Abstract
Obesity is associated with severe, poorly controlled asthma that does not respond as well to therapy as asthma in leaner asthmatics. Important insights gained from animal models of obesity and asthma suggests that different forms of obesity may lead to different manifestations of airway disease: obesity is associated with both innate increased airway reactivity and altered responses to aeroallergen and pollutant challenges. In humans, at least two broad groups of obese asthmatics have been recognized: one that is likely unique to obesity and another that is likely lean allergic asthma much complicated by obesity. This article will discuss what we have learned about the immunological and pathophysiological basis of asthma in obesity from animal and human studies, and how this might guide therapy.
Keywords: adiponectin, airway hyperreactivity, asthma, diet, leptin, obesity, phenotypes
Background
The world is in the midst of an epidemic of obesity [1], and this is having a dramatic effect on the disease of asthma. Obesity is a risk factor for the development of asthma: in the USA, with one-third of the population obese, approximately 250,000 cases of asthma per year are related to obesity [2]. There appears to be a particularly high prevalence of obesity in patients with severe asthma: a recent study reported that well over 50% of severe asthmatics were obese [3]. Not only is obesity having a dramatic effect on the epidemiology of asthma, but it appears to be changing the biologic basis of the disease. Obese patients tend to have more severe asthma that does not respond as well to therapy compared with lean asthmatics [4]. Asthma patients with obesity are not simply larger versions of lean patients with asthma, as obesity has profound effects on the immune system and physiological function. This review will focus on the latest in our understanding of the immunological underpinnings of asthma in obesity from studies in animal models and human populations, and the implications this has for clinical therapy.
Mouse models of obesity & asthma
Mouse models are useful in order to dissect the myriad of mediators that control weight gain and asthma phenotype: they facilitate control of when weight gain occurs, allow the addition or removal of specific factors from the diet and enable selective breeding with other mice containing metagenomics alterations (e.g., knockouts, transgenics, gene polymorphisms or microbiome alterations). These mouse models enable the study of the innate airway hyperreactivity that occurs in obesity, and also airway hyperreactivity and inflammation induced by exposure to pollutants and allergens [5–7].
Genetic mutations regulating metabolism & obesity
Leptin, adiponectin and insulin are hormones that regulate satiety and energy expenditure, aberrations in signaling of these hormones lead to obesity and metabolic dysfunction [8–11], and also appear to be involved in the pathogenesis of asthma in obesity. For example, mutations in leptin (ob/ob mutation) or its hypothalamic receptor (db/db mutation) lead to hyperphagia and early onset, severe obesity in mice [12–15]. Both ob/ob and db/db mice exhibit innate airway hyperreactivity (which occurs without challenge and in the absence of overt inflammation), exacerbated airway reactivity and inflammation in response to the air pollutant ozone [16–18], but dampened airway inflammatory responses to allergen challenge (TABLE 1) [19].
Table 1.
Findings in mouse models of obesity and metabolic abnormalities related to obesity.
| Mouse model | Innate airway reactivity | Response to allergen challenge | Response to ozone |
|---|---|---|---|
| ob/ob, lack leptin | ↑ | ↓ Inflammation ↑ AHR | ↑ Inflammation & AHR (IL-6 dependent) |
| db/db, lack leptin receptor | ↑ | ↓ Inflammation ↑ AHR | ↑ Inflammation ↑ AHR |
| Adiponectin deficiency | ↑ | ↑ Inflammation ↑ AHR | ↑ Inflammation & AHR (IL-6 & -17A dependent) |
| CPEfat | ↑ | ↑ Inflammation ↑ AHR | ↑ Inflammation ↑ AHR |
| High fat diet | ↑ | ↑↓ Inflammation† ↑ AHR | ↑ Inflammation ↑ AHR |
‘Inflammation’ in this context refers to increased airway inflammation documented most often by bronchoal-veolar lavage cell counts.
Both increased and decreased inflammation in response to aeroallergen have been reported in various strains, with different allergens.
AHR: Airway hyperreactivity.
The ob/ob and db/db mice are illustrative of innate airway hyperreactivity that arises as a consequence of altered lung development. These mice do not exhibit increased basal airway inflammation. However, both strains have reduced lung growth and subsequent reduction of end-expiratory lung volume. As has been reviewed previously [5], the smaller lung size in ob/ob and db/db mice could either be due to the lack of leptin as a growth factor or because the increased fat mass during early onset obesity restricts lung growth during development.
Adiponectin is decreased in obesity. Adiponectin induces anti-inflammatory IL-10 from resident macrophages; in the absence of adiponectin, IL-10 production decreases in favor of proinflammatory cytokine secretion [7,20]. Mice that lack adiponectin (Adpn−−) develop slightly increased airway reactivity [21], and have exaggerated responses to allergen challenge [21]. Adpn−/− mice also develop increased inflammation in response to subacute ozone, a response dependent on IL-6 [22]. Adiponectin infusion attenuates allergen-induced airway reactivity and inflammation in normal weight mice [23]. Deficiency of adiponectin may contribute to asthma in obesity, particularly in response to allergen or environmental challenge.
Carboxypeptidase E is an enzyme required for the processing of insulin and other neuropeptides, mutations in the Cpe gene lead to aberrant signaling and weight gain [24]. Cpefat mice develop innate airway hyperreactivity, and have exacerbated hyperreactivity and inflammation in response to both ozone and allergen exposure (TABLE 1) [25,26].
Diet-induced obesity
Diet-induced obesity in mice is achieved by a high fat or sucrose diet [6,27]. High fat diet-induced obesity alters pulmonary responses to both ozone and allergen challenges, although both increased and decreased allergic airway inflammation have been reported by groups using various aeroallergens and strains of mice (TABLE 1) [28,29].
A high fat diet also induces innate airway reactivity, and recent work suggests that IL-17A may be centrally involved in this process. C57BL/6 mice on a 60% high fat diet developed innate airway reactivity by 24 weeks, accompanied by increases in pulmonary IL-17A+ CD4+ and IL-17A+γδ+ T cells [30]. IL-17A has been implicated in the development of airway reactivity in lean mouse models of allergic asthma, and also in obese CPEfat mice [31,32]. Additionally, recent work links IL-17A directly to high fat diet-induced airway hyperreactivity: IL-17A−/− mice demonstrate significant weight gain in response to a 14-week high fat diet, but their airway resistance is significantly lower than wild-type littermates fed the same diet [33]. The source of IL-17A in the lung was innate lymphoid type 3, and this was dependent upon NLRP3 activation and secretion of IL-1β from pulmonary macrophages [33]. We and others have demonstrated the importance of IL-1 signaling in the development of Th17 responses in lean allergic mouse models of asthma [34,35]. A number of studies now suggest that IL-17A may be important in the innate airway hyperreactivity of mice with obesity related to a high fat diet, and the CPEfat mutation.
Metabolic inflammation in obesity
Adipocytes in obese individuals secrete proinflammatory cytokines, including TNF-α, IL-1β and IL-6 [36,37], whereas the production of anti-inflammatory mediators (such as adiponectin) is decreased. Changes in these mediators may contribute to exaggerated airways reactivity and inflammation in obesity. TNF-α, for example, may be involved in increased inflammatory airway responses in obesity. TNF-α is increased in obesity. It can interact with its receptor on airway smooth muscle to increase contractility and induce intracellular signaling and inflammation. In lean individuals, adiponectin inhibits TNF-α-induced NF-κB signaling, and Treg produce IL-10 that can suppress TNF-α production. However, Treg are not only less abundant in obese adipose tissue, but also less effective producers of IL-10, and therefore less effective inhibitors of TNF-α [38,39].
The importance of the TNF-α pathway is best understood in the context of innate airway reactivity of obesity. TNF-α receptor 1 (TNFR1) may protect against the spontaneous airway reactivity in obesity, as shown in a study of Cpefat mice crossed with TNFR1-deficient mice: obese (Cpefat) mice develop increased airway reactivity to inhaled methacholine, this is further increased in Cpefat xTNFR1−/− mice, and this is associated with increased lung IL-17A expression [40]. Conversely, lack of TNF-α receptor-2 abrogates airway reactivity in obese mice [32], with downstream effects on IL17A, endothelin and tropomyosin-related kinase B: TNF-α-induced airway reactivity in obesity requires signaling specifically through TNF-α receptor-2.
Neurogenic pathways
Cholinergic innervation of the airways regulates airway tone. Obesity may alter parasympathetic signaling: Nie et al. found evidence of increased vagally mediated bronchoconstriction related to hyperinsulinemia in a rat model [41]. Arteaga-Solis-et al. showed that leptin usually inhibits parasympathetic signaling to produce bronchodilation, and that leptin resistance in obesity may promote parasympathetic bronchoconstriction and airway hyperreactivity [42]. Changes in cholinergic signaling may contribute to innate increased airway reactivity in obesity.
Signaling through substance P may also be involved in both obesity and allergic inflammation. Ramalho et al. have shown that both obesity and allergen challenge increase serum substance P, pharmacological inhibition of substance P decreases both obesity and markers of allergic inflammation, and this is associated with changes in staining for lymphatic structures on airway epithelium [43–45]. BOX 1 shows a summary of mechanisms linked with innate airway hyperreactivity in obese animals.
Box 1. Mechanisms linked with innate airway hyperreactivity in obese animals.
Associated with ↑ in lung IL-17A (CPEfat) [32].
High fat diet increases IL-17A (+) γδ T & CD4(+) T cells in lung [30].
High fat diet increases macrophage IL-1β, which can induce IL-17A from ILC3 cells [33].
↑ in mice lacking TNF-α receptor 1 (CPEfat), associated with ↑ IL-17A [40].
↓ in mice lacking TNF-; receptor-2 (CPEfat) associated with ↓ IL-17A [32].
Increased parasympathetic bronchial tone (regulated by leptin and insulin) [41,42].
Common pathways leading to obesity & allergic airway disease
Chitinase 3-like-1 (CHI3L1) is an asthma susceptibility gene associated with lung function impairment, Th2 response development, allergic airway inflammation and methacholine hyper-responsiveness [46,47]. CHI3L1 may also be involved in the pathogenesis of obesity [48]. Chi3l1-deficient mice have less visceral obesity and smaller adipocytes that produce lower levels of cytokines than those from wild-type mice. On the other hand, the overexpression of Chi3l1 in airway epithelium causes obesity. A high fat diet induces Chi3l1 and increases the magnitude of allergic airway inflammation, whereas Chi3l1-deficient mice fed a high fat diet have a blunted inflammatory response to antigen challenge. Interestingly, antigen challenge of mice on a normal diet induces epididymal adipose tissue expansion. Data from the mouse model are complemented by those from human subjects demonstrating that the CHI3L1 gene product (YKL-40) is elevated in the serum of persistent asthmatics and correlates with truncal obesity and low lung function in obese asthmatics [48]. CHI3L1 appears to serve as a pathogenic mediator of both allergic airway disease and increased adiposity, suggesting common pathways can produce both allergic asthma and obesity.
Vitamins & gut microbiome
Animal models provide insight into the role of the pre- and perinatal environment in establishing a lifelong risk for obesity as well as airway hyperreactivity. Maternal intake of the fat-soluble vitamins A, D and E regulate development of the fetal lung, and deficiencies in these vitamins during gestation are linked to the development of asthma and low lung function [49–54]. These same vitamins modulate the development of a healthy gastrointestinal microbiome and impact the lifetime risk for obesity and metabolic disorders [55,56]. The gut microbiome of obese mice has preferential growth of bacterial species that take up more energy from the diet for storage as fat compared with lean mice [57]. Heterozygous leptin-deficient mothers (Lep+/ob) and their fully deficient (ob/ob) offspring share the same intestinal microbes at birth. However, the leptin-deficient mice consume >42% more food, and this is associated with a marked reduction in Bacteroides and a corresponding increase in Firmicutes species, compared with lean (wild-type) controls [58]. Transplantation of the Firmicutes-rich microbiota from ob/ob mice into lean, germ-free controls induced a significant increase in body fat, without a corresponding increase in chow consumption [59]. The makeup of the intestinal microbiome also controls the CD4+ T-cell phenotype in both the gut and in mouse models of allergic asthma [60]. The impact that the human resident lung microbiome has on the development of obese asthma is only beginning to be explored. BOX 2 shows a summary of vitamins, microbiome and asthma interactions.
Box 2. Vitamins, microbiome and asthma.
The numerous links between the development of obesity and the development of asthma in animal models provide insights into the immunological mechanisms that govern both syndromes (FIGURE 1). Obese mice have innate increases in airway reactivity (occurring without challenge or overt inflammation), but they have varying responses to ozone and allergen challenges. This suggests that while obesity and metabolic dysfunction produce innate airway reactivity, responses to pollutants and allergen challenge differ according to the mechanism of obesity and type of challenge. This observation has important implications for our understanding of the complexity of the obesity–asthma relationship in humans.
Figure 1. Shared mediators of obesity and airway reactivity (AHR) regarding early and late onset.
Mouse models of early obesity (ob/ob and db/db mice) exhibit developmental differences and share characteristic mediators with early onset airways hyperresponsiveness (AHR). Conversely, chronic inflammation appears to play a role in both obesity that develops over time as well as later-onset airway hyperreactivity.
Phenotypes of obese asthma in humans
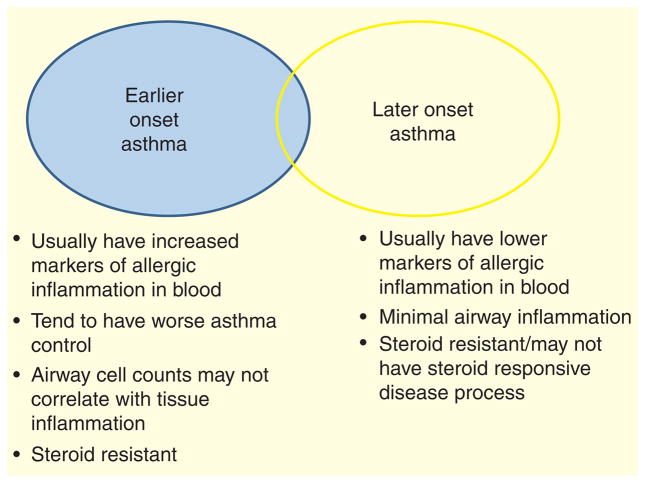
Distinct phenotypes of asthma are recognized in obese subjects (FIGURE 2). Holguin et al., in an analysis of more than 1000 participants from the “Severe Asthma Research Program”, divided participants into two groups based on the age of asthma onset: early onset asthma (age 11 years and under), and later onset asthma (age 12 and above). Obese patients with early onset asthma had a sixfold increased risk of intensive care unit admission for asthma in the preceding year compared with lean patients with early onset asthma, whereas those with late-onset asthma and obesity had a relatively modest 1.3-fold increased risk of intensive care unit admission compared with lean late-onset asthmatics [61]. The late-onset obese group had significantly lower serum IgE levels, and were less often atopic than the early onset obese group, though exhaled nitric oxide levels and sputum eosinophils levels were similar in both obese groups: obese patients with early onset asthma had particularly poorly controlled disease and increased systemic markers of Th2 inflammation compared with obese patients with late-onset disease. Sutherland et al., using an unbiased cluster analysis approach, identified two phenotypes of obese asthmatic among mild-to-moderate persistent asthmatics participating in ‘Asthmanet’ clinical trials [62]. One group had much worse asthma control than the other (Juniper asthma control score 1.8 vs 0.9, with 1.5 being suggestive of poor asthma control, and 0.75 suggestive of well-controlled asthma [63]). The group with worse asthma control were significantly younger at the time of asthma onset (10 vs 16 years), less often female, and had higher exhaled nitric oxide and IgE levels compared with the obese group with better controlled asthma [62]. Again, this suggests a group with early onset disease, worse control and increased markers of Th2 inflammation. Late-onset asthmatics with low IgE and early onset asthmatics with high IgE had differing responses to weight loss surgery: airway reactivity improved only in those with late-onset asthma and low IgE [64]. This suggests that those with late-onset disease and lower markers of allergic inflammation have disease that is most directly related to obesity and will improve with weight loss.
Figure 2. Currently, two major phenotypes of asthma in obesity are recognized: early onset asthma which is likely asthma complicated by obesity, and late-onset asthma which is likely asthma consequent to obesity.
This is likely a gross oversimplification, and further phenotypes will emerge as we gain a better understanding of the interrelationship between these complex syndromes.
These studies are consistent in that they suggest there are at least two phenotypes of obese asthmatics. But this is likely to be a gross oversimplification, as is readily apparent from our discussion of work in mice. Asthma itself is a disease of many phenotypes, and obesity is a multifaceted disease dependent on a complex interaction between factors ranging from genetic and epigenetic to socioeconomic and societal. We are just beginning to investigate the effects of obesity on adaptive and innate immune pathways in human asthmatics, and this work will likely uncover many different phenotypes of asthma in obesity.
Effect on adaptive & innate immune pathways in humans
Lymphocyte function
Overall, obesity is thought to favor a bias toward Th1 and Th17 adaptive lymphocyte responses rather than the Th2 responses typical of allergic asthma (TABLE 2) [65]. Rastogi et al. have shown that obese children with asthma tend to have increased CD4+ Th1 compared with Th2 cells in peripheral blood compared with lean allergic asthmatics [66]; this is consistent with a wealth of data suggesting increased Th1 responses in obese non-asthmatic patients, and suggests that enhanced Th2 pathways are not as common in obese as lean asthmatic children. Lymphocyte function is also altered in obesity; metabolic function is a critical factor in the generation of lymphocyte effector responses [67]. Stimulated lymphocyte cytokine responses tend to be dampened in the setting of severe morbid obesity and asthma in humans [64], a finding mirrored by responses of lymphocytes isolated from the mediastinal lymph nodes of mice fed a high fat diet [68]. Overall, obesity does not increase Th2 CD4 cell differentiation, and some data suggest that Th2 CD4 effector cell function may be decreased in the setting of obesity – this is likely consistent with the fact that many obese asthmatics do not have Th2 high asthma, but may have a form of asthma associated with increased Th1 and Th17 function.
Table 2.
Effect of obesity and asthma on cellular immune function.
| Effect of obesity | Findings in obese asthma | |
|---|---|---|
| Lymphocyte function | ↑ Th1 and ↑Th17 | ↑ CD4+ Th1 compared with Th2 cells ↑ cytokine production from CD4+ T cells after weight loss ↓ cytokine production from mediastinal lymph node cells with high fat diet |
| Eosinophil function | Increased chemotaxis and adhesion | Most studies show decreased airway eosinophilia with obesity, this may be related to abnormal trafficking from tissue |
| Neutrophil function | Increased circulating neutrophils in obesity | Increased sputum and circulating neutrophils in some obese asthmatics Weight loss reduces sputum neutrophilia in women |
| Macrophage function | Promotes development of M1 macrophages | Increased sputum macrophages Impaired efferocytosis Impaired response to glucocorticoids Altered response to lipopolysaccharide Increased macrophage activation in obese children |
While obesity overall may promote Th1 and Th17 responses, there are data to suggest that Th2-based allergic inflammation may actually promote the development of obesity. In vitro Th2 differentiation of naïve human CD4 cells is associated with the production of melanin-concentrating hormone, a peptide which increases appetite and causes obesity [69]. Although there have been no studies measuring melanin-concentrating hormone in asthma, this observation suggests there may be common pathogenic pathways involved in the development of both Th2 responses and the development of obesity [69]. As noted earlier, a recent study in mice showed that the CHI3L1 pathway could induce both allergic airway inflammation and visceral adiposity [48], again suggesting that there may be common pathways inducing early Th2 inflammation and obesity. This is likely most relevant to those with early onset allergic asthma.
Eosinophilic airway inflammation
Airway eosinophilia is a hallmark of allergic asthma. Most studies report either no difference [70,71], or even a decrease in airway eosinophilia [72,73] with obesity. However, these studies included both early and late-onset (Th2 high and low) asthma, and so it is a little difficult to know exactly the effect that obesity has on eosinophilic asthma per se. One interesting study from the UK collected sputum and performed airway biopsies on asthmatics of differing BMI; there was no difference in sputum eosinophils, but an increase in submucosal eosinophils in obesity [74]. The patient population appeared to have high serum IgE, and so this may have represented an obese population with Th2 high asthma. This study raised the intriguing possibility that perhaps obesity may alter eosinophil trafficking in Th2 high asthma. Indeed, there is some evidence to support this from in vitro studies: eosinophil chemotaxis and adhesion may be increased in obesity, and leptin may prime chemotaxis in response to eotaxin (TABLE 2) [75,76]. Obesity appears to alter eosinophil function, and sputum eosinophilia may be a poor measure of eosinophilic airway inflammation in obese asthmatics.
Neutrophil function
Neutrophils may be involved in the pathogenesis of asthma in obesity (TABLE 2). Telenga et al. found more blood and sputum neutrophils in obese compared with lean asthmatics, particularly obese female asthmatics [77]. Weight loss in obese asthmatic woman is directly correlated with a decrease in sputum neutrophils; men have a similar trend toward reduced sputum neutrophils with weight loss, but in men reduced saturated fatty acid intake correlates with decreasing sputum neutrophils [78]. These data suggest that sputum neutrophilia is associated with asthma in obesity, particularly in woman. The observation that fatty acid intake is related to sputum neutrophilia suggests that dietary intake can also have a significant effect on markers of inflammation in the airway. Wood et al. have shown that a high fat meal induces sputum neutrophilia, and impairs response to bronchodilator in normal weight asthmatics [79]. This suggests that neutrophils may be involved in the pathogenesis of asthma in obesity, this may be related to obesity itself and/or the effects of a high fat diet.
Macrophage function
Macrophage function is altered in obesity, with a predominance of proinflammatory M1 macrophages in adipose tissue in obesity, in comparison with the M2 predominance in lean adipose tissue [80]. M2 macrophages are important in controlling inflammation through removal of dying cells, a process called efferocytosis, and associated production of anti-inflammatory mediators such as IL-10, TGF-β and prostaglandin E2 [81]. There have been some recent studies addressing macrophage function in the airway in obesity (TABLE 2). Macrophage numbers tend to be increased in the sputum of obese compared with lean asthmatics [82,83]. Fernandez-Boyanapalli-et al. found that sputum macrophages and peripheral blood monocytes from obese asthmatics have reduced efferocytosis compared with lean asthmatics, and reduced markers of M2 macrophage function [82]. This is associated with increased oxidative stress and evidence of impaired response to corticosteroids [82]. Lugogo et al. found evidence that cytokine responses of bronchoalveolar lavage macrophages to lipopolysaccharide were enhanced in the presence of high-dose leptin in obese asthmatics [84], and leptin is known to have a number of effects on macrophage function in the lung [85]. Cytokine responses of bronchoalveolar macrophages to lipopolysaccharide increase following weight loss surgery [86]. Sutherland et al. have found that mononuclear cells from asthmatics express increasing levels of TNF-α, in direct proportion to increasing BMI [87]. Taken together, these studies suggest that altered macrophage number and function, perhaps related to effects of leptin on airway macrophages, may have an important role in the pathogenesis of asthma in obesity.
Rastogi et al. have shown that among obese asthmatic children on inhaled corticosteroids (presumably the more severely afflicted patients), there were changes in monocyte populations, with fewer classically activated (CD4+CD16−) and more patrolling (CD14−CD16+) monocytes [66]. These immune changes were associated with metabolic markers of insulin resistance. Periyalil et al. also found evidence of elevated circulating macrophage activation (measured by the serum marker soluble CD163a), particularly in obese female children [88]. Markers of monocyte lineage activation in children were associated with poor asthma control and low lung function. The differences between the study populations (an Australian pediatric population in the Periyalil study and an African-American and His-panic population in the Rasotgi study) may explain differences in detailed findings between the two studies, but the striking similarity of macrophage activation in these diverse pediatric populations suggest that macrophage activation and metabolic abnormalities are likely important in the pathogenesis of asthma in obese children.
Immunologic characteristics of late-onset non-allergic asthma in obesity
A subset of asthmatics develop late-onset asthma in the setting of obesity, it is likely that the immunological characteristics of this form of asthma are quite distinct from patients with early onset allergic asthma complicated by obesity. This is likely analogous to the innate increase in airway reactivity that has been described in obese mice. Classically, this form of asthma is more common in women, and these patients have low levels of markers of airway inflammation [89]. These type of asthmatics have minimal airway inflammation (as assessed by cellularity and cytokines in bronchoalveolar lavage), but have increased markers of metabolic inflammation in adipose tissue [86]. The functional significance of these metabolic abnormalities in adipose tissue is not known, although airway reactivity is correlated with visceral adipose leptin expression, suggesting metabolic factors are involved in the pathogenesis of this form of asthma [86]. Work in mice suggest that IL-17A-related pathways may be important, but as of yet, there are no reports addressing this in obese patients.
Markers of oxidative stress are noted to increase in proportion to BMI in patients with asthma. Komakula et al. reported that 8-isoprostanes in exhaled breath condensate increase in direct proportion to BMI, whereas exhaled nitric oxide decreases in proportion to BMI [90]. The precise effects of increased oxidative stress on the immune system in this late-onset asthma of obesity are not yet defined. One association is a decrease in exhaled nitric oxide. This may be related to oxidative conversion of nitric oxide to other nitrite species, and inhibition of nitric oxide synthases (Holguin et al. have shown that asymmetric dimethyl arginine, an inhibitor of nitric oxide synthases which is increased in obesity, is inversely related to lung function in these late-onset asthmatics [91]). Nitric oxide has important homeostatic functions in the airway, including bronchodilation [92], so decreased levels may be pathogenic in this form of asthma.
There are few studies in humans that have separated out these different phenotypes of obese asthma, but it is likely that some of the immunological changes described in the general population of obese asthmatics (such as macrophage dysfunction) may be playing a role in the late-onset asthma of obesity.
Glucocorticoid resistance
Obese patients appear to have reduced response to inhaled corticosteroids. This has been reported in many different populations [93,94]. There may be many reasons for this: different phenotypes with less steroid-responsive inflammation and different drug deposition related to altered drug delivery are both possibilities. Another reason appears to be intrinsic steroid resistance which can be measured at the cellular level. Sutherland et al. isolated monocytes and alveolar macrophages from asthmatics of differing BMI then studied steroid responsiveness of these cells, measured by induction of MAPK, a signaling molecule induced by steroids, which inhibits proinflammatory cytokine production. Obese asthmatics had lower levels of MAPK in response to dexamethasone [87]. In a follow-up study using a different patient population, Sutherland et al. found that expression of the glucocorticoid receptor α-subunit was reduced in obese subjects, this correlated with reduced expression of MAPK in response to dexamethasone [62]. Impaired intracellular signaling in response to glucocorticoids likely contributes to the steroid resistance that occurs in obesity.
Dietary factors
Dietary factors may influence the immune system, as noted earlier. Obesity is associated with low levels of vitamin D [95]. Much has been written about the potential effects of vitamin D on the immune system in asthma: vitamin D promotes the development of Treg cells, and can increase IL-10 and TGF-β, which might attenuate inflammatory responses in asthma [96]. However, there have been no prospective studies of vitamin D supplementation in obese asthmatics.
High intake of dietary fat may also have effects on the immune system, as noted above. Toll-like receptors can be activated by dietary fatty acid, and this is associated with increased NF-κB expression and increased production of reactive oxygen species in peripheral blood mononuclear cells [97–99]. Wood et al. found increased sputum neutrophilia associated with increased expression of TLR4 in sputum of asthmatics fed a high fat diet, compared with asthmatics fed a low fat diet [79].
Non-immunological factors
Non-immunological factors may also play an important role in obesity-associated asthma. The asthma that occurs in late-onset non-allergic asthma is associated with increased elastance in the periphery of the lung, the cause of this is not known [100], but could be related to changes in airway wall compliance or thickness [101]. The obese patients tend to have increased comorbidities such as depression and obstructive sleep apnea, which contribute to poor asthma control and perhaps to the pathogenesis of asthma in this population [102,103]. Sex hormones may also play a role: obesity is a greater risk factor for asthma in women than in men, although the exact reasons for this are not known [104].
Implications for treatment
Role of corticosteroids
Data from the mice models and humans show that obesity is associated with innate increased airway reactivity, without obvious airway inflammation; this is not related to challenge with allergen or pollutant. This is likely analogous to late-onset asthma in people with minimal markers of eosinophilic or neutrophilic inflammation. Given no data to suggest steroid-responsive pathways are active in this form of disease, it seems critical to avoid high-dose steroid therapy in these patients, and to consider other interventions.
The use of corticosteroid therapy in those individuals with early-onset allergic asthma complicated by obesity is likely to be more complex than in lean asthmatics. The work by Holguin and Sutherland suggest those with early onset asthma and increased markers of allergic inflammation may be particularly subject to poor asthma control, and so adequate treatment is critical in these patients. There does appear to be evidence of steroid resistance at the molecular level, and whether high-dose steroids are required to overcome this is not known. If high-dose systemic steroids are being contemplated, it may be prudent to at least confirm that a patient has uncontrolled airway inflammation and not a phenotype of asthma with minimal inflammation. Documenting airway inflammation may be even more complicated in the obese than in the lean patient – the work by Desai et al. suggests that it may be difficult to detect eosiniophilic airway inflammation by conventional non-invasive monitoring (sputum and exhaled nitric oxide) [74], whether this can be done using a circulating biomarker, or whether this requires endobronchial biopsy is not yet known.
Weight loss
Bariatric surgery does appear to improve asthma control; this has been shown in a number of studies [64,105]. However, weight loss surgery is an expensive, complex intervention, associated with morbidity and even mortality, so this is unlikely to be an option for many patients. Diet-induced weight loss may help: there are few published studies, but it seems likely that 5–10% weight loss is required to improve asthma control in obese asthmatics [78,106].
Dietary factors
Even if weight loss cannot be achieved, attention to dietary factors may be helpful. A high fat diet may worsen airway inflammation and reduce bronchodilator responsiveness even in normal weight patients with asthma [79]. There are no large multicenter trials addressing reduction of fat intake in people with asthma, but given the pleiotropic health benefits of such a diet, it is worth at least discussing dietary fat content with patients.
Low vitamin D is associated with obesity, and may contribute to both steroid resistance and poor asthma control. Although vitamin D does not improve asthma control in adult asthmatics overall [107], supplementing to at least a normal level is likely a simple intervention to consider in patients deficient in vitamin D.
Environmental exposures
Work in mice models suggest that environmental pollutants significantly worsen airway inflammation and airway reactivity in mouse models of asthma. Data in humans suggest that obese asthmatics may be more affected by air pollution than lean asthmatics [108,109]. Although there are no specific recommendations at this time for the treatment of such asthma, it makes sense to caution obese patients with asthma to be particularly cautious on days with poor air quality, and to consider the role that indoor air pollution may be playing in aggravating their disease.
Comorbidities
Comorbidities such as depression and sleep apnea are associated with poor asthma control in this patient population [102,103]. As of yet, there are no interventional clinical trials assessing whether treating these comorbidities might improve asthma control in obese asthmatics, although these comorbidities usually warrant treatment regardless of their efficacy for asthma. Given the association of poor asthma control in obesity with obstructive sleep apnea and depression, it would seem prudent to consider these diagnoses, and treat when appropriate.
Expert commentary
The majority of severe asthmatics in the USA are obese. Obesity fundamentally changes the nature of asthma through a myriad of effects on the immune system and pulmonary pathophysiology. No drug-treatment studies specifically targeting obese asthmatics have yet been published, and so we are currently dependent on our understanding of the disease from mouse models and observational studies in humans to guide management. These studies have provided us with critical insights into the pathogenesis of asthma in obesity, and some potential targets are beginning to emerge. One very important lesson is that there is a spectrum of disease, and phenotyping of obese asthmatics is likely to be critical as we move forward testing any new therapies in this patient population.
Five-year view
Over the coming 5 years, we will learn more about how changes in metabolism and the microbiome might influence changes in immune and physiological functions pertinent to obesity and asthma. Studies from mouse models will help illuminate new targets, and enable the testing of innovative interventions for weight loss and asthma. There will be studies focusing on weight loss and other dietary interventions for obese asthmatics. There will be investigations targeting oxidative stress and the role of some of the newer biologics in this patient population (although dosing considerations are more complicated, this is the patient population that is most likely to benefit from these therapies). Toward the end of the coming 5 years, we should be in position to develop specific treatment guidelines for obese patients with asthma.
Key issues.
Obesity is a complex disease, and the mode and type of obesity affect manifestations of disease in the airway.
Obesity produces fundamental changes in innate and adaptive immunity.
Obesity is associated with innate airway reactivity, in the absence of any provocative agent, and without obvious airway inflammation.
Two phenotypes of asthma have been identified in obese people with asthma (late-onset, non-allergic airway disease and early onset allergic airway disease), but there are likely to be many more.
These different phenotypes of asthma in obesity will require customized treatment based on the underlying pathophysiology of the disease.
Obese asthmatics have altered responses to medications, they do not respond as well to glucocorticoids. Treatment trials targeting specific phenotypes of obese asthmatic are needed.
Footnotes
Financial & competing interests disclosure
The authors were supported by Grants from the National Institutes of Health. AE Dixon serves on Data and Safety Monitoring Boards for Boehringer Ingelheim and Roche. AE Dixon receives grant funding from the National Institutes of Health, American Lung Association and Pfizer. ME Poynter receives grant funding from the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175(7):661–6. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Schatz M, Hsu JW, Zeiger RS, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133(6):1549–56. doi: 10.1016/j.jaci.2013.10.006. A cluster analysis of over 4000 patients with severe asthma. Five phenotypes were identified in adults; the prevalence of obesity ranged from 52 to 63% in these clusters. [DOI] [PubMed] [Google Scholar]
- 4.Dixon A. The treatment of asthma in obesity. Expert Rev Respir Med. 2012;6(3):331–40. doi: 10.1586/ers.12.22. [DOI] [PubMed] [Google Scholar]
- 5.Shore SA. Obesity and asthma: lessons from animal models. J Appl Physiol. 2007;102(2):516–28. doi: 10.1152/japplphysiol.00847.2006. [DOI] [PubMed] [Google Scholar]
- 6.Speakman J, Hambly C, Mitchell S, Krol E. The contribution of animal models to the study of obesity. Lab Anim. 2008;42(4):413–32. doi: 10.1258/la.2007.006067. [DOI] [PubMed] [Google Scholar]
- 7.Leiria LO, Martins MA, Saad MJ. Obesity and asthma: beyond T2 inflammation. Metabolism. 2015;64(2):172–81. doi: 10.1016/j.metabol.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 9.Considine RV, Caro JF. Leptin and the regulation of body weight. Int J Biochem Cell Biol. 1997;29(11):1255–72. doi: 10.1016/s1357-2725(97)00050-2. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto Y, Kihara S, Funahashi T, et al. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 2006;110(3):267–78. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- 11.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 12.Campfield LA, Smith FJ, Guisez Y, et al. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–9. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 13.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 14.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317–18. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 15.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127–8. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 16.Lu FL, Johnston RA, Flynt L, et al. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L856–65. doi: 10.1152/ajplung.00386.2005. [DOI] [PubMed] [Google Scholar]
- 17.Rivera-Sanchez YM, Johnston RA, Schwartzman IN, et al. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol (1985) 2004;96(6):2200–6. doi: 10.1152/japplphysiol.00960.2003. [DOI] [PubMed] [Google Scholar]
- 18.Lang JE, Williams ES, Mizgerd JP, Shore SA. Effect of obesity on pulmonary inflammation induced by acute ozone exposure: role of interleukin-6. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):L1013–20. doi: 10.1152/ajplung.00122.2007. [DOI] [PubMed] [Google Scholar]
- 19.Johnston RA, Zhu M, Rivera-Sanchez YM, et al. Allergic airway responses in obese mice. Am J Respir Crit Care Med. 2007;176(7):650–8. doi: 10.1164/rccm.200702-323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf AM, Wolf D, Rumpold H, et al. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323(2):630–5. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 21.Medoff BD, Okamoto Y, Leyton P, et al. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009;41(4):397–406. doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasahara DI, Kim HY, Mathews JA, et al. Pivotal role of IL-6 in the hyperinflammatory responses to subacute ozone in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2014;306(6):L508–20. doi: 10.1152/ajplung.00235.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shore SA, Terry RD, Flynt L, et al. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118(2):389–95. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Coleman DL, Eicher EM. Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered. 1990;81(6):424–7. doi: 10.1093/oxfordjournals.jhered.a111019. [DOI] [PubMed] [Google Scholar]
- 25.Johnston RA, Theman TA, Shore SA. Augmented responses to ozone in obese carboxypeptidase E-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R126–33. doi: 10.1152/ajpregu.00306.2005. [DOI] [PubMed] [Google Scholar]
- 26.Dahm PH, Richards JB, Karmouty-Quintana H, et al. Effect of antigen sensitization and challenge on oscillatory mechanics of the lung and pulmonary inflammation in obese carboxypeptidase E-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2014;307(6):R621–33. doi: 10.1152/ajpregu.00205.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang ZH, Miyahara H, Takeo J, Katayama M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetol Metab Syndr. 2012;4(1):32. doi: 10.1186/1758-5996-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge XN, Greenberg Y, Hosseinkhani MR, et al. High-fat diet promotes lung fibrosis and attenuates airway eosinophilia after exposure to cockroach allergen in mice. Exp Lung Res. 2013;39(9):365–78. doi: 10.3109/01902148.2013.829537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calixto MC, Lintomen L, Schenka A, et al. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol. 2010;159(3):617–25. doi: 10.1111/j.1476-5381.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathews JA, Wurmbrand AP, Ribeiro L, et al. Induction of IL-17A precedes development of airway hyperresponsiveness during diet-induced obesity and correlates with complement factor D. Front Immunol. 2014;5:440. doi: 10.3389/fimmu.2014.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson RH, Whitehead GS, Nakano H, et al. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180(8):720–30. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams AS, Chen L, Kasahara DI, et al. Obesity and airway responsiveness: role of TNFR2. Pulm Pharmacol Ther. 2013;26(4):444–54. doi: 10.1016/j.pupt.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HY, Lee HJ, Chang YJ, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20(1):54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ather JL, Foley KL, Suratt BT, et al. Airway epithelial NF-kappaB activation promotes the ability to overcome inhalational antigen tolerance. Clin Exp Allergy. 2015 doi: 10.1111/cea.12491. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin RA, Ather JL, Lundblad LK, et al. Interleukin-1 receptor and caspase-1 are required for the Th17 response in nitrogen dioxide-promoted allergic airway disease. Am J Respir Cell Mol Biol. 2013;48(5):655–64. doi: 10.1165/rcmb.2012-0423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60(3):349–56. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 37.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121(5):1087–93. doi: 10.1016/j.jaci.2008.03.004. quiz 1094-1085. [DOI] [PubMed] [Google Scholar]
- 38.Priceman SJ, Kujawski M, Shen S, et al. Regulation of adipose tissue T cell subsets by Stat3 is crucial for diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA. 2013;110(32):13079–84. doi: 10.1073/pnas.1311557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cipolletta D. Adipose tissue-resident regulatory T cells: phenotypic specialization, functions and therapeutic potential. Immunology. 2014;142(4):517–25. doi: 10.1111/imm.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu M, Williams AS, Chen L, et al. Role of TNFR1 in the innate airway hyperresponsiveness of obese mice. J Appl Physiol (1985) 2012;113(9):1476–85. doi: 10.1152/japplphysiol.00588.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nie Z, Jacoby DB, Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol. 2014;51(2):251–61. doi: 10.1165/rcmb.2013-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arteaga-Solis E, Zee T, Emala CW, et al. Inhibition of leptin regulation of parasympathetic signaling as a cause of extreme body weight-associated asthma. Cell Metab. 2013;17(1):35–48. doi: 10.1016/j.cmet.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramalho R, Almeida J, Beltrao M, et al. Substance P antagonist improves both obesity and asthma in a mouse model. Allergy. 2013;68(1):48–54. doi: 10.1111/all.12052. [DOI] [PubMed] [Google Scholar]
- 44.Ramalho R, Almeida J, Beltrao M, et al. Neurogenic inflammation in allergen-challenged obese mice: a missing link in the obesity-asthma association? Exp Lung Res. 2012;38(6):316–24. doi: 10.3109/01902148.2012.699589. [DOI] [PubMed] [Google Scholar]
- 45.Ramalho R, Almeida J, Fernandes R, et al. Neurokinin-1 receptor, a new modulator of lymphangiogenesis in obese-asthma phenotype. Life Sci. 2013;93(4):169–77. doi: 10.1016/j.lfs.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Ober C, Chupp GL. The chitinase and chitinase-like proteins: a review of genetic and functional studies in asthma and immune-mediated diseases. Curr Opin Allergy Clin Immunol. 2009;9(5):401–8. doi: 10.1097/ACI.0b013e3283306533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CG, Elias JA. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy Asthma Immunol Res. 2010;2(1):20–7. doi: 10.4168/aair.2010.2.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Ahangari F, Sood A, Ma B, et al. Chitinase 3-like-1 regulates both visceral fat accumulation and asthma-like Th2 inflammation. Am J Respir Crit Care Med. 2015;191(7):746–57. doi: 10.1164/rccm.201405-0796OC. This study found that chitinase 3-like-1 in mice is involved in the development of allergic airway inflammation and obesity. In humans, this molecule is increased in obese compared with lean asthmatics, and correlates with low lung function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allan KM, Prabhu N, Craig LC, et al. Maternal vitamin D and E intakes during pregnancy are associated with asthma in children. Eur Respir J. 2015;45(4):1027–36. doi: 10.1183/09031936.00102214. [DOI] [PubMed] [Google Scholar]
- 50.Cook-Mills JM, Avila PC. Vitamin E and D regulation of allergic asthma immunopathogenesis. Int Immunopharmacol. 2014;23(1):364–72. doi: 10.1016/j.intimp.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss ST, Litonjua AA. The in utero effects of maternal vitamin D deficiency: how it results in asthma and other chronic diseases. Am J Respir Crit Care Med. 2011;183(10):1286–7. doi: 10.1164/rccm.201101-0160ED. [DOI] [PubMed] [Google Scholar]
- 52.Weiss ST. Bacterial components plus vitamin D: the ultimate solution to the asthma (autoimmune disease) epidemic? J Allergy Clin Immunol. 2011;127(5):1128–30. doi: 10.1016/j.jaci.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen F, Marquez H, Kim YK, et al. Prenatal retinoid deficiency leads to airway hyperresponsiveness in adult mice. J Clin Invest. 2014;124(2):801–11. doi: 10.1172/JCI70291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paul G, Brehm JM, Alcorn JF, et al. Vitamin D and asthma. Am J Respir Crit Care Med. 2012;185(2):124–32. doi: 10.1164/rccm.201108-1502CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ly NP, Litonjua A, Gold DR, Celedon JC. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? J Allergy Clin Immunol. 2011;127(5):1087–94. doi: 10.1016/j.jaci.2011.02.015. quiz 1095-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonet ML, Ribot J, Felipe F, Palou A. Vitamin A and the regulation of fat reserves. Cell Mol Life Sci. 2003;60(7):1311–21. doi: 10.1007/s00018-003-2290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101(44):15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ley RE, Backhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102(31):11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 60••.Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–66. doi: 10.1038/nm.3444. Study in mice showing that composition of fiber in the gut can change the gut and lung microbiota, and then can alter Th2 effector cell development through effect on short chain fatty acids in dendritic cells. [DOI] [PubMed] [Google Scholar]
- 61•.Holguin F, Bleecker ER, Busse WW, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127(6):1486–1493. e1482. doi: 10.1016/j.jaci.2011.03.036. An analysis of participants in the Severe Asthma Research Program, which found that those with early and late-onset asthma represented two distinct asthma phenotypes in obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutherland ER, Goleva E, King TS, et al. Cluster analysis of obesity and asthma phenotypes. PLoS One. 2012;7(5):e36631. doi: 10.1371/journal.pone.0036631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Juniper EF, O’byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 64.Dixon AE, Pratley RE, Forgione PM, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128(3):508–15. e501–502. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mclaughlin T, Liu LF, Lamendola C, et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34(12):2637–43. doi: 10.1161/ATVBAHA.114.304636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rastogi D, Fraser S, Oh J, et al. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am J Respir Crit Care Med. 2015;191(2):149–60. doi: 10.1164/rccm.201409-1587OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fracchia KM, Walsh CM. Metabolic mysteries of the inflammatory response: T cell polarization and plasticity. Int Rev Immunol. 2015;34(1):3–18. doi: 10.3109/08830185.2014.974748. [DOI] [PubMed] [Google Scholar]
- 68.De Vries A, Hazlewood L, Fitch PM, et al. High-fat feeding redirects cytokine responses and decreases allergic airway eosinophilia. Clin Exp Allergy. 2009;39(5):731–9. doi: 10.1111/j.1365-2222.2008.03179.x. [DOI] [PubMed] [Google Scholar]
- 69.Sandig H, Mcdonald J, Gilmour J, et al. Human Th2 cells selectively express the orexigenic peptide, pro-melanin-concentrating hormone. Proc Natl Acad Sci USA. 2007;104(30):12440–4. doi: 10.1073/pnas.0705457104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Todd DC, Armstrong S, D’silva L, et al. Effect of obesity on airway inflammation: a cross-sectional analysis of body mass index and sputum cell counts. Clin Exp Allergy. doi: 10.1111/j.1365-2222.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 71.Sutherland TJ, Cowan JO, Young S, et al. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med. 2008;178(5):469–75. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 72.Van Veen IH, Ten Brinke A, Sterk PJ, et al. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63(5):570–4. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 73.Lessard A, Turcotte H, Cormier Y, Boulet LP. Obesity and asthma: a specific phenotype? Chest. 2008;134(2):317–23. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 74•.Desai D, Newby C, Symon FA, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Respir Crit Care Med. 2013;188(6):657–63. doi: 10.1164/rccm.201208-1470OC. A study comparing induced sputum and biopsy parameters in asthmatics of different BMI. This study found elevated IL-5 in sputum, and elevated endobronchial (though not sputum) eosinophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kato H, Ueki S, Kamada R, et al. Leptin has a priming effect on eotaxin-induced human eosinophil chemotaxis. Int Arch Allergy Immunol. 2011;155(4):335–44. doi: 10.1159/000321195. [DOI] [PubMed] [Google Scholar]
- 76.Grotta MB, Squebola-Cola DM, Toro AA, et al. Obesity increases eosinophil activity in asthmatic children and adolescents. BMC Pulm Med. 2013;13:39. doi: 10.1186/1471-2466-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Telenga ED, Tideman SW, Kerstjens HA, et al. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy. 2012;67(8):1060–8. doi: 10.1111/j.1398-9995.2012.02855.x. [DOI] [PubMed] [Google Scholar]
- 78.Scott HA, Gibson PG, Garg ML, et al. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy. 2013;43(1):36–49. doi: 10.1111/cea.12004. [DOI] [PubMed] [Google Scholar]
- 79.Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. 2011;127(5):1133–40. doi: 10.1016/j.jaci.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 80.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mccubbrey AL, Curtis JL. Efferocytosis and lung disease. Chest. 2013;143(6):1750–7. doi: 10.1378/chest.12-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fernandez-Boyanapalli R, Goleva E, Kolakowski C, et al. Obesity impairs apoptotic cell clearance in asthma. J Allergy Clin Immunol. 2013;131(4):1041–7. 1047.e1041–1043. doi: 10.1016/j.jaci.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. Eur Respir J. 2013;42(4):1012–19. doi: 10.1183/09031936.00124912. [DOI] [PubMed] [Google Scholar]
- 84.Lugogo NL, Hollingsworth JW, Howell DL, et al. Alveolar macrophages from overweight/ obese subjects with asthma demonstrate a proinflammatory phenotype. Am J Respir Crit Care Med. 2012;186(5):404–11. doi: 10.1164/rccm.201109-1671OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Mancuso P, Myers MG, Jr, Goel D, et al. Ablation of leptin receptor-mediated ERK activation impairs host defense against Gram-negative pneumonia. J Immunol. 2012;189(2):867–75. doi: 10.4049/jimmunol.1200465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sideleva O, Suratt BT, Black KE, et al. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186(7):598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87•.Sutherland ER, Goleva E, Strand M, et al. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178(7):682–7. doi: 10.1164/rccm.200801-076OC. Peripheral blood monocytes and alveolar macrophages from obese asthmatics have impaired induction of MAPK in response to dexamethasone stimulation, demonstrating cellular evidence of glucocorticoid resistance in obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Periyalil HA, Wood LG, Scott HA, et al. Macrophage activation, age and sex effects of immunometabolism in obese asthma. Eur Respir J. 2015;45(2):388–95. doi: 10.1183/09031936.00080514. [DOI] [PubMed] [Google Scholar]
- 89.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–23. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Komakula S, Khatri S, Mermis J, et al. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res. 2007;8:32. doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holguin F, Comhair SA, Hazen SL, et al. An association between L-arginine/ asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. Am J Respir Crit Care Med. 2013;187(2):153–9. doi: 10.1164/rccm.201207-1270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kacmarek RM, Ripple R, Cockrill BA, et al. Inhaled nitric oxide. A bronchodilator in mild asthmatics with methacholine-induced bronchospasm. Am J Respir Crit Care Med. 1996;153(1):128–35. doi: 10.1164/ajrccm.153.1.8542105. [DOI] [PubMed] [Google Scholar]
- 93.Peters-Golden M, Swern A, Bird SS, et al. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27(3):495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 94.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med. 2007;101(11):2240–7. doi: 10.1016/j.rmed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 95.Parikh SJ, Edelman M, Uwaifo GI, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89(3):1196–9. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 96.Chambers ES, Suwannasaen D, Mann EH, et al. 1alpha,25-dihydroxyvitamin D3 in combination with transforming growth factor-beta increases the frequency of Foxp3 (+) regulatory T cells through preferential expansion and usage of interleukin-2. Immunology. 2014;143(1):52–60. doi: 10.1111/imm.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aljada A, Mohanty P, Ghanim H, et al. Increase in intranuclear nuclear factor kappaB and decrease in inhibitor kappaB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. Am J Clin Nutr. 2004;79(4):682–90. doi: 10.1093/ajcn/79.4.682. [DOI] [PubMed] [Google Scholar]
- 98.Deopurkar R, Ghanim H, Friedman J, et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care. 2010;33(5):991–7. doi: 10.2337/dc09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patel C, Ghanim H, Ravishankar S, et al. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab. 2007;92(11):4476–9. doi: 10.1210/jc.2007-0778. [DOI] [PubMed] [Google Scholar]
- 100.Al-Alwan A, Bates JH, Chapman DG, et al. The nonallergic asthma of obesity. A matter of distal lung compliance. Am J Respir Crit Care Med. 2014;189(12):1494–502. doi: 10.1164/rccm.201401-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bates JH, Dixon AE. Potential role of the airway wall in the asthma of obesity. J Appl Physiol. 2015;118(1):36–41. doi: 10.1152/japplphysiol.00684.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kapadia SG, Wei C, Bartlett SJ, et al. Obesity and symptoms of depression contribute independently to the poor asthma control of obesity. Respir Med. 2014;108(8):1100–7. doi: 10.1016/j.rmed.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dixon AE, Clerisme-Beaty EM, Sugar EA, et al. Effects of obstructive sleep apnea and gastroesophageal reflux disease on asthma control in obesity. J Asthma. 2011;48(7):707–13. doi: 10.3109/02770903.2011.601778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen Y, Dales R, Jiang Y. The association between obesity and asthma is stronger in nonallergic than allergic adults. Chest. 2006;130(3):890–5. doi: 10.1378/chest.130.3.890. [DOI] [PubMed] [Google Scholar]
- 105.Boulet LP, Turcotte H, Martin J, Poirier P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med. 2012;106(5):651–60. doi: 10.1016/j.rmed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 106.Ma J, Strub P, Xiao L, et al. Behavioral weight loss and physical activity intervention in obese adults with asthma. A randomized trial. Ann Am Thorac Soc. 2015;12(1):1–11. doi: 10.1513/AnnalsATS.201406-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Castro M, King TS, Kunselman SJ, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311(20):2083–91. doi: 10.1001/jama.2014.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu KD, Breysse PN, Diette GB, et al. Being overweight increases susceptibility to indoor pollutants among urban children with asthma. J Allergy Clin Immunol. 2013;131(4):1017–23. 1023.e1011–1013. doi: 10.1016/j.jaci.2012.12.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dong GH, Qian Z, Liu MM, et al. Obesity enhanced respiratory health effects of ambient air pollution in Chinese children: the Seven Northeastern Cities study. Int J Obes. 2013;37(1):94–100. doi: 10.1038/ijo.2012.125. [DOI] [PubMed] [Google Scholar]