Abstract
The name Anopheles (Kerteszia) lepidotus Zavortink, commonly used for an important malaria vector in the eastern cordillera of the Andes, is here corrected to An. pholidotus Zavortink. We discovered that An. (Ker.) specimens from Peru, and reared-associated specimens from Ecuador, had unambiguous habitus characters that matched those on the male holotype of An. lepidotus. However, the specimens do not exhibit characters of the female allotype and female paratypes of An. lepidotus, which are actually An. pholidotus. Our specimens are the first correctly associated females of An. lepidotus, which allow us to provide a new morphological interpretation for the adult habitus of this species. This finding is also corroborated by molecular data from a portion of the Cytochrome Oxidase I (COI) gene and ribosomal DNA Internal Transcribed Spacer 2 (rDNA ITS2). The pupal stage of An. lepidotus is described for the first time, and additional larval characters are also noted. Diagnostic morphological characters for the adult, pupal, and larval stages of An. pholidotus are provided to separate the two species. All stages of An. lepidotus are easily separated from other currently known species in subgenus Kerteszia and a new key to the females of An. (Kerteszia) is given. Previously published distribution, bionomics, and medical significance data are corrected and enhanced.
Keywords: Anopheles lepidotus, Anopheles pholidotus, redescription, key, morphology, ITS2, COI, malaria
Introduction
Zavortink (1973) provided a preliminary review of current knowledge of the morphological diagnoses for species in subgenus Kerteszia Theobald, of genus Anopheles Meigen. In that publication he treated 10 species, including two new species, An. lepidotus Zavortink, and An. pholidotus Zavortink, and one provisional (unnamed) species called “Species 10. Auyan-Tepui Mesa form.” Subsequently three species have been described, i.e., An. rollai Cova Garcia, Pulido, & Escalante de Ugueto (1976, 1977b), An. gonzalezrinconesi Cova Garcia, Pulido, & Escalante de Ugueto (1977a), and An. auyantepuiensis Harbach & Navarro (1996) for “Species 10. Auyan-Tepui Mesa form” of Zavortink (1973). Of the two species described by Zavortink, An. lepidotus was based on distinctive characters he noted on two larval exuviae from two reared males collected in Restrepo, Meta, Colombia in 1935, which were previously identified as An. boliviensis Theobald by Komp & Orsono-Mesa (1936). Zavortink designated a female collected in 1943 in Villavicencio, Meta, Colombia as the allotype of An. lepidotus and 31 female paratypes from various collection dates from Meta, Colombia. Anopheles pholidotus was described based on a male holotype, female allotype, and paratypes including a male, a female, larval and pupal exuviae, and whole larvae collected in Bocas del Toro, Panama. He considered An. lepidotus and An. pholidotus as closely related to An. boliviensis because of dense scale patches on the abdominal terga. In a key to the females, he used only the width of scales on the proximal abdominal terga to separate his two new species. However, in discussing these two species Zavortink (1973, p. 18) qualified their differences and stated “Although lepidotus closely resembles pholidotus or is possibly even indistinguishable from it in the adult and male genitalia, I am recognizing it as a distinct species on the basis of the larval differences indicated in the key and description.” Subsequent malaria workers in Peru, Ecuador, Colombia, and Venezuela who used this female key couplet (p. 7) to separate the two species found judging whether the proximal tergal scales were “narrow to moderately broad” versus “moderately broad to broad” difficult, thus making identifications of females inconclusive. Specimens from one of these studies (Quiñones et al. 1984) were sent to one of us (BAH), who determined in 1987 that the species they reported from Tolima Department, Colombia, were actually An. pholidotus, not An. lepidotus. To our knowledge this unpublished determination, evidenced by a note in the unit tray in the NMNH collection containing them, was only recently researched and confirmed by Escobar et al. (2010).
In 1985–86, Hayes et al. (1987), working under the auspices of a National Research Council grant through the BOSTID (Board on Science and Technology for International Development) program, Washington, DC, collaborated with personnel from the Instituto Nacional de Salud, Ministerio de Salud, Lima, Peru, and the Walter Reed Biosystematics Unit (WRBU), Smithsonian Institution, and conducted two malaria surveillance trips in Junín Province, Peru. During those trips, three females of Anopheles (Kerteszia) were collected that initially were considered to be a new species (BAH) because of several obvious morphological characters not previously described in subgenus Kerteszia. However, resolving the correct identity and describing the three specimens was not considered appropriate at that time without additional specimens, particularly specimens reared with associated larval and pupal exuviae. In 1998, larvae were collected (RCW) from arboreal bromeliads in Ecuador and reared, producing a limited number of males and females with associated exuviae. Based on a larval character (seta 3-C stout) given in the description and key of Zavortink (1973), these specimens were given a tentative field identification as An. lepidotus. Later, one of us (BAH) noted that the females from Ecuador had morphological characters that matched those of the three females collected 12–13 years earlier in Peru. This initiated a thorough study of the unknown species from Peru and the putative An. lepidotus from Ecuador, including an examination of type specimens and other Kerteszia specimens deposited in the Smithsonian Institution. The results of this study are provided below.
Material and methods
Morphological
Terminology follows Harbach & Knight (1980, 1982). Costal and apical wing spot designations follow Wilkerson & Peyton (1990), which unlike Zavortink (1973), means that the apical fringe spots on Kerteszia species are not homologous with the apical pale (AP) spots on other Anopheles. Other terminology follows Peyton et al. (1992), who revised the position of seta 3-VI in Kerteszia pupae. Abbreviations for specimens follow: ♀, female; ♂, male; G, genitalia; Pe, pupal exuviae; Le, larval exuviae, and L, fourth-instar larva. The use of an asterisk by a stage abbreviation means the life stage is illustrated. We agree with Reinert (1990) in the use of “Pa” instead of “P” as the designation for the pupal paddle, which eliminates confusion with the “P” used for the larval prothorax. Larval and pupal chaetotaxy tables are not included because of limited specimens. Genus and subgenus abbreviations follow Reinert (2009). Pinned adults and whole adults in alcohol were examined with a Leica S8APO® dissection microscope and a cold fiber dual optics light source with a blue filter. We follow Tanaka et al. (1979), who recognized that all true palpomeres have scales, and Wood et al. (1979) who stated “The palpus is typically five-segmented, which is the standard number in the Nematocera.” Harbach & Kitching (1998) agreed with Tanaka et al. (1979) and stated that mosquitoes can have five palpomeres. Forattini (2002), however, claimed mosquitoes have four palpomeres, but our data (see below) support Tanaka et al. (1979), with Anopheles (Kerteszia) having five palpomeres. The few apical pale scales that normally occur on the tip of palpomere 5 of Kerteszia species (except An. bambusicolus) may be rubbed and missing. When this occurs on An. lepidotus, the apical pale scales on palpomeres 1–4 are still evident and clearly demonstrate the presence of five palpomeres. The specimens studied here are deposited in the National Museum of Natural History (NMNH = USNM), Smithsonian Institution, Washington, DC.
Molecular
DNA was extracted from five specimens of Anopheles lepidotus (EC166-102; EC151-5; EC168a; EC168b; EC151-1) and three An. pholidotus (VZ7-102, VZ11-4, VZ7-101), using the commercially available DNesy Blood & Tissue Kit (QIAgen®, Maryland, USA). Amplification of rDNA Internal Transcriber Spacer 2 (ITS2) was carried out following the protocol of Li & Wilkerson (2005). Barcode fragments (658bp) of the mtDNA Cytochrome Oxidase I (COI) gene were amplified using the standardized Mosquito Barcoding Initiative (MBI) protocol as described in Ruiz et al. (2010); the shorter internal barcode fragment (472bp) was amplified following Linton et al. (2001).
Sequencing reactions were carried out in both directions using the Big Dye Terminator Kit® on an ABI 3730 automated sequencer (PE Applied BioSystems, Warrington, England). Sequences were edited using Sequencher™ v.4.8 (Genes Codes Corporation, Ann Arbor, MI). The ITS2 sequences were aligned using MAFFT v.6.814b (Katoh et al., 2002) within Geneious Pro 5.0.2 software, and the COI sequences were aligned and translated into amino acids in MacClade v.4.06 (Maddison & Maddison 2003). Sequence similarities were compared with those available in GenBank using Basic Local Alignment Search Tool—BLAST (http://blast.ncbi.nlm.nih.gov). Sequence divergence was calculated in MEGA v.4.0 (Kumar et al. 2008) using the Kimura 2-parameter distance model (K2P) (Kimura 1980). A bootstrap Neighbor-Joining (NJ) tree (Saitou & Nei 1987) was generated in PAUP v.4.0 (Swofford 2003), using 1000 replicates of the K2P distance matrices.
Results
Morphological
Currently, there are 12 recognized species of An. (Kerteszia), and 10 of these were examined during this study. Specimens of An. gonzalezrinconesi and An. rollai were not available, thus we relied on the original descriptions and illustrations for those two species. All Kerteszia specimens deposited in the NMNH were examined regardless of species determinations, and no additional females were found that match the females of putative An. lepidotus collected in Peru and Ecuador. However, examination of the primary types of Kerteszia deposited in the NMNH revealed that the male holotype of An. lepidotus possesses the same unique habitus characters found on the Peru and Ecuador females (Table 1), as well as on the Ecuador males. Also, the larval exuviae of the Ecuador females and males possess the same distinct characters that Zavortink (1973) found on the larval exuviae of the holotype and single male paratype, and used as the basis for describing An. lepidotus. The many shared unique adult and larval characters among the Peru and Ecuador males, females, and larvae with the male and larval exuviae of the holotype of An. lepidotus, infers the specimens originally collected in Peru and Ecuador are An. lepidotus, and not a new species. As a consequence of this finding, 175 female specimens in the NMNH that Zavortink (1973) identified as An. lepidotus (including the allotype and 31 paratypes he designated as An. lepidotus) were assigned incorrectly and are not An. lepidotus. After examining the Colombian, Panamanian, and Venezuelan An. pholidotus specimens and finding considerable variation in wing spots and the width of abdominal scales, we are reassigning the females that Zavortink designated as An. lepidotus to An. pholidotus. At this point, since we do not have DNA from specimens of An. pholidotus from the type locality, we are not investigating the possibility introduced by Zavortink (1973) that An. pholidotus may represent more than one species. The pupa of An. lepidotus is described for the first time, and several diagnostic characters were found that will separate this stage of An. lepidotus from pupae of other Kerteszia species. Examination of larval and pupal exuviae of An. lepidotus from Ecuador not only confirmed the larval characters that Zavortink used to describe this species, but also revealed additional new diagnostic characters. Additional male genitalia characters were also found that will assist in the identification of An. lepidotus.
TABLE 1.
Diagnostic external morphological characters differentiating adults (both sexes) of An. lepidotus from other Anopheles (Kerteszia) species.
| Structure | Character |
|---|---|
| Head | Palpomere 1 with small patch of apical white scales |
| Palpus with white apical scales on all 5 palpomeres | |
| Antennal pedicel with small dorsal and lateral patches of white scales | |
| Median portion of proboscis with white or translucent scales | |
| Thorax | Ventral portion of postspiracular area with small patch of white scales |
| Wing | Vein M1+2 fork with small patch of white scales |
| Wing with 7 pale fringe spots (R2, R3, M1, M2, M3+4, CuA, and 1A) | |
| Leg | Hindtarsomeres 1 and 2 without apical white bands from dorsal view |
| Genitalia | Cerci covered with erect white scales |
| Gonocoxite covered with erect white scales |
Anopheles lepidotus possesses many unique morphological characters on females and males and is probably the easiest species in subgenus Kerteszia to identify in the adult stage. Although An. lepidotus and An. pholidotus share several unique characters not found on other Kerteszia species, morphological characters on females, males, larvae, and pupae will also easily differentiate An. lepidotus from An. pholidotus (see below).
Since the descriptions of An. lepidotus and An. pholidotus Zavortink (1973), differentiating females of these two species has been impossible because the original females assigned to An. lepidotus were incorrectly identified An. pholidotus. In addition to the unique female characters of An. lepidotus (Table 1), diagnostic characters for An. pholidotus are presented (Table 2) that will solve the problem of identifying females and other life stages of these two species. To help alleviate any problems with the identification of the An. (Kerteszia) females, a key is presented below.
TABLE 2.
Diagnostic characters differentiating An. pholidotus from other Anopheles (Kerteszia) species.
| Stage | Character |
|---|---|
| Female | Palpomeres 2–5 with apical white scales (4 pale bands on palpi) |
| Mesepimeron with one long ventrally projecting row of long white scales | |
| Vein M1 with pale fringe spot adjacent to tip | |
| Terga V–VII with distal transverse rows of long erect dark scales | |
| Hindtarsomeres 2 and 3 with narrow apical white bands from dorsal view | |
| Male | Internal spine of gonocoxite with flattened tip |
| Anterior lobe of ventral claspette broadly rounded, not bent downward | |
| Pupa | Trumpet pinna short (<0.33 of trumpet length) |
| Seta 1-VII inserted cephalad of segment posterior margin | |
| Seta 9-V approximately 0.5 length of 9-VI | |
| Apical 0.5 of lateral margin of paddle with spicules gradually increasing in length, most apical spicules very long and straight, not wavy | |
| Larva | Seta 3-A long and tapered, much longer than 2-A |
| Seta 3-C moderately long, slightly more stout than 2-C, with long attenuated tip | |
| Seta 11-P moderately developed, long | |
| Seta 1-I long, single | |
| Seta 1-II–VI with brush-like palmate setae (5 pairs) | |
| Seta 6-VI with several strong basal branches | |
| Seta 9-IV–VI single | |
| Pecten with two rows of spicules on each spine |
Key to the females of Anopheles (Kerteszia)
| 1 | Mesepimeron with 1 long, large and curved (C-shaped) scale-patch that extends ventrally from upper setae | 2 |
| – | Mesepimeron with 1 or 2 small scale-patches | 3 |
| 2(1) | Proboscis, pedicel, and palpomere 1 with white scales; hindtarsomeres 1and 2 without apical pale band (from dorsal view) (Fig. 3) | lepidotus |
| – | Proboscis, pedicel, and palpomere 1 without white scales; hindtarsomeres 1 and 2 with narrow apical pale band (from dorsal view) | pholidotus |
| 3(1) | Mesepimeron with 1 small scale-patch next to upper setae | 4 |
| – | Mesepimeron with 2 small scale-patches (upper and middle) | 7 |
| 4(3) | Abdominal terga II–VII with numerous dark decumbent scales; sterna with few white scales | boliviensis, gonzalezrinconesi, rollai (currently cannot be separated) |
| – | Abdominal terga and sterna without scales (except possibly on VII, VIII and cerci) | 5 |
| 5(4) | Hindtarsomere 5 entirely white-scaled; wing without pale fringe spot at tip of wing | bambusicolus |
| – | Hindtarsomere 5 with base dark, apical 0.35–0.60 pale; wing with large pale fringe spot at tip or rarely divided into 2 small pale fringe spots | 6 |
| 6(5) | Scutum with white scales on acrostichal area from anterior promontory to near prescutellar setae; hindtarsomeres 2–4 with narrow white band on distal 0.15–0.5 | auyantepuiensis |
| – | Scutum without white scales on acrostichal area; hindtarsomeres 2–4 with broad white band on distal 0.5–0.7 | neivai |
| 7(3) | Hindtarsomeres 2–4 with narrow apical pale band, 0.3 or less length of tarsomeres; hindtarsomere 5 usually entirely dark | bellator |
| – | Hindtarsomeres 2–5 with broad apical pale bands, 0.4–0.7 length of tarsomere | 8 |
| 8(7) | Scutum with anterior 0.3–0.4 of acrostichal and dorsocentral areas and middle of scutellum with few white scales; vein M entirely or mostly white-scaled basal to level of Cu fork | laneanus |
| – | Scutum without pale scales on acrostichal, dorsocentral, and scutellum; vein M with dark scales basal to level of Cu fork | 9 |
| 9(8) | Scales on palpomeres 3 and 4 predominately decumbent, those on base of 3 may be slightly erect | cruzii* |
| – | Scales on palpomere 3 covered with slightly erect scales, palpomere 4 with slightly erect to decumbent scales | homunculus* |
| *This character is only useful if the specimens are in good condition. Other previously described characters are too variable because An. cruzii is a sibling species complex of 2 or 3 undescribed species (Ramirez & Dessen 2000a,b; Rona et al. 2009, 2010), as is An. homunculus (Sallum et al. 2009). | ||
FIGURE 3.
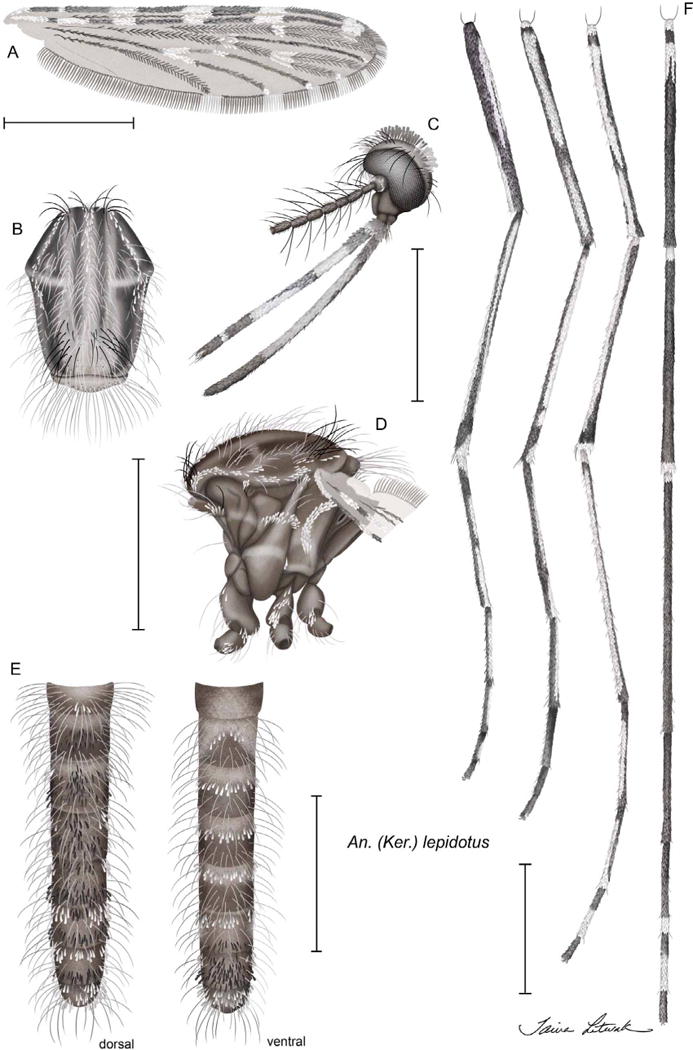
Anopheles (Kerteszia) lepidotus Zavortink, female habitus: A, wing; B, thorax, dorsal view; C, head, lateral view; D, thorax, lateral view; E, abdomen, dorsal and ventral views; F, (left to right) foreleg, anterior view; midleg, anterior view; hindleg, anterior view; hindleg, dorsal view.
Molecular
The ITS2 sequence analysis showed the presence of two divergent sets of sequences; An. lepidotus (447 bp, n = 5) and An. pholidotus (496 bp, n = 3). DNA alignment showed 343 identical sites (66.5%), 89 gaps, 45 transversions, and 39 transitions (Fig. 1). The nucleotide frequencies (%) were T: 26.2 %, C: 26.6 %, A: 19.6 % and G: 27.6 %, and the mean of K2P genetic divergence was 0.228.
FIGURE 1.

The ITS2 (rDNA) sequence alignments of Anopheles (Kerteszia) pholidotus (n = 3, Venezuela) and An. lepidotus (n = 5, Ecuador), using MAFFT (Katoh et al., 2002). A total of 343 nucleotides were identical; 45 transversions, 39 transitions, and 89 gaps were observed. Underlined bases show the ITS2 primers.
The COI barcode region (658 bp), and the shorter region (520 bp), showed seven different haplotypes for the samples analyzed (n = 7), with a mean intra-specific differentiation of 0.009 (An. lepidotus) and 0.003 (An. pholidotus), and a mean inter-specific divergence of 0.082 (range 0.076–0.087). Mean nucleotide frequencies were T: 39.0 %, C: 16.9 %, A: 28.7 % and G: 15.5 %. An analysis of nucleotide frequencies over all samples showed an average of 508 identical nucleotide pairs (174 first position; 177 second position; 157 third position), and 13 differences (ten transitions and three transversions). The NJ-K2P tree supported the presence of An. lepidotus and An. pholidotus as different lineages, with a 100% bootstrap value (Fig. 2). No stop codons were observed in the COI sequences, eliminating the presence of possible pseudogenes. All changes were synonymous except for the one at position 62 (G/A) [An. pholidotus (Valine) and An. lepidotus (Isoleucine), respectively].
FIGURE 2.
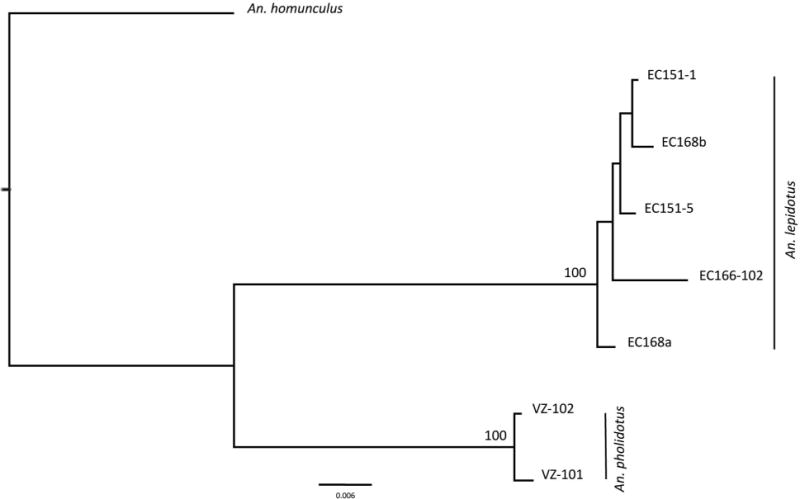
Bootstrap NJ-K2P tree of COI sequences belonging to Anopheles (Kerteszia) lepidotus and An. pholidotus from Ecuador (EC) and Venezuela (VZ). Bootstrap values below 70 % are not shown. Outgroup: An. (Ker.) homunculus Komp.
COI sequences generated formed part of the activities of the Mosquito Barcoding Initiative (project leaders Yvonne-Marie Linton & Richard Wilkerson) and complete specimen records and COI sequence data, including electropherograms, are available through the Barcode of Life database website at http://boldsystems.org/. GenBank accessions for COI and ITS2 sequences generated in this study are in Table 3.
TABLE 3.
Specimens from Venezuela and Ecuador sequenced with COI and ITS2 DNA markers with their locality data and GenBank accession numbers.
| Species | Country, locality | Coordinates | Date | Collection | GenBank | |
|---|---|---|---|---|---|---|
| ITS2 | COI | |||||
| An. lepidotus | Ecuador, Napo, Tiputini Biodiversity Station | −0.63806, −76.14500 | 4 Nov 1998 | EC151-1 | JN967763 | JQ041286 |
| EC151-5 | JN967764 | JQ041283 | ||||
| 7 Nov 1998 | EC166-102 | JN967765 | JQ041285 | |||
| EC168a | JN967766 | JQ041282 | ||||
| EC168b | JN967767 | JQ041284 | ||||
| An. pholidotus | Venezuela, Táchira, El Tama National Park | 7.63444, −72.43917 | 13 Jan 2001 | VZ7-102 | JN967768 | JQ041287 |
| 14 Jan 2001 | VZ7-101 | JN967769 | JQ041288 | |||
| VZ-11-4 | JN967770 | – | ||||
Taxonomy: Anopheles lepidotus Zavortink, 1973
Anopheles (Kerteszia) boliviensis Theobald, 1905, of Komp & Osorno-Mesa, 1936: 415 (♂*, L*); Komp, 1937: 500 (♂*, L); Lane, 1953: 279 (♂*, L*); Komp, 1956: 40 (♂, L, biology and distribution, in part); Stone, Knight & Starcke, 1959: 35 (♂, L, taxonomy, distribution, in part); Forattini, 1962: 448 (♂*, L); Aragão, 1964: 76 (biology and distribution, in part).
Anopheles (Kerteszia) lepidotus Zavortink 1973: 17 (♂*, ♀* [misidentification], L, key to females incorrect, biology and distribution, in part); Knight & Stone, 1977: 58 (only ♂, L, distribution, in part).
Overview
Because Komp & Orsono-Mesa (1936) assigned two males with larval skins to An. boliviensis, numerous publications before Zavortink (1973) were unknowingly addressing An. lepidotus instead of An. boliviensis. Numerous articles following Zavortink (1973) that addressed An. lepidotus were correct, in part, as the male, larva and genitalia are An. lepidotus. However, post-1973 articles addressing An. lepidotus females and their structures were incorrect and should now be interpreted as An. pholidotus. Also, post-1973 published discussions of the biology, distribution, medical significance, and the phylogenetics of An. lepidotus are only partially correct. A good example of the above can be found in Gonzalez & Carrejo (2009) where, as in Zavortink (1973), the larval and male genitalia keys and descriptions correctly separate the two species, but the adult female key and associated characters in the text do not. As in other publications, the distribution records in this report need to be re-assessed. Country records are revised here, but within country provincial and local records for the two species need to be re-evaluated.
Male and female diagnostic characters. (Figs. 3, 4A; Table 1)
FIGURE 4. Anopheles (Kerteszia) lepidotus.
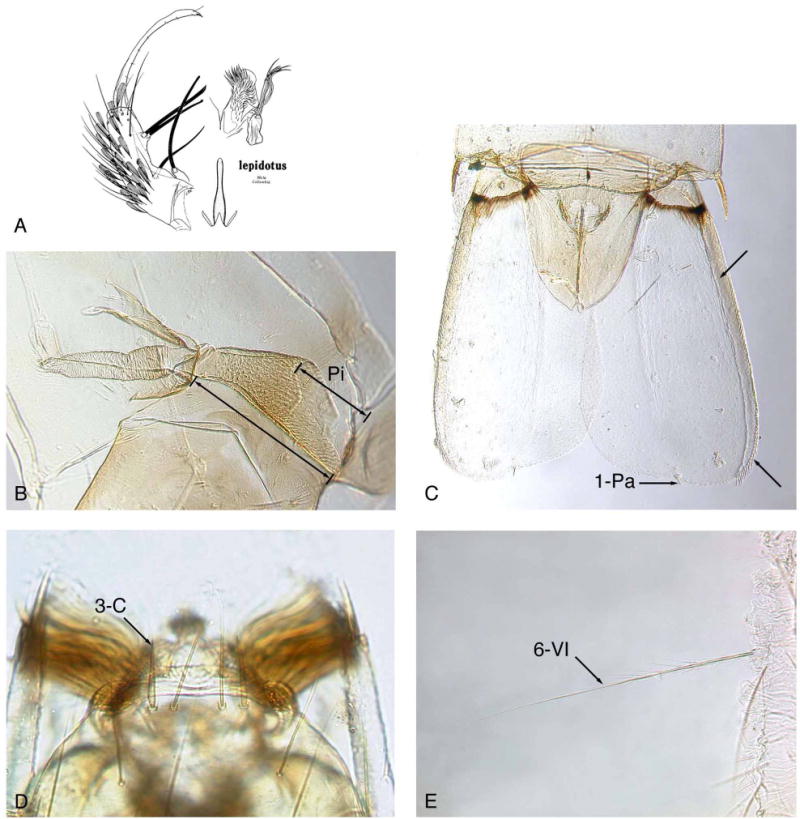
A, male genitalia (from Zavortink, 1973); B, An. lepidotus pupal trumpet, pinna (Pi) long, about 0.5 trumpet length; C, pupal paddle showing lateral margin exceptionally thick, lateral margin without long filamentous spicules and relatively straight apical margin at 1-Pa; D, seta 3-C very thick and short; E, seta 6-VI stout, long, with median length aciculae on basal 0.33 and shorter aciculae more distal, without strong basal branches.
Maxillary palpus with apical pale scales on all 5 palpomeres; palpomere 1 with apical white scales; pedicel with dorsal and ventrolateral patches of small white spatulate scales; mid-portion of proboscis with large area of variable pale scaling on dorsum, laterally, and/or on venter; postspiracular area with small ventral patch of white scales (may be present or absent on males); wing normally with 7 pale fringe spots; M1+2 fork with white scales (may be 1–3 scales or obscure on males); hindtarsomeres 1 and 2 without apical pale scales on dorsum; terga I, III–VIII normally with white scales; female cerci with erect white scales; male gonocoxite with erect white scales to apex; accessory setae on gonocoxite of unequal length; internal seta of gonocoxite with long gradually attenuated tip.
FEMALE (Fig. 3, Table 1)
Head. Pedicel with dorsal and ventrolateral patches of small white spatulate scales; palpomeres 1–5 with white scales on distal half or apex, palpomere 1 with several apical white scales, pal-pomere 2 with apical 0.6 pale-scaled, distal half of palpomere 3 with long white area ventrolaterally and long dark area dorsomesally before white apex, palpomeres 4,5 with very small white-scaled area at apex; palpomeres 1,2 with erect black scales; proboscis dark-scaled on basal 0.1, with white or translucent scales dorsally, laterally and/or ventrally (variable) on median 0.5, dark-scaled on apical 0.3. Thorax (Fig. 3). Scutal integument with 2 submedium and 2 lateral longitudinal dark lines; scutum with pale scales on acrostichal and dorsocentral rows on anterior 0.4; long erect setae on scutum pale except for stout dark setae at anterior ends of acrostichal and dorsocentral rows (rarely extending posteriorly) and dark setae over wing root and infrequently in prescutellar area; scutellum may have pale and dark setae, with several black scales on mid-region and infrequently laterally; antepronotum with long white scales, occasionally with small patch of black scales at mesal end; postpronotum with pale pruinose area posteroventrally; proepimeron and subspiracular areas with pale pruinose areas in line with that on postpronotum; postspiracular area with small ventral patch of white scales in line with anterior pruinose areas and white scales on upper mesokatepisternum; pruinose areas and connecting white scales form upper lateral white line on side of thorax from postpronotum to mesokatepisternum; lower lateral pale line on side of thorax incorporates pruinose areas on the metameron, lower mesepimeron, middle of mesokatepisternum, and propleuron; propleuron without scales, usually with 1 long seta; prespiracular area with setae and several white scales; mesepimeron with 1 long vertical “C-shaped” row of long white scales that extends downward from upper mesepimeral setae to middle of mesepimeron. Wing. (Fig. 3). Wing with 5 pale spots on costa—a small humeral spot that starts at or just distal to humeral crossvein and 4 large sector, accessory sector, subcostal, and preapical pale spots, preapical pale spot extends to apex of R1; pale subcostal spot extends to R2+3; pale fringe spots at wing apex usually consist of two small spots located at tips of R2 and R3, occasionally merged into one larger spot (combined R2 and R3 fringe spots); posterior fringe spots occur at tips of M1, M2, M3+4, CuA, and 1A, usually increasing in size from M1 to 1A; vein R4+5 with small pale spot at base, apex with dark fringe scales; base of vein M dark-scaled, pale-scaled at M1+2 fork; base of vein CuA usually dark-scaled before pale-scaled mcu crossvein; 1A dark-scaled to distal end, or rarely with 1–3 pale scales at tip. Legs. (Fig. 3). Fore- and midcoxae with white scales; foretarsomeres 4,5 normally dark-scaled, 4 occasionally with small dorsal pale patch; hindtarsomere 1 with small basal pale spot, small postbasal dark band, dark-scaled dorsally to apex, but pale-scaled ventrally from postbasal dark band to apical dark band; hindtarsomere 2 dark dorsally from base to apex, with long pale area on venter from near base to near apex; hindtarsomere 3 dark dorsally except for small apical pale band, pale ventrally from near base to apex; hindtarsomere 4 dark-scaled on basal 0.3–0.4 and pale-scaled to apex; hindtarsomere 5 dark-scaled; hindtarsomeres 1–3 dark from dorsal view except for small basal pale spot on Ta-III1 and small apical pale spot on Ta-III3; Ta-III4 dark basally and pale apically, and Ta-III5 dark; from ventral view Ta-III1 is nearly all pale from near base to small dark tip, Ta-III2 pale except for small dark areas at base and apex, Ta-III3 pale from near base to apex; Ta-III4 and Ta-III5 as described for dorsal view. Abdomen (Fig. 3). Terga and sterna with long, erect, spatulate, white and brown scales; tergum I with several long erect white scales on apicomesal area, sternum I without scales, tergum II with apicomesal patch of brown partially erect scales, sternum II with curved mesal row of long erect white scales, terga III–VII with long erect white scales either laterally (III–V) or lateral and basal (VI–VII), terga III–V with mesal patch of brown erect scales beyond base (III) or extending from base to apex (IV,V), those on V forming basal and apical transverse rows of long erect scales, sterna III–V with postbasal row of erect white scales, tergum VI with small patch of brown basomesal erect scales and distinct apical row of brown erect scales, sternum VI with basal row of erect white scales and apical row of erect brown scales, tergum VII with basal row of erect white scales and apical row of mixed erect white and brown scales, sternum VII nearly covered with erect long brown scales and few white lateral scales, tergum and sternum of VIII covered with long erect white scales.
MALE
Males possess the same diagnostic characters (Table 1) as females and generally have a habitus matching the females. Occasionally characters on males may be less evident or absent, these include the posterior extension of white scales in the acrostichal row on the scutum, absence of a small ventral patch of scales on the postspiracular area, fewer (1–3) white scales on wing fork M1+2, scales not so dense on wing veins, and very faint posterior fringe spots on the wing. Certain characters like the costal pale spots on the wing may appear larger on males. Genitalia. (Fig. 4A). Tergum VIII with median broad erect spatulate white scales; gonocoxite with erect white scales to apex; 2 accessory setae on gonocoxite of unequal length, longest seta flattened and broadened apically with a pointed tip, shortest seta with broad rounded tip; internal seta of gonocoxite with long gradually attenuated tip; aedeagus long slender without leaflets; ventral lobe of claspette with dense long spicules mesally near rounded bare apex, with shorter more scattered spicules basally and laterally, lateral margin with broad narrowing and bluntly rounded lobe joining basal stem at emarginate angle; dorsal lobe composed of 2 stems, each with 3–4 sinuous flattened setae.
Pupal diagnostic characters (Fig. 4B,C)
Pinna exceptionally long, 0.41–0.55 (mean 0.46) trumpet length; seta 1-IX short, very thick, acutely pointed; lateral margin of paddle (Fig. 4C) exceptionally thick, usually sclerotized and pigmented on basal portion; paddle with midrib absent or weakly developed; paddle asymmetrical, with relatively straight apical margin at seta 1-Pa, and without long filamentous spicules on distal 0.33 of lateral margin.
Pupa
Integument light to medium brown, most pigmentation on trumpet and segments I–IV; setae very thin and weak, single or with few branches. Cephalothorax. Trumpet medium brown, angusticorn, without meatal cleft, with wide opening, pinna exceptionally long, 0.41–0.55 (mean 0.46) trumpet length (n = 12); seta 13-CT or alveolus present. Abdomen. Setae 0-VII,VIII long, frail, nearly as long as seta 1-VII; 1-II–VII short, frail, single or with few distal branches; 1-IX very short, thick, sharply pointed; 9-I very short, 9-II,III weakly developed, 9-III slightly longer than 9-II, 9-IV,V length ratio 0.19–0.50 (mean 0.33), 9-V,VI length ratio 0.38–0.61 (mean 0.52), 9-V–VIII well developed, spine-like, darkly pigmented, with long sharp dorsolateral aciculae; 10-VI present; 14-III absent. Paddle. Base with distinct darkly pigmented transverse line; paddle asymmetrical, lateral half considerably longer than mesal half, paddle index (L/W) 1.72–2.06 (mean 1.92), apical margin straight near seta 1-Pa on both lateral and mesal sides; lateral edge exceptionally thick, sclerotized and pigmented, extending to near tip; midrib absent or very weakly developed; spicules on lateral margin small, wide, and acute, beginning on basal 0.33, with larger and longer stout spicules on distal 0.33 approximately equal length of 1-Pa; lateral spicules in 2 rows, one directed dorsally and the other ventrally, except in most distal row of longer stout spicules; distal 0.33 of lateral margin of paddle without long sinuous or straight spicules that are much longer than 1-Pa; seta 1-Pa short, stout, sharp pointed, not filamentous, inserted mesal to tip of paddle; 2-Pa short, filamentous, usually inserted cephalad and some distance from 1-Pa.
Larval diagnostic characters (Fig. 4D,E)
Seta 3-C very thick, short, usually sharply pointed; 11-P very short; 1-I small with 4 or 5 simple branches; palmate setae (1-II–VI) only on 5 segments, moderately open, not brush-like; 2-IV exceptionally long, equal in thickness to 6-IV, usually simple, rarely with aciculae; 3-VIII with few branches near base; 6-VI stout, long, with median length aciculae on basal 0.33 and shorter aciculae more distal, without strong basal branches; 9-IV–VI nearly equal length of 7-IV–VI, with 2–5 branches; pecten spines from side view with single mesal row of stout aciculae extending out to tip.
Larva
Integument light brown on head, abdominal plates, and sclerotized structures on segments VIII and X. Head. Antennal seta 3-A as long as or only slightly longer than 2-A; 3-C very thick, short, usually sharply pointed, infrequently tip split or with small aciculae; distance between both 2-C, measured between the outer adjacent margins of the two alveoli, narrow, not more than 2.5 times distance between outer adjacent alveoli margins of 2-C and 3-C on one side; 5-C simple; 11-C usually with 2–6 small distal branches. Thorax. Seta 11-P very short. Abdomen. Seta 1-I with 4–5 simple branches; palmate setae on five segments (II–VI); palmate setae moderately open and well formed, not brush-like; 1-VII short with 2 or 3 simple branches; 2-IV exceptionally long, stout, equal thickness to 6-IV, usually simple, rarely with aciculae; 3-VIII with 2 or 3 large branches on basal 0.33; 5-II–V plumose, with branches along main stem; 6-VI as stout and long as 6-V, with frail median length aciculae on basal 0.33, and short aciculae more distally, without strong branches near base; 9-IV–VI nearly equal length of 7-IV–VI, with 2–5 branches; 8-S present; pecten spines (18–23) of nearly equal length, from side view with single row of long stout aciculae; seta 1-X very long, attenuated, approximately 2 times saddle length; most anterior ventral brush seta (4a-X) short, usually <0.33 length of seta 4b-X; ventral brush (4-X) with 9 pairs of setae.
Egg
Unknown.
Specimens Examined (Anopheles lepidotus)
Seventy specimens were examined (19♀, 13♂, 14Le, 17Pe, 7G) from three countries as follows. COLOMBIA: Meta Department, Restrepo, holotype (specimen B, pinned), ♂, with slides of associated larval exuviae and genitalia, from leaf axil of bromeliad, XII-1935, Orsono-Mesa, in NMNH; paratype (specimen A mounted on slide), 1♂, with slides of associated larval exuviae and genitalia, from same locality and collection as holotype. ECUADOR: Napo Province, Yasuni National Park, Tiputini Biodiversity Station, EC104, -2, -3, 1♀, 2LePe, from bromeliad, 29-X-1998, R. Wilkerson; EC126(1), 1♀, biting human, 1-XI-1998, R. Wilkerson; EC151, -1 through -4, 4♂, 4LePe, 2G, from bromeliad, 4-XI-1998, R. Wilkerson; EC166, -101, -102, -2 through -4, -6, 3♀, 3♂, 3Le, 4Pe, 3G (2M in ETOH), from bromeliad, 7-XI-1998, R. Wilkerson; EC168, 5♀ (3 in ETOH), biting human, 7-XI-1998, R. Wilkerson; EC256, -100, -103, -105 through -109, -1 through -3, 6♀, 4♂, 3Le, 7Pe, from bromeliad, 26-V-1999, R. Wilkerson. PERU: Junín Province, Mission Cutivireni, PE349, 1♀, biting human in hut, 20-III-1985, Falcone and others; PE359, 1♀, biting human in canopy, 22-III-1985, Hayes, Harrison & Savage; Junín Province, Puerto Ocopa, PE346, 1♀, biting human in hut, 26-II-1986, Calderón & Hayes.
Bionomics
Anopheles lepidotus is a true “bromelicolus” species that occurs at relatively low elevations in remote or semi-remote tropical forests on the Amazonian slopes of the Andes in South America. It has been collected at elevations between 234 and 483 m, but not at elevations of 1,700 m like An. auyantepuiensis (Harbach & Navarro, 1996), or above 2,000 m like An. boliviensis, An. gonzalezrinconesi, An. pholidotus, and An. rollai (Navarro et al., 2010). Specimens were collected as larvae in arboreal bromeliads in Colombia and Ecuador and reared to adults with associated exuviae of the immature stages, and in human landing collections (HLC) in Ecuador and Peru at ground and canopy levels. To date, nine host-seeking An. lepidotus females were captured (out of 19 known females) in human landing collections. Seven of those females were captured in the canopy on open platforms at 15 m height (Peru) and at 34 m height (Ecuador), whereas two females were captured in Peru at ground level inside unscreened houses. One of the last two specimens was collected engorged while feeding on a human. The three females collected (1 canopy, 2 at ground level) in Peru in 1985–86 came to humans in February and March shortly after dark (1800–1900 hr), although HLC continued at ground level throughout the night. The remaining six females captured by HLC were taken in November 1998 at 34 m in the canopy in Ecuador between 1800–1900 hr. These collections during the dry summer months differ from 1942–43 collections of An. boliviensis in Villavicencio, Colombia, which were most common during the wet season between April and October (Bates, 1945). However, Bates qualified those data by stating, “The capture data must be viewed with some suspicion because the species has predominantly late diurnal and crepuscular habits, and the captures were made at midday.” Larval collections from bromeliad axils (species unknown) at varying heights up to 34 m resulted in 11 males and 16 females that were reared in Ecuador during October and November 1998, plus the two males (holotype and paratype) collected during December 1935 from bromeliad axils at an unspecified height in Colombia. Larvae collected during the Ecuador trip survived well after collection, but development of the successive instars and stages was lengthy.
Species associated with An. lepidotus and captured by HLC between 1600–2000 hr during a canopy (15 m) collection (PE359) in Mission Cutivireni, Peru (12° S, 74° W) in 1985 were: Aedes sp.; Ochlerotatus (Chrysoconops) fulvus (Wiedemann) (as Aedes (Och.) fulvus (Wiedemann); see Reinert et al. (2008)); Anopheles (Anopheles) fluminensis Root; An. (Nyssorhynchus) oswaldoi (Peryassú); An. (Nys.) rangeli Gabaldón, Cova Garcia & Lopez; Chagasia ablusa Harbach; Ch. bonneae Root; Haemagogus (Conopostegus) sp.; Hg. (Haemagogus) sp.; Psorophora (Janthinosoma) ferox (von Humboldt); Ps. (Grabhamia) dimidiata Cerqueira; and Sabethes (Sabethoides) chloropterus (von Humboldt). Species associated with An. lepidotus, captured at ground level by HLC between 1800–0600 hr inside a house (PE349) in Mission Cutivireni, Peru in 1985 were: Ochlerotatus fulvus (as Ae. fulvus); An. fluminensis; An. (Ano.) intermedius (Peryassú); An. (Nys.) nuneztovari Gabaldón; An. (Nys.) oswaldoi; An. (Nys.) rangeli; An. (Nys.) trinkae Faran; An. (Nys.) sp.; Ch. bonneae Root; Ps. (Gra.) cingulata (Fabricius); Ps. (Jan.) ?horrida (Dyar & Knab); and Ps. (Psorophora) lineata (von Humboldt). Taxa associated with An. lepidotus in larval collections from bromeliad axils at 34 m height in Napo, Ecuador (0° 38′17″S, 76° 08′ 42″ W) in 1998 were: EC104, Wyeomyia sp.; EC151and EC166, Culex spp.
Distribution
Anopheles lepidotus is only known from four localities in three countries on the Amazonian slopes of the Andes. Those sites are Colombia (Meta, Restrepo, and Buena Vista), Ecuador (Napo, Yasuní National Park, Tiputini Biodiversity Station), and Peru (Junín, Mission Cutivireni, and Puerto Ocopa). The Ecuador and Peru collections represent the first confirmed records of this species in those two countries. The female previously identified as An. lepidotus from Cochabamba, Bolivia (Zavortink, 1973) is actually An. pholidotus.
Medical significance
Prior to this study, An. lepidotus was considered a fairly abundant species in areas of Colombia where malaria transmission occurred. Quiñones et al. (1984) proposed that An. lepidotus females were the primary Kerteszia species biting humans and the probable vector in an area of high malaria endemicity in Colombia. However, subsequent to that study specimens were sent to us for examination (courtesy of Dr. Suarez, SNEM, Colombia) and they were not An. lepidotus, but An. pholidotus. Reared specimens from the above 1984 study area and examinations of dissected male genitalia also confirmed that the correct species was An. pholidotus, not An. lepidotus (Escobar et al., 2010). Since the holotype and paratype male of An. lepidotus were reared from larvae in 1935 in Meta, Colombia, there have been no other confirmed specimens of this species collected and preserved from the type locality or Colombia. During the last 28 years only an additional 30 specimens of An. lepidotus have been collected in three sites in Peru and Ecuador. Zavortink (1973) thought that An. lepidotus was the dominant and most important species in the Meta Department of Colombia, but we found that only two specimens (types) of An. lepidotus are known from Meta (and Colombia). Thus, the biological information and medical importance for the specimens previously identified as An. boliviensis and subsequently assigned to An. lepidotus by Zavortink actually apply to An. pholidotus or An. boliviensis (to a lesser extent). For all of the above reasons, we consider An. lepidotus an uncommon (or inaccessible in the forest canopy) or rare species, and unlikely to be involved in the transmission of human malaria parasites on a large scale. However, An. lepidotus was the only Kerteszia species collected biting humans in Ecuador and Peru, and it obviously has an affinity for human blood in the canopy and at ground level. This suggests it may become infected with malaria by feeding on primates in the canopy and then transmit the parasites to humans at a later time. Deane (1967, 1988) determined that in the State of Amazonas, Brazil, adjacent to Peru, Plasmodium malariae was the major simian malaria parasite, which occurred in 25 monkey species in Brazil. Sulzer et al. (1975) and Sulzer et al. (1978) conducted three malaria surveys in Mission Cutivireni, our 1985–86 collection site, and declared the locality a hyperendemic area for Plasmodium vivax and P. malariae, with 97.2% of the Ashaninka Amerindians infected in the initial studies. Hayes et al. (1987) conducted vector studies at Mission Cutivireni and determined that An. trinkae, a ground feeding species, was the primary malaria vector at the Mission and in Puerto Ocopa, but the Plasmodium species were not identified and no Kerteszia species dissected. Forattini (2002) and Collucci & Sallum (2003) discussed the role of several arboreal Kerteszia species in the transmission of malaria parasites, particularly P. malariae (= P. brasilianum) in primates and humans in Brazil and other areas of South America Thus, humans living in or moving into areas like Mission Cutivireni on the eastern slopes of the Andes might be exposed to P. malariae in a simian/arboreal Anopheles/human cycle like those described above by Forattini (2002) in Brazil, or similar to the macaque/Anopheles/human P. knowlesi cycles now known in peninsular Malaysia, Malaysian Borneo, and other parts of southeast Asia (Vythilingam et al., 2008; Tan et al., 2008).
Morphological discussion
Adult
Based on the adult diagnostic characters (Table 1), An. lepidotus is morphologically unique among species in subgenus Kerteszia. Those characters are visible using a 60–80 × magnification dissection microscope, even when examining adults stored in alcohol. At least two characters appear unique in genus Anopheles, i.e., white scales on the apex of palpomere 1, and white scales on the ventral portion of the postspiracular area. Linthicum (1988) mentioned scattered pale scales on palpomeres 1 and 2 of species in the Argyritarsis Section of subgenus Nyssorhynchus, but not at the apex of palpomere 1. Although the wings and legs of An. lepidotus are dark compared to some other Kerteszia species, this species has striking tendencies toward leucism as it displays more white scales and setae in places where they do not occur on any other species in the subgenus. Examples of leucism are: (1) white apical scales on all five palpomeres, (2) white scales on the proboscis, (3) two patches of white scales on the pedicel, (4) white scales on the ventral portion of the postspiracular area, (5) white erect scales on the abdominal terga (mixed with dark scales) and sterna, (6) small white scales on the anterior third of the acrostichal row on the scutum, (7) white setae covering most of the scutum, (8) more white fringe spots on the wing, (9) white scales on the cerci, and (10) white scales on the gonocoxite. Some of these characters suggest evidence of a relationship with subgenus Nyssorhynchus (3, 5, 6, 7), whereas the others (2, 4, 7, 9, 10) suggest a relationship with subgenus Cellia (2, 7, 9) or are apomorphic (1, 4, 10).
A major goal of this study was to clearly differentiate An. lepidotus from An. pholidotus. In this regard, adults of An. lepidotus possess five secondary diagnostic characters that are shared with only one other species in subgenus Kerteszia. Anopheles lepidotus and An. pholidotus share four of those characters: (1) one long vertically curved, white scale-patch on the mesepimeron; (2) four or more palpomeres with white apical scales (An. lepidotus with white apical scales on palpomeres 1–5, An. pholidotus with white apical scales on palpomeres 2–5, whereas the remaining species do not have white apical scales proximal to palpomere 3; (3) transverse apical rows of erect dark spatulate scales on terga V–VII; and (4) wing with a pale fringe spot next to the tip of vein M1 [Harbach & Navarro (1996) found this fringe spot uncommon on An. auyantepuiensis]. Based on the above four diagnostic characters shared by An. lepidotus and An. pholidotus, they likely share a common distant ancestor (see Molecular Discussion). Character (5), totally dark hindtarsomere 5, is shared with An. bellator Howard, Dyar, & Knab [Wilkerson & Peyton (1991) found one specimen of An. cruzii with hindtarsomere 5 entirely dark].
Both An. lepidotus and An. pholidotus possess small white scales along the anterior 0.4 of the acrostichal row, as do An. auyantepuiensis, An. laneanus, An. gonzalezrinconesi, and An. rollai (Harbach & Navarro, 1996; Sallum et al., 2000). Thus, this character occurs on half of the known An. (Kerteszia) species and is not unique on An. auyantepuiensis as originally stated in Harbach & Navarro (1996). Also, this study determined that An. gonzalezrinconesi, and An. rollai are not morphologically similar to An. lepidotus and An. pholidotus, but are much more similar to An. boliviensis, provided one or both of the two former species are not synonymous with An. boliviensis. Without reared specimens of An. gonzalezrinconesi, An. rollai, and the unknown males and unknown immature stages of An. boliviensis, more precise relationships of these three species cannot be determined. Komp & Orsono-Mesa (1936) described two males and their larval skins and identified them as An. boliviensis, which Zavortink (1973) subsequently used as the holotype and paratype for describing An. lepidotus. Thus, only the female of An. boliviensis is currently known. As noted above, the long vertically curved white scale-patch on the mesepimeron is found only on An. lepidotus and An. pholidotus. The other Kerteszia species, i.e., An. bellator, An. cruzii, An. homunculus Komp, and An. laneanus Correa & Cerqueira, have two small white patches of scales on the mesepimeron, one adjacent to the upper setae and a middle anterior patch, whereas An. auyantepuiensis, An. bambusicolus Komp, An. boliviensis, An. gonzalezrinconesi, An. neivai Howard, Dyar, & Knab, and An. rollai only have a single white scale-patch adjacent to the upper setae. The significance of these three different scale patterns on the mesepimeron cannot be overemphasized as they quickly (compared to variable hindtarsal and wing patterns) can be used to identify the three Kerteszia species assemblages mentioned above. The two unique patches of white scales on the pedicel of An. lepidotus are lacking on other species of Kerteszia. Also, two patches of scales on the pedicel are not found on species in the Albimanus or Argyritarsis Sections of subgenus Nyssorhynchus, which only have one patch of white scales on the pedicel (Faran, 1980; Linthicum, 1988). The presence of white scales on the proboscis, white scales at the M1+2 fork, numerous spotting differences on the wing veins and pale fringe spots, variable hindtarsal color patterns, are all characters that occur in some species of other subgenera of Anopheles. However, the presence of white erect scales covering the cerci and gonocoxites is much more unusual in Anopheles.
Pupa
The pupa of An. lepidotus is described for the first time. Generally, the pupa is lightly pigmented with the trumpet light brown, and has setae that are very fragile, short, with few branches. The trumpet of this species appears unique in the subgenus because the average length of the pinna is nearly half the trumpet length (occasionally >0.5). All other described Kerteszia species, including An. pholidotus, have a pinna approximately 0.15–0.33 the length of the trumpet. However, Wilkerson & Peyton (1991) found the pinna was variable on An. cruzii Dyar & Knab, with some specimens having a longer pinna. This noted difference on An. cruzii sensu lato, may be of value in differentiating the provisional molecular sibling species of An. cruzii currently recognized in Brazil (Collucci & Sallum, 2003, Malafronte et al., 2007, Rona et al. 2009, 2010). The presence of seta 1 immediately adjacent to the posterior margin of segment VII on An. lepidotus is similar to most Kerteszia species, except An. pholidotus which has 1-VII removed from the posterior margin. Seta 1-IX is very short, thick, sharply pointed on An. lepidotus, whereas it is longer, thin, and more visible on An. pholidotus. Anopheles lepidotus apparently has the largest paddle length/width ratio in the subgenus, i.e., x = 1.92, range 1.72–2.06. The midrib on the paddle is weakly developed (or absent), hence the development of a stronger, thick, sclerotized, and pigmented lateral margin needed for support. The two rows of small basal spicules on the lateral paddle margins become one row of more stouter, longer spicules on the apical 0.33, which are no longer than two times seta 1-Pa length. The longer spicules on the apical 0.33 do not extend to 1-Pa and are much shorter than the very long apical spicules occurring on the paddle of An. pholidotus. Seta 1-Pa on An. lepidotus is short and thick, whereas 2-Pa is frail, thin, and not inserted adjacent to, but usually cephalad of, 1-Pa.
Larva
Zavortink (1973) used seven characters found on larval exuviae of the male holotype and one male paratype as the basis for describing An. lepidotus, i.e., seta 3-C short, thick, usually pointed; seta 11-P weakly developed and short; palmate setae moderately developed, not brush-like; seta 5-II–V plumose; seta 5-VII short; seta 9-IV–VI usually branched, but only near the base; and pecten spines with one row of spicules restricted to basal portion of the external edge. Examination of the Ecuadorean larval specimens confirmed the seven characters described by Zavortink (1973), and also provided additional valuable characters for identifying An. lepidotus. The new characters include setae 2,3-A approximately equal in length (compare An. pholidotus); seta 1-II–VI palmate (five pairs), shared with An. pholidotus and uncommon specimens of An. bambusicolus; seta 1-I with 4 or 5 simple branches (compare An. pholidotus); seta 1-VII short, with 2 or 3 simple branches; seta 2-IV exceptionally long, development equal to 6-IV, rarely with aciculae; seta 6-VI long, without strong basal branches, with frail aciculae; seta 8-S present; and pecten with one row of spicules on spines, usually pointed mesally (compare An. pholidotus with two rows of spicules per spine).
Specimens examined (Anopheles pholidotus)
A total of 304 life stages and structures of An. pholidotus in NMNH were examined (256♀, 9♂, 14Le, 17Pe, 6L, 2G) from Bolivia, Colombia, Costa Rica, Ecuador, and Venezuela during this study. PANAMA (Type series): Bocas del Toro, La Zorra, PA173-110, holotype ♂, LePeG, collected in axil of terrestrial bromeliad, 5-IV-1963, A. Quiñones. Bocas del Toro, Caldera-Chirique Trail, 1,000m, allotype ♀, biting human in upper canopy of deep forest, 31-X-1955, P. Orguela. Paratypes PA173-104, ♂LePeG; PA173-111, Le5L; PA176, 1L, 6-IV-1963, collected in axil of epiphytic bromeliad, with same data as holotype (except PA176); ♀ with same data as allotype. COLOMBIA (Specimens misidentified as An. lepidotus by Zavortink (1973): Meta, Cuchilla, E. of Villavicencio, allotype ♀, 16-III-1943; Meta, near Buena Vista, paratype ♀, 4-VI-1942, W.H.W Komp; Meta, Restrepo. Paratypes with following data – 4♀, VIII-1935, W.H.W Komp; 3♀, 1935, W.H.W Komp; ♀, 20-XI-1936, W.H.W Komp; 2♀, KO-121A-10, W.H.W Komp. Other misidentified specimens follow: Meta, Restrepo, Retiro, 7♀, 10-VIII-1935, W.H.W Komp; Tolima, Villarrica, 12♀, various 1982–83 dates and collectors; 174♀, J.A. Kerr. Additional correctly identified specimens from Colombia include: Tolima, Villarrica, 8♀, various 1981,82,86 dates and collectors; Meta, Restrepo, 4♀, 20-XI-1936; Cajete, Cauca, ♀, 23-VI-1991, M. Barreto; Tolima, Icononzo, 6♀, X–XII-1982, C.M.; Tolima, Villarrica, 7♀, X-XII-1982, C.M. BOLIVIA: Camata, 3♀, 15-IV-1949; Cochabamba, Yungus d. Palmar, ♀, 1,200m, (second label – 4133); Cochabamba, Chapare, ♀, 30-IV-1944, Torres M.; Chapare, Palmar, 2♀, 1-V-1944. ECUADOR: Yumaza, Morone Santiago, 5♀, VIII-1967, Duret. VENEZUELA: Merida, 2♀, 10-VI-1938, P. J. Anduze; Tachira, El Tama National Park, near Mata Mula, 7°38.04 N, 72°25.81W, 1,727m elevation, VZ7, 8♀, 7♂, 11Le, 15Pe, from axils of bromeliad (Tillandsia fendleri), 13-I-2001, Wilkerson & Navarro, WRBU Acc. No. 1721.
Molecular discussion
DNA sequence divergences and bootstrap support confirmed An. lepidotus and An. pholidotus as two different taxa. These results corroborate the clear separation of these two species using morphological characters. The mean COI divergence (8.2%) is more than two-fold the threshold reported by Hebert et al. (2003) for species delimitation (3%), reinforcing the use of barcode sequence for effective species delimitation. Sequence of rDNA ITS2 gives a similar result as can be seen on the NJ K2P tree (Fig. 2), which has 100% support. The presence of indels in ITS2, resulting in a 49 bp size difference, could even be used in a simple PCR, or PCR/RFLP diagnostic for species identification without sequencing. In addition, a BLAST search in GenBank (http://blast.ncbi.nlm.nih.gov) did not reveal homologous sequence to ours for either species, which confirms our results as the first record of DNA sequences for these species. Sequences for ITS2 are available in GenBank for some species belonging to the subgenus Kerteszia – (An. homunculus, An. cruzii s.l., An. laneanus, An. neivai, and An. bellator) but these have less than 83% similarity with our sequences. Anopheles bellator and An. cruzii have been characterized at the COI locus by Sallum et al. (2002). However, the region used by them only partially overlaps at the 3′-terminal of the barcode region used here, for this reason no comparison can be made.
Conclusion
In summary, we, along with Escobar et al. (2010), have shown that the name, An. lepidotus, historically given (e.g. Quiñones et al., 1984) to the primary malaria vector in the Department of Tolima, Colombia, and most likely throughout this part of Colombia, is incorrect and should instead be An. pholidotus. Also, an entirely new adult habitus is described for An. lepidotus, and new characters and a key to the subgenus Kerteszia are provided to distinguish it from An. pholidotus. Furthermore, this study has affirmed the benefits of combined morphological and molecular studies, continued field collections of new material, preserved morphological voucher specimens that can be sub-sampled for DNA analysis, examining morphological type specimens when working on complex taxonomic problems, and the need for adequate time to resolve complex species identification problems. This study also demonstrated the risks or potential risks taken by not making certain that a holotype, allotype, and paratypes are confirmed as con-specific, relying on identified museum specimens without confirming them, and relying on only one methodology or life stage, whether it be morphology or molecular genetics.
Dedication
This paper is dedicated to Dr. Jack Hayes (deceased), a co-principal investigator on the BOSTID grant. Jack helped organize both trips to Peru, was devoted to helping local indigenous people suffering from mosquito-borne diseases, was very friendly, compassionate, an excellent traveling companion, and a tireless worker on those trips.
Acknowledgments
We gratefully acknowledge the National Research Council, National Academy of Science, Washington, DC, for support with BOSTID Grant No. MVR-PE-4-84-35, the Walter Reed Biosystematics Unit (WRBU), National Museum of Natural History, Smithsonian Institution, Washington, DC, National Institutes of Health grant 2R01AI054139 to Jan Conn, the Instituto Nacional de Salud, Ministerio de Salud, Lima, Peru, the Naval Medical Research Institute (NAMRID), Lima Detachment, Lima, Peru, and to Kelly Swing and the staff at the Tiputini Biodiversity Station, Universidad San Francisco de Quito, who made collections of crucial specimens for this study possible. We are very grateful to LCDR Chris H. Gardiner, for local assistance in Lima and in Junín Province. The hard field and laboratory work of Roberto Falcone and Victor Zambrano in Peru was of great value to the study. Special thanks are also due Taina Litwak for the beautiful illustrations, Judith Stoffer for the photomicrographs and their preparation for publication. We also appreciate the helpful and constructive edits and comments provided by Ralph Harbach, Anice Sallum, and Yvonne-Marie Linton.
This research was performed under a Memorandum of Understanding between the Walter Reed Army Institute of Research and the Smithsonian Institution, with institutional support provided by both organizations. The material to be published reflects the views of the authors and should not be construed to represent those of the Department of the Army or the Department of Defense.
Contributor Information
BRUCE A. HARRISON, Email: skeeterdoc@gmail.com.
FREDDY RUIZ-LOPEZ, Email: ruizj@si.edu.
GUILLERMO CALDERON FALERO, Email: calderon_entomol@hotmail.com.
HARRY M. SAVAGE, Email: hms1@cdc.gov.
JAMES E. PECOR, Email: pecorj@si.edu.
References cited
- Aragão MB. Distribuição geográfica e abundancia das especies de Anopheles (Kerteszia) (Diptera, Culicidae) Revista Brasileira de Malariologia e Doenças Tropicais. 1964;16:73–109. [PubMed] [Google Scholar]
- Bates M. Observations on climate and seasonal distribution of mosquitoes in eastern Colombia. The Journal of Animal Ecology. 1945;14:17–25. [Google Scholar]
- Collucci E, Sallum MAM. Phylogenetic analysis of the subgenus Kerteszia of Anopheles (Diptera: Culicidae: Anophelinae) based on morphological characters. Insect Systematics and Evolution. 2003;34:361–372. [Google Scholar]
- Cova-García P, Pulido-F J, Escalante de Ugueto C. Anopheles (Kerteszia) hilli (Diptera, Culicidae) una nueva specie de Venezuela. Boletín de la Dirección de Malariología y Saneamiento Ambiental. 1976;17:344–53. [Google Scholar]
- Cova-García P, Pulido-F J, Escalante de Ugueto C. Anopheles (Kerteszia) gonzalezrinconesi n. sp. (Diptera, Culicidae) de Venezuela. Boletín de la Dirección de Malariología y Saneamiento Ambiental. 1977a;17:140–149. [Google Scholar]
- Cova-García P, Pulido-F J, Escalante de Ugueto C. Anopheles (Kerteszia) rollai, nomen novum Anopheles (Kerteszia) hilli Cova Garcia, 1976 (non Anopheles hilli Woodhill & Lee 1944) Boletín de la Dirección de Malariología y Saneamiento Ambiental. 1977b;17:241. [Google Scholar]
- Deane LM. World Health Organization/Malaria (WHO/MAL) 1967. Monkey malaria in Brazil, a summary of studies performed in 1964–1966; p. 20. 67.603. [PubMed] [Google Scholar]
- Deane LM. Malaria studies and control in Brazil. American Journal of Tropical Medicine and Hygiene. 1988;38:223–230. doi: 10.4269/ajtmh.1988.38.223. [DOI] [PubMed] [Google Scholar]
- Escobar J, Gonzáles R, Quiñones ML, Wilkerson RC, Harrison B. Presence of Anopheles (Kerteszia) pholidotus in a malaria focus in Colombia. In: Clark GG, Rubio-Palis Y, editors. Journal of the American Mosquito Control Association; Mosquito vector biology and control in Latin America – a 20th symposium; 2010. pp. 315–316.pp. 306–320. [DOI] [PubMed] [Google Scholar]
- Faran ME. Mosquito studies (Diptera, Culicidae) XXXIV. A revision of the Albimanus Section of the subgenus Nyssorhynchus of Anopheles. Contributions of the American Entomological Institute (Ann Arbor) 1980;15:1–215. [Google Scholar]
- Forattini OP. Entomologia Médica Parte geral, Diptera, Anophelinae. Vol. 1. São Paulo, Brazil: 1962. p. 662. [Google Scholar]
- Forattini OP. Culicidologia Médica Identificação, biologia, epidemiologia. Vol. 2. São Paulo, Brazil: 2002. p. 864. [Google Scholar]
- González R, Carrejo N. Introdución al estudio taxonómico de Anopheles de Colombia, claves y notas de distribución. Programa Editorial Universidad del Valle; Cali, Colombia: 2009. p. 260. [Google Scholar]
- Harbach RE, Kitching IJ. Phylogeny and classification of the Culicidae (Diptera) Systematic Entomology. 1998;23:327–370. [Google Scholar]
- Harbach RE, Knight KL. Taxonomists’ glossary of mosquito anatomy. Plexus Publishing, Inc.; Marlton, N.J., U.S.A: 1980. p. 415. [Google Scholar]
- Harbach RE, Knight KL. Corrections and additions to Taxonomists’ glossary of mosquito anatomy. Mosquito Systematics. 1982;13:201–217. [Google Scholar]
- Harbach RE, Navarro JC. A new species of Anopheles subgenus Kerteszia (Diptera: Culicidae) from Venezuela. Entomologica Scandinavica. 1996;27:207–216. [Google Scholar]
- Hayes J, Calderón G, Falcon R, Zambrano V. New incriminated anopheline vectors of human malaria parasites in Junín Department, Peru. Journal of the American Mosquito Control Association. 1987;3:418–422. [PubMed] [Google Scholar]
- Hebert PD, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proceedings of the Royal Society of London, Series B. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 1980;6:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Knight KL, Stone A. A catalog of the mosquitoes of the world (Diptera: Culicidae) 2nd. Thomas Say Foundation, Entomological Society of America; 1977. pp. 6, xi–611. [Google Scholar]
- Komp WHW. The species of the subgenus Kerteszia of Anopheles. Annals of the Entomological Society of America. 1937;30:492–529. [Google Scholar]
- Komp WHW. Notes on mosquitoes from an area of endemic Yellow fever in Colombia. Proceedings of the Entomological Society of Washington. 1956;58:37–42. [Google Scholar]
- Komp WHW, Orsono-M E. The male and larva of Anopheles (Kerteszia) boliviensis Theobald. Annals of the Entomological Society of America. 1936;29:415–419. [Google Scholar]
- Kumar S, Dudley J, Nei M, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Briefings in Bioinformatics. 2008;9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J. Neotropical Culicidae. Vol. 1. São Paulo, Brazil: 1953. p. 548. [Google Scholar]
- Li C, Wilkerson RC. Identification of Anopheles (Nyssorhynchus) albitarsis complex species (Diptera: Culicidae) using rDNA ITS2-based PCR primers. Memorias do Institute Oswaldo Cruz. 2005;100:495–500. doi: 10.1590/s0074-02762005000500009. [DOI] [PubMed] [Google Scholar]
- Linthicum KJ. A revision of the Argyritarsis Section of the subgenus Nyssorhynchus of Anopheles (Diptera: Culicidae) Mosquito Systematics. 1988;20:98–271. [Google Scholar]
- Linton YM, Harbach RE, Chang MS, Anthony TG, Matusop A. Morphological and molecular identity of Anopheles (Cellia) sundaicus (Diptera: Culicidae), the nominotypical member of a malaria vector species complex in Southeast Asia. Systematic Entomology. 2001;26:357–366. [Google Scholar]
- Maddison DR, Maddison WR. MacClade v.4.06: Analysis of phylogeny and character evolution. Sinauer Associates; Sunderland, MA: 2003. [DOI] [PubMed] [Google Scholar]
- Malafronte RDS, Marrelli MT, Ramirez CL, Nassar MN, Marinotti O. Intraspecific variation of second internal transcribed spacer of nuclear ribosomal DNA among populations of Anopheles (Kerteszia) cruzii (Diptera: Culicidae) Journal of Medical Entomology. 2007;44:538–542. doi: 10.1603/0022-2585(2007)44[538:ivosit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Navarro JC, Ventura FD, Zorrilla A, Liria J. Registros de mayor altitud para mosquitos (Diptera: Culicidae) en Venezuela. Revista de Biologia Tropical (International Journal of Tropical Biology. ISSN-0034-7744) 2010;58:245–254. [PubMed] [Google Scholar]
- Peyton EL, Wilkerson RC, Harbach RE. Comparative analysis of the subgenera Kerteszia and Nyssorhynchus of Anopheles (Diptera: Culicidae) Mosquito Systematics. 1992;24:51–69. [Google Scholar]
- Quiñones ML, Suárez MF, Rodriguez A, Fleming GA, Galvis LE. Comportamiento de Anopheles (Kerteszia) lepidotus Zavortink, 1973, y su incriminación como posible vector de malaria en el Departamento del Tolima, Colombia. Biomedica. 1984;4:5–13. [Google Scholar]
- Ramirez CCL, Dessen BEM. Chromosomal evidence for sibling species of the malaria vector Anopheles cruzii. Genome. 2000a;43:143–151. doi: 10.1139/g99-103. [DOI] [PubMed] [Google Scholar]
- Ramirez CCL, Dessen BEM. Chromosome differentiated populations of Anopheles cruzii: evidence for a third sibling species. Genetica. 2000b;108:73–80. doi: 10.1023/a:1004020904877. [DOI] [PubMed] [Google Scholar]
- Reinert JF. Medical Entomology Studies – XVII. Biosystematics of Kenknightia, a new subgenus of the mosquito genus Aedes Meigen from the Oriental Region (Diptera: Culicidae) Contributions of the American Entomological Institute (Gainesville) 1990;26(2):1–119. [Google Scholar]
- Reinert JF. List of abbreviations for currently valid generic-level taxa in family Culicidae (Diptera) European Mosquito Bulletin. 2009;27:68–76. [Google Scholar]
- Reinert JF, Harbach RE, Kitching IJ. Phylogeny and classification of Ochlerotatus and allied taxa (Diptera: Culicidae: Aedini) based on morphological data from all life stages. Zoological Journal of the Linnean Society. 2008;153:29–114. [Google Scholar]
- Rona LDP, Carvalho-Pinto CJ, Gentile C, Grisard EC, Peixoto AA. Assessing the molecular divergence between Anopheles (Kerteszia) cruzii populations from Brazil using the timeless gene: Further evidence of a species complex. Malaria Journal. 2009;8:60. doi: 10.1186/1475-2875-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rona LDP, Carvalho-Pinto CJ, Peixoto AA. Molecular evidence for the occurrence of a new sibling species within the Anopheles (Kerteszia) cruzii complex in South-East Brazil. Malaria Journal. 2010;9:33. doi: 10.1186/1475-2875-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz F, Linton YM, Ponsonby DJ, Conn JE, Herrera M, Quiñones ML, Wilkerson RC. Molecular comparison of topotypic specimens confirms Anopheles (Nyssorhynchus) dunhami Causey (Diptera: Culicidae) in the Colombian Amazon. Memories do Institute Oswaldo Cruz. 2010;105:899–903. doi: 10.1590/s0074-02762010000700010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sallum MAM, Dos Santos CLS, Wilkerson RC. Studies on Anopheles (Kerteszia) homunculus Komp (Diptera: Culicidae) Zootaxa. 2009;2299:1–18. [Google Scholar]
- Sallum MAM, Forattini OP, Wilkerson RC. Redescription of the adult and larva and first description of the pupa of Anopheles (Kerteszia) laneanus. Journal of the American Mosquito Control Association. 2000;16:86–92. [PubMed] [Google Scholar]
- Sallum MAM, Schultz TR, Foster PG, Aronstein K, Wirtz RA, Wilkerson RC. Phylogeny of Anophelinae (Diptera: Culicidae) based on nuclear ribosomal and mitochondrial DNA sequences. Systematic Entomology. 2002;27:361–382. [Google Scholar]
- Stone A, Knight KL, Starcke H. A synoptic catalog of the mosquitoes of the world (Diptera, Culicidae). Thomas Say Foundation, Entomological Society of America. 1959;6:358. [Google Scholar]
- Sulzer AJ, Cantella R, Colichon A, Gleason NN, Walls KW. A focus of hyperendemic Plasmodium malariae – P. vivax with no P. falciparum in a primitive population in the Peruvian Amazon jungle. Bulletin of the World Health Organization. 1975;52:273–278. [PMC free article] [PubMed] [Google Scholar]
- Sulzer AJ, Sulzer KR, Cantella R, Colichon H, Latorre CR, Welch M. Study of coinciding foci of malaria and leptospirosis in the Peruvian Amazon area. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1978;72:76–83. doi: 10.1016/0035-9203(78)90305-x. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*: phylogenetic analysis using parsimony, version 4.0b 10. Sinauer Associates; Sunderland, MA: 2003. [Google Scholar]
- Tan CH, Vythilingam V, Matusop A, Chan ST, Singh B. Bionomics of Anopheles latens in Kapit, Sarawak, Malaysian Borneo in relation to the transmission of zoonotic simian malaria parasite Plasmodium knowlesi. Malaria Journal. 2008;7:52. doi: 10.1186/1475-2875-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Mizusawa K, Saugstad ES. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara Islands) and Korea (Diptera: Culicidae) Contributions of the American Entomological Institute (Ann Arbor) 1979;16:i–vii. 1–987. [Google Scholar]
- Theobald FV. A catalogue of the Culicidae in the Hungarian National Museum with descriptions of new genera and species. Annales Historico-Naturales Musei Nationalis Hungarici. 1905;3:61–120. [Google Scholar]
- Vythilingam I, Yusuf MNA, Tan CH, Adela IJ, Yusof MY, Abdul HA, Ismail NP, Abdullah NR, Sulaiman LH. Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasites and Vectors. 2008;1:26. doi: 10.1186/1756-3305-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson RC, Peyton EL. Standardized nomenclature for the costal wing spots of the genus Anopheles and the other spotted-wing mosquitoes (Diptera: Culicidae) Journal of Medical Entomology. 1990;27:207–224. [Google Scholar]
- Wilkerson RC, Peyton EL. The Brazilian malaria vector Anopheles (Kerteszia) cruzii: life stages and biology (Diptera: Culicidae) Mosquito Systematics. 1991;23:110–122. [Google Scholar]
- Wood DM, Dang PT, Ellis RA. The insects and arachnids of Canada part 6. The mosquitoes of Canada, Diptera: Culicidae. Vol. 1686. Biosystematics Research Institute, Canada Department of Agriculture Publication; 1979. p. 390. [Google Scholar]
- Zavortink TJ. Mosquito Studies (Diptera, Culicidae) XXIX. A review of the subgenus Kerteszia of Anopheles. Contributions of the American Entomological Institute (Ann Arbor) 1973;9:1–54. [Google Scholar]


