes differentiated from human pluripotent stem cells. Together with its extraordinary safety profile and the ability to support long-term transgene expression, these lines of evidence justify AAV to be considered as the preferred gene delivery modality for ex vivo gene therapy, to modify donor cardiomyocytes.
In addition to its pleiotropic tissue substrates, different serotype variants demonstrate preference toward certain tissues.9 This feature has been utilized in vivo for tissue-specific gene delivery to minimize off-target effects. AAV's serotype is largely determined by the variation of viral capsid proteins, which dictates the required cell surface receptors for AAV's attachment and internalization.9 For example, the primary receptor for AAV2 is heparin sulfate proteoglycans, while AAV1, 5, and 6 are recognized by N-linked or O-linked sialic acids.20 Widely shared cell surface receptors probably lack the tissue specificity required to account for the observed tissue tropism. It has been proposed that steps after viral internalization may be more important determinant of cellular permissiveness to AAV. The fact that all serotype variants demonstrate preference for postmitotic cells may be explained by these cells' attenuated DNA damage response—a critical step to eliminate foreign genetic material such as AAV.21 In our experiments, AAV's cardiac tissue tropism was reproduced with all tested serotypes, reflected by the preferential transduction of cardiomyocytes in comparison to noncardiomyocytes (e.g., smooth muscle and endothelial cells derived from a common cardiac precursor22) that retain mitotic activity. Thus, the observed cardiac tropism is consistent with previous report on neonatal rat cardiomyocytes,21 and may be adequately explained by the difference in mitotic capacity between cardiomyocytes and noncardiomyocytes at this developmental stage (6–8 weeks).
Insights gained from developmental biology have greatly improved the cardiac induction efficiency,7,23 but the consistent generation of high-purity cardiomyocytes from human pluripotent stem cells in large quantity still presents a major hurdle for various applications. Several enrichment methods have been described to selectively purify cardiomyocytes based on genetic selection,24 cell surface marker or mitochondria labeling,25 metabolic selection,26 or 3D cell culture.27 The observed intrinsic cardiac tropism of AAV prompted us to exploit this property, as a proof of concept, for cardiac cell enrichment. A neomycin resistance gene was administered via the most efficient AAV serotype AAV1 to facilitate G418 selection. Two-week G418 selection achieved 2-fold enrichment in one experiment, in which cardiomyocytes initially constitute 27% of total population. Compared to genetic selection,24 mitochondria labeling,25 or surface marker-based cell sorting,28–30 methods described here have several advantages. First, though incidences of human genome integration have been reported,31–34 rAAVs pose a lower risk for insertional tumorgenesis35 compared with other vehicles such as lentivirus. Second, in contrast to surface marker sorting and intracellular dye staining, no antibody or dye labeling is involved and the output is not constrained by flow cytometry. As a result, large-scale cardiomyocyte enrichment might be achieved in a cost-effective and timely manner. It is conceivable that the selection efficiency could be further optimized by combining cardiac-specific promoter into the current strategy, as suggested by a recent study utilizing AAV9 combined with cTnT promoter to precisely target Cre recombinase to cardiomyocytes following systemic delivery.36
Supplementary Material
Acknowledgments
This work is supported by the Muscular Dystrophy Association (MDA201127). X.G. is supported by American Heart Association Predoctoral Fellowship (14PRE18570042). Additional support by the Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center, Seattle (NIH U54AR065139).
Author Disclosure
No competing financial interests exist.
References
- 1.Ginn SL, Alexander IE, Edelstein ML, et al. Gene therapy clinical trials worldwide to 2012—an update. J Gene Med 2013;15:65–77 [DOI] [PubMed] [Google Scholar]
- 2.Penaud-Budloo M, Le Guiner C, Nowrouzi A, et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J Virol 2008;82:7875–7885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nowrouzi A, Penaud-Budloo M, Kaeppel C, et al. Integration frequency and intermolecular recombination of rAAV vectors in non-human primate skeletal muscle and liver. Mol Ther 2012;20:1177–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis BL, Hirsch ML, Barker JC, et al. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1-9) and one engineered adeno-associated virus serotype. Virol J 2013;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapti K, Stillitano F, Karakikes I, et al. Effectiveness of gene delivery systems for pluripotent and differentiated cells. Mol Ther Methods Clin Dev 2015;2;14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan X, Mack DL, Moreno CM, et al. Dystrophin-deficient cardiomyocytes derived from human urine: new biologic reagents for drug discovery. Stem Cell Res 2014;12:467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lian X, Hsiao C, Wilson G, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA 2012;109:E1848–E1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingston RE, Chen CA, Rose JK. Calcium phosphate transfection. Curr Protoc Mol Biol 2003;Chapter 9:Unit 9.1 [DOI] [PubMed] [Google Scholar]
- 9.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther 2012;20:699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zsebo K, Yaroshinsky A, Rudy JJ, et al. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ Res 2014;114:101–108 [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Chamberlain JS, Tapscott SJ, Storb R. Gene therapy in large animal models of muscular dystrophy. ILAR J 2009;50:187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiba Y, Fernandes S, Zhu WZ, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 2012;489:322–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510:273–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekine H, Shimizu T, Dobashi I, et al. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng Part A 2011;17:2973–2980 [DOI] [PubMed] [Google Scholar]
- 15.Xue T, Cho HC, Akar FG, et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation 2005;111:11–20 [DOI] [PubMed] [Google Scholar]
- 16.Plotnikov AN, Shlapakova I, Szabolcs MJ, et al. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation 2007;116:706–713 [DOI] [PubMed] [Google Scholar]
- 17.Shintani S, Kusano K, Ii M, et al. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat Clin Practice Cardiovasc Med 2006;3 Suppl 1:S123–S128 [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Methot D, Poppa V, et al. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol 2001;33:907–921 [DOI] [PubMed] [Google Scholar]
- 19.Cao F, Lin S, Xie X, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation 2006;113:1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zacchigna S, Zentilin L, Giacca M. Adeno-associated virus vectors as therapeutic and investigational tools in the cardiovascular system. Circ Res 2014;114:1827–1846 [DOI] [PubMed] [Google Scholar]
- 21.Lovric J, Mano M, Zentilin L, et al. Terminal differentiation of cardiac and skeletal myocytes induces permissivity to AAV transduction by relieving inhibition imposed by DNA damage response proteins. Mol Ther 2012;20:2087–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell 2006;11:723–732 [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Klos M, Wilson GF, et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res 2012;111:1125–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest 1996;98:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattori F, Chen H, Yamashita H, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Meth 2010;7:61–66 [DOI] [PubMed] [Google Scholar]
- 26.Tohyama S, Hattori F, Sano M, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2012;12:127–137 [DOI] [PubMed] [Google Scholar]
- 27.Nguyen Doan C, Hookway Tracy A, Wu Q, et al. Microscale generation of cardiospheres promotes robust enrichment of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep 2014;3:260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011;8:228–240 [DOI] [PubMed] [Google Scholar]
- 29.Uosaki H, Fukushima H, Takeuchi A, et al. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One 2011;6:e23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Hoof D, Dormeyer W, Braam SR, et al. Identification of cell surface proteins for antibody-based selection of human embryonic stem cell-derived cardiomyocytes. J Proteome Res 2010;9:1610–1618 [DOI] [PubMed] [Google Scholar]
- 31.Janovitz T, Oliveira T, Sadelain M, Falck-Pedersen E. Highly divergent integration profile of adeno-associated virus serotype 5 revealed by high-throughput sequencing. J Virol 2014;88:2481–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huser D, Gogol-Doring A, Chen W, Heilbronn R. Adeno-associated virus type 2 wild-type and vector-mediated genomic integration profiles of human diploid fibroblasts analyzed by third-generation PacBio DNA sequencing. J Virol 2014;88:11253–11263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janovitz T, Klein IA, Oliveira T, et al. High-throughput sequencing reveals principles of adeno-associated virus serotype 2 integration. J Virol 2013;87:8559–8568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosas LE, Grieves JL, Zaraspe K, et al. Patterns of scAAV vector insertion associated with oncogenic events in a mouse model for genotoxicity. Mol Ther 2012;20:2098–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gauttier V, Pichard V, Aubert D, et al. No tumour-initiating risk associated with scAAV transduction in newborn rat liver. Gene Ther 2013;20:779–784 [DOI] [PubMed] [Google Scholar]
- 36.Werfel S, Jungmann A, Lehmann L, et al. Rapid and highly efficient inducible cardiac gene knockout in adult mice using AAV-mediated expression of Cre recombinase. Cardiovasc Res 2014;104:15–23 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.