Abstract
Alport syndrome (AS) is one of the most common types of inherited nephritis caused by mutation in one of the glomerular basement membrane components. AS is characterized by proteinuria at early stage of the disease and glomerular hyperplastic phenotype and renal fibrosis at late stage. Here, we show that global deficiency of tumor suppressor p53 significantly accelerated AS progression in X-linked AS mice and decreased the lifespan of these mice. p53 protein expression was detected in 21-week-old wild-type mice but not in age-matched AS mice. Expression of proinflammatory cytokines and profibrotic genes was higher in p53+/− AS mice than in p53+/+ AS mice. In vitro experiments revealed that p53 modulates podocyte migration and positively regulates the expression of podocyte-specific genes. We established podocyte-specific p53 (pod-p53)-deficient AS mice, and determined that pod-p53 deficiency enhanced the AS-induced renal dysfunction, foot process effacement, and alteration of gene-expression pattern in glomeruli. These results reveal a protective role of p53 in the progression of AS and in maintaining glomerular homeostasis by modulating the hyperplastic phenotype of podocytes in AS.
Keywords: Alport-s syndrome, p53, podocyte, renal progression, p53 knockout, mouse
Alport syndrome (AS) is a hereditary glomerulonephritis that progressively declines the renal function. The most common mutation in AS is found in the COL4A5 gene located on the X-chromosome.1,2 AS starts with glomerular basement membrane (GBM) dysplasia associated with type IV collagen loss.3 Alteration of GBM matrix components caused by type IV collagen loss, mesangial cell invasion to the glomerular capillary tuft and podocyte foot process effacement lead to albuminuria and renal inflammation.4 Renal fibrosis and glomerular crescent formation caused by proliferation of glomerular epithelial cells (GECs) such as podocytes and parietal epithelial cells (PECs) are detected in late-stage AS.5,6 The phenotypic characteristics of AS involving dysplasia, mesangial expansion, and alteration in cytoskeletal organization suggest a dysregulation of factors involved in cellular homeostasis.
p53 protein is critical in maintaining cellular homeostasis. p53 inactivation or deletion leads to abnormal cell growth and metastasis while persistent p53 activation results in cell apoptosis or senescence.7 p53 is activated in the kidney of diabetic nephropathy and in drug-induced acute kidney injury, and this activation induces podocyte and proximal tubular cell apoptosis resulting in the progression of renal dysfunction.8–10 Conversely, the expression of p53 target gene, p21 is suppressed in the podocyte of renal diseases with hyperplastic phenotype of glomerular and tubule cells such as minimal change disease, focal segmental glomerulosclerosis, and AS.11 It was shown that p53 stabilization by treatment with nutlin-3α, an inhibitor of E3 ubiquitin ligase MDM2, improves adriamycin-induced podocyte mitotic catastrophe and glomerulosclerosis by cell cycle regulation and compensation of podocyte mitotic stress.12 Although p53-dependent renal cell apoptosis is an important step in the development of certain kidney diseases, the role of p53 in glomerular hyperplastic diseases is still unclear.
Here we found that p53 retards the progression of AS pathology, assessed using p53-deficient mouse crossed with mouse bearing X-linked Col4a5 mutation (g5X).13 p53 deletion in AS mouse showed significant aggravation of several renal pathologies. In vitro analyses using primary podocyte and mouse podocyte cell line MPC-5 revealed that p53 regulates podocyte migration and podocyte-specific gene expression. Furthermore, we established podocyte-specific p53-null AS mouse using podocin promoter-driven Cre-loxP system and found that p53 deletion in podocyte also promoted the progression of AS. The number of proliferating cell nuclear antigen (PCNA)-positive cells was increased and the number of foot processes was decreased in pod-p53−/− AS mouse kidneys. Taken together, our findings revealed a renoprotective function of p53 in the progression of AS, and that latent expression of p53 is essential for the maintenance of podocyte function in AS.
Results
p53 Deletion Impairs Renal Function and Survival of AS Mouse
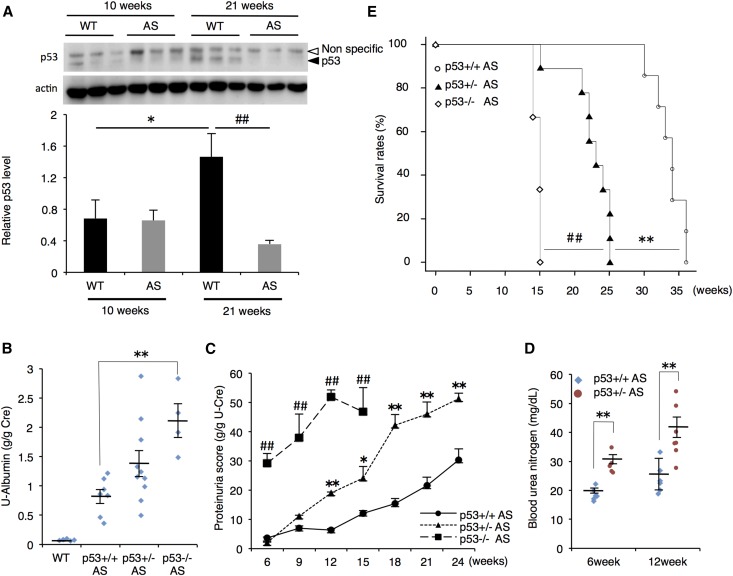
We first checked the expression level of p53 in the glomeruli of wild-type (WT) and AS mice. p53 protein expression was only slightly detected in both 10-week-old WT and AS mouse glomeruli, but it was clearly seen in 21-week-old WT glomeruli. However, p53 expression was not detected in the glomeruli of 21-week-old AS mice (Figure 1A). There was no difference in p53 mRNA expression between 21-week-old WT and AS mouse glomeruli (Supplemental Figure 1A). To determine the role of p53 in the development of AS, we crossed p53-deficient mice with AS mice to generate p53 heterozygous (+/−) and null homozygous (−/−) AS mice. Whereas p53-deletion had no effect on the renal function of non-AS mice groups (Supplemental Figure 1B–D), urine-albumin score was increased correspondingly with the absence of p53 in AS mice (Figure 1B). Progressive increase in proteinuria score was significantly exacerbated in p53+/− AS compared with p53+/+ AS mice, and p53−/− AS mice showed a more severe phenotype (Figure 1C). Because of the low fertility rate and high mortality of p53−/− AS mice, we mainly compared the renal phenotypes between p53+/+ AS and p53+/− AS in the following experiments. Consistent with the urine data, blood urea nitrogen (BUN) score was higher in p53+/− AS mice than in p53+/+ AS mice at 6 and 12 weeks (Figure 1D). These data indicated that p53 retards the progression of renal disorder in AS mice. Notably, the loss of p53 dramatically decreased the survival rate of AS mice (Figure 1E), suggesting that the renoprotective function of p53 is important for prolongation of lifespan in AS mice.
Figure 1.
Whole-body p53 deletion promotes the progression of renal dysfunction in X-linked AS mouse model. (A) Glomeruli were isolated from WT or AS mice in early (10 weeks) or late (21 weeks) stage of AS. Proteins were extracted from glomeruli and p53 protein was analyzed by immunoblotting. Actin was used as loading control. Relative amount of p53 was quantified and normalized to actin (mean±SEM, n=3). **P<0.01 versus WT. (B) Urine samples were obtained from 12-week-old mice of each genotype, and urinary albumin score was measured. U-Albumin scores were normalized with urine creatinine score (mean±SEM, n=4–10). (C) Urine samples were collected at 6, 9, 12, 15, 18, 21, and 24 weeks, and protein concentration was measured. Proteinuria score was calculated based on the urine protein concentration and urine creatinine score (mean±SEM, n=3–8). Measuring proteinuria score of p53−/− AS group was terminated at 15 weeks due to the death of all mice in this group. (D) BUN score of p53+/+ AS and p53−/− AS mice was measured at 6 and 12 weeks (mean±SEM, n=5–7). (E) Survival rate of p53+/+, +/−, −/− AS mice was measured and analyzed by Kaplan–Meier method. Log-rank test was used for statistical analysis (n=3–11). *P<0.05; **P<0.01 versus p53+/+ AS; ##P<0.01 versus p53+/− AS.
p53 Deletion Enhances Renal Inflammation, Glomerular Injury and Fibrosis in AS Mouse
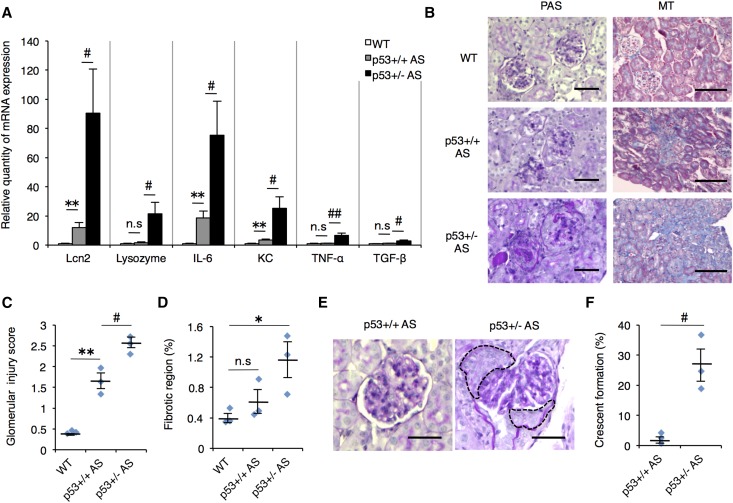
Renal inflammation and fibrosis are critical steps in the development of kidney failure.14,15 To clarify the role of p53 in renal inflammation, we analyzed the mRNA expression level of kidney injury markers and inflammatory cytokines in whole kidneys of 12-week-old WT, p53+/+ AS, and p53+/− AS mice. The kidney injury markers, Lcn-2 and Lysozyme,16 and inflammatory cytokines expression were up-regulated in p53+/− AS mouse compared with p53+/+ AS (Figure 2A). In the p53+/− AS kidney, glomerular collapse was observed and gaps between glomeruli and Bowman’s capsule disappeared in almost all renal corpuscle, whereas p53+/+ AS glomeruli showed only mesangial cell expansion (Figure 2, B and C). Masson’s trichrome (MT) staining revealed that there were no overt differences between WT and p53+/+ AS mouse kidney. But in p53+/− AS mouse, the renal fibrotic region was broader compared with WT (Figure 2, B and D). Furthermore, 15-week-old p53−/− AS mouse showed advanced renal fibrosis and severe tubule dilation (Supplemental Figure 2). These results suggest that p53 deletion influences multiple progressive phenotypes of AS.
Figure 2.
p53-deficient AS mice showed enhanced renal inflammation, glomerular injury, and fibrosis. (A) Total RNA was isolated from renal tissue of 12-week-old WT, p53+/+ AS, and p53+/− AS mice. Quantitative RT-PCR was performed to analyze the expression of indicated renal injury marker genes and cytokines. Gapdh (glyceraldehyde-3-phosphate dehydrogenase) was used as internal control (mean±SEM, n=6–7). (B) Staining of renal sections from 12-week-old mice was performed using PAS or MT staining. Scale bars, 50 μm (PAS) and 100 μm (MT). (C) Glomerulosclerosis scores were calculated by counting the level of glomerular injury in PAS-stained kidney sections. (D) Tubulointerstitial fibrosis scores were evaluated by measuring the region of fibrosis in MT-stained sections. (E) Representative images of glomerular crescent formation in p53+/− AS mouse in PAS-stained kidney sections. Broken line indicates the crescent formation regions. Scale bars, 50 μm. (F) Quantitative results of glomerular crescent formation in p53+/+ or +/− AS groups. (C, D, F) mean±SEM, n=3. *P<0.05; **P<0.01 versus WT; #P<0.05; ##P<0.01 versus p53+/+ AS. n.s., not significant.
p53 Regulates Migration of Podocytes and The Expression of Podocyte-Specific Genes
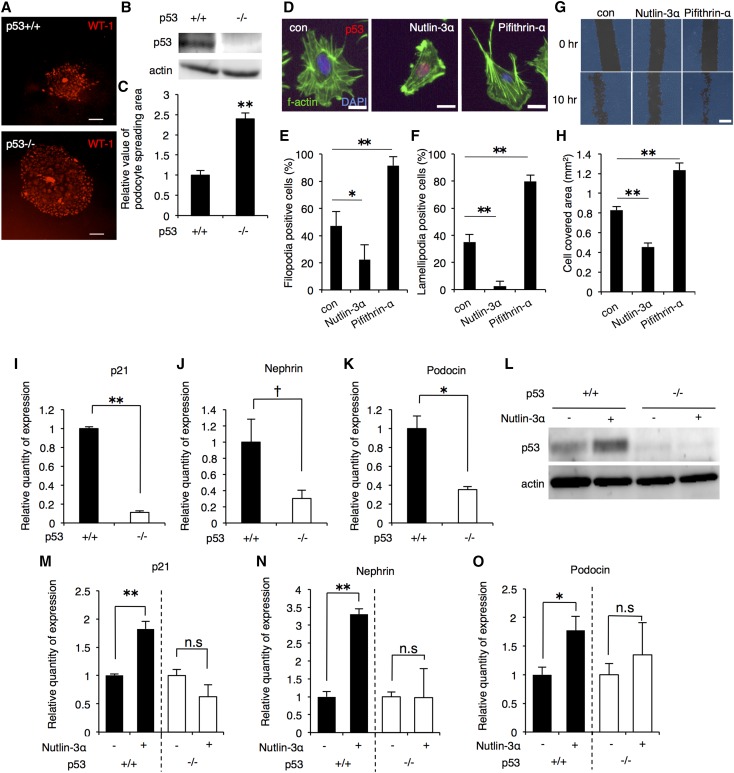
Intriguingly, we observed higher frequency of crescent formation in p53+/− AS compared with p53+/+ AS mouse (Figure 2, E and F). Crescent formation is an important step in glomerulosclerosis and is caused by migration and proliferation of PECs and podocytes.5 Because p53 modulates cell migration and growth, p53 deficiency might have caused the dysregulated podocyte migration and proliferation. We performed podocyte-spreading assay and compared the spreading area of podocytes in the presence or absence of p53. The spreading area of p53−/− mouse-derived podocytes was broader than that of p53+/+ mouse-derived podocytes (Figure 3, A–C), indicating that p53 affects migration of podocytes. To further investigate the role of p53 in the migration of podocyte, differentiated MPC-5 was treated with p53 activator nutlin-3α or p53 inhibitor pifithrin-α. Nutlin-3α treatment induced p53 accumulation in the nucleus and decreased the formation of filopodia and lamellipodia, which are the hallmarks of cell migration (Figure 3, D–F).17 In contrast, pifithrin-α treatment increased the filopodia and lamellipodia formation twice as those of control (Figure 3, D–F). Moreover, in vitro scratch assay results showed that in nutlin-3α-treated cells, less area was covered by migrating cells after 10 hours compared with control whereas pifithrin-α induced more area coverage (Figure 3, G and H), indicating that p53 modulates the migration of podocyte in vitro.
Figure 3.
p53 suppressed podocyte migration in vitro and positively regulated podocyte-specific genes. (A) Glomeruli were isolated from p53+/+ or −/− mice and cultured for 5 days. Podocytes migrate from glomeruli and proliferate as colonies.52 Migrating GECs were fixed with formalin, stained with WT-1, and visualized by immunofluorescence. Scale bars, 200 μm. (B) p53 expression in the glomeruli of p53+/+ and −/− mouse was confirmed by immunoblotting. Actin was used as loading control. (C) Podocyte-spreading area in (A) was quantified (mean±SEM, n=67–98). (D) MPC-5 cells were treated with 25 μM nutlin-3α or 50 μM pifithrin-α for 18 hours. After treatment, cells were fixed, stained with antibodies against p53 (red), F-actin (green), and DAPI (blue) and visualized by immunofluorescence. Scale bars, 20 μm. (E, F) Filopodia- and lamellipodia-positive cells were counted and ratio was calculated (mean±SEM, 33–55 cells per sample, n=4). (G) Differentiated MPC-5 cells were treated for 24 hours with 25 μM nutlin-3α or 50 μM pifithrin-α. After washout, in vitro scratch assay was performed, and cells were re-incubated for additional 10 hours. Acquisition of phase-contrast images at 0 and 10 hours was done using Bio-Revo. Scale bars, 300 μM. (H) The area covered by cells (blue field) was quantified and analyzed using Bio-Revo imaging and analysis software. Values shown are mean±SEM (con, n=7; nutlin-3α, n=7; pifithrin-α, n=9). (I-K) Primary GECs were isolated from p53+/+ or −/− mouse and cultured. Basal mRNA expression of the indicated genes was analyzed by quantitative RT-PCR (mean±SEM, n=3). (L) Primary GECs from p53+/+ or −/− mouse were treated with 25 μM nutlin-3α for 24 hours and protein lysate was extracted. p53 expression was analyzed by western blotting. Actin was used as a loading control. (M-O) Total RNA was extracted from p53+/+ or −/− primary GECs after treatment with 25 μM nutlin-3α for 24 hours. Expressions of the indicated genes were analyzed by quantitative RT-PCR. Gapdh (glyceraldehyde-3-phosphate dehydrogenase) was used as internal control. (mean±SEM, n=3). †P<0.1; *P<0.05; **P<0.01.
Next, we focused on the role of p53 in the regulation of podocyte-specific genes. The mRNA expression levels of p21, Nephrin, Podocin, and Wt-1 were evaluated in differentiated MPC-5 cells treated with varying concentrations of nutlin-3α.18 Nutlin-3α up-regulated p21 and podocyte-specific genes in dose- and time-dependent manner (Supplemental Figure 3, A and B). We isolated and cultured primary GECs from p53+/+ or −/− mice and compared the basal expression levels of p21, Nephrin, and Podocin. The expression levels of these genes were lower in p53−/− GECs than in p53+/+ GECs (Figure 3, I–K). Functional inhibition of p53 by pifithrin-α also down-regulated the mRNA expression of these genes in p53+/+ GECs (Supplemental Figure 3C). To check the p53-dependency of nutlin-3α-induced increase of podocyte-associated genes, we treated p53+/+ and p53−/− GECs with nutlin-3α (Figure 3L). The mRNA expression of each gene was increased by treatment with nutlin-3α in p53+/+ but not in p53−/− GEC (Figure 3, M–O). These data suggest that maintenance of podocyte-specific genes expression is dependent in part on p53.
Podocyte-Specific p53 Knockout Exacerbates Renal Dysfunction in AS Mouse
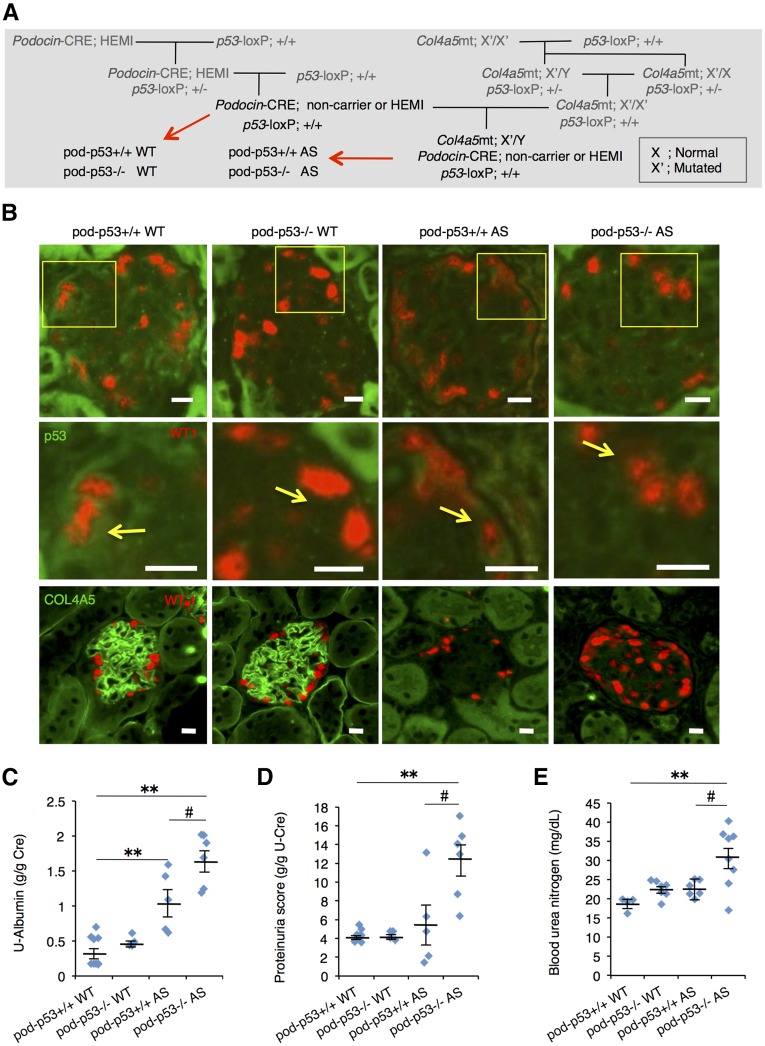
To determine whether p53 in the podocyte in vivo has impact on the progression of AS renal dysfunction, we generated podocyte-specific p53 knockout (KO) (pod-p53−/−) mouse. Artificial genetic modification of Podocin-Cre mouse has no effect on the renal function (Supplemental Figure 4A). Pod-p53−/− mice were born at the expected Mendelian frequency and appeared healthy. Pod-p53−/− mouse showed decreased expression of p53 in the glomeruli compared with pod-p53+/+ mouse (Supplemental Figure 4B). We generated pod-p53+/+ or −/− AS mice by crossing pod-p53−/− mouse and p53loxP/loxP AS mouse (Figure 4A). Low level p53 expression was detected in the cytoplasm of WT-1-positive cells in pod-p53+/+ WT and pod-p53+/+ AS but not in pod-p53−/− WT and pod-p53−/− AS mice (Figure 4B; yellow arrows). Co-localization of p53 and WT-1 in the nucleus was also detected in a few podocytes of pod-p53+/+ WT and AS mouse (Supplemental Figure 4C). The level of p53 expression is consistent with the fact that p53 is normally kept low due to degradation by MDM2.19 We checked type IV collagen expression in glomeruli of each genotype and found loss of type IV collagen only in AS groups (Figure 4B; lower panels). These results showed that pod-p53−/− AS mouse was established. Notably, pod-p53 deletion significantly enhanced the parameters of renal dysfunction in AS groups but not in WT groups (Figure 4, C–E). These results indicated that loss of pod-p53 has no effect on normal renal function but it accelerates renal dysfunction in AS.
Figure 4.
Podocyte-specific p53 deletion promotes AS-induced renal dysfunction. (A) Mating procedure to generate podocyte-specific p53-deficient WT and AS mice. Each littermate group of pod-p53+/+ or −/− WT and pod-p53+/+ or −/− AS was used for the following experiments. (B) Frozen sections of renal cortex harvested from 15-week-old mice were stained for immunofluorescence with antibodies against p53 (green) and WT-1 (red) or with type IV collagen A5 (green) and WT-1 (red). Fields in yellow box are magnified in the panel below. Yellow arrows indicate p53 expression in WT-1-positive cells. Scale bars, 10 μm. (C, D) Urine samples from 15-week-old mice were assessed for (C) U-Albumin and (D) proteinuria scores. (mean±SEM, n=4–9). (E) BUN score in 15-week-old mice was measured (mean±SEM, n=4–8). *P<0.05, **P<0.01 versus pod-p53+/+ WT; #P<0.05 versus pod-p53+/+ AS.
Deletion of Pod-p53 Promotes Glomerular Damage and Renal Fibrosis in AS Mouse
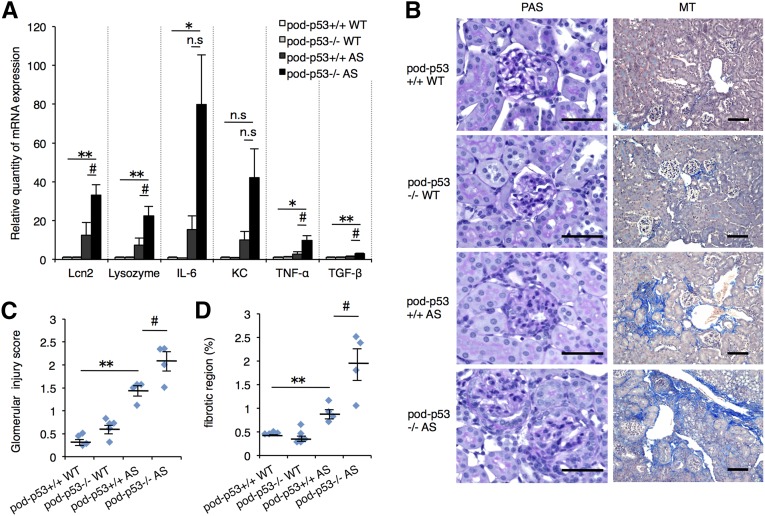
The mRNA expressions of renal injury markers and inflammatory cytokines such as Tnfa and Tgfb were significantly increased by the deletion of pod-p53 in AS groups (Figure 5A). Histologic analysis revealed that glomerular injury and fibrosis were also exacerbated by the loss of pod-p53 in 15-week-old AS groups (Figure 5, B–D). These results suggested that pod-p53 limits the severity of AS. terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay revealed that a few apoptotic cells were concentrated on the atrophic region and were not increased significantly by pod-p53 deletion in AS mouse (Supplemental Figure 5, A and B).
Figure 5.
Renal pathology of AS mouse was exacerbated by podocyte-specific p53 deletion. (A) Total RNA was isolated from renal tissues of 15-week-old mice. Quantitative RT-PCR was performed to analyze the expression of the indicated renal injury marker genes and cytokines. Gapdh (glyceraldehyde-3-phosphate dehydrogenase) was used as internal control (mean±SEM, n=4–6). (B) PAS and MT staining of renal sections in 15-week-old mice were performed. Scale bars, 50 μm. (C, D) Glomerulosclerosis and tubulointerstitial fibrosis scores were quantified from the PAS and MT staining results (mean±SEM, n=4–6). *P<0.05; **P<0.01 versus pod-p53+/+ WT; #P<0.05 versus pod-p53+/+ AS. n.s., not significant.
AS-Induced Alteration of Gene-Expression Pattern in Mouse Glomeruli is Enhanced by Podocyte-Specific Deletion of p53
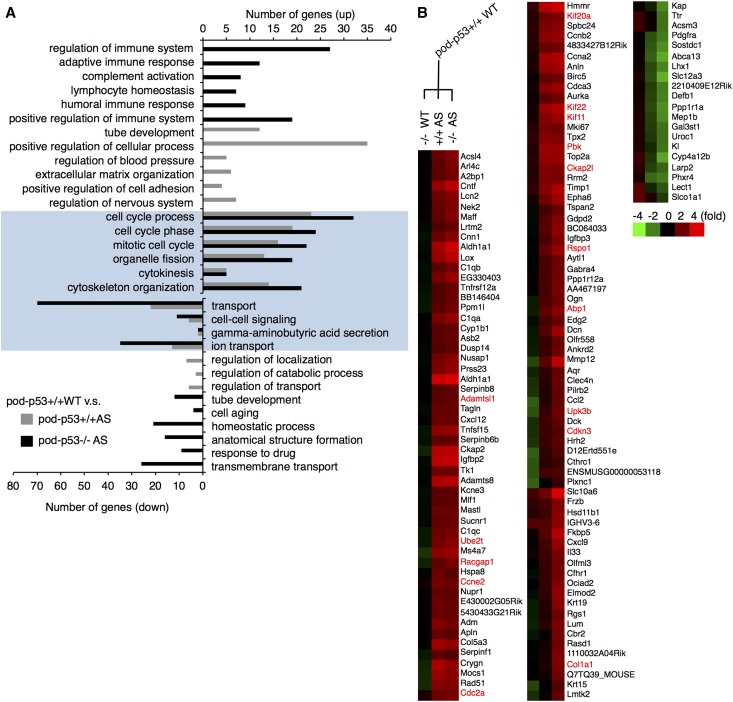
We performed microarray analysis on the glomeruli of pod-p53+/+ or −/− WT and AS mice to determine the gene-expression pattern underlying the hyperplastic phenotype of AS glomeruli. Gene ontology analysis revealed that among the up-regulated genes, the cell cycle-related genes were enriched in the pod-p53+/+ and −/− AS groups compared with pod-p53+/+ WT (Figure 6A). To clarify the role of pod-p53, we sorted the genes whose AS-induced alteration was enhanced by the deletion of pod-p53 in the cluster analysis (Supplemental Figure 6A, Figure 6B). We selected the genes whose protein expression was confirmed in the human glomeruli by the Human Protein Atlas Project,20 and assessed their mRNA expression in the kidneys of pod-p53+/+ and −/− WT or AS (Supplemental Figure 6B). Fifteen genes whose expression was three-fold higher in pod-p53−/− AS compared with pod-p53+/+ WT are highlighted in red in Figure 6B. Cell proliferation-regulating genes (Cdc2a, Pbk, Rspo1, Abp1, Cdkn3) and cytoskeletal-regulating genes (Kif20a, Kif22, Kif11, Racgap1, Ckap2l) were up-regulated in AS and this increase was enhanced by pod-p53 deletion. Interestingly, up-regulation of genes such as Cdc2a, Cdkn3, Pbk, Rspo1, Kif20a, Kif22, Kif11, Racgap1, and Ube2t correspond to several types of tumors.21–28 These results suggested that the alteration toward a more proliferative gene-expression pattern underlies the development of glomerular hyperplastic phenotype in AS mice, and that pod-p53 may directly or indirectly influence this change.
Figure 6.
Microarray analysis of gene-expression pattern in glomeruli of pod-p53+/+ and pod-p53−/− mice. Total RNA was isolated from the glomeruli of 12-week-old pod-p53+/+ or −/− WT and AS mice and analyzed by 3D-Gene DNA chip microarray (TORAY, Japan). (A) Gene ontology analysis was performed for the gene data of pod-p53+/+ AS and pod-p53−/− AS groups that were up-regulated or down-regulated statistically compared with pod-p53+/+ WT group using DAVID functional annotation. Gene categories were lined up by P value (P<0.05) and the top six categories that include up- or down-regulated genes in pod-p53+/+ AS group (gray bar), or in pod-p53−/− AS group (black bar) and common category between pod-p53+/+ and −/− AS groups (highlighted in blue) were picked up. (B) Genes were classified according to the changes in expression pattern. Clusters of genes whose AS-induced alteration of expression was enhanced by pod-p53 deletion were picked up. The red text indicates genes that are up-regulated three-fold in the kidneys of pod-p53−/− AS mice compared with pod-p53+/+ WT mice as determined by quantitative PCR shown in Supplement Figure 6B.
Pod-p53−/− AS Mice Show Significant Increase of Proliferating GEC and Foot Process Effacement Including Filopodia Formation
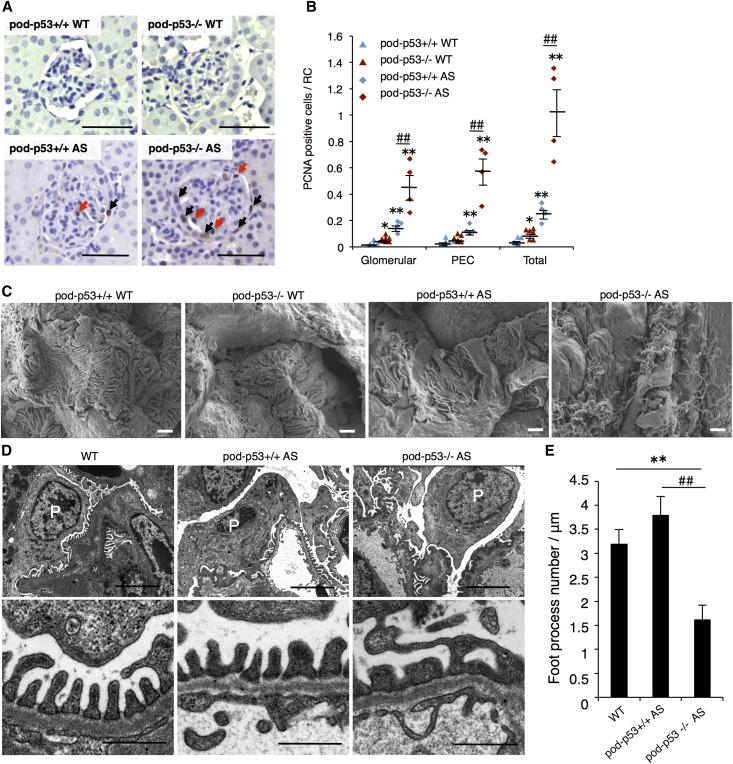
We assessed the podocyte proliferation and morphology of foot process in the presence or absence of pod-p53 in WT or AS mice. Renal cortex sections were stained with PCNA, a marker of proliferation. Pod-p53−/− AS mice showed higher number of proliferating glomeruli than pod-p53+/+ AS (Figure 7A; red arrows, and B). Consistently, the number of WT-1-positive cells was increased in the pod-p53−/− AS group compared with the pod-p53+/+ AS group (Supplemental Figure 7A). In the parietal layer of the Bowman’s capsule, more PECs stained for PCNA in pod-p53−/− than in pod-p53+/+ AS mice (Figure 7A; black arrows, and B). Moreover, the tubular region in the renal cortex exhibited more PCNA staining in pod-p53−/− AS mice than in other groups (Supplemental Figure 7C). These data suggest that p53 in podocyte is required for the suppression of proliferation in AS mice. Podocyte foot process thickening and secondary foot process fusion were observed in AS mice but not in WT mice (Figure 7C). Compared with pod-p53+/+ AS mice, pod-p53−/− AS mice showed more severe foot process effacement, and numerous filopodia spread out on the zipper-like structures (Figure 7C). Consistent with these results, transmission electron microscopy (TEM) images revealed that deletion of pod-p53 enhances foot process enlargement and/or fusion in AS mouse resulting in fewer foot processes (Figure 7D and E). Piecing together these in vivo data, p53 suppresses cell proliferation and migration of podocytes that are induced in AS. Thus, we firstly showed here that p53 is a factor that attenuates rapid AS progression.
Figure 7.
p53 modulates podocyte abnormal growth and foot process effacement in AS. (A) Kidney sections from 15-week-old pod-p53+/+ or −/− WT or AS mice were immunostained with cell proliferation marker protein, PCNA. Sections were counterstained with hematoxylin. PCNA-positive cells in the renal corpuscle (RC) were counted and distinguished by histologic localization as glomerular, PEC or total. Red arrows show PCNA-positive cells in glomerular region and black arrows show PCNA-positive cells in Bowman’s capsule region. Scale bars, 50 μm. (B) PCNA-positive cells in RC were counted and analyzed in glomerular region (Glomerular) and in Bowman’s capsule region (PEC) or counted as total PCNA-positive cells in RC (Total). PCNA count was performed in 27–90 glomeruli per sample (mean±SEM, n=4–6). (C, D) Structure of podocyte foot process in 15-week-old mice was analyzed by SEM and TEM. High-magnification micrographs for the indicated mouse genotype are shown. (C) Scale bars, 1 μm. (D) Scale bars, 4 μm (upper panels) and 0.5 μm (lower panels). Podocyte is marked with “P”. (E) Morphometric analysis of the foot process. The foot processes were manually counted in four random glomeruli in each mouse genotype. Values shown are mean±SEM **P<0.01 versus pod-p53+/+ WT. ##P<0.01 versus pod-p53+/+ AS.
Discussion
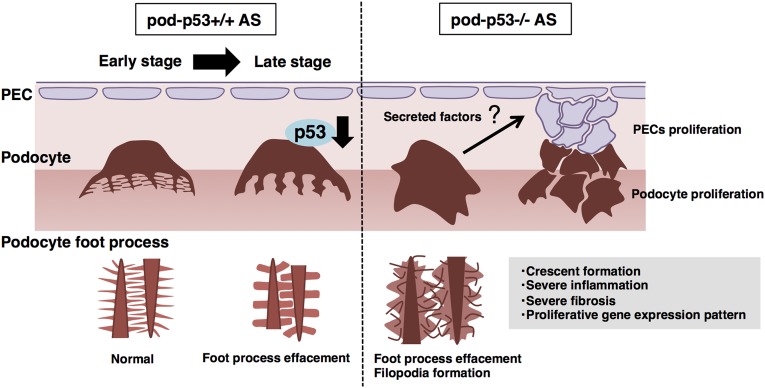
The present study revealed that p53 affected podocyte foot process effacement and abnormal proliferation in AS pathology, and whole-body and podocyte-specific p53 deletion accelerated AS progression (Figure 8). Previous reports demonstrated that whole-body p53−/− mice appeared normal but are prone to spontaneously develop a variety of neoplasm by 6 months of age,29 indicating that low but latent function of p53 before 6 months of age is necessary to maintain tissue homeostasis. p53 expression was low in young (10 weeks) mouse glomeruli, but was more detectable in the majority of 21-week-old mice. In contrast, p53 expression was not detected in 21-week-old AS mouse glomeruli (Figure 1A), suggesting that AS progression suppresses p53 and that latently functional p53 in the podocyte is necessary to retard the progression of AS (Figure 4C-E). p53 is activated by a variety of stimuli to maintain cell homeostasis but p53 expression could also be down-regulated by chronic inflammation, loss of cell adherence, and other various chronic stimuli.30 We speculate that the reduction of p53 protein but not the mRNA level in the 21-week-old AS mouse glomeruli occurred due to the chronic inflammatory milieu and/or anoikis caused by progressive GBM matrix degradation.31,32
Figure 8.
Pod-p53 regulates the glomerular hyperplastic phenotypes in the progression of AS. Podocyte foot process structures are maintained in early stage of AS. Progressive disruption of foot process structures gradually induces renal dysfunction. Pod-p53 deletion in AS mouse enhances podocyte foot process effacement and induces aberrant filopodia formation. Pod-p53−/− AS mouse had increased number of proliferative PECs and podocytes. Gene-expression patterns were altered, and secreted type of factors that modify cell proliferation and migration were enhanced in pod-p53−/− AS mouse. These secreted factors may influence proliferation of PECs and crescent formation in AS progression. Furthermore, p53 expression is suppressed in the glomeruli of AS mouse at late stage and this contributes to the hyperplastic glomerular disorder and progression of renal dysfunction in AS.
Although AS progression was accelerated in both whole-body and podocyte-specific deficiency of p53, more severe phenotypes were seen in whole-body p53−/− AS mice than in pod-p53−/− AS mice. Moreover, whole-body p53−/− AS mice had reduced lifespan. The severity of the phenotypes seen in whole-body p53−/− AS mice could be due to the developmental abnormality of whole-body p53−/− kidney because p53 activation is essential for nephrogenic niche and normal tubular differentiation in the developing kidney.33,34 Aside from the role of p53 in kidney development, p53 functions as a suppressor of inflammatory response in several inflammation models via attenuation of NF-κB pathway.35 The loss of p53 could result in the removal of the modulatory effect of p53 on NF-κB rendering an imbalance of this negative feedback signaling axis. Previous works on Alport renal disease have revealed the critical involvement of proinflammatory cytokines, profibrotic protein TGF-β1, and matrix metalloproteinases (MMPs) in the progressive ultrastructural changes and deteriorating function of the GBM. These studies have helped to identify potential therapeutic targets.4 Notably, the regulation of these molecules involves the NF-κB signaling pathway. In addition, it was recently demonstrated that laminin-α2, which is normally restricted to mesangial cells but progressively accumulates in the GBM, induces activation of focal adhesion kinase (FAK) and NF-κB-dependent up-regulation of MMP-9, -10, and -12.36 p53 attenuates not only NF-κB pathway as mentioned above but also FAK protein and MMPs.37,38 Consistently, our microarray analysis data showed that MMP-10 and MMP-12 expression was increased in pod-p53−/− AS mouse glomeruli (data not shown). The multi-targeted inhibitory role of p53 in podocyte as well as in immune-related cells might contribute to the retardation of AS progression.
Glomeruli and GECs derived from whole-body p53−/− mouse showed broader spreading area of podocytes and downregulation of Nephrin and Podocin gene expression (Figure 3, C and I–K), but it did not produce overt renal dysfunction in vivo (Supplemental Figure 1, B–D). However, pod-p53 deletion enhanced the AS phenotypes (Figure 4, C–E). These results signify that p53 contributes to the prevention of podocyte dysfunction but not to the prevention of disease initiation. The cross-talk of secretory proteins between glomerulus and tubule is important for the maintenance of kidney homeostasis during renal disease progression.39 Based on our findings that the gene expressions of some secreted factors such as Abp1, Cntf, and Rspo1 were considerably changed in pod-p53−/− AS mice (Figure 6B),40–42 we speculate that the loss of pod-p53 might lead to increased number of proliferating PECs and tubular epithelial cells via a paracrine manner (Figure 8).
p53 is extensively investigated as a therapeutic target of several cancers, mostly with the aim of activating p53 to induce cell cycle arrest, metastasis inhibition, and cell apoptosis. Here we showed a renal protective role of p53 in AS mouse. Our data suggest that maintenance of normal p53 function in podocytes could retard AS development. That p53 has renoprotective function is not unique to Alport nephritis. p53 KO mice had more severe kidney injury compared with WT mice in ischemic AKI and functional attenuation of p53 by pifithrin-α aggravated renal fibrosis in the rat model of ischemic AKI.43,44 p53 conferred protection by reducing the extent and duration of inflammation, implying that as a suppressor of proinflammatory cytokines p53 helps attenuate ischemic AKI. It is also possible that the function of p53 as suppressor of hyperplastic phenotype in podocyte might apply in other glomerular hyperplastic diseases such as rapid progressive glomerulonephritis and focal segmental glomerulosclerosis. But overactivation of pod-p53 could induce apoptosis and detachment of podocytes from GBM and adversely enhance renal dysfunction. Therefore, to target p53 in AS and other renal diseases with hyperplastic glomerular phenotype, optimized p53 activation that does not induce apoptosis is needed. Although it has been proposed that p53 suppression could be a therapeutic strategy for kidney failure,45,46 our findings suggest that excessive p53 attenuation especially in podocyte hyperplastic disease like AS might exacerbate renal disorder. Depending on the apoptotic or hyperplastic pathology of podocyte in respective diseases, there is a need to critically evaluate p53-based therapy.
Concise Methods
Cell Culture
Mouse podocyte cell line MPC-5 was kindly provided by Dr. Peter Mundel, and cells were maintained as described previously.47 Glomeruli were isolated from kidneys of each mouse genotype by metal sieve filtration method and cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and antibiotics. Primary GECs were obtained by gathering migrated cells from glomeruli and re-culturing in culture media for 5 days to induce differentiation. Differentiated cells were used for the experiments.
Animals
X-linked AS mouse model (Col4a5tm1Yseg G5X mutant, C57BL/6 background) were generated by Col4a5 gene exon 1 transversion, 213G→T, to convert codon 5 from glycine to stop codon (G5X).13 p53 loxP mouse (Trp53fl/fl, C57BL/6 background) was genetically edited at loxP sites flanking p53 exons 2 through 10. Podocin-Cre mouse (NPHS2-Cre, C57BL/6 background) was generated using human NPHS2 promoter including the entire 5′ untranslated region to drive Cre recombinase expression in podocyte.48 These mice were obtained from The Jackson Laboratory (Bar Harbor, ME). p53 KO mouse (p53−/−, C57BL/6 background), characterized previously,49 was kindly provided by S. Aizawa from the Center for Developmental Biology, RIKEN, Tokyo, Japan. p53−/− mouse was obtained by homologous recombination of DNA vector with Neor (neomycin phosphotransferase) gene inserted to the second exon in the opposite orientation with the p53 gene to disrupt the translation initiation codon. Whole-body p53+/− AS mice were established by mating AS female mice and p53−/− male mice. p53+/− AS (Col4a5tm1Yseg G5X homozygote) female and p53−/− male mice were crossed to obtain p53−/− AS mice. Age-matched AS mice of each p53 genotype were used for experiments. WT and AS mice with podocyte-specific p53 deletion were established as shown in Figure 4A Age-matched littermate mice of each group were used for the experiments. Male mice were used for all experiments to avoid sex difference because of the sex-linked inheritance of Col4a5tm1Yseg G5X mutation. Mice were fed with food and water ad libitum.
Glomeruli Isolation and Primary Glomerular Epithelial Cell Culture
For western blotting and microarray analysis, glomeruli were isolated by magnetic beads method.50 Anesthetized mice were perfused with 40 ml Hanks Balanced Salt Solution containing 8×107 magnetic beads (14013; Life Technologies). Kidneys were harvested and physically and enzymatically disassembled by 37°C incubation with collagenase (10103578001; Roche Diagnostics) and deoxyribonuclease (18047–019; Invitrogen) for 30 minutes. Collected glomeruli were filtered using mesh with 100 μm pore size and washed three times with ice-cold Hanks Balanced Salt Solution. For podocyte-spreading assay and primary GEC culture, glomeruli were isolated by metal sieve filtration method using three types of pore size metal sieve (71, 100, 180 μm) as described previously.51 Isolated glomeruli were cultured with RPMI supplemented with 10% FBS and antibiotics in rat-tail collagen-coated dish. Primary GECs were collected 5 days after glomerular culture and re-plated on collagen-coated dishes. Primary GECs were cultured for another 5 days in the same condition as glomerular culture.
Western Blotting Analysis
Isolated glomeruli and primary GECs were lysed in lysis buffer and subjected to SDS- PAGE and western blot analysis.51 Blots were reacted for 2 hours with monoclonal mouse anti-p53 (#2524; Cell Signaling Technology) or for 1 hour with polyclonal goat anti-Actin (sc-1616; Santa Cruz Biotechnology) diluted at 1:1000, and with the respective HRP-conjugated secondary antibodies diluted at 1:5000. After each antibody reaction, membranes were washed three times with 0.1% PBS-Tween and blots were visualized with Super Signal WestPico chemiluminescence substrate (Thermo Fisher Scientific). Images were captured by LAS-4000 (GE HealthCare).
Proteinuria Score and Urinary Albumin Score
Urine collection for 24 hours was performed using mouse metabolism cage (AS ONE Corporation). Urinary protein and creatinine were measured by Bradford method (Bio-Rad) and Jaffe’s method (Wako Pure Chemicals), respectively. Urinary protein concentration was normalized with urinary creatinine concentration, and presented as proteinuria score. Urinary albumin was measured by indirect competitive albumin ELISA kit, Albuwell M (Exocell). Urinary albumin concentration was normalized with urinary creatinine concentration.
Blood Urea Nitrogen Score
Mouse blood samples were obtained from abdominal aorta. Fresh blood samples were centrifuged at 3000 rpm, 4°C for 15 minutes, and blood plasma was collected. Blood urea nitrogen score of murine plasma was measured using Urea Nitrogen B Test (Wako Pure Chemicals). The evaluation of samples was carried out according to the manufacturer’s recommended protocol.
Survival Analysis
Survival rates of p53+/+, +/−, −/− AS mice were analyzed by Kaplan–Meier method. Statistical significance analysis was performed with Log-rank test.
Real-Time RT-PCR
Quantitative RT-PCR was carried out as described previously by using RNA isolated from renal tissue or cultured MPC-5 and primary GECs.51 After reverse transcription, we performed real-time PCR with iQ5 (Bio-Rad) in a mix containing DNA polymerase and SYBR Green (PR820; TaKaRa). The sequence of primers used for real-time PCR are shown in Table 1.
Table 1.
Primer sequence for real-time quantitative RT-PCR
| Gene | Sense | Antisense |
|---|---|---|
| Lcn-2 | 5′-CAGAAGGCAGCTTTACGATG-3′ | 5′-CCTGGAGCTTGGAACAAATG-3′ |
| Lysozyme | 5′-CCAGTGTCACGAGGCATTCA-3′ | 5′-TGATAACAGGCTCATCTGTCTCA-3′ |
| Il-6 | 5′-GAGGATACCACTCCCAACAGACC-3′ | 5′-AAGTGCATCATCGTTGTTCATACA-3′ |
| Il-8 (KC) | 5′-TGTCAGTGCCTGCAGACCAT-3′ | 5′-GAGCCTTAGTTTGGACAGGATCTG-3′ |
| Tnfa | 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ | 5′-TGGGAGTAGACAAGGTACAACCC-3′ |
| Tgfb | 5′-CACCTGCAAGACCATCGACAT-3′ | 5′-GAGCCTTAGTTTGGACAGGATCTG-3′ |
| p21 | 5′-CCCTCTATTTTGGAGGGTTAATCT-3′ | 5′-GTACCCTGCATATACATTCCCTTC-3′ |
| Nephrin | 5′-AGGATCGAATCAGGAATGAAT-3′ | 5′-CTGTGAAGGCTTGGCGATATGACA-3′ |
| Podocin | 5′-GTGTCCAAAGCCATGCAGTT-3′ | 5′-GCAATGCTCTTCCTTTCCAG-3′ |
| p53 | 5′-ACTGCATGGACGATCTGTTG-3′ | 5′-GCCATAGTTGCCCTGGTAAG-3′ |
| Wt1 (+KTS) | 5′-GAGACACACAGGTGTGAAACCATTC-3′ | 5′-GACAGCTGAAGGGCTTTTCACTTG-3′ |
| Wt1 (total) | 5′-GAGACACACAGGTGTGAAACCATTC-3′ | 5′-GCGCCACGTGGAGTTTGGTCATG-3′ |
| Gapdh | 5′-CCTGGAGAAACCTGCCAAGTATG-3′ | 5′-GGTCCTCAGTGTAGCCCAAGATG-3′ |
Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Microarray Analysis
RNA was recovered from freshly isolated glomeruli with RNeasy kit (Qiagen) and microarray analysis was performed (TORAY) using 3D-Gene Mouse Oligo chip 24k. Gene ontology analysis was performed using DAVID Bioinformatic Resources 6.7 (http://david.abcc.ncifcrf.gov). Expression of genes that were changed compared with WT p53+/+ mice was confirmed by quantitative RT-PCR. The sequences of primers for the genes are shown in Supplemental Table 1.
Evaluation of Glomerulosclerosis Score and Tubulointerstitial Fibrosis Score
Glomerulosclerosis score and tubulointerstitial score were evaluated as described previously.51 Briefly, for glomerulosclerosis score, more than 100 periodic acid schiff (PAS)-stained random glomeruli per mouse were evaluated and scored from 0 to 4 (0, no lesion; 1, expansion of mesangial area; 2, expansion of Bowman’s epithelial cells and adhesion of glomeruli and Bowman’s capsule; 3, sclerotic area in 50–75% of glomerulus; 4, sclerotic area in 75–100% of glomerulus). Double-blinded scoring was performed and average value was used. For tubulointerstitial score, 10–14 MT-stained random fields of cortex region per sample were evaluated by calculating MT-stained area versus unstained area with ImageJ software. Mean scores were obtained and calculated and presented as percentage.
Immunofluorescence
Renal tissues were snap frozen with liquid nitrogen in OCT compound immediately after harvesting. Frozen tissues were sliced at 4-μm thickness and attached onto glass slides. Samples were thawed and fixed with ice-cold acetone at −20°C for 10 minutes and 4% paraformaldehyde at 4°C for 10 minutes. After blocking with 3% BSA in PBS for 1 hour, samples were reacted with primary antibody diluted at 1:50 with 3% BSA in PBS and secondary antibody diluted at 1:500 with PBS. Polyclonal rabbit anti-WT1 (sc-192; Santa Cruz Biotechnology), monoclonal mouse anti-p53 conjugated with Alexa Fluor 488 (#2015; Cell Signaling Technology), FITC-anti-collagen IV α5 (IV) + Texas Red-anti-collagen IV α2 (IV) chain (CFT-45325; SGE), polyclonal rabbit anti-Podocin (ab50339; Abcam, Inc.), and anti-rabbit Alexa Fluor 546 (Molecular Probes) were used for antibody reactions. Samples were incubated with 1 μg/ml of 4′,6-diamidino-2-phenylindole (DAPI) solution for 15 minutes. PBS-wash was performed three times between each procedure except fixing. Stained samples were mounted with Vectashield mounting medium (Vector Laboratories). Images were obtained and analyzed by using Bio-Revo imaging and analysis software (Keyence).
Podocyte-Spreading Assay
Glomeruli were collected from 12-week-old p53+/+ and p53−/− mice using metal sieve filtration method and cultured in RPMI-1640 supplemented with 10% FBS and antibiotics. After 5 days, cultured glomeruli and migrated GECs were fixed with 10% paraformaldehyde at room temperature (RT) for 30 minutes and incubated with 0.1% Triton-X, 3% BSA, and 10% goat serum in PBS at RT for 1 hour. Samples were reacted for 3 hours with rabbit monoclonal anti-WT-1 (sc-192; Santa Cruz Biotechnology) and for 90 minutes with anti-rabbit Alexa Flour 546. PBS-wash was performed three times between each procedure except fixing. Samples were mounted with Vectashield mounting medium (Vector Laboratories). Photo images were captured using Bio-Revo (Keyence), and podocyte-spreading area was calculated by ImageJ software (Fujifilm). n=67 (p53+/+) and n=98 (p53−/−) glomeruli were scored.
Filopodia- and Lamellipodia-Positive Cell Count
Differentiated MPC-5 cells were treated with 25 μM nutlin-3α (430–128; Enzo Life Science) or 50 μM pifithrin-α (#506132; Calbiochem) for 24 hours. After treatment, MPC-5 were fixed with 10% formalin (or 4% paraformaldehyde) for 30 minutes and permeabilized with 0.1% Triton-X in PBS for 20 minutes. After blocking with 5% BSA in PBS for 30 minutes, cells were immunostained with polyclonal rabbit anti-p53 (Fl-393; Santa Cruz Biotechnology) for 2 hours and anti-rabbit Alexa Fluor 546 for 1 hour. Cells were co-stained with Alexa Fluor 488 phalloidin (A12379; Invitrogen) for 20 minutes and 1 μg/ml DAPI (340–07971; Dojindo) in PBS for 10 minutes. All reactions were carried out at room temperature. Samples were washed with PBS three times between each procedure. Photo images were captured using Bio-Revo (Keyence). Cells were scored as filopodia-positive when presenting at least five filopodia and scored as lamellipodia-positive when approximately 50% of cell surfaces were covered with lamellipodia. Thirty to eighty cells were scored and analyzed for each group.
Migration Assay
Differentiated MPC-5 cells plated in six-well plates were treated with 25 μM nutlin-3α or 50 μM pifithrin-α for 24 hours. In vitro scratch assay was performed following the protocol reported by Liang et al.35 Specifically, cell monolayer was scraped in a straight line with a p-200 pipet tip. The debris was removed by washing the cell monolayer, and fresh culture medium was added after washout. Cells were re-incubated in media without nutlin-3α or pifithrin-α and cultured for additional 10 hours. Images were acquired using Bio-Revo (Keyence). Cell migration was assessed by quantifying the area covered by cells at 0 and 10 hours using Bio-Revo imaging and analysis software.
PCNA Staining
Paraffin-embedded renal samples were sliced at 6-μm thickness and de-paraffinized. Samples were soaked in citrate buffer (pH 6.0) and heated in a microwave three times for 3 minutes for antigen retrieval. For blocking, samples were incubated with 10% goat serum and 0.3% Triton-X in PBS at RT for 30 minutes. Samples were reacted for 2 hours with monoclonal mouse anti-PCNA antibody (Clone PC10; Dako) diluted to 1:100 with 5% goat serum in 0.3% Triton-X in PBS. Endogenous peroxidase in samples were removed by incubation with 6% H2O2 in PBS at RT for 10 minutes. Samples were reacted with HRP-conjugated anti-mouse antibody diluted to 1:500 with 0.3% Triton-X in PBS. After washing three times with PBS, samples were reacted with 3,3′-diaminobenzidine tetrahydrochloride (Dojindo) solution. Samples were counterstained with Gill 3 hematoxylin solution (Sigma-Aldrich) and mounted after dehydration. Images were obtained and PCNA-positive cells/hematoxylin-stained cells were quantified using Bio-Revo imaging and analysis software (Keyence).
TUNEL Staining
Glomerular apoptosis was evaluated by staining paraffin sections with Dead End Fluorometric TUNEL System (Promega) following the manufacturer’s protocol. Sections were counterstained with DAPI solution (1 mg/ml). For assessment of TUNEL-positive cells, more than 10 fields of cortex region per mouse were captured using Bio-Revo microscope (Keyence), and apoptotic cell number was divided by the assessed renal area (mm2).
Scanning Electron Microscopy (SEM)
Renal cortex tissues of each genotype mouse were harvested and fixed with 2% glutaraldehyde in PBS for 24 hours at 4°C, cut into 1 mm3 small pieces, and post-fixed with 1% osmium tetroxide at RT for 1 hour. After dehydration in a graded series of ethanol solutions and t-butyl solution, t-butyl-embedded tissues were freeze-dried and sputter coated with platinum. Samples were analyzed using JSM-7600F microscope (JEOL Ltd.).
Transmission Electron Microscopy (TEM)
Renal cortex tissues of each genotype mouse were harvested and fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer for 1 hour, cut into small pieces and post-fixed in 1% osmium tetroxide. After dehydration in a graded series of ethanol solutions and propylene oxide and embedding in Epon 812, ultrathin sections were cut by using Ultratome, then stained with uranyl acetate and lead citrate. Samples were observed using Hitachi H-7500 electron microscope (Hitachi).
Statistical Analysis
All data are expressed as mean±SEM. Significance of the difference between two groups was assessed using Student’s unpaired two-tailed t-test. For three or more group comparisons, we used one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test or with Tukey-Kramer. Survival rate was analyzed by Kaplan–Meier method, and Log-rank test was used for statistical analysis. In vitro experiments were performed in triplicate unless otherwise indicated. Statistical analysis was performed by Statcel3 3rd edition (OMS Publication). A P value of <0.05 is considered statistically significant.
Study Approval
All experiments were performed according to the protocol approved by the Animal Welfare Committee of Kumamoto University (#25–230E).
Disclosures
None
Supplementary Material
Acknowledgments
We are grateful to Dr. Shinichi Aizawa (Laboratory for Animal Resources and Genetic Engineering, Center for Developmental Biology, RIKEN) for the p53 KO mice. This work was supported by Grants-in-Aid for Science Research from the Ministry of Education, Science, Sports, and Culture of Japan (MEXT) (#25117721 to H.K., #20363525 to M.A., and #13J08535 to R.F.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014111109/-/DCSupplemental.
References
- 1.Lemmink HH, Schröder CH, Monnens LA, Smeets HJ: The clinical spectrum of type IV collagen mutations. Hum Mutat 9: 477–499, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Martin P, Heiskari N, Zhou J, Leinonen A, Tumelius T, Hertz JM, Barker D, Gregory M, Atkin C, Styrkarsdottir U, Neumann H, Springate J, Shows T, Pettersson E, Tryggvason K: High mutation detection rate in the COL4A5 collagen gene in suspected Alport syndrome using PCR and direct DNA sequencing. J Am Soc Nephrol 9: 2291–2301, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove D: Glomerular pathology in Alport syndrome: a molecular perspective. Pediatr Nephrol 27: 885–890, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB: Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol 15: 61–67, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Ryu M, Migliorini A, Miosge N, Gross O, Shankland S, Brinkkoetter PT, Hagmann H, Romagnani P, Liapis H, Anders HJ: Plasma leakage through glomerular basement membrane ruptures triggers the proliferation of parietal epithelial cells and crescent formation in non-inflammatory glomerular injury. J Pathol 228: 482–494, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Muller PA, Vousden KH, Norman JC: p53 and its mutants in tumor cell migration and invasion. J Cell Biol 192: 209–218, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE: AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem 285: 37503–37512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ: Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol 16: 2615–2625, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, Dong Z: Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol 25: 2278–2289, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava T, Garola RE, Whiting JM, Alon US: Cell-cycle regulatory proteins in podocyte cell in idiopathic nephrotic syndrome of childhood. Kidney Int 63: 1374–1381, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Liapis H, Romagnani P, Anders HJ: New insights into the pathology of podocyte loss: mitotic catastrophe. Am J Pathol 183: 1364–1374, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rheault MN, Kren SM, Thielen BK, Mesa HA, Crosson JT, Thomas W, Sado Y, Kashtan CE, Segal Y: Mouse model of X-linked Alport syndrome. J Am Soc Nephrol 15: 1466–1474, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Silverstein DM: Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol 24: 1445–1452, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Liu Y: Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moldavsky M: Lysozyme immunostaining in renal tubular dysgenesis. Pediatr Dev Pathol 3: 200–201, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Huttenlocher A, Sandborg RR, Horwitz AF: Adhesion in cell migration. Curr Opin Cell Biol 7: 697–706, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Kreidberg JA: Podocyte differentiation and glomerulogenesis. J Am Soc Nephrol 14: 806–814, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Chao CC: Mechanisms of p53 degradation. Clin Chim Acta 438: 139–147, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Pontén F, Jirström K, Uhlen M: The Human Protein Atlas—a tool for pathology. J Pathol 216: 387–393, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Nishida T, Matsushima T, Tsujimoto M, Takahashi T, Kawasaki Y, Nakayama S, Omori T, Yamamura M, Cho H, Hirota S, Ueshima S, Ishihara H: Cyclin-dependent kinase activity correlates with the prognosis of patients who have gastrointestinal stromal tumors [published online ahead of print February 24, 2015]. Ann Surg Oncol 10.1245/sl0434-015-4438-y [DOI] [PubMed] [Google Scholar]
- 22.Li T, Xue H, Guo Y, Guo K: CDKN3 is an independent prognostic factor and promotes ovarian carcinoma cell proliferation in ovarian cancer. Oncol Rep 31: 1825–1831, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Lei B, Qi W, Zhao Y, Li Y, Liu S, Xu X, Zhi C, Wan L, Shen H: PBK/TOPK expression correlates with mutant p53 and affects patients’ prognosis and cell proliferation and viability in lung adenocarcinoma. Hum Pathol 46: 217–224, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Choi EJ, Yun JA, Jeon EK, Won HS, Ko YH, Kim SY: Prognostic significance of RSPO1, WNT1, P16, WT1, and SDC1 expressions in invasive ductal carcinoma of the breast. World J Surg Oncol 11: 314, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniuchi K, Furihata M, Saibara T: KIF20A-mediated RNA granule transport system promotes the invasiveness of pancreatic cancer cells. Neoplasia 16: 1082–1093, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y, Wang XY, Sun L, Wang YL, Wan YF, Li XQ, Feng YM: Inhibition of KIF22 suppresses cancer cell proliferation by delaying mitotic exit through upregulating CDC25C expression. Carcinogenesis 35: 1416–1425, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Valensin S, Ghiron C, Lamanna C, Kremer A, Rossi M, Ferruzzi P, Nievo M, Bakker A: KIF11 inhibition for glioblastoma treatment: reason to hope or a struggle with the brain? BMC Cancer 9: 196, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imaoka H, Toiyama Y, Saigusa S, Kawamura M, Kawamoto A, Okugawa Y, Hiro J, Tanaka K, Inoue Y, Mohri Y, Kusunoki M: RacGAP1 expression, increasing tumor malignant potential, as a predictive biomarker for lymph node metastasis and poor prognosis in colorectal cancer. Carcinogenesis 36: 346–354, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A: Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356: 215–221, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Vousden KH, Lu X: Live or let die: the cell’s response to p53. Nat Rev Cancer 2: 594–604, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Hodge DR, Peng B, Cherry JC, Hurt EM, Fox SD, Kelley JA, Munroe DJ, Farrar WL: Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer Res 65: 4673–4682, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Ghosh A, Chen TC, Kapila YL: Anoikis triggers Mdm2-dependent p53 degradation. Mol Cell Biochem 343: 201–209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hilliard SA, Yao X, El-Dahr SS: Mdm2 is required for maintenance of the nephrogenic niche. Dev Biol 387: 1–14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aboudehen K, Hilliard S, Saifudeen Z, El-Dahr SS: Mechanisms of p53 activation and physiological relevance in the developing kidney. Am J Physiol Renal Physiol 302: F928–F940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gudkov AV, Gurova KV, Komarova EA: Inflammation and p53: A Tale of Two Stresses. Genes Cancer 2: 503–516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delimont D, Dufek BM, Meehan DT, Zallocchi M, Gratton MA, Phillips G, Cosgrove D: Laminin α2-mediated focal adhesion kinase activation triggers Alport glomerular pathogenesis. PLoS ONE 9: e99083, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golubovskaya VM, Cance WG: FAK and p53 protein interactions. Anticancer Agents Med Chem 11: 617–619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer E, Vollmer JY, Bovey R, Stamenkovic I: Matrix metalloproteinases 9 and 10 inhibit protein kinase C-potentiated, p53-mediated apoptosis. Cancer Res 65: 4261–4272, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Tanaka M, Asada M, Higashi AY, Nakamura J, Oguchi A, Tomita M, Yamada S, Asada N, Takase M, Okuda T, Kawachi H, Economides AN, Robertson E, Takahashi S, Sakurai T, Goldschmeding R, Muso E, Fukatsu A, Kita T, Yanagita M: Loss of the BMP antagonist USAG-1 ameliorates disease in a mouse model of the progressive hereditary kidney disease Alport syndrome. J Clin Invest 120: 768–777, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linker RA, Mäurer M, Gaupp S, Martini R, Holtmann B, Giess R, Rieckmann P, Lassmann H, Toyka KV, Sendtner M, Gold R: CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med 8: 620–624, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Su B, Zhao W, Shi B, Zhang Z, Yu X, Xie F, Guo Z, Zhang X, Liu J, Shen Q, Wang J, Li X, Zhang Z, Zhou L: Let-7d suppresses growth, metastasis, and tumor macrophage infiltration in renal cell carcinoma by targeting COL3A1 and CCL7. Mol Cancer 13: 206, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chassot AA, Gregoire EP, Lavery R, Taketo MM, de Rooij DG, Adams IR, Chaboissier MC: RSPO1/β-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS ONE 6: e25641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutton TA, Hato T, Mai E, Yoshimoto M, Kuehl S, Anderson M, Mang H, Plotkin Z, Chan RJ, Dagher PC: p53 is renoprotective after ischemic kidney injury by reducing inflammation. J Am Soc Nephrol 24: 113–124, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dagher PC, Mai EM, Hato T, Lee SY, Anderson MD, Karozos SC, Mang HE, Knipe NL, Plotkin Z, Sutton TA: The p53 inhibitor pifithrin-α can stimulate fibrosis in a rat model of ischemic acute kidney injury. Am J Physiol Renal Physiol 302: F284–F291, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E: siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol 20: 1754–1764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujino T, Muhib S, Sato N, Hasebe N: Silencing of p53 RNA through transarterial delivery ameliorates renal tubular injury and downregulates GSK-3β expression after ischemia-reperfusion injury. Am J Physiol Renal Physiol 305: F1617–F1627, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of Cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Tsukada T, Tomooka Y, Takai S, Ueda Y, Nishikawa S, Yagi T, Tokunaga T, Takeda N, Suda Y, Abe S, et al. : Enhanced proliferative potential in culture of cells from p53-deficient mice. Oncogene 8: 3313–3322, 1993 [PubMed] [Google Scholar]
- 50.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koga T, Kai Y, Fukuda R, Morino-Koga S, Suico MA, Koyama K, Sato T, Shuto T, Kai H: Mild electrical stimulation and heat shock ameliorates progressive proteinuria and renal inflammation in mouse model of Alport syndrome. PLoS ONE 7: e43852, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bollée G, Flamant M, Schordan S, Fligny C, Rumpel E, Milon M, Schordan E, Sabaa N, Vandermeersch S, Galaup A, Rodenas A, Casal I, Sunnarborg SW, Salant DJ, Kopp JB, Threadgill DW, Quaggin SE, Dussaule JC, Germain S, Mesnard L, Endlich K, Boucheix C, Belenfant X, Callard P, Endlich N, Tharaux PL: Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med 17: 1242–1250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.