Abstract
Novel therapeutic interventions are required to prevent or treat AKI. To expedite progress in this regard, a consensus conference held by the Acute Dialysis Quality Initiative was convened in April of 2014 to develop recommendations for research priorities and future directions. Here, we highlight the concepts related to renal hemodynamics in AKI that are likely to reveal new treatment targets on investigation. Overall, we must better understand the interactions between systemic, total renal, and glomerular hemodynamics, including the role of tubuloglomerular feedback. Furthermore, the net consequences of therapeutic maneuvers aimed at restoring glomerular filtration need to be examined in relation to the nature, magnitude, and duration of the insult. Additionally, microvascular blood flow heterogeneity in AKI is now recognized as a common occurrence; timely interventions to preserve the renal microcirculatory flow may interrupt the downward spiral of injury toward progressive kidney failure and should, therefore, be investigated. Finally, development of techniques that permit an integrative physiologic approach, including direct visualization of renal microvasculature and measurement of oxygen kinetics and mitochondrial function in intact tissue in all nephron segments, may provide new insights into how the kidney responds to various injurious stimuli and allow evaluation of new therapeutic strategies.
Keywords: acute renal failure, clinical nephrology, hemodynamics and vascular, regulation
There is an unmet need to develop effective treatments aimed at decreasing the morbidity and mortality of AKI, which continues to impose a substantial health care burden. AKI is a complex clinical syndrome encompassing a wide spectrum of etiologies, such as sepsis, volume depletion, inflammation, ischemia-reperfusion, exogenous and endogenous toxins, surgery, obstruction, heart failure, and others. These various insults often coexist in a given patient, thus making it very challenging to identify unique pathophysiologic mechanism(s) and universal relevant therapeutic targets. The 13th Acute Dialysis Quality Initiative (ADQI) Consensus Conference focused on identifying major mechanisms and targets for therapeutics in AKI, and this paper highlights the workgroup that specifically focused on hemodynamic mechanisms and targets in AKI. The conference was held in Charlottesville, Virginia on April 20, 2014. The consensus panel made recommendations on the major gaps between incomplete understanding of mechanisms that underlie AKI, target discovery, and viable AKI treatments. Given the wide heterogeneity of etiologies for AKI, for the purpose of research and conceptualization of the role of renal hemodynamics in AKI, sepsis, ischemia, and nephrotoxic insults were focused on as major model disorders for the discussion of the pathophysiology and treatment targets related to renal macrocirculatory and microcirculatory alterations. We present recommendations for future research to bridge gaps in knowledge as well as potential targets for therapy, which should hopefully result in effective therapeutic interventions for AKI.
Methods
The 13th ADQI Consensus Conference on Therapeutic Targets of Human AKI held in Charlottesville, Virginia in April of 2014 (www.adqi.net) was attended by an international group of experts and focused on an objective scientific review of the current literature, development of a consensus of opinion, with evidence where possible, to distill current literature, and articulate a research agenda to address important unanswered questions. Similar to other ADQI meetings, a modified Delphi approach was followed. Details of the methods can be found in the supplement of the introduction and summary by Okusa et al.1 in this issue of the Journal of the American Society of Nephrology.
Results
Total Renal Blood Flow in AKI
Global renal ischemia caused by compromised kidney perfusion has traditionally been recognized as one of the most common pathogenic factors leading to AKI in acutely ill patients. However, studies in large mammals, including humans, suggest that global renal hypoperfusion cannot be invoked as the sole etiology of AKI.2–5 The causative contribution of renal blood flow (RBF) to kidney dysfunction is particularly puzzling in sepsis, and there is widespread disagreement as to whether RBF is reduced, normal, or even increased.2,3,6–8 In the few large animal and clinical studies, the patterns of RBF in AKI and its relation with systemic hemodynamics are highly variable.3,6 Absence of benefit of vasodilator therapy in human sepsis–associated AKI9 may be explained by variability in blood flow. Unfortunately, more specific knowledge about the relationship between RBF and AKI in sepsis is limited by the lack of reliable methods to monitor continuous RBF in human AKI. Nevertheless, recent studies allow for several important insights. First, renal circulatory changes might behave differently in sepsis with AKI as opposed to sepsis alone.3 Second, renal hemodynamics in subjects with sepsis and AKI cannot be predicted reliably from systemic hemodynamics.3 Third, sepsis-associated AKI may be accompanied by dissociation between systemic and renal vascular resistance. These differences in systemic and renal vascular resistances may relate to both species differences and most importantly, the time periods of observations after the induction of sepsis.3,6 Regardless the pattern of total RBF, key questions are whether the interplay between the changes in global renal flow, renal oxygenation, and function is causally linked, just epiphenomena, or even protective in most common types of AKI (i.e., sepsis and surgery) and what their role is in different phases of AKI (i.e., early versus established AKI). Of critical importance is that an isolated determination of RBF outside the context of the complex interaction with glomerular and peritubular microcirculation and the tissue energy state may not be representative of downstream processes and may downplay the existence of widespread, albeit patchy renal tissue ischemia (see below). Thus, total RBF is of limited value in predicting AKI, and its causative role in AKI is difficult to determine given the complexities of the renal microcirculation.
Renal Microcirculation
Glomerular Hemodynamics in AKI
The reduction in GFR is a hallmark of AKI. The precise knowledge of glomerular hemodynamics and its determinants during AKI may have therapeutic implications. Animal studies show that, in severe prerenal insults (e.g., profound hypovolemia, low BP, and reduced cardiac output), there is a homogenous and intense reduction in single-nephron GFR in virtually all nephrons, primarily as a result of reduced renal plasma flow (RPF) and Kf.10 In contrast to this scenario, glomerular dynamics in the course of septic AKI are controversial.7,8,11 The widely held concept of a fall in transcapillary hydraulic pressure caused by afferent arteriolar vasoconstriction leading to the reduction in GFR in sepsis has been largely derived from rodents challenged with large endotoxin bolus.7,8 Recent experimental data from models of hyperdynamic sepsis in sheep questioned this paradigm, and data introduced the concept of decreased rather than increased glomerular vascular resistance, at least in early sepsis in ruminants.11,12 Although the decline in GFR is mainly attributable to a profound lowering of the intraglomerular capillary pressure, the potential contributions of elevation of Bowman’s pressure secondary to tubular obstruction and/or increased tubular flow in hyperfiltrating nephrons, impaired hydraulic permeability, and reduction of filtration surface area should be addressed by other studies in relevant animal models. Collectively, it is conceivable that a comparison of determinants of hypofiltration among progressors and nonprogressors of AKI as well as precise (semi)continuous monitoring of changes in GFR over extended periods of time should allow more sensitive investigations of the role of intrarenal hemodynamics during the development of AKI.
Regardless of the nature of glomerular loss of filtration capacity, another key question remains of what the net consequence is of strategies aimed at enhancing GFR. The reversal of functional vasoconstriction and restoration of GFR with hemodynamic optimization strategies may be beneficial during early, transient prerenal events, such as volume depletion and low BP, because the reduction in RPF is caused by systemic or extrarenal events. In most cases, if systemic alterations can be corrected, the RPF and GFR in the kidney will be improved or normalized, because this is a normal renal response to systemic changes. However, during AKI with tubular epithelial cell injury and vasoconstriction, the net effects of a hemodynamic optimization approach are far less straightforward. This renal vasoconstriction is in response to intrarenal events, such as tubular injury, and mediated largely by tubuloglomerular feedback activation. Under these conditions, such reduction in GFR may contribute to a form of renal hibernation that acts to protect injured tubules from further injury by reducing the filtered load and thereby, the reabsorptive workload. Studies performed in rat models of renal ischemia and reperfusion have shown that maneuvers aimed at ameliorating the decrease in GFR may, in fact, produce more tubular damage in the long run.13,14 In addition, transgenic mice lacking adenosine A1 receptors, through which tubuloglomerular feedback is modulated, were more sensitive to ischemia-reperfusion injury.15 Thus, although restoration of RBF and GFR might be a logical strategy in early prerenal states, hypofiltration mediated by the persistence of the tubuloglomerular feedback system may be protective in the presence of significant renal injury.16 It must be stressed, however, that the importance of tubuloglomerular feedback in mediating the link between prominent decline in GFR and focal tubular injury has been suggested for ischemia-reperfusion and nephrotoxic AKI17,18 but remains unproven in models of septic AKI and clinical AKI caused by multiple insults. Finally, internephron heterogeneity, the focal distribution of lesions and accessibility limited to a subgroup of superficial nephrons, should be kept in mind when interpreting the results of micropuncture techniques. Combinations of micropuncture with new imaging techniques, such as multiphoton microscopy, may offer new opportunities in studying real–time acute renal pathophysiology.19 In summary, uncertainty about the nature, time course, and magnitude of glomerular hemodynamics, its regulation, and how it can be effectively modulated represents an important gap of knowledge that may hinder the appropriate design of hemodynamically oriented clinical trial. The relationship and timing of glomerular hemodynamic changes to evidence of major tubular damage must also be better correlated and understood. It has been shown repeatedly that tubuloglomerular feedback adapts temporally, and the reduction of RBF during acute tubular injury may not be persistent and may only be during the acute injury phase. Clearly, the total reduction in GFR cannot be explained entirely by renal vasoconstriction and reductions in RPF rates. Other factors, including reduced glomerular permeability coefficient, tubular backleak of solutes, and tubular obstruction, remain critical factors to the total reduction in GFR. In fact, renal vasoconstriction may be transient and an appropriate response to tubular injury, inducing a form of renal hibernation or protection of injured tubules. Absolute reductions in RBF are probably more dramatic and quantitatively significant during prerenal states, which one encounters in severe volume depletion or congestive heart failure, although the latter has not been proven unequivocally. Again, species- and model-specific differences in glomerular dynamics must seriously be taken into consideration when extrapolating the findings to humans.
Peritubular Microcirculation in AKI
The postglomerular peritubular microvasculature has been increasingly recognized as a crucial compartment in different forms of AKI.20–22 Both ischemia and sepsis have profound effects on the integrity and function of peritubular capillaries.23 Rodent models of ischemia-reperfusion show compromised peritubular perfusion characterized by sluggish and retrograde blood flow that develops within minutes after reperfusion24,25 followed by restoration of normal flow within the first 4 hours, only to dramatically worsen over the next 20 hours.21,26 Similarly, rodent models of acute endotoxemia suggest that cortical peritubular capillaries are among the first renal structures injured.27–30 Interestingly, despite an apparent full recovery of renal function at 48 hours, functional capillary density recovered only partially.27 Moreover, areas of compromised cortical microvascular perfusion have been shown to correlate with renal tubular cell stress in corresponding regions, suggesting important links between altered peritubular microcirculation and epithelial cell dysfunction.27 These findings were corroborated by a study by Gupta et al.,31 in which quantitative two–photon intravital microscopy revealed markedly reduced peritubular capillary blood flow in an endotoxemia model in rats. Additionally, renal ischemia and reperfusion were associated with reduced capillary blood flow and loss of glycocalyx integrity in human kidney transplantation.32 The resulting tubular hypoxic stress serves as a robust stimulus for the amplification of local inflammatory and profibrotic factors, oxidative stress, and possibly, adaptive cellular metabolic downregulation with reprioritization of energy pathways because of hypoxia.33–35 These data collectively support the emerging evidence that tubular hypoxia and inflammation resulting from renal microvascular dysfunction are critical contributing steps in the progression of AKI. Peritubular capillaries are not only among the first structure affected during an acute insult, but also, their damage may determine both functional and structural reversibility of AKI. Indeed, microvascular injury may persist even after resolution of the initial insult, resulting in chronic microvascular alterations, fibrogenesis, and rarefaction of cells.36 In fact, persistent peritubular capillary failure and subsequent microvascular dropout predispose survivors of AKI to development of CKD and increase their risk for recurrent AKI.36,37
Mechanisms of Peritubular Capillary Dysfunction
The unique microvascular architecture of the kidney (Figure 1) and its role as a set of resistors in series as well as in parallel make the study of renal microvasculature challenging but essential to understanding the pathogenesis of AKI. Having both series and parallel components allows for continued flow if microvascular obstruction or constriction occurs in certain areas. This likely is the underlying reason for the focal nature of cortical injury during ischemia-reperfusion and injury during sepsis. The mechanisms that contribute to peritubular capillary dysfunction in both ischemia and sepsis may be multifactorial. Because peritubular capillaries are derived from the efferent glomerular arterioles, any disturbance of glomerular blood flow will impair peritubular perfusion. In addition, because efferent arterioles in the middle and inner cortex of the kidney supply not only their parent nephron, zonal peritubular ischemia involving several adjacent nephrons might occur. Further mechanisms include direct inflammatory injury of endothelial cells and activation of the epithelial-endothelial axis by inflammatory signals released from tubular cells exposed to toxicity of filtered danger signals.23 As a result, the balance is strongly tipped toward increased microvascular permeability, endothelial cell inflammation, imbalance between vasodilating and vasoconstricting factors, and activation of coagulation. The intricate molecular pathways within the local microenvironment of endothelial cells fall outside the scope of this work, and the reader is referred to recent in–depth reviews.23,38
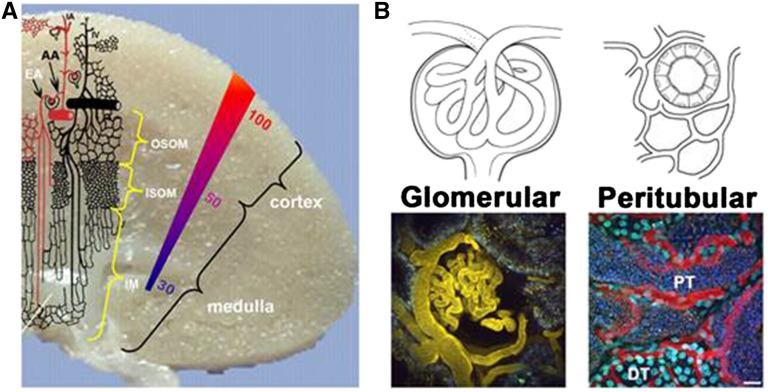
Figure 1.
(A) Schematic representation of the microvasculature within the kidney. The colored gradient represents the oxygen tension in mmHg across the kidney. Note the lack of arterial blood in the outer medullary region. AA, afferent arteriole; EF, efferent arteriole; IA, interlobular artery; IM, inner medulla; ISOM, inner stripe of the outer medulla; OSOM, outer stripe of the outer medulla. Modified from work by Aird,60 with permission. (B) Drawings and three–dimensional two–photon images of glomerular and cortical tubular areas. Glomerular: Intravial two–photon three–dimensional micrograph showing fluorescent albumin (yellow) localized within the capillary loops of a glomerulus (center) and the microvasculature surrounding proximal tubules (PTs). Peritubular: Intravital two–photon three–dimensional micrograph showing a large molecular weight fluorescent dextran (red) localized within the peritubular capillaries (red) surrounding both PTs and distal tubules (DTs). Hoechst 33342 labels the nuclei of all cells types (cyan), with brighter binding occurring within the DTs. Note that the space between tubular structures, known as the interstitial space, is largely filled by the peritubular microvasculature under physiologic conditions. Scale bar, 20 µm.
Interestingly, microcirculatory perfusion defects are not uniform throughout the kidney during acute injury. Persistent perfusion deficits and diminished tissue oxygenation have been shown to be of greater magnitude in the highly vulnerable outer medulla compared with the cortex in a variety of experimental models, including total ischemia, radiocontrast nephropathy, and nonsteroidal anti–inflammatory drug– and other drug–induced AKI etiologies, including fluid therapy.23,39 The renal cortex microcirculation is significantly compromised in sepsis models3,40 and humans,41 where inhomogeneous patchy areas of microischemia can occur (Figure 2). These heterogeneous areas of hypoxia and normal oxygenation define the nature of hemodynamic alterations leading to inflammation, because it is expected that there is increased reactive oxygen species production associated with hypoxia-normoxia interactions.42 The increased incidence of AKI in patients with CKD is likely, in part, because of the existing underlying microvascular alterations and marginal areas of oxygenation existing in patients with CKD before the new ischemic or septic event.23 In endotoxemic rats, microvascular hypoxic areas were identified using a partial pressure of oxygen (pO2) histogram analysis. However, mean microcirculatory pO2 showed no changes; a demonstration of the overall heterogeneity of ischemia is in Johannes et al.43 Similar results were obtained in an ischemia-reperfusion model of AKI, where mean tissue pO2 measured using oxygen electrodes showed no change, whereas the hypoxia–sensitive pimonidazole adduct immunohistochemistry was able to identify microheterogenous hypoxic areas.44 In addition to these effects, the patchy distribution of microvascular changes might affect both the glomerular and peritubular circulations. As a result, zones of nephrons with glomerular hypofiltration and peritubular capillary occlusion might coexist with clusters of normal or even hyperfiltrating at–risk nephrons. Hypoxic zones might coexist with intact regions with preserved tissue pO2. Hypothetically, the hypoxic nephrovascular units may signal surrounding functional nephrons in a paracrine but undefined fashion, thereby disseminating the injurious stimulus. In summary, such hypoxic areas in the cortex have been shown to coexist in the presence of normal or even supranormal arterial RBF. These findings have important consequences for clinical monitoring, because diagnostic tools will have to be developed that allow for simultaneous and dynamic determination of total RBF, differential zone flow, and tissue oxygenation.45 Without this, we will find it very difficult to translate preclinical data into therapeutic success given the heterogeneity of the disease syndrome. Currently, various functional magnetic resonance imaging (MRI) techniques (e.g., cine phase–contrast MRI, arterial spin labeling, and blood oxygen level–dependent MRI) and to some extent, contrast-enhanced ultrasound are the only clinically available research tools allowing noninvasive quantification and assessment of renal perfusion and intraorgan flow distribution during AKI.46
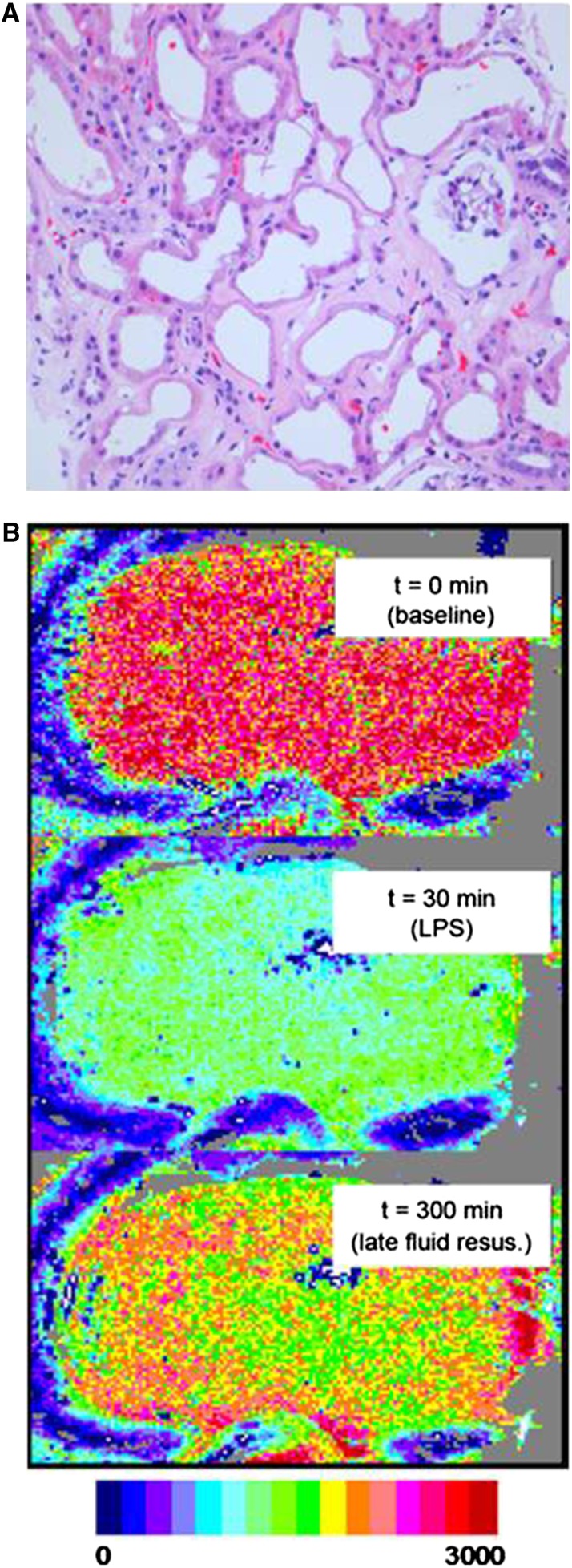
Figure 2.
(A) Histopathology of human kidney biopsy from the cortical area in a patient with AKI. Note the enlarged interstitial space with markedly reduced peritubular capillaries, ongoing cellular plugging of existing capillaries, deteriorating tubules, and an ischemic shrunken glomerulus. This is a later stage of ischemic AKI likely resulting in microvascular dropout, CKD development, and/or acceleration of CKD progression. Reprinted from Steve Bonsid (Nephropath, Little Rock, AK), with permission. (B) Shown is a speckle imaging perfusion map of the surface of a rat kidney61 at baseline, during septic shock, and after fluid resuscitation after a 30-minute delay. Fluid resuscitation corrected systemic hemodynamic variables but induced heterogeneous areas of hypoperfusion in the renal cortex. Reprinted from Legrand et al.,40 with permission.
Although compromised renal peritubular microcirculation seems to have a central and possibly causative role in the pathogenesis of AKI, often profound reduction in GFR is unlikely to be solely an effect of the focal nature of microvascular changes, indicating a central role for altered glomerular hemodynamics. Hypothetically, increased filtration of danger signals in areas of initially preserved nephrovascular units can lead to subcellular renal tubular cell injury/activation, thus driving the activation of the tubuloglomerular feedback mechanism. However, there is no clear proof showing the trafficking between tubular and glomerular compartments linking tubular function to renal hemodynamics in human AKI. Along the same vein, a question remains whether the renal microvascular alterations are always the primary and predominant event leading to tubular stress and injury.47 Alternatively, tubular cell injury might trigger reflex microvascular vasoconstriction at the single-nephron level. This reasoning is supported by observations derived from models of single–nephron tubular obstruction48 and localized microinjection of Escherichia coli into early proximal tubule lumens.49–51 Therefore, there seems to be a bidirectional and possibly synergistic crosstalk within the complex tubule-capillary microenvironment, with the sequence of events depending on the nature of insult.23 Although microcirculatory changes may lead to tubular injury, the reverse may also be true.
Targets to Protect the Nephrovascular Units
The maintenance of peritubular microcirculation, thus, seems to be an important therapeutic target to improve the acute and chronic renal outcomes in patients with AKI. However, multiple knowledge gaps need to be addressed to improve our capacity to transfer basic science knowledge into clinical practice. Many of pathways implicated in renal microvascular failure may serve dual roles—damaging endothelial and tubular cells acutely but triggering adaptive response or even enhancing tissue repair later. Future studies must dissect these dual mechanisms. Furthermore, limited blood flow can modulate downstream molecular mechanisms (evoking local inflammatory response) or act directly as executioners of cell death. Likewise, augmenting the blood flow may increase influx of danger molecules and amplify the danger response. Dissection of which phenomenon prevails in which phase of AKI might dictate the treatment efficacy. In addition, any therapeutic intervention aimed at restoring glomerular perfusion and filtration may be counterproductive unless accompanied by the restoration of peritubular circulation and oxygenation. We also need to better understand the effect and mechanisms of aging and major comorbidities on renal vascular vulnerability to insults and how these mechanisms affect the effectiveness of novel therapies. Another recognized problem is that the majority of animal models copy neither the entire spectrum of clinical AKI (combination of relevant insults; e.g., hypovolemia, sepsis, and toxicity) nor relevant AKI population (predisposing comorbidities).
Therapeutic strategies that target the microvasculature in AKI can be broadly divided into several categories (Table 1). Oxidative stress–mediated cellular injury has been implicated in the pathogenesis of AKI as a central element of the network of danger response, affecting both endothelial and epithelial functions as well as cellular energetics.33 Therapeutic interventions aimed at maintaining the homeostatic balance between oxygen radicals, nitric oxide, and oxygen have been shown to be effective in several studies in rodent models of septic AKI27–31 and ischemia-reperfusion injury52 as well as in large animal models of septic AKI.53,54 The lack of effective inducible nitric oxide synthase inhibitors for clinical use can be considered an important area for research.
Table 1.
Promising hemodynamic–based therapeutic targets to treat AKI
| Alteration and Target | Setting | Mechanisms | Potential Downside | Stage |
|---|---|---|---|---|
| Global RBF | ||||
| MAP | Shock states, sepsis | Improved early renal perfusion | Vasopressor load, medullary hypoxia, tubular workload | Two small clinical studies, one phase 3 clinical |
| ANP low dose | Cardiac surgery | Improved renal perfusion | Ineffective in late AKI, hypotension in large dose | One small RCT |
| Renal (de)congestion | Heart failure, sepsis, ICU AKI | Improved renal perfusion, tissue edema | Induction of prerenal response | Observational trials |
| Glomerular hemodynamics | ||||
| Selective renal adenosine 1 receptors agonists | I/R, sepsis AKI | Activation of tubuloglomerular feedback, prevention of tubular cell danger load and medullary hypoxia | Reduced cortical perfusion | Mice I/R, sepsis AKI |
| Selective renal adenosine 1 receptors antagonists | Radiocontrast AKI, nephrotoxic AKI | Suppression of tubuloglomerular feedback, improved GFR and postglomerular perfusion | Medullary hypoxia, tubular workload | Small animals studies |
| Angiotensin II | Early hyperemic sepsis AKI | Increased glomerular filtration pressure | Systemic effects, tubular danger load, renal ischemia | Sheep hyperdynamic sepsis and AKI |
| Vasopressin/terlipressin | Early sepsis AKI | Increased glomerular filtration pressure | Systemic effects, tubular danger load, renal ischemia | Small clinical, post hoc RCT analysis (VASST), preclinical large animals |
| Glomerular inflammation | Sepsis AKI | Glomerular endothelial protection | Ineffectiveness of anti-TNF strategies in human trials | TNFR1 knockout mice |
| Peritubular microcirculation | ||||
| NOS-targeted therapy (e.g., iNOS inhibition, eNOS preservation) | Sepsis AKI, I/R | Preserved microvascular perfusion, suppression of local inflammation, oxidative stress and bioenergetic failure | Unclear timing, both damaging and protective consequences | Preclinical studies, limited human evidence |
| RNOS-targeted therapy | Sepsis AKI, I/R | Preserved microvascular perfusion, suppression of local inflammation and bioenergetic failure | Unclear timing, variety of drugs, both damaging and protective consequences | Preclinical studies |
| IκB kinase inhibition | Sepsis AKI | Attenuation of iNOS while increasing eNOS | Biphasic roles—limiting inflammation early, limiting recovery later on? Proapoptotic effects? | Mice sepsis and endotoxemia |
| Endothelin-1 antagonism | I/R, sepsis, progression to CKD | Improved renal microcirculation, reduction in oxidative stress and inflammation | Role of other receptors and their distribution unknown | Mouse I/R, porcine endotoxemia |
| Vascular integrity | ||||
| Toll–like receptors 4 manipulation | I/R, sepsis, inflammation | Limiting DAMPS–induced vascular injury | Biphasic roles—limiting inflammation early, limiting recovery later on? Immune suppression? | Small animal I/R, Tx, endotoxemia |
| S1P1R agonists | Prevention of I/R | Prevention of endothelial barrier dysfunction | Unclear | Human trial in Tx ongoing, mice I/R, cell culture |
| Vasculotrophic strategies (VEGF, EPC, MSC, angiopoietin-1) | Microvascular regeneration | Limiting vascular dropout | Safety limits? | Small animal I/R |
| ENT inhibition | I/R | A2B adenosine receptors–mediated prevention of post–ischemic no reflow phenomenon | Unclear | Mice I/R |
| Soluble thrombomodulin | I/R | Attenuated endothelial permeability, cellular adhesion | Unclear | Rat I/R |
| Others | ||||
| Supplemented resuscitation fluids (ethylpyruvate analog) | Sepsis, shock, inflammation | Glycocalyx-protecting interventions, antioxidant effects | Unclear | Small animal sepsis |
| HIF activators | I/R | Tolerance to tissue hypoxia | Only pretreatment? Risk of fibrogenesis? | Small animal models |
Because multiple mechanisms are involved in the development of microvascular dysfunction, it is unlikely that a single-pathway intervention would be effective. Evaluation of effectiveness of combined therapies compared with single therapy and determination of the optimal timing of the components of combination therapy are required. MAP, mean arterial pressure; ANP, atrial natriuretic factor; NOS, nitrogen oxygen species; iNOS, inducible nitric oxide synthase; eNOS, endothelial nitric oxide synthase; RNOS, reactive nitrogen oxygen species; IκB, inhibitor of kappa B kinase; S1P1R, sphingosine-1 phosphate 1 receptor; VEGF, vascular endothelial growth factor; EPC, endothelial progenitor cell; MSC, mesenchymal stem cell; ENT, equilibrative nucleoside transporter; HIF, hypoxia-induced factor; ICU, intensive care unit; I/R, ischemia-reperfusion; DAMPS, damage-associated molecular pattern molecules; A2B, adenosine A2B receptor; RCT, randomized controlled trial; VASST, vasopressin and septic shock trial; TNFR1, tumor necrosis factor receptor 1; Tx, transplantation.
Recent discoveries of the critical physiologic role of endothelial glycocalyx in controlling vascular permeability and limiting interaction between endothelium and circulating cells as well as its role in propagating inflammation and tissue edema if injured give hope for the development of new strategies aimed at enhancement of vascular integrity and amelioration of vascular leak in AKI.55 Another emerging possibility to act therapeutically on endothelial vascular integrity involves Toll–like receptor 4–dependent activation of endothelial cells56 and modulation thrombomodulin–driven pathways.57 From the long-term perspective, postinsult recovery of the peritubular capillary network may prevent chronic tubulointestinal hypoxia and fibrosis.58 Therefore, the therapeutic potential of strategies targeting vascular repair and regeneration, such as angiogenic factors, and stem or progenitor cell populations is worthy of further preclinical testing.
In addition to identifying new treatment targets, there is also a pressing need to assess more completely the consequences of current therapeutics. Most interventions that are used in the resuscitation of critically ill patients may influence the renal microcirculation in a deleterious manner. For example, the exact mechanisms by which different fluid compositions (such as normal saline and colloid or buffered solutions) affect renal microcirculation are not well understood, and emerging experimental and clinical data suggest that use of inappropriate fluids or fluid replacement strategies may exert deleterious effects on the microcirculation and renal outcomes.40,59 Strategies allowing real-time evaluation of microcirculatory fluid and vasopressors responsiveness might be expected to optimize resuscitation effectiveness while limiting the potential to cause harm.
Research Agenda
We have developed the following research agenda in relation to the understanding and treatment of AKI from a renal hemodynamic point of view. Issues that require further investigation are listed.
The identification of pathway(s) implicated in renal microvascular stress that should be targeted for therapeutic intervention (functional versus structural changes).
Understanding the nature, time course, and magnitude of glomerular hemodynamics and the effect of bidirectional tubuloglomerular feedback signal on injury and recovery in various AKI models. Development of animal models depicting the phenomenon of uncoupling severe depression of glomerular filtration and limited focal structural tubular cell dysfunction.
The identification of an optimal time window for interventions as well as development of novel real–time monitoring methods to ensure that emerging therapies reach their specific cellular targets within the renal vasculature.
An integrative preclinical approach involving simultaneous determination of RBF, direct visualization of microcirculation and oxygen kinetics, and assessment of mitochondrial function and mediators involved in regulation of renal hemodynamics represents a comprehensive monitoring platform to identify AKI, individualize therapy, and follow the response to therapy. Imaging the microcirculation will need to take into account the heterogeneity of the microcirculation and its dysfunction.
New technologies that are most likely to improve our understanding in the clinical research setting includefunctional MRI (allowing noninvasive quantification and assessment of intraorgan distribution of renal perfusion and oxygen kinetics during AKI) and contrast-enhanced ultrasound.
Because of the inaccessibility of the kidney under clinical conditions, future research will need to determine to what extent distant microcirculatory alterations (e.g., sublingual microcirculation) reflect microcirculatory defects occurring in the kidney.
To bridge the gap between preclinical and clinical studies, better models of AKI that recapitulate the complex nature of human AKI, including myriad etiologies, are needed. Mechanistic studies are required to better understand how comorbidities (such as CKD) alter the renal hemodynamic response, enhance the intrinsic susceptibility of the (micro)vascular system to insults, and affect the effectiveness of novel therapies.
Disclosures
M.M., L.S.C., R.B., B.A.M., M.H.R., M.D.O., and C.R. report no conflicts of interest. C.I. has received honoraria and independent research grants from Fresenius-Kabi, Baxter Health Care, and AM Pharma. C.I. has developed sidestream dark field (SDF) imaging and is listed as inventor on related patents commercialized by MicroVision Medical (MVM) under a license from the Academic Medical Center. C.I. has been a consultant for MVM in the past but has not been involved with this company for >5 years, except that he still holds shares. Braedius Medical, a company owned by a relative of C.I., has developed and designed a handheld microscope called CytoCam-IDF (incident dark field illumination) imaging. C.I. has no financial relation with Braedius Medical of any sort (i.e., never owned shares or received consultancy or speaker fees from Braedius Medical). J.A.K. has received consulting fees from Abbott, Aethlon, Alere, Alung, AM Pharma, Astute Medical, Atox Bio, Baxter, Cytosorbents, venBio, Gambro, Grifols, Roche, Spectral Diagnostics, Sangart, and Siemens. J.A.K. has also received research grants from Alere, Astute Medical, Atox Bio, Bard, Baxter, Cytosorbents, Gambro, Grifols, Kaneka, and Spectral Diagnostics and has licensed technologies through the University of Pittsburgh to Astute Medical, Cytosorbents, and Spectral Diagnostics.
Supplementary Material
Acknowledgments
Insights related to this paper have been obtained with Dutch Kidney Foundation Grants C09-2290 (to C.I.) and 14OIP11 (to C.I.). M.M. was supported by National Sustainability Program I No. LO1503 and by the project No. CZ.1.07/2.3.00/30.0061 co-financed by the European Social Fund and the state budget of the Czech Republic.
A complete list of participants is provided in Supplemental Appendix.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030234/-/DCSupplemental.
References
- 1.Okusa MD, Rosner MH, Kellum JA, Ronco C: Therapeutic targets of human AKI: Harmonizing human and animal AKI. J Am Soc Nephrol 26: 44–48, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langenberg C, Wan L, Egi M, May CN, Bellomo R: Renal blood flow and function during recovery from experimental septic acute kidney injury. Intensive Care Med 33: 1614–1618, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Benes J, Chvojka J, Sykora R, Radej J, Krouzecky A, Novak I, Matejovic M: Searching for mechanisms that matter in early septic acute kidney injury: An experimental study. Crit Care 15: R256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saotome T, Ishikawa K, May CN, Birchall IE, Bellomo R: The impact of experimental hypoperfusion on subsequent kidney function. Intensive Care Med 36: 533–540, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Parekh DJ, Weinberg JM, Ercole B, Torkko KC, Hilton W, Bennett M, Devarajan P, Venkatachalam MA: Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol 24: 506–517, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prowle JR, Molan MP, Hornsey E, Bellomo R: Measurement of renal blood flow by phase-contrast magnetic resonance imaging during septic acute kidney injury: A pilot investigation. Crit Care Med 40: 1768–1776, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Schrier RW, Wang W: Acute renal failure and sepsis. N Engl J Med 351: 159–169, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Lugon JR, Boim MA, Ramos OL, Ajzen H, Schor N: Renal function and glomerular hemodynamics in male endotoxemic rats. Kidney Int 36: 570–575, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Kellum JA: Impaired renal blood flow and the ‘spicy food’ hypothesis of acute kidney injury. Crit Care Med 39: 901–903, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Blantz RC, Singh P: Analysis of the prerenal contributions to acute kidney injury. Contrib Nephrol 174: 4–11, 2011 [DOI] [PubMed] [Google Scholar]
- 11.May CN, Calzavacca P, Ishikawa K, Langenberg C, Wan L, Ramchandra R, Bellomo R: Novel targets for sepsis-induced kidney injury: The glomerular arterioles and the sympathetic nervous system. Exp Physiol 97: 1168–1177, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Langenberg C, Wan L, Egi M, May CN, Bellomo R: Renal blood flow in experimental septic acute renal failure. Kidney Int 69: 1996–2002, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Bird JE, Milhoan K, Wilson CB, Young SG, Mundy CA, Parthasarathy S, Blantz RC: Ischemic acute renal failure and antioxidant therapy in the rat. The relation between glomerular and tubular dysfunction. J Clin Invest 81: 1630–1638, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bird JE, Evan AP, Peterson OW, Blantz RC: Early events in ischemic renal failure in the rat: Effects of antioxidant therapy. Kidney Int 35: 1282–1289, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J: Mediation of tubuloglomerular feedback by adenosine: Evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A 98: 9983–9988, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chawla LS, Kellum JA, Ronco C: Permissive hypofiltration. Crit Care 16: 317, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson OW, Gabbai FB, Myers RR, Mizisin AP, Blantz RC: A single nephron model of acute tubular injury: Role of tubuloglomerular feedback. Kidney Int 36: 1037–1044, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Singh P, Blantz RC, Rosenberger C, Gabbai FB, Schoeb TR, Thomson SC: Aberrant tubuloglomerular feedback and HIF-1α confer resistance to ischemia after subtotal nephrectomy. J Am Soc Nephrol 23: 483–493, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall AM, Molitoris BA: Dynamic multiphoton microscopy: Focusing light on acute kidney injury. Physiology (Bethesda) 29: 334–342, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molitoris BA: Renal blood flow in sepsis: A complex issue. Crit Care 9: 327–328, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molitoris BA, Sutton TA: Endothelial injury and dysfunction: Role in the extension phase of acute renal failure. Kidney Int 66: 496–499, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Ergin B, Kapucu A, Demirci-Tansel C, Ince C: The renal microcirculation in sepsis. Nephrol Dial Transplant 30: 169–177, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Molitoris BA: Therapeutic translation in acute kidney injury: The epithelial/endothelial axis. J Clin Invest 124: 2355–2363, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto T, Tada T, Brodsky SV, Tanaka H, Noiri E, Kajiya F, Goligorsky MS: Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am J Physiol Renal Physiol 282: F1150–F1155, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, Goligorsky MS: Endothelial dysfunction in ischemic acute renal failure: Rescue by transplanted endothelial cells. Am J Physiol Renal Physiol 282: F1140–F1149, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA: Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol 285: F191–F198, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Gokden N, Mayeux PR: Evidence for the role of reactive nitrogen species in polymicrobial sepsis-induced renal peritubular capillary dysfunction and tubular injury. J Am Soc Nephrol 18: 1807–1815, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Holthoff JH, Wang Z, Seely KA, Gokden N, Mayeux PR: Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int 81: 370–378, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Holthoff JH, Seely KA, Pathak E, Spencer HJ, 3rd, Gokden N, Mayeux PR: Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am J Pathol 180: 505–516, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiwari MM, Brock RW, Megyesi JK, Kaushal GP, Mayeux PR: Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: Role of nitric oxide and caspases. Am J Physiol Renal Physiol 289: F1324–F1332, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Gupta A, Rhodes GJ, Berg DT, Gerlitz B, Molitoris BA, Grinnell BW: Activated protein C ameliorates LPS-induced acute kidney injury and downregulates renal INOS and angiotensin 2. Am J Physiol Renal Physiol 293: F245–F254, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Snoeijs MG, Vink H, Voesten N, Christiaans MH, Daemen JW, Peppelenbosch AG, Tordoir JH, Peutz-Kootstra CJ, Buurman WA, Schurink GW, van Heurn LW: Acute ischemic injury to the renal microvasculature in human kidney transplantation. Am J Physiol Renal Physiol 299: F1134–F1140, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Aksu U, Demirci C, Ince C: The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide. Contrib Nephrol 174: 119–128, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Sutton TA, Fisher CJ, Molitoris BA: Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 1539–1549, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA: A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 41: 3–11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basile DP: The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int 72: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Hörbelt M, Lee SY, Mang HE, Knipe NL, Sado Y, Kribben A, Sutton TA: Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol 293: F688–F695, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johannes T, Mik EG, Nohé B, Unertl KE, Ince C: Acute decrease in renal microvascular PO2 during acute normovolemic hemodilution. Am J Physiol Renal Physiol 292: F796–F803, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Legrand M, Bezemer R, Kandil A, Demirci C, Payen D, Ince C: The role of renal hypoperfusion in development of renal microcirculatory dysfunction in endotoxemic rats. Intensive Care Med 37: 1534–1542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS: Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 187: 509–517, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Legrand M, Mik EG, Johannes T, Payen D, Ince C: Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med 14: 502–516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johannes T, Mik EG, Ince C: Nonresuscitated endotoxemia induces microcirculatory hypoxic areas in the renal cortex in the rat. Shock 31: 97–103, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Abdelkader A, Ho J, Ow CP, Eppel GA, Rajapakse NW, Schlaich MP, Evans RG: Renal oxygenation in acute renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 306: F1026–F1038, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Okusa MD, Jaber BL, Doran P, Duranteau J, Yang L, Murray PT, Mehta RL, Ince C: Physiological biomarkers of acute kidney injury: A conceptual approach to improving outcomes. Contrib Nephrol 182: 65–81, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider AG, Goodwin MD, Bellomo R: Measurement of kidney perfusion in critically ill patients. Crit Care 17: 220, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkatachalam MA, Weinberg JM: The tubule pathology of septic acute kidney injury: A neglected area of research comes of age. Kidney Int 81: 338–340, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arendshorst WJ, Finn WF, Gottschalk CW: Nephron stop-flow pressure response to obstruction for 24 hours in the rat kidney. J Clin Invest 53: 1497–1500, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Månsson LE, Melican K, Boekel J, Sandoval RM, Hautefort I, Tanner GA, Molitoris BA, Richter-Dahlfors A: Real-time studies of the progression of bacterial infections and immediate tissue responses in live animals. Cell Microbiol 9: 413–424, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Melican K, Boekel J, Månsson LE, Sandoval RM, Tanner GA, Källskog O, Palm F, Molitoris BA, Richter-Dahlfors A: Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis. Cell Microbiol 10: 1987–1998, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Choong FX, Sandoval RM, Molitoris BA, Richter-Dahlfors A: Multiphoton microscopy applied for real-time intravital imaging of bacterial infections in vivo. Methods Enzymol 506: 35–61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Legrand M, Almac E, Mik EG, Johannes T, Kandil A, Bezemer R, Payen D, Ince C: L-NIL prevents renal microvascular hypoxia and increase of renal oxygen consumption after ischemia-reperfusion in rats. Am J Physiol Renal Physiol 296: F1109–F1117, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Matejovic M, Krouzecky A, Martinkova V, Rokyta R, Jr., Kralova H, Treska V, Radermacher P, Novak I: Selective inducible nitric oxide synthase inhibition during long-term hyperdynamic porcine bacteremia. Shock 21: 458–465, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Matejovic M, Krouzecky A, Martinkova V, Rokyta R, Jr., Radej J, Kralova H, Treska V, Radermacher P, Novak I: Effects of tempol, a free radical scavenger, on long-term hyperdynamic porcine bacteremia. Crit Care Med 33: 1057–1063, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Chelazzi C, Villa G, Mancinelli P, De Gaudio AR, Adembri C: Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care 19: 26, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Hartono JR, John R, Bennett M, Zhou XJ, Wang Y, Wu Q, Winterberg PD, Nagami GT, Lu CY: Early interleukin 6 production by leukocytes during ischemic acute kidney injury is regulated by TLR4. Kidney Int 80: 504–515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharfuddin AA, Sandoval RM, Berg DT, McDougal GE, Campos SB, Phillips CL, Jones BE, Gupta A, Grinnell BW, Molitoris BA: Soluble thrombomodulin protects ischemic kidneys. J Am Soc Nephrol 20: 524–534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chade AR: Renal vascular structure and rarefaction. Compr Physiol 3: 817–831, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aksu U, Bezemer R, Yavuz B, Kandil A, Demirci C, Ince C: Balanced vs unbalanced crystalloid resuscitation in a near-fatal model of hemorrhagic shock and the effects on renal oxygenation, oxidative stress, and inflammation. Resuscitation 83: 767–773, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Aird WC, editor: Endothelial Biomedicine, Chapter 138, Cambridge, United Kingdom, Cambridge University Press, 2007, p 1271 [Google Scholar]
- 61.Bezemer R, Legrand M, Klijn E, Heger M, Post IC, van Gulik TM, Payen D, Ince C: Real-time assessment of renal cortical microvascular perfusion heterogeneities using near-infrared laser speckle imaging. Opt Express 18: 15054–15061, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.